Marine mudflat actinomycetes as a novel natural products source
- 1Department of Convergence Study on the Ocean Science and Technology, Korea Maritime and Ocean University, Busan, Republic of Korea
- 2Department of Chemistry and Nanoscience, Ewha Womans University, Seoul, Republic of Korea
A mudflat is a type of intertidal zone that is alternately affected by terrestrial and marine environments. We searched for examples of research related to the discovery of secondary metabolites in actinomycetes originating from mudflats. In total, we found 16 studies describing 42 natural products. The recognized bioactivities of the secondary metabolites were digested. We also performed a phylogenetic analysis of mudflat-derived actinomycetes. Most of the actinomycete strains belong to the genus Streptomyces. This review underscores mudflat as promising environment for discovering novel actinomycete strains that produce unique bioactive secondary metabolites. This highlights the imperative to explore this distinct environment for marine natural product research.
1 Introduction
An intertidal zone is an area of seashore that is exposed during low tide and submerged during high tide (Dyer et al., 2000). This process creates a unique biome that survives in such varying conditions. Intertidal zones may include rocky shorelines, bays, estuaries, or sandy beaches (Dyer, 1998). Within the wide range of different intertidal environments, areas characterized by unsolidified sedimentary deposits of fine mud are called mudflats. The distribution of mudflats worldwide is influenced by multiple factors, including geography, climate, and local geology (Dissanayake et al., 2018). For instance, Asian countries where mudflats can be found include China, Korea, Japan, Thailand, Malaysia, and India. In North America, this intertidal environment covers the eastern coasts of Canada and the United States. In Europe, mudflats are present along the shores of the Netherlands, Germany, and Denmark, where they are connected to the open sea and bordered by sand. The Amazon delta in South America represents a different type of intertidal habitat, in which sediment is primarily composed of sand (Yan et al., 2018). The ecological significance of this intertidal zone lies in providing a habitat for diverse organisms that have adapted to survive in the harsh conditions of this unique ecosystem; these include microorganisms, algae, barnacles, crabs, clams, and many other species (Satyam and Thiruchitrambalam, 2018).
Actinobacteria, one of the major bacterial phyla, are characterized by their Gram-positive cell wall structure and high GC content (Barka et al., 2016). These bacteria are widely distributed in both aquatic and terrestrial environments where they exhibit extensive morphological diversity, forming mycelial structures and producing spores for reproduction (Barka et al., 2016). Additionally, actinomycetes have a robust secondary metabolism, producing almost two-thirds of all naturally derived antibiotics currently in clinical use, as well as numerous anthelmintic, antifungal, and anticancer compounds (Ventura et al., 2007; Satyam and Thiruchitrambalam, 2018). Actinomycetes-inspired drug discovery has resulted in a host of novel antibacterials and anticancers such as the abysomicins (Riedlinger et al., 2004) and salinosporamide A, respectively (Williams et al., 2005).
Because of the extensive biotechnological significance of actinomycetes-derived natural products, many recent studies have employed different strategies for isolating these groups of bacteria from diverse environments and organic habitats (Jose et al., 2021). Actinomycetes are often found in association as symbionts with plants, insects and animals providing various functions (van der Meij et al., 2017). They are associated with insects and occur as endophytes in plant tissues where they contribute to defend the host and to recycle the nutrients, respectively (Kaltenpoth, 2009; Trujillo et al., 2015). Actinomycetes have also been found in co-epiphytic relations with marine organisms such as sponges, corals, and ascidians (Chen et al., 2021a). They are also found existing in other unique environments such as deep oceans where they recycle nutrients under harsh conditions (Colquhoun et al., 1998; Bull and Goodfellow, 2019) or the mangrove habitat which is also characterized by large fluctuations of salinity and tidal gradients (Hong et al., 2009), similar to mudflats. The ability to adapt to live in different habitats and association with other organisms have greatly impacted the biosynthetic potentials of these organisms; thus, they can produce a wide range of secondary metabolites (Lewin et al., 2016; van der Meij et al., 2017; Sivakala et al., 2021).
As earlier mentioned, actinomycetes are highly evolved and well-suited to thrive in dynamic environments (Siro et al., 2023). Similar to mangrove habitat, the peculiarity and complexity of mudflats trigger certain adaptative strategies, genomic evolution, and metabolism in actinomycetes (Tian et al., 2016), which can result in the production of secondary metabolites with important biological activities and unique chemical scaffolds as templates for drug development. The daily tidal action of submersion and exposure also allows more organic carbon, oxygen, and nutrients to dissolve into intertidal sediment (Pierre et al., 2014). The severe environmental changes in salinity, sunlight, temperature, oxygen, and water pressure, caused by tidal differences make mudflat a highly diverse ecosystem (Moon et al., 2015; Jeong et al., 2022) and have attracted the attention of natural product chemists and microbiologists over the last decade as a valuable source of secondary metabolites and microbes, particularly actinobacteria.
Although some studies have reported the isolation of compounds from marine mudflat-derived actinomycetes, this unique ecosystem is still underexplored (Li et al., 2009). The review therefore highlights the discovery of secondary metabolites with bioactivities from marine mudflat-derived actinomycetes for the benefit of further research into mudflat bioprospecting.
2 Mudflat
The unconsolidated, fine sediment deposition terrain that forms the boundary between land and sea is globally prevalent. However, there is a lack of a consistent term for this terrain. Various combinations of academic terms like intertidal, mud, and tidal mud are used to refer to this terrain with the “-flat”. For the use of clear academic terminology, many scientific databases, including the American Chemical Society, Science Direct, Google Scholar, MDPI, and PubMed, were utilized to investigate examples of research related to mudflats. The frequency of the term “mudflat” was determined by examining the status of research articles using several keywords such as “mudflat”, “mud flat”, “mud-flat”, “intertidal mudflat”, “intertidal mud flat”, “intertidal mud-flat”, “tidal mudflat”, “tidal mud flat”, and “tidal mud-flat”. The results are as below.
Among the nine search terms, Google Scholar yielded the highest number of results with 685,000 hits for “mud flat”, making it the most comprehensive search. “Tidal mud flat” ranked second, with 173,000 results, followed by “intertidal mud flat” with 70,900 hits, “mudflat” with 70,000 hits, “tidal mudflat” with 49,900 results, “intertidal mudflat” with 41,300 hits, “mud-flat” with 25,100 hits, “tidal mud-flat” with 16,200 results, and “intertidal mud-flat” with 15,900 hits. In the MDPI database, the most frequently occurring term was “mudflat” with 97 results, followed by 21 results for “intertidal mudflat”, 20 results for “tidal mudflat”, and 15 results for “mudflat”. No results were found for the terms “mud-flat”, “tidal mud-flat”, “tidal mud flat”, “intertidal mud flat”, or “intertidal mud-flats”. In the ACS database, “tidal mudflat” produced 147,199 hits, “mud flat” generated 672 hits, “tidal mud-flat” yielded 139 hits, “mudflat” resulted in 121 hits, “intertidal mud-flat” produced 43 hits, “tidal mud flat” had 41 hits, “intertidal mudflat” had 1 hit, and “intertidal mud flat” also had 1. No results were found for “mud flat”. In the Science Direct and PubMed databases, “mudflat” had 1317 and 646 results, “mud flat” had 796 and 115 results, “mud-flat” had 796 and 73 results, “tidal mudflat” had 445 and 113 results, “tidal mud flat” had 365 and 40 results, “tidal mud-flat” had 365 and 0 results, “intertidal mudflat” had 796 and 218 results, “intertidal mud flat” had 268 and 45 results, and “intertidal mud-flat” had 268 and 0 results, respectively.
Based on our findings, we concluded that the term “mudflat” is a more suitable terminology to use for this research. While “mudflat” itself did not yield the highest number of results in the database search, it encompasses both “intertidal mudflat” and “tidal mudflat.” Additionally, “mudflat” is more appropriate for indicating the specific terrain compared to “mud flat” or “mud-flat.”
After establishing the terminology for “mudflat,” we studied secondary metabolites of actinomycetes derived from mudflats using other terms that could be utilized as search keywords. For example, “mudflat actinomycetes,” “mudflat metabolites,” and “mudflat natural products” yielded appropriate results. However, a search for “mudflat bacteria” produced results that were too broad and deviated from the focus of the paper.
3 Mudflat actinomycetes
3.1 Microbial biodiversity of mudflat and actinomycetes
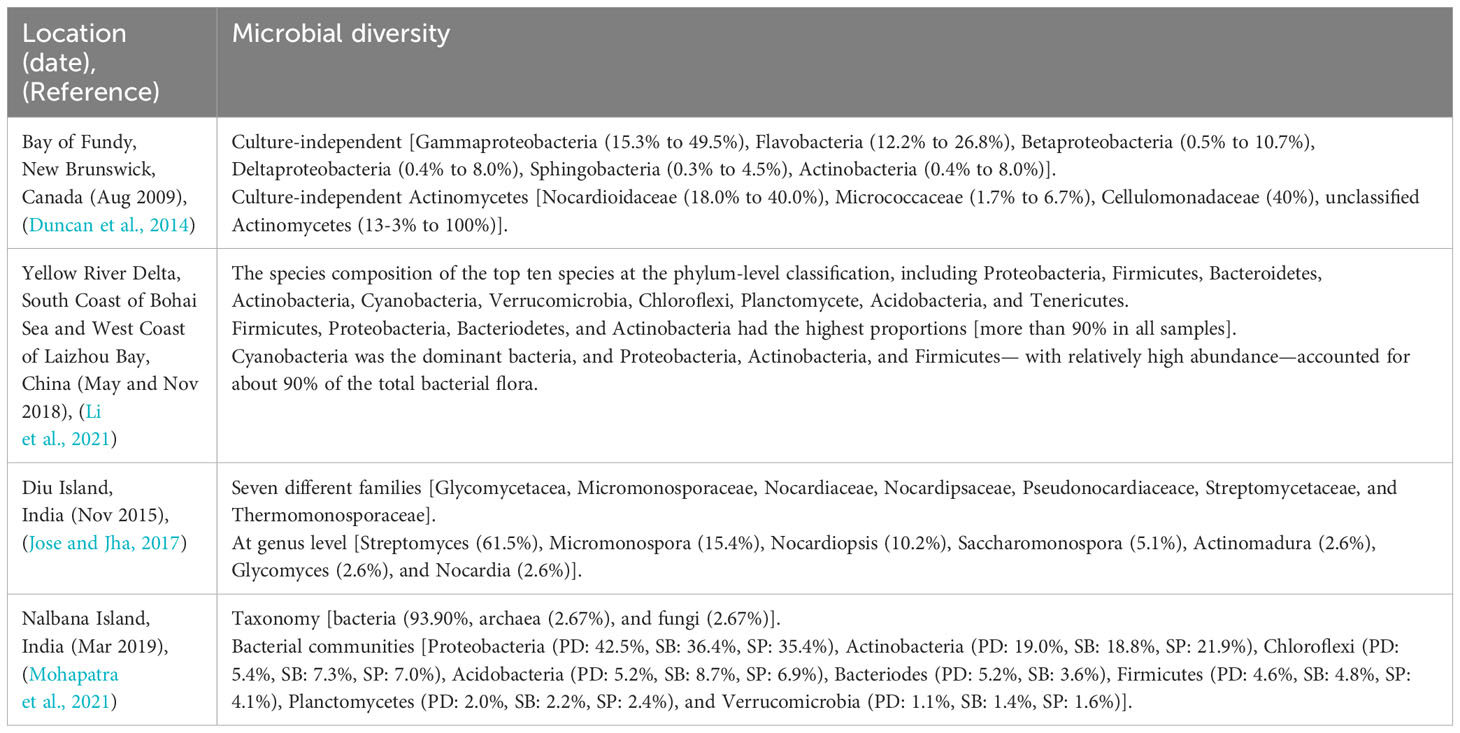
Biodiversity or biological diversity refers to the diversity of life on Earth at all levels, from genes to ecosystems, and includes the ecological, cultural, and evolutionary processes that support life (Swingland, 2013). The microbial biodiversity of mudflat focused on actinomycete in several countries, including Canada, China, and India, as revealed by the studies has been summarized in Table 1.
In Canada, Duncan and other researchers obtained a total of eight sediments from four stations in the Bay of Fundy, New Brunswick. The diversity of culture-independent and culture-dependent microorganisms in each area was then investigated. Notably, when considering the diversity of actinomycetes, the most dominant actinomycetes was found to be Nocardioidaceae, not Streptomycetaceae (Duncan et al., 2014). Biodiversity investigations were also conducted at locations in China and India (Diu Island and Nalbana Island). In China, Li and others collected a total of thirty-nine samples by repeating three times at thirteen stations in the Yellow River Delta, South Coast of Bohai Sea and West Coast of Laizhou Bay (Li et al., 2021). In India, Jose and Jha got a total of eighteen sediments from three stations on Diu Island in the Arabic Sea. As a result of the biodiversity investigation, Streptomyces was found to be the most common among the actinomycetes discovered (Jose and Jha, 2017). In another case study, a total of eighteen sediments collected from the mudflat of Nalbana Island in Chilika Lagoon resulted in actinomycetes as the second most prevalent bacteria found per station after proteobacteria (Mohapatra et al., 2021).
3.2 The culture conditions of the mudflat actinomycetes
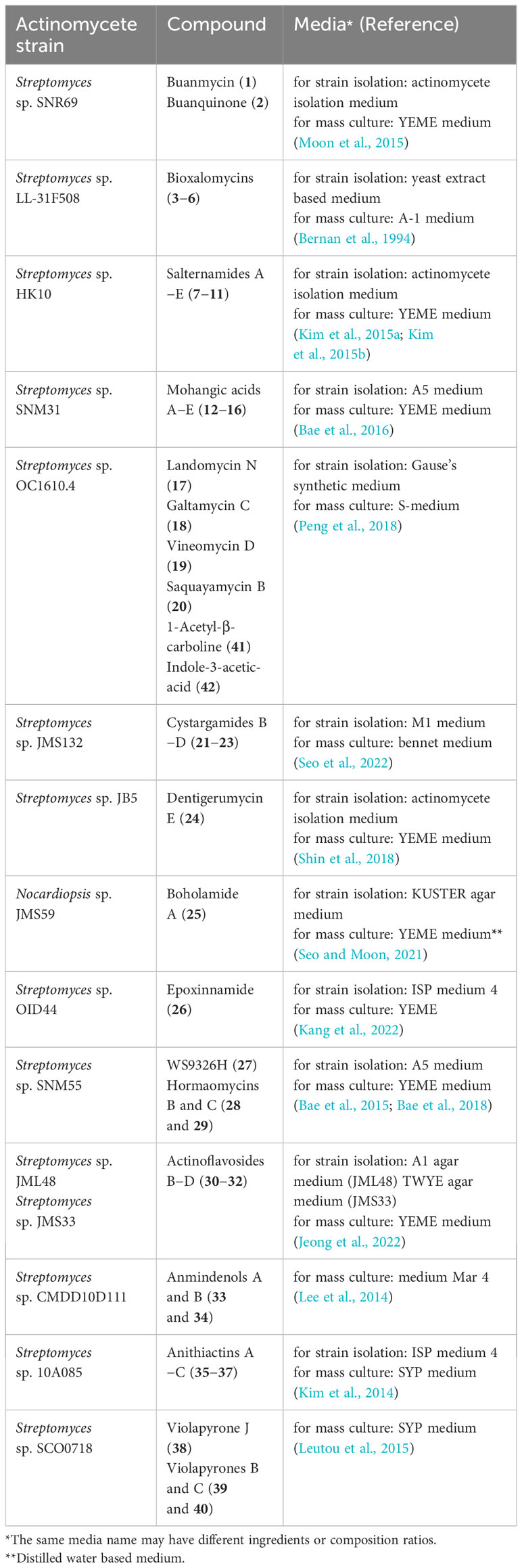
A strain that originates from a mudflat may not be endemic to the mudflat because of the alternating characteristic of mudflat between terrestrial and marine environments. However, because this point can be a mechanism to increase the longitudinal biological diversity of mudflats, it can be useful for the discovery of new natural products. The information about the media employed for the isolation and culture of the mudflat-derived actinomycetes from which the underlisted compounds originated, is shown in Table 2. Most researchers used separate media compositions for the isolation and mass culture of these actinomycetes. Most of the culture media employed either natural or artificial seawater, or added sodium chloride to maintain the salt concentration, ranging from 75 to 100% of seawater. Only one exception was reported, employing a distilled water-based medium for mass culture (Seo and Moon, 2021). The information listed in Table 2 can be used as a reference when studying the production of natural products from mudflat-derived actinomycetes, as well as for determining the best conditions for isolating actinomycetes from mudflats.
4 Natural products
4.1 Phenols
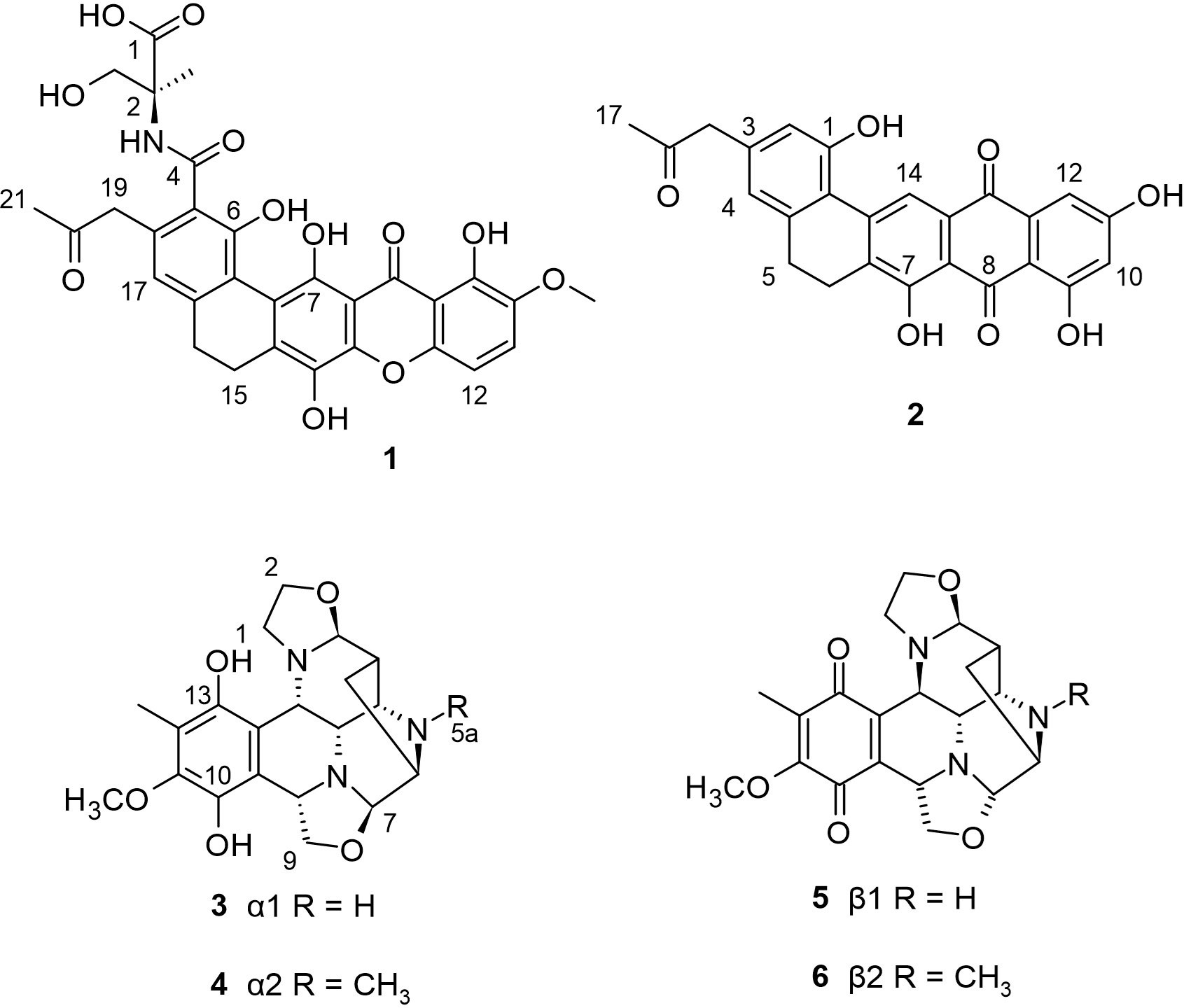
A marine Streptomyces strain which was collected from a tidal mudflat in Buan, Republic of Korea, was found to produce two novel compounds named buanmycin (1) and buanquinone (2). Compound 1 is a pentacyclic xanthone while 2 is a pentacyclic anthraquinone. While compound 2 showed no significant antibacterial and antifungal properties, the pentacyclic xanthone 1 showed significant antibacterial activity against Bacillus subtilis and Salmonella enterica, with an equal IC50 value of 0.7 μM and a moderate antifungal activity against Candida albicans with MIC value of 21.1 μM. In addition, compound 1 displayed significant bacteriostatic activity against Staphylococcus aureus by inhibiting sortase A, an important enzyme for adhesion and host invasion by Gram-positive bacteria, with a reported IC50 value of 43.2 μM. Furthermore, compound 1 exhibited potent cytotoxicity against the human carcinoma cell lines MDA-MB231 (breast cancer), SNU638 (gastric cancer), A549 (lung cancer), SK-HEP1 (liver cancer), and HCT116 (colon cancer) with IC50 values of 0.8 to 1.9 μM, but not against K562 (leukemia). On the other hand, the pentacyclic anthraquinone 2 had no significant activity against all tested cell lines but with moderate cytotoxicity to K562 with IC50 value of 18.3 μM (Moon et al., 2015). No other study reports the isolation of both compounds from literature.
In addition, a Streptomyces strain LL-31F508 was isolated from an intertidal sediment sample collected in Key West, Florida (Bernan et al., 1994). Bioxalomycins (3−6) were isolated from this strain exhibiting potent antimicrobial activity against Staphyloccoccus and Enterococcus sp. Compounds 5 and 6 are the quinone form of compounds 3 and 4 respectively. These bioxalomycins are similar to naphthyridinomycin, but for the additional oxazolidine ring formed from C-7 and C-9. The excellent antibacterial activity of 4 suggests that the reduction of the ketone group at positions C-10 and C-13 together with the CH3 group at the C-5a position plays a critical role in the improved activity of 4 compared to other bioxalomycins. This is the only study that reports the isolation of the bioxalomycins. The chemical structures of 1−6 are shown in Figure 1.
4.2 Polyketides
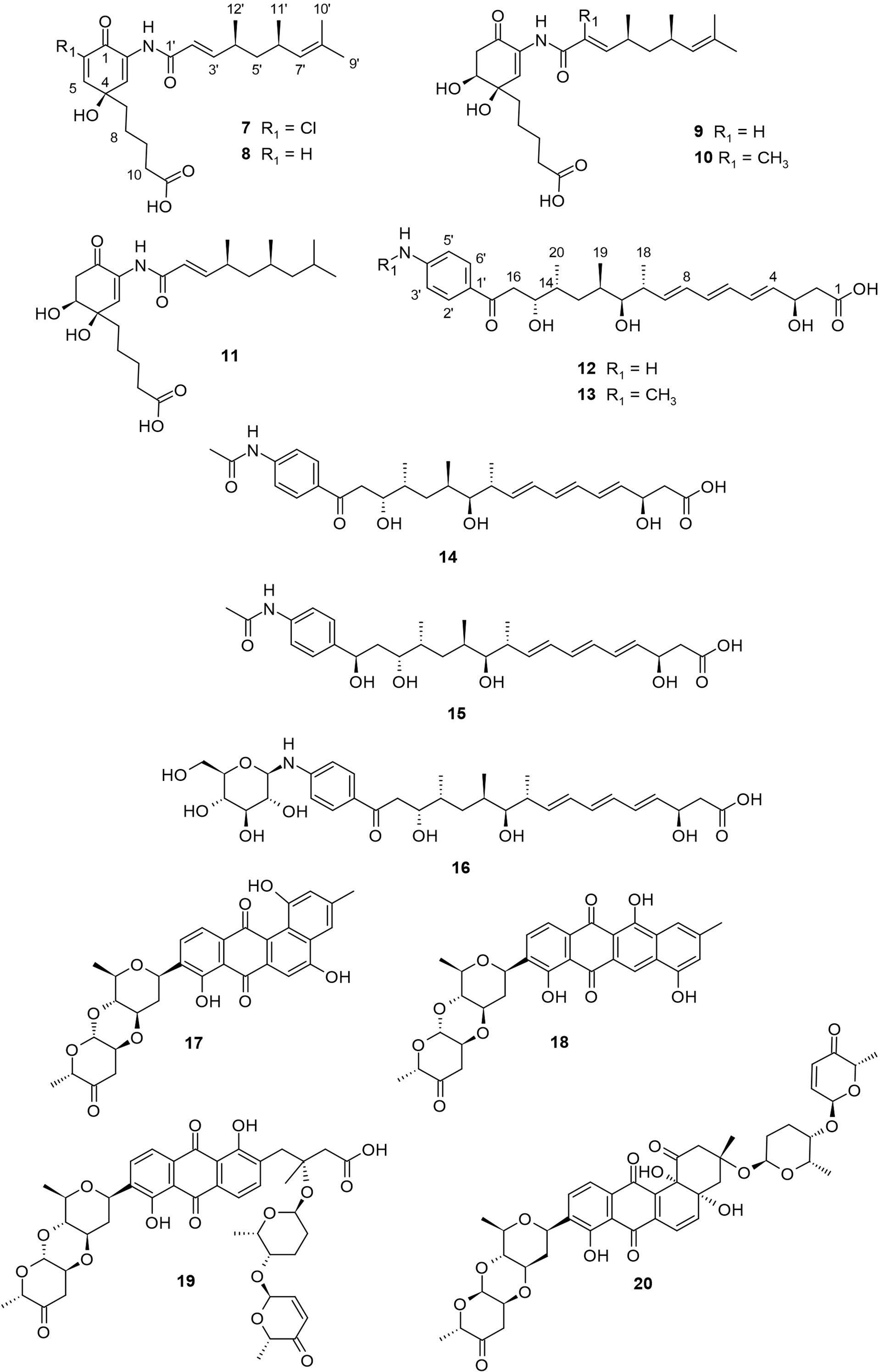
Streptomyces sp. HK10 was collected from Shinui Island, Republic of Korea, and was found to produce salternamides A−E (7−11, Figure 2). Salternamides are monocyclic compounds belonging to the munamycin family, with compound 7 as the first known chlorinated member. They have characteristic pentanoic acid and trimethyl nonadienamide moieties, except for 11 which has a tetramethyl nonadienamide moiety. The salternamides exhibited cytotoxicity against various cancer cell lines (Kim et al., 2015a; Kim et al., 2015b). Notably, compound 7 displayed a significant antiproliferative effect against HCT116 (colon cancer) and SNU638 (gastric cancer) cell lines, each with an IC50 value of 0.96 μM. In addition, compounds 7 and 10 exhibited weak inhibitory activity against Na+/K+ ATPase (Kim et al., 2015a). Furthermore, compound 11 exhibited weak cytotoxicity against the human carcinoma cell lines HCT116, SK-HEP1, A549, SNU638, K562, and MDA-MB231, with reported IC50 values of 85, 91, 83, 75, 70, and 54 μM, respectively (Kim et al., 2015b). Compared to others, the significant cytotoxicity of 7 can be attributed to chlorine substitution at C-6. Similarly, the stronger inhibitory activity of compound 10 against Na+/K+ ATPase suggests the importance of the methyl-substitution at C-2′. No other study reports the isolation of salternamides from literature.
Mohangic acids A−E (12−16, Figure 2) are p-aminoacetophenonic acids with a conjugated diene moiety. These compounds were isolated from the culture broth of a marine Streptomyces sp. collected from a mudflat in Buan, Republic of Korea (Bae et al., 2016). Compounds 12−16 were tested for their cytotoxicity activity against various human cancer cell lines, including SK-HEP1 (liver cancer), A549 (lung cancer), SNU638 (gastric cancer), MDA-MB231 (breast cancer), HCT116 (colon cancer), and K562 (leukemia). The results showed that compounds 12−16 did not exhibit significant activity against tested cancer lines with IC50 values > 10 μM. In a different study, compound 12 was isolated from a deep-sea bacterium Alcanivorax dieselolei BC-5 together with mohangic acids F and G, where both showed no cytotoxic activity against PC-3 and HCT-116 cancer cell lines with a reported IC50 >10 μM (Zhao et al., 2022). This was congruent with the works of Bae and colleagues, who also reported the lack of cytotoxic action for mohangic acids (Bae et al., 2016). Contrary to compounds 12−16, mohangic acids F and G possess a conjugated triene moiety. Furthermore, compounds 12−16 showed no significant effects (MIC > 128 μM) against pathogenic fungi and bacteria. On the other hand, 16 displayed a significant (20 µM) and concentration-dependent quinone-reductase activity in the cultured Hepa-1c1c7 murine hepatoma cell line, suggesting the function of 16 as a cancer chemopreventive (Bae et al., 2016). The quinone-reductase activity of 16 suggests the importance of the β-glucose moiety attached to the amine at C-4′ of the p-aminoacetophenonic acid.
Other reported polyketides from mudflat-derived actinomycetes include four angucycline glycosides, landomycin N (17), galtamycin C (18), and vineomycin D (19) and saquayamycin B (20), which were isolated from a culture broth of Streptomyces sp. OC1610.4, obtained from intertidal sediment from Xiaoshi Island in Weihai, China (Peng et al., 2018). Among these angucycline glycosides, compounds 17−19 are novel but without significant cytotoxic activity. The known compound 20 exhibited potent cytotoxic activity against hepatoma carcinoma cells HepG-2, SMMC-7721, and plc-prf-5, with IC50 values between 0.033−0.244 µM respectively. According to Shaaban and colleagues, cytotoxic activities of angucyline glycosides are better for compounds containing more sugar moiety (Shaaban et al., 2012). The presence of two disaccharides and aquayamycin aglycone thus explains the cytotoxicity of 20. Also, compound 20 showed a better cytotoxic profile on PC3 cells compared to other saquayamycins (Shaaban et al., 2012), indicating the importance of the connected sugar type. The compounds are shown in Figure 2.
4.3 Peptides
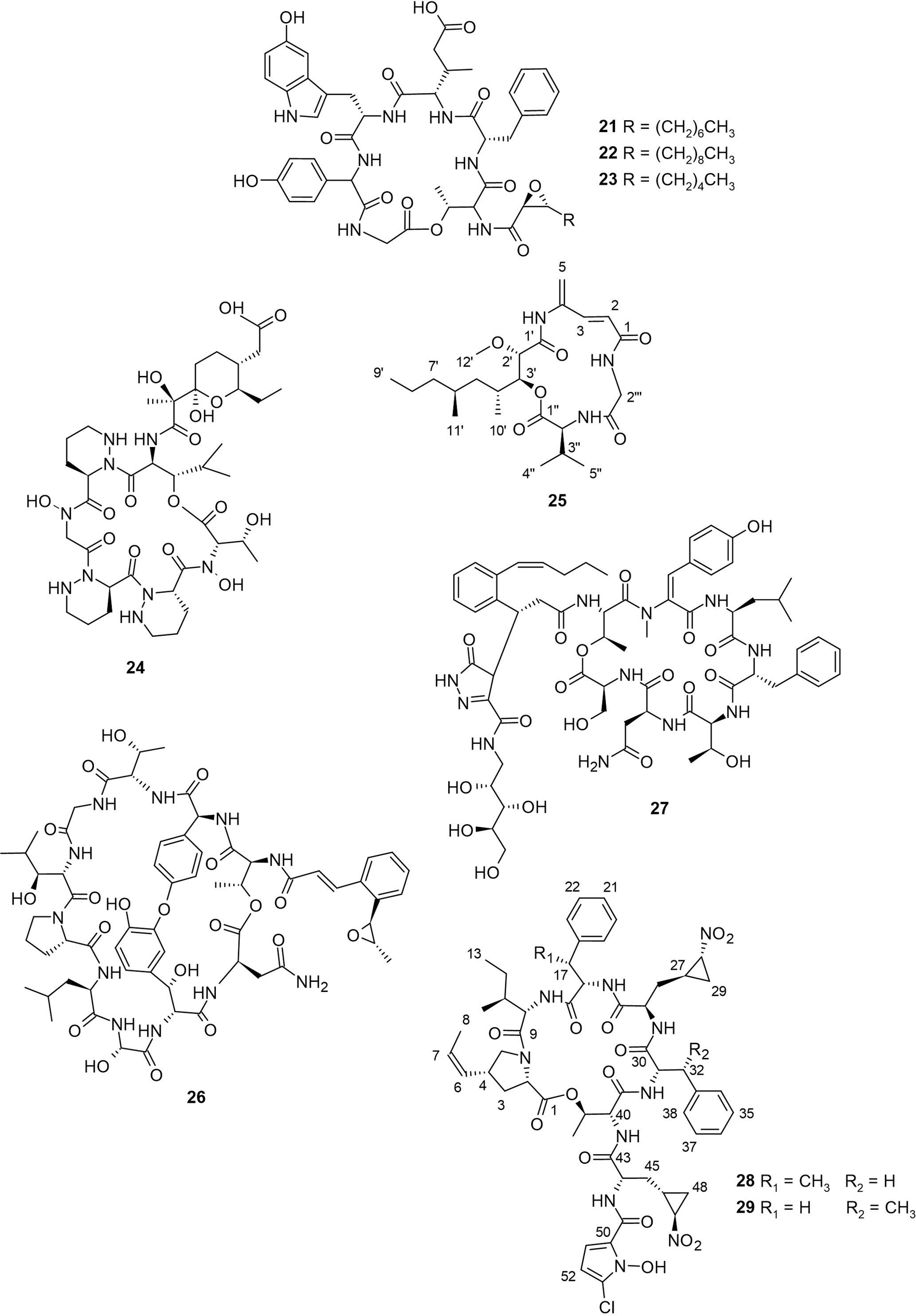
Cystargamides B−D (21−23, Figure 3) were produced from Streptomyces sp. JMS132, which was obtained from tidal-flat sediment collected at Beolgyo, South Korea (Seo et al., 2022). Cystargamides are cyclic lipopeptides having six amino acids and an oxidized fatty acid chain. The first cystargamide was obtained from the fermentation broth of another actinomycete, Kitasatospora cystarginea (Gill et al., 2014). Compound 21 has been previously isolated from a Streptomyces strain derived from Kaempferia galanga rhizome where it inhibited NS2BglyNS3 protease by 67% and 44% at concentrations of 200 and 100 μg/mL respectively (Kitani et al., 2018). Meanwhile, the study of Seo and colleagues represents the first and only report on the isolation of compounds 22 and 23. Furthermore, antioxidant evaluation results showed that compounds 21−23 exhibited strong radical scavenging ability in both DPPH free-radical and ABTS cation-radical assays. Notably, compound 23 reduced ABTS free radicals by 100% at 200 µg/mL and compound 22 decreased DPPH free radicals by about 53% at 200 µg/mL (Seo et al., 2022). The results of the study revealed that the length of the epoxy fatty acid side chain influences the antioxidant activity of cystargamides.
Another cyclic hexapeptide, dentigerumycin E (24, Figure 3) was discovered through co-cultivation of Streptomyces sp. JB5, derived from a mud sample collected from Wando Island, Republic of Korea, with a marine Bacillus sp. GN1 (Shin et al., 2018). It is composed of unusual amino acid piperazic acids and a pyran-bearing polyketide acyl chain. Compound 24 was found to exhibit moderate antiproliferative activities against human cancer cell lines HCT116, SK-HEP-1, MDA-MB-231, A549, and SNU638 with reported IC50 between 27−39 µM. In the wound healing assay, compound 24 inhibited cell migration at 20 and 40 µM by 20% and 48%, respectively, revealing the antimetastatic potential of this compound (Shin et al., 2018). Compared to the 2-N,16-N-deoxy and methyl ester derivatives of 24, it can be deduced that both the N-OH and carboxylic acid functional groups are essential for biological activity. Although there are reports of other dentigerumycins from other sources (Oh et al., 2009; Bae et al., 2021), this is the only report on the isolation of dentigerumycin E.
The cyclodepsipeptide, boholamide A (25), which had been previously isolated from a Nocardiopsis sp. 3158H.R.1a.03 obtained from the tissue of a mollusc (Torres et al., 2020), was isolated from Nocardiopsis dassonvillei collected from a tidal mudflat in Muan, Republic of Korea (Seo and Moon, 2021). The chemical structure of 25 was determined using NMR spectral analysis, as shown in Figure 3. This compound is a 4-amido-2,4-pentadieneoate (APD)-class peptide. The antimicrobial activity of 25 against Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, Candida albicans, and Erwinia rhapontici revealed that compound 25 did not exhibit significant activity, but it did demonstrate inhibition against B. subtilis, with an IC50 of 0.08 mM (Seo and Moon, 2021). Cytotoxic tests done at both normal and hypoxic levels revealed that 25 is hypoxic-selective, similar to other APD-peptides such as vinylamycin and rakicidin A (Torres et al., 2020). The lack of cytotoxicity displayed by vinylamycin (Igarashi et al., 1999; Torres et al., 2020) suggests that the cytotoxicity of compound 25 against mammalian cell lines could be due to a lack of hydroxyl group at position C-2′ of the polyketide part of compound 25.
A nonribosomal peptide known to contain cinnamoyl was isolated from a mudflat-derived Streptomyces sp. OID44, which was collected from an intertidal mudflat at Oido, Siheung, Gyeonggi-do, the Republic of Korea and identified as epoxinnamide (26, Figure 3) (Kang et al., 2022). Compound 26 is composed of a bicyclic deca-depsipeptide backbone attached to a cinnamoyl acyl chain. The unique cinnamoyl acyl chain of this group of peptides has been implicated in their biological activities (Shi et al., 2019). Due to the partial structural similarity between compound 26 and nyuzenamide C, Kang and colleagues evaluated the quinone reductase (QR) activity and antiangiogenic potential of both compounds. Compound 26 induced quinone reductase (QR) activity in murine Hepa-1c1c7 cells by 1.6-fold at 5 µM and also inhibited angiogenesis in human umbilical vein endothelial cells, exhibiting an IC50 value of 13.4 µM. Altogether, compound 26 was stronger for QR-inducing activity while the antiangiogenic effect of nyuzenamide C was better (Kang et al., 2022), suggesting that the biological activity of compound 26 is related to its amino acid composition.
From a sediment sample collected in Mohang, Buan, Republic of Korea, a mudflat-derived Streptomyces sp. SNM55 was isolated, which led to the discovery of a new cyclic heptapeptide, WS9326H (27, Figure 3), and novel hormaomycins B and C (28 and 29, Figure 3) (Bae et al., 2015; Bae et al., 2018). These are the only existing studies reporting the isolation of compounds 27−29. The unprecedented pyrazolone ring structure of 27 and its acyl chain modification not only distinguish 27 from other nonribosomal WS9326 peptide family but also highlights it as a novel chemotype. Notably, compound 27 exhibited a significant angiogenetic effect by inhibiting the formation of VEGF-induced human umbilical vein endothelial cells (HUVECs) at a concentration of 20 µM, albeit without any significant cytotoxicity to human carcinoma cell lines, antibacterial, and antifungal activities (Bae et al., 2018). Compounds 28 and 29 bear a structurally unique 4-(Z)-propenylproline, 3-(2-nitrocyclopropyl) alanine, β-methylphenylalanine, and 5-chloro-1-hydroxypyrrol-2-carboxylic acid in their planar structure. On the other hand, compounds 28 and 29 exhibited potent inhibitory activities particularly against tested Gram-positive bacteria. Interestingly, the antibacterial activity of 28 and 29 compared to the known hormaomycin A suggests that the presence of the methyl groups at C-17 and C-32 are important to the antibacterial properties of hormaomycins (Bae et al., 2015).
4.4 Others
4.4.1 Flavonoids
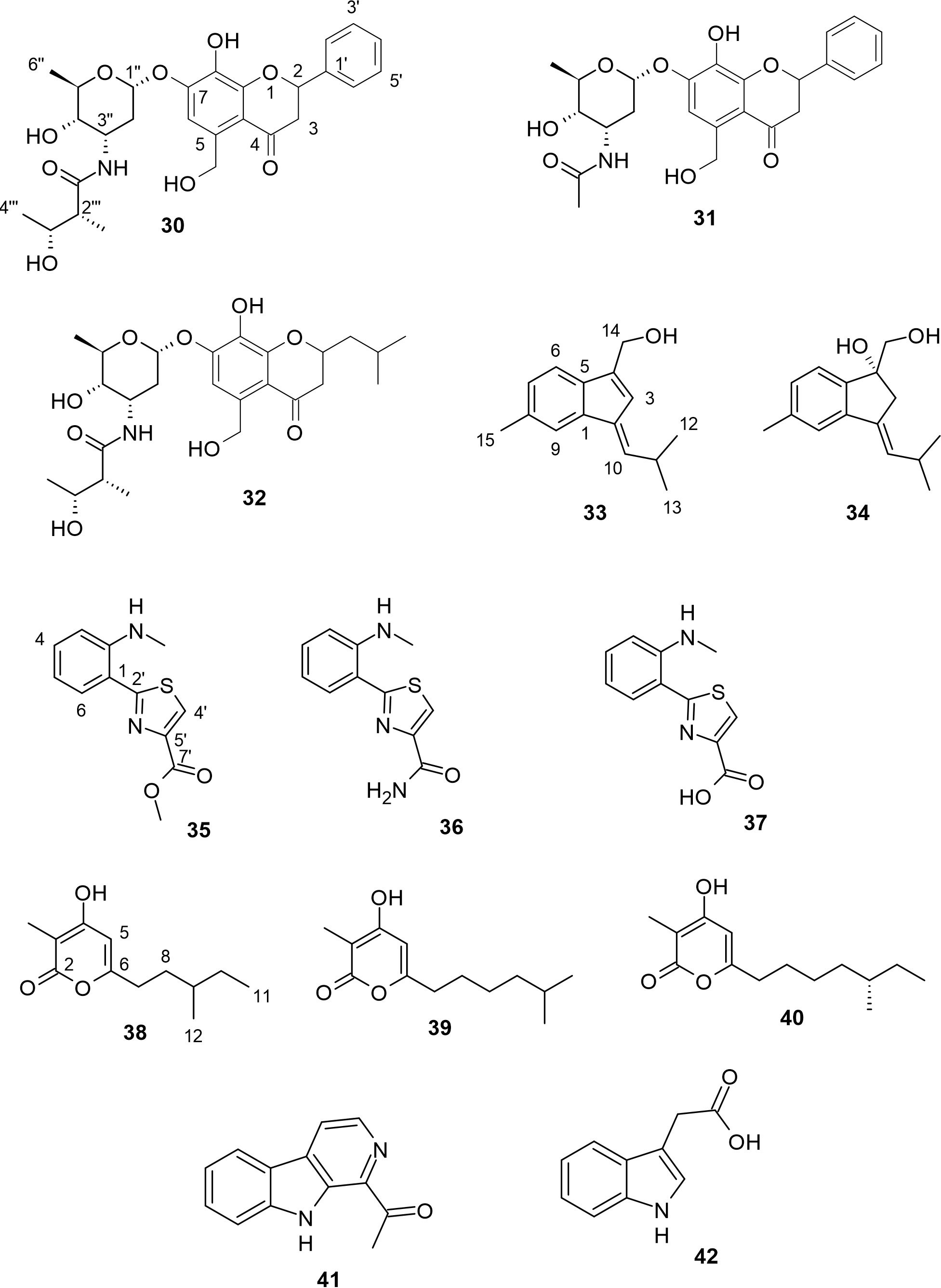
Actinoflavosides B−D (30−32, Figure 4) are three new flavonoid-type glycosides which were obtained from Streptomyces sp. strains JML48 and JMS33 isolated from tidal mudflat sediment in Muan, Republic of Korea (Jeong et al., 2022). Compound 30 has a similar structure to the known actinoflavoside A, except for the stereochemistry of the proton at C-3′′′. Jeong and other researchers reported that compound 30 inhibited the Gram-positive B. subtilis with a MIC value of 0.14 mM and Jiang and colleagues stated that actinoflavoside A, which was isolated from an estuarine-derived Streptomyces strain, showed antibacterial activity against all test Gram-positive bacteria with MIC values of 0.12 mM (Jiang et al., 1997; Jeong et al., 2022). MIC values of 0.29 and 0.30 mM inhibited the growth of Gram-positive P. aeruginosa for compounds 30 and 32 respectively (Jeong et al., 2022). These results further suggest that the 2-methyl-3-hydroxy-butyramide moiety is significant to the displayed antibacterial activity, compared to compound 31. Also, compound 32 significantly increased interleukin-2 (IL-2) production, a cytokine essential for T-cell proliferation and differentiation in mouse splenocytes. The ability of 32 to increase IL-2 production showed the effect of the substitution of the aromatic ring at C-2 in 30 with 2-methyl propyl in 32.
4.4.2 Terpenoids
A marine-derived Streptomyces strain which was isolated from tidal-flat sediments collected at Dongmak, on the west coast of the Republic of Korea yielded two new sesquiterpenoids, anmindenols A and B (33 and 34) with a characteristic indene moiety (Lee et al., 2014). The unique 6,5-indene ring system in compounds 33 and 34 has never been reported for sesquiterpenoids from actinomycetes. Both compounds showed no significant cytotoxic activity against human pancreatic and renal cancer cell lines at concentrations of up to 100 μM. However, both 33 and 34 were found to inhibit NO production in lipopolysaccharide (LPS)-activated mouse macrophage RAW264.7 cells, with IC50 values of 23 μM and 19 μM respectively. The novel skeleton of these anmindenols therefore makes them suitable as templates for developing iNOS inhibitors (Lee et al., 2014). Also, the additional hydroxyl groups in compound 34 at position C-4 may be attributed to the improved inhibitory activity on NO production. These compounds are illustrated in Figure 4.
4.4.3 Thiazoles
Anithiactins A−C (35−37, Figure 4) were the first aniline-thiazole natural products produced from Streptomyces sp. 10A085 which was obtained from the mudflat at Jaebu Island, GyeongGi-Do, the Republic of Korea, in 2010 (Kim et al., 2014). Interestingly, at about the same time, compound 35 was isolated from another marine Actinomycetospora strain with the name thiasporine C (Fu and Macmillan, 2015). These natural products have an unusual aniline moiety which differentiates them from other members of the 2-phenylthiazoline class such as pulicatins and aeruginoic acid (Kim et al., 2014). The AChE-inhibitory and cytotoxicity activities of these compounds indicated that compounds 35−37 exhibited moderate AChE-inhibitory effects (IC50 = 63, 53, and 68 µM, respectively), but did not show any significant cytotoxic effects against human cancer cell lines ACHN and A-498 (Kim et al., 2014). The lack of cytotoxicity shown by compound 35 agrees with the cytotoxicity reports from Fu and Macmillan for compound 35 against non-small-cell lung cancer cell lines HCC44, H2122, A549 and HCC366, (Fu and Macmillan, 2015). The increase in AChE inhibitory effect for compound 37 can be attributed to the nucleophilicity offered by the attached amine group. Also, compounds 35−37 were evaluated for their inhibitory activity on monoamine oxidase isoforms, MAO-A and MAO-B, which are drug targets for neuropsychiatric disorders. While compounds 36 and 37 were either weak inhibitors or inactive, compound 35 displayed selective inhibition for MAO-A only, with an IC50 value of 13.0 µM. The hydrophobic methyl substituent in 35 may be crucial to the inhibition of MAO-A (Lee et al., 2015b).
4.4.4 α-Pyrones
Violapyrone J (38), a novel α-pyrone derivative, was isolated from the culture broth of marine-derived Streptomyces sp. SC0718, along with known violapyrones B and C (39 and 40), as shown in Figure 4. The strain was obtained from mudflat sediment at Suncheon Bay, in the South Sea of the Republic of Korea (Leutou et al., 2015). Compounds 39 and 40 were first discovered from Streptomyces strain isolated from fresh feces excreted by healthy adult Hylobates hoolock in Yunnan Wild Animal Park, Kunming, Yunnan Province, China (Zhang et al., 2013). Both 39 and 40 were also rediscovered from another Streptomyces strain isolated from crown-of-thorns starfish, Acanthaster planci, which was collected from Chuuk, Federated States of Micronesia (Lee et al., 2015a). Also, compound 38 was later rediscovered from the fermentation broth of a Streptomyces strain obtained from the rhizosphere of Radix stellariae, which showed no known cytotoxicity to MCF-7/TamR cells (Yang et al., 2017). Both 39 and 40 were found to exhibit antibacterial activity against Bacillus subtilis and Staphylococcus aureus, with MIC values ranging from 4 to 16 μg/mL (Zhang et al., 2013). This result suggests that the antibacterial potential of these 3,4,6-trisubstituted α-pyrone derivatives reduces with increasing alkyl chain (Leutou et al., 2015). Zhang and others deduced that the antibacterial potential of α-pyrones might not necessarily be directly proportional to the alkyl side chain length, but that their antibacterial activity weakens when the alkyl side chain is oxygenated (Zhang et al., 2013).
4.4.5 Alkaloids
Two known alkaloids, containing an indole moiety were obtained from mudflat-derived Streptomyces sp. OC1610.4 originating from intertidal sediment collected at Xiaoshi Island, Weihai, China. These alkaloids were identified as 1-acetyl-β-carboline (41) and indole-3-acetic acid (42), as shown in Figure 4 (Peng et al., 2018). Compound 41 has previously been reported as a potent inhibitor of methicillin-resistant Staphylococcus aureus (MRSA), while compound 42 is an important plant hormone that plays a crucial role in plant development.
5 Phylogenetic analysis
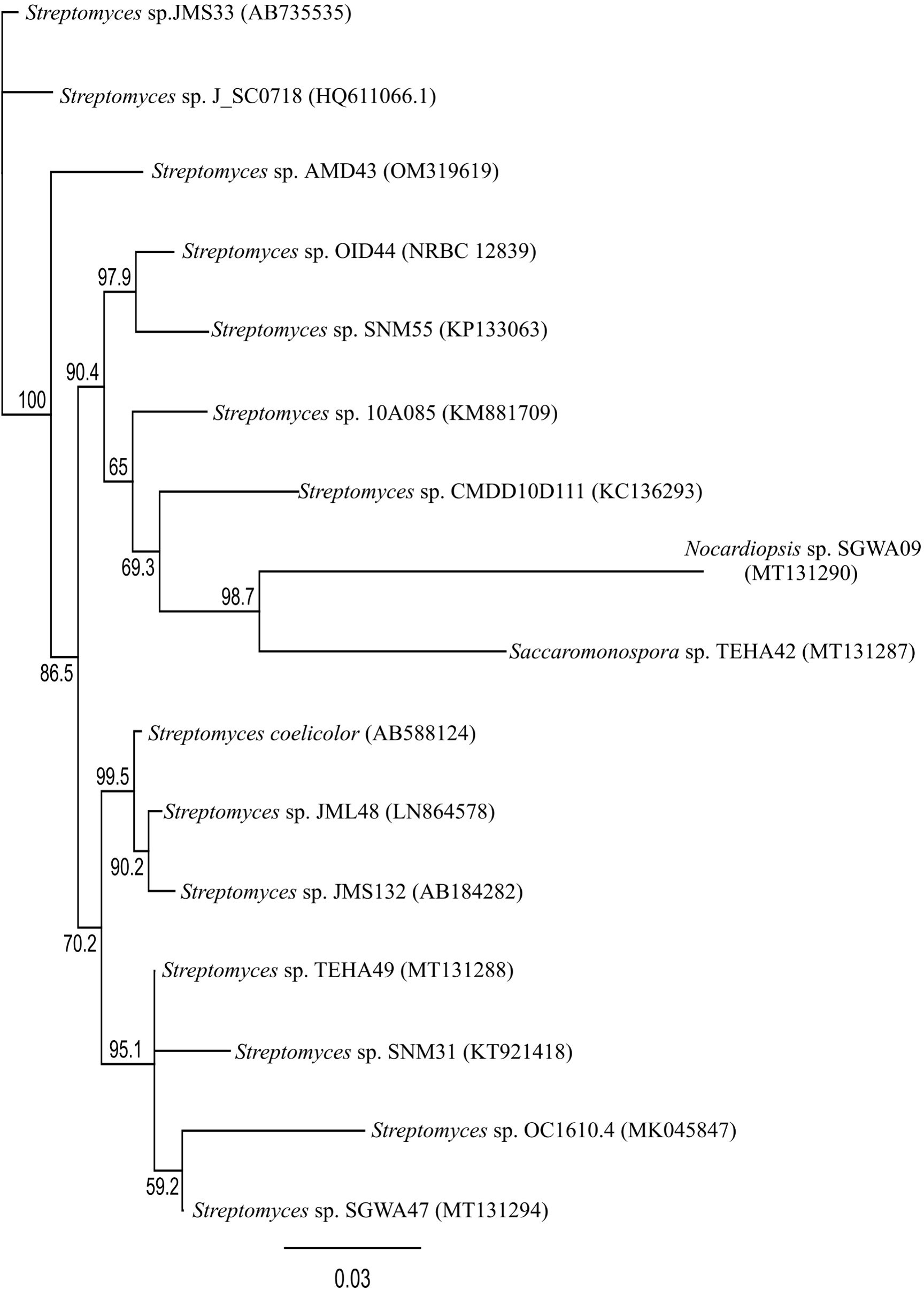
For phylogenetic analysis, sequencing data of the actinomycetes reported in previous sections were collected. The 16S rRNA gene sequence data was collected using accession numbers registered in GenBank. All sequence data collected for the phylogenetic tree were produced using the Geneious prime program. Multiple sequences were aligned using MUSCLE (Edgar, 2004). The phylogenetic tree was obtained by the neighbor-joining method using Geneious prime and the bootstrap value was 1000 x replicated (Saitou and Nei, 1987). The phylogenetic tree result is shown in Figure 5.
There were 44 cases in which the species names of microorganisms originating from mudflats have been mentioned previously. Several steps were required for phylogenetic analysis. Firstly, 15 microorganisms other than actinomycetes were excluded from the total of 44 cases (Tambadou et al., 2014; Chen et al., 2021b). Three strains for which sequence information was not input were also removed (Bernan et al., 1994; Seo and Moon, 2021). Finally, 11 strains were excluded because their base pairs were less than 800 bp (Kim et al., 2015b; Moon et al., 2015; Shin et al., 2018; Chen et al., 2021b).
As shown in Figure 5, a total of 16 strain sequences were used for phylogenetic analysis. Streptomyces coelicolor A3(2) (Accession number: AB588124) was included for comparison (Hoskisson and van Wezel, 2019). Contrary to the fact that the marine environment is known to be inhabited by various microorganisms, Streptomyces species are mentioned mostly in Figure 5, indicating narrow phylogenetic diversity recovered (Duncan et al., 2014), and further suggesting that more research is needed concerning the diversity of mudflat actinomycetes.
6 Conclusion
In this article, we have highlighted the secondary metabolites derived from mudflats actinomycetes, as well as their biological activities. We have also summarized the culture media used for the cultivation of each metabolite-producing actinomycete strain. In total, mudflat actinomycetes have yielded forty-two compounds, which include six phenolic compounds, fourteen polyketides, nine peptides, three flavonoids, two terpenoids, three thiazoles, three α-pyrones, and two alkaloids.
Although most of the mudflat-derived actinomycete strains belong to the genus Streptomyces, the greater diversity of mudflat-derived natural products compared to species diversity could be attributed to techniques employed for strain isolation. The isolation of chemical structures with unique scaffolds, such as the unprecedented WS9326H, anmindenols A and B, and anithiactins A-C, as well as compounds like saquayamycin B with significant bioactivity, could serve as templates for drug discovery. This demonstrates that the mudflat habitat possesses unique ecological features that can stimulate the biosynthesis of distinctive natural products It is also noteworthy that all the previous research lacks a biosynthetic gene cluster study, despite the chemical structure novelty of the isolated secondary metabolites.
As shown in this review, more research efforts are required to obtain a wide range of actinomycetes from this distinct marine environment. The isolation of a cyclodepsipeptide-producing Nocardiopsis shows the need to employ culturing conditions that can facilitate the isolation of rare actinomycetes which could prove pivotal in the search for interesting natural products.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
DR: Conceptualization, Data curation, Investigation, Writing – original draft. PH: Data curation, Investigation, Writing – original draft. GA: Data curation, Writing – original draft. S-JN: Resources, Supervision, Writing – review & editing. IY: Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1C1C1008465). Additionally, the research was backed by High seas bioresources program of Korea Institute of Marine Science & Technology (KIMST) funded by the Ministry of Oceans and Fisheries (KIMST-20210646).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bae M., Chung B., Oh K. B., Shin J., Oh D. C. (2015). Hormaomycins B and C: New antibiotic cyclic depsipeptides from a marine mudflat-derived Streptomyces sp. Mar. Drugs 13, 5187–5200. doi: 10.3390/md13085187
Bae M., Mevers E., Pishchany G., Whaley S. G., Rock C. O., Andes D. R., et al. (2021). Chemical exchanges between multilateral symbionts. Org. Lett. 23, 1648–1652. doi: 10.1021/acs.orglett.1c00068
Bae M., Moon K., Kim J., Park H. J., Lee S. K., Shin J., et al. (2016). Mohangic Acids A-E, p-Aminoacetophenonic Acids from a Marine-Mudflat-Derived Streptomyces sp. J. Nat. Prod. 79, 332–339. doi: 10.1021/acs.jnatprod.5b00956
Bae M., Oh J., Bae E. S., Oh J., Hur J., Suh Y. G., et al. (2018). WS9326H, an antiangiogenic pyrazolone-bearing peptide from an intertidal mudflat actinomycete. Org. Lett. 20, 1999–2002. doi: 10.1021/acs.orglett.8b00546
Barka E. A., Vatsa P., Sanchez L., Gaveau-Vaillant N., Jacquard C., Klenk H.-P., et al. (2016). Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 80, 1–43. doi: 10.1128/mmbr.00019-15
Bernan V. S., Montenegro D. A., Korshalla J. D., Steinberg D. A., Greenstein M., Maiese W. M. (1994). Bioxalomycins, new antibiotics produced by the marine streptomyces sp. LL-31F508: taxonomy and fermentation. J. Antibiot. (Tokyo) 47, 1417–1424. doi: 10.7164/antibiotics.47.1417
Bull A. T., Goodfellow M. (2019). Dark, rare and inspirational microbial matter in the extremobiosphere: 16 000 m of bioprospecting campaigns. Microbiol. (United Kingdom) 165, 1252–1264. doi: 10.1099/mic.0.000822
Chen J., Xu L., Zhou Y., Han B. (2021a). Natural products from actinomycetes associated with marine organisms. Mar. Drugs 19, 629. doi: 10.3390/md19110629
Chen L., Wang Z., Du S., Wang G. (2021b). Antimicrobial activity and functional genes of actinobacteria from coastal wetland. Curr. Microbiol. 78, 3058–3067. doi: 10.1007/s00284-021-02560-3
Colquhoun J. A., Mexson J., Goodfellow M., Ward A. C., Horikoshi K., Bull A. T. (1998). Novel rhodococci and other mycolate actinomycetes from the deep sea. Antonie van Leeuwenhoek 74, 27–40. doi: 10.1023/a:1001743625912
Dissanayake N. G., Frid C. L. J., Drylie T. P., Caswell B. A. (2018). Ecological functioning of mudflats: Global analysis reveals both regional differences and widespread conservation of functioning. Mar. Ecol. Prog. Ser. 604, 1–20. doi: 10.3354/meps12728
Duncan K., Haltli B., Gill K. A., Kerr R. G. (2014). Bioprospecting from marine sediments of New Brunswick, Canada: Exploring the relationship between total bacterial diversity and actinobacteria diversity. Mar. Drugs 12, 899–925. doi: 10.3390/md12020899
Dyer K. R. (1998). The typology of intertidal mudflats. Geol. Soc. 139, 11–24. doi: 10.1144/GSL.SP.1998.139.01.02
Dyer K. R., Christie M. C., Wright E. W. (2000). The classification of intertidal mudflats. Cont Shelf Res. 20, 1039–1060. doi: 10.1016/S0278-4343(00)00011-X
Edgar R. C. (2004). MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinf. 5:113. doi: 10.1186/1471-2105-5-113
Fu P., Macmillan J. B. (2015). Thiasporines A-C, thiazine and thiazole derivatives from a marine-derived Actinomycetospora chlora. J. Nat. Prod. 78, 548–551. doi: 10.1021/np500929z
Gill K. A., Berrué F., Arens J. C., Kerr R. G. (2014). Isolation and structure elucidation of cystargamide, a lipopeptide from Kitasatospora cystarginea. J. Nat. Prod. 77, 1372–1376. doi: 10.1021/np500122s
Hong K., Gao A. H., Xie Q. Y., Gao H., Zhuang L., Lin H. P., et al. (2009). Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 7, 24–44. doi: 10.3390/md7010024
Hoskisson P. A., van Wezel G. P. (2019). Streptomyces coelicolor. Trends Microbiol. 27, 468–469. doi: 10.1016/j.tim.2018.12.008
Igarashi M., Shida T., Sasaki Y., Kinoshita N., Naganawa H., Hamadaand M., et al. (1999). Vinylamycin, a new depsipeptide antibiotic, from streptomyces sp. J. Antibiot. (Tokyo) 52, 873–879. doi: 10.7164/antibiotics.52.873
Jeong H., Jo S. J., Bae M., Kim Y. R., Moon K. (2022). Actinoflavosides B–D, flavonoid type glycosides from tidal mudflat-derived actinomyces. Mar. Drugs 20, 565. doi: 10.3390/md20090565
Jiang Z.-D., Jensen P., Fenical W. (1997). Actinoflavoside, a novel flavonoid-like glycoside produced by a marine bacterium of the genus Streptomyces. Tetrahedron Lett. 38, 5065–5068. doi: 10.1016/S0040-4039(97)01127-1
Jose P. A., Jha B. (2017). Intertidal marine sediment harbours Actinobacteria with promising bioactive and biosynthetic potential. Sci. Rep. 7, 1–15. doi: 10.1038/s41598-017-09672-6
Jose P. A., Maharshi A., Jha B. (2021). Actinobacteria in natural products research: Progress and prospects. Microbiol. Res. 246:126708. doi: 10.1016/j.micres.2021.126708
Kaltenpoth M. (2009). Actinobacteria as mutualists: general healthcare for insects? Trends Microbiol. 17, 529–535. doi: 10.1016/j.tim.2009.09.006
Kang S., Han J., Jang S. C., An J. S., Kang I., Kwon Y., et al. (2022). Epoxinnamide: an epoxy cinnamoyl-containing nonribosomal peptide from an intertidal mudflat-derived streptomyces sp. Mar. Drugs 20:455. doi: 10.3390/md20070455
Kim S. H., Shin Y., Lee S. H., Oh K. B., Lee S. K., Shin J., et al. (2015a). Salternamides A-D from a halophilic streptomyces sp. Actinobacterium. J. Nat. Prod. 78, 836–843. doi: 10.1021/acs.jnatprod.5b00002
Kim S. H., Shin Y., Lee S. K., Shin J., Oh D. C. (2015b). Salternamide E from a saltern-derived marine actinomycete Streptomyces sp. Nat. Prod. Sci. 21, 273–277. doi: 10.20307/nps.2015.21.4.273
Kim H., Yang I., Patil R. S., Kang S., Lee J., Choi H., et al. (2014). Anithiactins A-C, modified 2-phenylthiazoles from a mudflat-derived Streptomyces sp. J. Nat. Prod 2716–2719. doi: 10.1021/np500558b
Kitani S., Yoshida M., Boonlucksanawong O., Panbangred W., Anuegoonpipat A., Kurosu T., et al. (2018). Cystargamide B, a cyclic lipodepsipeptide with protease inhibitory activity from Streptomyces sp. J. Antibio. 71, 662–666. doi: 10.1038/s41429-018-0044-0
Lee H. S., An B. J., Kim H. J., Cho Y. H., Kim D. I., Jang J. Y., et al. (2015a). Anti-inflammatory effect of violapyrones B and C from a marine-derived Streptomyces sp. Nat. Prod. Sci. 21, 251–254. doi: 10.20307/nps.2015.21.4.251
Lee H. W., Jung W. K., Kim H. J., Jeong Y. S., Nam S. J., Kang H., et al. (2015b). Inhibition of monoamine oxidase by anithiactins from Streptomyces sp. J. Microbiol. Biotechnol. 25, 1425–1428. doi: 10.4014/jmb.1505.05020
Lee J., Kim H., Lee T. G., Yang I., Won D. H., Choi H., et al. (2014). Anmindenols A and B, inducible nitric oxide synthase inhibitors from a marine-derived Streptomyces sp. J. Nat. Prod. 77, 1528–1531. doi: 10.1021/np500285a
Leutou A. S., Yang I., Seong C. N., Ko J., Nam S. J. (2015). Violapyrone J, α-pyrone derivative from a Marine-derived actinomycetes, Streptomyces sp. Nat. Prod. Sci. 21, 248–250. doi: 10.20307/nps.2015.21.4.248
Lewin G. R., Carlos C., Chevrette M. G., Horn H. A., McDonald B. R., Stankey R. J., et al. (2016). Evolution and ecology of actinobacteria and their bioenergy applications. Annu. Rev. Microbiol. 70, 235–254. doi: 10.1146/annurev-micro-102215-095748
Li J., Chen Q., Li Q., Zhao C., Feng Y. (2021). Influence of plants and environmental variables on the diversity of soil microbial communities in the Yellow River Delta Wetland, China. Chemosphere 274, 129967. doi: 10.1016/j.chemosphere.2021.129967
Li M. Y., Xiao Q., Pan J. Y., Wu J. (2009). Natural products from semi-mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 26, 281–298. doi: 10.1039/b816245j
Mohapatra M., Yadav R., Rajput V., Dharne M. S., Rastogi G. (2021). Metagenomic analysis reveals genetic insights on biogeochemical cycling, xenobiotic degradation, and stress resistance in mudflat microbiome. J. Environ. Manage 292, 112738. doi: 10.1016/j.jenvman.2021.112738
Moon K., Chung B., Shin Y., Rheingold A. L., Moore C. E., Park S. J., et al. (2015). Pentacyclic antibiotics from a tidal mud flat-derived actinomycete. J. Nat. Prod. 78, 524–529. doi: 10.1021/np500736b
Oh D. C., Poulsen M., Currie C. R., Clardy J. (2009). Dentigerumycin: A bacterial mediator of an ant-fungus symbiosis. Nat. Chem. Biol. 5, 391–393. doi: 10.1038/nchembio.159
Peng A., Qu X., Liu F., Li X., Li E., Xie W. (2018). Angucycline glycosides from an intertidal sediments strain Streptomyces sp. and their cytotoxic activity against hepatoma carcinoma cells. Mar. Drugs 16, 470. doi: 10.3390/md16120470
Pierre G., Zhao J. M., Orvain F., Dupuy C., Klein G. L., Graber M., et al. (2014). Seasonal dynamics of extracellular polymeric substances (EPS) in surface sediments of a diatom-dominated intertidal mudflat (Marennes-Oléron, France). J. Sea Res. 92, 26–35. doi: 10.1016/j.seares.2013.07.018
Riedlinger J., Reicke A., Zahner H., Krismer B., Bull A. T., Maldonado L. A., et al. (2004). Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine verrucosispora strain AB-18-032. J. Antibiot. (Tokyo) 57, 271–279. doi: 10.7164/antibiotics.57.271
Saitou N., Nei M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molcular Biol. Evol. 4, 406–425. doi: 10.1093/oxfordjournals.molbev.a040454
Satyam K., Thiruchitrambalam G. (2018). “Habitat Ecology and Diversity of Rocky Shore Fauna,” in Biodiversity and Climate Change Adaptation in Tropical Islands (Elsevier Inc, Port Blair, India), 187–215. doi: 10.1016/B978-0-12-813064-3/00007-7
Seo J., Moon K. (2021). Structure and bioactivity of boholamide A from a tidal mudflat actinomycete. Korean J. Pharmacognosy 52, 203–207. doi: 10.22889/KJP.2021.52.4.203
Seo J., Shin Y. H., Jo S. J., Du Y. E., Um S., Kim Y. R., et al. (2022). Cystargamides C and D, new cyclic lipopeptides from a tidal mudflat-derived streptomyces sp. JMS132. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.904954
Shaaban K. A., Ahmed T. A., Leggas M., Rohr J. (2012). Saquayamycins G-K, cytotoxic angucyclines from Streptomyces sp. including two analogues bearing the aminosugar rednose. J. Nat. Prod. 75, 1383–1392. doi: 10.1021/np300316b
Shi J., Liu C. L., Zhang B., Guo W. J., Zhu J., Chang C. Y., et al. (2019). Genome mining and biosynthesis of kitacinnamycins as a STING activator. Chem. Sci. 10, 4839–4846. doi: 10.1039/c9sc00815b
Shin D., Byun W. S., Moon K., Kwon Y., Bae M., Um S., et al. (2018). Coculture of marine Streptomyces sp. with Bacillus sp. produces a new piperazic acid-bearing cyclic peptide. Front. Chem. 6. doi: 10.3389/fchem.2018.00498
Siro G., Donald L., Pipite A. (2023). The diversity of deep-sea actinobacteria and their natural products: an epitome of curiosity and drug discovery. Diversity (Basel) 15, 30. doi: 10.3390/d15010030
Sivakala K. K., Gutiérrez-García K., Jose P. A., Thinesh T., Anandham R., Barona-Gómez F., et al. (2021). Desert environments facilitate unique evolution of biosynthetic potential in Streptomyces. Molecules 26:588. doi: 10.3390/molecules26030588
Swingland I. R. (2013). “Definition of Biodiversity,” in Encyclopedia of Biodiversity, 2nd ed. Ed. Levin S. A. (Elsevier Inc, New York), 399–410. doi: 10.1016/B978-0-12-384719-5.00009-5
Tambadou F., Lanneluc I., Sablé S., Klein G. L., Doghri I., Sopéna V., et al. (2014). Novel nonribosomal peptide synthetase (NRPS) genes sequenced from intertidal mudflat bacteria. FEMS Microbiol. Lett. 357, 123–130. doi: 10.1111/1574-6968.12532
Tian X., Zhang Z., Yang T., Chen M., Li J., Chen F., et al. (2016). Comparative genomics analysis of Streptomyces species reveals their adaptation to the marine environment and their diversity at the genomic level. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00998
Torres J. P., Lin Z., Fenton D. S., Leavitt L. U., Niu C., Lam P. Y., et al. (2020). Boholamide A, an APD-class, hypoxia-selective cyclodepsipeptide. J. Nat. Prod. 83, 1249–1257. doi: 10.1021/acs.jnatprod.0c00038
Trujillo M. E., Riesco R., Benito P., Carro L. (2015). Endophytic actinobacteria and the interaction of Micromonospora and nitrogen fixing plants. Front. Microbiol. 6. doi: 10.3389/fmicb.2015.01341
van der Meij A., Worsley S. F., Hutchings M. I., van Wezel G. P. (2017). Chemical ecology of antibiotic production by actinomycetes. FEMS Microbiol. Rev. 41, 392–416. doi: 10.1093/femsre/fux005
Ventura M., Canchaya C., Tauch A., Chandra G., Fitzgerald G. F., Chater K. F., et al. (2007). Genomics of actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 71, 495–548. doi: 10.1128/mmbr.00005-07
Williams P. G., Buchanan G. O., Feling R. H., Kauffman C. A., Jensen P. R., Fenical W. (2005). New cytotoxic salinosporamides from the marine actinomycete. Salinispora tropica 70:6196–6203. doi: 10.1021/jo050511
Yan Y. W., Jiang Q. Y., Wang J. G., Zhu T., Zou B., Qiu Q. F., et al. (2018). Microbial communities and diversities in mudflat sediments analyzed using a modified metatranscriptomic method. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00093
Yang R.-M, Zhang X.-L., Wang L., Huang J.-P., Yang J., Yan Y.-J., et al. (2017). a-Pyrone Derivatives from a Streptomyces Strain Resensitize Tamoxifen Resistance in Breast Cancer Cells. Nat. Rep. Bioprospect. 7, 329-334. doi: 10.1007/s13659-017-0136-8
Zhang J., Jiang Y., Cao Y., Liu J., Zheng D., Chen X., et al. (2013). Violapyrones A-G, α-pyrone derivatives from Streptomyces violascens isolated from Hylobates hoolock feces. J. Nat. Prod. 76, 2126–2130. doi: 10.1021/np4003417
Keywords: marine actinomycetes, intertidal actinomycetes, tidal actinomycetes, marine natural products, marine microbial secondary metabolites, tidal flat actinomycetes
Citation: Ryu D, Hillman PF, Akinniyi G, Nam S-J and Yang I (2023) Marine mudflat actinomycetes as a novel natural products source. Front. Mar. Sci. 10:1297446. doi: 10.3389/fmars.2023.1297446
Received: 20 September 2023; Accepted: 17 November 2023;
Published: 08 December 2023.
Edited by:
Bo Wang, Shandong University of Science and Technology, ChinaReviewed by:
Mallique Qader, University of Illinois at Chicago, United StatesDaniela Giordano, National Research Council (CNR), Italy
Copyright © 2023 Ryu, Hillman, Akinniyi, Nam and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang-Jip Nam, sjnam@ewha.ac.kr; Inho Yang, ihyang@kmou.ac.kr
 Dohee Ryu
Dohee Ryu Prima F. Hillman2
Prima F. Hillman2  Ganiyu Akinniyi
Ganiyu Akinniyi Sang-Jip Nam
Sang-Jip Nam Inho Yang
Inho Yang