Autoimmune bullous diseases during COVID-19 pandemic: 2022 update on rituximab and vaccine
- 1Molecular and Cell Biology Laboratory, Istituto Dermopatico dell’Immacolata (IDI)-IRCCS, Rome, Italy
- 2Dermatology Unit, Istituto Dermopatico dell’Immacolata (IDI)-IRCCS, Rome, Italy
Autoimmune bullous diseases (AIBDs) are a heterogeneous group of life-threatening disorders associated with subepidermal or intraepidermal blistering. Skin barrier alterations and prolonged immunosuppressive treatments increase the risk of infections in patients with AIBDs, who are considered fragile. COVID-19 pandemic had a heavy impact on these patients. Although advances have been made in terms of prevention and treatment of COVID-19, this topic remains significant as the pandemic and its waves could last several years and, so far, a relevant proportion of the population worldwide is not vaccinated. This review is a 2022 update that summarizes and discusses the pandemic’s burden on AIBD patients mainly considering relevant studies in terms of: (i) sample dimension; (ii) quality of control populations; (iii) possible standardization by age, gender and country. The findings show that: (i) the risk of COVID-19 infection and its severe course were comparable in AIBD patients and in the general population, except for rituximab-treated patients that presented a higher risk of infection and severe disease; (ii) the mortality rate in COVID-19-infected bullous pemphigoid patients was higher than in the general population, (iii) 121 cases of AIBD onset and 185 cases of relapse or exacerbation occurred after COVID-19 vaccination and a causal relationship has not been demonstrated so far. Altogether, acquired knowledge on COVID-19 pandemic could also be important in possible, albeit undesirable, future pandemic scenarios.
Introduction
Since December 2019 an outbreak of the novel coronavirus (SARS-CoV-2) disease has quickly spread worldwide. The infectious disease and pneumonia caused by this virus is called 2019 coronavirus diseases (COVID-19). Globally, as of 25 November 2022, there have been 636,440,663 confirmed cases of COVID-19 (7.96% of the world population), including 6,606,624 deaths (1.04% of confirmed cases), reported to the World Health Organization (1). Hospitalization is needed for 3–20% of COVID-19 patients; among these, 10–30% require intensive care (2, 3). Age, obesity, male sex, and comorbidities such as hypertension, heart disease, diabetes, kidney failure, and chronic pulmonary disease are considered as risk factors (4–10). Autoimmune bullous diseases (AIBDs) are a heterogeneous group of disorders including bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), pemphigus, pemphigoid gestationis (PG), linear IgA bullous dermatosis (LABD). These disorders are associated with subepidermal or intraepidermal blistering and their treatment usually involves specific immunosuppressive therapies. Due to skin barrier alterations and chronic immunosuppressive treatments, patients are potentially at greater risk of infections, and they are considered fragile.
This review aims to discuss the pandemic’s burden on AIBD patients in terms of (i) susceptibility to COVID-19 infection; (ii) the relationship between infection and disease course and treatment; (iii) possible effects of vaccination in terms of induction, exacerbation or relapse of autoimmune disease.
Methods
To analyze the prevalence of COVID-19 in AIBD patients, and the effect exerted by immunosuppressive treatments in AIBD patients we qualitatively selected studies based on: (i) sample size; (ii) quality of control populations; (iii) standardization by age, gender, and country. To illustrate the spectrum of reported cases of new onset, relapse and/or worsening of AIBDs after COVID-19 vaccination all published cases found in PubMed searches up to November 2022 have been included in the present review.
Prevalence of COVID-19 in AIBDs patients
A recent systematic review analyzed the proportion of AIBD patients with COVID-19 symptoms and positive molecular test (11). This review included 732 subjects affected by AIBDs (409 not specified, 211 pemphigus, 112 pemphigoid). Almost all information was collected via phone/telemedicine visits. COVID-19 symptoms were reported in 70 (9.5%) patients, and the diagnosis was confirmed in 16 (2.2%) patients (11). At present, 11 relevant studies reported prevalence data on SARS-CoV-2 infection in AIBD patients, with a pooled total of 10,060 patients (Table 1). COVID-19 symptoms were observed in 256 patients (2.5%), of whom 162 (1.6%) had a confirmed diagnosis (12–22) (Table 1). In order to compare prevalence data between AIBD patients and the general population, two recent studies performed a standardization by age, gender, country, or region of interest and period involved (12, 13). Kridin et al. carried out a retrospective cohort study on 3,081 patients with BP and pemphigus from the data set of Clalit Health Services in Israel. The adjusted hazard ratio (HR) between confirmed COVID-19 patients and controls was 1.1 [95% confidence interval (CI):0.71–1.71] for BP, 0.79 (95% CI: 0.43–1.46) for pemphigus (13). In parallel, 49 dermatology departments in France enrolled 5,180 AIBD patients and found that 29 (0.6%) had a confirmed COVID-19 diagnosis while 59 (1.1%) had possible, probable, or confirmed COVID-19 diagnosis. The standardized incidence ratio of hospitalized confirmed COVID-19 infection was 0.42 (95% CI: 0.20–0.80) for BP, 1.02 (95% CI: 0.37–2.26) for pemphigus, and 1.18 (95% CI: 0.55–2.23) for MMP (12). Altogether, these studies confirmed that the risk of COVID-19 infection in AIBDs patients was not higher than in the general population.
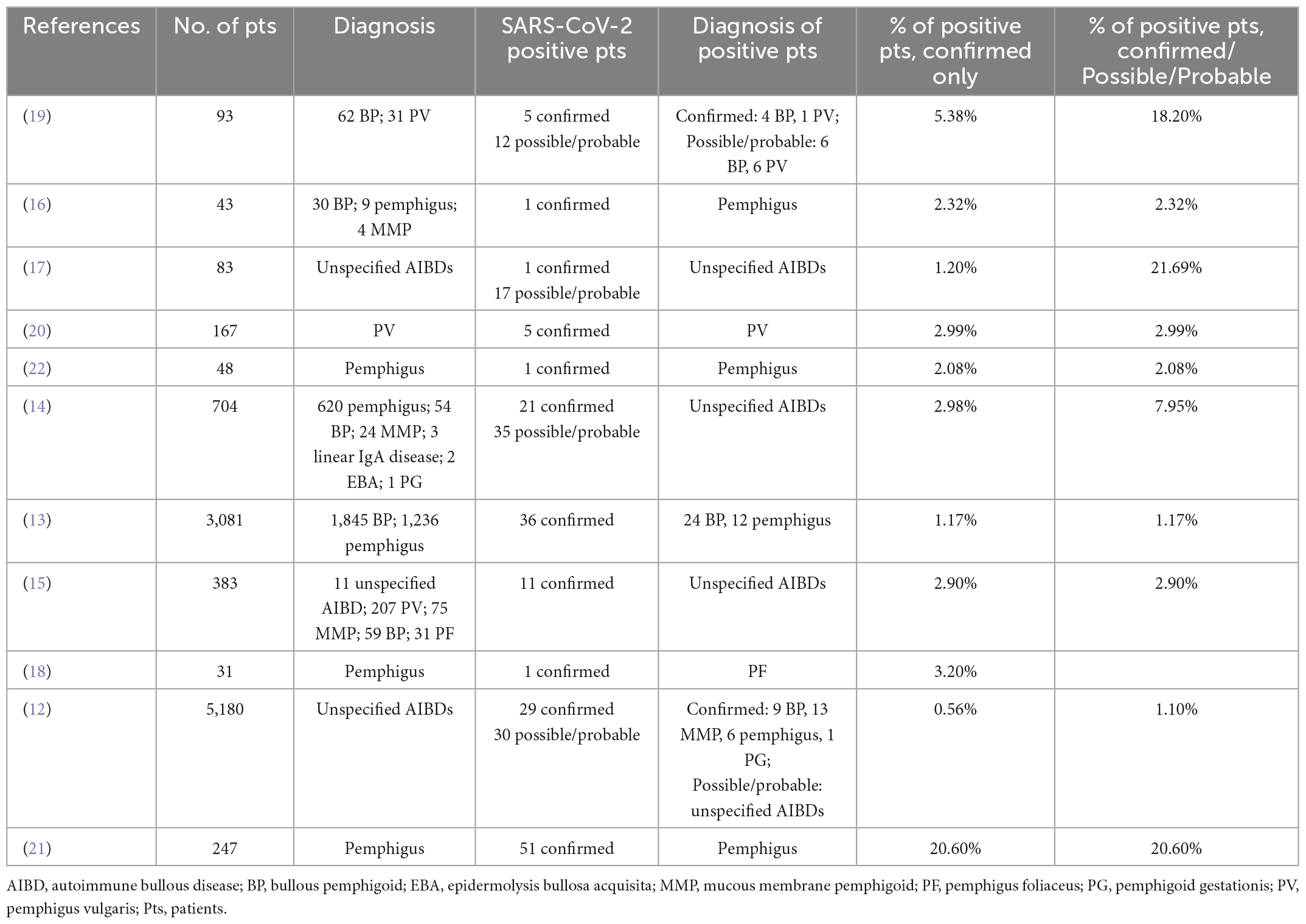
Table 1. Summary of relevant published studies reporting prevalence data on SARS-CoV-2 infection in AIBDs patients.
Although AIBD patients receiving rituximab (RTX) are more prone to viral infections than the normal population (23), only few studies investigated the possible influence of RTX-induced B cell depletion on the SARS-CoV-2 infection rate. A cohort study on 704 AIBD patients reported that among 21 COVID-19 confirmed cases, 14 (66.7%) received RTX within the last 12 months. Interestingly, the relative risk (RR) of COVID-19 infection and hospitalization decreased by 38% (95% CI: 18–57%) and 45% (95% CI: 15–72%), respectively, with each passing month from the last RTX infusion (14). In line with this observation, Joly and French Study Group on Auto Immune Bullous Skin Diseases, and the French Network of Rare Diseases in Dermatology [FIMARAD] reported an incidence ratio of COVID-19 in 23 RTX-treated patients ranging from 3.62 (95% CI: 1.29–8.85) in hospitalized patients with a confirmed diagnosis to 5.37 (95% CI: 3.15–8.96) in patients with a confirmed, probable, or possible diagnosis of COVID-19 (12). On the other hand, Breglio et al. found no difference in the rate of positive SARS-CoV-2 tests between 11 tests collected from RTX-treated patients and 66 tests collected from patients that were either never treated with RTX or received the last infusion at least 1 year before the onset of the pandemic (9.1 vs. 12.1%). However, the number of positive tests does not necessarily match the number of positive patients, since one patient could have been tested multiple times. In addition, the incidence could have been overestimated because patients performing SARS-CoV-2 test are usually symptomatic (24). On the contrary, Özgen et al. reported that among 51 of 247 pemphigus patients with COVID-19, 33% were treated with RTX while among non-COVID-19 patients only 21% were RTX-treated. These findings once more suggested that RTX increases the risk of COVID-19 (21). Of note, among AIBD patients, 45% of SARS-CoV-2 infected and 78% of non-infected were vaccinated; interestingly, only 29% of infected and RTX-treated patients were vaccinated (21), suggesting a role of the vaccine in preventing infection.
Thus, even though these results should be interpreted with some caution due to their relatively small sample size, the risk of SARS-CoV-2 infection seemed higher in RTX-treated patients than in those who did not receive a B cell depletion therapy.
The influence of AIBD and its immunosuppressive treatment on COVID-19 clinical course and vice versa
Two recent studies investigated the risk of severe COVID-19 outcomes in patients with immune-mediated inflammatory diseases (25, 26), reporting a similar risk of COVID-19-related death and hospitalization in patients and the general population. In addition, some evidence suggests that immunocompromised patients do not have a higher incidence or complications from COVID-19 than the general population (27, 28). In line with these studies, a systematic review suggested that immunomodulatory treatments of AIBDs do not increase the risks of poor outcomes in COVID-19 patients (11). Later, other studies investigated the course of COVID-19 in AIBD patients. Özgen in Turkey enrolled 51 patients having COVID-19 of whom 40 (78%) had a non-serious disease, 11 (22%) required hospitalization, and one (2%) died (21). In Iran Mahmoudi et al. followed 21 AIBD patients with confirmed COVID-19 diagnosis: 15 (71%) had been hospitalized, 7 (33%) needed intensive care facilities and 3 (14%) died. Of note, two studies found that COVID-19 infection and hospitalization rates were higher with prednisolone dosages > 10 mg/die (14, 29). In parallel, Kridin et al. in Israel followed 36 confirmed COVID-19 cases in a cohort of 3,081 AIBD patients, of whom 17 (47.2%) were hospitalized, and 7 (19.4%) died (13). Similar results were observed in a larger French cohort of 5,180 AIBD patients where Joly and French Study Group on Auto Immune Bullous Skin Diseases, and the French Network of Rare Diseases in Dermatology [FIMARAD] identified 59 possible, probable, or confirmed COVID-19 cases of whom 30 (50.8%) were hospitalized, 7 (11.9%) were admitted to an intensive care unit, and 15 (25.4%) died (12). The latter two studies on the largest cohorts analyzed indicated that the risk of hospitalization was comparable to the general population (13) and that exposure to systemic corticosteroids and immunosuppressive agents was not significantly associated with increased severity of COVID-19 (12, 13). However, both studies reported a higher mortality rate for BP patients. In particular, Kridin et al. reported that COVID-19-associated mortality was higher in BP patients (adjusted HR 2.81) compared to the control population, while in pemphigus patients it was similar (adjusted HR 1.15) to their controls (13). Similar results are stated in Joly and French Study Group on Auto Immune Bullous Skin Diseases, and the French Network of Rare Diseases in Dermatology [FIMARAD]’s study, where mortality risk in confirmed COVID-19 patients was 1.63-fold higher in hospitalized patients with AIBDs than in the aged-matched population (12).
RTX could affect the COVID-19 disease course
A major concern in management of AIBDs during the pandemic is the safety of RTX treatment. In patients with rheumatologic disease, RTX treatment increased the hospitalization and mortality rate and led to an unfavorable disease course (30–35). Recently, a study on a very large cohort of patients affected by immune-mediated inflammatory diseases found that RTX therapy was associated with increased COVID-19-related death (HR 1.68, 95% CI: 1.11–2.56) (25). To evaluate the effect of RTX treatment on the COVID-19 disease course several studies were conducted also on AIBD patients. Few studies assessed a favorable COVID-19 disease course in RTX-treated patients (20, 22). However, none of the reported patients were infected close to the time of RTX infusion, when B cell depletion was profound. In this context, Özgen et al. on a cohort of 51 patients, found a higher percentage of hospitalization in RTX-treated vs. non-treated patients (33.35 vs. 23.52%), but not a higher mortality (21). In line with this study, Mahmoudi et al. found that the RR of being infected with COVID-19 and being hospitalized decreased by 38% (95% CI: 18–57%) and 45% (95% CI: 15–72%), respectively, with each passing month from the last RTX infusion (14). Hwang and Tomayko reported that among 19 patients with AIBDs and COVID-19 infection two patients, treated with RTX < 5 months before, deceased. However, in both cases, they were elderly (74 and 82 years old) and affected by several comorbidities (36). In our experience, recent treatment with RTX could correlate with a more severe course, but not necessarily with an unfavorable prognosis (18). Most of these studies involved unvaccinated patients, but COVID-19 course could be less severe in vaccinated patients. In fact, circulating anti-microbial antibodies appear to arise from long-lived plasma cells. RTX does not abrogate these cells, as they are not dependent on CD20 positive memory B cells, such as reported in mice where maintenance of the plasma cell pool is independent of memory B cells (37). A recent study from Moghadam et al. evaluated 93 AIBD patients with a confirmed diagnosis of COVID-19. Interestingly, the authors found that vaccination and each passing month from the last RTX dose attenuated disease severity (29).
Disease worsening or induction following SARS-CoV-2 infection
There is evidence that SARS-CoV-2 could hyper-stimulate the immune system inducing autoantibodies’ synthesis, and possibly triggering autoimmune diseases (38). Despite this, very few cases of COVID-19-induced AIBDs have been published (39). More frequently, COVID-19 infection seems to trigger AIBD worsening/flare. Ghalamkarpour and Pourani described a case of pemphigus vulgaris (PV) flare after COVID-19 infection (40). According to Özgen et al. among 51 patients 10 (19.6%) experienced disease flare after COVID-19, and five of them (50%) needed further treatment or therapy adjustment due to pemphigus flare (21). In accordance, Kasperkiewicz et al. found a moderate to severe AIBD worsening in 35.2% of patients through a web-based survey (15). Recently, Moghadam et al. reported that 18 out of 93 (19.3%) AIBD patients experienced a disease flare following COVID-19 infection (29).
New-onset of AIBDs associated with COVID-19 vaccines
Several case reports, case series, and a recent Italian multicenter study proposed a possible, so far unproven, causal relationship between COVID-19 vaccination and AIBD onset (Supplementary Table 1) (41–91). To our knowledge 51 studies reporting new-onset AIBDs, with a total of 121 patients that developed BP (92 cases), anti-p200 pemphigoid (1 case), pemphigus (1 case), PV (15 cases), pemphigus foliaceus (PF) (7 cases), pemphigus vegetans (1 case), and LABD (4 cases) following SARS-CoV-2 vaccination (Supplementary Table 1) were published. Information about age and sex was not reported for 17 patients. The remaining cases included 58 men and 46 women (M/F = 1.3), ranging from 23 to 97 years (median age: 76 years). Information about administered vaccines was not reported for five patients, and latency to lesions was not reported for 17 patients. According to its greater employment, AIBD onset was more frequently associated with the Pfizer vaccine (61.2%). The onset of AIBDs followed the first dose in 44 patients, the second dose in 47 patients, and the third dose in 13 patients; median latency time was 9, 7, and 14 days from the first, second, and third dose, respectively. Information about the diagnosis was not reported for eight patients. The remaining 113 subjects had a diagnosis confirmed by consistent histopathological and/or serological findings. All patients were treated with corticosteroids and/or immunosuppressive drugs, and the vast majority showed a good clinical response (Supplementary Table 1). The largest cohort belongs to an Italian multicenter study that enrolled 21 patients with COVID-19 vaccine-associated BP (65). The authors concluded that BP patients did not differ essentially from idiopathic BP; however, a male predominance and a reduced autoantibody response to BP230 were considered unique features of vaccinated BP cases. Of note, a recent study by Birabaharan et al. on a very large cohort of individuals found no difference in the risk of BP onset among persons receiving the mRNA COVID-19 vaccine compared to an unvaccinated matched control cohort (92), suggesting that in a period with global mass vaccinations, the association between AIBDs occurrence and vaccine could be a random coincidence. A limitation of the study is the only use of International Classification of Diseases (ICD) codes from database sources for BP diagnosis.
A possible speculation that may bring to an agreement among the real-life clinical observations on patients with a new-onset of an AIBD and the convincing data from Birabaharan et al. considers the vaccination as a precipitating factor. Specifically, the vaccine may induce autoimmunity in genetically predisposed individuals by stimulating a pre-existent, and sub-clinical autoreactivity against hemidesmosomal components. This phenomenon could slightly anticipate BP development without significantly modifying the incidence of the disease. Additional investigations on this controversial topic are needed.
Relapses and worsening of AIBD after SARS-CoV-2 vaccination
Several vaccinations such as tetanus and influenza have been reported to exacerbate AIBDs (93, 94). The mechanism mentioned above for AIBD onset after vaccination could also be considered for vaccine-associated relapse or worsening of a pre-existing disease. In fact, even in patients who achieve disease control or remission, clinical or sub-clinical autoreactivity against hemidesmosomal components is probably present. Kasperkiewicz and Woodley analyzed 30 published papers on AIBD during the pandemic and reported that 10% of patients had a flare or worsening following vaccination (95). To date, relapse/worsening after COVID-19 vaccine has been described in 185 patients (21, 42, 55, 61, 69, 75, 79, 82, 83, 96–100) (Table 2). Considering cases with known gender and age, the M/F ratio was 0.8 (8 M, 10 F). Ages ranged from 28 to 83 years, and the median age was 62 years. Similar to new-onsets, relapse and worsening were more frequently associated with the Pfizer vaccine (44.4%), as expected. The relapse/worsening of AIBDs followed the first dose in 27 patients, the second dose in 10 patients and the third dose in three patients; mean latency time was 7.5, 11.2, and 15.1 days from the first, second, and third dose, respectively (Table 2). Most patients were treated with corticosteroids and/or immunosuppressive drugs and showed a good clinical response (Table 2). Of note, it should be considered that exacerbation of the disease after the first dose does not preclude the administration of a second dose. In fact, very few patients presented disease exacerbation after both doses (83, 96) (Table 2). In general, disease relapse/worsening events can be reduced if the AIBD is stable at the time of vaccination (83). Of note, the risk of exacerbation should not dissuade from getting the vaccine.
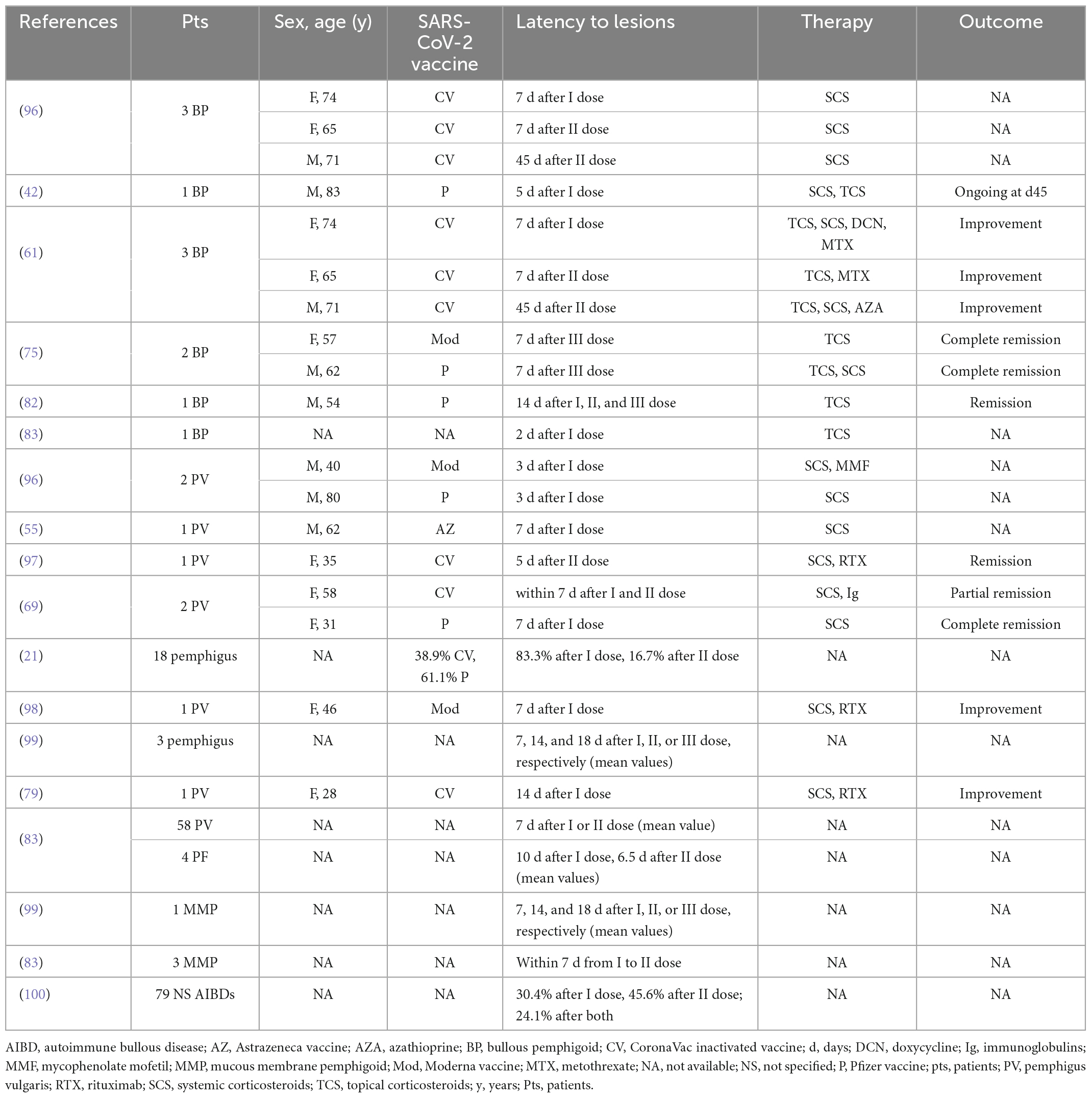
Table 2. Demographics, vaccines, latency time, and clinical features of AIBD patients reporting disease flare or worsening after COVID-19 vaccination.
COVID-19 vaccination in AIBD patients
Kasperkiewicz and Woodley reported that vaccination did not negatively affect the clinical course of AIBDs in most patients. Only about 6% of post-SARS-CoV-2-vaccinal cases presented clinically with de novo AIBDs, and 10% had a flare or worsening of pre-existing AIBDs usually well controlled with standard immune-suppressive treatment (95). However, the fact that vaccination could be less effective during immunosuppressive therapy should be considered. Despite the ongoing treatment with oral corticosteroid, all five AIBD patients analyzed by Damiani et al. developed IgG antibodies against SARS-CoV-2 (> 150 UI) 1 month after receiving the second dose (96). In this context, to achieve maximum efficacy and protection by vaccination, patients should be preferably vaccinated during disease remission or at least under low immunosuppression condition (101, 102). Nonetheless, lowering the corticosteroid dosage before or during the vaccination is not advisable due to the risk of exacerbations (15). Different considerations should be made in case of RTX therapy, especially in unvaccinated patients. B cell-depleting treatments have the most prominent effect on anti-SARS-CoV-2 IgG neutralization titers (103). Specifically, RTX-treated patients have failed to mount any humoral anti-SARS-CoV-2 response, even with a 4–12-month interval between RTX infusion and vaccination (104). Thus, a panel of experts advises that in these patients, vaccination should be performed either before therapy initiation or 4–6 months following RTX infusion (105).
Conclusion
In COVID-19 and AIBD research area further well-designed investigations are needed. In fact, there are only two studies on large cohorts with standardized findings on prevalence and disease course and very few studies on RTX treatment and COVID-19 disease. In particular, most of the studies do not consider the time between the last RTX treatment and COVID-19, that is the most important parameter to evaluate B-cell population at the time of SARS-CoV-2 infection. Moreover, few studies compare the impact of COVID-19 infection in vaccinated and not-vaccinated patients so far. However, despite of these limitations some important advances can be underlined.
The risks of COVID-19 infection and its severe course were comparable in AIBD patients and in the general population, except for RTX-treated patients. Of note, among COVID-19-infected BP patients the mortality rate was higher than in general population, while for pemphigus data were inconclusive. Thus, AIBD patients during the COVID-19 pandemic, especially if unvaccinated, should be subjected to risk-benefit assessments when considering RTX as a therapeutic option. The causal relationship between vaccination and AIBD onset, relapse or exacerbation has not been demonstrated so far. It could be speculated that vaccination may induce an autoimmune response in genetically predisposed persons by stimulation of pre-existent and sub-clinical autoreactivity. Additional investigations on this controversial topic should be performed. Of course, the risk of these rare adverse events should not discourage vaccination itself, although it should be performed during disease remission or at least while under low immunosuppression.
Finally, the impact of the pandemic on patients and especially on fragile ones has been heavy, but the precious acquired knowledge could also be used in possible, albeit undesirable, future pandemic scenarios.
Author contributions
AP and GD designed the manuscript. AP, JS, FrM, FeM, and GD wrote the manuscript. GD supervised the work. All authors contributed to the preparation of the manuscript and approved the submitted version.
Funding
This study was supported by the “Progetto Ricerca Corrente” and “Ricerca Finalizzata” (No. 12367807) of the Italian Ministry of Health, Rome, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1112823/full#supplementary-material
References
1. World Health Organization [WHO]. COVID-19. (2022). Available online at: https://covid19.who.int/ (accessed November 28, 2022).
2. Mahajan S, Caraballo C, Li SX, Dong Y, Chen L, Huston SK, et al. SARS-CoV-2 infection hospitalization rate and infection fatality rate among the non-congregate population in connecticut. Am J Med. (2021) 134:812–6.e2. doi: 10.1016/j.amjmed.2021.01.020
3. Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. (2020) 20:e238–44. doi: 10.1016/S1473-3099(20)30484-9
4. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. (2020) 584:430–6. doi: 10.1038/s41586-020-2521-4
5. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
6. O’Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. (2021) 590:140–5. doi: 10.1038/s41586-020-2918-0
7. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. (2020) 8:853–62. doi: 10.1016/S2213-2600(20)30316-7
8. Zhou F, Yu T, Du R, Fan G, Liu Y. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
9. Gallo Marin B, Aghagoli G, Lavine K, Yang L, Siff EJ, Chiang SS, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. (2021) 31:1–10. doi: 10.1002/rmv.2146
10. Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. (2020) 92:2188–92. doi: 10.1002/jmv.26031
11. Kasperkiewicz M. COVID-19 outbreak and autoimmune bullous diseases: a systematic review of published cases. J Am Acad Dermatol. (2021) 84:563–8. doi: 10.1016/j.jaad.2020.08.012
12. Joly P, French Study Group on Auto Immune Bullous Skin Diseases, and the French Network of Rare Diseases in Dermatology [FIMARAD]. Incidence and severity of COVID-19 in patients with autoimmune blistering skin diseases: a nationwide study. J Am Acad Dermatol. (2022) 86:494–7. doi: 10.1016/j.jaad.2021.10.034
13. Kridin K, Schonmann Y, Weinstein O, Schmidt E, Ludwig RJ, Cohen AD, et al. The risk of COVID-19 in patients with bullous pemphigoid and pemphigus: a population-based cohort study. J Am Acad Dermatol. (2021) 85:79–87. doi: 10.1016/j.jaad.2021.02.087
14. Mahmoudi H, Farid AS, Nili A, Dayani D, Tavakolpour S, Soori T, et al. Characteristics and outcomes of COVID-19 in patients with autoimmune bullous diseases: a retrospective cohort study. J Am Acad Dermatol. (2021) 84:1098–100. doi: 10.1016/j.jaad.2020.12.043
15. Kasperkiewicz M, Yale M, Strong R, Zillikens D, Woodley DT, Recke A, et al. COVID-19 pandemic and autoimmune bullous diseases: a cross-sectional study of the International Pemphigus and Pemphigoid Foundation. J Eur Acad Dermatol Venereol. (2021) 35:e418–21. doi: 10.1111/jdv.17228
16. Balestri R, Rech G, Girardelli CR. Occurrence of SARS-CoV-2 during mycophenolate mofetil treatment for pemphigus. J Eur Acad Dermatol Venereol. (2020) 34:e435–6. doi: 10.1111/jdv.16578
17. Di Altobrando A, Patrizi A, Abbenante D, Bardazzi F. Should SARS-CoV-2 influence immunosuppressive therapy for autoimmune blistering diseases? J Eur Acad Dermatol Venereol. (2020) 34:e295–7. doi: 10.1111/jdv.16491
18. Sinagra JL, Vedovelli C, Binazzi R, Salemme A, Moro F, Mazzanti C, et al. Case report: complete and fast recovery from severe COVID-19 in a pemphigus patient treated with rituximab. Front Immunol. (2021) 12:665522. doi: 10.3389/fimmu.2021.665522
19. Carugno A, Sena P, Raponi F, Robustelli Test E, Vezzoli P. Patients with bullous skin disease in a high-epidemic COVID-19 area, Bergamo, Italy. Br J Dermatol. (2020) 183:589–91. doi: 10.1111/bjd.19266
20. Shahidi-Dadras M, Abdollahimajd F, Ohadi L, Tabary L, Araghi F, Mozafari N, et al. COVID-19 in pemphigus vulgaris patients with previous rituximab therapy: a tele-medicine experience. J Dermatolog Treat. (2022) 33:1181–2. doi: 10.1080/09546634.2020.1789041
21. Özgen Z, Aksoy H, Akın Çakıcı O, Koku Aksu AE, Erdem O, Polat AK, et al. COVID-19 severity and SARS-Cov-2 vaccine safety in pemphigus patients. Dermatol Ther. (2022) 35:e15417. doi: 10.1111/dth.15417
22. Uzuncakmak TK, Özkoca D, Askin O, Kutlubay Z. Can rituximab be used in the treatment of pemphigus vulgaris during the COVID-19 pandemic? Dermatol Ther. (2021) 34:e14647. doi: 10.1111/dth.14647
23. Baum S, Raviv T, Gilboa S, Pavlotsky F, Barzilai A. Efficacy of repeated courses of rituximab as treatment for pemphigus vulgaris. Acta Dermatol Venereol. (2020) 100:adv00286. doi: 10.2340/00015555-3649
24. Breglio KF, Sarver MM, Hall RP III, Marano AL. SARS-CoV-2 infections in patients with autoimmune blistering disorders: a case series and retrospective analysis. J Am Acad Dermatol Int. (2022) 7:38–43. doi: 10.1016/j.jdin.2022.01.003
25. MacKenna B, Kennedy NA, Mehrkar A, Rowan A, Galloway J, Matthewman J, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. (2022) 4:e490–506. doi: 10.1016/S2665-9913(22)00098-4
26. Boekel L, Stalman EW, Wieske L, Hooijberg F, van Dam KPJ. Breakthrough SARS-CoV-2 infections with the delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies. Lancet Rheumatol. (2022) 4:e417–29. doi: 10.1016/S2665-9913(22)00102-3
27. Favalli EG, Monti S, Ingegnoli F, Balduzzi S, Caporali R, Montecucco C, et al. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol. (2020) 72:1600–6. doi: 10.1002/art.41388
28. Michelena X, Borrell H, López-Corbeto M, López-Lasanta M, Moreno E, Pascual-Pastor M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. (2020) 50:564–70. doi: 10.1016/j.semarthrit.2020.05.001
29. Moghadam FS, Kianfar N, Dasdar S, Samii R, Farimani Z, Azar PM, et al. Adverse outcome and severity of COVID-19 in patients with autoimmune bullous diseases: a historical cohort study. Dermatol Ther. (2022) 35:e15672. doi: 10.1111/dth.15672
30. Kow CS, Hasan SS. Use of rituximab and the risk of adverse clinical outcomes in COVID-19 patients with systemic rheumatic disease. Rheumatol Int. (2020) 40:2117–8. doi: 10.1007/s00296-020-04715-0
31. Guilpain P, Le Bihan C, Foulongne V, Taourel P, Pansu N, Maria ATJ, et al. Response to: ‘Severe COVID-19 associated pneumonia in 3 patients with systemic sclerosis treated with rituximab’ by Avouac et al. Ann Rheum Dis. (2021) 80:e38. doi: 10.1136/annrheumdis-2020-217955
32. Guilpain P, Le Bihan C, Foulongne V, Taourel P, Pansu N, Maria ATJ, et al. Rituximab for granulomatosis with polyangiitis in the pandemic of COVID-19: lessons from a case with severe pneumonia. Ann Rheum Dis. (2021) 80:e10. doi: 10.1136/annrheumdis-2020-217549
33. Loarce-Martos J, Garcia-Fernandez A, Lopez-Gutierrez F, Garcia-Garcia V, Calvo-Sanz L. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. (2020) 40:2015–21. doi: 10.1007/s00296-020-04699-x
34. Rondaan C, Furer V, Heijstek MW, Agmon-Levin N, Bijl M, Breedveld FC, et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. (2019) 5:e001035. doi: 10.1136/rmdopen-2019-001035
35. Strangfeld A, Schafer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. (2021) 80:930–42. doi: 10.1136/annrheumdis-2020-219498
36. Hwang E, Tomayko MM. COVID-19 outcomes in patients with autoimmune blistering disease. Br J Dermatol. (2021) 185:1048–50. doi: 10.1111/bjd.20571
37. Di Lillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. (2008) 180:361–71. doi: 10.4049/jimmunol.180.1.361
38. Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y, et al. The SARS-CoV-2 as an instrumental trigger Of autoimmunity. Autoimmun Rev. (2021) 20:102792. doi: 10.1016/j.autrev.2021.102792
39. De Medeiros VLS, Monteiro-Neto AU, França DDT, Castelo Branco R, de Miranda Coelho EO, Takano DM, et al. Pemphigus vulgaris after COVID-19: a case of induced autoimmunity. SN Compr Clin Med. (2021) 3:1768–72. doi: 10.1007/s42399-021-00971-8
40. Ghalamkarpour F, Pourani MR. Aggressive course of pemphigus vulgaris following COVID-19 infection. Dermatol Ther. (2020) 33:e14398. doi: 10.1111/dth.14398
41. Solimani F, Mansour Y, Didona D, Dilling A, Ghoreschi K, Meier K, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. (2021) 35:e649–51. doi: 10.1111/jdv.17480
42. Tomayko M, Damsky W, Fathy R, McMahon DE, Turner N, Valentin MN, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. (2021) 148:750–1. doi: 10.1016/j.jaci.2021.06.026
43. Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH, et al. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol. (2022) 49:34–41. doi: 10.1111/cup.14104
44. Coto-Segura P, Fernández-Prada M, Mir-Bonafé M, García-García B, González-Iglesias I, Alonso-Penanes P, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. (2022) 47:141–3. doi: 10.1111/ced.14835
45. McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. (2022) 86:113–21. doi: 10.1016/j.jaad.2021.09.002
46. Nakamura K, Kosano M, Sakai Y, Saito N, Takazawa Y, Omodaka T, et al. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J Dermatol. (2021) 48:e606–7. doi: 10.1111/1346-8138.16170
47. Agharbi FZ, Eljazouly M, Basri G, Faik M, Benkirane A, Albouzidi A, et al. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann Dermatol Venereol. (2022) 149:56–7. doi: 10.1016/j.annder.2021.07.008
48. Young J, Mercieca L, Ceci M, Pisani D, Betts A, Boffa MJ, et al. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J Eur Acad Dermatol Venereol. (2022) 36:e13–6. doi: 10.1111/jdv.17676
49. Thongprasom K, Pengpis N, Phattarataratip E, Samaranayake L. Oral pemphigus after COVID-19 vaccination. Oral Dis. (2022) 28(Suppl. 2):2597–8. doi: 10.1111/odi.14034
50. Schmidt V, Blum R, Möhrenschlager M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in an 84-year-old female with polymorbidity and polypharmacy. J Eur Acad Dermatol Venereol. (2022) 36:e88–90. doi: 10.1111/jdv.17722
51. Pérez-López I, Moyano-Bueno D, Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Med Clin (Engl Ed). (2021) 157:e333–4. doi: 10.1016/j.medcle.2021.05.004
52. Hali F, Kerouach A, Alatawna H, Chiheb S, Lakhdar H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. (2022) 47:611–3. doi: 10.1111/ced.15007
53. Lua ACY, Ong FLL, Choo KJL, Yeo YW, Oh CC. An unusual presentation of pemphigus foliaceus following COVID-19 vaccination. Australas J Dermatol. (2022) 63:128–30. doi: 10.1111/ajd.13755
54. Dell’Antonia M, Anedda S, Usai F, Atzori L, Ferreli C. Bullous pemphigoid triggered by COVID-19 vaccine: rapid resolution with corticosteroid therapy. Dermatol Ther. (2022) 35:e15208. doi: 10.1111/dth.15208
55. Hatami P, Balighi K, Nicknam Asl H, Aryanian Z. COVID vaccination in patients under treatment with rituximab: a presentation of two cases from Iran and a review of the current knowledge with a specific focus on pemphigus. Dermatol Ther. (2022) 35:e15216. doi: 10.1111/dth.15216
56. Koutlas IG, Camara R, Argyris PP, Davis MDP, Miller DD. Development of pemphigus vulgaris after the second dose of the mRNA-1273 SARS-Cov-2 vaccine. Oral Dis. (2022) 28(Suppl. 2):2612–3. doi: 10.1111/odi.14089
57. Bostan E, Yel B, Akdogan N, Gokoz O. New-onset bullous pemphigoid after inactivated COVID-19 vaccine: synergistic effect of the COVID-19 vaccine and vildagliptin. Dermatol Ther. (2022) 35:e15241. doi: 10.1111/dth.15241
58. Knechtl GV, Seyed Jafari SM, Berger T, Rammlmair A, Feldmeyer L, Borradori L, et al. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J Eur Acad Dermatol Venereol. (2022) 36:e251–3. doi: 10.1111/jdv.17868
59. Pauluzzi M, Stinco G, Errichetti E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: a report and brief literature review. J Eur Acad Dermatol Venereol. (2022) 36:e257–9. doi: 10.1111/jdv.17891
60. Gambichler T, Hamdani N, Budde H, Sieme M, Skrygan M, Scholl L, et al. Bullous pemphigoid after SARS-CoV-2 vaccination: spike-protein-directed immunofluorescence confocal microscopy and T-cell-receptor studies. Br J Dermatol. (2022) 186:728–31. doi: 10.1111/bjd.20890
61. Afacan E, Edek YC, Ýlter N, Gülekon A. Can COVID-19 vaccines cause or exacerbate bullous pemphigoid? A report of seven cases from one center. Int J Dermatol. (2022) 61:626–7. doi: 10.1111/ijd.16086
62. Hung WK, Chi CC. Incident bullous pemphigoid in a psoriatic patient following mRNA-1273 SARS-CoV-2 vaccination. J Eur Acad Dermatol Venereol. (2022) 36:e407–9. doi: 10.1111/jdv.17955
63. Koumaki D, Krueger-Krasagakis SE, Papadakis M, Katoulis AC, Gkiaouraki I, Zografaki K, et al. Psoriasis flare-up after AZD1222 and BNT162b2 COVID-19 mRNA vaccines: report of twelve cases from a single centre. J Eur Acad Dermatol Venereol. (2022) 36:e411–5. doi: 10.1111/jdv.17965
64. Yıldırıcı S, Yaylı S, Demirkesen C, Vural S. New onset of pemphigus foliaceus following BNT162b2 vaccine. Dermatol Ther. (2022) 35:e15381. doi: 10.1111/dth.15381
65. Maronese CA, Caproni M, Moltrasio C, Genovese G, Vezzoli P, Sena P, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). (2022) 9:841506. doi: 10.3389/fmed.2022.841506
66. Alshammari F, Abuzied Y, Korairi A, Alajlan M, Alzomia M, AlSheef M, et al. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) COVID-19 vaccine: a case report. Ann Med Surg (Lond). (2022) 75:103420. doi: 10.1016/j.amsu.2022.103420
67. Calabria E, Canfora F, Mascolo M, Varricchio S, Mignogna MD, Adamo D, et al. Autoimmune mucocutaneous blistering diseases after SARS-Cov-2 vaccination: a case report of pemphigus vulgaris and a literature review. Pathol Res Pract. (2022) 232:153834. doi: 10.1016/j.prp.2022.153834
68. Hali F Sr., Araqi L Jr., Marnissi F, Meftah A, Chiheb S. Autoimmune bullous dermatosis following COVID-19 vaccination: a series of five cases. Cureus. (2022) 14:e23127. doi: 10.7759/cureus.23127
69. Akoglu G. Pemphigus vulgaris after SARS-CoV-2 vaccination: a case with new-onset and two cases with severe aggravation. Dermatol Ther. (2022) 35:e15396. doi: 10.1111/dth.15396
70. Saffarian Z, Samii R, Ghanadan A, Vahidnezhad H. De novo severe pemphigus vulgaris following SARS-CoV-2 vaccination with BBIBP-CorV. Dermatol Ther. (2022) 35:e15448. doi: 10.1111/dth.15448
71. Singh A, Bharadwaj SJ, Chirayath AG, Ganguly S. Development of severe pemphigus vulgaris following ChAdOx1 nCoV-19 vaccination and review of literature. J Cosmet Dermatol. (2022) 21:2311–4. doi: 10.1111/jocd.14945
72. Maronese CA, Di Zenzo G, Genovese G, Barei F, Monestier A, Pira A, et al. Reply to “New-onset bullous pemphigoid after inactivated COVID-19 vaccine: synergistic effect of the COVID-19 vaccine and vildagliptin”. Dermatol Ther. (2022) 35:e15496. doi: 10.1111/dth.15496
73. Desai AD, Shah R, Haroon A, Wassef C. Bullous pemphigoid following the moderna mRNA-1273 vaccine. Cureus. (2022) 14:e24126. doi: 10.7759/cureus.24126
74. Han J, Russo G, Stratman S, Psomadakis CE, Rigo R, Owji S, et al. Toxic epidermal necrolysis-like linear IgA bullous dermatosis after third Moderna COVID-19 vaccine in the setting of oral terbinafine. J Am Acad Dermatol Case Rep. (2022) 24:101–4. doi: 10.1016/j.jdcr.2022.04.021
75. Bardazzi F, Carpanese MA, Abbenante D, Filippi F, Sacchelli L, Loi C, et al. New-onset bullous pemphigoid and flare of pre-existing bullous pemphigoid after the third dose of the COVID-19 vaccine. Dermatol Ther. (2022) 35:e15555. doi: 10.1111/dth.15555
76. Bailly-Caillé B, Jouen F, Dompmartin A, Morice C. A case report of anti-P200 pemphigoid following COVID-19 vaccination. JAAD Case Rep. (2022) 23:83–6. doi: 10.1016/j.jdcr.2022.03.011
77. Zhang Y, Lang X, Guo S, He H, Cui H. Bullous pemphigoid after inactivated COVID-19 vaccination: case report. Dermatol Ther. (2022) 35:e15595. doi: 10.1111/dth.15595
78. Shanshal M. Dyshidrosiform bullous pemphigoid triggered by COVID-19 vaccination. Cureus. (2022) 14:e26383. doi: 10.7759/cureus.26383
79. Shakoei S, Kalantari Y, Nasimi M, Tootoonchi N, Ansari MS, Razav Z, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. (2022) 35:e15651. doi: 10.1111/dth.15651
80. Gui H, Young PA, So JY, Pol-Rodriguez M, Rieger KE, Lewis MA, et al. New-onset pemphigus vegetans and pemphigus foliaceus after SARS-CoV-2 vaccination: a report of 2 cases. JAAD Case Rep. (2022) 27:94–8. doi: 10.1016/j.jdcr.2022.07.002
81. Avallone G, Cavallo F, Astrua C, Caldarola G, Conforti C, De Simone C, et al. Cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: a real-life multicentre experience. J Eur Acad Dermatol Venereol. (2022) 36:e876–9. doi: 10.1111/jdv.18386
82. Alelq NA, Kubieniec ME, French LE, Prinz JC. Influence of COVID-19 vaccination on immune-mediated skin diseases. J Eur Acad Dermatol Venereol. (2022) 36:e965–8. doi: 10.1111/jdv.18388
83. Kianfar N, Dasdar S, Farid AS, Balighi K, Mahmoudi H, Daneshpazhooh M, et al. Exacerbation of autoimmune bullous diseases after severe acute respiratory syndrome coronavirus 2 vaccination: is there any association? Front Med (Lausanne). (2022) 9:957169. doi: 10.3389/fmed.2022.957169
84. Daines B, Madigan LM, Vitale PA, Khalighi M, Innes M. A new eruption of bullous pemphigoid following mRNA COVID-19 vaccination. Dermatol Online J. (2022) 28:11.
85. Corrá A, Barei F, Genovese G, Zussino M, Spigariolo CB, Mariotti EB, et al. Five cases of new-onset pemphigus following vaccinations against coronavirus disease 2019. J Dermatol. (2022) 17:10.1111/1346–8138.16554. doi: 10.1111/1346-8138.16554
86. Nahm WJ, Juarez M, Wu J, Kim RH. Eosinophil-rich linear IgA bullous dermatosis induced by mRNA COVID-19 booster vaccine. J Cutan Pathol. (2022) 50:24–8. doi: 10.1111/cup.14305
87. Pouran M, Bidari-Zerehpoosh F, Ayatollahi A, Robati RM. New onset of pemphigus foliaceus following BBIBP COVID-19 vaccine. Dermatol Ther. (2022) 35:e15816. doi: 10.1111/dth.15816
88. Al Maashari RS, Al Mahmood Noaf S, Tausif S. Vesiculo-bullous eruption following COVID vaccination. Saudi J Med. (2022) 7:501–4.
89. Chao YC, Liu KL. New-onset bullous pemphigoid triggered by AstraZeneca COVID-19 vaccine. Dermatol Sin. (2022) 40:245–6.
90. Wan V, Chen D, Shiau CJ, Jung GW. Association between COVID-19 vaccination and bullous pemphigoid–a case series and literature review. SAGE Open Med Case Rep. (2022) 10:2050313X221131868. doi: 10.1177/2050313X221131868
91. Baffa ME, Maglie R, Montefusco F, Pipitò C, Senatore S. Severe bullous pemphigoid following COVID-19 vaccination resistant to rituximab and successfully treated with dupilumab. J Eur Acad Dermatol Venereol. (2022): [Epub ahead of print]. doi: 10.1111/jdv.18673
92. Birabaharan M, Kaelber DC, Orme CM, Paravar T, Karris MY. Evaluating risk of bullous 115 pemphigoid after mRNA COVID-19 vaccination. Br J Dermatol. (2022) 187:271–3. doi: 10.1111/bjd.21240
93. Korang K, Ghohestani R, Krieg T, Uitto J, Hunzelmann N. Exacerbation of pemphigus foliaceus after tetanus vaccination accompanied by synthesis of auto-antibodies against paraneoplastic pemphigus antigens. Acta Dermato Venereol. (2002) 82:482–3. doi: 10.1080/000155502762064755
94. De Simone C, Caldarola G, D’Agostino M, Zampetti A, Amerio P. Exacerbation of pemphigus after influenza vaccination. Clin Exp Dermatol. (2008) 33:718–20. doi: 10.1111/j.1365-2230.2008.02835.x
95. Kasperkiewicz M, Woodley DT. Association between vaccination and immunobullous disorders: a brief, updated systematic review with focus on COVID-19. J Eur Acad Dermatol Venereol. (2022) 36:e498–500. doi: 10.1111/jdv.18030
96. Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. (2021) 35:e645–7. doi: 10.1111/jdv.17472
97. Saleh MA, Saleh NA. Pemphigus vulgaris relapse during the coronavirus disease pandemic. Dermatol Ther. (2022) 35:e15354. doi: 10.1111/dth.15354
98. Ong SK, Darji K, Chaudhry SB. Severe flare of pemphigus vulgaris after first dose of COVID-19 vaccine. J Am Acad Dermatol Case Rep. (2022) 22:50–2. doi: 10.1016/j.jdcr.2022.01.027
99. Sprow G, Afarideh M, Dan J, Feng R, Keyes E, Grinnell M, et al. Autoimmune skin disease exacerbations following COVID-19 vaccination. Front Immunol. (2022) 13:899526. doi: 10.3389/fimmu.2022.899526
100. Kasperkiewicz M, Strong R, Mead K, Yale M, Zillikens D, Woodley DT, et al. COVID-19 vaccine acceptance and hesitancy in patients with immunobullous diseases: a cross-sectional study of the international pemphigus and pemphigoid foundation. Br J Dermatol. (2022) 186:737–9. doi: 10.1111/bjd.20906
101. Kasperkiewicz M, Schmidt E, Fairley JA, Joly P, Payne AS, Yale ML, et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. (2020) 34:e302–3. doi: 10.1111/jdv.16525
102. Seree-Aphinan C, Chanprapaph K, Rattanakaemakorn P, Setthaudom C, Suangtamai T, Pomsoong C, et al. Inactivated COVID-19 vaccine induces a low humoral immune response in a subset of dermatological patients receiving immunosuppressants. Front Med. (2021) 8:769845. doi: 10.3389/fmed.2021.769845
103. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, El-Qunni AA, et al. Glucocorticoids and B cell depleting agents substantially impair immunogenicity of mRNA vaccines to SARS-CoV-2. medRxiv [Preprint]. (2021): doi: 10.1101/2021.04.05.21254656
104. Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. (2021) 80:1355–6. doi: 10.1136/annrheumdis-2021-220408
105. European Reference Network [ERN] Skin,. Vaccination Advices. (2022) Available online at: https://ern-skin.eu/vaccination-advices/# (accessed November 30, 2022).
Keywords: COVID-19, SARS-CoV-2, autoimmune bullous diseases, bullous pemphigoid, pemphigus, vaccine, rituximab
Citation: Pira A, Sinagra JLM, Moro F, Mariotti F and Di Zenzo G (2023) Autoimmune bullous diseases during COVID-19 pandemic: 2022 update on rituximab and vaccine. Front. Med. 10:1112823. doi: 10.3389/fmed.2023.1112823
Received: 30 November 2022; Accepted: 04 January 2023;
Published: 19 January 2023.
Edited by:
Emanuele Cozzani, University of Genoa, ItalyReviewed by:
Takashi Hashimoto, Osaka City University, JapanFarzan Solimani, Charité – Universitätsmedizin Berlin, Germany
Copyright © 2023 Pira, Sinagra, Moro, Mariotti and Di Zenzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Di Zenzo,  g.dizenzo@idi.it
g.dizenzo@idi.it
 Anna Pira
Anna Pira Jo Linda Maria Sinagra
Jo Linda Maria Sinagra Francesco Moro1,2
Francesco Moro1,2  Feliciana Mariotti
Feliciana Mariotti Giovanni Di Zenzo
Giovanni Di Zenzo