Successful treatment of acute respiratory distress syndrome caused by hypervirulent Klebsiella pneumoniae with extracorporeal membrane oxygenation and continuous renal replacement therapy: A case report and literature review
- 1Department of Respiratory Medicine, National Key Clinical Specialty, Branch of National Clinical Research Center for Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China
- 2Center of Respiratory Medicine, Xiangya Hospital, Central South University, Changsha, China
- 3Clinical Research Center for Respiratory Diseases in Hunan Province, Xiangya Hospital, Central South University, Changsha, China
- 4Hunan Engineering Research Center for Intelligent Diagnosis and Treatment of Respiratory Disease, Xiangya Hospital, Central South University, Changsha, China
- 5National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Hypervirulent Klebsiella pneumoniae (hvKP) causes invasive infections and leads to high morbidity and mortality rates. Here, we report the case of a Chinese man with diabetes mellitus who developed acute respiratory distress syndrome and septic shock due to hvKP belonging to the K1 strain. The patient was treated with venovenous extracorporeal membrane oxygenation and continuous renal replacement therapy, in combination with antibiotics and recovered well. Clinicians should be aware of fatal infections caused by hvKP and investigate the best treatment options for patients at various stages of infection.
Background
The emergence of hypervirulent Klebsiella pneumoniae (hvKP) poses a significant challenge to public health (1). Additionally, hvKP can cause fatal systemic infections. In contrast to classical KP, hvKP displays hypervirulent phenotypic and genotypic characteristics, namely, a hypermucoviscous phenotype and the presence of different virulence genes (2).
Hypervirulent K. pneumoniae can cause community-acquired or nosocomial infections in both relatively healthy individuals (3) and those with underlying diseases such as diabetes mellitus (4). It may result in septic shock and multi-organ failure, which may be life-threatening.
Here, we report the case of a patient infected with hvKP belonging to serotype K1 and with virulence-associated genes iutA and rmpA. He developed septic shock, acute respiratory distress syndrome (ARDS), and acute renal failure within a short time, and was successfully treated with veno-venous extracorporeal membrane oxygenation (vv-ECMO) and continuous renal replacement therapy (CRRT) in combination with antibiotics.
Case presentation
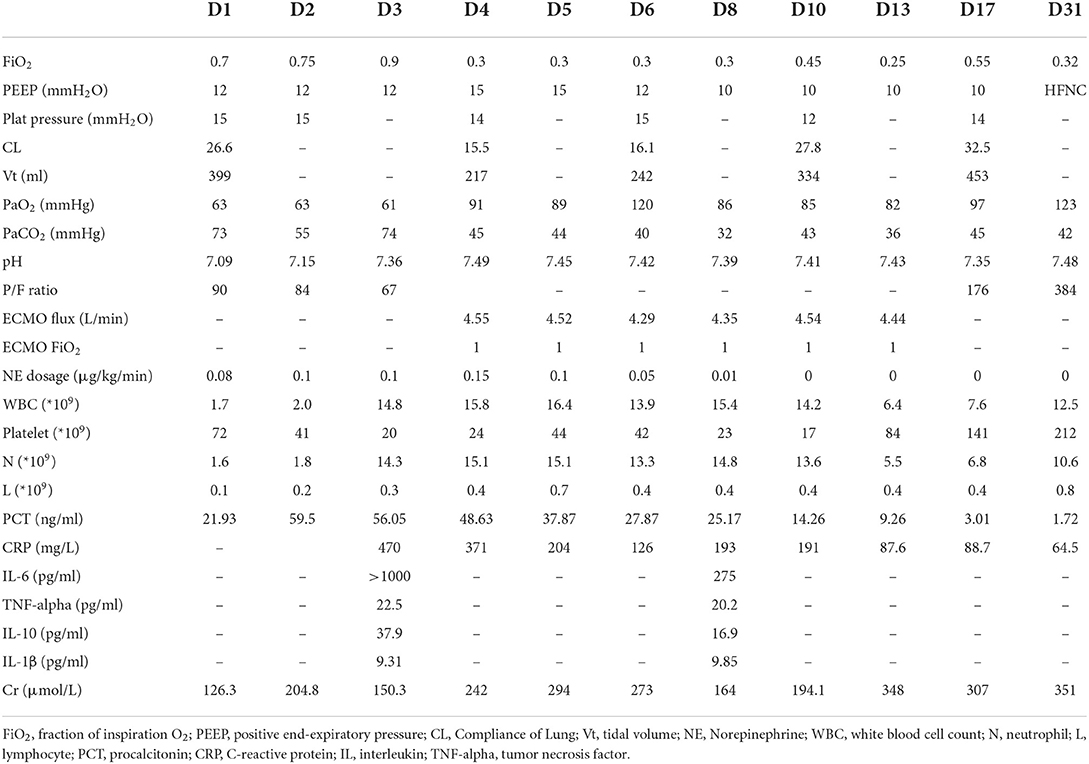
The patient was a 52-year-old man who was admitted to the emergency room because of fever for 4 days and chest pain for 1 day. The patient had type 2 diabetes mellitus. He developed dyspnea with reduced oxygen saturation, and his blood pressure also declined the next morning; therefore, he was admitted to the respiratory intensive care unit (RICU). His temperature was 36.6°C, pulse rate 128 beats/min, respiratory rate 26 times/min, and blood pressure 113/62 mmHg (with a norepinephrine dose of 0.05 μg/kg/min). Coarse crackles were heard in both lungs. Endophthalmitis in the left eye was verified during ophthalmological consultation. Laboratory examinations revealed the following: white blood cell count, 1.7 × 109/L; neutrophil counts 1.6 × 109/L; lymphocyte counts, 0.1 × 109/L; platelets, 72 × 109/L; procalcitonin, 59.5 ng/ml; IL-6 > 1,000 pg/ml; TNF-α, 22.5 pg/ml; IL-1β, 9.31 pg/ml; IL-10, 37.9 pg/ml; and C-reactive protein, 470 mg/L. K. pneumoniae with a hypermucoviscous phenotype was isolated from the blood and bronchoalveolar lavage fluid (BALF) cultures. PCR and metagenomic next-generation sequencing (mNGS) of BALF identified a sequence type (ST) 23 serotype K1 strain with virulence-associated genes iutA (aerobactin) and rmpA (regulator of mucoid phenotype), the drug resistance gene blaSH was also positive. CT scan of the abdomen and brain revealed no abscess. Tracheal intubation and mechanical ventilation were performed promptly. Oliguria, a rapid decline in renal function (serum creatinine increase >100% within 48 h), and severe acidosis (pH 7.08–7.15) occurred; hence, CRRT was initiated on the 2nd day. Prone position and recruitment maneuver were attempted to improve oxygenation, but arterial blood gas analysis indicated continuous deterioration in the P/F ratio, pH, and hypercapnia.
On the 3rd day after admission, vv-ECMO was performed following the assessment of cardiac function by ultrasonography, and hypoxemia and hypercapnia were corrected promptly. The prone position, CRRT, and antibiotics (meropenem and levofloxacin based on antimicrobial susceptibility test findings) were continued for the next few days. The patient's condition gradually improved, and ECMO was withdrawn successfully on the 17th day. He underwent tracheostomy on the 21st day, was supported by a ventilator and high flow nasal cannula (HFNC) in turn, and subsequently moved to full HFNC support on the 29th day. He was transferred out of the RICU on the 41st day (Table 1; Figure 1). In the follow-up, his lung (Figure 1) and renal functions were gradually restored, and his serum creatinine level dropped to 143 μmol/L on 26 April 2022, but the vision of the left eye was lost.
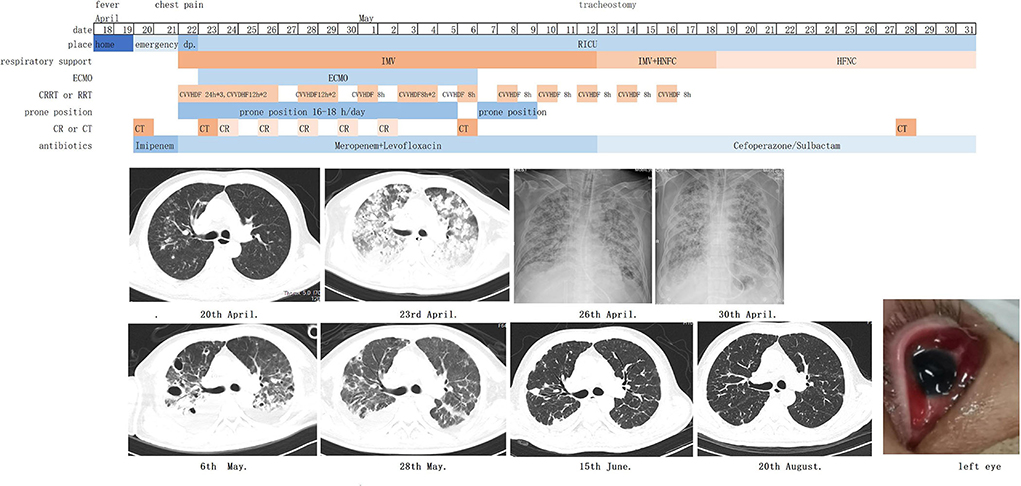
Figure 1. Timeline of courses and treatments. IMV, invasive mechanical ventilation; HFNC, high-flow nasal cannula oxygen therapy; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy; CVVHDF, continuous venovenous hemodiafiltration; CT, computerized tomography; CR, Chest radiography.
Discussion
Here, we present the case of a diabetic patient infected with hvKP in the community. The patient developed ARDS and septic shock in a short time. After 41 days of intensive care and support, he survived with good recovery of the lung and kidney functions but lost vision in his left eye.
Hypervirulent K. pneumoniae was defined as the hypermucoviscosity phenotype (string test showing a positive result) (5). Other features associated with hvKP include K1 or K2 serotype, overexpression of rmpA (regulator of mucoid phenotype), and stealth siderophore biosynthesis (5). The hvKP strain in our case belonged to serotype K1 with virulence genes iutA and rmpA. K1 and K2 strains are the dominant strains causing community-onset infections (6). K1 is more prevalent in Asian countries whereas K2 is more prevalent in Europe (7, 8). The K1 strain is associated with a higher incidence of liver abscess (8). Numerous studies have identified virulence genes of hvKP over the past few decades (6, 9). Since not all hvKP have the hypermucoviscous phenotype, some studies have defined the hvKP based on the positivity of virulence genes, namely, the combination of peg-344, iroB, iucA, rmpA, and rmpA2 (6, 9). RmpA is a gene that regulates the synthesis of extracellular polysaccharide capsules and is responsible for hypermucoviscosity and is found in 95.1–99.4% of hvKP (6, 10). IutA is a gene encoding the aerobactin system, which is crucial for growth and infection (11), and is present in 85.3–90.7% of hvKP (10, 12). Concurrence of rmpA and iutA may increase the risk of developing a severe form of infection (13). In our case, we confirmed the presence of virulence genes in BALF by NGS. In the conventional method, we should obtain a positive culture of the specimen, and PCR is needed for the identification of the serotype, sequence type, drug resistance genes, and virulence genes, which is time-consuming. NGS can be used to analyze capsular serotypes and identify virulence-associated genes and drug resistance genes simultaneously (14). Deoxyribonucleic acid extracted from clinical specimens can directly be processed for NGS analysis. Therefore, metagenomics is advantageous for the identification of the strain and virulence of the hvKP in contrast to traditional cultivation (15). The use of NGS may help in the early and accurate identification of hvKP, ultimately improving patients' outcomes.
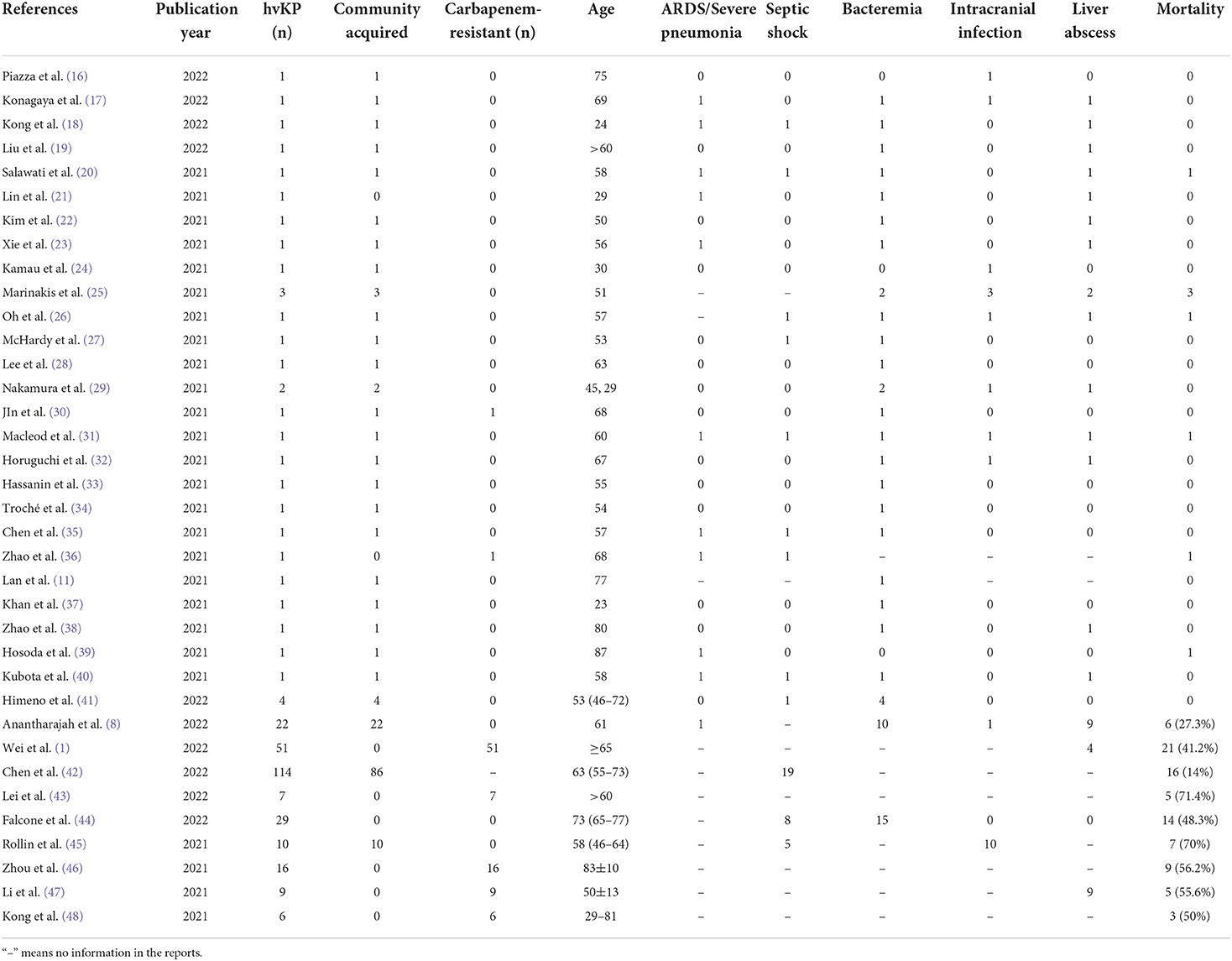
Hypervirulent K. pneumoniae has spread across Asian countries such as China, Japan, and South Korea, with sporadic but increasing rates reported elsewhere. We have summarized the reports on the hvKP from 1 January 2021 to 30 April 2022 in Table 2. The hvKP can cause severe community or hospital-acquired infections (49, 50). The risk factors include renal insufficiency (42, 51), diabetes (52), age ≥ 65 years (1), and chronic alcoholism (53). HvKP causes tissue invasive infection, often involving multiple sites. Septic shock and multi-organ failure are more common in patients infected with hvKP than in those infected by classical K. pneumoniae (cKP). The mortality rate of hvKP infection is higher in the elderly and those who are infected with carbapenem-resistant strains, but it appears to be similar in the general population between carbapenem-sensitive hvKP and cKP groups (12). In our study, the patient had type 2 diabetes without taking any hypoglycemics. He developed disseminated infections in the lungs and left eye. The patient progressed to septic shock and multi-organ failure. He also manifested a surge in inflammatory markers and prolonged thrombocytopenia.
Veno-venous extracorporeal membrane oxygenation is increasingly used in patients with severe ARDS to correct life-threatening hypoxemia and serves as a bridge for recovery. However, vv-ECMO may not always be appropriate in patients with ARDS and septic shock. In the Piotr Suwalski study (54), 7.1% of patients on vv-ECMO due to ARDS caused by COVID-19 were converted to venoarterial (va)-ECMO when septic shock developed. Han (55) reported 23 patients with refractory septic shock treated with va-ECMO, and five patients survived. Falk et al. (56) showed that patients with septic shock with or without left ventricular failure benefited from va-ECMO more than vv-ECMO, but in their study, 6 of the 10 patients with vv-ECMO survived, possibly because vv-ECMO could improve heart function by increasing cardiac oxygenation. In our case, the patient experienced ARDS and septic shock due to hvKP. His heart function was assessed using echocardiography, and the results showed no left or right ventricular dysfunction. His norepinephrine dose was <0.2 μg/kg/min. Therefore, we decided to establish vv-ECMO, and the patient improved and avoided transitioning to V-A or hybrid ECMO. Patients with refractory septic shock (high dose of vasopressin, and inability to sustain mean arterial pressure ≥65 mmHg) should be treated with va-ECMO; however, for those with a low dose of vasopressin and no heart dysfunction, vv-ECMO may also be adequate to improve the patients' outcome.
Approximately 41.4–64.4% of critically ill patients with severe sepsis or septic shock develop acute kidney injury (57, 58). Renal replacement therapy (RRT) is widely used for these patients. However, the optimal RRT modality remains controversial. A recent meta-analysis showed no significant difference in patients and kidney survival among patients receiving CRRT, sustained low-efficiency dialysis, or intermittent hemodialysis (59). Studies on early vs. late initiation of RRT revealed no benefit for patients who start early RRT (60, 61). However, for patients with complications such as obvious fluid overload, acute pulmonary edema, severe acidosis, and severe hyperkalemia, RRT may be performed urgently. Regarding CRRT, investigators had to provide treatment continuously for 24 h with a change of membranes at least every 72 h, and a minimum ultrafiltration (or dialysate) rate of 25 ml/kg/h was recommended (61, 62). For intermittent hemodialysis, the length of the session had to be 4–6 h or more, and the frequency had to be at least once every 48 h. Recommendations were to set a blood flow rate of 150–250 ml/min, and a dialysate flow rate of 300–500 ml/min (61, 62). Across the studies, RRT dependence among survivors was about 5.9–23.1% in 28 days, lowering to 2–13.4% in 90 days (61–63). In our case, the patient developed oliguria, pulmonary edema, and severe acidosis on the 1st day after admission to the RICU. The average fluid intake of the patient was >2,500 ml/day in the first week, and early initiation of CRRT could control the fluid disturbances and prevent the deterioration of lung oxygenation and heart function. We treated the patient with continuous techniques in the first 72 h and changed to intermittent hemodialysis for 8–12 h each time on the next days (Figure 1). The patient's kidney functions gradually recovered and RRT was discontinued on the 25th day. Like COVID-19 infection, inflammatory mediators play an important role in sepsis and septic shock, exacerbating organ damage and correlating with disease severity (64). Many clinical strategies have been attempted to reduce inflammatory damage. CRRT with a specific filter may successfully lower the levels of cytokines such as TNF-α, IL-6, IL-8, and IFNγ (65). In our case, hvKP led to a severe cytokine storm with IL-6 > 1,000 pg/ml, and an extended duration of continuous venovenous hemodiafiltration seemed to remove the inflammatory mediators and restore the homeostasis of the patient. Whether the combination of CRRT and ECMO can improve the outcome of patients with septic shock and ARDS, and when and which modality should be initiated requires further study.
Conclusion
This is a successful case of treating severe infection and multi-organ failure caused by hvKP, demonstrating that vv-ECMO and CRRT may improve the outcomes in this group of patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Boards (IRBs) in Xiangya Hospital, Central South University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WP and YW reviewed the lectures and wrote the manuscript. RL and YZ collected and arranged the materials. JC and PP viewed the complete manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Key R&D Program of China (No. 2016YFC1304204); Key Program of Hunan Province (No. 2022SK2038); the Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant No. 2020LNJJ05).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wei T, Zou C, Qin J, Tao J, Yan L, Wang J, et al. Emergence of hypervirulent ST11-K64 Klebsiella pneumoniae poses a serious clinical threat in older patients. Front Public Health. (2022) 10:765624. doi: 10.3389/fpubh.2022.765624
2. Arabaghian H, Salloum T, Alousi S, Panossian B, Araj GF, Tokajian S. Molecular characterization of carbapenem resistant Klebsiella pneumoniae and Klebsiella quasipneumoniae isolated from Lebanon. Sci Rep. (2019) 9:531. doi: 10.1038/s41598-018-36554-2
3. Shankar C, Veeraraghavan B, Nabarro LEB, Ravi R, Ragupathi NKD, Rupali P. Whole genome analysis of hypervirulent Klebsiella pneumoniae isolates from community and hospital acquired bloodstream infection. BMC Microbiol. (2018) 18:6. doi: 10.1186/s12866-017-1148-6
4. Li J, Ren J, Wang W, Wang G, Gu G, Wu X, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis. (2018) 37:679–89. doi: 10.1007/s10096-017-3160-z
5. Mike LA, Stark AJ, Forsyth VS, Vornhagen J, Smith SN, Bachman MA, et al. A systematic analysis of hypermucoviscosity and capsule reveals distinct and overlapping genes that impact Klebsiella pneumoniae fitness. PLoS Pathog. (2021) 17:e1009376. doi: 10.1371/journal.ppat.1009376
6. Liao CH, Huang YT, Hsueh PR. Multicenter surveillance of capsular serotypes, virulence genes, and antimicrobial susceptibilities of Klebsiella pneumoniae causing bacteremia in Taiwan, 2017-2019. Front Microbiol. (2022) 13:783523. doi: 10.3389/fmicb.2022.783523
7. Talebzadeh H, Mellali H, Solgi H. Association of fluoroquinolone resistance and ESBL production in hypervirulent Klebsiella pneumoniae ST11 and ST893 in Iran. Acta Microbiol Immunol Hung. (2022). doi: 10.1556/030.2022.01638
8. Anantharajah A, Deltombe M, de Barsy M, Evrard S, Denis O, Bogaerts P, et al. Characterization of hypervirulent Klebsiella pneumoniae isolates in Belgium. Eur J Clin Microbiol Infect Dis. (2022). doi: 10.1007/s10096-022-04438-z
9. Bulger J, MacDonald U, Olson R, Beanan J, Russo TA. Metabolite transporter PEG344 is required for full virulence of hypervirulent Klebsiella pneumoniae strain hvKP1 after pulmonary but not subcutaneous challenge. Infect Immun. (2017) 85:e00093-17. doi: 10.1128/IAI.00093-17
10. Zhang S, Zhang X, Wu Q, Zheng X, Dong G, Fang R, et al. Clinical, microbiological, and molecular epidemiological characteristics of Klebsiella pneumoniae-induced pyogenic liver abscess in southeastern China. Antimicrob Resist Infect Control. (2019) 8:166. doi: 10.1186/s13756-019-0615-2
11. Lan P, Zhao D, Gu J, Shi Q, Yan R, Jiang Y, et al. Genome-based analysis of a sequence type 1049 hypervirulent Klebsiella pneumoniae causing bacteremic neck abscess. Front Microbiol. (2020) 11:617651. doi: 10.3389/fmicb.2020.617651
12. Li L, Yuan Z, Chen D, Xie X, Zhang B. Clinical and microbiological characteristics of invasive and hypervirulent Klebsiella pneumoniae infections in a teaching hospital in China. Infect Drug Resist. (2020) 13:4395–403. doi: 10.2147/IDR.S282982
13. Khaertynov KS, Anokhin VA, Davidyuk YN, Nicolaeva IV, Khalioullina SV, Semyenova DR, et al. Case of meningitis in a neonate caused by an extended-spectrum-beta-lactamase-producing strain of hypervirulent Klebsiella pneumoniae. Front Microbiol. (2017) 8:1576. doi: 10.3389/fmicb.2017.01576
14. Yang P, Wu Z, Liu C, Zheng J, Wu N, Wu Z, et al. Clinical outcomes and microbiological characteristics of sequence type 11 Klebsiella pneumoniae infection. Front Med (Lausanne). (2022) 9:889020. doi: 10.3389/fmed.2022.889020
15. Liu J, Xu Z, Li H, Chen F, Han K, Hu X, et al. Metagenomic approaches reveal strain profiling and genotyping of Klebsiella pneumoniae from hospitalized patients in China. Microbiol Spectr. (2022) 2022:e0219021. doi: 10.1128/spectrum.02190-21
16. Piazza A, Perini M, Mauri C, Comandatore F, Meroni E, Luzzaro F, et al. Antimicrobial susceptibility, virulence, and genomic features of a hypervirulent serotype K2, ST65 Klebsiella pneumoniae causing meningitis in Italy. Antibiotics. (2022) 11:261. doi: 10.3390/antibiotics11020261
17. Konagaya K, Yamamoto H, Suda T, Tsuda Y, Isogai J, Murayama H, et al. Ruptured emphysematous prostatic abscess caused by K1-ST23 hypervirulent Klebsiella pneumoniae presenting as brain abscesses: a case report and literature review. Front Med (Lausanne). (2021) 8:768042. doi: 10.3389/fmed.2021.768042
18. Kong L, Wang Y, Ji H, Li Z, Sun Y, Liu Y, et al. A case of forearm soft tissue infection caused by hypervirulent K. pneumoniae in an otherwise healthy 24-year-old woman. Infect Drug Resist. (2022) 15:63–8. doi: 10.2147/IDR.S342019
19. Liu Y, Zhu H, Yin Y, Yan Z. Left eye enucleation caused by multi-systemic Klebsiella pneumoniae invasive syndrome. J Int Med Res. (2022) 50:3000605211069284. doi: 10.1177/03000605211069284
20. Salawati EM. Fatal disseminated pyogenic infection due to hypermucoviscous hypervirulent Klebsiella pneumoniae: a case report and literature review. Clin Case Rep. (2021) 9:e04754. doi: 10.1002/ccr3.4754
21. Lin YC, Cao X, Mo YC, Xie CP, Zhang YF Li N, et al. Successful treatment of hypervirulent Klebsiella pneumoniae bacteremia with combination carbapenem and rifampicin. IDCases. (2021) 26:e01276. doi: 10.1016/j.idcr.2021.e01276
22. Kim M, Yoo JR, Oh H, Kim YR, Lee KH, Heo ST. The first case of abdominal mycotic aneurysm caused by K1 hypervirulent Klebsiella pneumoniae in a healthy adult. Acute Crit Care. (2021) 36:390–4. doi: 10.4266/acc.2021.00010
23. Xie J, Zhu Z. A case report of pyogenic liver abscess caused by hypervirulent Klebsiella pneumoniae diagnosed by metagenomic next-generation sequencing. J Int Med Res. (2021) 49:3000605211032793. doi: 10.1177/03000605211032793
24. Kamau E, Allyn PR, Beaird OE, Ward KW, Kwan N, Garner OB, et al. Endogenous endophthalmitis caused by ST66-K2 hypervirulent Klebsiella pneumoniae, United States. Emerg Infect Dis. (2021) 27:2215–8. doi: 10.3201/eid2708.210234
25. Marinakis G, Kassianidis G, Kafkoula E, Stamatopoulou C, Kavallieratos F, Patrani M, et al. Community-acquired Klebsiella spp meningitis/invasive infection in filipino-descent patients living in Greece: a case series. Eur J Case Rep Intern Med. (2021) 8:002576. doi: 10.12890/2021_002576
26. Oh H, Heo ST, Kim M, Kang CH, Yoo JR. Devastating community-acquired bacterial meningitis caused by hypervirulent Klebsiella pneumoniae in an immunocompetent patient. J Clin Neurol. (2021) 17:484–6. doi: 10.3988/jcn.2021.17.3.484
27. McHardy JA, Selvaganeshapillai V, Khanna P, Whittington AM, Turton J, Gopal Rao G, et al. case of neck abscess caused by rare hypervirulent Klebsiella pneumoniae, capsular type K20 and sequence type 420. Ann Clin Microbiol Antimicrob. (2021) 20:46. doi: 10.1186/s12941-021-00453-8
28. Lee SE, Mushtaq A, Gitman M, Paniz-Mondolfi A, Chung M, Obla A, et al. Lemierre's syndrome associated with hypervirulent Klebsiella pneumoniae: a case report and genomic characterization of the isolate. IDCases. (2021) 25:e01173. doi: 10.1016/j.idcr.2021.e01173
29. Nakamura K, Nomoto H, Harada S, Suzuki M, Yomono K, Yokochi R, et al. Infection with capsular genotype K1-ST23 hypervirulent Klebsiella pneumoniae isolates in Japan after a stay in East Asia: two cases and a literature review. J Infect Chemother. (2021) 27:1508–12. doi: 10.1016/j.jiac.2021.05.011
30. Jin X, Chen Q, Shen F, Jiang Y, Wu X, Hua X, et al. Resistance evolution of hypervirulent carbapenem-resistant Klebsiella pneumoniae ST11 during treatment with tigecycline and polymyxin. Emerg Microbes Infect. (2021) 10:1129–36. doi: 10.1080/22221751.2021.1937327
31. Macleod CK, Khokhar FA, Warne B, Wick R, Butcher R, Cassimon B, et al. Rapid whole genome sequencing of serotype K1 hypervirulent Klebsiella pneumoniae from an undocumented Chinese Migrant. Case Rep Infect Dis. (2021) 2021:6638780. doi: 10.1155/2021/6638780
32. Horiguchi K, Tsurutani Y, Sasaki T, Sunouchi T, Hirose R, Watanabe S, et al. Hypervirulent Klebsiella pneumoniae infection in an elderly patient with diabetes mellitus. Geriatr Gerontol Int. (2021) 21:590–1. doi: 10.1111/ggi.14179
33. Hassanin F, Khawjah D, Elkhamary S, Al Hussain H. Renal abscesses and endogenous endophthalmitis due to hypermucoviscous hypervirulent Klebsiella pneumoniae (HVKP). IDCases. (2021) 24:e01130. doi: 10.1016/j.idcr.2021.e01130
34. Troché G, Henry A, Sarfati F, Hickel C, Amara M, Bruneel F, et al. 54-year-old healthy patient with meningitis and conjunctivitis. J Am Coll Emerg Phys Open. (2021) 2:e12425. doi: 10.1002/emp2.12425
35. Chen Y, Chen J, Zheng N, Chen Y. Contrast-enhanced ultrasound (CEUS) guided drainage in the treatment of a patient with lung abscess secondary to hypervirulent Klebsiella pneumoniae (hvKP) infection: a case report. Respir Med Case Rep. (2021) 32:101343. doi: 10.1016/j.rmcr.2021.101343
36. Zhao J, Zhang Y, Fan Y, Han J, Xiong Z, Liu X, et al. Characterization of an NDM-5-producing hypervirulent Klebsiella pneumoniae sequence type 65 clone from a lung transplant recipient. Emerg Microbes Infect. (2021) 10:396–9. doi: 10.1080/22221751.2021.1889932
37. Khan SF, Lacey JA, Gorrie C, Tong SYC. Genomic sequencing of hypervirulent Klebsiella pneumoniae with novel patterns of virulence and global epidemiological linkage. Pathology. (2021) 53:682–5. doi: 10.1016/j.pathol.2020.10.011
38. Zhao B, Hu R, Gong L, Wang X, Zhu Y, Wu G. Pyogenic liver abscess and endogenous endophthalmitis due to K64-ST1764 hypervirulent Klebsiella pneumoniae: a case report. Infect Drug Resist. (2021) 14:71–7. doi: 10.2147/IDR.S289088
39. Hosoda T, Harada S, Okamoto K, Ishino S, Kaneko M, Suzuki M, et al. COVID-19 and fatal sepsis caused by hypervirulent Klebsiella pneumoniae, Japan, 2020. Emerg Infect Dis. (2021) 27:556–9. doi: 10.3201/eid2702.204662
40. Kubota Y, Ishioka H, Harada S, Suzuki M, Shiotsuka J, Lefor AK, et al. Septic shock with emphysematous cholecystitis and disseminated infection caused by hypervirulent Klebsiella pneumoniae capsular genotype K2-ST65 in a Japanese man with diabetes mellitus: a case report. J Infect Chemother. (2021) 27:350–3. doi: 10.1016/j.jiac.2020.09.017
41. Himeno D, Matsuura Y, Maruo A, Ohtori S. A novel treatment strategy using continuous local antibiotic perfusion: a case series study of a refractory infection caused by hypervirulent Klebsiella pneumoniae. J Orthop Sci. (2022) 27:272–80. doi: 10.1016/j.jos.2020.11.010
42. Chen D, Zhang Y, Wu J, Li J, Chen H, Zhang X, et al. Analysis of hypervirulent Klebsiella pneumoniae and classic Klebsiella pneumoniae infections in a Chinese hospital. J Appl Microbiol. (2022) 132:3883–90. doi: 10.1111/jam.15476
43. Lei J, Zhou WX, Lei K, Chen D, Zhang PQ, Xue L, et al. Analysis of molecular and clinical characteristics of carbapenem-resistant hypervirulent Klebsiella pneumoniae in the intensive care unit. Zhonghua Yu Fang Yi Xue Za Zhi. (2022) 56:63–8. doi: 10.3760/cma.j.cn112150-20210812-00781
44. Falcone M, Tiseo G, Arcari G, Leonildi A, Giordano C, Tempini S, et al. Spread of hypervirulent multidrug-resistant ST147 Klebsiella pneumoniae in patients with severe COVID-19: an observational study from Italy, 2020-21. J Antimicrob Chemother. (2022) 77:1140–5. doi: 10.1093/jac/dkab495
45. Rollin G, Rossi B, Brisse S, Decré D, Leflon-Guibout V, Bert F, et al. Spontaneous and postsurgical/traumatic Klebsiella pneumoniae meningitis: two distinct clinico-microbiological entities. Int J Infect Dis. (2021) 114:185–91. doi: 10.1016/j.ijid.2021.11.013
46. Zhou C, Wu Q, He L, Zhang H, Xu M, Yuan B, et al. Clinical and molecular characteristics of carbapenem-resistant hypervirulent Klebsiella pneumoniae isolates in a tertiary hospital in Shanghai, China. Infect Drug Resist. (2021) 14:2697–706. doi: 10.2147/IDR.S321704
47. Li Y, Hu D, Ma X, Li D, Tian D, Gong Y, et al. Convergence of carbapenem resistance and hypervirulence leads to high mortality in patients with postoperative Klebsiella pneumoniae meningitis. J Glob Antimicrob Resist. (2021) 27:95–100. doi: 10.1016/j.jgar.2021.02.035
48. Kong ZX, Karunakaran R, Abdul Jabar K, Ponnampalavanar S, Chong CW, Teh CSJ. The detection of hypermucoviscous carbapenem-resistant Klebsiella pneumoniae from a tertiary teaching hospital in Malaysia and assessment of Hypermucoviscous as marker of hypervirulence. Microb Drug Resist. (2021) 27:1319–27. doi: 10.1089/mdr.2020.0096
49. Wu H, Li D, Zhou H, Sun Y, Guo L, Shen D. Bacteremia and other body site infection caused by hypervirulent and classic Klebsiella pneumoniae. Microb Pathog. (2017) 104:254–62. doi: 10.1016/j.micpath.2017.01.049
50. Wang X, Xie Y, Li G, Liu J, Li X, Tian L, et al. Whole-genome-sequencing characterization of bloodstream infection-causing hypervirulent Klebsiella pneumoniae of capsular serotype K2 and ST374. Virulence. (2018) 9:510–21. doi: 10.1080/21505594.2017.1421894
51. Zhou S, Ren G, Liu Y, Liu X, Zhang L, Xu S, et al. Challenge of evolving Klebsiella pneumoniae infection in patients on hemodialysis: from the classic strain to the carbapenem-resistant hypervirulent one. Int J Med Sci. (2022) 19:416–24. doi: 10.7150/ijms.69577
52. Ding Z, Li Z, Tang M, Zeng Z, Song M, Yang K, et al. The molecular characteristics, clinical manifestations, and risk factors of hypervirulent Klebsiella pneumoniae infections in a large teaching hospital in southwest China. Microb Pathog. (2022) 162:105152. doi: 10.1016/j.micpath.2021.105152
53. Melot B, Brisse S, Breurec S, Passet V, Malpote E, Lamaury I, et al. Community-acquired meningitis caused by a CG86 hypervirulent Klebsiella pneumoniae strain: first case report in the Caribbean. BMC Infect Dis. (2016) 16:736. doi: 10.1186/s12879-016-2065-2
54. Suwalski P, Staromłyński J, Braczkowski J, Bartczak M, Mariani S, Drobiński D, et al. Transition from simple V-V to V-A and hybrid ECMO configurations in COVID-19 ARDS. Membranes (Basel). (2021) 11:e0434. doi: 10.3390/membranes11060434
55. Han L, Zhang Y, Zhang Y, Wu W, He P. Risk factors for refractory septic shock treated with VA ECMO. Ann Transl Med. (2019) 7:476. doi: 10.21037/atm.2019.08.07
56. Falk L, Hultman J, Broman LM. Extracorporeal membrane oxygenation for septic shock. Crit Care Med. (2019) 47:1097–105. doi: 10.1097/CCM.0000000000003819
57. Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, Frei U, et al. Acute renal failure in patients with severe sepsis and septic shock—a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant. (2008) 23:904–9. doi: 10.1093/ndt/gfm610
58. Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. (2009) 35:871–81. doi: 10.1007/s00134-008-1367-2
59. Nash DM, Przech S, Wald R, O'Reilly D. Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care. (2017) 41:138–44. doi: 10.1016/j.jcrc.2017.05.002
60. Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. (2020) 383:240–51. doi: 10.1056/NEJMoa2000741
61. Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. (2018) 379:1431–42. doi: 10.1056/NEJMoa1803213
62. Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. (2016) 315:2190–9. doi: 10.1001/jama.2016.5828
63. Li X, Liu C, Mao Z, Li Q, Zhou F. Timing of renal replacement therapy initiation for acute kidney injury in critically ill patients: a systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Crit Care. (2021) 25:15. doi: 10.1186/s13054-020-03451-y
64. Wang X, Zhang Q, Yan Y, Yang Y, Shang X, Li Y. Clinical significance of pro-inflammatory cytokines and their correlation with disease severity and blood coagulation in septic patients with bacterial co-infection. Shock. (2021) 56:396–402. doi: 10.1097/SHK.0000000000001735
Keywords: extracorporeal membrane oxygenation (ECMO), continuous renal replacement therapy (CRRT), hypervirulent Klebsiella pneumoniae (hvKP), acute respiratory distress syndrome (ARDS), septic shock
Citation: Peng W, Wu Y, Lu R, Zheng Y, Chen J and Pan P (2022) Successful treatment of acute respiratory distress syndrome caused by hypervirulent Klebsiella pneumoniae with extracorporeal membrane oxygenation and continuous renal replacement therapy: A case report and literature review. Front. Med. 9:936927. doi: 10.3389/fmed.2022.936927
Received: 05 May 2022; Accepted: 29 July 2022;
Published: 24 August 2022.
Edited by:
John-David Aubert, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Wenjian Liao, The First Affiliated Hospital of Nanchang University, ChinaPin-Kuei Fu, Taichung Veterans General Hospital, Taiwan
Copyright © 2022 Peng, Wu, Lu, Zheng, Chen and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pinhua Pan, pinhuapan668@csu.edu.cn
 Wenzhong Peng
Wenzhong Peng Yanhao Wu1,2,3,4,5
Yanhao Wu1,2,3,4,5  Jie Chen
Jie Chen Pinhua Pan
Pinhua Pan