Changes in High-Risk HPV Infection Prevalence and Associated Factors in Selected Rural Areas of China: A Multicenter Population-Based Study
- 1Department of Cancer Epidemiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Public Health and Preventive Medicine, Baotou Medical College, Baotou, China
- 3School of Public Health, Fujian Medical University, Fuzhou, China
- 4School of Public Health, Dalian Medical University, Dalian, China
- 5Chinese Academy of Medical Sciences and Peking Union Medical College School of Population Medicine and Public Health, Beijing, China
Background: The Chinese government has taken action to prevent cervical cancer by implementing the National Cervical Cancer Screening Programme in Rural Areas (NACCSPRA), which was launched in 2009. Numerous studies have demonstrated that long-term cervical cancer screening alters human papillomavirus (HPV) infection rates and cervical disease detection. Nearly 80 million women have been screened over 10 years, representing <30% of the target population; however, in some rural areas, such as Ordos City of Inner Mongolia Autonomous Region, Xiangyuan County of Shanxi Province, and Jinyun County, and Jingning County of Zhejiang Province, programs for prevention and treatment of cervical cancer have been implemented. Numerous studies have demonstrated that long-term cervical cancer screening alters rates of human papillomavirus (HPV) infection and cervical disease detection. In this study, we aimed to determine the infection rates of high-risk HPV (hrHPV) and the detection rate of cervical lesions; and changes in factors associated with cervical cancer, to provide scientific data to inform efforts to eliminate cervical cancer in rural areas.
Methods: This was a cross-sectional, population-based, and multi-center survey. Populations from three rural areas of China (Ordos City of Inner Mongolia Autonomous Region, Xiangyuan County of Shanxi Province, and Jinyun County and Jingning County of Zhejiang Province) were selected and 9,332 women aged 20–64 years old were invited to participate in cervical cancer screening by both cytology and HPV testing. The outcomes assessed were: infection rates with hrHPV, HPV16, 18, 16/18, and other 12 hrHPV types (HPV 31,33,35,39,45,51,52,56,58,59,66 and 68); detection rates of cytological and histological lesions; and factors associated with HPV infection.
Results: A total of 9,217 women aged 45.62 ± 8.02 years were included in this study. Infection rates with hrHPV, HPV 16, 18, 16/18, and other 12 hrHPV types were 16.3%, 3.0%, 1.5%, 4.3%, and 13.6%, respectively. There were significant differences among the age-specific HPV infection rates (P < 0.05). Infection rates with hrHPV, 16, 18, 16/18, and the other 12 hrHPV types showed a single peak infection mode, with a peak age of 56–65 years old. Age, marital status, number of live births, education level, reproductive disease history, and a history of alcohol consumption were risk factors for hrHPV infection. The detection rate of cytological abnormalities was 12.98% in the study and was higher in women older than 56 years old. The detection rates of cervical intraepithelial neoplasia CIN2+ and CIN3+ in the population were 1.45% and 0.77%, respectively. The highest incidence rates of CIN2+ and CIN3+ were 32.12% and 17.51%, respectively, in the 41–45 years old group.
Conclusion: Infection rates with hrHPV, HPV16, and cervical lesions among our screening population were lower than the mean level in rural areas of China. Infection rates with hrHPV, HPV16, 18, and 16/18 showed a single-peak infection pattern, with the peak age of infection being 56-65 years old. Risk factors for hrHPV infection were age, history of alcohol consumption, marital status, reproductive diseases, education level, and the number of live births. Based on these data, we recommend that cervical cancer screening be offered to women older than 30 years in rural areas, particularly those aged 41–45 years.
Introduction
Cervical cancer is ranked third among gynecological malignancies in terms of both estimated new cases and deaths of women worldwide. An estimated 604,000 new cervical cancer cases and 342,000 deaths were reported globally in 2020 (1). In China, there were up to 110,000 and 60,000 of new cases and deaths from cervical cancer, respectively, in 2020 (2), representing increases of 3.5% and 23.0% relative to 2018 (3). Hence, prevention and treatment of cervical cancer are urgent, particularly in China.
There has been heavy investment in cervical cancer prevention and control in China in recent years; however, the goal of eliminating cervical cancer, especially in rural areas, remains some way from being achieved (4). The Chinese government has taken action to prevent cervical cancer by implementing the National Cervical Cancer Screening Program in Rural Areas (NACCSPRA), which was launched in 2009 to provide free annual screening for 10 million women aged 35–64 in rural China (5, 6). Over the past decade, screening areas and population coverage have been expanding, with a screening rate for rural women from 2016 to 2018 of 26% (7), which remains far from the 70% screening target proposed by the World Health Organization (WHO). Due to imbalances in economic development, health levels, HPV infection rates, risk factors, and disease detection rates in rural areas of China, successful implementation of the cervical cancer elimination plan for China is challenging in these areas, particularly the prevention and treatment of cervical cancer. The detection and screening rates for precancerous cervical lesions were raised in rural China (8). Good systems for prevention and treatment of cervical cancer have been achieved in some rural areas of China, such as Ordos City, which has high rates of cervical cancer incidence. As the first city in China to implement a policies of screening for cervical cancer in all women aged 35–64 years and to conduct HPV vaccine immunization for all girls aged 13–18 years, the WHO considered the Ordos of the city in China likely to be first eliminate cervical cancer (9)1, and the region has a high population of people with Mongol ethnicity, who have a higher incidence of cervical cancer. Further, due to its implementation of cervical cancer screening for almost 30 years, Xiangyuan County in Shanxi Province is a rural area that demonstrates the potential for the prevention and treatment of cervical cancer in China, and this rural area was also a clinical experimental site for the bivalent, quadrivalent, and 9-valent HPV vaccines (10). Jinyun County and Jingning County in Zhejiang Province also have a good record of cervical cancer prevention and treatment. In addition, it was the clinical experimental site for the HPV screening kit.
HPV infection rates, particularly the HPV 16 infection rate, were altered by the implementation of cervical cancer screening measures after ten years follow-up (11). The prevention and treatment experience of cervical cancer in Australia and other countries have confirmed that cervical cancer screening and HPV vaccination reduce the detection rate of cervical lesions. Compared with similar published studies, this study focused on the change of selected rural areas, where successful implementation of a cervical cancer elimination plan was introduced and had higher cervical cancer incidence and mortality rates in rural areas of China. There were unreported changes in HPV infection and cervical lesion detection rates and factors influencing these selected rural areas, especially after HPV vaccination in the market. Therefore, we conducted this study to explore the extent of prevention and treatment of cervical cancer in rural China by comparing how HPV infection rates, cervical lesions detection rates, and factors influencing HPV infection have changed. Furthermore, we investigated the effectiveness and relationship with age of cervical cancer screening for women in areas with high incidence rates of cervical cancer to provide a basis for design of follow-up cervical cancer screening strategies. The primary purpose of this study was to evaluate changes in HPV infection and cervical lesion detection rates and factors influencing these in screened women aged 20–65 years in rural areas of China with high incidence of cervical cancer: Ordos City of Inner Mongolia Autonomous Region, Xiangyuan County of Shanxi Province, and Jinyun County, and Jingning County of Zhejiang Province.
Methods
Setting
This was a multicenter, population-based, and cross-sectional study conducted in rural areas of China from 2016 to 2019. Three rural areas were chosen based on their high incidence rates of cervical cancer, including the Ordos, Inner Mongolia (Hang jin banner and Yi jinholo banner), Shanxi Province (Xiangyuan Country), and Zhejiang Province (Jinyun County and Jingning County). A total of three tertiary hospitals and five maternal and child health hospitals were selected. Ethics approval was obtained from the Cancer Hospital, Chinese Academy of Medical Sciences (No. 16-013/1092), and the study was approved by all institutional review boards of the participating hospitals.
Study Population
In the initial stage of the study, 9,332 women participated, while 9,217 eligible women aged 20 to 64 years who lived in villages or sub-districts participated in the questionnaire survey, gynecological examination, and laboratory testing. All eligible women provided informed consent before enrolment. The investigators carried out procedures, inspecting whether the women complete the questionnaires or met the inclusion and exclusion criteria (Figure 1).
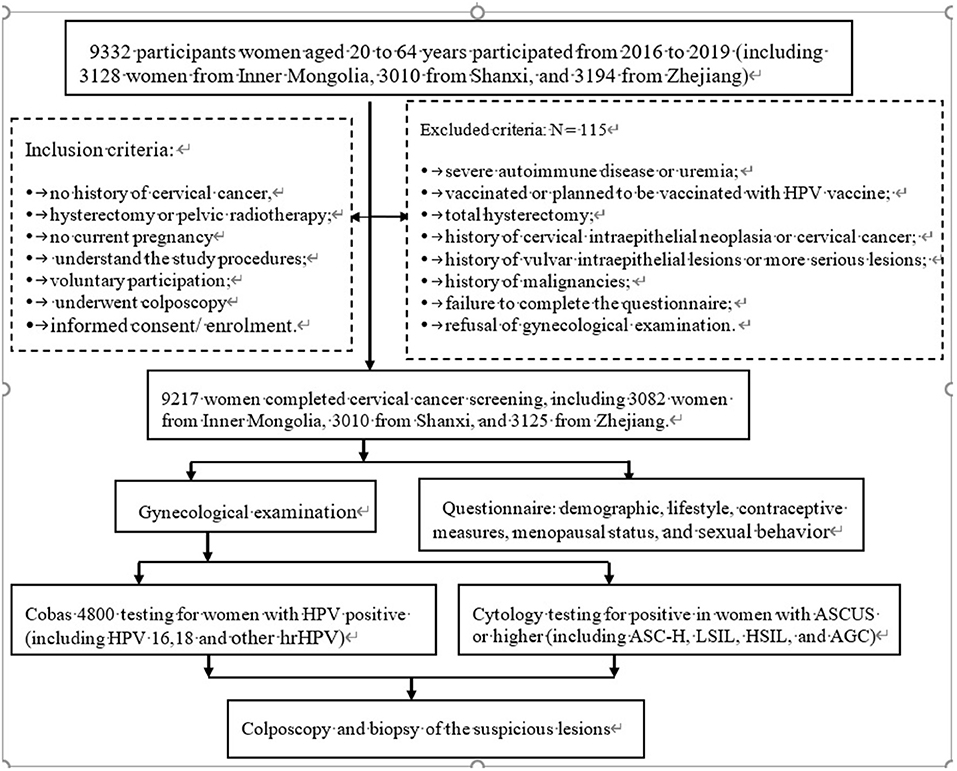
Figure 1. The flow chart of the study procedures carried out on the sites. ASCUS, atypical squamous cells of undetermined significance; ASC-H, higher including low-grade squamous cell-cannot exclude high-grade squamous intraepithelial lesion; LSIL, low-grade intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; AGC, atypical glandular cell; NILM, negative for intraepithelial lesion or malignancy.
Procedure
The survey was conducted face-to-face by trained interviewers. If patients had difficulty reading and completing the scales, trained interviewers helped them read and explain, or family members helped them answer questions. Information collected included: demographic (birth date, sex, location, occupational situation, marital status, education, and annual household income); and other factors, including a history of disease, pregnancy, reproduction, lifestyle (smoking and alcohol consumption), contraceptive measures, menopausal status, and sexual behavior. A strict quality control scheme was adhered to throughout the entire investigation process, including data collection, filing, entry, and checking, revision, and data security. The trained interviewers checked the questionnaires immediately on completion to avoid missing items and logical errors. If the questionnaires had missing items or obvious logical mistakes (such as missing items and errors), the trained interviewers called the patient to amend them and check the information. All procedures were performed by trained local physicians, while the materials for and results of cytology and hrHPV analyses were provided by central hospitals.
Cervical Cancer Screening Process
All women were tested by cytology and for hrHPV using Thin Prep medium and the Cobas 4,800 test (Roche Diagnostics). Women positive for either HPV 16/18 or other HPV genotypes with positive cytology results were deemed to have screened positive; colposcopy and biopsy of suspicious lesions were performed if necessary. Women positive for other HPV genotypes and negative on cytology, and those negative for HPV genotypes, were deemed to have screened negative. The results of cytology show atypical squamous cells of undetermined significance (ASCUS) or higher (including low-grade squamous cell-cannot exclude high-grade squamous intraepithelial lesion (ASC-H), low-grade intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and atypical glandular cell (AGC), among others) indicated the need to undergo colposcopy and biopsy of the suspicious lesions. Women negative for intraepithelial lesion or malignancy (NILM) were screened negative, with follow-up observation to be carried out in 3 years.
According to CIN terminology, CIN was diagnosed as one of four stages: NILM, CIN grade 1 (CIN1), CIN2, CIN3; and cervical cancer as micro-invasive carcinoma, invasive carcinoma, and others.
Precancerous lesions diagnosed by cytology were classified into four stages: NILM, ASCUS, LSIL, and HSIL+.
Statistical Analysis
A database was established using Microsoft Access 2007 software. Statistical analyses were performed in SPSS, version 28.0. A χ2 test was performed to compare proportions in subjects with specific characteristics and incidence rates of hrHPV, HPV16, 18, 16/18, and another 12 high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) and cytology. Linear trend tests were used to compare infection rates with total hrHPV, HPV16, 18, 16/18, and the 12 other hrHPV genotypes; detection rates of abnormal cells; and detection rates of cervical precancerous lesions, according to age group.
Results
Sociodemographic Information
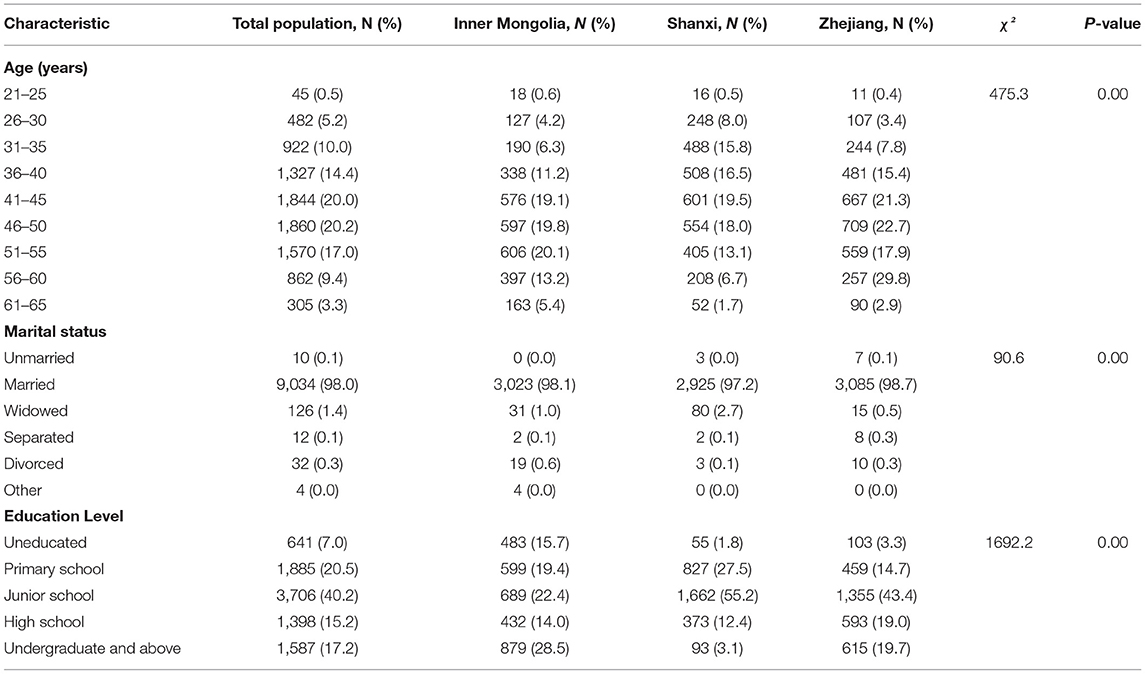
Initial screening for cervical cancer was conducted in 9,332 women from rural areas, of which 115 were excluded for various reasons, including an unwillingness to consent, disapproval of gynecological examination, previous uterine surgery, and incomplete data. Finally, 9,217 women completed cervical cancer screening, including 3,082 women from Inner Mongolia, 3,010 from Shanxi, and 3,125 from Zhejiang. The mean age of the 9,217 women was 45.15 ± 8.74 years, and women aged 41–45-years-old accounted for 20.0% of the screened population. The largest age groups from Inner Mongolia, Shanxi Province, and Zhejiang Province in the screened populations were those aged 51–55 years-old (20.1%), 41–45 years old (19.5%), and 56–60 years old (9.4%), respectively. There were significant differences in age distribution, marital status, and educational level (P < 0.001) (Table 1).
Distribution of HPV Status
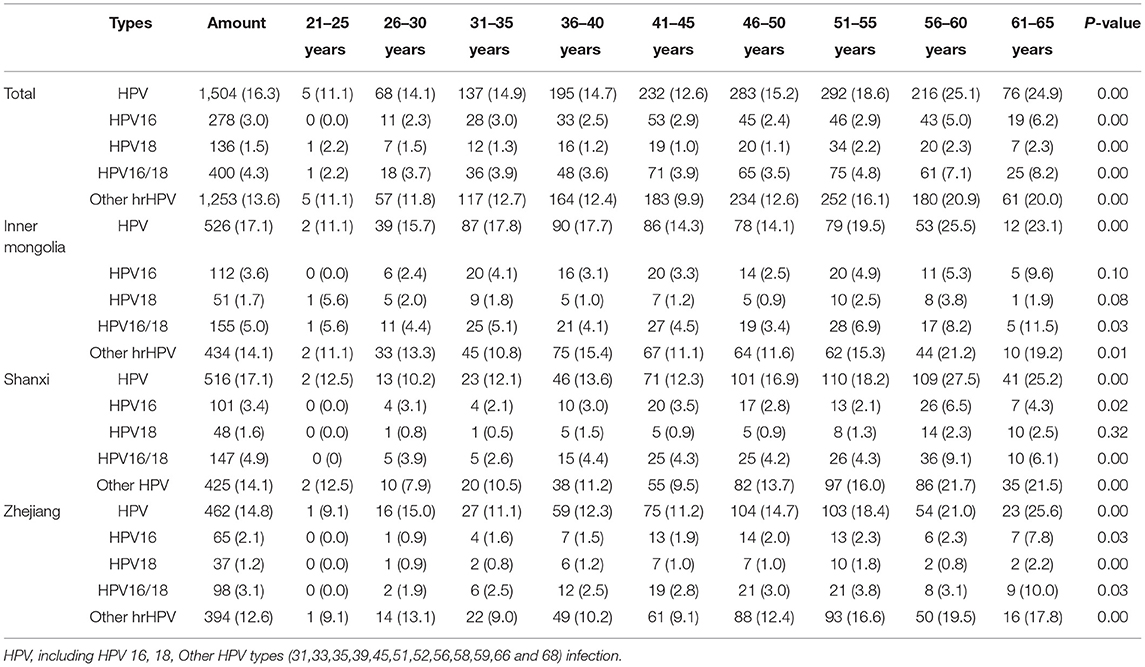
Among the women screened for cervical cancer, the positive rates for the hrHPV types, 16, 18, 16/18, and others (12 high-risk types) were 16.3%, 3.0%, 1.5%, 4.3%, and 13.6%, respectively. Further, the infection rates of HPV16, 18, 16/18, and other types in the total population differed significantly among age groups (P < 0.05), with the peak age of infection at 56–65 years old (Table 2). The infection rate of hrHPV (17.1% vs. 17.1%), HPV 16/18 (5.0% vs. 4.9%) and other 12 high-risk types (14.1% vs. 21.7%) among the screening population in Inner Mongolia and Shanxi Province were higher than those in Zhejiang Province (14.8%), HPV16 (1.6%), HPV16/18 (3.1%) and other 12 high-risk types (12.6%) (P < 0.05).There was no significant difference in infection rate of HPV 18 among the selected areas. Comparing the age groups, the infection rates of hrHPV and HPV 16/18 were statistically different among age groups in Inner Mongolia Autonomous Region and Shanxi Province (P < 0.05), except for the infection rates of HPV16. While infection rates of HPV16, 18, 16/18, and other types in the Zhejiang Province were statistically significant (P < 0.05), with the peak age of infection at 61–65 years old.
Risk Factors Associated With HrHPV Infection
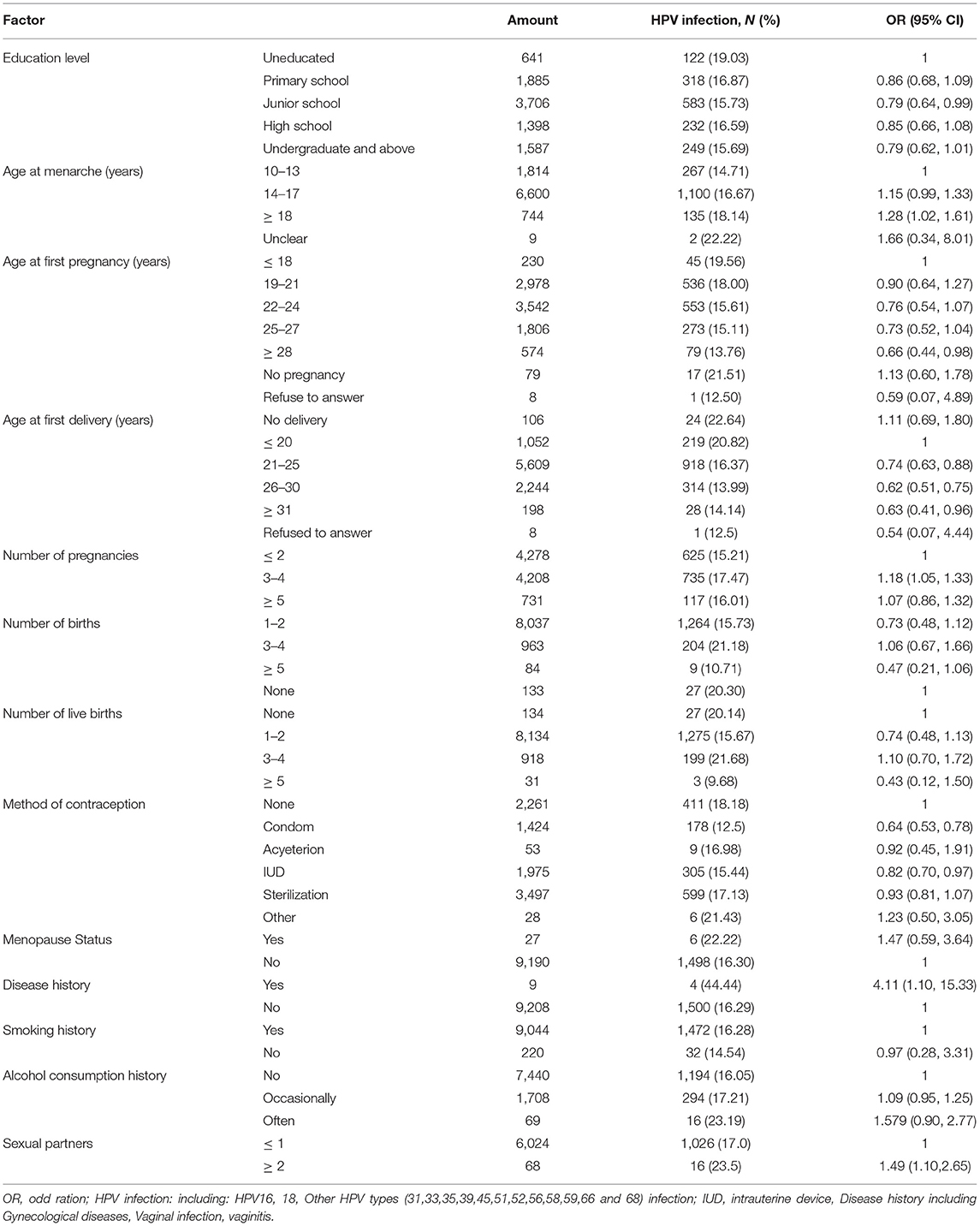
Among the women screened for cervical cancer, HrHPV infection rates did not differ significantly according to disease history, age at menarche, menopause status, alcohol consumption and smoking (P > 0.05). However, the hrHPV infection rate differed significantly according to age at first pregnancy, age at first delivery, number of births and live births, method of contraception, menopause status, and the number of sexual partners (P < 0.05). Infection rates were higher in women who were younger at first pregnancy and delivery, had given birth 3–4 times, had 3–4 live births, had disease history, had ≥ 2 sexual partners, and used other method of contraception (including external ejaculation, vaginal medication, and fallopian tube blockage) (Table 3).
Logistic Regression Analysis of Factors Associated With HrHPV Infection
Seven factors were candidate predictors that were associated with hrHPV infection on univariate analyses. HrHPV infection rates differed significantly according to age [odds ratio (OR) = 1.124, P < 0.0001], education level (OR = 1.068, P = 0.025), number of births (OR = 0.601, P = 0.039), marital status (OR = 1.476, P < 0.0001), number of live births (OR = 1.751, P = 0.032), age at first delivery (OR = 0.815, P = 0.001), and alcohol consumption history (OR = 1.164, P = 0.024).
Multivariate analysis confirmed that five variables were independently associated with hrHPV infection, including age, marital status, number of live births, education level, and alcohol consumption history (Table 4; Supplementary Table 1).
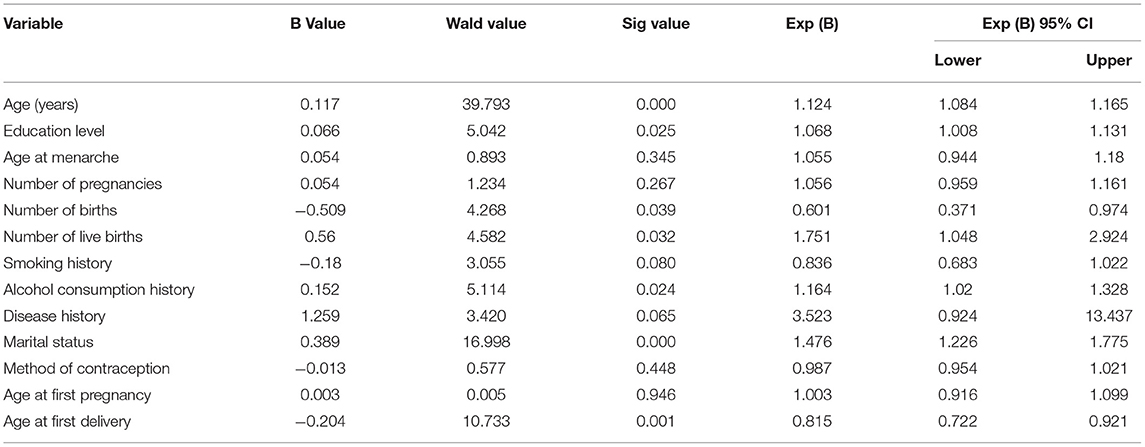
Table 4. Multivariate logistic regression analysis of factors associated with high-risk HPV infection.
Detection Rates of Cytological Abnormalities in Different Age Groups
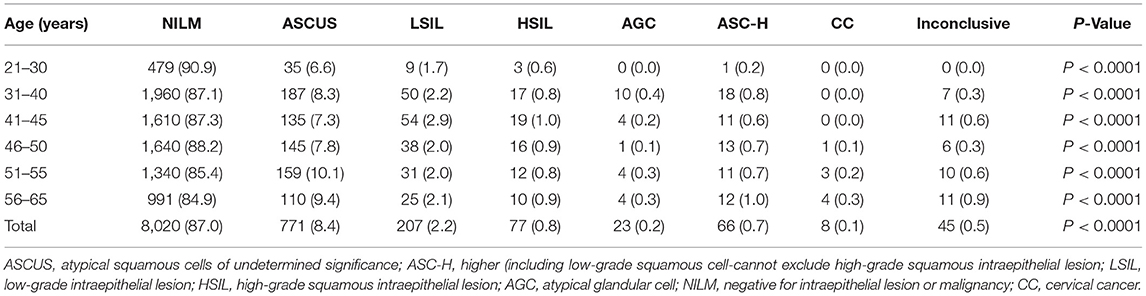
The cytological results for the 9,217 women included in the study were as follows: NILM (n = 8,020), inconclusive (n = 45), ASCUS (n = 771, 8.4%), LSIL (n = 207, 2.2%), HSIL (n = 77, 0.8%), AGC (n = 23, 0.2%), ASC-H (n = 66, 0.7%), and cervical cancer (n = 8, 0.1%). ASCUS or above was not detected in the 21–25 year-old age group, and the detection rate of abnormal cells differed significantly among age groups (P < 0.05), with the highest in women aged > 56 years (Table 5).
Detection Rates of Histological Abnormalities in Different Age Groups
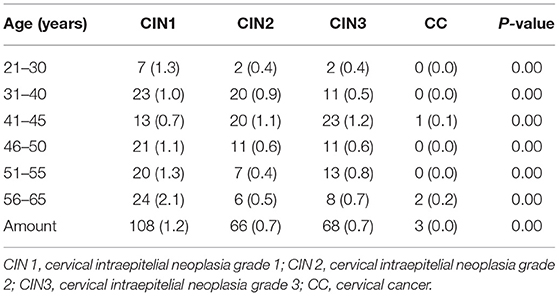
Of the 9,217 women who underwent cervical cancer screening, 711 were referred for colposcopy, of whom 250 had abnormal histological findings. We found 108 cases of CIN1 (including 18 cases in Ordos City, 48 cases in Xiangyuan County, and 42 cases in Jinyun County, and Jingning County), 66 cases of CIN2 (including 25 cases in Ordos City, 28 cases in Xiangyuan County, and 15 cases in Jinyun County and Jingning County), 68 cases of CIN3 (including 21 cases in Ordos City, 24 cases in Xiangyuan County, and 23 cases in Jinyun County, and Jingning County), 3 cases of cervical cancer, and 3 cases of vulvar intraepithelial neoplasia among those women with abnormal histological findings. The overall CIN2+ detection rate was 1.45%, of which 0.04%, 0.33%, 0.48%, 0.24%, 0.22%, and 0.17% were in the 21–30, 31–40, 41–45, 46–50, 51–55, and > 56 years age groups, respectively; there was an overall CIN3+ detection rate of 0.77%, with 0.02%, 0.12%, 0.26%, 0.26%, 0.26%, and 0.12% in each age group, respectively. CIN 2/3+ was not detected in women aged 21–25 years, and the highest CIN 2/3+ detection rates were found in the 41–45 years age group, accounting for 32.12% and 17.51% of all cases respectively. The detection rate of abnormal cervical histology differed significantly among the different age groups (P < 0.05) (Table 6).
Discussion
HrHPV infection is closely associated with genital warts and penile, anal, oropharyngeal, and cervical cancers. The elimination of HPV-related cancers, particularly cervical cancer, has attracted global attention as a public health problem (12). The WHO launched the “Global Strategy for accelerating the Elimination of Cervical Cancer as a Public Health Problem” on November 17, 2020, and 194 countries, including China, committed to eliminating cervical cancer for the first time (13). There remains much to achieve to reach the goal of eliminating cervical cancer in China (14). A major problem was low coverage of cervical cancer screening and vaccination with the HPV vaccine; however, some regions, including rural areas, of China, have taken effective measures to prevent and control HPV, such as Ordos City of Inner Mongolia Autonomous Region, Xiangyuan County of Shanxi Province, Jinyun County, and Jingning County of Zhejiang Province. Ordos was the first city in China to implement the national screening plan for women aged 35–64 years and HPV vaccine immunization for girls aged 13–18 years. Based on their experience and study findings, two “National Demonstration Base for Early Diagnosis and Treatment of Cervical Cancer” programs were set up in Xiangyuan County Maternal and Child Health Hospital (rural type) in Shanxi Province in February 2005 (15). Furthermore, Xiangyuan and the counties of Jinyun and Jingning) were the sites for clinical trials of the HPV vaccine and HPV testing kit. Compared with other regions, these areas could be expected to have superior outcomes in terms of prevention and control of cervical cancer, due to the implementation of screening. A 10-year cohort study cervical cancer screening reported reduced rates of HPV infection, particularly infection with HPV16 (16). To assess how the HPV infection rate, the rate of cervical lesion detection, and risk factors influencing the HPV infection rate have changed in these rural areas, we conducted this multicenter, population-based study focused on rural areas, including Ordos, Xiangyuan, Jinyun, and Jingning County, to provide theoretical guidance to further the realization of the plan for the elimination of cervical cancer in rural areas.
In our study, we found that the hrHPV infection rate was 16.3% in the screened population, which was consistent with the findings of Zhao et al. regarding the national screened population but lower than rates reported in other parts of the world, such as Africa (20.9%–23.4%) (17, 18). Combined with our results regarding risk factors, the observed differences may be related to poor health status, early age of marriage, and higher numbers of births, among other factors, associated with hrHPV infection. The HPV 16 infection rate was 3.0%, which was consistent with the findings of the ATHENA study in the USA (2.8%), but it was lower than that reported by the ICO (19, 20). The HPV 18 infection rate was 1.5%, higher than that reported by the ATHENA study (1.0%) (21). The infection rates with HPV 16/18 and the other 12 hrHPV types were 4.3 and 13.6%, respectively. Overall, infection rates with hrHPV, HPV16, 18, 16/18, and the other 12 hrHPV types were consistent with the findings from the national screening population, indicating declining infection rates in these rural areas (Ordos, Xiangyuan, Jinyun, and Jingning County). Except for the infection rates of HPV18, the infection rates of hrHPV, HPV16, 16/18 and other 12 hrHPV types in Inner Mongolia and Shanxi Province were higher than those of in Zhejiang Province, which was indicated that the distribution of hrHPV, HPV16, 16/18 and the other 12 hrHPV types were regional. This may be related to the fact that the women living in pastoral areas of Inner Mongolia or rural areas in Shanxi Province had poor sanitary conditions and premarital sexual behavior (19). In our study, the infection rates of hrHPV, HPV16, 16/18 were higher in Inner Mongolia, Shanxi Province and Zhejiang Province than that in the western region (18) and ICO (20), which may be related to the research subjects coming from the areas with high incidence and mortality of cervical cancer in China. The change in the HPV18 infection rate was not obvious, which may be related to the low infection rate in China. It was suggested that the government should pay attention to the prevention and control of high incidence cervical cancer areas.
Infection rates with hrHPV, HPV16, 18, 16/18, and the other 12 hrHPV types differed significantly according to age (P < 0.05), with the peak age of infection at 56–65-years-old in the total mount, Inner Mongolia, Shanxi Province and Zhejiang Province. These findings are inconsistent with the double peak HPV infection rate phenomenon previously reported in rural areas of China (22), which described two peak HPV infection rates in the 25–29 years (14.2%) and 55–59 years (19.3%) age groups. This can likely be attributed to the fact that, in these rural areas (which were clinical trial sites for the HPV vaccine and HPV testing kit), women in the 25–35 years age group underwent HPV vaccine injection, while those aged 35–64 were screened for cervical cancer. Furthermore, the 21–24 years age group was relatively small in our rural population and women tended to marry earlier in rural areas, which may have led to an earlier peak in the HPV infection rate; however, HPV infection in this age group was transient and had no clinical significance. It is established that infection rates exhibit one peak in women aged 21–24 years, which was the highest rate (30.5%) in the ATHENA and ARTISTIC (39.9%) studies (23, 24), subsequently decreasing in women aged ≥ 55 years. Our findings differ from those reports mentioned above; we found that women aged 56–65 years had the highest rate of HPV infection, possibly associated with decreased immunity, a lower natural rate of HPV clearance, and an increased possibility of hrHPV infection in women experiencing menopause or perimenopause (25). Therefore, it is more clinically meaningful to conduct HPV testing for older than younger women, aiming for cervical cancer prevention and treatment in these rural areas (Ordos, Xiangyuan, Jinyun, and Jingning County).
We found that risk factors for hrHPV infection in our population were age, marital status, number of live births, education level, disease history, and alcohol consumption history, consistent with the findings of numerous domestic studies (26, 27). To date, studies conducted in China have unanimously recognized that many sexual partners, more pregnancies, more births, reproductive system diseases, and other factors can increase the risk of infection with hrHPV (28).
In this study, we found that a high number of live births is a risk factor for hrHPV infection, likely due to stimulation and damage of the cervical mucosa, resulting in cervical cell metaplasia or abnormal hyperplasia and changes in estrogen and progesterone levels in the body leading to reduced immunity and increased risk of HPV infection (29, 30). We found that a history of reproductive system infection and gynecological diseases were major risk factors for hrHPV infection, which may be related to the destruction of the cervical mucosal barrier by reproductive system infection, making it easier for HPV to invade and infect the epithelial basal layer, and reducing local immunity in the vagina, causing abnormal differentiation or proliferation of cervical cells (31). Reproductive tract infection with hrHPV is necessary for the development of cervical cancer and CIN; therefore, a key step in cervical cancer prevention is to prevent reproductive tract infections with hrHPV. Menopause was a risk factor for HPV infection in our study, consistent with a report from Smith et al. (32) on postmenopausal women with 7 years of follow-up observation. The proportion of women aged 46–65 years (per-menopause or menopause) in our study was high, and hormone levels in women of this age are relatively disordered, while physiological immunity begins to decline. We found an association between contraceptive methods with HPV infection, which was inconsistent with published literature reports (33). These results suggest that women in our study were insufficiently knowledgeable about contraceptive measures, such as condoms and intrauterine devices, leading to misunderstandings and making it impossible to determine the relationship between contraception and HPV infection rate.
Our data confirm that women with higher education levels had lower hrHPV infection rates, likely because women with higher education levels pay more attention to their health and maintain good personal life and hygiene habits. Previous reports indicate that drinking alcohol is not associated with increased HPV infection rates (34). The women in our study came from areas with large ethnic minority populations. In particular, people with Mongolian ethnicity drink alcohol more frequently than those from other regions. We found that drinking alcohol was a risk factor for hrHPV infection, which warrants further research.
HPV infection rates are higher in married, divorced, and widowed women, which may be related to the number of sexual partners of women in these groups. The HPV infection rate was higher in younger women, consistent with the literature (35). Women aged <20 years may be more likely to be infected with HPV due to immature cervical epithelium repair function and imperfect immune function, as well as early age of initial sexual behavior, which increases the opportunity for HPV infection (36). Due to decreased immune system function and weakened virus clearance ability, the HPV infection rate was higher in older women. Furthermore, women who had ≥ 2 sexual partners had more opportunities to be infected with HPV, consistent with a previous report (37).
We found that the total detection rate of ≥ASCUS was 12.98%, which was higher than those reported by the national “two cancers” screening report (3.93%) (38) and the national special industry project report (5.63%) (39). The detection rates of ASCUS, LSIL, HSIL, AGC, ASC-H, and cervical cancer were 8.4, 2.2, 0.8, 0.2, 0.7, and 0.1%, respectively. Further, the rate of detection of abnormal cytology was high in this study, which may have had several possible causes. First, the cytological diagnoses were all made by doctors with professional training in cytology working at grade A hospitals. Second, the population came from agricultural and pastoral areas, with relatively concentrated ethnic minority populations, or from rural areas that are economically underdeveloped. There were differences rates of abnormal cytology detection among age groups, with the highest rate found in the 56–65 years old group. These data suggest that screening for cervical cancer in older women in rural China should be strengthened. As no abnormal cervical cells were detected in the 21–25 years old group, cervical cancer screening is not advisable for this group. The rate of abnormal cytology detection in the 25–30 years old group was 0.4%, and these women may decide whether to carry out cervical cancer screening according to their economic status.
We found three cases of vulvar intraepithelial neoplasia, which were not analyzed according to age group; however, rates of abnormal cervical tissue differed among age groups. The detection rates of CIN2+ and CIN3+ were 1.45 and 0.77%, respectively, which were lower than those previously published in the literature for rural areas (40, 41). The highest CIN2+ and CIN3+ detection rates were in the 41–45-year-old group, which was similar to the peak age group (40–49 years old). This may be related to differences in geographical regions, methods for cervical cancer screening (liquid-based cytology, HPV detection), tools for screening, population characteristics, age structure, study design, classification used in cytology, and pathology and principles of biopsy sampling, as these factors influence the results of studies of the prevalence of CIN (42). It is clear that the prevalence of cervical lesions is lower in populations with higher rates of screening than in those with lower rates of screening (43). Our study indicates that programs for the prevention and treatment of cervical cancer in these areas are generating initial results. No CIN2+ cases were found in the 21–30 years age group, indicating that cervical cancer screening is not suitable for women in this age group. The detection rates of CIN2+ and CIN3+ were 0.8 and 0.4%, respectively, among women aged 25–30 years, and were highest (32.12 and 17.51%) in women aged 41–50 years. These findings are broadly consistent with the literature (44, 45) and indicate that there should be more focus on cervical cancer screening for middle-aged and older women in rural areas of China.
Strengths and Limitations
This study has some strengths. The study population was large and a multicenter design was applied, using HPV genotype testing for cervical cancer screening in minority or rural areas of mainland China. To confirm the effects of HPV infection, we chose doctors at local hospitals to perform all screenings, permitting assessment by a superior hospital. Cervical cancer screening has been implemented in rural areas of China for more than ten years; however, the associated risk factors, HPV infection rates, rates of abnormal cervical cytology, and rates of abnormal histology and precancerous lesions in these areas are unclear, and elimination of cervical cancer by screening and prevention programs is expected. Second, our data provide a reference for real-world assessment of the effects of prevention and treatment programs for cervical cancer in rural areas of China.
The study also has some limitations. There was an inevitable loss to follow-up, with a rate of 18.74% in this study; however, analysis of the basic characteristics, main risk factors, and main outcome indicators of the lost population demonstrated that it is unlikely to have influenced our analyses. Furthermore, differences in the cytology, histology, and diagnosis findings of gynecologists in different regions may have had certain impacts on our results.
Conclusions
The rates of hrHPV and HPV16 infections and cervical lesions in the screened population included in this study were lower than the mean rates in rural China. The infection rates of hrHPV, HPV16, 18, and 16/18 showed a single-peak infection pattern, with a peak infection age of 56–65 years. Risk factors associated with hrHPV infection were: age, history of alcohol consumption, marital status, reproductive diseases, education level, and number of live births. Cervical cancer screening is not recommended for women aged 21–25 years in rural areas, while women aged 26–30 years may decide to undergo screening, according to their economic status. Older women (>30 years old), particularly those aged 41–45 years, are recommended to undergo cervical cancer screening.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
Central Ethics Approval was obtained from the Cancer Hospital, Chinese Academy of Medical Sciences (No. 16-013/1092). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-QY, M-YJ, R-MF, MB, WC, and Y-LQ participated in study design, data analysis and visualization, validation of the entire study, and preparation of the manuscript. Y-QY and M-YJ conducted data collection and supervision. Y-QY analyzed the data and write the article. All authors read and approved the final manuscript.
Funding
The study was funded by Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (Nos. 2017-I2M-B&R-03 and 2021-I2M-1-004). The authors declare that this study received funding from Roche company. Roche was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
Y-LQ and WC received grants from the Ministry of Science and Technology of the People's Republic of China during the conduct of the study, and personal fees and non-financial support from Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful the patients, their families, all of the investigators, and Roche for contributing to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.911367/full#supplementary-material
Abbreviations
AGC, atypical glandular cell; ASC-H, low-grade squamous cell-cannot exclude high-grade squamous intraepithelial lesion; ASCUS, atypical squamous cell of undetermined significance; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, Human papillomavirus; hrHPV, high risk HPV; HSIL, high grade squamous intraepithelial lesion; LSIL, low grade squamous intraepithelial lesion; NACCSPRA, National Cervical Cancer Screening Programme in Rural Areas; NILM, negative for intraepithelial lesion or malignancy; OR, odds ratio; WHO, World Health Organization.
Footnotes
1. ^Available online at: https://m.gmw.cn/baijia/2021-04/21/1302246124.html
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lu Y, Li P, Luo G, Liu D, Zou H. Cancer attributable to human papillomavirus infection in China: burden and trends. Cancer. (2020) 126:3719–32. doi: 10.1002/cncr.32986
3. Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. (2020) 395:575–90. doi: 10.1016/S0140-6736(20)30068-4
4. Xia CF, Qiao YL, Zhang Y, Zhao FH. WHO's global strategy of cervical cancer elimination and the challenges and initiatives in China. Zhonghua Yi Xue Za Zhi. (2020) 100:3484–8. doi: 10.3760/cma.j.cn112137-20200909-02606
5. Di J, Rutherford S, Chu C. Review of the cervical cancer burden and population-based cervical cancer screening in China. Asian Pac J Cancer Prev. (2015) 16:7401–7. doi: 10.7314/APJCP.2015.16.17.7401
6. Qiao Y, Wang A, Wang X, Wang Q, Fang L, Wang L. Detection and associated factors for cervical precancerous lesions among HIV-positive women from high HIV-burden areas - China, 2015-2016. China CDC Wkly. (2020) 2:355–61. doi: 10.46234/ccdcw2020.092
7. Zhang M, Zhong Y, Zhao Z, Huang Z, Zhang X, Li C, et al. Cervical cancer screening rates among Chinese women-China,2015. China CDC Wkly. (2020) 2:481–6. doi: 10.46234/ccdcw2020.128
8. Liu L, Wang D, Dong H, Jin C, Jiang L, Song H, et al. Characteristics of carcinogenic HPV genotypes in North China plain and the association with cervical lesions. Med. (2019) 98:e17087. doi: 10.1097/MD.0000000000017087
9. Guili Y. The “Two Cancers” Free Examination Project for School-Age Women in the City Was Fully Launched [n] Erdos daily (2010).
10. Wen C. China's plans to curb cervical cancer. Lancet Oncol. (2005) 6:139–41. doi: 10.1016/S1470-2045(05)01761-4
11. Dong L, Hu SY, Zhang Q, Feng RM, Zhang L, Zhao XL, et al. Changes in genotype prevalence of human papillomavirus over 10-year follow-up of a cervical cancer screening cohort. Zhonghua Liu Xing Bing Xue Za Zhi. (2017) 38:20–5. doi: 10.3760/cma.j.issn.0254-6450.2017.01.004
12. Zhao FH, Ren WH. Accelerating the elimination of cervical cancer in China and building a paradigm for “Healthy China” cancer prevention. Zhonghua Yi Xue Za Zhi. (2021) 101:1831–4. doi: 10.3760/cma.j.cn112137-20210310-00602
13. World Health Organization. Cervical Cancer: An NCD We Can Overcome. Geneva: WHO (2018). Available online at: https://www.who.int/dg/speeches/detai l/cervical cancer and we can overcome
14. World Health Organization. Draft: Global Strategy Towards Eliminating Cervical Cancer as a Public Health Problem. Geneva: WHO (2020).
15. Zhang S, Xu H, Zhang L, Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. (2020) 32:720–8. doi: 10.21147/j.issn.1000-9604.2020.06.05
16. Li M, Liu T, Luo G, Sun X, Hu G, Lu Y, et al. Incidence, persistence and clearance of cervical human papillomavirus among women in Guangdong, China 2007-2018: a retrospective cohort study. Infect Public Health. (2021) 14:42–9. doi: 10.1016/j.jiph.2020.11.011
17. Zhao FH, Lewkowitz AK, Hu SY, Chen F, Li LY, Zhang QM, et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: a pooled analysis of 17 population-based studies. Int J Cancer. (2012) 131:2929–38. doi: 10.1002/ijc.27571
18. Zhao P, Liu S, Zhong Z, Hou J, Lin L, Weng R, et al. Prevalence and genotype distribution of human papillomavirus infection among women in northeastern Guangdong Province of China. BMC Infect Dis. (2018) 18:123–35. doi: 10.1186/s12879-018-3105-x
19. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. (2015) 136:189–97. doi: 10.1016/j.ygyno.2014.11.076
20. Bruni LAGSB. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in China. Summary Report 10 December 2018. Summary Report. (2019) (accessed December 10, 2018).
21. Cox JT, Castle PE, Behrens CM, Sharma A, Wright TCJr, Cuzick J, et al. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. (2013) 208:184.e1–11. doi: 10.1016/j.ajog.2012.11.020
22. Kang LN, Castle PE, Zhao FH, Jeronimo J, Chen F, Bansil P, et al. A prospective study of age trends of high-risk human papillomavirus infection in rural China. BMC Infect Dis. (2014) 14:96. doi: 10.1186/1471-2334-14-96
23. Wright TCJr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. (2012) 206:41–6. doi: 10.1016/j.ajog.2011.07.024
24. Mchome B, Linde DS, Manongi R, Waldstroem M, Lftner T, Wu C, et al. Incident detection of human papillomavirus - a prospective follow-up study among Tanzanian women with a focus on HIV status. Int J Infect Dis. (2021) 110:165–70. doi: 10.1016/j.ijid.2021.07.011
25. Lee CY, Tseng CJ, Chang CC, Lee MC, Yang SF. Postpartum HPV vaccination rate and differences in background characteristics between HPV vaccinated and unvaccinated postpartum women: strict monitoring and follow-up of postpartum HPV vaccination program. Front Immunol. (2021) 12:626582. doi: 10.3389/fimmu.2021.626582
26. Lu W, Chen T, Yao Y, Chen P. Prevalence of high-risk human papillomavirus and cervical lesion risk factors: a population-based study in Zhejiang, China 2010-2019. Med Virol. (2021) 93:5118–25. doi: 10.1002/jmv.27034
27. Mchome BL, Kjaer SK, Manongi R, Swai P, Waldstroem M, Iftner T, et al. HPV types, cervical high-grade lesions and risk factors for oncogenic human papillomavirus infection among 3416 Tanzanian women. Sex Transm Infect. (2021) 97:56–62. doi: 10.1136/sextrans-2019-054263
28. Kasamatsu E, Rodríguez Riveros MI, Soilan AM, Ortega M, Mongelós P, Páez M, et al. Factors associated with high-risk human papillomavirus infection and high-grade cervical neoplasia: a population-based study in Paraguay. PLoS ONE. (2019) 14:e0218016. doi: 10.1371/journal.pone.0218016
29. Itarat Y, Kietpeerakool C, Jampathong N, Chumworathayi B, Kleebkaow P, Aue-Aungkul A, et al. Sexual behavior and infection with cervical human papillomavirus types 16 and 18. Int J Womens Health. (2019) 11:489–94. doi: 10.2147/IJWH.S218441
30. Mukanyangezi MF, Sengpiel V, Manzi O, Tobin G, Rulisa S, Bienvenu E, et al. Screening for human papillomavirus, cervical cytological abnormalities and associated risk factors in HIV-positive and HIV-negative women in Rwanda. HIV Med. (2018) 19:152–66. doi: 10.1111/hiv.12564
31. Chen MX, Zhou ZY, Qing W, Li H, Zhou HW. The cervical microbiota characteristics in patients with human papillomavirus infection. Zhonghua Yu Fang Yi Xue Za Zhi. (2021) 55:867–74. doi: 10.3760/cma.j.cn112150-20210224-00184
32. Smith EM, Johnson SR, Ritchie JM, Feddersen D, Wang D, Turek LP, et al. Persistent HPV infection in postmenopausal age women[J]. Int J Gynaecol Obstet. (2004) 87:131–7. doi: 10.1016/j.ijgo.2004.07.013
33. Wang X, Zhuang J, Wu K, Xu R, Li M, Lu Y. Human semen: the biological basis of sexual behaviour to promote human papillomavirus infection and cervical cancer. Med Hypotheses. (2010) 74:1015–6. doi: 10.1016/j.mehy.2010.01.009
34. Seo SS, Oh HY, Kim MK, Lee DO, Chung YK, Kim JY, et al. Combined effect of secondhand smoking and alcohol drinking on risk of persistent human papillomavirus infection. Biomed Res Int. (2019) 2019:5829676. doi: 10.1155/2019/5829676
35. Bao HL, Jin C, Wang S, Song Y, Xu ZY, Yan XJ, et al. Prevalence of cervicovaginal human papillomavirus infection and genotypes in the pre-vaccine era in China: a nationwide population-based study. Infect. (2021) 82:75–83. doi: 10.1016/j.jinf.2021.02.017
36. Bergqvist L, Kalliala I, Aro K, Auvinen E, Jakobsson M, Kiviharju M, et al. Distribution of HPV genotypes differs depending on behavioural factors among young women. Microorganisms. (2021) 9:750. doi: 10.3390/microorganisms9040750
37. Bennett KF, Waller J, Ryan M, Bailey JV, Marlow LAV. Concerns about disclosing a high-risk cervical human papillomavirus (HPV) infection to a sexual partner: a systematic review and thematic synthesis. BMJ Sex Reprod Health. (2020) 47:17–26. doi: 10.1136/bmjsrh-2019-200503
38. Zhang M, Bao HL, Wang LM, Zhao ZP, Huang ZJ, Zhang X, et al. Analysis of cervical cancer screening and related factors in China. Zhonghua Yi Xue Za Zhi. (2021) 101:1869–74. doi: 10.3760/cma.j.cn112137-20210108-00054
39. Zhang J, Zhao Y, Dai Y, Dang L, Ma L, Yang C, et al. Effectiveness of high-risk human papillomavirus testing for cervical cancer screening in china: a multicenter, open-label, randomized clinical trial. JAMA Oncol. (2021) 7:263–70. doi: 10.1001/jamaoncol.2020.6575
40. Li X, Ding L, Song L, Gao W, Wang L, Wang J. Effects of exposure to polycyclic aromatic hydrocarbons combined with high-risk human papillomavirus infection on cervical intraepithelial neoplasia: a population study in Shanxi Province, China. Int J Cancer. (2020) 146:2406–12. doi: 10.1002/ijc.32562
41. Zhao S, Zhao X, Hu S, Lu J, Duan X, Zhang X, et al. High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. Gynecol Oncol. (2017) 28:e30. doi: 10.3802/jgo.2017.28.e30
42. Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. preventive services task force. Ann Intern Med. (2011) 155:687–97. doi: 10.7326/0003-4819-155-10-201111150-00376
43. Zhang R, Velicer C, Chen W, Liaw KL, Wu EQ, Liu B, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grades 1 or worse among 4215 Chinese women in a population-based study. Cancer Epidemiol. (2013) 37:939–45. doi: 10.1016/j.canep.2013.10.005
44. Tom JJ, Vaz C, Nisha C. Screening for cervical dysplasia and reproductive tract infections in Kerala, India: a multicentric study. Family Med Prim Care. (2020) 9:4107–11. doi: 10.4103/jfmpc.jfmpc_514_20
Keywords: high-risk human papillomavirus, cervical cancer, genotype, risk factors, China demonstration rural areas
Citation: Yu Y-Q, Jiang M-Y, Dang L, Feng R-M, Bangura MS, Chen W and Qiao Y-L (2022) Changes in High-Risk HPV Infection Prevalence and Associated Factors in Selected Rural Areas of China: A Multicenter Population-Based Study. Front. Med. 9:911367. doi: 10.3389/fmed.2022.911367
Received: 02 April 2022; Accepted: 13 June 2022;
Published: 12 July 2022.
Edited by:
Redhwan Ahmed Al-Naggar, National University of Malaysia, MalaysiaCopyright © 2022 Yu, Jiang, Dang, Feng, Bangura, Chen and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You-Lin Qiao, qiaoy@cicams.ac.cn; Wen Chen, chenwen@cicams.ac.cn
 Yan-Qin Yu
Yan-Qin Yu Ming-Yue Jiang1
Ming-Yue Jiang1  Rui-Mei Feng
Rui-Mei Feng Wen Chen
Wen Chen You-Lin Qiao
You-Lin Qiao