- 1Parasite and Vector Research Unit (PAVRU), Department of Microbiology and Parasitology, University of Buea, Buea, Cameroon
- 2Research Foundation for Tropical Diseases and the Environment (REFOTDE), Buea, Cameroon
- 3Institute of Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn (UKB), Bonn, Germany
- 4German Centre for Infection Research (DZIF), Partner Site Bonn-Cologne, Bonn, Germany
- 5German-West African Centre for Global Health and Pandemic Prevention (G-WAC), Partner Site Bonn, Bonn, Germany
Introduction: Mouse models of human filarial infections are not only urgently needed to investigate the biology of the nematodes and their modulation of the host’s immunity, but will also provide a platform to screen and test novel anti-filarial drugs. Recently, murine Loa loa infection models have been stablished using immunocompromised mouse strains, whereas murine Mansonella perstans infections have not been implemented until now.
Methods: Therefore, we aim to establish experimental M. perstans infections using the immunocompromised mouse strains RAG2IL-2Rγ-/- (lack B, T and natural killer cells), IL-4Rα/IL-5-/- (impaired IL-4/5 signalling and eosinophil activation) and NOD.Cg-PrkdcscidIl2rgtm1Wj l/SzJ (NOD scid gamma, NSG) BALB/c mice (lack mature lymphocytes) through subcutaneous (s.c.) or intraperitoneal (i.p.) inoculation of infective stage 3 larvae (L3) isolated from engorged vectors.
Results: In total, 145 immunocompromised mice have been inoculated with 3,250 M. perstans, 3,337 O. volvulus, and 2,720 Loa loa L3 to comparatively analyse which immunocompromised mouse strain is susceptible to human filarial infections. Whereas, no M. perstans and O. volvulus L3 could be recovered upon 2-63 days post-inoculation, a 62-66% Loa loa L3 recovery rate could be achieved in the different mouse strains. Gender of mice, type of inoculation (s.c. or i.p.) or time point of analysis (2-63 days post inoculation) did not interfere with the success of L3 recovery. In addition, administration of the immune suppressants hydrocortisone, prednisolone and cyclophosphamide did not restore M. perstans L3 recovery rates.
Discussion: These findings show that RAG2IL-2Rg-/-BALB/c and C57BL/6, IL-4Rα/IL-5-/- BALB/c and NSG mice were not susceptible to M. perstans and O. volvulus L3 inoculation using the applied methods, whereas Loa loa infection could be maintained. Further studies should investigate if humanized immunocompromised mice might be susceptible to M. perstans. and O. volvulus.
Introduction
The human filariae Mansonella perstans, Loa loa and Onchocerca volvulus affect more than 240 million individuals. Whereas Loa loa is only prevalent in Western and Central Africa, M. perstans and O. volvulus are endemic throughout Sub-Saharan Africa and parts of South and Central America (1, 2). All three parasites are vector-borne diseases and transmission depend on Culicoides midges for M. perstans (3, 4), Chrysops flies for Loa loa (5) and Simulium black flies for O. volvulus (6). These vectors transmit infective stage 3 larvae (L3), which develop into adult worms that reside in body cavities (M. perstans) (7), subcutaneous tissue (Loa loa) (8) and subcutaneous nodules (O. volvulus) (9). Fertile female adult worms produce the microfilariae (MF) that circulate in the peripheral blood (M. perstans and Loa loa) (7, 8) or subcutaneous tissue (O. volvulus) (10), which can be taken up by the corresponding vectors during another blood meal. Whereas no distinct severe clinical symptoms have been associated with M. perstans (1), loiasis is associated with Calabar swelling, pruritis, arthralgia and eye worm (11) and onchocerciasis can cause papular dermatitis, skin hyperpigmentation or depigmentation (leopard skin) and vision loss (12, 13). Nevertheless, the majority of filarial infections remain asymptomatic due to the strong modulation of the host immunity which promotes survival of the parasites (14–16). The asymptomatic nature of filarial infections has resulted in a shortfall of knowledge, but the understanding of the biology of the nematodes and their evasion tactics within the host is important to fulfil the goals of control and elimination programmes. Thus, preclinical models are essential to study the biology of filariae and to test novel anti-filarial drugs. Although, the murine model of filariasis Litmosoides sigmodontis is widely used for drug screening (17), results from this model cannot be directly translated to human filarial infections. In recent years, in vitro culture models for M. perstans (18–20), Loa loa (21–23), and O. volvulus (24, 25) have been established. However, in vitro models do not take into account the complexity of the different niches, in which the parasite resides, and the internal environment, which is important that a drug can reach its target. Thus, in vivo rodent models for Loa loa and O. volvulus have been established using immunocompromised rodents (26–30), whereas no in vivo model for M. perstans has been implemented until now. Since RAG2- (27, 28, 31, 32) and IL-4/5-deficient (33–35), as well as NOD scid gamma mouse (NSG) strains (26, 36), have been proven to enhance susceptibility to filarial infections, we elucidate if M. perstans infections can be maintained in these mouse strains in comparison to Loa loa and O. volvulus infection.
Methods
Ethics
Ethical clearance was obtained from the National Institutional Review Board, Yaoundé (REF: N° 2022/12/1506/CE/CNERSH/SP) and administrative clearance from the Delegation of Public Health, South West Region (Re: R11/MINSANTE/SWR/RDPH/PS/259/382) after approval of the protocol. Special consideration was taken to minimize any health risks to the participant and involvement was strictly voluntary. The objectives of the study were explained in detail to each individual willing to participate after which they signed an informed consent form. The participant’s documents were given a code to protect the privacy of the study subject. O. volvulus and M. perstans microfilariae+ volunteers were followed up with 200mg doxycycline daily for 28 days, while albendazole 400mg daily for three weeks was administered to L. loa MF+ volunteers (37).
Animals
In this study, female and male IL-4Rα/IL-5-deficient BALB/c, Rag2tm1FwaII2rgtm1WjlRAG2IL-2Rγ-deficient BALB/c and C57BL/6 (RAG2IL-2Rγ-/-), and NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD scid gamma, NSG) BALB/c mice were used. These mouse strains were provided by the Institute of Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn (UKB) in Germany. RAG2IL-2Rγ-/- mice were purchased from Taconic Biosciences Inc (Cologne, Germany) and the other two strains were bred at the IMMIP and UKB animal facilitates, but originally, IL-4Rα/IL-5-/- were a gift from Prof. Dr. Klaus Matthaei (Matthaei, Stem Cell & Gene Targeting Laboratory, ANU College of Medicine, Biology and Environment, Canberra, Australia) and NSG mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Mice were kept under SPF conditions following German animal protection laws and EU guidelines 2010/63/E4 and had access to food and water ad libitum. Mice were shipped in filter topped boxes to the Research Foundation for Tropical Diseases and the Environment (REFOTDE), Buea, Cameroon in agreement with the veterinary office in Bonn, Germany. Upon arrival, mice were housed in the animal facility of the Research Foundation for Tropical Diseases and the Environment, University of Buea under SPF conditions following Cameroonian animal protection laws.
Parasite material
To infect the different mouse strains with the filarial species, infective stage 3 larvae (L3) were obtained from different batches of the collected vectors as previously described (18, 25, 33). In short, Culicoides midges (M. perstans), Simulium damnosum (O. volvulus) and Chrysops silacea (Loa loa) were collected upon a blood meal on microfilariae positive volunteers (at least 2 volunteers per filarial species). The blood-feed vectors were maintained in captivity under controlled conditions for up to 14 days to allow the development of L3. Then, L3 were isolated in RPMI-1640 medium (Sigma-Aldrich, Munich, Germany) supplemented with a 2% antibiotic cocktail (penicillin-streptomycin-neomycin; Thermo Fisher Scientific, Schwerte, Germany) by dissecting the head, the thorax and the abdomen allowing the migration of L3 into the dissecting medium. Finally, L3 were washed twice in RPMI-1640 medium to get rid of fly debris and counted using a dissecting microscope (Leica, Wetzlar, Germany). Then, the motility of the isolated L3 was assessed. Only motile L3 were considered viable and directly used for the inoculation of the different immunocompromised mouse strains.
Experimental mouse infections
Mice were infected with the isolated alive L3 subcutaneously (s.c.) or intraperitoneally (i.p.) and the efficiency of L3 inoculation was confirmed by flushing the needle and investigating the flushed-out fluid under the microscope. Furthermore, subcutaneous implantation was performed during some inoculation experiments. In detail, mice were s.c. anaesthetized by a combination of 1µg/kg ketamine (WDT, Garbsen, Germany) and 10µg/kg medetomidine (Orion Pharma, Espoo, Finland) in combination with prophylactic antibiotic penicillin G (Sigma-Aldrich). Anaesthetised mice were arranged on sterile drapes on top of heat pads and shaved on the upper left abdomen that were swabbed with iodine (Ecolab, Monheim am Rhein, Germany). Then, a small incision was performed through the skin with sterile surgical instruments and M. perstans L3 worms were implanted into the subcutaneous cavity with 200µl RPMI medium (Sigma-Aldrich). Finally, mice were sutured through the skin and an α2-antagonist (atipamezole; Orion Pharma) was administered. Until recovery mice were placed on heat pads and finally placed back into IVC cages.
Mouse dissection and worm recovery
Upon 2-63 days post-infection (p.i.) mice were euthanized by exposure to increasing concentrations of CO2. Cardiac blood was collected by cardiac puncture and stored in non-heparinised 1.5 ml microcentrifuge tubes to obtain sera, which was stored at -20°C. Then, heart, lung, intestine, skin and muscles were gently excised and placed in separate Petri dishes containing RPMI-1640 medium (Sigma-Aldrich) supplemented with 2% antibiotic cocktail (penicillin-streptomycin-neomycin; Thermo Fisher Scientific). Muscle tissues were teased gently to ease worm migration into the medium. Then, all tissues were incubated at 37°C for 2h to allow migration of the parasites from tissues into the medium. Finally, Petri dishes were then observed under a dissecting microscope (Leica, Wetzlar, Germany) for the presence of parasites.
Administration of immune suppressants
The used immunocompromised mouse strains lack crucial immune cells and cell signalling mechanisms. However, to further inhibit the immune cells and responses immune suppressants were used like the corticosteroids hydrocortisone (Vinco Pharmaceutical Ltd, Lagos, Nigeria) and prednisolone (Jenapharm GmbH & Co.KG, Jena, Germany), which inhibits inflammatory transcription factors and promotes anti-inflammatory genes (37), and cyclophosphamide (Thermo Fisher Scientific), which is an antitumor agent that triggers the death of hematopoietic stem cells leading to a loss of different immune cells like neutrophils, monocytes, B and T cells (38, 39). Hydrocortisone and prednisolone (10mg/kg) were administered intramuscular (i.m.) and i.p. respectively, one day before filarial infection, whereas cyclophosphamide was administered i.p. twice, one day before infection (150mg/kg) and 4 days p.i. (100mg/kg).
Results
Overview of the mouse infections with different L3 species
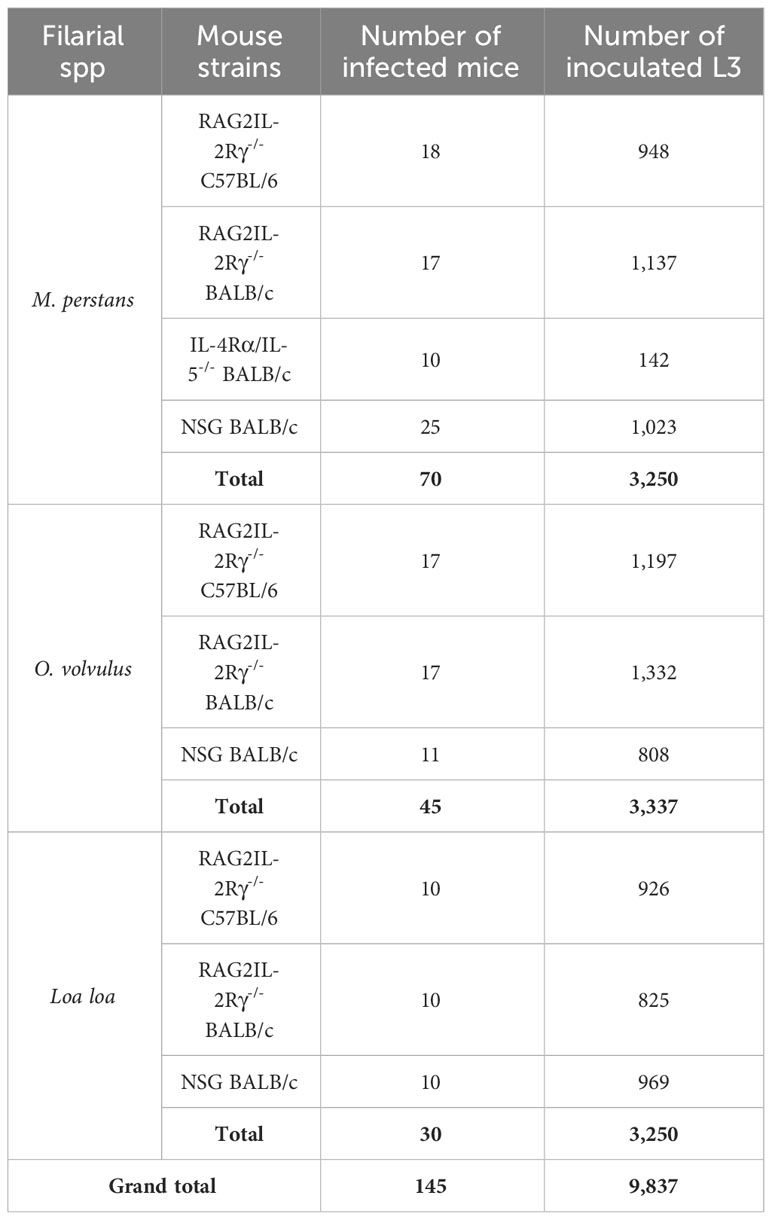
To establish experimental filarial infections of M. perstans, O. volvulus and Loa loa, the immunocompromised mouse strains RAG2IL-2Rγ-/- (lack B, T and natural killer cells), IL-4Rα/IL-5-/- (impaired IL-4/5 signalling and eosinophil activation) and NSG (lack mature lymphocytes) were used. In total, 3,250 M. perstans, 3,337 O. volvulus, and 2,720 Loa loa L3 were isolated to infect in total of 145 immunocompromised mice. Table 1 shows a summary of mouse strains and the number of L3 that were used in this study.
Parasite recovery rates
Since no mouse infection of M. perstans has been established so far, we first inoculated the different mouse strains with M. perstans L3 and analysed the recovery rate of the larvae 2-63 p.i. As shown in Table 2, no L3 could be recovered from any of the mouse strains independently of the type of administration (s.c. or i.p.), number of inoculated L3 (10-123/mouse), time point of analysis (2-63 days p.i.) or sex of mice. Similarly, no O. volvulus L3 could be recovered from the different immunocompromised mouse strains 2-7 days p.i. (Table 3).
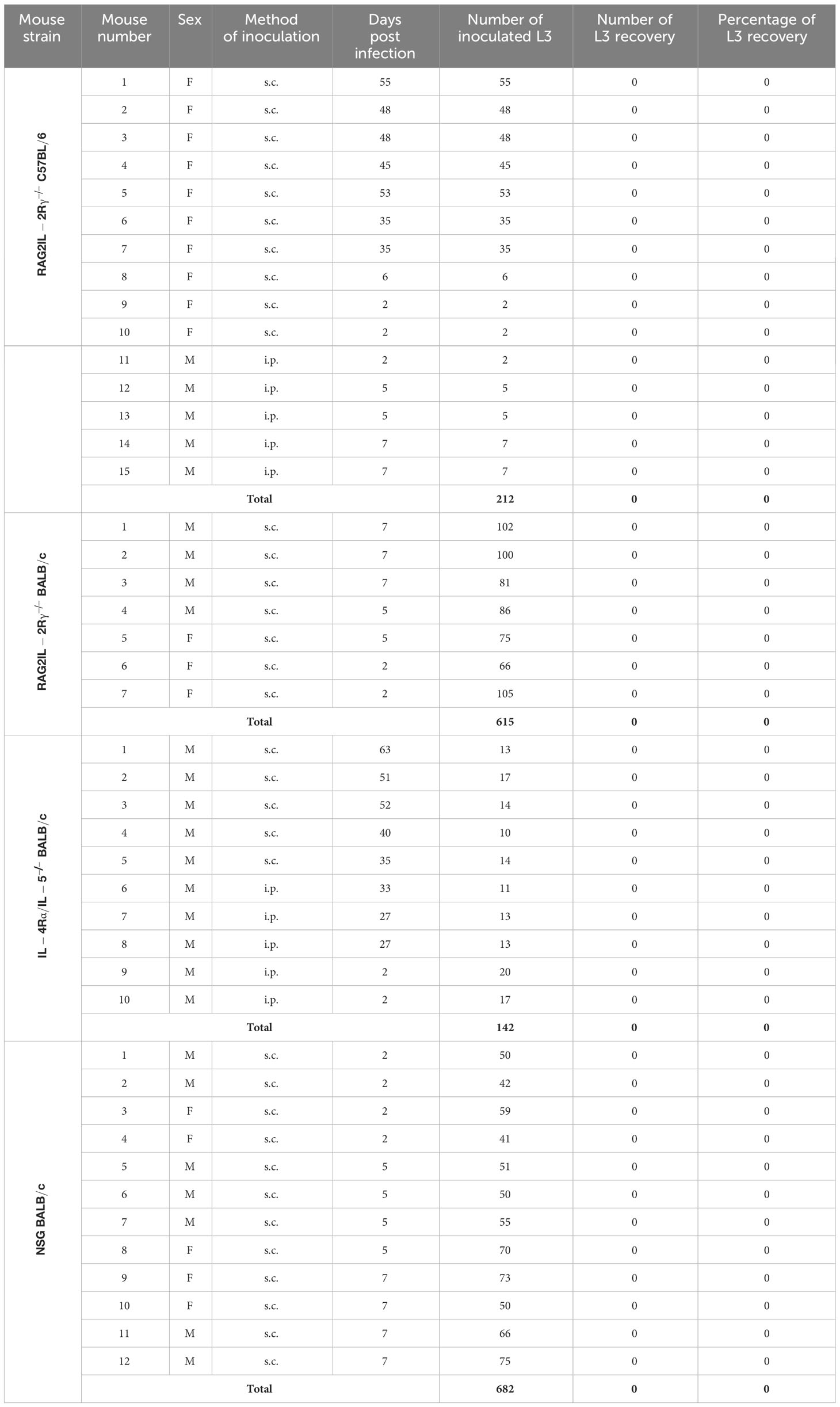
Table 2 Summary of M. perstans L3 inoculation experiments and L3 recovery rates from the different mouse strains (F, Female; M, Male; s.c., subcutaneous; i.p., intraperitoneal).
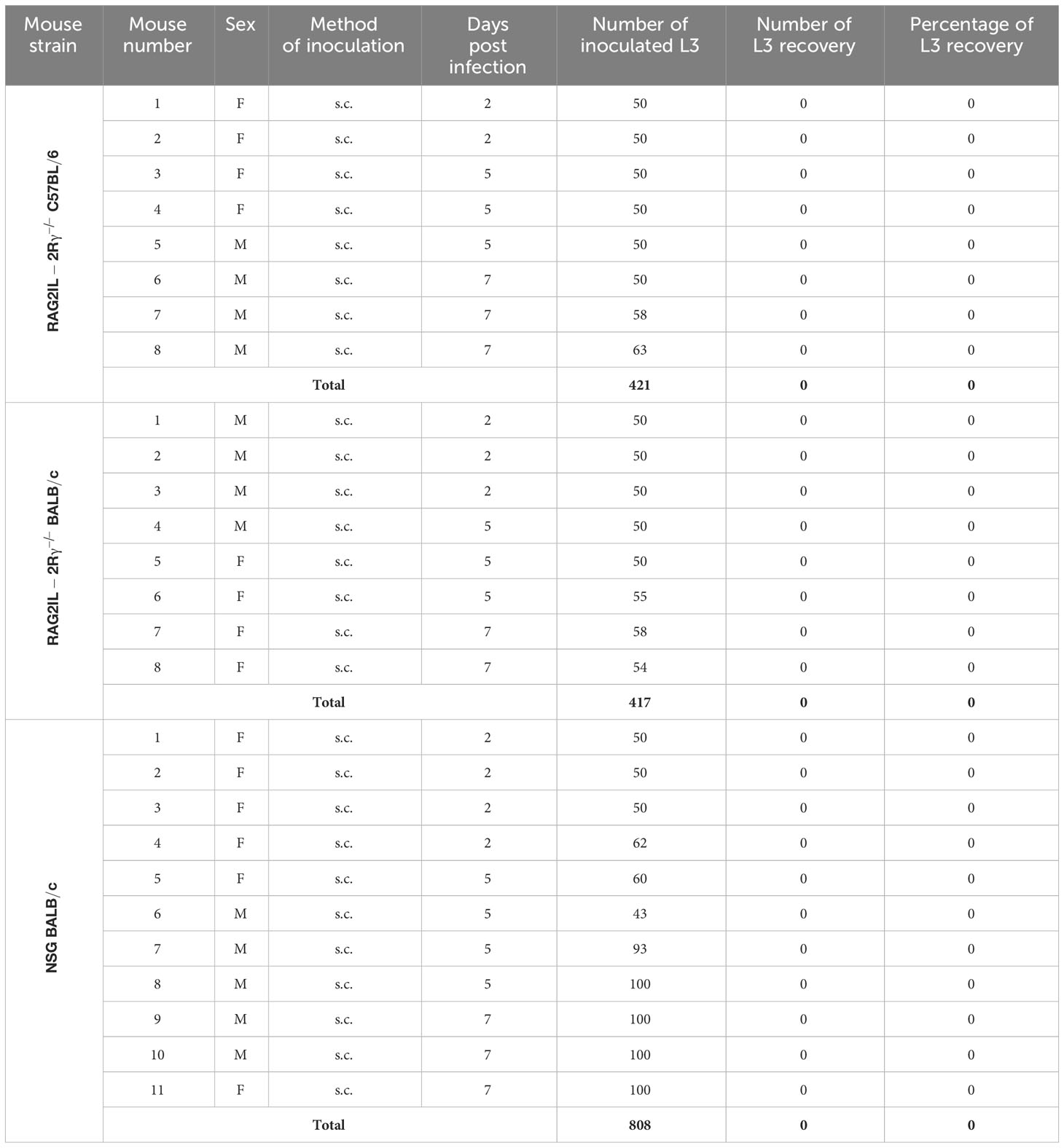
Table 3 Summary of O. volvulus L3 inoculation experiments and L3 recovery rates from the different mouse strains (F, Female; M, Male; s.c., subcutaneous).
Since no L3 could be recovered from M. perstans and O. volvulus inoculated mice, we were wondering if the isolated L3 were suitable for mouse infection experiments. However, previous studies showed that immunocompromised mice were susceptible to Loa loa L3 (27–29). Thus, we isolated Loa loa L3 from engorged Chrysops flies to inoculate the different immunocompromised mice with Loa loa L3. Indeed, we revealed that Loa loa L3 inoculation led to a recovery rate of L3 from 66% in RAG2IL-2Rγ-/- C57BL/6, 65.7% RAG2IL-2Rγ-/- BALB/c, and 61.8% in NSG BALB/c mice upon 2-7 days p.i., independently of sex of mice or number of inoculated L3 (Table 4). These results show that the isolated L3 were suitable to infect immunocompromised mice, but highlight that M. perstans and O. volvulus L3 did not survive in the immunocompromised mouse strains using the applied methods.
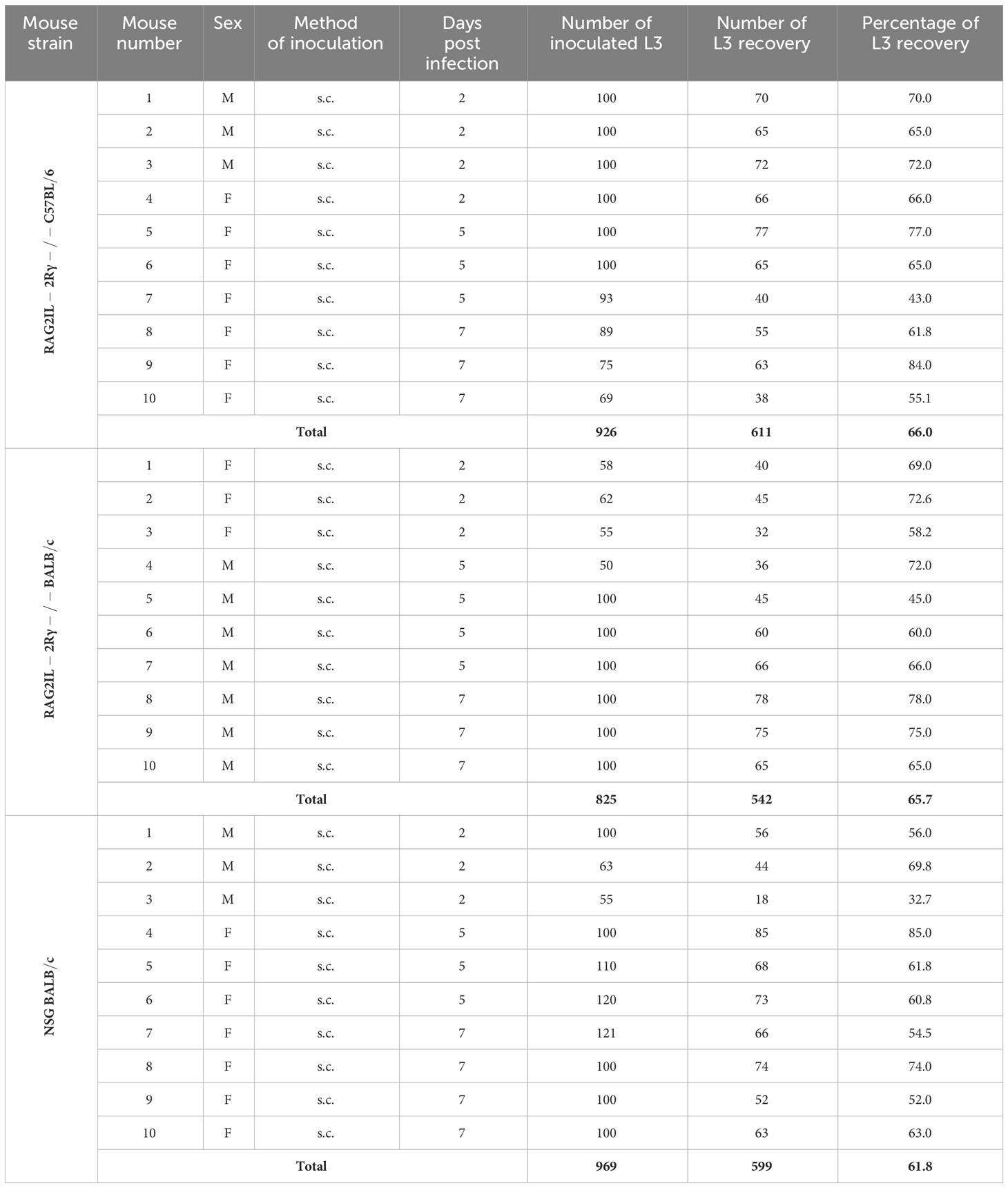
Table 4 Summary of Loa loa L3 inoculation experiments and L3 recovery rates from the different mouse strains (F, Female; M, Male; s.c., subcutaneous).
Since no L3 larvae could be recovered from M. perstans and O. volvulus L3 inoculated mice, we test if further immune suppression will increase susceptibility to M. perstans and O. volvulus. Therefore, we treated RAG2/IL-2Rγ-/- mice with hydrocortisone, prednisolone and cyclophosphamide and analysed L3 recovery rate 2-7 days p.i., but again no L3 could be obtained independently of the number of inoculated larvae or sex of mice (Table 5). Since hydrocortisone, prednisolone and cyclophosphamide treatment did not improve parasite recovery in RAG2/IL-2Rγ-/- mice, we consequently did not proceed with this approach with the other mouse strains to fulfil the 3R principles, especially the reduction of mouse numbers.
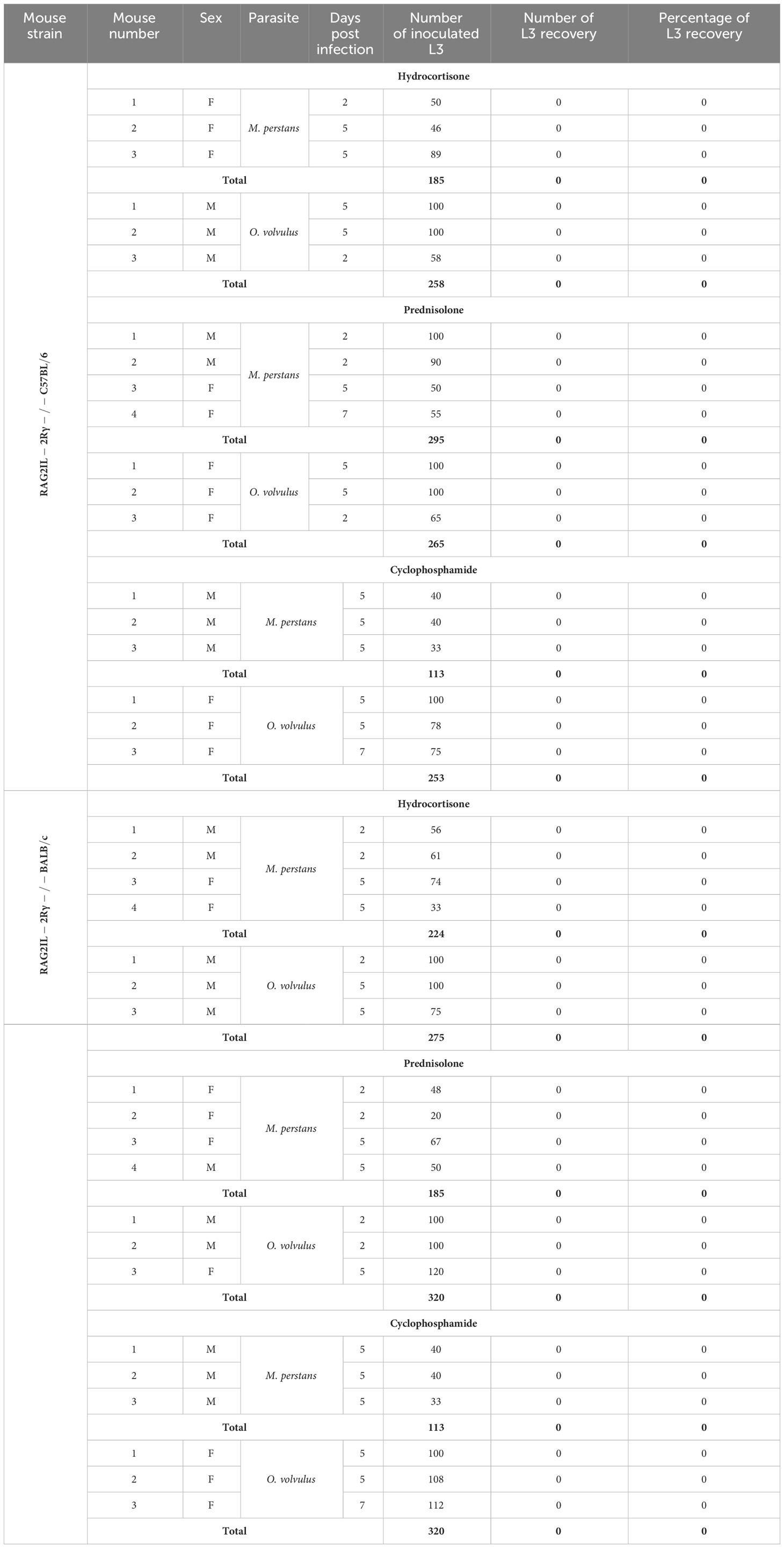
Table 5 Summary of M. perstans and O. volvulus L3 recovery rates from the RAG2/IL-2Rγ-/- C57BL/6 and BALB/c mice (F, Female; M, Male; L3 larvae were inoculated subcutaneously).
In conclusion, these findings suggest that RAG2IL-2Rγ-/-BALB/c and C57BL/6, IL-4Rα/IL-5-/- BALB/c and NSG mice were not susceptible to M. perstans and O. volvulus L3 inoculation using the applied protocols and methods, whereas Loa loa infection can be established in these immunocompromised mouse strains.
Discussion
Research about human filarial infections is often hindered due to the complex life cycle and difficulties in obtaining the life stages of the parasites. Thus, the mouse model of human filariasis, Litomosoides sigmodontis, is a suitable tool to investigate the biology of filarial infections (40, 41) and filarial-driven immune modulation of the host (42), and can be used as a preclinical model for drug testing (17). Nevertheless, findings obtained from the murine model of filariasis cannot be directly translated to the human situation. Thus, in vitro models of lymphatic filariasis (43, 44), onchocerciasis (24, 25, 45), loiasis (21–23) and mansonelliosis (18–20) have been established to investigate the biology of the filarial nematodes and screen for anti-filarial drugs. Although long-term cultivation of the parasites and even the development of larvae to young adults have been achieved in some of these in vitro culture systems (19, 24, 25, 45), the development of L3 into fertile adult worms and consequently the production of microfilariae has not been achieved until now. However, in vitro filarial cultures are important for initial drug screening, but the findings need to be taken with caution due to variation in stage- and species-specific expression of filarial drug targets and possible involvement of tissue-specific responses and host immunity, which cannot be depicted in vitro culture systems. Thus, in vivo models for human filariae are needed and rodent models using immunocompromised animals have been established for Loa loa, O. volvulus and Brugia spp. (26–30, 46), but not for Mansonella spp.
Therefore, we aim to establish a murine model of M. perstans based on the knowledge of the established animal models of onchocerciasis, lymphatic filariasis and loiasis. Immunocompromised mouse strains that lack adaptive immunity pathways like RAG2-/- (27, 28), IL-4/5-/- (32) and NSG strains (26, 36) have been proven suitable for human filarial infections and recently, NSG mice have been also proven to be susceptible to infection with the dog heartworm Dirofilaria immits (47, 48). Indeed, a 62-66% L3 recovery rate could be obtained upon Loa loa L3 inoculation, whereas M. perstans and O. volvulus L3 were absent in all immunocompromised mouse strains. This was independent of the route of administration (i.p. or s.c.), gender of the mice, or day of analysis (day 2-63 p.i.). In addition, subcutaneous implantation of 50 M. perstans L3/mouse was performed in 3 NSG BALB/c mice, but also no L3 could be recovered upon 2-7 days p.i. Moreover, inhibition of immune responses by immune suppressants, which have been shown to promote Loa loa survival in BALB/c mice (4), did not increase susceptibility to M. perstans or O. volvulus L3. Indeed, previous studies already revealed that several immunologically intact mouse strains were not susceptible to O. volvulus L3 (49) and only transplantation of adult worms into SCID mice allowed worm survival for more than 20 weeks (36). It seemed that O. volvulus L3 can only survive in chimpanzees or mangabey monkeys (50–54), but recently it has been shown that NSG mice humanized with human immune cells allowed survival and maturation of O. volvulus L3 into L4 within 12 weeks of infection (26). Of note, low recovery rates (1.7-2.3%) of O. volvulus parasites have been also observed 4-8 weeks upon infection in non-humanized NSG mice (26), which could not be confirmed here. This might be because of the different analysis time points (2-7 days p.i. vs 4-8 weeks p.i.) and applied recovery methods, which do not include qPCR approaches and overnight incubation of muscle, skin and organs into RPMI. We suggest that increased incubation time and application of molecular approaches will increase the L3 recovery rate and detection sensitivity of the parasites, respectively and thus should be applied in future experiments. Nevertheless, parasite recovery rates increased 3-4 times when NSG mice were humanized with multiple human cell lines (26). Indeed, the addition of feeder cells that provide nutrition and support development and survival through the secretion of different factors has been proven to be important for the growth, survival and development of filariae in vitro (18–25, 44, 45). Humanized rodent models open up a novel perspective to establish human filarial infections not only to screen for novel drugs but also to investigate human immune cells and their immune responses towards the parasite in vivo, which cannot be compiled with in situ and in vitro restimulation experiments (55). Nevertheless, the question remains why Loa loa L3 infections can be established in the different immunocompromised mouse strains using the applied methods that were based on previously published studies (5, 23, 28, 33, 55), whereas M. perstans and O. volvulus infection were not successful. We doubt that the applied medium including antibiotics that have been used for the L3 isolation might be the cause of this phenomenon, since this medium has been also used for the successful Loa loa infection experiments and long-term M. perstans L3 in vitro cultures (18–20), showing that the medium does not interfere with the viability of the isolated L3. One explanation could be that Loa loa does not harbor Wolbachia endosymbionts (56, 57), which activate innate and adaptive immune responses and inflammatory pathways (58, 59) and thus can limit infection efficacy (60). Although the used immunocompromised mice lack important adaptive immune cells and signalling pathways, innate immune responses can be initiated by the Wolbachia endosymbionts from M. perstans (61) and O. volvulus (62) leading to the observed resistance of the mouse strains towards these filarial nematodes. In addition, filarial infections need human metabolites, cells or tissue for development and indeed, it has been shown that threonine transport from the human host is essential for O. volvulus but not for Loa loa, which can produce its own threonine (63). To overcome these obstacles humanized immunocompromised mouse model might fill the gap of the missing human factors in rodents and promising results with O. volvulus L3 inoculation in humanized SCID mice (26) highlight that future studies should use humanized NSG mice to establish a mouse model of M. perstans and O. volvulus. In addition, Mongolian gerbils (Meriones unguiculatus) are susceptible to the human filarial nematode Brugia malayi (64) and thus might be also considered as an alternative for future infection experiments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by National Institutional Review board, Yaoundé (REF: N° 2022/12/1506/CE/CNERSH/SP) and administrative clearance from the Delegation of Public Health, South West Region (Re: R11/MINSANTE/SWR/RDPH/PS/259/382). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by German animal protection laws and EU guidelines 2010/63/E4 and Cameroonian animal protection laws. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
VC: Investigation, Writing – original draft, Formal analysis, Methodology. FF: Investigation, Methodology, Writing – review & editing. CK: Investigation, Methodology, Writing – review & editing. REb: Investigation, Methodology, Writing – review & editing. FE: Investigation, Methodology, Writing – review & editing. AN: Investigation, Methodology, Writing – review & editing. EO: Investigation, Methodology, Writing – review & editing. NG: Formal analysis, Investigation, Methodology, Writing – review & editing. REk: Investigation, Methodology, Writing – review & editing. FN: Investigation, Methodology, Writing – review & editing. LN: Investigation, Methodology, Writing – review & editing. CM: Investigation, Methodology, Writing – review & editing. AN: Formal analysis, Investigation, Methodology, Writing – original draft. PE: Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. AH: Supervision, Writing – review & editing. SW: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. MR: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing..
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is funded through a grant awarded to SW and MR from the Deutsche Forschungsgemeinschaft (DFG) within the “African-German Cooperation Projects in Infectiology” (RI 3036/1-1). AH is additionally supported by the DFG under Germany’s Excellence Strategy – EXC2151 – 390873048 and SW is the Senior Fellow Plus of the European Developing Clinical Trial Partnership (EDCTP2).
Acknowledgments
The authors would like to thank all the consented microflaremic donors who took part in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ritter M, Hoerauf A, Hubner MP. Human filariasis. In: Encyclopedia of infection and immunity, vol. 2. Amsterdam, Netherlands: Elsevier (2021). p. 602–21. doi: 10.1016/B978-0-12-818731-9.00192-0
2. Portela CS, Mendes de Araújo CP, Moura Sousa P, Gomes Simão CL, Silva de Oliveira JC, Crainey JL. Filarial disease in the Brazilian Amazon and emerging opportunities for treatment and control. Curr Res Parasitol Vector Borne Dis. (2023) 5:100168. doi: 10.1016/j.crpvbd.2023.100168
3. Wanji S, Tayong DB, Ebai R, Opoku V, Kien CA, Ndongmo WPC, et al. Update on the biology and ecology of Culicoides species in the South-West region of Cameroon with implications on the transmission of Mansonella perstans. Parasit Vectors. (2019) 12:166. doi: 10.1186/s13071-019-3432-9
4. Ebai R, Kien CA, Fombad FF, Esofi F, Ouam E, Ntuh AN, et al. Culicoides species of the Rain Forest Belt of the Littoral Region of Cameroon: Their Incrimination in the Transmission of Mansonella perstans. Pathogens. (2024) 13:146. doi: 10.3390/pathogens13020146
5. Wanji S, Tendongfor N, Esum ME, Enyong P. Chrysops silacea biting densities and transmission potential in an endemic area of human loiasis in south-West Cameroon. Trop Med Int Health. (2002) 7:371–7. doi: 10.1046/j.1365-3156.2002.00845.x
6. Duke BO. The population dynamics of Onchocerca volvulus in the human host. Trop Med Parasitol. (1993) 44:61–8.
7. Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. (2011) 120:S109–20. doi: 10.1016/j.actatropica.2010.01.014
8. Padgett JJ, Jacobsen KH. Loiasis: African eye worm. Trans R Soc Trop Med Hyg. (2008) 102:983–9. doi: 10.1016/j.trstmh.2008.03.022
9. Schulz-Key H. Observations on the reproductive biology of Onchocerca volvulus. Acta Leiden. (1990) 59:27–44.
10. Hoerauf A, Büttner DW, Adjei O, Pearlman E. Onchocerciasis. BMJ. (2003) 326:207–10. doi: 10.1136/bmj.326.7382.207
12. Murdoch ME, Hay RJ, Mackenzie CD, Williams JF, Ghalib HW, Cousens S, et al. A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br J Dermatol. (1993) 129:260–9. doi: 10.1111/j.1365-2133.1993.tb11844.x
13. Abiose A. Onchocercal eye disease and the impact of Mectizan treatment. Ann Trop Med Parasitol. (1998) 92 Suppl 1:S11–22. doi: 10.1080/00034989859519
14. Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis—Beyond Th1 vs. Th2. Trends Parasitol. (2002) 18:25–31. doi: 10.1016/s1471-4922(01)02173-0
15. Ritter M, Ndongmo WPC, Njouendou AJ, Nghochuzie NN, Nchang LC, Tayong DB, et al. Mansonella perstans microfilaremic individuals are characterized by enhanced type 2 helper T and regulatory T and B cell subsets and dampened systemic innate and adaptive immune responses. PloS Negl Trop Dis. (2018) 12:e0006184. doi: 10.1371/journal.pntd.0006184
16. Ricciardi A, Nutman TB. IL-10 and its related superfamily members IL-19 and IL-24 provide parallel/redundant immune-modulation in Loa loa infection. J Infect Dis. (2021) 223:297–305. doi: 10.1093/infdis/jiaa347
17. Risch F, Ritter M, Hoerauf A, Hübner MP. Human filariasis-contributions of the Litomosoides sigmodontis and Acanthocheilonema viteae animal model. Parasitol Res. (2021) 120:4125–43. doi: 10.1007/s00436-020-07026-2
18. Njouendou AJ, Ritter M, Ndongmo WPC, Kien CA, Narcisse GTV, Fombad FF, et al. Successful long-term maintenance of Mansonella perstans in an in vitro culture system. Parasit Vectors. (2017) 10:563. doi: 10.1186/s13071-017-2515-8
19. Njouendou AJ, Kien CA, Esum ME, Ritter M, Chounna Ndongmo WP, Fombad FF, et al. In vitro maintenance of Mansonella perstans microfilariae and its relevance for drug screening. Exp Parasitol. (2019) 206:107769. doi: 10.1016/j.exppara.2019.107769
20. Njouendou AJ, Ritter M, Kien CA, Esum ME, Ndongmo WPC, Fombad FF, et al. Dataset on in vitro maintenance of Mansonella perstans microfilariae and drug testing. Data Brief. (2019) 28:104930. doi: 10.1016/j.dib.2019.104930
21. Njouendou AJ, Fombad FF, O'Neill M, Zofou D, Nutting C, Ndongmo PC, et al. Heterogeneity in the in vitro susceptibility of Loa loa microfilariae to drugs commonly used in parasitological infections. Parasit Vectors. (2018) 11:223. doi: 10.1186/s13071-018-2799-3
22. Zofou D, Fombad FF, Gandjui NVT, Njouendou AJ, Kengne-Ouafo AJ, Chounna Ndongmo PW, et al. Evaluation of in vitro culture systems for the maintenance of microfilariae and infective larvae of Loa loa. Parasit Vectors. (2018) 11:275. doi: 10.1186/s13071-018-2852-2
23. Fombad FF, Njouendou AJ, Ndongmo PC, Ritter M, Chunda VC, Metuge HM, et al. Effect of flubendazole on developing stages of Loa loa in vitro and in vivo: A new approach for screening filaricidal agents. Parasit Vectors. (2019) 2:14. doi: 10.1186/s13071-018-3282-x
24. Malkmus C, Jawahar S, Tricoche N, Lustigman S, Hansmann J. Preliminary evaluations of 3-dimensional human skin models for their ability to facilitate in vitro the long-term development of the debilitating obligatory human parasite Onchocerca volvulus. PloS Negl Trop Dis. (2020) 14:e0008503. doi: 10.1371/journal.pntd.0008503
25. Gandjui NVT, Njouendou AJ, Gemeg EN, Fombad FF, Ritter M, Kien CA, et al. Establishment of an in vitro culture system to study the developmental biology of Onchocerca volvulus with implications for anti-Onchocerca drug discovery and screening. PloS Negl Trop Dis. (2021) 15:e0008513. doi: 10.1371/journal.pntd.0008513
26. Patton JB, Bennuru S, Eberhard ML, Hess JA, Torigian A, Lustigman S, et al. Development of Onchocerca volvulus in humanized NSG mice and detection of parasite biomarkers in urine and serum. PloS Negl Trop Dis. (2018) 12:e0006977. doi: 10.1371/journal.pntd.0006977
27. Pionnier NP, Sjoberg H, Chunda VC, Fombad FF, Chounna PW, Njouendou AJ, et al. Mouse models of Loa loa. Nat Commun. (2019) 10:1429. doi: 10.1038/s41467-019-09442-0
28. Ndzeshang LB, Fombad FF, Njouendou AJ, Chunda VC, Gandjui NVT, Akumtoh DN, et al. Generation of Loa loa infective larvae by experimental infection of the vector, Chrysops silacea. PloS Negl Trop Dis. (2020) 14:e0008415. doi: 10.1371/journal.pntd.0008415
29. Wanji S, Chunda VC, Fombad FF, Njouendou AJ, Gandjui NVT, Ritter M, et al. Advances in preclinical platforms of Loa loa for filarial neglected tropical disease drug and diagnostics research. Front Trop Dis. (2021) 2:778724. doi: 10.3389/fitd.2021.778724
30. Ayiseh RB, Mbah GE, Monya E, Ndi EM, Sakanari J, Lustigman S, et al. Development and validation of small animal models for onchocerciasis and loiasis microfilaricide discovery. PloS Negl Trop Dis. (2023) 17:e0011135. doi: 10.1371/journal.pntd.0011135
31. Layland LE, Ajendra J, Ritter M, Wiszniewsky A, Hoerauf A, Hübner MP. Development of patent Litomosoides sigmodontis infections in semi-susceptible C57BL/6 mice in the absence of adaptive immune responses. Parasit Vectors. (2015) 8:396. doi: 10.1186/s13071-015-1011-2
32. Wiszniewsky A, Layland LE, Arndts K, Wadephul LM, Tamadaho RSE, Borrero-Wolff D, et al. Adoptive transfer of immune cells into RAG2IL-2Rγ-deficient mice during litomosoides sigmodontis infection: A novel approach to investigate filarial-specific immune responses. Front Immunol. (2021) 12:777860. doi: 10.3389/fimmu.2021.777860
33. Tendongfor N, Wanji S, Ngwa JC, Esum ME, Specht S, Enyong P, et al. The human parasite Loa loa in cytokine and cytokine receptor gene knock out BALB/c mice: survival, development and localization. Parasit Vectors. (2012) 5:43. doi: 10.1186/1756-3305-5-43
34. Ritter M, Tamadaho RS, Feid J, Vogel W, Wiszniewsky K, Perner S, et al. IL-4/5 signalling plays an important role during Litomosoides sigmodontis infection, influencing both immune system regulation and tissue pathology in the thoracic cavity. Int J Parasitol. (2017) 47:951–60. doi: 10.1016/j.ijpara.2017.06.009
35. Frohberger SJ, Ajendra J, Surendar J, Stamminger W, Ehrens A, Buerfent BC, et al. Susceptibility to L. sigmodontis infection is highest in animals lacking IL-4R/IL-5 compared to single knockouts of IL-4R, IL-5 or eosinophils. Parasit Vectors. (2019) 12:248. doi: 10.1186/s13071-019-3502-z
36. Rajan TV, Nelson FK, Cupp E, Schultz LD, Greiner DL. Survival of Onchocerca volvulus in nodules implanted in immunodeficient rodents. J Parasitol. (1992) 78:160–3. doi: 10.2307/3283709
37. Klion AD, Massougbodji A, Horton J, Ekoue S, Lanmasso T, Ahouissou NL, et al. Albendazole in human loiasis: result of a double-blind, placebo-controlled trial. J Infect Dis. (1993) 168:202–6. doi: 10.1093/infdis/168.1.202
38. Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. (2016) 78:661–71. doi: 10.1007/s00280-016-3152-1
39. Stackowicz J, Jönsson F, Reber LL. Mouse models and tools for the in vivo study of neutrophils. Front Immunol. (2020) 10:3130. doi: 10.3389/fimmu.2019.03130
40. Petit G, Diagne M, Maréchal P, Owen D, Taylor D, Bain O. Maturation of the filaria Litomosoides sigmodontis in BALB/c mice; comparative susceptibility of nine other inbred strains. Ann Parasitol Hum Comp. (1992) 67:144–50. doi: 10.1051/parasite/1992675144
41. Hübner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus). Exp Parasitol. (2009) 123:95–8. doi: 10.1016/j.exppara.2009.05.009
42. Karunakaran I, Ritter M, Pfarr K, Klarmann-Schulz U, Debrah AY, Debrah LB, et al. Filariasis research –from basic research to drug development and novel diagnostics, over a decade of research at the Institute for Medical Microbiology, Immunology and Parasitology, Bonn, Germany. Front Trop Dis. (2023) 4:1126173. doi: 10.3389/fitd.2023.1126173
43. Riberu WA, Atmosoedjono S, Purnomo, Tirtokusumo S, Bangs MJ, Baird JK. Cultivation of sexually mature Brugia malayi in vitro. Am J Trop Med Hyg. (1990) 43:3–5. doi: 10.4269/ajtmh.1990.43.3
44. Falcone FH, Zahner H, Schlaak M, Haas H. In vitro cultivation of third-stage larvae of Brugia malayi to the young adult stage. Trop Med Parasitol. (1995) 46:230–4.
45. Voronin D, Tricoche N, Jawahar S, Shlossman M, Bulman CA, Fischer C, et al. Development of a preliminary in vitro drug screening assay based on a newly established culturing system for pre-adult fifth-stage Onchocerca volvulus worms. PloS Negl Trop Dis. (2019) 13:e0007108. doi: 10.1371/journal.pntd.0007108
46. Nelson FK, Greiner DL, Shultz LD, Rajan TV. The immunodeficient scid mouse as a model for human lymphatic filariasis. J Exp Med. (1991) 173:659–63. doi: 10.1084/jem.173.3.659
47. Hess JA, Eberhard ML, Kracher B, Shryock J, Harrington J, Abraham D. A rodent model for Dirofilaria immitis, canine heartworm: parasite growth, development, and drug sensitivity in NSG mice. Sci Rep. (2023) 13:976. doi: 10.1038/s41598-023-27537-z
48. Marriott AE, Dagley JL, Hegde S, Steven A, Fricks C, DiCosty U, et al. Dirofilariasis mouse models for heartworm preclinical research. Front Microbiol. (2023) 14:1208301. doi: 10.3389/fmicb.2023.1208301
49. Kozek WJ, Figueroa Marroquin H. Attempts to establish Onchocerca volvulus infection in primates and small laboratory animals. Acta Trop. (1982) 39:317–24.
50. Duke BO. Observations on Onchocerca volvulus in experimentally infected chimpanzees. Tropenmed Parasitol. (1980) 31:41–54.
51. Greene BM. Primate model for onchocerciasis research. Ciba Found Symp. (1987) 127:236–43. doi: 10.1002/9780470513446.ch16
52. Eberhard ML, Dickerson JW, Boyer AE, Tsang VC, Zea-Flores R, Walker EM, et al. Experimental Onchocerca volvulus infections in mangabey monkeys (Cercocebus atys) compared to infections in humans and chimpanzees (Pan troglodytes). Am J Trop Med Hyg. (1991) 44:151–60. doi: 10.4269/ajtmh.1991.44.151
53. Soboslay PT, Dreweck CM, Taylor HR, Brotman B, Wenk P, Greene BM, et al. Experimental Onchocerciasis in chimpanzees. Cell-mediated immune responses, and production and effects of IL-1 and IL-2 with Onchocerca volvulus infection. J Immunol. (1991) 147:346–53. doi: 10.4049/jimmunol.147.1.346
54. Soboslay PT, Weiss N, Dreweck CM, Taylor HR, Brotman B, Schulz-Key H, et al. Experimental Onchocerciasis in chimpanzees. Antibody response and antigen recognition after primary infection with Onchocerca volvulus. Exp Parasitol. (1992) 74:367–80. doi: 10.1016/0014-4894(92)90199-K
55. Chunda VC, Ritter M, Bate A, Gandjui NVT, Esum ME, Fombad FF, et al. Comparison of immune responses to Loa loa stage-specific antigen extracts in Loa loa-exposed BALB/c mice upon clearance of infection. Parasit Vectors. (2020) 13:51. doi: 10.1186/s13071-020-3921-x
56. McGarry HF, Pfarr K, Egerton G, Hoerauf A, Akue JP, Enyong P, et al. Evidence against Wolbachia symbiosis in Loa loa. Filaria J. (2003) 2:9. doi: 10.1186/1475-2883-2-9
57. Büttner DW, Wanji S, Bazzocchi C, Bain O, Fischer P. Obligatory symbiotic Wolbachia endobacteria are absent from Loa loa. Filaria J. (2003) 2:10. doi: 10.1186/1475-2883-2-10
58. Hise AG, Daehnel K, Gillette-Ferguson I, Cho E, McGarry HF, Taylor MJ, et al. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J Immunol. (2007) 178:1068–76. doi: 10.4049/jimmunol.178.2.1068
59. Turner JD, Langley RS, Johnston KL, Gentil K, Ford L, Wu B, et al. Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J Biol Chem. (2009) 284:22364–78. doi: 10.1074/jbc.M901528200
60. Zhang D, Wang Y, He K, Yang Q, Gong M, Ji M, et al. Wolbachia limits pathogen infections through induction of host innate immune responses. PloS One. (2020) 15:e0226736. doi: 10.1371/journal.pone.0226736
61. Keiser PB, Coulibaly Y, Kubofcik J, Diallo AA, Klion AD, Traoré SF, et al. Molecular identification of Wolbachia from the filarial nematode Mansonella perstans. Mol Biochem Parasitol. (2008) 160:123–8. doi: 10.1016/j.molbiopara.2008.04.012
62. Kozek WJ, Marroquin HF. Intracytoplasmic bacteria in Onchocerca volvulus. Am J Trop Med Hyg. (1977) 26:663–78. doi: 10.4269/ajtmh.1977.26.663
63. Cotton JA, Bennuru S, Grote A, Harsha B, Tracey A, Beech R, et al. The genome of Onchocerca volvulus, agent of river blindness. Nat Microbiol. (2016) 2:16216. doi: 10.1038/nmicrobiol.2016.216
Keywords: Mansonella perstans, Loa loa, Onchocerca volvulus, murine models of human filariasis, immunocompromised mice
Citation: Chunda VC, Fombad FF, Kien CA, Ebai R, Esofi F, Ntuh AN, Ouam E, Gandjui NVT, Ekanya R, Nietcho F, Nchang LC, Magha C, Njouendou AJ, Enyong P, Hoerauf A, Wanji S and Ritter M (2024) Comparative development of human filariae Loa loa, Onchocerca volvulus and Mansonella perstans in immunocompromised mouse strains. Front. Trop. Dis 5:1293632. doi: 10.3389/fitd.2024.1293632
Received: 13 September 2023; Accepted: 25 March 2024;
Published: 15 April 2024.
Edited by:
Kaio Cesar Chaboli Alevi, Sao Paulo State University, BrazilReviewed by:
James Lee Crainey, Oswaldo Cruz Foundation (Fiocruz), BrazilNicolas Pionnier, Manchester Metropolitan University, United Kingdom
Copyright © 2024 Chunda, Fombad, Kien, Ebai, Esofi, Ntuh, Ouam, Gandjui, Ekanya, Nietcho, Nchang, Magha, Njouendou, Enyong, Hoerauf, Wanji and Ritter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel Ritter, manuel.ritter@ukbonn.de
†These authors share last authorship
 Valerine C. Chunda
Valerine C. Chunda Fanny Fri Fombad
Fanny Fri Fombad Chi Anizette Kien
Chi Anizette Kien Rene Ebai
Rene Ebai Anna Ning Ntuh
Anna Ning Ntuh Narcisse Victor Tchamatchoua Gandjui
Narcisse Victor Tchamatchoua Gandjui Franck Nietcho
Franck Nietcho Lucy Cho Nchang
Lucy Cho Nchang Abdel Jelil Njouendou
Abdel Jelil Njouendou Samuel Wanji
Samuel Wanji Manuel Ritter
Manuel Ritter