Sentinel node mapping in thyroid cancer: an overview
- 1Department of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy
- 2Endocrine Surgery Unit, Department of Surgery, University Hospital of Pisa, Pisa, Italy
In this paper we describe the current status of sentinel node mapping (SNM) in thyroid tumors and its potential perspectives. SNM in thyroid cancer has been tested since the end of the twentieth century, mainly in papillary thyroid cancer (PTC) and in medullary thyroid cancer (MTC). In PTC, it has been employed to find occult lymph node metastases in the central compartment of the neck as an alternative or indication for prophylactic dissection, by several methods. All of them have proven effective in spotting sentinel nodes, but the results have been somewhat diminished by uncertainty about the clinical significance of occult metastases in differentiated thyroid cancer. SNM in MTC has also been used to find occult lymph node metastases in the lateral compartments of the neck, also with excellent results hindered by a similar doubt about the real clinical significance of MTC micrometastases. Well designed, adequately sized randomized controlled trials are lacking, so SNM in thyroid tumors remains an interesting yet experimental methodology. New technology is emerging that could facilitate such studies, which could add solid information about the clinical significance of occult neck metastases in thyroid cancer.
Introduction
Sentinel node mapping (SNM) is a widely used surgical technique to find subclinical lymph node metastases in apparently unaffected node basins in a variety of primary tumors. Here we report the current status of SNM in thyroid tumors and present our updated experience with it in medullary thyroid carcinoma.
Sentinel node mapping in thyroid cancer
The sentinel node concept has been known since the second half of the twentieth century (1); the sentinel node is thought to represent the first lymphatic station draining from a primary tumor. This concept has been then applied to a variety of solid tumors, including thyroid tumors (2). The rationale for its use is to identify clinically occult metastases without resorting to a prophylactic lymph node dissection, which is associated with non-neglectable rates of complications and higher costs.
The rationale for SNM in thyroid cancer
Preoperative evaluation of lymph nodal status in the neck relies on imaging, especially on preoperative high-frequency ultrasounds (US), which is, in experienced hands, effective in evaluating lateral compartments of the neck, but less so for the central compartment (3–5), which often is the first echelon of node metastasis from thyroid tumors. In his review, Xue et al. (3) reported that the accuracy of US is only 48.3%, while the sensitivity for predicting central lymph node metastasis is only 22.6–55%. The intraoperative exploration of neck compartments by the surgeon is also less than satisfying in detecting micrometastases (6, 7).
Since aim of thyroid cancer surgery is removal of the thyroid and affected lymph nodes, three alternative options exists for thyroid tumors with no clinical evidence of node metastases (cN0): (a) avoid dissection of nodes in favor of observation, with a certain risk of disease persistence/recurrence in case of occult metastases; (b) perform a prophylactic neck dissection, based on specific risk factors, with the risk of surgical overtreatment and related increase in morbidity; (c) SNM, with SN biopsy and therapeutic compartmental lymphadenectomy if the SN harbor a metastasis.
The concept of SNM has been excellently summarized by Dr. Expòsito Rodriguez: sentinel node mapping is the intraoperative complement of a negative US scan (8). In thyroid cancer, the SNM is a clue to accurate N staging while reducing surgical dissection, associated costs, and morbidity (9), while SNM has nearly null rates of related complications (10). A positive SNB could guide the extent of surgery, limiting lateral compartmental dissection only for therapeutic purposes (10–13) and personalize postoperative radiometabolic treatment (8, 14–17). Furthermore, SNM provides the ability to visualize altered lymphatic pathways and individual lymphatic patterns (18).
The methods of SNM in thyroid cancer
The methods commonly used for harvesting the sentinel nodes in thyroid cancer can be schematized as follows:
(A) Visual tracers (e.g., blue dye, carbon nanoparticles and indocyanine green)
(B) Radioactive tracers (e.g., 99mTc saline solution, 99mTc-phytate and 99mTc-nanocolloid albumin)
(C) Hybrid tracers (e.g., nanocolloid albumin and methylene blue, indocyanine green and nanocolloid albumin); and
(D) Magnetic tracers, such superparamagnetic iron oxide (SPIO) nanoparticles (19, 20).
Besides, new tracers and techniques are currently under development and evaluation, such (68)Ga-tilmanocept PET/CT (21). These methods differ in the tracer employed, instruments necessary to identify the sentinel nodes, and logistics between the tracer injection and the surgical operation. Even studies using a similar method can differ in tracer amount and injection sites, timing from injection to optimal node spotting, and other variables. As a consequence, the comparison of the results between the various studies should be cautious, since small differences in detail could explain different results between the studies.
Nevertheless, some attempts have been made to compare the various techniques. Garau et al. (22) presented a meta-analysis, which included 45 studies, comparing 4 kind of procedure: vital-dye (VD) alone, Tc-nanocolloid planar lymphoscintigraphy with the use of intraoperative hand-held gamma probes (LS), both Tc-nanocolloid planar lymphoscintigraphy with intraoperative gamma probe and VD (LS + VD), Tc-nanocolloid planar lymphoscintigraphy with preoperative SPECT/CT, and intraoperative gamma probe (LS-SPECT/CT). Tc nanocolloid was superior to methylene blue, and the addition of SPECT/CT improved identification of metastatic SLNs outside the central neck (22). Gelmini et al. (23) conducted a prospective, non-randomized study comparing blue dye SNM (40 patients), lymphoscintigraphy (5 patients) and the combined technique (40 patients) with apparently better results with the single tracer techniques. In the recent years some surgeons have abandoned patent blue dye in favor of nano colloidal albumin: blue dye is economically advantageous and does not need a Nuclear Medicine facility, but is more difficult to manipulate intraoperatively since it can easily stain the operative field, gloves, swabs, parathyroid glands, and finally hide from sight the recurrent laryngeal nerve. Radioisotope SNM, on the other hand, localizes better SN in the lateral compartments, does not fix in parathyroid glands and does not blur the vision of operative field, so it is preferred, when available.
A new technical adjunct to limit the false negative rate of SNM on frozen sections is one-step nucleic acid amplification (OSNA), which allows real-time (intraoperative) detection of mRNA encoding for cytokeratin 19; OSNA has been tested for papillary thyroid carcinoma (24, 25), with satisfying results.
The results of SNM in differentiated thyroid cancer
Overall, although large prospective RCT are lacking, data reported in the literature attest that SNM is an effective method for intercepting most clinically occult metastases in approximately 30–40% of cases (8, 10–13, 15–17, 22, 23), both in the central and lateral neck, so that SNM could serve as an objective indicator for RAI or to modulate radioactivity doses, or for therapeutic compartmental dissection. The controversy arises when we look at the clinical usefulness of SNM, since no differences appear in observed relapse rates and disease specific survival when applying SNM, prophylactic dissection, or simple observation (26). We have tested SNM in a more aggressive subset of differentiated thyroid carcinomas, those harboring a V600E BRAF mutation (27). Even in this group with a potentially higher loco-regional relapse rate, we observed no structural or biological relapse after a long follow-up (the current mean follow-up is 7 years).
Opponents of SNM in thyroid cancer report a high false negative rate compared with prophylactic dissection (5); another estimation study argued that the higher costs for implementing SNM outweighed potential savings (28).
SNM in medullary thyroid cancer
We found it particularly interesting to apply SNM in medullary thyroid cancer (MTC) for several reasons: MTC is a lymphophilic tumor with early nodal spread; the most effective therapy is initial surgery, while RAI has no effect on this C-cell derived tumor; and persistence of disease can be detected by a specific circulating marker, calcitonin (CT), which can also reveal minimal residual disease after calcium infusion stimulation. Since central compartment dissection is considered an integral part of initial surgery even for cN0 cases (29, 30), SNM in MTC applies to lateral compartments of the neck, for which a prophylactic dissection remains controversial (30). Some surgeons perform lateral dissection according to one or more risk factors for the presence of lateral neck metastases form MTC, such preoperative CT levels, tumor diameter, presence and number of central compartment nodal metastases, postoperative basal and stimulated CT levels, and histologic features of the primary tumor including desmoplastic reaction; however most surgeons consider lateral dissection only in the presence of clinically evident metastatic nodes, since prophylactic lateral dissection carries a potential risk of surgical complications and related morbidity from unnecessary LND. A recent study comparing prophylactic lateral neck dissection vs. no lateral dissection in cN0 MTC patients with CT levels > 200 pg/ml found that prophylactic dissection is not associated with improved survival (31).
The results of SNM in medullary thyroid cancer
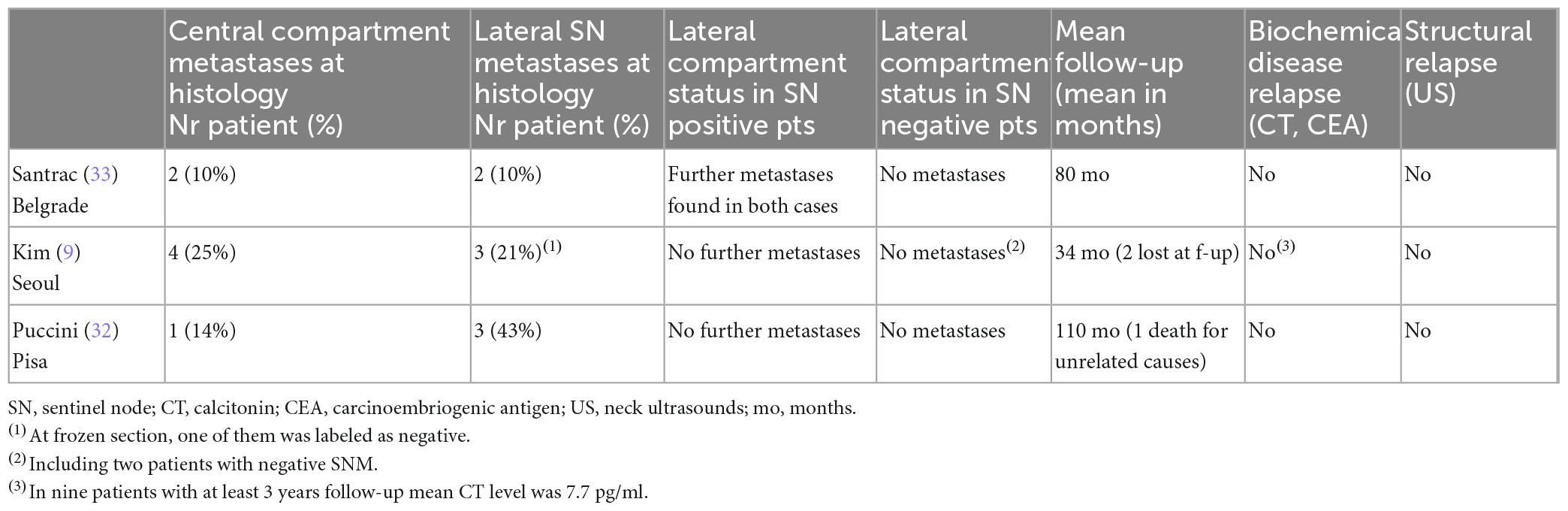
We found only two studies on SNM for cN0 MTC, besides ours (9, 32, 33). In this paper we report an update of our cases. All three studies are limited in number of patients, and used different methods for SNM: two centers used a radiotracer (Pisa: 99mTc-nanocolloid-albumin; Seoul: 99mTc-Phytate), while Belgrade used blue dye. Tumor characteristics were relatively similar. The sentinel node identification rate was excellent, but the rate of metastatic SN was higher in Pisa. The biopsy was analyzed by definitive histology in Pisa, and by frozen section in the other two centers. After the biopsy, the surrounding tissue (the level in which the sentinel node was detected) was also removed in Pisa and Belgrade (in case of negative SN on frozen section), the whole compartment in case of metastatic sentinel at frozen section in Belgrade, while in Seoul the biopsy was always followed by a lateral compartment dissection, regardless of frozen section results. The studies differ also in terms of non-sentinel metastatic nodes and in central compartment metastases (Table 1). Of note, in all cases, when the sentinel node was non-metastatic, no other metastases were found in the lateral neck (as in the two Korean cases where a sentinel node was not detected).

Table 1. Studies evaluating sentinel node mapping (SNM) of lateral compartment in medullary thyroid cancer – methods.
The effects of these studies are shown in Table 2: there is no evidence of relapse even at the longer follow-up in our series (mean, 110 months). CT and CEA levels are undetectable both in Pisa and in Belgrade, while Kim et al. (9) report low but detectable (probably non-stimulated) CT levels for 9 patients with a mean follow-up of 3 years. This should be evaluated with caution, but it is not clearly representative of a residual disease. In our series, the original plan was to practice a therapeutic lateral dissection in case of metastatic sentinel node, but until now no patient has undergone salvage surgery since biological evidence of relapse is lacking (according to basal and stimulated CT levels). We registered one death due to the rupture of a cerebral aneurism. In these limited experiences, no occult metastases were detected when the sentinel node was tumor-free.
The considerations regarding SNM in medullary thyroid cancer are surprisingly similar to those regarding differentiated cancer: SNM is very efficient in identifying occult metastases in the lateral compartment, and apparently if the sentinel nodes are unaffected, the risk of occult metastases in other nodes seems extremely low. The data published suggest that SNM of the lateral compartments with therapeutic dissection only in case of positive sentinel node could be equivalent to prophylactic dissection, but the limited volume of patients tested and the structure of the studies represent strong bias and prevent any conclusions, and impose the need for prospective, randomized multicenter studies with a standardized technique and a sufficient statistical power to eventually include SNM in the guidelines for surgical management of MTC.
In conclusion, SNM for thyroid cancer remains an interesting yet experimental methodology, which has been proven to be safe and effective in identifying occult metastases, and has potential applications whose clinical impact has still to be demonstrated.
The ideal method should be economically and logistically affordable, relatively easy to practice, with a quick learning curve, the identification of sentinel nodes should have minimal interobserver variation, and should be safe for patients. It should be non-inferior to prophylactic resections, and superior to simple observation, especially in terms of long-term oncologic outcomes.
The continuing advances in technology are offering new tracers and methods for performing practical and cost-effective SNM, but the main challenge remains to demonstrate a consistent impact on the clinical management of thyroid cancer patients.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MP: concept and writing of the manuscript. CA and LD: corrections and data extraction. LR: literature search. GM: supervision and critical revision. All authors contributed to the article and approved the submitted version.
Funding
This manuscript received funding by the University of Pisa, Direzione Area di Medicina.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gould EA, Winship T, Philbin PH, Kerr HH. Observations on a “sentinel node” in cancer of the parotid. Cancer. (1960) 13:77–8. doi: 10.1002/1097-0142(196001/02)13:13.0.co;2-d
2. Kelemen PR, Van Herle AJ, Giuliano AE. Sentinel lymphadenectomy in thyroid malignant neoplasms. Arch Surg. (1998) 133:288–92. doi: 10.1001/archsurg.133.3.288
3. Xue S, Wang P, Hurst ZA, Chang YS, Chen G. Active surveillance for papillary thyroid microcarcinoma: challenges and prospects. Front Endocrinol (Lausanne). (2018) 9:736. doi: 10.3389/fendo.2018.00736
4. Zhao H, Li H. Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: Diagnosis of central and lateral compartment nodal metastases. Eur J Radiol. (2019) 112:14–21. doi: 10.1016/j.ejrad.2019.01.006
5. Huang O, Wu W, Wang O, You J, Li Q, Huang D, et al. Sentinel lymph node biopsy is unsuitable for routine practice in younger female patients with unilateral low-risk papillary thyroid carcinoma. BMC Cancer. (2011) 11:386. doi: 10.1186/1471-2407-11-386
6. Scherl S, Mehra S, Clain J, Dos Reis LL, Persky M, Turk A, et al. The effect of surgeon experience on the detection of metastatic lymph nodes in the central compartment and the pathologic features of clinically unapparent metastatic lymph nodes: what are we missing when we don’t perform a prophylactic dissection of central compartment lymph nodes in papillary thyroid cancer? Thyroid. (2014) 24:1282–8. doi: 10.1089/thy.2013.0600
7. Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. (1999) 229:880–7; discussion 887–8. doi: 10.1097/00000658-199906000-00016
8. Expósito Rodríguez A, Corta Gómez I, Domínguez Ayala M, García Carrillo M, González García AI, Gutiérrez Rodríguez MT, et al. Sentinel lymph node biopsy in papillary thyroid cancer: Accuracy and application in clinical practice. Cir Esp (Engl Ed). (2022) 100:416–21. doi: 10.1016/j.cireng.2022.04.022
9. Kim MJ, Back K, Choe JH, Kim JH, Kim JS. Feasibility of lateral sentinel lymph node biopsy in medullary thyroid cancer: Surrogate tool for determining prophylactic lateral neck dissection-A pilot study. Head Neck. (2021) 43:3276–86. doi: 10.1002/hed.26808
10. Cunningham DK, Yao KA, Turner RR, Singer FR, Van Herle AR, Giuliano AE. Sentinel lymph node biopsy for papillary thyroid cancer: 12 years of experience at a single institution. Ann Surg Oncol. (2010) 17:2970–5. doi: 10.1245/s10434-010-1141-x
11. Lee SK, Kim SH, Hur SM, Choe JH, Kim JH, Kim JS. The efficacy of lateral neck sentinel lymph node biopsy in papillary thyroid carcinoma. World J Surg. (2011) 35:2675–82. doi: 10.1007/s00268-011-1254-9
12. Delgado-Oliver E, Vidal-Sicart S, Martínez D, Squarcia M, Mora M, Hanzu FA, et al. Applicability of sentinel lymph node biopsy in papillary thyroid cancer. Q J Nucl Med Mol Imaging. (2020) 64:400–5. doi: 10.23736/S1824-4785.18.03097-2
13. Pelizzo MR, Boschin IM, Toniato A, Bernante P, Piotto A, Rinaldo A, et al. The sentinel node procedure with Patent Blue V dye in the surgical treatment of papillary thyroid carcinoma. Acta Otolaryngol. (2001) 121:421–4. doi: 10.1080/000164801300103012
14. Cranshaw IM, Carnaille B. Micrometastases in thyroid cancer. An important finding? Surg Oncol. (2008) 17:253–8. doi: 10.1016/j.suronc.2008.04.005
15. Roh JL, Park CI. Sentinel lymph node biopsy as guidance for central neck dissection in patients with papillary thyroid carcinoma. Cancer. (2008) 113:1527–31. doi: 10.1002/cncr.23779
16. Saliba J, Payne RJ, Varshney R, Sela E, Maniakas A, Rahme E, et al. Sentinel lymph node biopsy status correlates with postoperative stimulated thyroglobulin levels in low-risk papillary thyroid cancer patients. Endocr Pract. (2014) 20:399–404. doi: 10.4158/EP13121.OR
17. Zhao J, Zhao Y, Ling Y, Kang H. Risk factors of central lymph node metastasis in papillary thyroid microcarcinoma and the value of sentinel lymph node biopsy. Front Surg. (2021) 8:680493. doi: 10.3389/fsurg.2021.680493
18. Süslü NS, Katar O, Tuncel M. Role of indocyanine green combined with radiotracer-Technetium 99m in neck surgery for primary and recurrent head and neck cancer: preliminary results of a tertiary cancer center. Eur Arch Otorhinolaryngol. (2022) 279:1549–60. doi: 10.1007/s00405-021-06931-1
19. Baena Fustegueras JA, González FH, Calderó SG, de la Fuente Juárez MC, López SR, Riu FR, et al. Magnetic detection of sentinel lymph node in papillary thyroid carcinoma: The MAGIC-PAT study results. Eur J Surg Oncol. (2019) 45:1175–81. doi: 10.1016/j.ejso.2019.03.017
20. Ríos A, Rodríguez JM, Ibañez N, Piñero A, Parrilla P. Detection of the sentinel node using a magnetic tracer in thyroid cancer. A technical pilot study. Cir Esp (Engl Ed). (2019) 97:169–74. doi: 10.1016/j.ciresp.2018.12.003
21. de Vries LH, Lodewijk L, de Keizer B, Borel Rinkes IH, Vriens MR. Sentinel lymph node detection in thyroid carcinoma using (68)Ga-tilmanocept PET/CT: a proof-of-concept study protocol. Future Oncol. (2022) 18:3493–9. doi: 10.2217/fon-2022-0165
22. Garau LM, Rubello D, Morganti R, Boni G, Volterrani D, Colletti PM, et al. Sentinel lymph node biopsy in small papillary thyroid cancer: a meta-analysis. Clin Nucl Med. (2019) 44:107–18. doi: 10.1097/RLU.0000000000002378
23. Gelmini R, Campanelli M, Cabry F, Franceschetto A, Ceresini G, Ruffini L, et al. Role of sentinel node in differentiated thyroid cancer: a prospective study comparing patent blue injection technique, lymphoscintigraphy and the combined technique. J Endocrinol Invest. (2018) 41:363–70. doi: 10.1007/s40618-017-0756-1
24. Iglesias Felip C, Zafon Llopis C, Temprana-Salvador J, García-Burillo A, Serres Créixams X, Caubet Busquet E, et al. One-step nucleic acid amplification for intraoperative analysis of sentinel lymph node in papillary thyroid carcinoma. Eur J Endocrinol. (2019) 180:21–9. doi: 10.1530/EJE-18-0624
25. Kaczka KA, Pomorski L. One-step nucleic acid amplification analysis of sentinel lymph nodes in papillary thyroid cancer patients. Arch Med Sci. (2017) 13:1416–26. doi: 10.5114/aoms.2017.65466
26. Ahn JH, Kwak JH, Yoon SG, Yi JW, Yu HW, Kwon H, et al. A prospective randomized controlled trial to assess the efficacy and safety of prophylactic central compartment lymph node dissection in papillary thyroid carcinoma. Surgery. (2022) 171:182–9. doi: 10.1016/j.surg.2021.03.071
27. Puccini M, Manca G, Neri CM, Boni G, Coli V, Garau LM, et al. Effect of sentinel node biopsy in clinically N0, BRAF V600E-mutated, small papillary thyroid carcinoma: a pilot study. Clin Nucl Med. (2019) 44:359–64. doi: 10.1097/RLU.0000000000002465
28. Balasubramanian SP, Brignall J, Lin HY, Stephenson TJ, Wadsley J, Harrison BJ, et al. Sentinel node biopsy in papillary thyroid cancer–what is the potential? Langenbecks Arch Surg. (2014) 399:245–51. doi: 10.1007/s00423-014-1168-8
29. Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. (2010) 95:2655–63. doi: 10.1210/jc.2009-2368
30. Patel KN, Yip L, Lubitz CC, Grubbs EG, Miller BS, Shen W, et al. The American association of endocrine surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. (2020) 271:e21–93. doi: 10.1097/SLA.0000000000003580
31. Spanheimer PM, Ganly I, Chou JF, Capanu M, Nigam A, Ghossein RA, et al. Prophylactic lateral neck dissection for medullary thyroid carcinoma is not associated with improved survival. Ann Surg Oncol. (2021) 28:6572–9. doi: 10.1245/s10434-021-09683-8
32. Puccini M, Manca G, Ugolini C, Candalise V, Passaretti A, Bernardini J, et al. Interest of sentinel node biopsy in apparently intrathyroidal medullary thyroid cancer: a pilot study. J Endocrinol Invest. (2014) 37:829–34. doi: 10.1007/s40618-014-0112-7
Keywords: sentinel lymph node, thyroid cancer, medullary thyroid cancer, papillary thyroid cancer, radioguide, indocyanine green, lymph node dissection
Citation: Puccini M, Ambrosini CE, Rossi L, De Napoli L and Materazzi G (2023) Sentinel node mapping in thyroid cancer: an overview. Front. Med. 10:1163151. doi: 10.3389/fmed.2023.1163151
Received: 10 February 2023; Accepted: 30 May 2023;
Published: 20 June 2023.
Edited by:
Ramin Sadeghi, Mashhad University of Medical Sciences, IranReviewed by:
Nada Santrac, Institute of Oncology and Radiology of Serbia, SerbiaGian Luigi Canu, University of Cagliari, Italy
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), Switzerland
Copyright © 2023 Puccini, Ambrosini, Rossi, De Napoli and Materazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Puccini, marco.puccini@unipi.it
 Marco Puccini
Marco Puccini Carlo Enrico Ambrosini2
Carlo Enrico Ambrosini2  Leonardo Rossi
Leonardo Rossi Luigi De Napoli
Luigi De Napoli