Sex Disparity in Severity of Lung Lesions in Newly Identified Tuberculosis Is Age-Associated
- 1Shanghai Key Laboratory of Tuberculosis, School of Medicine, Clinical and Research Centre of Tuberculosis, Shanghai Pulmonary Hospital, Tongji University, Shanghai, China
- 2Department of Radiology, School of Medicine, Shanghai Pulmonary Hospital, Tongji University, Shanghai, China
- 3Key Laboratory of Environment Pollution Monitoring and Disease Control, School of Public Health, Guizhou Medical University, Ministry of Education, Guiyang, China
Background: The age-associated characteristic of computed tomography (CT) images of tuberculosis (TB) and the reason for male bias in TB are still not clear.
Methods: We compared the CT images, clinical inflammatory indices and sputum bacterial counts between 594 non-smoking men and women with newly diagnosed TB with matched large span of ages from 15 to 92 years old. Logistic regression analyses were used to identify the cavity-associated factors of men and women, separately and in combination.
Results: Sputum bacterial counts, ratio of cavities, lung injury scores, and level of C reactive protein were significantly higher in men than in women with ages from 15 to 74, but not in cases older than 75. In CT images, thick walled cavity, cicatricial emphysema and parenchymal bands were present in men at ages of 15–74 more than matched women. Ratios of cases with lobular emphysema and pleural effusion were higher in men after age of 56. While ratios of cases with parenchymal bands, calcification, pleural effusion, pleural thickening, lobular emphysema and bronchovascular distortion increased with aging, those of centrilobular nodules, micronodules and tree in bud decreased with aging in men. Erythrocyte sedimentation rate (ESR) increased with aging, but no differences were found between men and women in ESR or T-SPOT TB tests. Higher complement C4 and lower body mass index in men and positive result in anti-TB antibody test in women were strongly associated with the presence of cavity.
Conclusions: The sex bias in TB is age-associated. TB prevention, treatment and research should take differences of sex and age into account.
Background
Tuberculosis (TB), which is induced by Mycobacterium tuberculosis (Mtb.), resulted in approximately 1.3 million deaths in 2017 and remains one of the top 10 causes of death and the leading cause of a single infectious agent worldwide (1). A male bias in case notification was noticed with a man to woman ratio of TB cases of around 1.5–2.1 in all regions of the world (1, 2), and the influence of different levels of accessibility to healthcare services between men and women has been excluded in a two-stage random sampling population survey through interviews of men and women in pairs (3).
In the human population, the gap between male and female reported TB cases seems to start after puberty (1) (2017-annext II). Consistently, the exacerbated pulmonary pathology and increased morbidity and mortality in male mice can be prevented by castration (4, 5). These observations indicated that age-associated factors, e.g., changed levels of sex hormones, may play key roles in pathogenesis of TB, and testosterone could be a TB susceptibility factor. Except for sex hormones, however, sex–related genetic background and regulation, and sex-specific metabolic features are also suggested to be correlated with the sex bias in TB pathogenesis (6–9). The underlying mechanisms of the sex bias in TB are still uncertain.
Sex hormone-associated differences in both the immune and the endocrine systems are influenced greatly by aging (10). In this study, we hypothesize that, through comparison of the lung lesions with computed tomography (CT) images and inflammatory indices between new cases of well-matched men and women with TB over a large span of ages (adolescent and adult, middle aged and gerontic aged), we can get clues for the interplay of sex-associated endocrines with the aging immune system on sex bias of TB pathogenesis.
Methods
Study Subjects
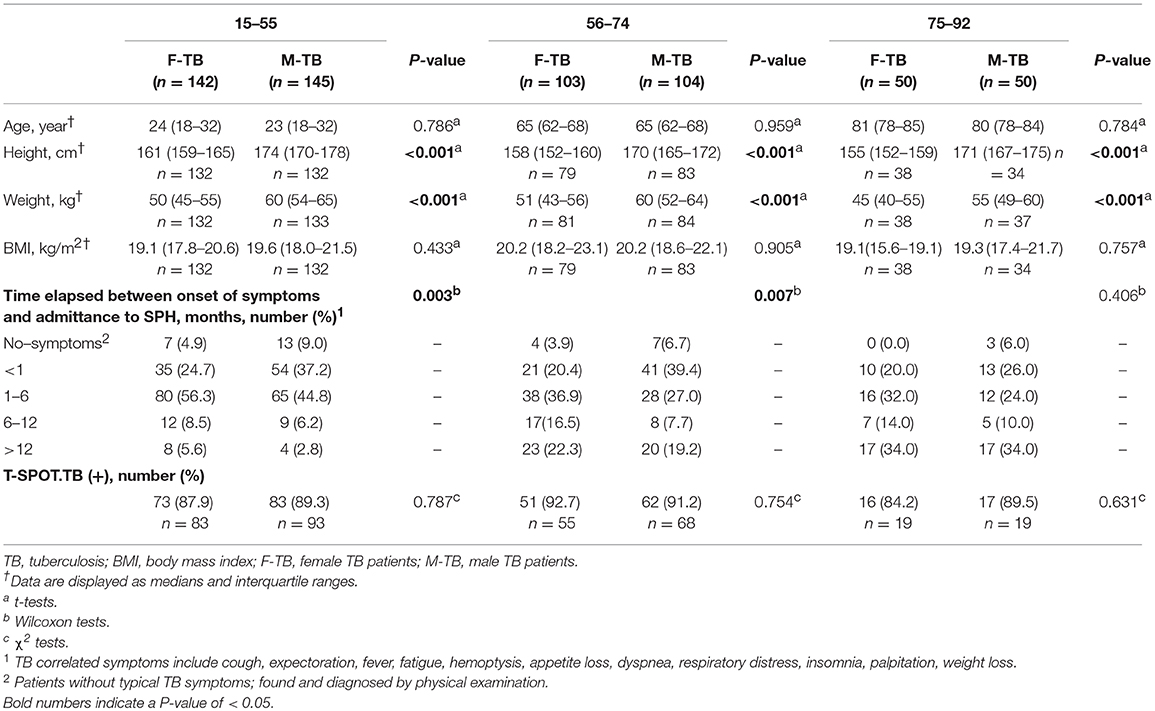
We included in a total of 594 newly diagnosed pulmonary TB (299 men, 295 women) with large span of ages (15 to 92 years old) from Shanghai Pulmonary Hospital (SPH) between Feb. 2012 and Nov. 2018. TB was diagnosed based on acid-fast bacilli (AFB) staining and culture; patients whose cultures yielded non-tuberculous mycobacteria were excluded from the study. We retrospectively reviewed medical records and excluded patients who had a history of any of the following: smoking, excessive alcohol drinking, human immunodeficiency virus infection, immunosuppressive drug therapy, hormone therapy, cancer, diabetes, pneumoconiosis, silicosis and Hepatitis B and C viruses infection (11). None of the patients had received anti-TB therapy for more than 1 week before registration. All the enrolled female (F) and male (M) TB subjects were matched for age (±3 years) and were classified according to their ages: adolescents and adults TB (F-TB15−55 and M-TB15−55, footnotes represent the range of ages), middle aged and early elderly TB (F-TB56−74 and M-TB56−74), and gerontic aged patients (F-TB75−92 and M-TB75−92).
Review of Clinical Findings and Laboratory Tests
Routine inflammatory, hematological and biochemical parameters were reviewed. Grading of AFB, T-SPOT.TB tests and anti-TB Ig G antibody (TBAb) detection were carried out according to previous description (11).
Computed Tomography Evaluation
In all 594 patients, high-resolution CT scans from 223 female and 223 male patients performed within the first week of admittance were collected and evaluated as in a previous report (11). In brief, lungs were divided into 6 zones (low, middle and high zones for left and right lungs) and the presence of the abnormalities including nodule, micronodule, cavity, consolidation, parenchymal bands, ground glass opacity and bronchial lesion were noted (12–15). The scans were assessed by two specialists who were blinded to the groups of patients. The total weighted profusion score was calculated as profusion score ×100 / 24 (total score) +40 if cavitation was present.
Ethical Approval
This study was conducted in accordance with the amended Declaration of Helsinki and the ethical guidelines of the institutional review board of Tongji University (Project approval number: K17-043). All participants gave written consent for the use of their clinical information for research purposes. Clinical data were anonymized.
Statistical Analyses
We performed χ2 test for categorical variables, Wilcoxon rank sum test for nominal variables, and t tests for continuous variables. To identify the parameters associated with the extent of lung lesions (cavity), 53 physiological, hematological and biochemical indices were involved in multivariate logistic regression with males and females analyzed separately and in combination. Statistical significance was determined at p < 0.05. All analyses were performed using SPSS (version 19, SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
The physiological characteristics of the age-matched groups of men and women with TB are shown in Table 1. The paired groups showed no significant differences in BMI at the time of their first registration in SPH.
In patient groups with ages of 15–55 and 56–74, the ratios of men (37.2 and 39.4%) who were admitted to hospital within 1 month after onset of the symptoms were higher than those of women (24.7, 20.4%, respectively); no differences were found in this case between men and women patients older than 75.
In T-SPOT.TB tests, there were no statistical difference between men and women in all matched ages on both the positive ratios (Table 1) and the counts of the spots (Supplementary Figure 1). In an antibody test for Mtb. antigens (LAM and 38 kD), postmenopausal women showed periodical decrease in positive ratio in F-TB56−74 compared to the age–matched M-TB56−74 and F-TB15−55 (Figure 1), but no difference was found between men and women in results of these two tests of Mtb. infection at other stages of age.
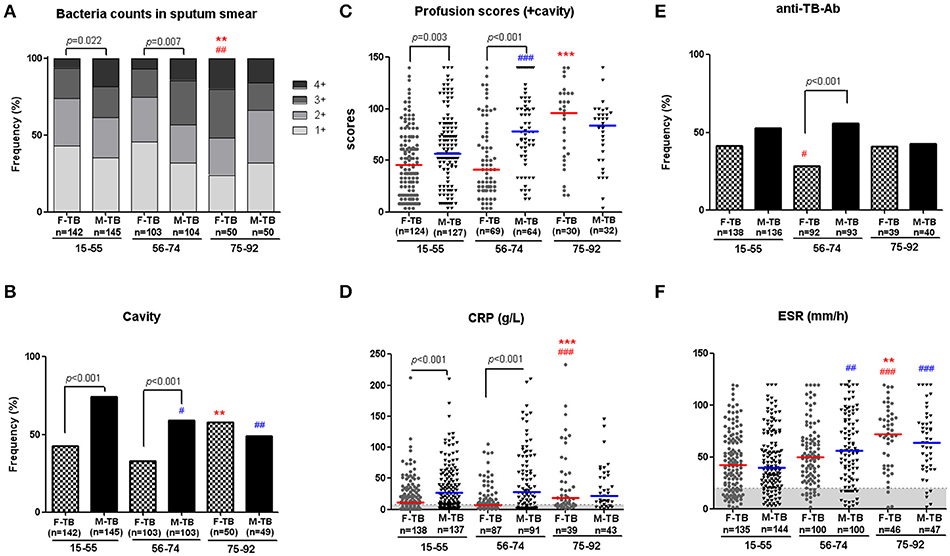
Figure 1. Age-associated male bias in active TB patients without treatment. Disparity on grades of sputum bacterial counts (A), cavity (B), weighted profusion scores (C), and CRP levels (D) were found between M-TB15−74 and matched F-TB15−74, but not between the genders with ages older than 75. Results of anti-TB antibody response (E) and ESR (F) in men and women with TB at different age stages are also shown. The χ2 tests were used to compare variables displayed as percentages. Differences between groups of C,D,F were analyzed by Mann–Whitney tests. Horizontal lines represent median values. #compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). **P < 0.01; ***P < 0.001.
Male Bias in Lung Lesions Is Age-Associated
Higher sputum bacterial counts (3+ and 4+) were observed in men with TB at ages of 15 to 74 years (F-TB15−55 vs. M-TB15−55, 26.1 vs. 38.6%; F-TB56−74 vs. M-TB56−74, 25.3 vs. 43.3%), but not in patients after the age of 75 (F-TB75−92 vs. M-TB75−92, 52 vs. 34%) (Figure 1), as sputum bacterial counts significantly increased in gerontic aged women.
The analysis of the CT images revealed that both men and women with TB showed age-dependent increase in the injured zones of lung (Supplementary Figure 2) and in ratios of pleural thickening, pleural effusion, calcification and lobular emphysema (Figure 2). Aging-associated increase of bronchiectasis, bronchial wall thickening, cicatricial emphysema, parenchymal bands and bronchovascular distortion were only found in women with TB; cases with these indices decreased in M-TB75−92. Men with centrilobular nodules, micronodules, and tree in bud reduced after age of 55 (Figure 2).
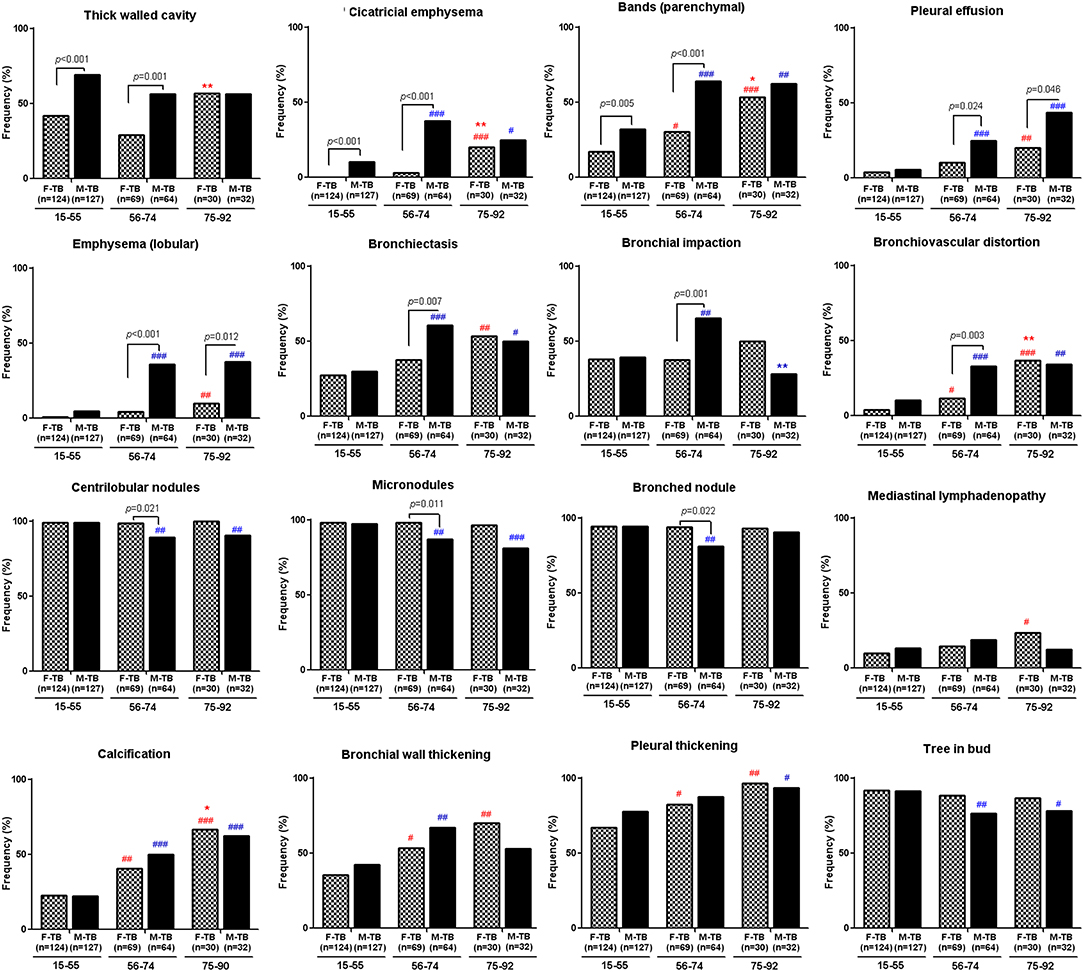
Figure 2. Differential age-associated changes in indices of CT images in men and women with TB. Data shown as positive ratios in each group. #compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). The χ2 tests were used to compare variables displayed as percentages. #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). *P < 0.05; **P < 0.01.
Significantly higher ratios of cases with bronchiectasis, bronchial impaction, bullae, hilar lymphadenopathy and bronchovascular distortion with lower ratios of centrilobular nodules, micronodules, and bronched nodules were found in M-TB56−74 compared to the matched women while ratios of pleural effusion and lobular emphysema were significantly higher in men after the age of 55 than the matched women.
Consistent with the pattern of difference in sputum bacterial counts, the ratios of cavity (Figure 1), especially thick-walled cavity, and those of cicatricial emphysema, parenchymal bands (Figure 2) and weighted profusion scores were significantly higher only in M-TB15−74 than the matched women. The disparities of these indices were not found between men and women older than 75.
Differential Critical Indices Associated With Cavity in Men and Women With TB
Comparison of the clinical indices indicated that the age/sex-associated changes of C reactive protein (CRP), counts of neutrophils, monocytes and white blood cells (WBC), percentages of neutrophils and lymphocytes, ratios of Neu/Lym, Mon/Lym and PLT/Lym, and level of antithrombin III (AT3) were highly consistent with that of cavity and the sputum bacterial counts; the sex disparities of these indices were observed only in patients at the ages of 15-74 (Figure 3). The erythrocyte sedimentation rate (ESR) increased with aging in both men and women, especially in women after the age of 75, but no statistically significant difference of ESR was observed in all the paired groups (Figure 1).
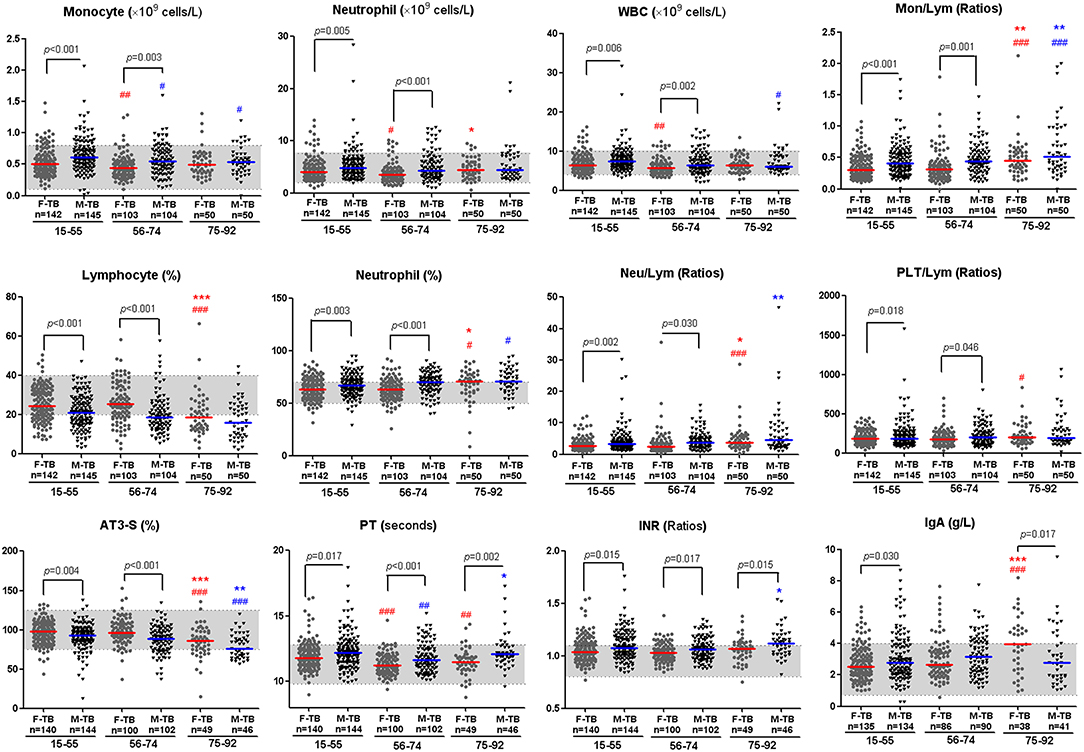
Figure 3. Inflammatory and coagulation indices with significant disparity between men and women with TB. Horizontal lines represent median values. Gray areas represent the normal ranges of the indices. WBC, white blood cells; Mon/Lym, monocyte to lymphocyte ratio; Neu/Lym, neutrophil to lymphocyte ratio; PLT/Lym, platelet to lymphocyte ratio; AT3, antithrombin III; PT, prothrombin time; INR, international normalized ratio; IgA, Immunoglobulin A. The differences between groups were analyzed by Mann–Whitney tests. #Compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). *P < 0.05; **P < 0.01; ***P < 0.001.
The aging-associated changes and disparities of other indices between men and women with TB are shown in Figure 3, Supplementary Figures 1, 3, 4. Significant difference in coagulation-correlated prothrombin time (PT) and PT-associated international normalized ratio (INR) exists between men and women in the whole age span (Figure 3).
In multivariate logistic regression analysis including both men and women cases, male gender included in the model was highly associated with cavity with odds ratio (OR = 2.70). Higher age was associated with decreased cavity on the whole (OR = 0.5); while mean corpuscular hemoglobin concentration and ESR showed weak association (ORs were closed to 1) (Table 2). However, in a separate analysis, cavity in men was strongly and positively associated with complement component 4 (C4) (OR = 4092.06, P < 0.001) and negatively with BMI (OR = 0.84, P = 0.026). In women with TB, compared to the 15-55 age range, ages 56-74 were negatively associated with cavity (OR = 0.16), but ages 75-92 were positively associated with cavity (OR = 18.07). Positive response in anti-TB antibody test [TBAb(+)] (OR = 3.05, P = 0.023) had medium-strength association with cavity (Table 2).
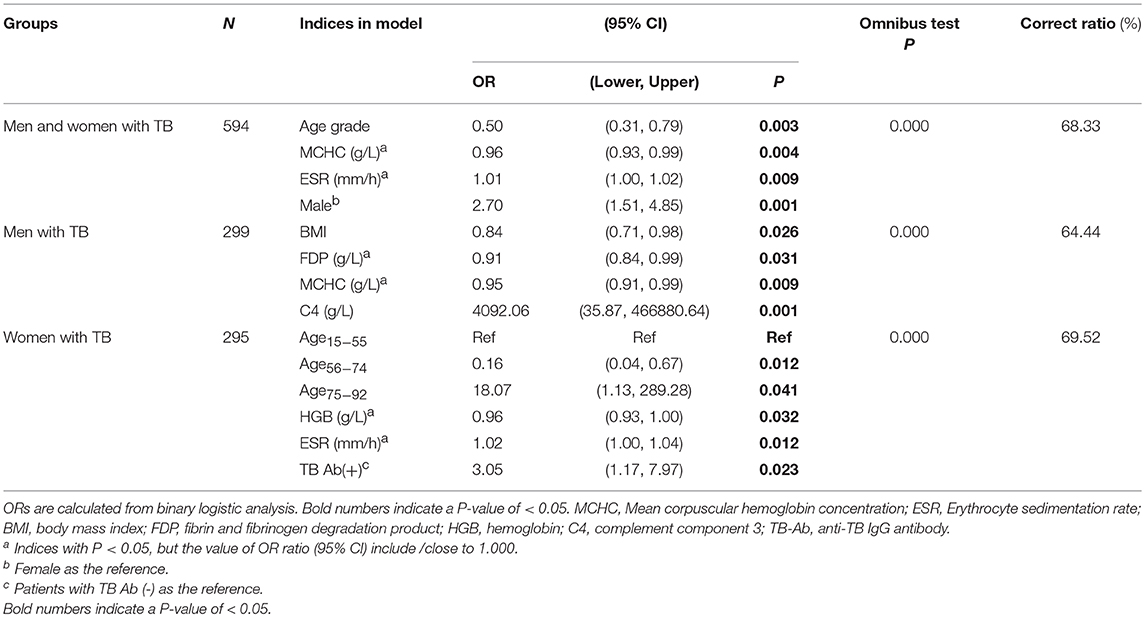
Table 2. Indices associated with cavity in multivariate logistic regression analysis in men and women with TB separately and in combination.
Discussion
This study characterized the radiological and clinical indices in men and women with newly diagnosed TB, with a long age span from adolescent and adult to elderly ages. As we have excluded the cases with common confounding factors which bias the pathogenesis of TB (16, 17), the significant difference of lung lesions between men and women may reflect the overall impact of age-associated differential endocrines, especially sex hormones, sex-related genetic regulation, and metabolism on the host response to the infection.
On the whole, men with TB had higher ratios of cases presented with inflammatory/infection-associated indices either at ages of 15-74 (cavity, esp. thick-walled cavity, cicatricial emphysema, parenchymal bands), after age of 56 (pleural effusion, lobular emphysema), or periodically at 56-74 (bronchiectasis, bronchial impaction, bronchiovascular distortion, bullae, and hilar lymphadenopathy).
The accumulation of these detailed disparity results in the first intriguing finding that the male bias in TB pathogenesis is age-associated. The indices which reflect the severity of lung lesion on the whole directly (the ratios of cavity, especially thick-walled cavity), and indirectly (weighted profusion scores, sputum bacterial loads), as well as the typical indices which reflect inflammatory status (CRP, counts of WBC, neutrophil, monocytes, etc.) all were significantly higher in M-TB15−74 than in F-TB15−74, but not in men with TB older than 75.
It is intriguing that even the gender differences of time from onset of TB-associated symptoms to seeking healthcare in this study were consistent with the similar age-associated pattern of changes as mentioned above. A previous population-based survey which assessed health-seeking behavior in adults indicated a significant delay in women (41 vs. 19 days in men) with a cough of more than 3 weeks (18, 19). The author suggested that socio-economic and culture-associated sex inequalities may lead to poorer access to health care and delays to diagnosis of TB in women. However, this possibility has been excluded in a following survey based on large populations in Bangladesh (3). Our results from this and a previous study (20) with sputum smear positive patients may suggest a biological explanation of the age stage-associated delay of health-seeking behavior in women with TB: TB-induced lung lesions might be mild and less severe and therefore endurable in women compared to in men during adolescent and adult ages after onset of symptoms, while they become more severe in women than in men at elderly ages.
Age-associated changes in levels of sex steroid hormones may play key roles in this age-associated sex disparity in TB-induced lung lesions (10). In our study, two age points, 55 and 75, were set to divide the patients into three groups according to their age-associated differential levels of sex hormones. In general, at age 55, most women are postmenopausal; estradiol production in the ovaries ceases (21). Thereafter, only basal levels of progesterone are being synthetized by the adrenal glands. In aged women, dehydroepiandrosterone and testosterone levels decrease, yet follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels rise from the 4th decade onwards (22). In men, the concentration of serum testosterone starts declining steadily at ~30–40 years of age at a rate of about 1% per year (23); the level of free testosterone decreases by about 2–3% per year displaying no clear turning point (24). Accordingly, in men at ages of 75 and elder, the level of free testosterone will decline to about 1/3 of the level of the testosterone in men in their forties, although the average testosterone level remains within the normal range in most men. In turn, estradiol, estrone, LH, and FSH gradually increase (25).
In general, estradiol exhibits an enhancing effect, while testosterone exhibits an inhibitory effect on both the adaptive and innate immune systems (10). However, our data indicated that menopause in women did not change the bias of lung pathogenesis instantly, while the reducing cavities in aged men were seemly consistent with their theoretically decreasing testosterone levels. Moreover, low BMI in men but not in women was significantly correlated with cavity. As BMI and the associated obesity are regulated by androgen-estrogen balance (26, 27), the interplay between all the above-mentioned sex steroid hormones, rather than each isolated hormone, with aging immunity, may decide the differential extent of TB pathogenesis in men and women (28, 29).
Data from the WHO's annual report (1) (2017-annext II) compensate the limitation of our lacking data of TB with ages before puberty ( ≤ 14 years old): in most countries with high TB burden, remarkable sex bias in estimated TB incidence was found only in cases after puberty. The male/female ratios of estimated TB incidence in cases before 14 in these countries were mostly close to 1. Consistently, two analyses based on registering 31,358 new smear positive pediatric cases with TB in China (30), and on 10,744 patients in Tuscany (31) showed close notification ratios between boys and girls from the age of 0 to 14 years. These data further substantiated our hypothesis about the decisive role of sex hormones in sex disparity of TB pathogenesis.
We should emphasize, however, that this retrospective study was simplified by excluding the cases with confounding factors. In reality, although men and women non-smokers have similar proportions of LTBI in TB endemic area, according to a cross-sectional study in Taiwan (32), smoking may influence latent TB infection (LTBI) (32) and severity of active TB (33). Moreover, aging-associated chronic diseases, such as diabetes, hypertension, cardiac, hepatic or renal failure, chronic obstructive lung disease, and inflammatory arthritis, as well as medications, and even biological age-associated weight gain may change the levels of sex steroid hormones (34) and influence the susceptibility to TB (11). Stratified epidemiology research in the elderly by sex will reveal the overall influence of these factors on TB pathogenesis.
In addition, while male gender was identified as the only factor which is significantly associated with cavity in analysis of all 53 indices in all men and women patients, higher C4 level and positive anti-TB antibody response were identified as associated factors in men and women, respectively. Our data suggest that indiscriminate analysis of male and female cases may mask the key factors associated with pathogenesis. Very few studies have assessed the results of men and women separately (35); likewise, differential response to Mtb. infection and anti-TB treatment in either young or old men patients, and pre- or post-menopausal women patients have not yet been well-recognized. Therefore, our study emphasizes the importance of stratifying the analysis by both the sex and age in TB research to get an unbiased conclusion (36, 37).
Finally, as increasing levels of circulating antibody/antigen complexes with activation of classical complement (38, 39) characterized early disease in TB, understanding the influence of sex-associated endocrines on the complement and humoral immune response in TB progression (40) may help to reveal the mechanisms of the immune pathogenesis of TB in men and women. Further research is required to gain a better understanding of the differences in immunity to TB between men and women for future TB prevention and forecasting (41) at the community level (41, 42) and developing individualized treatment concepts for severe TB cases that take sex/age-specific host factors into account.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics Statement
This study was conducted in accordance with the amended Declaration of Helsinki and the ethical guidelines of the institutional review board of Tongji University (Project approval number: K17-043). All participants gave written consent for the use of their clinical information for research purposes; written informed consent was obtained from the parents or guardians of participants under the age of 16. Clinical data were anonymized.
Author Contributions
YF and JS designed research and wrote the article. JS and AS-L analyzed and interpreted radiological data. YC, FC, JH, GY, and XH collected clinical data. HY and WS interpreted the clinical data. YC, AS-L, and YF analyzed the data. All authors read and approved the final manuscript.
Funding
This work was supported by grants from National Natural Science Foundation of China (grant Nos. 81172806, 81471563, 81771692, 81760578).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00163/full#supplementary-material
Supplementary Figure 1. Age-associated inflammatory and coagulation indices without significant disparity between matched men and women with TB. Horizontal lines represent median values. Gray areas represent the normal ranges of the indices. Each dot from T-SPOT counts represents the higher count from the two counts of the test for each case. RDW-SD, red blood cell distribution width Standard Deviation; C3, complement component 3; FDP, fibrin and fibrinogen degradation product; PCT, plateletcrit; IgG, Immunoglobulin G. The differences between groups were analyzed by Mann–Whitney tests. #Compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Figure 2. Injured zones and indices of CT images without significant disparity between men and women with TB. The χ2 tests were used to compare variables displayed as percentages. #Compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). *P < 0.05.
Supplementary Figure 3. Age-associated change and disparity in transferrin, IgM and coagulation indices in men and women with TB. Horizontal lines represent median values. Gray areas represent the normal ranges of the indices. IgM, Immunoglobulin A; FIB, fibrinogen; C4, complement component 4; TT, thrombin time; PDW, platelet distribution width; MPV, mean platelet volume; P-LCR, platelet-large cell ratio; APTT, activated partial thromboplastin time. The differences between groups were analyzed by Mann–Whitney tests. #Compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). *P < 0.05; **P < 0.01.
Supplementary Figure 4. Age-associated changes of red blood cell indices between men and women with TB. Horizontal lines represent median values. Gray areas represent the normal ranges of the index in women, the area between dashed lines represents the normal ranges of the index in men. The differences between groups were analyzed by Mann-Whitney U tests. RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCH, mean corpuscular hemoglobin; MCV, erythrocyte mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration. The differences between groups were analyzed by Mann–Whitney tests. #Compared with F-TB15−55 (red mark) or M-TB15−55 (blue mark). #P < 0.05, ##P < 0.01; ###P < 0.001. *Compared with F-TB56−74 (red mark) or M-TB56−74 (blue mark). *P < 0.05; **P < 0.01; ***P < 0.001.
Abbreviations
AFB, acid-fast bacillus; BMI, body mass index; C4, complement component 4; CRP, C reactive protein; FIB, fibrinogen; HRCT, high resolution computed tomography; INR, international normalized ratios; OR, odds ratio; PT, prothrombin time; TB, tuberculosis; TB-Ab, anti-TB IgG antibody.
References
2. Horton KC, MacPherson P, Houben RM, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. (2016) 13:e1002119. doi: 10.1371/journal.pmed.1002119
3. Hamid Salim MA, Declercq E, Van Deun A, Saki KA. Gender differences in tuberculosis: a prevalence survey done in Bangladesh. Int J Tuberc Lung Dis. (2004) 8:952–7.
4. Dibbern J, Eggers L, Schneider BE. Sex differences in the C57BL/6 model of Mycobacterium tuberculosis infection. Sci Rep. (2017) 7:10957. doi: 10.1038/s41598-017-11438-z
5. Bini EI, Mata Espinosa D, Marquina Castillo B, Barrios Payán J, Colucci D, Cruz AF, et al. The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PLoS ONE. (2014) 9:e93831. doi: 10.1371/journal.pone.0093831
6. Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. (2009) 6:e1000199. doi: 10.1371/journal.pmed.1000199
7. Salie M, Daya M, Lucas LA, Warren RM, van der Spuy GD, van Helden PD, et al. Association of toll-like receptors with susceptibility to tuberculosis suggests sex-specific effects of TLR8 polymorphisms. Infect Genet Evol. (2015) 34:221–9. doi: 10.1016/j.meegid.2015.07.004
8. Nhamoyebonde S, Leslie A. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis. (2014) 209:S100–6. doi: 10.1093/infdis/jiu147
9. Hertz D, Schneider B. Sex differences in tuberculosis. Semin Immunopathol. (2019) 41:225–37. doi: 10.1007/s00281-018-0725-6
10. Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. (2015)14:309–21. doi: 10.1111/acel.12326
11. Dong Z, Shi J, Dorhoi A, Zhang J, Soodeen-Lalloo AK, Tan W, et al. Hemostasis and lipoprotein indices signify exacerbated lung injury in tuberculosis with diabetes comorbidity. Chest. (2018) 153:1187–200. doi: 10.1016/j.chest.2017.11.029
12. Ors F, Deniz O, Bozlar U, Gumus S, Tasar M, Tozkoparan E, et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging. (2007) 22:154–9. doi: 10.1097/01.rti.0000213590.29472.ce
13. Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. (2010) 65:863–9. doi: 10.1136/thx.2010.136242
14. Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, et al. Pulmonary tuberculosis: CT findings–early active disease and sequential change with antituberculous therapy. Radiology. (1993) 186:653–60. doi: 10.1148/radiology.186.3.8430169
15. Im JG, Itoh H, Han MC. CT of pulmonary tuberculosis. Semin Ultrasound CT MR. (1995) 16:420–34. doi: 10.1016/0887-2171(95)90029-2
16. Berg RD, Levitte S, O'Sullivan MP, O'Leary SM, Cambier CJ, Cameron J, et al. Lysosomal disorders drive susceptibility to tuberculosis by compromising macrophage migration. Cell. (2016) 165:139–52. doi: 10.1016/j.cell.2016.02.034
17. Lin HH, Wu CY, Wang CH, Fu H, Lönnroth K, Chang YC, et al. Association of obesity, diabetes, and risk of tuberculosis: two population-based cohorts. Clin Infect Dis. (2018) 66:699–705. doi: 10.1093/cid/cix852
18. Thorson A, Hoa NP, Long NH. Health-seeking behaviour of individuals with a cough of more than 3 weeks. Lancet. (2000) 356:1823–4. doi: 10.1016/S0140-6736(00)03241-4
19. Borgdorff MW, Maher D. Health-seeking behaviour for cough. Lancet. (2001) 357:1532–3. doi: 10.1016/S0140-6736(00)04690-0
20. Tan W, Soodeen-Lalloo AK, Chu Y, Xu W, Chen F, Zhang J, et al. Sex influences the association between haemostasis and the extent of lung lesions in tuberculosis. Biol Sex Differ. (2018) 9:44. doi: 10.1186/s13293-018-0203-9
21. Takahashi TA, Johnson KM. Menopause. Med Clin North Am. (2015) 99:521–34. doi: 10.1016/j.mcna.2015.01.006
22. Al-Azzawi F, Palacios S. Hormonal changes during menopause. Maturitas. (2009) 63:135–7. doi: 10.1016/j.maturitas.2009.03.009
23. Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. (2011) 96:2430–9. doi: 10.1210/jc.2010-3012
24. Huhtaniemi I, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat Rev Urol. (2011) 8:335–44. doi: 10.1038/nrurol.2011.47
25. Jasuja GK, Travison TG, Davda M, Murabito JM, Basaria S, Zhang A, et al. Age trends in estradiol and estrone levels measured using liquid chromatography tandem mass spectrometry in community-dwelling men of the framingham heart study. J. Gerontol A Biol Sci Med Sci. (2013) 68:733–40. doi: 10.1093/gerona/gls216
26. Liu Y, Ding Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction. (2017) 154:R123–31. doi: 10.1530/REP-17-0161
27. Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. (2011) 22:24–33. doi: 10.1016/j.tem.2010.10.002
28. van Koeverden ID, de Bakker M, Haitjema S, van der Laan SW, de Vries JPM, Hoefer IE, et al. Testosterone to estradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res. (2018) 115:453–62. doi: 10.1093/cvr/cvy188
29. Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. (2007) 211:173–80. doi: 10.1002/path.2110
30. Cheng SM, Du X, Xu M. Surveillance and analysis of the pediatric pulmonary tuberculosis in new smear positive cases from 1992 to 2004 in China. Chin J Pediatr. (2006) 44:257–61.
31. Stival A, Chiappini E, Montagnani C, Orlandini E, Buzzoni C, Galli L, et al. Sexual dimorphism in tuberculosis incidence: children cases compared to adult cases in Tuscany from 1997 to 2011. PLoS ONE. (2014) 9:e105277. doi: 10.1371/journal.pone.0105277
32. Ting WY, Huang SF, Lee MC, Lin YY, Lee YC, Feng JY, et al. Gender disparities in latent tuberculosis infection in high-risk individuals: a cross-sectional study. PLoS ONE. (2014) 9:e110104. doi: 10.1371/journal.pone.0110104
33. Gleeson LE, O'Leary SM, Ryan D, McLaughlin AM, Sheedy FJ, Keane J. Cigarette smoking impairs the bioenergetic immune response to mycobacterium tuberculosis infection. Am J Respir Cell Mol Biol. (2018) 59:572–9. doi: 10.1165/rcmb.2018-0162OC
34. Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J Clin Endocrinol Metab. (2008) 93: 2737–45. doi: 10.1210/jc.2007-1972
35. Schurz H, Kinnear CJ, Gignoux C, Wojcik G, van Helden PD, Tromp G, et al. A sex-stratified genome-wide association study of tuberculosis using a multi-ethnic genotyping array. Front Genet. (2019)9:678. doi: 10.3389/fgene.2018.00678
36. Heidari S, Bachelet VC. Sex and gender analysis for better science and health equity. Lancet. (2018) 392:1500–2. doi: 10.1016/S0140-6736(18)32619-9
37. van Lunzen J, Altfeld M. Sex differences in infectious diseases-common but neglected. J Infect Dis. (2014)209:S79–80. doi: 10.1093/infdis/jiu159
38. Scriba TJ, Penn-Nicholson A, Shankar S, Hraha T, Thompson EG, Sterling D, et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. (2017)13:e1006687. doi: 10.1371/journal.ppat.1006687
39. Esmail H, Lai RP, Lesosky M, Wilkinson KA, Graham CM, Horswell S, et al. Complement pathway gene activation and rising circulating immune complexes characterize early disease in HIV-associated tuberculosis. Proc Natl Acad Sci USA. (2018)115:E964–73. doi: 10.1073/pnas.1711853115
40. Li H, Javid B. Antibodies and tuberculosis: finally coming of age? Nat Rev Immunol. (2018) 18:591–6. doi: 10.1038/s41577-018-0028-0
41. Rhines AS, Rhines AS. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis. (2013)93:104–7. doi: 10.1016/j.tube.2012.10.012
Keywords: tuberculosis, sex bias, computed tomography, complement C4, BMI
Citation: Chu Y, Soodeen-Lalloo AK, Huang J, Yang G, Chen F, Yin H, Sha W, Huang X, Shi J and Feng Y (2019) Sex Disparity in Severity of Lung Lesions in Newly Identified Tuberculosis Is Age-Associated. Front. Med. 6:163. doi: 10.3389/fmed.2019.00163
Received: 01 May 2019; Accepted: 02 July 2019;
Published: 17 July 2019.
Edited by:
Zisis Kozlakidis, International Agency For Research On Cancer (IARC), FranceReviewed by:
Roberta Karla Barbosa De Sales, Heart Institute, Clinical Hospital, University of São Paulo, BrazilGeorgia Karpathiou, Centre Hospitalier Universitaire (CHU) de Saint-Étienne, France
Copyright © 2019 Chu, Soodeen-Lalloo, Huang, Yang, Chen, Yin, Sha, Huang, Shi and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghong Feng, feng_yonghong@tongji.edu.cn; Jingyun Shi, shijingyun89179@126.com
†These authors have contributed equally to this work
 Yue Chu
Yue Chu Adiilah K. Soodeen-Lalloo
Adiilah K. Soodeen-Lalloo Jin Huang
Jin Huang Guanghong Yang
Guanghong Yang Fengfang Chen
Fengfang Chen Hongyun Yin
Hongyun Yin Wei Sha1
Wei Sha1  Jingyun Shi
Jingyun Shi Yonghong Feng
Yonghong Feng