Case Report: Unclassified Renal Cell Carcinoma With Medullary Phenotype and SMARCB1/INI1 Deficiency, Broadening the Spectrum of Medullary Carcinoma
- 1Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy
- 2Department of Pathology, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Humanitas Clinical and Research Hospital, Rozzano, Italy
- 3Department of Urology, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Humanitas Clinical and Research Hospital, Rozzano, Italy
Renal medullary carcinoma (RMC) is a rare entity with poor prognosis bearing inactivating genomic alterations in SMARCB1/INI1 resulting in the loss of expression of INI1 and occurring in young patients with sickle cell trait or sickle cell disease. Recently, rare examples with histological characteristics of RMC have been described in older patients without hemoglobinopathies and provisionally termed “Renal cell carcinoma unclassified with medullary phenotype” (RCCU-MP). Fluorescence in situ Hybridization (FISH) can detect alterations in SMARCB1/INI1 consisting mostly in inactivating translocation of one allele and deletion of the second. To date, only seven further cases of RCCU-MP have been described in the literature. Here we report the second Italian case of RCCU-MP, a 62-year-old man presenting with persistent dull back pain and incidentally discovering a 13 cm mass in the right kidney. The nomenclature of this entity is still debated and might be updated as a variant of medullary carcinoma in the upcoming WHO classification. In the meantime, we encourage awareness of these extraordinarily rare neoplasms with poor outcomes.
Introduction
Renal medullary carcinoma (RMC) is an aggressive neoplasm accounting for <0.5% of renal cell carcinomas (RCC) (1, 2). This rare entity occurs mainly in the third decade of life and almost all (> 95%) patients have sickle cell trait, sickle cell disease, or associated hemoglobinopathies (2). RMC is characterized by inactivating genomic alterations in SMARCB1/INI1, a tumor suppressor gene (3), resulting in the loss of immunohistochemical expression of INI1 in all cases (4–6). Recently, exceedingly rare tumors sharing morpho-phenotypic features with RMC, but occurring in older patients without hemoglobinopathy have been reported in a few case reports and our recent small case series (7–9) and provisionally termed “RCC unclassified with medullary phenotype” (RCCU-MP) (7, 10). Alterations in SMARCB1/INI1 can be detected by Fluorescence in situ Hybridization (FISH). Almost 85% of patients with RMC show a genetic loss, most commonly due to inactivating translocation of one SMARCB1 allele and deletion of the second allele. Less frequently, deletion of both SMARCB1 alleles, deletion of one SMARCB1 allele and inDel of the second SMARCB1 allele, or deletion of one SMARCB1 allele and truncating non-sense mutation of the second SMARCB1 allele might occur (11). Here we report the second Italian case of RCCU-MP, a fascinating entity whose definition might be further clarified by the new upcoming WHO classification.
Case Description
A 62-year-old Italian man presented with persistent dull back pain. The patient was on anticoagulant therapy for recent pulmonary thromboembolism, had no previous history of malignancy or surgery, and presented no comorbidities. Ultrasound abdominal examination detected a renal mass, confirmed by a CT scan that documented a 13 × 10.8 × 5 cm solid tumor in the upper pole of the right kidney (Figure 1A), renal vein, and inferior vena cava thrombosis.
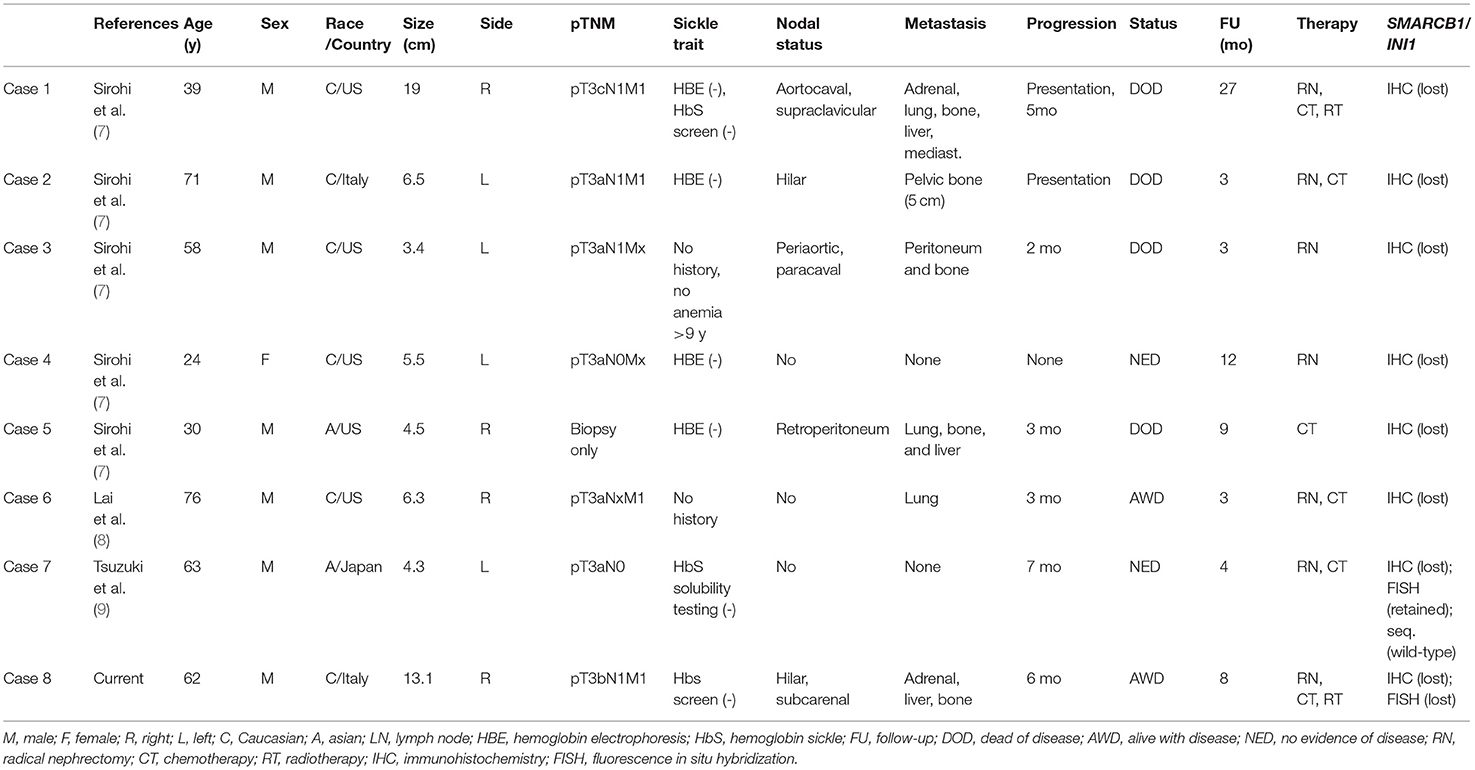
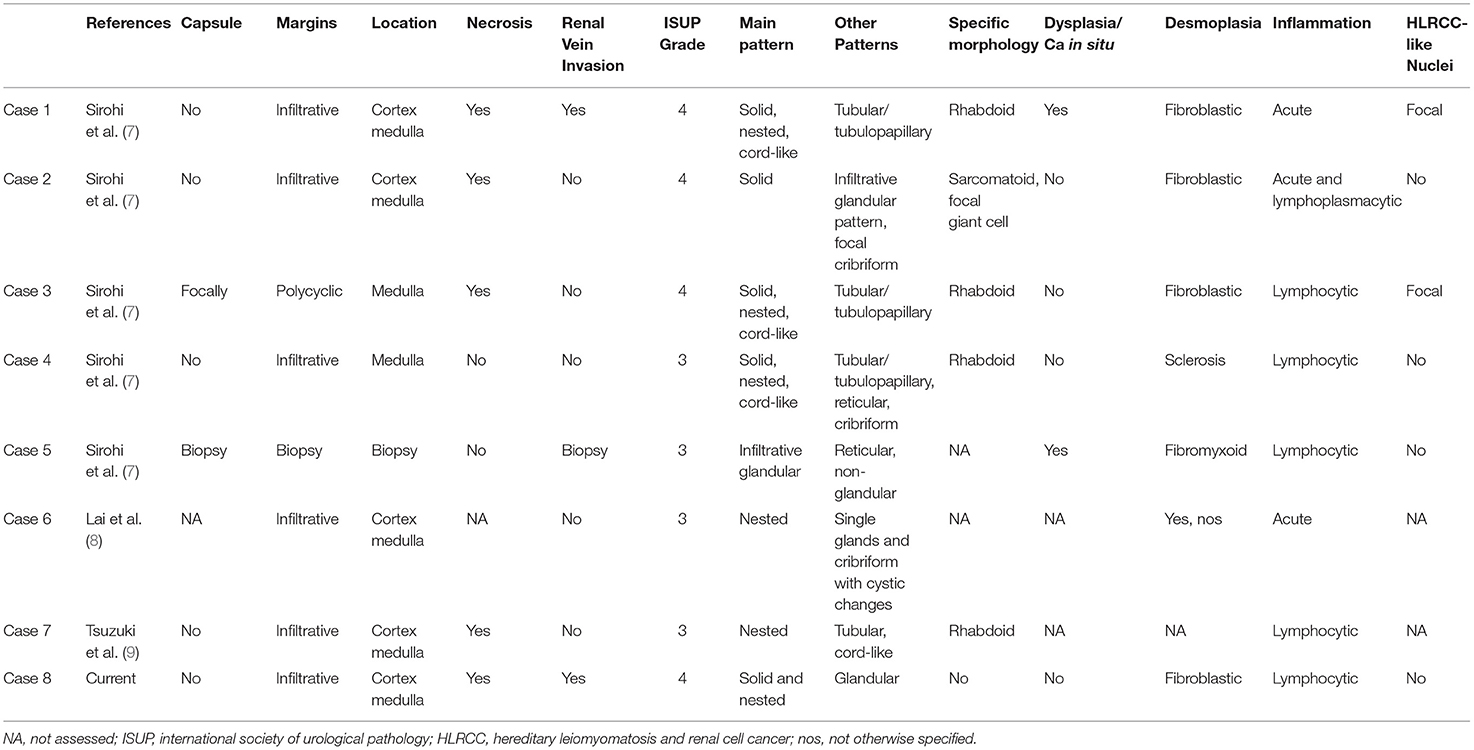
Figure 1. Abdominal CT scan showing a bulky renal mass in the upper pole of the right kidney (A). Grossly, renal parenchyma was partially replaced by a whitish, solid, and necrotic mass (B). Histological view showing a solid proliferation of eosinophilic, highly pleomorphic, epithelioid cells, with nested growth pattern and focal pseudo-glandular differentiation, associated with desmoplastic stromal response. Multiple foci of necrosis were present (30% of tumor). Notably, many cells had a high nuclear-cytoplasmic ratio with vesicular nuclei and prominent nucleoli (C,D). Immunohistochemically, the tumor was CK7+ in almost all cells (E) and fumarate hydratase (FH) expression was retained (F); neoplastic cells showed the characteristic loss of INI1 (G). Fluorescence in situ hybridization (FISH) with SPEC SMARCB1/22q12 Dual Color Probe detected, in a representative tumoral area, a loss of one SMARCB1 allele (single green and orange signal per cell) in almost half of the cells (H).
Right radical nephrectomy with cavotomy, thrombectomy, and regional lymphadenectomy was performed.
At gross examination, the tumor presented as a whitish, extensively necrotic mass of 13.1 cm in greatest dimension, infiltrating into the perinephric and renal sinus adipose tissue; neoplastic thrombosis was confirmed (Figure 1B). Incidentally, a metastatic nodule was documented in the ipsilateral adrenal gland.
Histologically, the tumor consisted of a proliferation of epithelioid cells, with enlarged and pleomorphic nuclei and eosinophilic cytoplasm, showing predominant solid and nested growth pattern, focally glandular, infiltrative borders, and extensive replacement of renal medulla (Figures 1C,D). The neoplasm was associated with desmoplastic stromal response, inflammatory lymphocytic infiltrate, and multiple foci of necrosis (~30% of the tumor). One metastatic hilar lymph node was observed. Non-neoplastic kidney showed chronic interstitial nephritis and mild glomerulosclerosis. Histological features, together with medullary involvement, prompted us to the hypothesis of medullary carcinoma.
The tumor cells showed an immunophenotype specific of the proximal renal tubule, with immunopositivity for cytokeratin 7, PAX8, and FH (retained), and absence of GATA3 and OCT3/4. Focal immunoreactivity for CA IX and Racemase was found. Expression of INI1 was lost, as expected in the suspicion of RMC (Figures 1E–G). Blood tests did not show evidence of sickle cell trait, sickle cell disease, or any other hemoglobinopathy, therefore the diagnosis of RCCU-MP was made. To detect SMARCB1/INI1 alterations, FISH was performed on paraffin sections of both tumoral areas and adjacent normal tissue using a commercial SPEC SMARCB1/22q12 Dual Color Probe (ZytoLight®, according to the manufacturer's protocol). Loss of one SMARCB1 allele was found in 42% of cells in the tumoral area (hemizygous deletion) (Figure 1H). Six months later, the patient experienced mediastinal nodal, hepatic, and bone metastases. Therefore, systemic therapy (Pembrolizumab + Axitinib and Radiotherapy on bone lesions) was administered. After 8 months of follow-up, the patient was alive with the disease.
Discussion
In this study, we described the eighth case reported so far of RCCU-MP (7–9). The provisional diagnostic terminology of “RCCU-MP” has been recently proposed by international genitourinary pathologists for extraordinarily rare tumors with morphological and phenotypical characteristics of RMC but without sickle cell trait nor sickle cell disease (10, 12). However, the definite designation of these neoplasms is still debated (2).
In the past, this entity has been mislabeled as other RCC subtypes, such as unclassified RCC or Collecting duct carcinoma (CDC). Therefore, it has been possibly under-reported in the literature. Indeed, RCCU-MP is often a challenging diagnosis. In the absence of hemoglobinopathies, the differential diagnosis of RCCU-MP comprises CDC (HMWK+, OCT3/4-, INI1+), upper tract urothelial carcinoma (UTUC) (GATA3+, p63+, OCT3/4-, INI1+), Fumarate-Hydratase deficient RCC (FH-d RCC) (FH-, OCT3/4-, INI1+), ALK-rearranged RCC (ALK+, INI1+), and metastatic carcinoma (2). Rarely, some rhabdoid features might raise the differential diagnosis with rhabdoid tumors of the kidney (9).
Genes related to hypoxia have been advocated in the pathogenesis of SMARCB1 abnormalities in RMC, triggered by red blood cell sickling and subsequent ischemia. SMARCB1 has been suggested to be located in a hotspot region for de novo mutations susceptible to hypoxic stress mediated by peculiar medullary microvascular physiology (13). Given the overlapping features between RMC and RCCU-MP, Sirohi et al. recently suggested that a genetic predisposition unrelated to hemoglobinopathies might lead to SMARCB1 abnormalities mediated by the same vascular mechanisms (14). Although interesting, this hypothesis needs further validation.
To the best of our knowledge, only seven further cases of RCCU-MP have been systematically collected and reported in the literature (7–9), whose clinical features are summarized in Table 1. The patients were mostly Caucasian and men, with a mean age of 52 years, and almost all cases were locally advanced (pT3) with distant metastases. Interestingly, in only one case genetic alterations were investigated, with a negative result (9). All cases were centered in the renal medulla; the main morphological pattern was solid, either nested or cord-like, often with necrosis and diffuse polymorphism. Four cases showed rhabdoid features while desmoplasia and inflammation were documented in almost all patients. Morphological features are summarized in Table 2. All cases were INI1-(lost), PAX8+ and FH+(retained), 4/5 cases were CK7+, and 5/7 cases were OCT3/4+.
This is the eighth case of RCCU-MP reported so far. The current classification of this rare entity is still debated: RCCU-MP and RMC share both morphological and phenotypical features so they might be regarded as variants of the same disease in the next future.
Waiting for the new WHO classification, awareness of the diagnosis of these rare entities should be encouraged since they identify patients with poor prognoses and might reveal unacknowledged hemoglobinopathies.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written consent was acquired from the patient.
Author Contributions
PC and MV conceived, designed the study, wrote, and revised the final manuscript. LT, MC, GE, and CD reviewed the histological slides and revised the final manuscript. NR performed the FISH analysis. GL and NB performed surgery and provided clinical data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swartz MA, Karth J, Schneider DT, Rodriguez R, Beckwith JB, Perlman EJ. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology. (2002) 60:1083–9. doi: 10.1016/S0090-4295(02)02154-4
2. Baniak N, Tsai H, Hirsch MS. The differential diagnosis of medullary-based renal masses. Arch Pathol Lab Med. (2021) 145:1148–70. doi: 10.5858/arpa.2020-0464-RA
3. Hollmann TJ, Hornick JL. INI1-deficient tumors: diagnostic features and molecular genetics. Am J Surg Pathol. (2011) 35:47–63. doi: 10.1097/PAS.0b013e31822b325b
4. Ohe C, Smith SC, Sirohi D, Divatia M, De Peralta-Venturina M, Paner GP, et al. Reappraisal of morphologic differences between renal medullary carcinoma, collecting duct carcinoma, and fumarate hydratase-deficient renal cell carcinoma. Am J Surg Pathol. (2018) 42:279–92. doi: 10.1097/PAS.0000000000001000
5. Liu Q, Galli S, Srinivasan R, Marston W, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol. (2020) 37:368–74. doi: 10.1097/PAS.0b013e3182770406
6. Calderaro J, Moroch J, Pierron G, Pedeutour F, Grison C, Maillé P, et al. SMARCB1/INI1 inactivation in renal medullary carcinoma. Histopathology. (2012) 61:428–35. doi: 10.1111/j.1365-2559.2012.04228.x
7. Sirohi D, Smith SC, Ohe C, Colombo P, Divatia M, Dragoescu E, et al. Renal cell carcinoma, unclassified with medullary phenotype: poorly differentiated adenocarcinomas overlapping with renal medullary carcinoma. Hum Pathol. (2017) 67:134–45. doi: 10.1016/j.humpath.2017.07.006
8. Lai JZ, Lai HH, Cao D. Renal cell carcinoma, unclassified with medullary phenotype and synchronous renal clear cell carcinoma present in a patient with no sickle cell trait/disease: diagnostic and therapeutic challenges. Anticancer Res. (2018) 38:3757–61. doi: 10.21873/anticanres.12657
9. Tsuzuki S, Kataoka TR, Ito H, Ueshima C, Asai S, Yokoo H, et al. A case of renal cell carcinoma unclassified with medullary phenotype without detectable gene deletion. Pathol Int. (2019) 69:710–4. doi: 10.1111/pin.12858
10. Amin MB, Smith SC, Agaimy A, Argani P, Compérat EM, Delahunt B, et al. Collecting duct carcinoma versus renal medullary carcinoma: an appeal for nosologic and biological clarity. Am J Surg Pathol. (2014) 38:871–4. doi: 10.1097/PAS.0000000000000222
11. Msaouel P, Malouf GG, Su X, Yao H, Tripathi DN, Soeung M, et al. Comprehensive molecular characterization identifies distinct genomic and immune hallmarks of renal medullary carcinoma. Cancer Cell. (2021) 37:720–34. doi: 10.1016/j.ccell.2020.04.002
12. Colombo P, Smith SC, Massa S, Renne SL, Brambilla S, Peschechera R, et al. Unclassified renal cell carcinoma with medullary phenotype versus renal medullary carcinoma: lessons from diagnosis in an Italian man found to harbor sickle cell trait. Urol Case Rep. (2015) 3:215–8. doi: 10.1016/j.eucr.2015.07.011
13. Msaouel P, Tannir NM, Walker CL. A model linking sickle cell hemoglobinopathies and SMARCB1 loss in renal medullary carcinoma. Clin Cancer Res. (2018) 24:2044–9. doi: 10.1158/1078-0432.CCR-17-3296
Keywords: renal medullary carcinoma, renal cell carcinoma unclassified with medullary phenotype, SMARCB1/INI1, sickle cell trait, kidney, case report
Citation: Valeri M, Cieri M, Elefante GM, De Carlo C, Rudini N, Lughezzani G, Buffi NM, Terracciano LM and Colombo P (2022) Case Report: Unclassified Renal Cell Carcinoma With Medullary Phenotype and SMARCB1/INI1 Deficiency, Broadening the Spectrum of Medullary Carcinoma. Front. Med. 9:835599. doi: 10.3389/fmed.2022.835599
Received: 14 December 2021; Accepted: 10 January 2022;
Published: 07 February 2022.
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
Rahul Mannan, University of Michigan, United StatesDeepika Sirohi, The University of Utah, United States
Copyright © 2022 Valeri, Cieri, Elefante, De Carlo, Rudini, Lughezzani, Buffi, Terracciano and Colombo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piergiuseppe Colombo, piergiuseppe.colombo@hunimed.eu
 Marina Valeri1,2
Marina Valeri1,2  Miriam Cieri
Miriam Cieri Nicolò Maria Buffi
Nicolò Maria Buffi Piergiuseppe Colombo
Piergiuseppe Colombo