Primary diffuse large B-cell lymphoma of orbit: A population-based analysis
- 1Department of Ophthalmology, Changzheng Hospital of Naval Medicine University, Shanghai, China
- 2Shenzhen Eye Hospital, Jinan University, Shenzhen, China
- 3Eye Hospital, Nanjing Medical University, Nanjing, China
Objective: Primary orbital lymphoma (POL) accounts for an essential part of adult orbital malignancies. Nevertheless, it remains a relatively rare lymphoid malignancy, accounting for <1% of all non-Hodgkin's lymphoma (NHL) cases. Orbital diffuse large B-cell lymphoma (DLBCL) is one of the most prevalent subtypes of POL that confers the worst outcomes. The prognostic determinants of orbital DLBCL remain unknown. Therefore, a retrospective analysis was conducted by investigating the Surveillance, Epidemiology, and End Results (SEER) database for independent predictive factors for the prognosis of orbital DLBCL.
Materials and methods: Using the SEER program, we acquired patient data including demographics, clinical characteristics, and treatment strategies. Our cohort included cases of primary orbital DLBCL diagnosed from 2000 to 2017. We conducted Kaplan-Meier analyses to visualize the overall survival (OS) and cause-specific survival (CSS). The Cox proportional hazard regression models were applied to assess the effects of these prognostic factors on OS and CSS.
Results: The present cohort included 332 patients with orbital DLBCL. Age was the most impacted variable by orbital DLBCL. Three independent prognostic variables of orbital DLBCL were identified on diagnosis: advanced age, no radiation treatment, and late-stage (Stage IV). Moreover, patients who underwent chemotherapy demonstrated a greater OS when compared with those who did not. In orbital DLBCL, being unmarried was also a poor prognostic factor.
Conclusion: The current study is the largest population-based case series of orbital DLBCL. The age at the time of diagnosis, marital status, absence of chemotherapy or radiotherapy, and tumor stage were all found to be correlated with worse prognosis.
Introduction
Primary orbital lymphoma (POL) is a common and the most prevalent orbital malignancy affecting older adults (1). Nevertheless, among all primary sites of the extra-nodal lymphoma, the orbit remains a relatively rare one, accounting for <1% of all non-Hodgkin's lymphoma (NHL) cases (2). Diffuse large B-cell lymphoma (DLBCL) is one of the most common histological subtypes of POL associated with the worst outcomes (3). However, the prognostic determinants of orbital DLBCL remain to be fully elucidated.
Since the orbital DLBCL is uncommon, efforts to clarify its clinical characteristics and the prognostic outcomes mostly rely on case reports and the collections of patients' cohorts from across the world (4–10). The clinical presentation, treatment strategies, and survival in orbital DLBCL have not been adequately analyzed at a large population level. Consequently, we sought to identify prognostic factors and the overall survival (OS) trends in orbital DLBCL by searching a national registry database—The SEER database. The National Cancer Institute developed the SEER program, which collects valuable information on cancer incidence and survival outcomes in USA, encompassing ~28% of all USA residents (11, 12). As of May 30, 2022, this research is the largest population-based case series of orbital DLBCL aimed at evaluating the clinical features and OS characteristics as well as determining the correlated prognostic factors in orbital DLBCL.
Methods
Data source and cohort selection
We adopted the 3rd edition of the International Classification of Diseases for Oncology [ICD-O-3] histology codes (9680/3, 9684/3, 9688/3, 9735/3, 9737/3, and 9738/3) to identify cases of orbital DLBCL and then accessed the interesting cohort. The original sites of DLBCL were determined by clinical physicians. We identified the primary orbital DLBCL by searching site number C69.6 (orbit, not otherwise specified [NOS]). We carried out this study following the standard guidelines and under the approval of the Ethics Committee of Shanghai Changzheng Hospital, Second Military Medical University.
All enrolled cases were diagnosed via valid histological identification, and cases that were diagnosed solely based on clinical characteristics, radiographic examination, autopsy, or death certificate were excluded. In addition, we excluded patients whose survival time was no more than 1 month. A resembling screen procedure was then conducted to acquire the comparative cohort of nodal DLBCL and extra-nodal DLBCL (without orbit).
The relevant data on survival, outcome, chemotherapy, radiotherapy, surgeries, the 6th edition of stage group-derived American Joint Committee on Cancer (AJCC), the laterality of orbit involvement, sex, race, age of diagnosis, and marital status were also queried. AJCC-6 staging was conducted instead of the updated AJCC-7 staging and AJCC-8 staging, as the former contains more data on DLBCL patients. Data on grading was sparse or unavailable. Surgical inventions were grouped as follows: surgery (codes: 10-80, 90, 98), no surgery (codes: 00), or unknown (codes: 99). Chemotherapy, as well as radiotherapy, were separately grouped as “yes” or “no/unknown”.
Outcomes and statistical analyses
The demographic characteristics and treatments of patients in the cohort were computed using descriptive statistics, and the differences among extra-nodal DLBCL, nodal DLBCL, and orbital DLBCL were analyzed by ANOVA, Chi-squared test, and Kruskal-Wallis test. We evaluated the survival outcomes, including OS and CSS, through Kaplan-Meier survival models and log-rank test. We determined the prognostic factors by using the univariate and multivariate Cox proportional hazard regression models. Hazard ratio (HR) with 95% confidence intervals (95% CI) were applied to explore the prognostic factors on OS and CSS. RStudio (RStudio, Inc., Boston, MA) was conducted for statistical analyses, and the p < 0.05 was considered significant.
Results
Incidence in primary orbital DLBCL
From 2000 to 2017, we collected 2024 cases of primary orbital NHL, including 332 (16.40%) cases of orbital DLBCL. Mucosal-Associated Lymphoid Tissue (MALT) Lymphoma (n = 1,048, 51.78%), and Follicular Lymphoma (n = 237, 11.71%) were the other top 3 histological subtypes. From 2000 to 2017, 332 cases of orbital DLBCL accounted for 0.34% of primary DLBCL (n = 97,607) and 1.00% of primary extra-nodal DLBCL (n = 33,060).
Clinical features and treatment
We enrolled 332 patients with primary orbital DLBCL meeting the inclusion criteria in our study. Table 1 summarizes the clinical and demographical features of the orbital, nodal, and other extra-nodal (non-orbit) DLBCL. The average age (68.9 years) of orbital DLBCL patients was greater than that of nodular DLBCL patients (64.1 years) and other extra-nodal DLBCL patients (65.4 years). There were equivalent cases of women (50.0%) diagnosed with orbital DLBCL (n = 166, sex-ratio: 1.00). However, DLBCL was more likely to affect men (about 55%) than the other two types of DLBCLs. The majority of the orbital DLBCL patients (82.8%) were Caucasians, which was consistent with the result for extra-nodal and nodal DLBCL patients. More than half of the patients in the entire cohort, as well as in the other two equivalents, were married. According to the Derived AJCC Stage Group, 6th Edition, the majority of the patients were classified into the following four stages: stage I (43.1%), stage IV(17.5%), stage II (4.2%), and stage III (1.8%). Chemotherapy was the primary treatment administered to patients with orbital DLBCL (66.0%), but it was also the least common among these 3 types of malignancies. Radiotherapy was the preferred treatment for 159 (47.9%) patients with orbital DBLCL, which is much higher than that for nodal DLBCL (17.9%) and other extra-nodal DLBCL (27.8%). Finally, only 131 (39.5%) patients received the surgical interventions in orbital DLBCL.
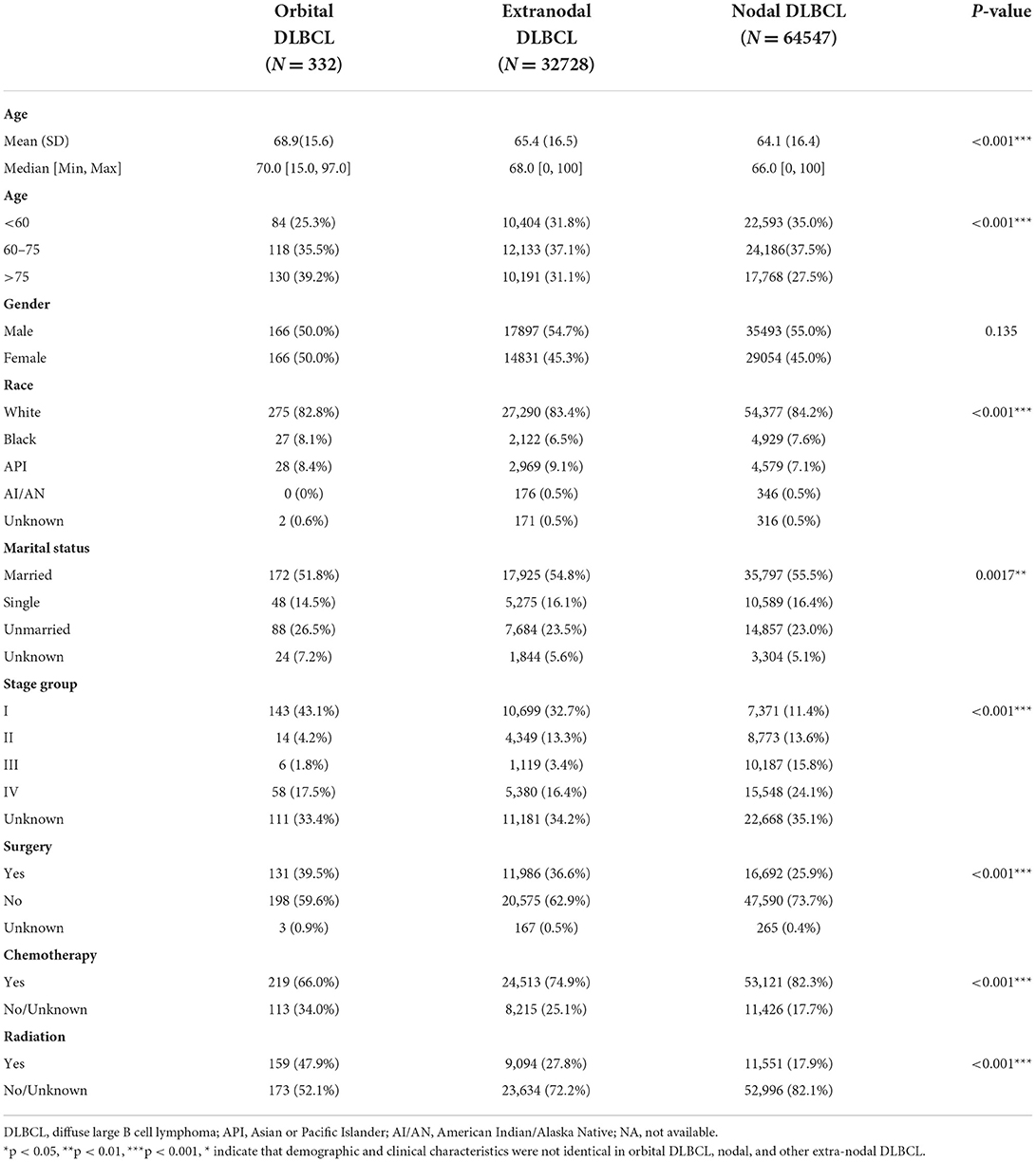
Table 1. Demographics and clinical features of patients with orbital DLBCL, nodal, and other extra-nodal sites as diagnosed in the SEER database (2000–2017).
Survival
Figure 1 shows the comparison of the OS (Figure 1A) and CSS (Figure 1B) of the orbital DLBCL patients to nodal/other extra-nodal DLBCL patients, demonstrating that the primary sites may affect the survival of DLBCL patients. All cases with orbital DLBCL in our cohort had a 3-, 5-, and 10-year OS of 65.99, 57.85, and 43.29%, respectively. Table 2 depicts statistical data on the OS probability of orbital DLBCL, extra-nodal DLBCL (without orbit), and nodal DLBCL. When compared to the other two types of DLBCL, patients with orbital DLBCL demonstrated a superior survival rate. Not only did orbital DLBCL confer the greatest OS but also provided the greatest CSS after 3-, 5-, and 10- years (77.67, 73.86, and 69.14%, respectively). Figure 1 displays the Kaplan–Meier curves of OS and CSS among these 3 DLBCLs.
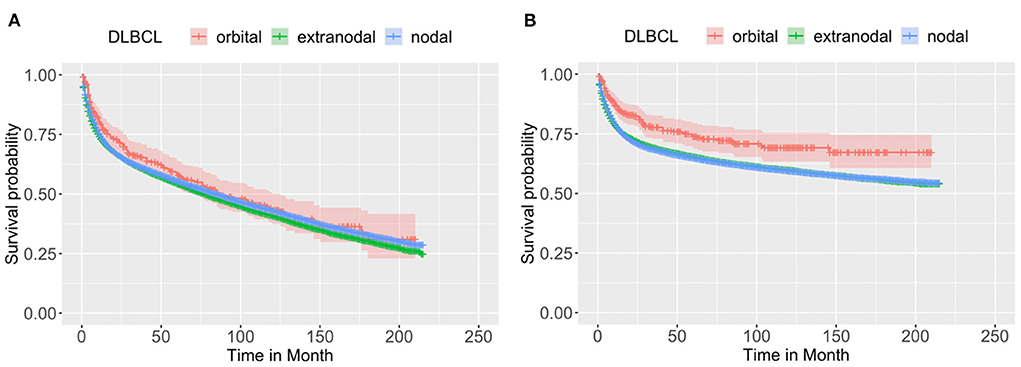
Figure 1. The Kaplan–Meier survival curve of (A) OS and (B) CSS of patients with DLBCL by the primary sites: orbit (n = 332), other extra-nodal sites (n = 32,728), and nodal site (n = 64,547). Through log-rank test, it was revealed that the OS and CSS were not identical among the three groups, with a p < 0.0001 and 0.0012, respectively, indicating the primary sites may affect the survival of DLBCL patients.

Table 2. Univariate and multivariate analysis of factors related to the risks of OS for patients with DLBCL in the SEER Program database from 2000 to 2017.
Univariate/multivariate analysis for predicting independent prognostic factors
We adopted univariate survival analysis to demonstrate increased age at diagnosis as a prognostic factor for OS (60–75 vs. <60 years, HR = 2.628, 95% CI 1.549–4.458, p < 0.001; >75 vs. <60 years, HR = 6.026, 95% CI 3.654–9.937, p < 0.001) (Figure 2A). Moreover, the Cox proportional hazard regressions indicated that patients age ≥75 years served as a crucial prognostic factor for CSS (HR = 2.324, 95% CI 1.265–4.270, p = 0.007) (Figure 3A). Furthermore, we noted remarkable differences in OS and CSS between married and unmarried (widowed/divorced) groups of orbital DLBCL patients (OS: unmarried vs. married, HR = 1.997, 95% CI 1.420–2.808, p < 0.001; CSS: unmarried vs. married, HR = 2.002, 95% CI 1.218–3.290, p = 0.006) (Figures 2B, 3B). When we compared OS by the AJCC stages in orbital DLBCL, we found that stage IV orbital DLBCL was related to a higher risk of death [OS: stage IV vs. stage I, HR = 2.022, 95% CI 1.336–3.062, p < 0.001 (Figure 2C); CSS: stage IV vs. stage I, HR = 2.612, 95% CI 1.529–4.462, p < 0.001 (Figure 3C)] (Tables 3, 4). In terms of treatment strategies, chemotherapy was also a prognostic factor for higher OS (HR = 0.683, 95% CI 0.501–0.932, p = 0.016) (Figure 2D), but not CSS (HR = 0.796, 95% CI 0.508–1.245 p = 0.317) (Table 4); similarly, patients with radiotherapy demonstrated greater OS (HR = 0.636, 95% CI 0.466–0.866, p = 0.004) (Figure 2E) than those without it, but not CSS (HR = 0.737, 95% CI 0.475–1.143, p = 0.173) (Table 4). However, we found little evidence supporting that surgical interventions improved the survival outcomes. The gender and race of the patients, as well as the laterality of the malignancy, were not linked with mortality (Tables 3, 4).
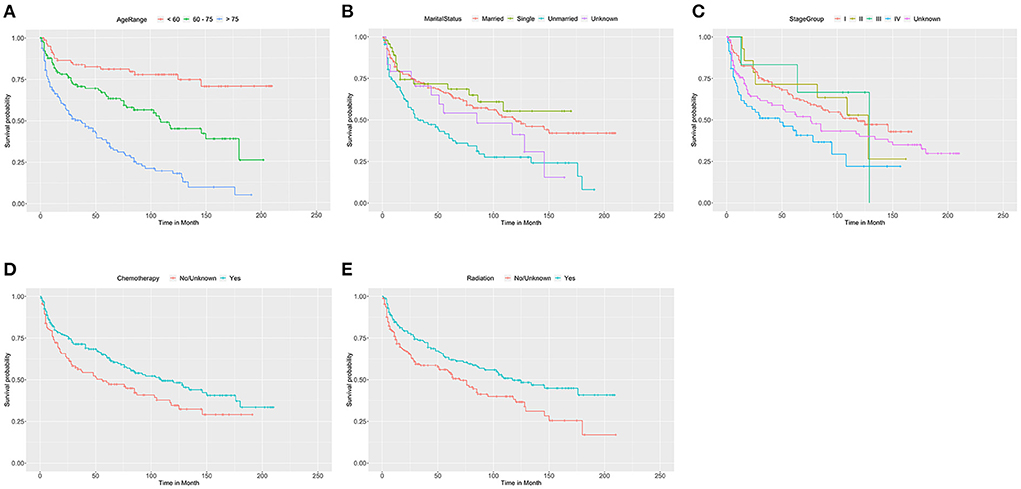
Figure 2. The Kaplan-Meier survival curve of OS in patients with orbital DLBCL stratifed by (A) age at diagnosis, (B) marital status, (C) stage, (D) chemotherapy, and (E) radiation. It was revealed that older age, unmarried status and stage IV were associated with worse OS compared by log-rank test. In (A), 60-75 vs< 60 years (p < 0.001); >75 vs. <60 years (p < 0.001). In (B), Unmarried vs. Married (p < 0.001); Single vs. Married (p = 0.520); Unknown vs. Married (p = 0.221). In (C), stage IV vs. stage I (p = 0.001); stage II vs. stage I (p = 0.919); and stage III vs. stage I (p = 0.853), and unknown vs. stage I (p = 0.089). In (D,E), chemotherapy (p = 0.016) and radiation therapy (p = 0.004) had significantly better OS than those without it.
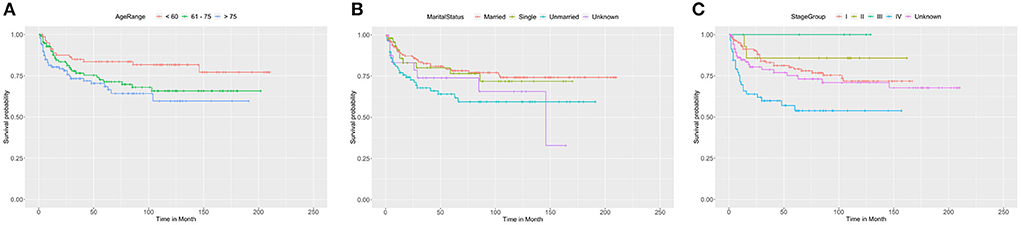
Figure 3. The Kaplan-Meier survival curve of CSS in patients with orbital DLBCL stratifed by (A) age at diagnosis, (B) marital status, (C) stage. It was revealed that old age, unmarried status, and stage IV were associated with worse CSS compared by log-rank test. In (A), >75 vs. <60 years (p = 0.007); 60-75 vs< 60 years (p = 0.074). In (B), Unmarried vs. Married (p = 0.006); Single vs. Married (p = 0.787); Unknown vs. Married (p = 0.161). In (C), stage IV vs. stage I (p < 0.001); stage II vs. stage I (p = 0.460); stage III vs. stage I (p = 0.994); unknown vs. stage I (p = 0.345).
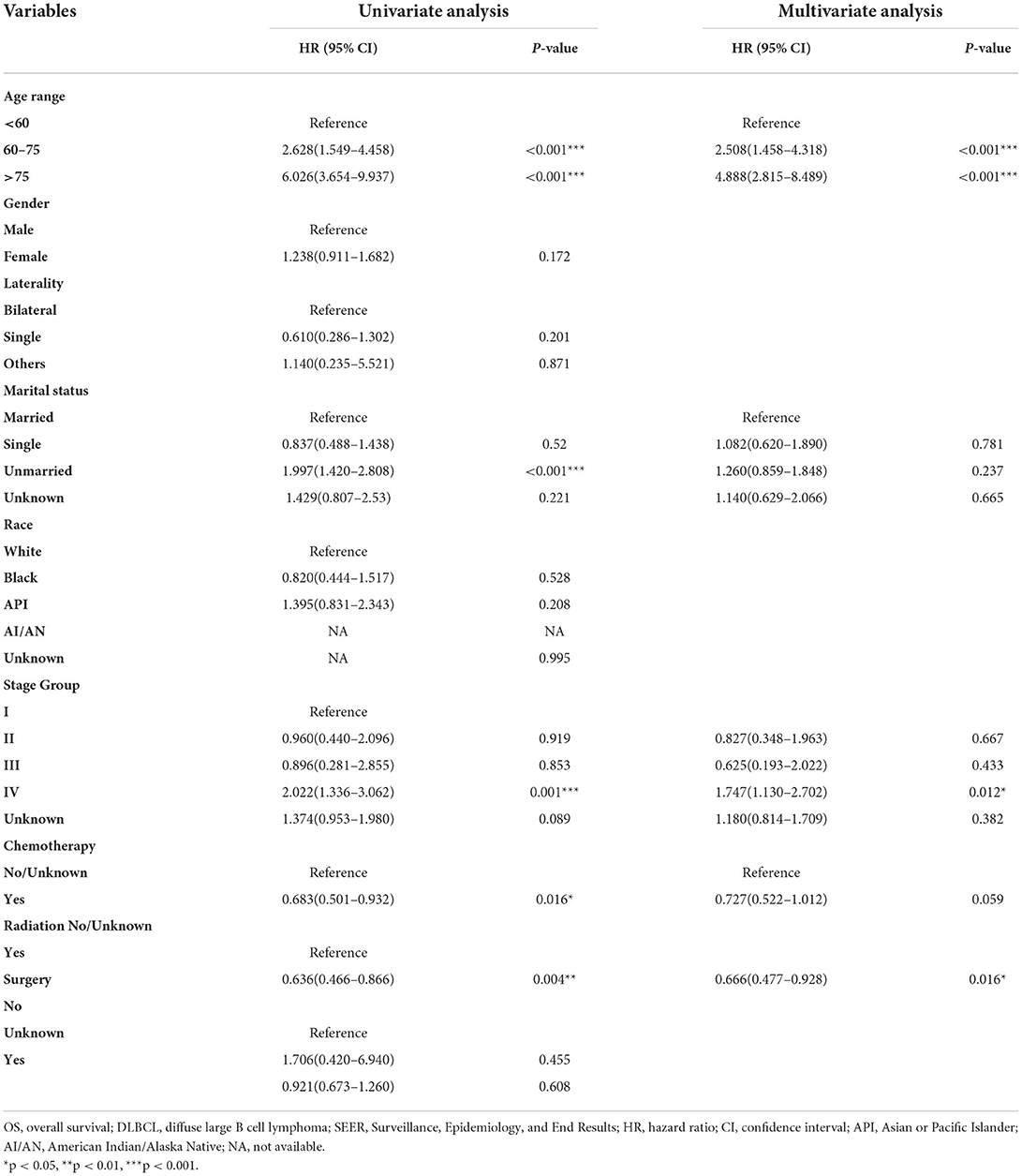
Table 3. Univariate and multivariate analysis of factors with the risk of OS for orbital DLBCL patients identified in the SEER Program database from 2000 to 2017.
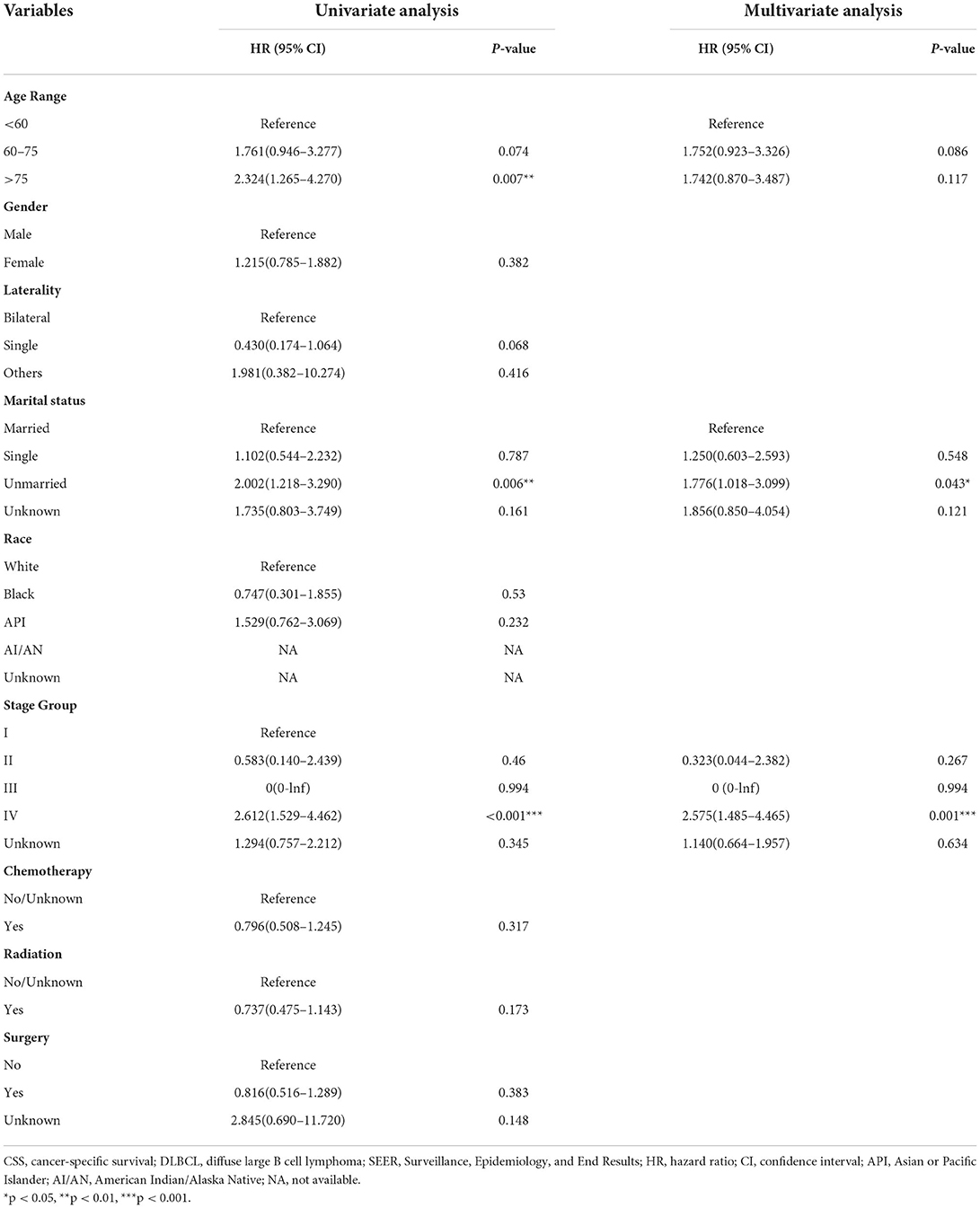
Table 4. Univariate and multivariate analysis of factors with the risk of CSS for orbital DLBCL patients identified in the SEER Program database from 2000 to 2017.
Tables 3, 4 display the results of multivariate survival analysis in summary. The advanced age of diagnosis (dichotomized at <60, 61–75, and >75 years) acted as an independent prognostic factor of OS (61–75 vs. <60 years, HR = 2.508, 95% CI 1.458–4.318, p = 0.001; >75 vs. <60 years, HR = 4.888, 95% CI 2.815–8.489, p < 0.001). Furthermore, stage IV orbital DLBCL patients were more likely to experience worse survival than stage I patients; we found that in both OS (HR = 1.747, 95% CI 1.130–2.702, p = 0.012) (Table 3) and CSS (HR = 2.575, 95% CI 1.485–4.465, p = 0.001) (Table 4). Radiotherapy was an independent prognostic factor indicating greater OS (HR = 0.666, 95% CI 0.477–0.928, p = 0.016) (Table 3). In addition, widowed or divorced patients showed lower CSS (HR = 1.776, 95% CI 1.018–3.099, p = 0.043) than married patients (Table 4).
Discussion
DLBCL is acknowledged as a malignancy of mature B lymphocytes and is one of the most prevalent subtypes of NHL, accounting for 25% of all NHL cases (13). DLBCL has been shown to be more frequent among male patients (56.0%) and Whites (85.5%) in the US (14). The predominant primary site of DLBCL was nodal in ~58% of the cases, extra-nodal in 42%, and extra-nodal extramedullary in 40% (15, 16). Orbital DLBCL is the second-most common NHL in orbit and a high-grade aggressive malignancy with the worst prognosis of any POL subtype (10). Several primary sites of extra-nodal DLBCL involvement, including the lung, kidney, adrenal, and ovary, are frequently aggressive and indicate disseminated diseases. Thyroid, gastric, and orbital DLBCL, on the other hand, indicate a relatively better prognosis under standard treatments (17). Our study is the first to investigate the clinical characteristics and prognosis of orbital DLBCL in such a large cohort.
It was found that the mean age of orbital DLBCL patients (68.9 years) exceeded the mean age of both nodal DLBCL (64.1 years) and other extra-nodal DLBCL patients (65.4 years) in this study. Thus, it is reasonable to suspect orbital DLBCL in these elderly patients. These results revealed that patients aged more than 60 years were more tend to have orbital DLBCL. In addition, the age range of our cohort patients was 15 to 97years (median age: 70 years), which is older than the median age of nodal DLBCL and extra-nodal DLBCL (66.0 and 68.0 years, respectively). We found that patients with extra-nodal DLBCL were remarkably older than those nodal DLBCL patients (15). Accordingly, we speculated that patients with orbital DLBCL represent one of the subgroups of extra-nodal DLBCL that arises later in life. Several factors might have contributed to this situation. First, the rate of inflammatory progression may have increased over time, which is currently recognized as a prominent risk for morbidity and mortality in the elderly (18). Moreover, it has been demonstrated that older age was an independent predictor of worse CSS in primary testicular DLBCL (19). It has been proven, particularly, that older age is an important predictor for patients with orbital lymphomas (3). Past studies have shown that advanced age conferred a poor prognosis in orbital lymphomas. The 5-year OS rate in this research was 57.85%, which is consistent with the findings in the former DLBCL research, wherein ocular adnexal DLBCL patients received the same treatments and showed a 5-year OS rate of 54–55.9% (20, 21). The outcomes of the univariate analysis revealed that patients above the age of 60 years had significantly worse OS and CSS. Furthermore, the result of multivariate analysis showed that older age was an independent predictor for worse OS in orbital DLBCL patients, but not for worse CSS.
Moreover, we observed that unmarried status was an independent predictor for poor outcomes in orbital DLBCL patients, which supported the theory that unmarried, widowed, or divorced patients had a greater risk of disease-related death and tumor metastases than married patients (22). Widowed Hodgkin lymphoma (HL) patients had poorer survival outcomes than married or others, and their marital status indicated an essential role in HL prognosis (23). Psychosocial factors might explain why marital status is relevant with a worse prognosis in lymphoma patients. Stress has been demonstrated to influence physical health (24, 25). Insufficient psychosocial support and excessive psychological stress were discovered to jeopardize the immune functions and resulted in death (26, 27). As psychosocial stress may be one of the predominant factors contributing to the high mortality rate of widowed or divorced patients, greater social support and assistance are needed for these patients.
Notably, the tumor stage can affect the survival outcome of orbital DLBCL. In both univariate and multivariate analyses, the advanced stage (stage IV) was related to a worse prognosis in our current investigation. Patients with stage IV orbital DLBCL showed lower OS and CSS than those with stage I lymphoma (20).
Orbital DLBCL, like other types of lymphoma, is treated with chemotherapy, radiotherapy, and surgery (1, 28). Chemotherapy is the most commonly utilized treatment in this cohort and notably improved prognosis. These findings were consistent with the findings of other extra-nodal DLBCL types, such as primary renal DLBCL (29), urinary tract DLBCL (30), adrenal DLBCL (31), and intestinal DLBCL (32). Furthermore, chemotherapy is the predominantly used treatment option for nodal DLBCL (33). Nevertheless, a former study demonstrated that the effectiveness of chemotherapy was determined by the primary site of extra-nodal NHL; they found no significant improvement among genitourinary DLBCL patients who received chemotherapy treatment (34). However, the combination of surgery and chemotherapy may cause a variance, and there is no specific data illustrating the application of other treatments in this case. Therefore, we need to conduct more valid studies in the future to elucidate the benefit of chemotherapy on the survival of patients with orbital DLBCL.
Furthermore, radiation was found to be advantageous for the survival of orbital DLBCL patients in our investigation. The patients who received radiation manifested more significant outcomes than those who did not. More than half of the orbital lymphoma patients with high-grade DLBCL (56.0%) received radiotherapy compared to 47.9% in our cohort (1). Nevertheless, our data lacked specific information on the frequency and extent of the radiation. Thus, additional studies are warranted to determine the influence of radiation treatment on the prognosis of orbital DLBCL.
We also observed that surgery did not influence the survival outcomes of patients with orbital DLBCL. Surgery is majorly performed in conjunction with other treatment modalities, particularly chemotherapy or radiotherapy. Surgery is not always employed in the treatment of orbital lymphoma and seldom used as the only treatment modality. It was reported that 30% of DLBCLs receive an excision (total or partial) of the neoplasm (1). The data we collected from the SEER database did not describe the specific surgical types, and it was difficult to clarify the survival disparities noted in the current cohort. Since orbitotomy surgery includes biopsy, the majority of the procedures in our cohort were most likely merely biopsies that did not affect the prognosis.
There were several limitations in our study. First, it was a retrospective analysis, the retrospective data has intrinsic reporting biases. Second, chemotherapy is the mainstay of treatment for the management of orbital DLBCL. However, records on chemotherapy delivery are sparse, and recovery of these missing data may potentially upgrade our study's prediction model. There were also main limitations of the SEER chemotherapy and radiotherapy data, such as the completeness of the variables, and the biases associated with who receives treatment. The other limitation of the study is the unavailability of data about the laboratory values, clinical course, and the International Prognostic Index (IPI) (35). Furthermore, the disease's natural history was not complete in all cases (36). It, therefore, remains ambiguous whether the difference in our cohort's survival outcomes was influenced by these factors or by other unaccounted confounding factors. Thus, cohort studies with a larger sample size are warranted to validate the present findings.
Conclusion
In summary, the present study is the largest cohort to clarify the influences of demographic characteristics and clinical presentations on the prognosis of patients with orbital DLBCL. We hope that, through comparison of orbital DLBCL to nodal DLBCL and extra-nodal DLBCL (without the orbit) patients, we may assist clinical ophthalmologists and oncologists in better managing patients with orbital DLBCL. Older age, unmarried status, and stage IV were identified as the crucial factors conferring a poor prognosis of primary orbital DLBCL. Patients treated with chemotherapy or radiation therapy showed a longer life expectancy than those who did not. Further studies are however needed to validate the present observations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further enquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-QC, FT, and R-LW contributed to the conception, design, and drafted the manuscript. Y-QC, Z-FY, and S-NC analyzed the data. Y-QC, W-HY, and R-LW contributed with a critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants from the National Natural Science Foundation of China (81770959 and 81570885), Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP014), and Sanming Project of Medicine in Shenzhen (SZSM202011015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol. (2019) 64:45–66. doi: 10.1016/j.survophthal.2018.08.002
2. Fitzpatrick PJ, Macko S. Lymphoreticular tumors of the orbit. Int J Radiat Oncol Biol Phys. (1984) 10:333–40. doi: 10.1016/0360-3016(84)90051-8
3. Ahmed OM, Ma AK, Ahmed TM, Pointdujour-Lim R. Epidemiology, outcomes, and prognostic factors of orbital lymphoma in the United States. Orbit. (2020) 39:397–402. doi: 10.1080/01676830.2019.1704032
4. Martinet S, Ozsahin M, Belkacemi Y, Landmann C, Poortmans P, Oehlere C, et al. Outcome and prognostic factors in orbital lymphoma: a Rare Cancer Network study on 90 consecutive patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. (2003) 55:892–8. doi: 10.1016/S0360-3016(02)04159-7
5. Kiesewetter B, Lukas J, Kuchar A, Mayerhoefer ME, Streubel B, Lagler H, et al. Clinical features, treatment and outcome of mucosa-associated lymphoid tissue (MALT) lymphoma of the ocular adnexa: single center experience of 60 patients. PLoS ONE. (2014) 9:e104004. doi: 10.1371/journal.pone.0104004
6. Karlin J, Peck T, Prenshaw K, Portell CA, Kirzhner M. Orbital mantle cell lymphoma presenting as myasthenia gravis. Orbit. (2017) 36:365–9. doi: 10.1080/01676830.2017.1337202
7. Sriram PR. A Rare Case of Aggressive, Huge Primary Orbital Lymphoma with Intracranial Extension and Bone Invasion. Asian J Neurosurg. (2017) 12:766–8. doi: 10.4103/1793-5482.185055
8. Eckardt AM, Lemound J, Rana M, Gellrich NC. Orbital lymphoma: diagnostic approach and treatment outcome. World J Surg Oncol. (2013) 11:73. doi: 10.1186/1477-7819-11-73
9. Freedman K, Shenoy S. Mucosa-associated lymphoid tissue lymphoma with intraocular and orbital involvement: case presentation and review of the literature. Orbit. (2018) 37:243–7. doi: 10.1080/01676830.2017.1383479
10. Olsen TG, Holm F, Mikkelsen LH, Rasmussen PK, Coupland SE, Esmaeli B, et al. Orbital Lymphoma-An International Multicenter Retrospective Study. Am J Ophthalmol. (2019) 199:44–57. doi: 10.1016/j.ajo.2018.11.002
11. Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer. (2014) 120 Suppl 23:3755–7.
12. Altekruse SF, Rosenfeld GE, Carrick DM, Pressman EJ, Schully SD, Mechanic LE, et al. SEER cancer registry biospecimen research: yesterday and tomorrow. Cancer Epidemiol Biomarkers Prev. (2014) 23:2681–7. doi: 10.1158/1055-9965.EPI-14-0490
13. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
14. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. (2006) 107:265–76. doi: 10.1182/blood-2005-06-2508
15. Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation–a population-based study of 1575 cases. Br J Haematol. (2004) 124:151–9. doi: 10.1046/j.1365-2141.2003.04749.x
16. Lopez-Guillermo A, Colomo L, Jimenez M, Bosch F, Villamor N, Arenillas L, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. (2005) 23:2797–804. doi: 10.1200/JCO.2005.07.155
17. Ollila TA, Olszewski AJ. Extranodal diffuse large B cell lymphoma: molecular features, prognosis, and risk of central nervous system recurrence. Curr Treat Options Oncol. (2018) 19:38. doi: 10.1007/s11864-018-0555-8
18. Fulop T, Witkowski JM, Olivieri F, Larbi A. The integration of inflammaging in age-related diseases. Semin Immunol. (2018) 40:17–35. doi: 10.1016/j.smim.2018.09.003
19. Gundrum JD, Mathiason MA, Moore DB, Go RS. Primary testicular diffuse large B-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol. (2009) 27:5227–32. doi: 10.1200/JCO.2009.22.5896
20. Madge SN, McCormick A, Patel I, Hatef E, Menon V, Prabhakaran VC, et al. Ocular adnexal diffuse large B-cell lymphoma: local disease correlates with better outcomes. Eye (Lond). (2010) 24:954–61. doi: 10.1038/eye.2009.283
21. Precupanu CM, Validire P, Levy C, Plancher C, Vincent-Salomon A, Dendale R, et al. Primary high-grade ocular adnexal lymphoma: clinicopathological characteristics and prognostic factors of a single-centre series. Am J Hematol. (2010) 85:372–5. doi: 10.1002/ajh.21673
22. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. (2013) 31:3869–76. doi: 10.1200/JCO.2013.49.6489
23. Wang F, Xie X, Yang X, Jiang G, Gu J. The influence of marital status on the survival of patients with Hodgkin lymphoma. Oncotarget. (2017) 8:51016–23. doi: 10.18632/oncotarget.16879
24. Molloy GJ, Stamatakis E, Randall G, Hamer M. Marital status, gender and cardiovascular mortality: behavioural, psychological distress and metabolic explanations. Soc Sci Med. (2009) 69:223–8. doi: 10.1016/j.socscimed.2009.05.010
25. Thoits PA. Stress and health: major findings and policy implications. J Health Soc Behav. (2010) 51:S41–53. doi: 10.1177/0022146510383499
26. Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. (2010) 6:1863–81. doi: 10.2217/fon.10.142
27. Jaremka LM, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology. (2013) 38:2713–9. doi: 10.1016/j.psyneuen.2013.06.031
28. Bhatia S, Paulino AC, Buatti JM, Mayr NA, Wen BC. Curative radiotherapy for primary orbital lymphoma. Int J Radiat Oncol Biol Phys. (2002) 54:818–23. doi: 10.1016/S0360-3016(02)02966-8
29. Chen J, Peng J, Zheng Y, Li S, Yang P, Wu X, et al. Primary renal lymphoma: a population-based study in the United States, 1980-2013. Sci Rep. (2019) 9:15125. doi: 10.1038/s41598-019-51635-6
30. Liu ZH, Yang LC, Song P, Fang K, Zhou J, Peng ZF, et al. Primary diffuse large B-cell lymphoma of the urinary tract: a population-based analysis. Front Oncol. (2021) 11:609882. doi: 10.3389/fonc.2021.609882
31. Li S, Wang Z, Wu Z, Zhuang H, Xu Y. Clinical characteristics and outcomes of primary adrenal diffuse large B cell lymphoma in a large contemporary cohort: a SEER-based analysis. Ann Hematol. (2019) 98:2111–9. doi: 10.1007/s00277-019-03740-9
32. Wang M, Ma S, Shi W, Zhang Y, Luo S, Hu Y. Surgery shows survival benefit in patients with primary intestinal diffuse large B-cell lymphoma: a population-based study. Cancer Med. (2021) 10:3474–85. doi: 10.1002/cam4.3882
33. Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. (2007) 110:29–36. doi: 10.1182/blood-2007-01-041871
34. Olszewski AJ, Winer ES, Castillo JJ. Improved survival with rituximab-based chemoimmunotherapy in older patients with extranodal diffuse large B-cell lymphoma. Leuk Res. (2014) 38:866–73. doi: 10.1016/j.leukres.2014.04.009
35. Rasmussen PK, Ralfkiaer E, Prause JU, Sjo LD, Toft PB, Siersma VD, et al. Diffuse large B-cell lymphoma of the ocular adnexal region: a nation-based study. Acta Ophthalmol. (2013) 91:163–9. doi: 10.1111/j.1755-3768.2011.02337.x
36. Meunier J, Lumbroso-Le Rouic L, Vincent-Salomon A, Dendale R, Asselain B, Arnaud P, et al. Ophthalmologic and intraocular non-Hodgkin's lymphoma: a large single centre study of initial characteristics, natural history, and prognostic factors. Hematol Oncol. (2004) 22:143–58. doi: 10.1002/hon.741
Keywords: diffuse large B-cell lymphoma, primary orbital lymphoma, survival, prognosis, SEER
Citation: Chen Y-Q, Yue Z-F, Chen S-N, Tong F, Yang W-H and Wei R-L (2022) Primary diffuse large B-cell lymphoma of orbit: A population-based analysis. Front. Med. 9:990538. doi: 10.3389/fmed.2022.990538
Received: 10 July 2022; Accepted: 19 August 2022;
Published: 15 September 2022.
Edited by:
Dario Rusciano, Consultant, Catania, ItalyReviewed by:
Stefania Marsili, Georgia Institute of Technology, United StatesMassimo Dal Monte, University of Pisa, Italy
Copyright © 2022 Chen, Yue, Chen, Tong, Yang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Li Wei, ruiliwei@smmu.edu.cn; Wei-Hua Yang, benben0606@139.com
†These authors have contributed equally to this work and share first authorship
 Yu-Qing Chen
Yu-Qing Chen Zi-Fan Yue
Zi-Fan Yue Sai-Nan Chen
Sai-Nan Chen Fei Tong
Fei Tong Wei-Hua Yang
Wei-Hua Yang Rui-Li Wei
Rui-Li Wei