- 1Center for Academic Global Ophthalmology, Wills Eye Hospital, Philadelphia, PA, United States
- 2Truhlsen Eye Institute, University of Nebraska Medical Center, Omaha, NE, United States
- 3Global Center for Health Security, University of Nebraska Medical Center, Omaha, NE, United States
- 4Department of Ophthalmology, Emory University School of Medicine, Atlanta, GA, United States
Viral hemorrhagic fevers (VHFs) are a diverse group of RNA virus-mediated systemic diseases with significant morbidity and mortality and represent a significant public health concern. Given the high systemic morbidity and mortality in a number of these entities, delays in diagnosis can lead to downstream public health consequences. Many viral hemorrhagic fevers have ophthalmic manifestations and ophthalmologists thus play a key role in disease recognition and the management of ocular complications associated with specific hemorrhagic fevers. This review summarizes the key ophthalmic consequences of viral hemorrhagic fevers, viral disease pathogenesis, disease findings, and areas of unmet research need.
Introduction
Viral hemorrhagic fevers (VHFs) are a diverse group of RNA virus-mediated systemic diseases with significant morbidity and mortality and represent a significant public health concern. Symptoms of disease can vary widely from mild flu-like illness to severe multi-organ dysfunction caused by fever, vascular permeability, decreased plasma volume, coagulation abnormalities, and varying degrees of hemorrhage (1). Given the high systemic morbidity and mortality in a number of these entities, delays in diagnosis from unusual clinical presentations and differing levels of community and regional laboratory preparedness may lead to delayed outbreak response, infectious disease transmission, and downstream public health consequences.
Many viral hemorrhagic fevers have ophthalmic manifestations which can include self-limited conditions such as conjunctival injection/ocular surface disease as well as conditions causing permanent vision loss such as ocular inflammation. Ophthalmologists thus play a role in disease recognition and the management of ocular surface disease, ocular inflammation and retinal pathologies that may lead to long-term visual complications associated with specific hemorrhagic fevers.
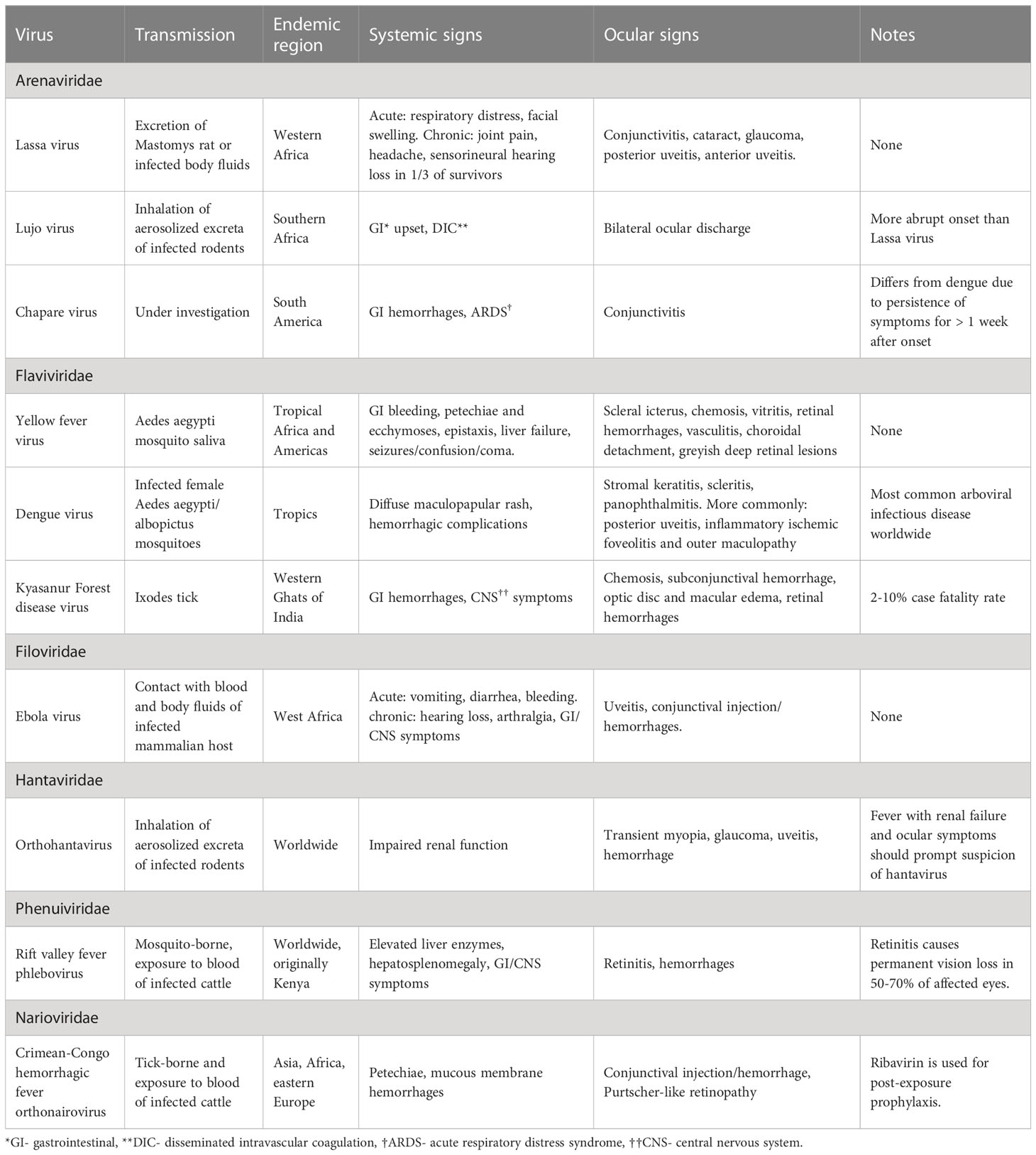
This review synthesizes existing literature regarding ophthalmic manifestations and pathophysiology of VHFs organized by taxonomic family and including Arenaviridae (Lassa fever, Lujo hemorrhagic fever, Chapare Hemorrhagic fever), Flaviviridae (Kyasanur Forest disease, Dengue fever, Yellow fever), Filoviridae (Ebola virus disease), Hantaviridae (Orthohantavirus), Nairoviridae (Crimean-Congo hemorrhagic fever) and Phenuiviridae (Rift Valley fever). A summary of the general considerations, systemic and ophthalmic features of these viruses can be found on Table 1.
Methods
We performed an online PubMed search for the articles cited in this review. Search terms related to viral hemorrhagic fever were combined with terms related to clinical disease, ophthalmic manifestations, treatment and pathophysiology. Ophthalmic search terms used included the following: ophthalmic findings, ophthalmology, eye, ocular, retina, uveitis, conjunctiva, cornea, and retinopathy. This review summarizes the key ophthalmic consequences of viral hemorrhagic fevers, viral disease pathogenesis, disease findings, and areas of unmet research need
Arenaviridae
Lassa fever
Lassa fever (LF) is an acute hemorrhagic illness caused by Lassa virus (LASV), an Arenavirus endemic to the West African countries of Benin, Ghana, Liberia, Guinea, Mali, Sierra Leone, and Nigeria (1). Humans become infected by LASV through exposure to excretions of the Mastomys rat species or direct contact with bodily fluids of another infected human (1). Up to 80% of those infected present asymptomatically or with mild symptoms, which may complicate the diagnostic process (2). A smaller subset of patients develops acute disease with more severe symptoms, including hemorrhage, respiratory distress, and facial swelling (1, 3, 4).
Ocular involvement in patients with acute LF may manifest as conjunctivitis and conjunctival edema (5). There are a few reported cases of transient blindness experienced during convalescence (6). In a cohort of LF survivors in Sierra Leone, visual acuity was worse among LF patients with ophthalmic findings suggestive of anterior or posterior segment disease. These pathologies included cataract, glaucoma, and findings suggestive of previous episodes of uveitis, such as chorioretinal scarring, retinal fibrosis, and vitreous opacity (7, 8). Histological examination of eyes from guinea pigs 2-3 weeks after being infected with LASV showed staining for LASV glycoprotein 2 most prominent in the anterior uvea, mainly in the filtration angle, ciliary body, iris, vessels of the bulbar conjunctiva, and peripheral cornea. Among animals that survived infection, no LASV staining was seen six weeks after infection, however the potential for virus persistence in the human eye is not understood (9). Long-term systemic sequelae of LF include joint pain, hearing loss and headache but further studies are needed to evaluate long-term ophthalmic manifestations (7).
Several areas require further investigation to improve our understanding of disease pathogenesis, facilitate the appropriate response to outbreaks, and optimize disease surveillance. Longitudinal studies may help determine the association between LF and long-term effects on vision, although current findings have shown minimal effects (10). In addition, LF survivors should continue to undergo careful observation for ophthalmic findings. From a public health standpoint, areas of interest include expediting recognition of distinctive disease features, initiating early treatment, and targeted responses towards high-risk contacts (11). Presently, there are no approved vaccines for LASV, and the only treatment, ribavirin is controversial; requiring use early in the disease course to be associated with improved outcomes (12).
Lujo hemorrhagic fever
Lujo virus (LUJV) is known to cause Lujo hemorrhagic fever (LUHF). LUJV was discovered in 2008 following a nosocomial outbreak of a LUHF in Johannesburg, South Africa, which had an alarmingly high case-fatality rate of 80% (13).
Clinical features of viral hemorrhagic fever caused by LUJV include initial flu-like symptoms that progress to gastrointestinal involvement and high rates of disseminated intravascular coagulation (DIC) (14, 15). Use of a strain 13/N guinea pig model infected with LUJV demonstrated development of disseminated infection in various organs that eventually led to uniform lethality 11-16 days post-infection (16). In this model, the authors observed an incubation period of 5 to 6 days from the time of inoculation to the first clinical signs of illness (fever and weight loss). Over the next 24 to 48 h, the animals began to display signs of progressive illness (bilateral ocular discharge, continued fever, weight loss, and dehydration) until they were found dead or humanely euthanized when moribund. By day 5 post infection, significant hematological changes began to occur, including hypoproteinemia, thrombocytopenia, and lymphopenia. During the 2008 outbreak of LUHF in Johannesburg, South Africa, three out of the five patients developed subconjunctival injection and hemorrhage within the first week of infection, otherwise no other ophthalmic consequences were noted (16).
A potential area of interest for future research includes establishment of a standardized animal LUJV model to better understand pathogenesis and to work towards developing vaccines and antiviral drugs. A more detailed funduscopic ophthalmic examination of infected patients would also be beneficial to determine whether LUHF can cause posterior segment findings.
Chapare hemorrhagic fever
Chapare virus (CHAPV) is known to cause Chapare hemorrhagic fever (CHHF). There is a low number of documented CHHF cases, which limits understanding of symptom progression and stages of this illness. Infectivity is hypothesized to result from a zoonotic reservoir (potentially O. microtis, although further investigation is needed) or human-to-human contact (17). Discovery of the virus was associated with a small outbreak of severe hemorrhagic fever in Bolivia in 2003 (18). Another series of CHAPV infection reported in Bolivia occurred in 2019 and had a significant case fatality rate of 60% (19).
CHAPV RNA was detected using RT-PCR in conjunctiva, along with other human body fluid samples, including blood, urine, nasopharyngeal, oropharyngeal, and semen (17). No specific ophthalmic symptoms associated with CHHF have been reported. Reported symptoms of CHAPV include fever, upper gastrointestinal hemorrhage and acute respiratory distress syndrome (ARDS) (19, 20). Although the presentation of CHHF may be similar to other endemic agents such as dengue and Bolivian hemorrhagic fever in the absence of molecular testing, a defining feature of CHHF may be continuation of fever, myalgia, weakness, or neurologic symptoms with hemorrhagic manifestations persisting more than seven days after symptom onset (17). The specific mechanism of CHAPV pathogenesis is not clearly defined, although postulated mechanisms of arenavirus infectivity include factors that increase viral replication capacity and suppression of the host’s innate immunity leading to severe hemorrhagic disease (21).
Further data on the degree of viral persistence is necessary. Given the low numbers of patients and lack of understanding of pathogenesis, a critical unmet research need is an animal model to serve as a proxy for human disease in the absence of human data. Furthermore, it may be useful to examine the long-term sequelae of CHHF, as persistence of ocular, neurologic, and auditory symptoms have been observed in individuals who recovered from other viral hemorrhagic illnesses (17).
Flaviviridae
Yellow fever
Yellow fever virus (YFV) causes yellow fever (YF) which is endemic to tropical regions of Africa and the Americas. It is a mosquito-borne illness with mortality rates of 20%-50% (22). Transmission of the virus to humans occurs in 3 cycles: sylvatic, intermediate, and urban. The most concerning of these is the urban cycle which is initiated when YFV carried in the saliva of Aedes aegypti mosquitoes is introduced into areas with high human population density and limited vaccine coverage resulting in large-scale epidemics. The clinical course of the disease starts with an incubation period of 3-7 days, with most patients having a mild flu-like illness and about 15% of cases have severe symptoms including chills, low back pain, headache, and fever. Next is a period of remission for 24-48 hours which may then be followed by a return of symptoms and marked intoxication, prostration, dehydration, petechiae, ecchymoses, epistaxis and the characteristic “black vomit” (gastrointestinal bleeding) (23). Yellow fever is distinguished from other VHFs by the characteristic severity of liver damage and eventual jaundice. Late central nervous system manifestations, such as confusion, seizure, and coma, presage death, which typically follows within 7 to 10 days of onset (22).
Ocular manifestations include conjunctival icterus and chemosis, vitritis, retinopathy (superficial and deep hemorrhages, macular exudates, retinal vasculitis, retinal venous congestion and choroidal detachments) (23–27). Studies of two recent outbreaks of yellow fever in Minas Gerais, Brazil (2016 and 2017) allowed for a characterization of retinal changes associated with the disease (24). Twenty percent of patients with confirmed yellow fever had evidence of retinopathy. Elevated aspartate aminotransferase (AST) levels, elevated total bilirubin levels, and renal failure were associated with higher levels of retinopathy. The most common fundus changes were retinal nerve fiber layer (RNFL) infarcts (55%), superficial hemorrhages (35%), and grayish deep lesions that appeared to be at the level of the outer retina or choroid (30%). The superficial retinal microangiopathic changes are associated with occlusion of RNFL precapillary arterioles due to immune-mediated endothelial damage and coagulopathy. Although the pathophysiology of the grayish deep lesions is unknown, similar lesions have been described during the convalescent stage of Ebola virus disease, suggestive of an immunologic mechanism.
Although live virions have been isolated from bodily fluids, there has been no evidence of direct ocular infection of YFV (28). Further research is needed to determine a protocol for ophthalmic screening of patients with yellow fever and potential avenues of treatment for ophthalmic manifestations. Additionally, more research is needed to determine the ophthalmic sequelae in vaccinated populations/experimental models.
Dengue fever
Dengue Fever (DF) is caused by the Dengue virus which is a mosquito-borne flavivirus that is endemic to the tropics. There are four known serotypes of the virus, but currently no studies showing one is more prone to causing ophthalmic manifestations than another serotype. It is transmitted by infected female Aedes aegypti/albopictus mosquitoes and is the most common arboviral infectious disease worldwide. The incubation period usually lasts 3-14 days and the initial infection may either cause a mild febrile illness or may produce a distinctive sudden onset high fever, headache, myalgia/arthralgias, nausea, vomiting and a diffuse maculopapular rash. Although most infections are self-limited, a small portion of patients may develop the lethal Dengue hemorrhagic fever syndrome (DHF) or Dengue shock syndrome (29). Highlighting the need for early diagnosis, a survey of European tourists showed an estimated 1% to 3% were exposed to Dengue virus after traveling to a tropical region for 1 month (30).
Ocular manifestations of DF and DHF are common, bilateral and associated with thrombocytopenia, inflammation and ischemia. Adnexal involvement is rare and can include orbital inflammation and retrobulbar hemorrhage (31). Subconjunctival hemorrhages are common and self-limited. Cases of stromal keratitis, necrotizing scleritis (32), anterior uveitis and panophthalmitis are infrequent but have been described. Numerous posterior segment findings have been noted, including vitritis, retinal hemorrhage, retinal vasculitis (33), choroiditis (34), foveolitis, yellow subretinal lesions, optic neuritis and neuroretinitis (35). Fluorescein angiography can reveal vascular leakage and occlusion while indocyanine green angiography can show hypocyanescent spots corresponding to the yellow subretinal lesions and large areas of choroidal hypercyanescence. Ocular coherence tomography (OCT) is useful in detecting and monitoring dengue induced inflammatory ischemic foveolitis and outer maculopathy (DIII-FOM) as well as for diagnosing serous retinal detachments and macular edema. OCT angiography (OCTA) can show ischemia of the deep retinal capillary plexus (36). Laboratory diagnosis involves serological tests to detect Dengue virus structural protein 1 and IgG/IgM antibodies directed against Dengue virus (29).
Future studies linking the serotype of Dengue virus to ophthalmic manifestations might be important in screening these individuals as well as to avoid loss of follow up after resolution of acute systemic manifestations.
Kyasanur forest disease
Kyasanur Forest disease (KFD) or “monkey fever” is a tick-borne, arboviral illness endemic to the Western Ghats of India. Transmission is usually through tick bites and the disease is self-limited in around 80 percent of patients, but the remaining 20 percent will develop severe hemorrhagic complications with neurological involvement. Although KFD had been limited to a small region of India for the past 60 years, evidence over the last 5 years shows an increase in disease burden and geographic distribution within India, necessitating an increased awareness by health care workers and effective diagnostic testing (37).
After being bitten by an infected Ixodes petauristae/ceylonensis tick, an incubation period of 3-8 days is followed by a biphasic, or occasionally quadriphasic, course of illness. The first phase involves a sudden onset of fever, headache, generalized myalgias localized to the neck/upper and lower back and extremities, gastrointestinal symptoms, lethargy and occasionally lymphadenopathy/hepatosplenomegaly. Hemorrhagic complications usually start 3-4 days after the onset of symptoms. 20 percent of patients further develop a second phase of illness which is accented by neurological symptoms and fever. The case-fatality rate of KFD is 2-10 percent but fortunately survivors have rarely shown long term complications (23).
Ocular complications include conjunctival injection in almost all cases during the initial phase followed by chemosis and serous discharge in more than half of all patients. Subconjunctival hemorrhages are common and up to 13 percent of cases can involve optic disc edema, macular edema with exudates and retinal/vitreous hemorrhage (38).
Long term ophthalmic follow up studies need to be performed in the future as well as testing of ocular fluids to determine whether live virus can be isolated from previously exposed patients as this can be a potential source of transmission.
With the evidence of ocular involvement from YF, DF and KFD other flaviviruses should also be investigated for any ophthalmic consequences. For example, only one study has been published regarding ocular manifestations in Omsk hemorrhagic fever, which noted subconjunctival hemorrhages in the early phases and scleral injection in the later phases (38). Since the second phase of illness includes meningo-encephalitis, it seems probable that this phase could manifest with posterior segment inflammation or hemorrhage (39, 40). Similarly, Alkhurma hemorrhagic fever has been known to cause ocular symptoms including orbital pain, photophobia and conjunctival injection, but comprehensive ophthalmic exams in exposed patients would be useful to establish whether inflammatory changes are present within the eye and orbit, as photophobia and orbital pain usually indicate an inflammatory or uveitic process (41, 42).
Filoviridae
Ebola virus disease
Ebola virus disease (EVD) in humans can be caused by four different viruses each with varying levels of mortality including: Bundibugyo virus (BDBV), Sudan virus (SUDV), Taï Forest virus (TAFV) and Ebola virus (EBOV, formerly Zaire Ebola virus). Out of these four viruses, EBOV infection is associated with the highest number of outbreaks. EVD is an acute and serious illness that is rapidly progressive and fatal if left untreated (43). The unprecedented outbreak of EVD in West Africa from 2014-2016 that unfortunately affected over 28,600 patients and resulted in over 11,300 deaths in the countries of Liberia, Guinea, and Sierra Leone was declared a Public Health Emergency of International Concern by the World Health Organization. The public and global health threat in West Africa, recent sporadic outbreaks in the Democratic Republic of the Congo, and the ongoing EVD outbreak in Uganda are critical reminders of the paramount importance of outbreak preparedness throughout the world (44).
Initial symptoms of EVD include fever, myalgia, and fatigue that progress to vomiting, rash, diarrhea, and bleeding (8). Ophthalmic findings in the early stages of disease include conjunctival injection, conjunctivitis, and subconjunctival hemorrhage (8, 45). Uveitis is the most common complication reported to occur during EVD convalescence and can cause severe vision loss in 40% of those affected (46, 47). Other EVD-associated ocular complications leading to vision impairment include cataract, optic neuropathy, retinal scarring, hypotony, and phthisis bulbi (48). Ophthalmic sequelae such as color vision deficits, low intraocular pressure (i.e., hypotony), vitreous cells, macular scars, and uveitis were more likely to be seen in EVD survivors compared to non-infected close contacts (49).
Furthermore, survivors have reported symptoms of a post-Ebola virus disease syndrome including arthralgia, abdominal pain, neurologic complications, and hearing loss (48, 50). The pathogenesis of this persistent disease during convalescence is uncertain but may involve severe inflammation and tissue edema (47). Analysis of aqueous humor sample from an EVD survivor three months after recovery showed Ebola virus persistence, as well as five point mutations compared to an earlier sample, suggesting continued viral replication in the eye during convalescence (51). Hypotheses for the disease pathogenesis include a reaction similar to cytokine storm or the formation of auto-antibodies. An autoimmune process is believed to be involved in the development of post-Ebola virus disease syndrome (52).
Areas of future research may involve addressing gaps in understanding the mechanism of viral pathogenesis. Further investigation regarding the timing and mechanism of uveitis development, effective management strategies, and treatment with antivirals or anti-inflammatory medications may be necessary as well (50).
Hantaviridae
Orthohantavirus
Orthohantaviruses are zoonoses transmitted via inhalation of aerosolized excreta of infected rodents (53–55). The incubation period typically ranges from 10 to 14 days following which systemic infection can occur and lead to bleeding or shock manifestations (53). The common clinical pattern associated with orthohantavirus infection is hemorrhagic fever with renal syndrome (HFRS), which is characterized by acute onset of fever, headache, abdominal pain, nausea, diarrhea, impaired renal function, reduced vision, and hemorrhagic complications (54).
Ocular involvement is common in patients with HFRS with up to 87% experiencing reduced vision during the acute infection (54). Several ocular manifestations including transient myopia, acute glaucoma attacks, decrease in intraocular pressure (IOP), anterior uveitis, lid edema, conjunctival chemosis, conjunctival hemorrhages, retinal edema and hemorrhages, and pupillary defect have been reported in patients with HFRS (55). Transient myopia has been reported to occur from 41% to 78% of patients with HFRS (54, 56, 57). Shallowing of the anterior chamber is also common and occurs up to 93% of patients suffering from myopic shift (58). It was speculated that relaxation of lens zonules and anterior displacement of the lens might have explained the occurrence of transitory myopia and shallowing of the anterior chamber (56). Another proposed mechanism describes the changes of osmolarity of the aqueous humor and crystalline lens, and edema of ciliary body due to increased capillary leakage (58). Contradictory results of IOP have been reported in patients with HFRS with several cases reports showing acute glaucoma attacks and several studies showing a decline in IOP (53–64). The decrease in IOP may be explained by the diminished aqueous formation and filtration in the ciliary body due to damage of the capillary endothelial cells (55). Eyelid edema, conjunctival chemosis, conjunctival hyperemia, retinal edema, and retinal hemorrhages have also been reported in patients with HFRS, and these are likely related to the increased capillary permeability in these patients (54, 56, 57, 59, 64). Although rare, uveitis has been reported in a few patients with HFRS (57, 59). However, these cases were reported to have resolved without treatment.
Ocular symptoms are common and usually present during the acute phase of orthohantavirus infection. In the setting of pyrexia of unknown origin and unexplained renal failure, ocular symptoms should prompt the suspicion of orthohantavirus infection, especially in the endemic areas and potentially avert severe systemic manifestations if timely treatment is undertaken.
Future research can evaluate the temporal relationship between transient myopia and onset systemic symptom as this could be a potent screening tool in endemic regions. Further studies evaluating ocular fluids (aqueous, vitreous and tear samples) for live virus are needed to determine whether transmission can occur via exposure to ocular fluids.
Phenuiviridae
Rift valley fever
Rift valley fever (RVF), caused by Rift valley fever phlebovirus, is a mosquito-borne, viral hemorrhagic fever associated with significant morbidity and mortality. The first documented outbreak occurred in Kenya in 1930 but has since spread across Africa and the Middle East. Transmission occurs via contact with blood or organs of infected animals (typically cattle) or via mosquito bite. Following an incubation period of 2-6 days, a diverse array of systemic presentations of RVF can occur (65). The classical presentation is that of a generalized febrile/flu-like illness with hepatic syndrome (elevated liver enzymes, hepatosplenomegaly, liver failure). Other body systems that may be affected include the gastrointestinal (nausea/vomiting/diarrhea/epigastric pain), renal (renal failure, acute kidney injury), neurological (encephalitis, meningismus, asthenia, hallucinations, confusion), hemorrhagic (pallor, maculopapular rash, diffuse hemorrhages), obstetric (abortion/miscarriage), cardio-pulmonary (syncope, check pain, cough, myocarditis, pneumonia) and finally ophthalmic (66).
Ophthalmic findings have been documented previously and include posterior segment manifestations that may lead to vision loss. A cross-sectional study of the 2000 outbreak in Saudi Arabia described retinal hemorrhages (40%), vitreous reactions (26%), optic disc edema (15%), retinal vasculitis (7%), and anterior uveitis (31%) (66). Retinitis which has a predilection for the macula can lead to vascular occlusion and chorioretinal scarring causing permanent vision loss in 50-70% of affected eyes (10, 67).
Future directions of study include establishing the temporal relationship between the course of systemic illness and the onset of ophthalmic manifestations. Furthermore, ocular fluid sampling should be performed to determine whether live virus can be found and whether transmission can occur via contact with ocular fluids.
Nairoviridae
Crimean-Congo hemorrhagic fever
Crimean-Congo hemorrhagic fever (CCHF), caused by Crimean-Congo hemorrhagic fever orthonairovirus, is a tick-borne viral illness endemic to Asia, Africa and Eastern Europe. It is transmitted to humans via bite of infected ticks (generally Hyalomma spp) or exposure to blood/tissues of infected livestock or humans and has a fatality rate of 3-30% percent. After an incubation period of 1-9 days, symptoms consist of a febrile flu-like illness which later progress to a hemorrhagic stage with petechiae, hemoptysis and mucous membrane hemorrhages. Death can occur from multi-system failure or bleeding diathesis (68).
Ocular manifestations include conjunctival injection, subconjunctival and retinal hemorrhages and Purtscher- like retinopathy (68). One prospective study in children affected with CCHF noted increased retinal vascular tortuosity (69).
Although treatment is primarily supportive, ribavirin is commonly used in practice during outbreaks because it has been shown to reduce the spread of the virus, disease severity and mortality as a post-exposure prophylaxis (70). Future research can evaluate the onset of retinal vascular tortuosity to determine whether this can be used as a screening tool to detect early CCHF during the incubation phase.
Conclusion
Given recent viral hemorrhagic fever outbreaks with ophthalmic findings that may threaten vision, as well as implications related to viral persistence and immune response, understanding the eye remains an important aspect of disease surveillance. The acute and long-term implications of specific VHF syndromes (e.g., Rift Valley fever, Dengue fever, EVD) require further research, given their status as World Health Organization high priority pathogens with the potential for broad transmission.
Ophthalmologists have previously responded to prior outbreaks for eye disease detection and management, given the unique instrumentation required for anterior segment and posterior segment biomicroscopic examination and retinal imaging. Understanding the signs and symptoms, as well as rigorous clinical ophthalmic phenotyping in viral hemorrhagic fevers will provide useful information for public health workers as well as patients. Increased collaboration between different medical disciplines, as well as with public health officials will be needed to avert organ-specific damage and to deploy programs at scale where needed in the future.
Author contributions
SK and SY contributed to the design and outline of the review. SK wrote the first draft of the manuscript. YH and NN wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the National Eye Institute of the National Institutes of Health under award number R01 EY029594 (SY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported by the Macula Society Retina Research Foundation, ARVO Mallinckrodt Young Investigator Grant, and the Stanley M. Truhlsen Family Foundation, Inc.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Lassa fever (2017). Available at: https://www.who.int/news-room/fact-sheets/detail/lassa-fever.
2. Centers for Disease Control and Prevention. Lassa fever: Signs and symptoms (2014). Available at: https://www.cdc.gov/vhf/lassa/symptoms/index.html.
3. Bavinger JC, Shantha JG, Yeh S. Ebola, COVID-19, and emerging infectious disease: lessons learned and future preparedness. Curr Opin Ophthalmol (2020) 31:416–22. doi: 10.1097/ICU.0000000000000683
4. Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. Bmj (2003) 327:1271–5. doi: 10.1136/bmj.327.7426.1271
5. White HA. Lassa fever. a study of 23 hospital cases. Trans R Soc Trop Med Hyg (1972) 66:390–401. doi: 10.1016/0035-9203(72)90269-6
6. McCormick JB, King IJ, Webb PA, Johnson KM, O'Sullivan R, Smith ES, et al. A case-control study of the clinical diagnosis and course of lassa fever. J Infect Dis (1987) 155:445–55. doi: 10.1093/infdis/155.3.445
7. Li AL, Grant D, Gbakie M, Kanneh L, Mustafa I, Bond N, et al. Ophthalmic manifestations and vision impairment in lassa fever survivors. PloS One (2020) 15:e0243766. doi: 10.1371/journal.pone.0243766
8. Kuthyar S, Anthony CL, Fashina T, Yeh S, Shantha JG. World health organization high priority pathogens: Ophthalmic disease findings and vision health perspectives. Pathogens (2021) 10(4):442. doi: 10.3390/pathogens10040442
9. Gary JM, Welch SR, Ritter JM, Coleman-McCray J, Huynh T, Kainulainen MH, et al. Lassa virus targeting of anterior uvea and endothelium of cornea and conjunctiva in eye of Guinea pig model. Emerg Infect Dis (2019) 25:865–74. doi: 10.3201/eid2505.181254
10. Venkatesh A, Patel R, Goyal S, Rajaratnam T, Sharma A, Hossain P. Ocular manifestations of emerging viral diseases. Eye (Lond) (2021) 35:1117–39. doi: 10.1038/s41433-020-01376-y
11. Kofman A, Choi MJ, Rollin PE. Lassa fever in travelers from West Africa, 1969-2016. Emerg Infect Dis (2019) 25:245–8. doi: 10.3201/eid2502.180836
12. Asogun DA, Günther S, Akpede GO, Ihekweazu C, Zumla A. Lassa fever: Epidemiology, clinical features, diagnosis, management and prevention. Infect Dis Clin North Am (2019) 33(4):933–51. doi: 10.1016/j.idc.2019.08.002
13. Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, et al. Genetic detection and characterization of lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PloS Pathog (2009) 5:e1000455. doi: 10.1371/journal.ppat.1000455
14. Sewlall NH, Richards G, Duse A, Swanepoel R, Paweska J, Blumberg L, et al. Clinical features and patient management of lujo hemorrhagic fever. PloS Negl Trop Dis (2014) 8:e3233. doi: 10.1371/journal.pntd.0003233
15. Kunz S, de la Torre JC. Breaking the barrier: Host cell invasion by lujo virus. Cell Host Microbe (2017) 22:583–5. doi: 10.1016/j.chom.2017.10.014
16. Bird BH, Dodd KA, Erickson BR, Albariño CG, Chakrabarti AK, McMullan LK, et al. Severe hemorrhagic fever in strain 13/N guinea pigs infected with lujo virus. PloS Negl Trop Dis (2012) 6:e1801. doi: 10.1371/journal.pntd.0001801
17. Loayza Mafayle R, Morales-Betoulle ME, Romero C, Cossaboom CM, Whitmer S, Alvarez Aguilera CE, et al. Chapare hemorrhagic fever and virus detection in rodents in Bolivia in 2019. N Engl J Med (2022) 386:2283–94. doi: 10.1056/NEJMoa2110339
18. Delgado S, Erickson BR, Agudo R, Blair PJ, Vallejo E, Albariño CG, et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PloS Pathog (2008) 4:e1000047. doi: 10.1371/journal.ppat.1000047
19. Escalera-Antezana JP, Rodriguez-Villena OJ, Arancibia-Alba AW, Alvarado-Arnez LE, Bonilla-Aldana DK, Rodríguez-Morales AJ. Clinical features of fatal cases of chapare virus hemorrhagic fever originating from rural la paz, Bolivia, 2019: A cluster analysis. Travel Med Infect Dis (2020) 36:101589. doi: 10.1016/j.tmaid.2020.101589
20. Delgado S, Erickson BR, Agudo R, Blair P, Vallejo E, Albarino CG, et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLOS Pathogens (2008) 4(4):e1000047.
21. Shao J, Liang Y, Ly H. Human hemorrhagic fever causing arenaviruses: molecular mechanisms contributing to virus virulence and disease pathogenesis. Pathogens (2015) 4:283–306. doi: 10.3390/pathogens4020283
22. Gardner CL, Ryman KD. Yellow fever: A reemerging threat. Clinics Lab Med (2010) 30:237–60. doi: 10.1016/j.cll.2010.01.001
23. Singh S, Farr D, Kumar A. Ocular manifestations of emerging flaviviruses and the blood-retinal barrier. Viruses (2018) 10:530. doi: 10.3390/v10100530
24. Brandão-de-Resende C, Cunha LHM, Oliveira SL, Pereira LS, Oliveira JGF, Santos TA, et al. Characterization of retinopathy among patients with yellow fever during 2 outbreaks in southeastern Brazil. JAMA Ophthalmol (2019) 137:996. doi: 10.1001/jamaophthalmol.2019.1956
25. Merle H, Donnio A, Jean-Charles A, Guyomarch J, Hage R, Najioullah F, et al. Ocular manifestations of emerging arboviruses: Dengue fever, chikungunya, zika virus, West Nile virus, and yellow fever. J Fr Ophtalmol (2018) 41:e235–43. doi: 10.1016/j.jfo.2018.05.002
26. Vianello S, de Souza GláucioS, Rubens Belfort MaurícioM, de Oliveira Dias JoãoR. Ocular findings in yellow fever infection. JAMA Ophthalmol (2019) 137:300. doi: 10.1001/jamaophthalmol.2018.6408
27. Zin OA, Medina FMC, da Costa DS, Barcaui HS, Belfort R. Retinal maculopathy in an adult with yellow fever. Lancet Infect Dis (2019) 19:216. doi: 10.1016/S1473-3099(18)30431-6
28. Shantha JG, Yeh S, Acharya N. Insights from 2 outbreaks in southeastern Brazil: Yellow fever retinopathy. JAMA Ophthalmol (2019) 137:1003. doi: 10.1001/jamaophthalmol.2019.1936
29. Abroug N, Khairallah M, Zina S, Ksiaa I, Amor HB, Attia S, et al. Ocular manifestations of emerging arthropod-borne infectious diseases. J Curr Ophthalmol (2021) 33(3):227. doi: 10.4103/joco.joco_134_21
30. Ratnam I, Leder K, Black J, Torresi J. Dengue fever and international travel. J Travel Med (2013) 20:384–93. doi: 10.1111/jtm.12052
31. Vijitha V, Dave T, Murthy S, Ali MJ, Dave VP, Pappuru RR, et al. Severe ocular and adnexal complications in dengue hemorrhagic fever: A report of 29 eyes. Indian J Ophthalmol (2021) 69(3):617. doi: 10.4103/ijo.IJO_1588_20
32. Kamoi K, Mochizuki M, Ohno-Matsui K. Dengue fever-associated necrotizing scleritis: A case report with long-term follow-up. Medicine (2018) 97(32):e11875. doi: 10.1097/MD.0000000000011875
33. Agarwal L, Agrawal N. Retinal vasculitis with macular infarction: A dengue-related ophthalmic complication. IMCRJ (2020) 13:363–6. doi: 10.2147/IMCRJ.S264324
34. Yadav HM, Dutta Majumder P, Biswas J. Dengue associated choroiditis: a rare entity. J Ophthal Inflammation Infect (2017) 7(1):14. doi: 10.1186/s12348-017-0132-5
35. Somkijrungroj T, Kongwattananon W. Ocular manifestations of dengue. Curr Opin Ophthalmol (2019) 30(6):500–5. doi: 10.1097/ICU.0000000000000613
36. Akanda M, Gangaputra S, Kodati S, Melamud A, Sen HN. Multimodal imaging in dengue-Fever-Associated maculopathy. Ocular Immunol Inflammation (2018) 26(5):671–6. doi: 10.1080/09273948.2017.1351571
37. Munivenkatappa A, Sahay R, Yadav P, Viswanathan R, Mourya D. Clinical & epidemiological significance of kyasanur forest disease. Indian J Med Res (2018) 148(2):145. doi: 10.4103/ijmr.IJMR_688_17
38. Raja H, Starr MR, Bakri SJ. Ocular manifestations of tick-borne diseases. Survey Ophthalmol (2016) 61(6):726–44. doi: 10.1016/j.survophthal.2016.03.011
39. Růžek D, Yakimenko VV, Karan LS, Tkachev SE. Omsk haemorrhagic fever. Lancet (2010) 376(9758):2104–13. doi: 10.1016/S0140-6736(10)61120-8
40. Wagner E, Shin A, Tukhanova N, Turebekov N, Nurmakhanov T, Sutyagin V, et al. First indications of omsk haemorrhagic fever virus beyond Russia. Viruses (2022) 14(4):754. doi: 10.3390/v14040754
41. Al-Tawfiq JA, Memish ZA. Alkhurma hemorrhagic fever virus. Microbes Infection (2017) 19(6):305–10. doi: 10.1016/j.micinf.2017.04.004
42. Shah SZ, Jabbar B, Ahmed N, Rehman A, Nasir H, Nadeem S, et al. Epidemiology, pathogenesis, and control of a tick-borne disease- kyasanur forest disease: Current status and future directions. Front Cell Infect Microbiol (2018) 8:149. doi: 10.3389/fcimb.2018.00149
43. World Health Organization. Ebola Virus disease (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease.
44. World Health Organization. Disease outbreak news; Ebola disease caused by Sudan ebolavirus. Uganda (2022). Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON423.
45. Shantha JG, Yeh S, Nguyen QD. Ebola Virus disease and the eye. Curr Opin Ophthalmol (2016) 27:538–44. doi: 10.1097/ICU.0000000000000313
46. Connors DB, Shantha JG, Yeh S. Emerging causes of viral-associated uveitis. Int Ophthalmol Clin (2015) 55:103–13. doi: 10.1097/IIO.0000000000000068
47. Shantha JG, Crozier I, Yeh S. An update on ocular complications of Ebola virus disease. Curr Opin Ophthalmol (2017) 28:600–6. doi: 10.1097/ICU.0000000000000426
48. Shantha JG, Crozier I, Hayek BR, Bruce BB, Gargu C, Brown J, et al. Ophthalmic manifestations and causes of vision impairment in Ebola virus disease survivors in Monrovia, Liberia. Ophthalmology (2017) 124:170–7. doi: 10.1016/j.ophtha.2016.10.011
49. Eghrari AO, Bishop RJ, Ross RD, Davis B, Larbelee J, Amegashie F, et al. Characterization of Ebola virus-associated eye disease. JAMA Netw Open (2021) 4:e2032216. doi: 10.1001/jamanetworkopen.2020.32216
50. Yeh S, Shantha JG, Hayek B, Crozier I, Smith JR. Clinical manifestations and pathogenesis of uveitis in Ebola virus disease survivors. Ocul Immunol Inflammation (2018) 26:1128–34. doi: 10.1080/09273948.2018.1484493
51. Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med (2015) 372(25):2423–7. doi: 10.1056/NEJMoa1500306
52. Rojas M, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Ansari AA, et al. Ebola Virus disease: An emerging and re-emerging viral threat. J Autoimmun (2020) 106:102375. doi: 10.1016/j.jaut.2019.102375
53. Mehta S, Jiandani P. Ocular features of hantavirus infection. Indian J Ophthalmol (2007) 55(5):378–80. doi: 10.4103/0301-4738.33827
54. Hautala N, Kauma H, Vapalahti O, Mähönen SM, Vainio O, Vaheri A, et al. Prospective study on ocular findings in acute puumala hantavirus infection in hospitalised patients. Br J Ophthalmol (2011) 95(4):559–62. doi: 10.1136/bjo.2010.185413
55. Hautala N, Partanen T, Kubin AM, Kauma H, Hautala T. Central nervous system and ocular manifestations in puumala hantavirus infection. Viruses (2021) 13(6):1–8. doi: 10.3390/v13061040
56. Saari KM, Luoto S. Ophthalmological findings in nephropathia epidemica in Lapland. Acta Ophthalmol (Copenh) (1984) 62(2):235–43. doi: 10.1111/j.1755-3768.1984.tb08400.x
57. Kontkanen M, Puustjarvi T, Kauppi P, Lahdevirta J. Ocular characteristics in nephropathia epidemica or puumala virus infection. Acta Ophthalmol Scand (1996) 74(6):621–5. doi: 10.1111/j.1600-0420.1996.tb00748.x
58. Kontkanen M, Puustjarvi T, Lahdevirta J. Myopic shift and its mechanism in nephropathia epidemica or puumala virus infection. Br J Ophthalmol (1994) 78(12):903–6. doi: 10.1136/bjo.78.12.903
59. Saari KM. Acute glaucoma in hemorrhagic fever with renal syndrome (nephropathia epidemica). Am J Ophthalmol (1976) 81(4):455–61. doi: 10.1016/0002-9394(76)90301-9
60. Saari M, Alanko H, Jarvi J, Vetoniemi-Korhonen SL, Rasanen O. Nephropathia epidemica. Scandinavian form hemorrhagic fever Renal syndrome JAMA (1977) 238(8):874–7. doi: 10.1001/jama.1977.03280090038018
61. Zimmermann A, Lorenz B, Schmidt W. [Bilateral acute angle-closure glaucoma due to an infection with hantavirus]. Ophthalmologe (2011) 108(8):753–8. doi: 10.1007/s00347-010-2311-8
62. Cho IH, Chang JH, Choo EJ. Bilateral simultaneous angle-closure glaucoma associated with septic condition of Korean hemorrhagic fever (KHF). J Glaucoma (2015) 24(1):81–3. doi: 10.1097/IJG.0b013e318287ac5d
63. Baillieul A, Le TL, Rouland JF. Acute angle-closure glaucoma with choroidal effusion revealing a hantavirus infection: Description of ultrasound biomicroscopy imagery and optical coherence tomography visante. Eur J Ophthalmol (2021) 31(1):NP4–8. doi: 10.1177/1120672119858895
64. Kontkanen M, Puustjarvi T. Hemorrhagic fever (Puumala virus infection) with ocular involvement. Graefes Arch Clin Exp Ophthalmol (1998) 236(9):713–6. doi: 10.1007/s004170050146
65. Anywaine Z, Lule SA, Hansen C, Warimwe G, Elliott A. Clinical manifestations of rift valley fever in humans: Systematic review and meta-analysis. McElroy AK Ed PloS Negl Trop Dis (2022) 16(3):e0010233. doi: 10.1371/journal.pntd.0010233
66. Al-Hazmi A, Al-Rajhi AA, Abboud EB, Ayoola EA, Al-Hazmi M, Saadi R, et al. Ocular complications of rift valley fever outbreak in Saudi Arabia. Ophthalmology (2005) 112(2):313–8. doi: 10.1016/j.ophtha.2004.09.018
67. Fouad YA, Mekkawy MO, Sallam AB. Bilateral macular retinitis in patients with presumed rift valley fever from Sudan: A case series. Eur J Ophthalmol (2023) 33(1):377–81. doi: 10.1177/11206721221096305
68. Yalinbas D, Bozali E, Vural A, Kocak H, Erdogan H. Purtscher-like retinopathy associated with Crimean-Congo hemorrhagic fever: A case report. Ocul Immunol Inflamm (2021) 30(4):1016–9. doi: 10.1080/09273948.2020.1841805
69. Yalinbas D, Komurluoglu A, Bozali E. Increased retinal vessel tortuosity associated with Crimean-Congo hemorrhagic fever in children. Pediatr Infect Dis J (2021) 40(10):880–4. doi: 10.1097/INF.0000000000003187
Keywords: viral hemorrhagic fever, viral uveitis, viral conjunctivitis, emerging viral diseases, yellow fever, Ebola virus, Lassa fever
Citation: Karnam S, Huang Y, Nguyen N and Yeh S (2023) Ophthalmic consequences of viral hemorrhagic fevers: Insights from the clinic and laboratory. Front. Trop. Dis 4:1107786. doi: 10.3389/fitd.2023.1107786
Received: 25 November 2022; Accepted: 22 February 2023;
Published: 13 March 2023.
Edited by:
David Safronetz, Public Health Agency of Canada (PHAC), CanadaReviewed by:
Lauren Garnett, Public Health Agency of Canada (PHAC), CanadaCopyright © 2023 Karnam, Huang, Nguyen and Yeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven Yeh, syeh@unmc.edu
 Santi Karnam
Santi Karnam Ye Huang2
Ye Huang2 Nam Nguyen
Nam Nguyen