Continuous production-degradation of dissolved organic matter provides signals of biogeochemical processes from terrestrial to marine end-members
- 1School of Earth System Science, Tianjin University, Tianjin, China
- 2Tianjin Key Laboratory of Earth Critical Zone Science and Sustainable Development in Bohai Rim, Tianjin University, Tianjin, China
- 3Graduate School of Biosphere Science, Department of Environmental Dynamics and Management, Hiroshima University, Higashi-Hiroshima, Japan
- 4College of Resources and Environment, Xingtai University, Xingtai, Hebei, China
- 5Dip.to di Scienze del Suolo, della Pianta e degli Alimenti, Università degli Studi di Bari “Aldo Moro”, Bari, Italy
- 6Department of Environmental Science and Disaster Management, Noakhali Science and Technology University, Noakhali, Bangladesh
- 7Università degli Studi di Torino, Dipartimento di Chimica, Torino, Italy
- 8Centro Interdipartimentale NatRisk, Grugliasco, Italy
Introduction
Photosynthesis powered by sunlight, which involves plants and microorganisms in terrestrial soils and phytoplankton in fresh and marine waters, is one of the most important biogeochemical processes occurring in the environment. It constantly controls the production of most natural organic matter (NOM), which is a fundamental constituent of all ecosystems of our planet. Dissolved organic matter (DOM) is generated from NOM in terrestrial soils depending on three key sets of properties, i.e. physical, including temperature and moisture, chemical, which comprise nutrient availability, amount of available oxygen and redox activity, and microbial, such as microfloral succession patterns and availability of aerobic and anaerobic microorganisms. Terrestrial DOM is then partially transferred to surface waters through surface runoff and groundwater leaching (Kritzberg et al., 2004; Catalán et al., 2016; Zark and Dittmar, 2018; Mostofa et al., 2019; Mohinuzzaman et al., 2020; Yi et al., 2021). Differently, autochthonous aquatic DOM originates from phytoplankton in surface water (Yamashita and Tanoue, 2004; Zhang et al., 2009; Guidi et al., 2016; Flemming et al., 2016; Shammi et al., 2017a; Igarza et al., 2019; Yang et al., 2021). Thus, a complex mixture of terrestrial and aquatic DOM occurs in surface-water environments (Kritzberg et al., 2004; Zark and Dittmar, 2018; Yi et al., 2021), which can be efficiently characterized on the basis of fluorescence properties (FDOM) and discriminated by excitation-emission matrix (EEM) fluorescence spectroscopy coupled with parallel factor (PARAFAC) modeling (Yamashita and Tanoue, 2004; Zhang et al., 2009; Shammi et al., 2017a; Mohinuzzaman et al., 2020; Yang et al., 2021; Yi et al., 2021). However, a clear, holistic understanding of terrestrial (allochthonous) and autochthonous aquatic DOM components is still lacking, as well as that of their sources and simultaneous production-degradation processes and pathways during transport from soil to sea through freshwater bodies, and their biogeochemical links.
Transformation of DOM from land source to marine environments
Based on the most recent research results, a detailed picture is provided in Figure 1 and summarized below. Soil DOM is universally recognized to be mostly composed of humic substances (HS), including humic acids (HA), fulvic acids (FA) and protein-like substances (PLS) (Figures 1A–C, forest soil), of which the fluorescence peaks are discussed in detail elsewhere (Mohinuzzaman et al., 2020). Terrestrial HS are partially released into ambient freshwaters via groundwater leaching and surface runoff (Figures 1D, E, F). In particular, the two terrestrial components FA and PLS are entirely degraded (Mostofa et al., 2019) due to their lability in photochemical and microbial degradation processes (Figure 1), and only the HA fraction persists during transport from soil to streams to coastal seawater (Mostofa et al., 2019), possibly due to its macromolecular structure (Senesi and Loffredo, 1999; Mohinuzzaman et al., 2020).
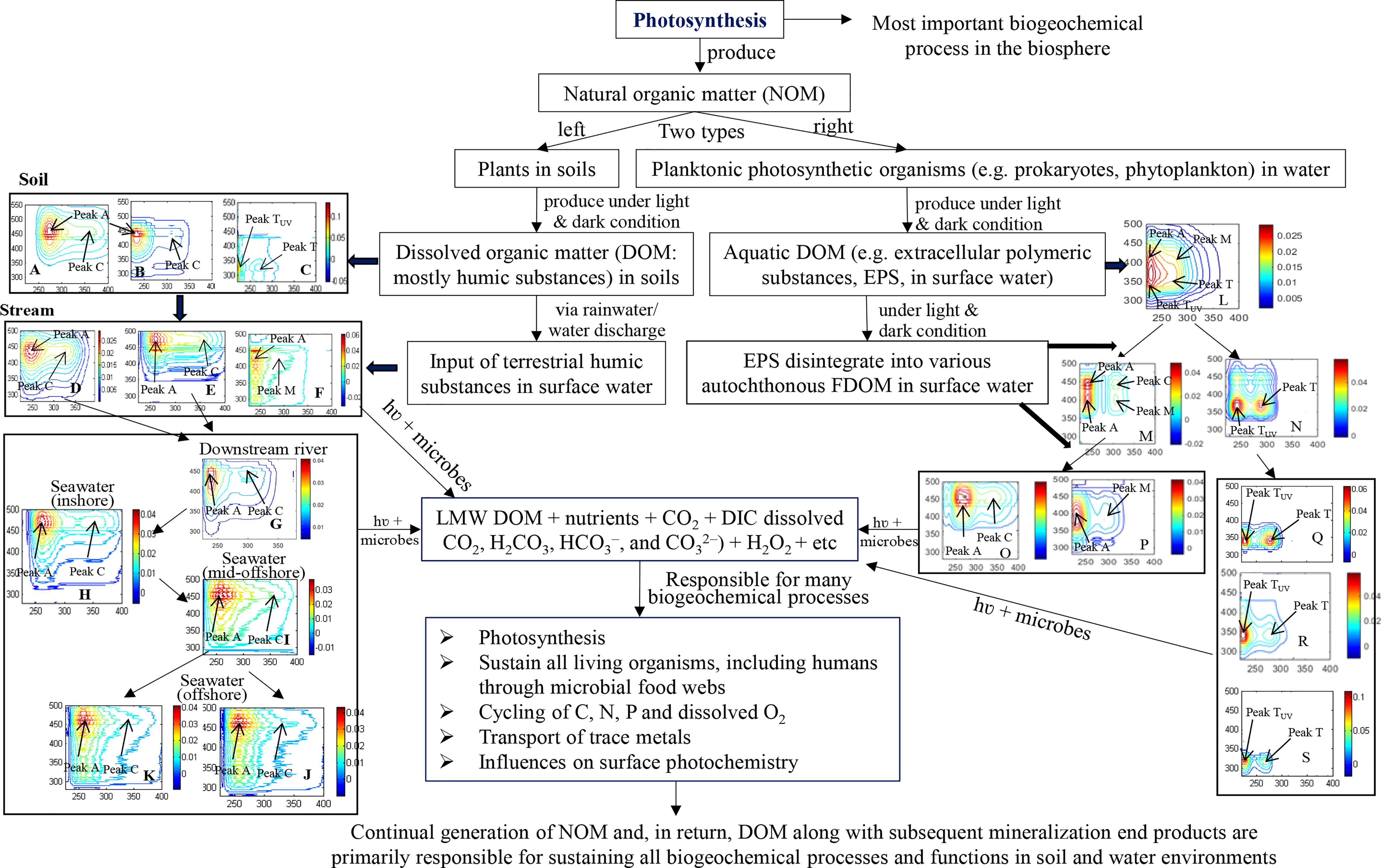
Figure 1 Flow diagram of the overall sequential constant photosynthetic production of natural organic matter (NOM) from terrestrial plants, animals and microorganisms in soils, and from planktonic photosynthetic organisms in waters. Figure 1, left: origin of soil humic substances [humic acids-HA (A), fulvic acids-FA (B) and protein-like substances-PLS (C)] from terrestrial NOM and their subsequent runoff/leaching into surface waters, first into streams [terrestrial HA-like (D), terrestrial FA-like (E), terrestrial PLS-like (F)], then into downstream river (only terrestrial HA-like substances, (G), then into inshore seawater ((H), 0-10 m depth) and mid-offshore seawater ((I), 0-10 m depth), and finally into offshore upper ((J), 0-15 m) and deeper (K), 20-300 m) seawaters in Seto Inland Sea. Figure 1, right: origin of extracellular polymeric substances (EPS, (L) from phytoplankton and their subsequent degradation, first into a combined form of autochthonous HA-like substances (AHLS) of C- and M-types (M) and a newly-released autochthonous protein-like substances (APLS, (N), then into their individual components, i.e. AHLS into C-type (O) and M-type (P) and APLS into protein-like substances (Q), tryptophan-like substances (R), tyrosine-like substances (S), etc. During all transformation described above for the two DOM systems mineralization end products are constantly produced, with the exception of terrestrial HA-like substances that ultimately did not degrade entirely, whereas autochthonous DOM is entirely degraded over one diurnal 24-h cycle by daytime sunlight-induced and nighttime microbial degradation.
In a study on the fluorescence behavior of the terrestrial HA fractions of DOM along their flow (soil → stream → river → sea), a red-shift from shorter to longer wavelength excitation-emission (Ex/Em) peak maxima has been measured when reaching the sea (peak C: stream 330/455; river 315/402; sea 350/473; peak A: stream 250/455; river 240/402; sea 260/473 nm) (Figure 1) (Mostofa et al., 2019; Mohinuzzaman et al., 2020). This red-shift can be ascribed to salinity effects occurring when terrestrial HAs reach seawater, possibly due to the formation of stable complexes with metal ions (Wu et al., 2004; Plaza et al., 2006; Mostofa et al., 2013; Mostofa et al., 2019). After reaching the sea, the water flows in the offshore direction determine a gradual blue shift of fluorescence peaks (C and A) toward wavelengths (325/461 and 255/461 nm in surface waters and 345/462 and 255/462 nm in deeper waters, respectively) that are shorter than those appearing for inshore to mid-offshore sea waters (345/461 and 260/461 nm, respectively). Apparently, a gradual transformation of terrestrial HA-like substances occurs along their transport in soil, stream, river, and then to coastal, mid-shore and offshore seawaters, where HA reach a relatively chemically recalcitrant nature. This behavior is confirmed by radio-carbon dating, which shows an increase of 14C ages from soil to inland waters and then to marine waters (Catalán et al., 2016). Importantly, photochemical and microbial degradation processes along with hydrological processes, particularly carbonate and silicate weathering, would play important roles in overall transformation processes along with transport of chemical species (Catalán et al., 2016; Igarza et al., 2019; Mostofa et al., 2019; Liu et al., 2020; Zhong et al., 2020; Yi et al., 2021).
Origin of autochthonous DOM from planktonic communities and its transformation
Differently, autochthonous DOM originates from photochemical processes and microbial respiration from planktonic photosynthetic organisms (Yamashita and Tanoue, 2004; Zhang et al., 2009; Flemming et al., 2016; Guidi et al., 2016; Shammi et al., 2017a; Yang et al., 2021; Yi et al., 2021). The latter are thought to be responsible for approximately 50% of oceanic primary production and fuel the global biological carbon pump in marine environments (Guidi et al., 2016). Extracellular polymeric substances (EPS) are primarily originated from the plankton community and are considered the early-stage of newly-formed DOM (Flemming et al., 2016; Shammi et al., 2017a). EPS are not yet converted into individual organic components and are composed mainly of polysaccharides, proteins, nucleic acids, lipids, surfactants and humic-like substances (Flemming et al., 2016). The fluorescence moieties, mostly protein-like and humic-like fractions in EPS are firstly transformed into the following components, which can be monitored/detected by EEM-PARAFAC: (i) a combined form of autochthonous humic-like substances (AHLS) of C- and M-types, and (ii) newly-released autochthonous protein-like substances (APLS) (Shammi et al., 2017a; Yang et al., 2021). Successively, AHLS evolve into their individual forms, i.e. C- and M-types, whereas APLS are gradually transformed into individual protein-like, tryptophan-like, tyrosine-like and phenylalanine-like substances (Figure 1) (Yang et al., 2021). All these substances are commonly detected in surface waters, particularly in stagnant waterbodies such as ponds and lakes, but also in estuaries and oceans (Yamashita and Tanoue, 2004; Shammi et al., 2017a; Yang et al., 2021; Yi et al., 2021). Finally, all individual components are photochemically and microbially degraded into low molecular weight (LMW) DOM and mineralized end-products, including gaseous CO2, dissolved inorganic carbon (DIC) and nutrients (e.g., NO3− and PO43−) (Figure 1) (Igarza et al., 2019; Yang et al., 2021; Yi et al., 2021). Differently, the carbohydrates and lipids moieties in EPS do not show any fluorescence, but undergo hydrolysis along with photochemical and microbial degradation/mineralization in surface waters (Zhang and Bishop, 2003; Adav et al., 2008; Shammi et al., 2017b). Details about the origin of autochthonous DOM and its subsequent daytime photoinduced and nighttime microbial degradation are extensively discussed elsewhere (Yang et al., 2021).
Discussion
On the basis of results described above, the features of the two mentioned DOM systems and their biogeochemical transformation processes can be summarized as follows. Terrestrial FA-like and PLS-like fractions are extensively degraded along their transport, whereas terrestrial HA-like substances are only partially degraded by both photochemical and microbial processes (Amador et al., 1989; Catalán et al., 2016; Mostofa et al., 2019), due to their macromolecular size (Senesi and Loffredo, 1999; Mohinuzzaman et al., 2020). Therefore, the macromolecular size of HA is only one feature that would possibly account for its recalcitrant nature, and so can be used to determine the radio-carbon dating (Catalán et al., 2016; Tadini et al., 2018). Furthermore, various long-chain aliphatic and aromatic organic acids are produced by the photoinduced degradation of humic substances extracted from lakes and prolonged irradiation, which leads to a decrease of their concentrations and concomitant mineralization to end-products (Corin et al., 1996). Similarly, many aliphatic and aromatic byproducts were found to derive from aquatic DOM upon pyrolysis (Leenheer and Croué, 2003). Studies also shows that various carboxylic acids (oxalic, malonic, formic, acetic, etc) are often major byproducts of the photoinduced degradation of DOM, which amounts to approximately 25.0–34.4% in surface waters (Bertilsson et al., 1999; Bertilsson and Tranvik, 2000; Ma and Green, 2004). Simultaneously, EPS of planktonic origin are rapidly converted into AHLS and APLS, which are further transformed into individual components that are finally degraded to produce LMW DOM and mineralized end-products (Yang et al., 2021).
The entire degradation of autochthonous aquatic FDOM has been shown to occur within a 24-h cycle under high air/water temperatures during summer, but not during low-temperature months (Yang et al., 2021). Similar results have been obtained for Antarctic glacial environments where exudates from primary production are utilized by heterotrophs within 24 h and support bacterial growth demands (Smith et al., 2017). As well, similar complete photoinduced degradation of humic-like fractions to EPS have been detected experimentally within 58 h,together with a decreasing DOC concentration of approximately 38.4%, from a very high (308.97 ± 1.20 mgL−1) initial DOC concentration (Shammi et al., 2017b).
Thus, the key difference between terrestrial (allochthonous) and aquatic (autochthonous) DOM is that the latter is entirely degraded within a 24-h diurnal period during summer, whereas in the case of terrestrial DOM only the FA-like and PLS-like fractions (but not the HA-like ones) can be completely degraded in waterbodies. The mineralization end-products of both allochthonous and autochthonous DOM are produced continuously under both daytime sunlight-induced degradation and nighttime microbial degradation. In particular, in-situ solar irradiation experimental studies showed that the dissolved inorganic carbon (DIC: dissolved CO2, H2CO3, HCO3-, and CO32-) photoproduction rate is much lower in river waters (0.04–0.22 mg/L) than in lake waters (0.21–0.73 mgC/L), whereas DOC concentrations vary respectively from 33.22-33.22 mg/L and 1.88-2.40 mg/L (Ma and Green, 2004). Similar results for DIC have been obtained for humic lake waters (0.086–0.41 mg C/L day) for in-situ photo-irradiation samples (Granéli et al., 1996). Moreover, several experimental studies also showed that autochthonous fluorescent aromatic amino acids (e.g. tryptophan, tyrosine and phenylalanine), PLS and humic-like substances can produce dissolved inorganic nitrogen such as NH4+ and NOx- in surface waters (Bushaw et al., 1996; Berman and Bronk, 2003; Stedmon et al., 2007; Zhang et al., 2021). As these end-products act as nutrients, they continuously fuel the reproduction of photosynthetic planktonic organisms in surface waters and simultaneously contribute to global carbon cycling as well as to climate change (Mopper et al., 1991; Bushaw et al., 1996; Moran and Zepp, 1997; Berman and Bronk, 2003; Zhong et al., 2020; Mostofa et al., 2013). In conclusion, the continuous production of NOM and its transformation/mineralization represent the primary backbone process in the cycles of carbon, nitrogen, phosphorus, dissolved oxygen and trace metals and in sustaining life of all living organisms, including humans, thus it is of key importance to deepen their understanding, in particular because climate warming determines longer stratification periods to occur in surface waters during summer.
Author contributions
KMGM designed and conceived the project. HS, KMGM, C-QL contributed the key data sources. JY, MM, YL, XY conducted the EEM-PARAFAC analysis. KMGM, NS wrote the manuscript. DV and S-LL reviewed & edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the National Natural Science Foundation of China (42221001, 41925002) and the Haihe Laboratory of Sustainable Chemical Transformations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adav S. S., Lee D.-J., Tay J–H. (2008). Extracellular polymeric substances and structural stability of aerobic granule. Water Res. 42 (6–7), 1644–1650. doi: 10.1016/j.watres.2007.10.013
Amador J. A., Alexander M., Zika R. G. (1989). Sequential photochemical and microbial degradation of organic molecules bound to humic acid. App Environ Microb 55, 2843–2849.
Berman T., Bronk D. A. (2003). Dissolved organic nitrogen: a dynamic participant in aquatic ecosystems. Aquat. Microb. Ecol. 31 (3), 279–305. doi: 10.3354/ame031279
Bertilsson S., Stepanauskas R., Cuadros-Hansson R., Granéli W., Wikner J., Tranvik L. J. (1999). Photochemically induced changes in bioavailable carbon and nitrogen pools in a boreal watershed. Aquat Microb Ecol 19, 47–56.
Bertilsson S., Tranvik L. J. (2000). Photochemical transformation of dissolved organic matter in lakes. Limnol. Oceanogr. 45, 753–762
Bushaw K. L., Zepp R. G., Tarr M. A., Schulz-Jander D., Bourbonniere R. A., Hodson R. E., et al. (1996). Photochemical release of biologically available nitrogen from aquatic dissolved organic matter. Nature 381 (6581), 404–407. doi: 10.1038/381404a0
Catalán N., Marcé R., Kothawala D. N., Tranvik L. J. (2016). Organic carbon decomposition rates controlled by water retention time across inland waters. Nat. Geosci. 9, 501–506. doi: 10.1038/ngeo2720
Corin N., Backlund P., Kulovaara M. (1996). Degradation products formed during UV-irradiation of humic waters. Chemosphere 33, 245–255
Flemming H. C., Wingender J., Szewzyk U., Steinberg P, Rice S. A., Kjelleberg S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. doi: 10.1038/nrmicro.2016.94
Granéli W., Lindell M., Tranvik L. (1996). Photo-oxidative production of dissolved inorganic carbon in lakes of different humic content. Limnol Oceanogr 41, 698–706
Guidi L., Chaffron S., Bittner L., Eveillard D., Larhlimi A., Roux S., et al. (2016). Plankton networks driving carbon export in the oligotrophic ocean. Nature 532, 465–470. doi: 10.1038/nature16942
Igarza M., Dittmar T., Graco M., Niggemann J. (2019). Dissolved organic matter cycling in the coastal upwelling system off central Peru during an “El niño” year. Front. Mar. Sci. 6, 198. doi: 10.3389/fmars.2019.00198
Kritzberg E. S., Cole J. J., Pace M. L., Granéli W., Bade D. L. (2004). Autochthonous versus allochthonous carbon sources of bacteria: Results from whole-lake 13C addition experiments. Limnol. Oceanogr. 49, 588–596. doi: 10.4319/lo.2004.49.2.0588
Leenheer J. A., Croué J. P. (2003). Characterizing aquatic dissolved organic matter. Environ Sci Technol 37, 18–26.
Liu J., Zhong J., Ding H., Yue F.-J., Li C., Xu S., et al (2020). Hydrological regulation of chemical weathering and dissolved inorganic carbon biogeochemical processes in a monsoonal river. Hydrol. Process. 34, 2780–2792. doi: 10.1002/hyp.13763
Ma X., Green S. A. (2004). Photochemical transformation of dissolved organic carbon in Lake Superior-an in-situ experiment. J Great Lakes Res 30 (suppl 1), 97–112
Mohinuzzaman M., Yuan J., Yang X., Senesi N., Mostofa K. M.G., Liu C. Q. (2020). Insights into solubility of soil humic substances and their fluorescence characterisation in three characteristic soils. Sci. Total Environ. 720, 137395. doi: 10.1016/j.scitotenv.2020.137395
Mopper K., Zhou X., Kieber R. J., Kieber D. J., Sikorski R. J., Jones R. D. (1991). Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle. Nature 353, 60–62. doi: 10.1038/353060a0
Moran M. A., Zepp R. G. (1997). Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnol Oceanogr 42, 1307–1316. doi: 10.4319/lo.1997.42.6.1307
Mostofa K. M. G., Jie Y., Sakugawa H., Liu C.-Q. (2019). Equal treatment of different EEM data on PARAFAC modeling produces artifact fluorescent components that have misleading biogeochemical consequences. Environ. Sci. Technol. 53, 561–563. doi: 10.1021/acs.est.8b06647
Mostofa K. M. G., Yoshioka T., Mottaleb M. A., Vione D. (2013). Photobiogeochemistry of organic matter: Principles and practices in water environments (Berlin Heidelberg: Springer).
Plaza C., Brunetti G., Senesi N., Polo A. (2006). Molecular and quantitative analysis of metal ionbinding to humic acids from sewage sludge and sludge-amended soils by fluorescence spectroscopy. Environ. Sci. Technol. 40, 917–923. doi: 10.1021/es051687w
Senesi N., Loffredo E. (1999). “The chemistry of soil organic matter,” in Soil physical chemistry. Ed. Sparks D. L. (Boca Raton: CRC Press), 239–370.
Shammi M., Pan X., Mostofa K. M. G., Zhang D., Liu C. Q. (2017a). Seasonal variation and characteristic differences in the fluorescent components of extracellular polymeric substances from mixed biofilms in saline lake. Sci. Bull. 62, 764–766. doi: 10.1016/j.scib.2017.04.016
Shammi M., Pan X. L., Mostofa K. M. G., Zhang D., Liu C. Q., Song W. (2017b). Investigating extracellular polymeric substances from microbial mat upon exposure to sunlight. Polymer Degradation Stabil 146, 192–200. doi: 10.1016/j.polymdegradstab.2017.10.011
Smith H., Foster R. A., McKnight D. M., Lisle J. T., Littmann S., Kuypers M. M. M., et al. (2017). Microbial formation of labile organic carbon in Antarctic glacial environments. Nat. Geosci. 10, 356–360. doi: 10.1038/ngeo2925
Stedmon C. A., Markager S., Tranvik L., Kronberg L., Slatis T., Martinsen W. (2007). Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea. Mar. Chem. 104, 227–240. doi: 10.1016/j.marchem.2006.11.005
Tadini A. M., Nicolodelli G., Senesi G. S., Ishida D. A., Montes C. R., Lucas Y., et al. (2018). Soil organic matter in podzol horizons of the Amazon region: humification, recalcitrance, and dating. Sci. Total Environ. 613–614, 160–167. doi: 10.1016/j.scitotenv.2017.09.068
Wu F. C., Cai Y. R., Evans D., Dillon P. (2004). Complexation between Hg(II) and dissolved organic matter in stream waters: an application of fluorescence spectroscopy. Biogeochemistry 71, 339–351. doi: 10.1007/s10533-004-0058-5
Yamashita Y., Tanoue E. (2004). In situ production of chromophoric dissolved organic matter in coastal environments. Geophys. Res. Lett. 31, L14302. doi: 10.1029/2004GL019734
Yang X., Yuan J., Yue F.-J., Li S., Wang B., Mohinuzzaman M., et al. (2021). New insights into mechanisms of sunlight- and dark-mediated high-temperature can accelerate diurnal production-degradation transformation of lake fluorescent DOM. Sci. Total Environ. 760, 143377. doi: 10.1016/j.scitotenv.2020.143377
Yi Y., Zhong J., Bao H., Mostofa K. M.G., Xu S., Xiao H.-Y., et al. (2021). The impacts of reservoirs on the sources and transport of riverine organic carbon in the karst area: A multi-tracer study. Water Res. 194, 116933. doi: 10.1016/j.watres.2021.116933
Zark M., Dittmar T. (2018). Universal molecular structures in natural dissolved organic matter. nat. comm. 9, 3178 zhang x., bishop p., biodegradability of biofilm extracellular polymeric substances. Chemosphere 50 (2003), 63–69. doi: 10.1038/s41467-018-05665-9
Zhang X., Bishop P. (2003). Biodegradability of biofilm extracellular polymeric substances. Chemosphere 50, 63–69.
Zhang Y., van Dijk M. A., Liu M., Zhu G., Qin B. (2009). The contribution of phytoplankton degradation to chromophoric dissolved organic matter (CDOM) in eutrophic shallow lakes: Field and experimental evidence. Water Res. 43, 4685–4697. doi: 10.1016/j.watres.2009.07.024
Zhang Y., Zhang R., Li S. L., Mostofa K. M. G., Fu X., Ji H., et al. (2021). Photo-ammonification of low molecular weight dissolved organic nitrogen by direct and indirect photolysis. Sci. Total Environ. 764, 142930. doi: 10.1016/j.scitotenv.2020.142930
Keywords: Natural dissolved organic matter, fluorescent dissolved organic matter, freshwater, Marine water, photodegradation, microbial degradation
Citation: Mostofa KMG, Sakugawa H, Yuan J, Liu C-Q, Senesi N, Mohinuzzaman M, Liu Y, Yang X, Vione D and Li S-L (2022) Continuous production-degradation of dissolved organic matter provides signals of biogeochemical processes from terrestrial to marine end-members. Front. Mar. Sci. 9:1044135. doi: 10.3389/fmars.2022.1044135
Received: 14 September 2022; Accepted: 31 October 2022;
Published: 14 November 2022.
Edited by:
Jeng-Wei Tsai, China Medical University, TaiwanReviewed by:
Chen Xu, Texas A&M University at Galveston, United StatesYanbin Li, Ocean University of China, China
Copyright © 2022 Mostofa, Sakugawa, Yuan, Liu, Senesi, Mohinuzzaman, Liu, Yang, Vione and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khan M. G. Mostofa, mostofa@tju.edu.cn
 Khan M. G. Mostofa
Khan M. G. Mostofa Hiroshi Sakugawa3
Hiroshi Sakugawa3  Nicola Senesi
Nicola Senesi Mohammad Mohinuzzaman
Mohammad Mohinuzzaman Xuemei Yang
Xuemei Yang Davide Vione
Davide Vione Si-Liang Li
Si-Liang Li