Commentary: Association between systemic immuneinflammation index and psoriasis: a population-based study
- 1Ninth Clinical College of Medicine, Shanxi Medical University, Taiyuan, China
- 2Department of Dermatology, Taiyuan Central Hospital, Shanxi Medical University, Taiyuan, China
- 3Key Laboratory of Stem Cells for Immunologic Skin Diseases, Taiyuan Central Hospital, Taiyuan, China
Background: The systemic immune-inflammation index (SII),as measured by lymphocyte, neutrophil and platelet counts in peripheral blood, is regarded as a favorable indicator of both inflammatory state and immune response. Psoriasis is an immune-mediated disease notable for its chronic inflammation of the entire system. Our research sought to explore the latent link between psoriasis and SII.
Methods: We performed a cross-sectional investigation utilizing data extracted from the National Health and Nutrition Examination Survey (NHANES, 2009-2014). Employing multivariate linear regression models and subgroup analysis, we sought to uncover the association between SII and psoriasis.
Results: This study enrolled a total of 17,913 participants as part of its research cohort. Our multivariate linear regression analysis revealed a notable and positive correlation between SII and psoriasis [1.013 (1.000, 1.026)]. As SII tertiles increased, the risk of psoriasis demonstrated an upward trend. The significant dependence on this positive association were maintained in women, BMI(≥ 30 kg/m2),non-stroke and non-cancer subjects in subgroup analysis and interaction tests. Furthermore, we identified a significant association between SII and psoriasis, characterized by two consecutive inverted U-shaped patterns. Notably, the analysis revealed the most prominent inflection point at a specific value of 797.067.
Conclusions: The results indicate a significant correlation between elevated SII levels and the presence of psoriasis. However, to corroborate and strengthen these results, additional large-scale prospective studies are required.
1 Background
Psoriasis, a genetic skin disorder mediated by the immune system, impacts approximately 2-3% of the global population (1, 2). It typically affects the skin, but may also affects different organ systems such as the joints (3). The risk factors of psoriasis can be divided into extrinsic(trauma, drugs, infections, vaccination, lifestyle)and intrinsic(obesity, diabetes mellitus, dyslipidemia, hypertension, mental stress)groups with a view to prevent (4). Innate and adaptive immune responses are involved in the progression of psoriatic inflammation (5). Antimicrobial peptides (AMPs), such as LL-37, β-defensin and S100 protein, trigger and maintain inflammatory pathways in psoriasis (6). In the maintenance phase of psoriasis, the TNF-α/IL-23/IL-17 axis assumes a crucial role (7).
Recent studies have reported that psoriasis shares an underlying chronic inflammatory basis with comorbidities such as metabolic syndrome and cardiovascular disease (8–11). Uncovering the role of adipose inflammation in psoriasis is being intensively investigated (12, 13). Biologics such as efolizumab can delay the onset of systemic inflammatory psoriasis (14, 15). Other studies have suggested that clinical parameters of plasma cytokines and inflammation may serve as novel strategies to monitor the progression of psoriasis (16, 17). Elevated levels of various inflammatory and immune-based indices, such as the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) have been observed and found to correlate with Psoriasis Area and Severity Index (PASI) scores in individuals with psoriasis (18, 19).
SII has become a prognostic biomarker for a variety of malignant tumors, including gastric cancer (20, 21), non-small cell lung cancer (22, 23), esophageal cancer (24, 25) and colorectal cancer (26). It provides a comprehensive reflection of the body’s equilibrium between inflammatory factors and immune responses. Besides, the new score has been established as an effective predictive indicator of cardiovascular events (27). Some recent findings suggest that elevated SII levels are associated with hepatic steatosis and increased urinary albumin excretion (28, 29). The differences in this study are, first, that the study population was a sample of NHANES and that the sample size was increased, as were confounders for psoriasis or general health. Second, SII and the likelihood of psoriasis were plotted as a curve-fitted graph for analysis.
As a result, our objective was to investigate the correlation between the SII and psoriasis within the United States population. To accomplish this, we utilized the NHANES dataset in this study.
2 Methods
2.1 Study population
The NHANES is a comprehensive survey conducted biennially by the National Center for Health Statistics (NCHS), providing cross-sectional data that represents the entire US population. The survey using the continuous cycle NHANES data set of 2009-2014.Initially, a cohort of 30,468 participants was enrolled for the study. We excluded 5,219 participants with missing SII data (neutrophil, lymphocyte, and platelet count) and 7,336 with missing psoriasis data. The study ultimately included 17913 eligible participants. Figure 1 presents the visual representation of our sample selection process.
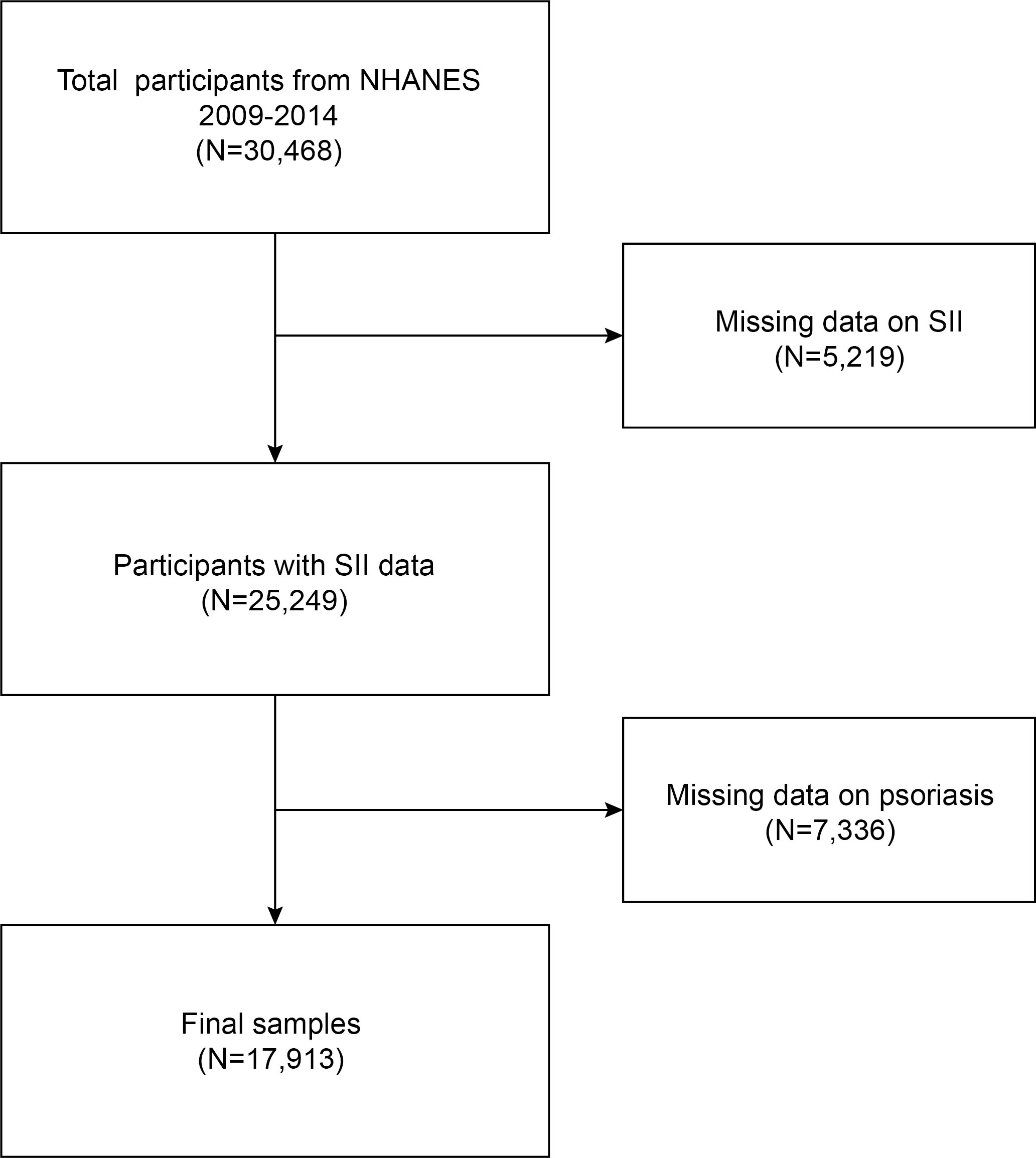
Figure 1 Flow chart of participants selection. NHANES, National Health and Nutrition Examination Survey.
2.2 Definition of systemic immune-inflammation index
The Systemic Immunoinflammatory Index (SII), a new biomarker of local immune response and systemic inflammation throughout the body, has been shown to correlate with the prognosis of cancer patients. Regarded as a continuous variable, The calculation of SII for each participant was performed utilizing the formula:
Lymphocyte, neutrophil and platelet count using automatic hematology analysis equipment (Coulter ® DxH analyzer 800) determination of complete blood count, expressed as x 103 cells/ml. we designated SII as the exposure variable and psoriasis condition as an outcome variable in this study. Since the effect size was not significant, SII/100 was used to amplify the effect size by a factor of 100.
2.3 Selection of covariates
The covariates considered in this study encompassed various factors, including sex (men/women), age (years), race (Mexican American/other Hispanic/non-Hispanic White/non-Hispanic Black/other races), educational attainment (less than high school, high school or equivalent/above high school), body mass index (BMI, kg/m2), smoking status (yes/no), alcohol consumption (yes/no), presence of coronary artery disease (yes/no), diabetes mellitus (yes/no), congestive heart failure (yes/no), history of stroke (yes/no), and presence of cancer (yes/no). Individuals were categorized into three groups based on their body mass index (BMI):<25 kg/m2 indicating normal weight, 25-29.9 kg/m2 indicating overweight, and ≥30 kg/m2 indicating obesity.
2.4 Statistical analysis
The statistical analyses were carried out following the guidelines of the Disease Control and Prevention (CDC), taking into account the appropriate NHANES sampling weights and employing complex multi-stage cohort surveys. Continuous variables are expressed as means with standard deviation (SD) and categorical variables as proportions. We used multivariate logistic regression analysis between SII and psoriasis to construct multivariate tests that resulted in beta values and 95% confidence intervals. The multivariate test involved three models: model 1 (unadjusted), model 2 (adjusted for gender, age, and race), and model 3 (adjusted for all covariates).Smooth curve fitting was performed simultaneously by adjusting the variables. A threshold effect analysis model was used to investigate the relationship and inflection point between SII and psoriasis. Subgroup analysis of the SII-psoriasis relationship was conducted considering stratification factors including gender (man/woman), BMI (normal weight/overweight/obesity), stroke (yes/no), cancer (yes/no). In the process, we used the statistical calculation and graphics software R(version 4.1.3) and the Irrigation Statistics Software (version 2.0) for statistical research. Significance was established at a threshold of P< 0.05.
3 Results
3.1 Baseline characteristics
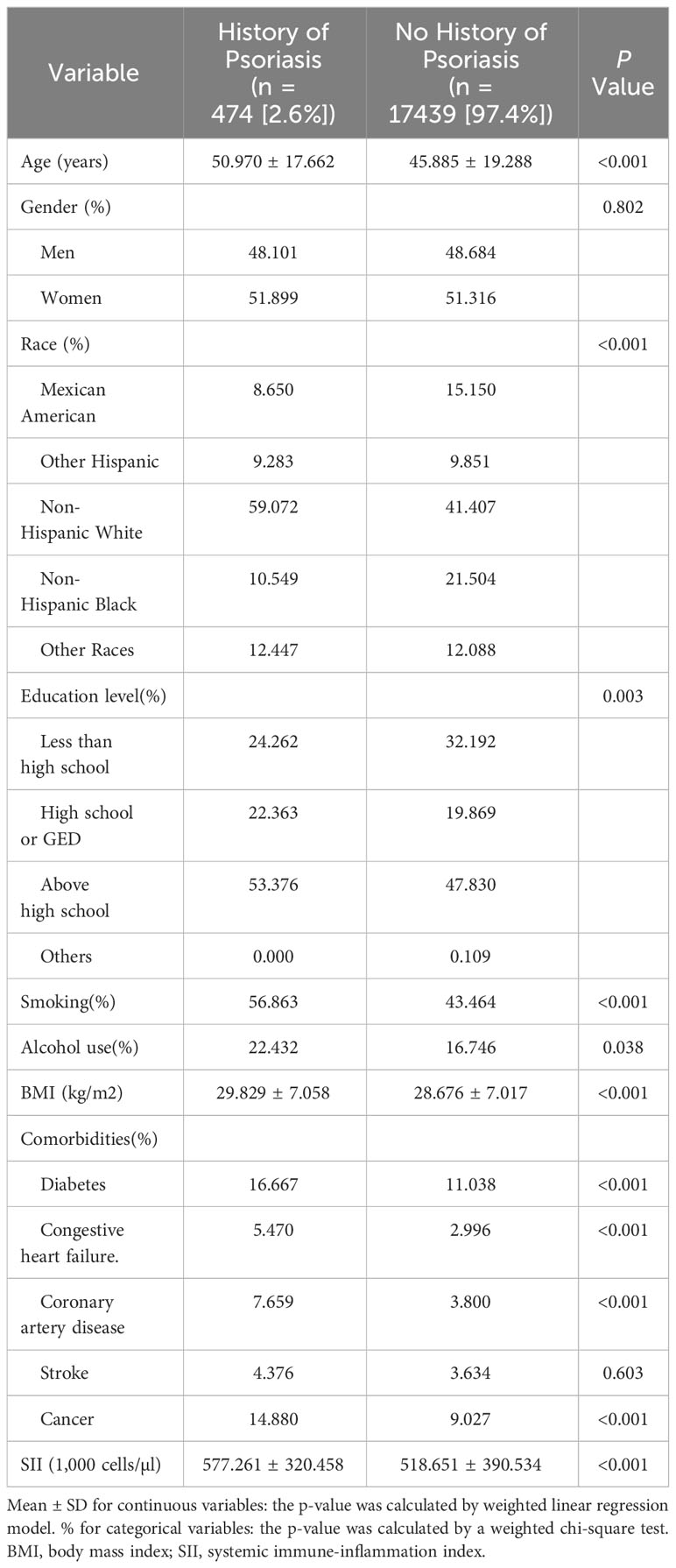
This study involved 17913 participants with a mean age of (46.02 ± 19.26) years. Of these, 48.67% were men and 51.33% were women. Among these participants, 14.98% were Mexican American, 9.84% were other Hispanic,41.87% were non-Hispanic white, 22.33%were non-Hispanic black, and 12.10% were from other races. The mean SII score was (520.20 ± 388.94) for all participants. In comparison to the no history of psoriasis group, the history of psoriasis group is significantly more likely to be older and have comorbidities including stroke, coronary artery disease,diabetes,congestive heart failure and cancer with a higher proportion of non-Hispanic white and education above high school; with higher smoking and alcohol status; and higher levels of BMI and SII.The statistical analysis did not demonstrate a significant difference in gender and stroke between the two groups (p > 0.05) (Table 1).
3.2 Association between systemic immune-inflammation index and psoriasis
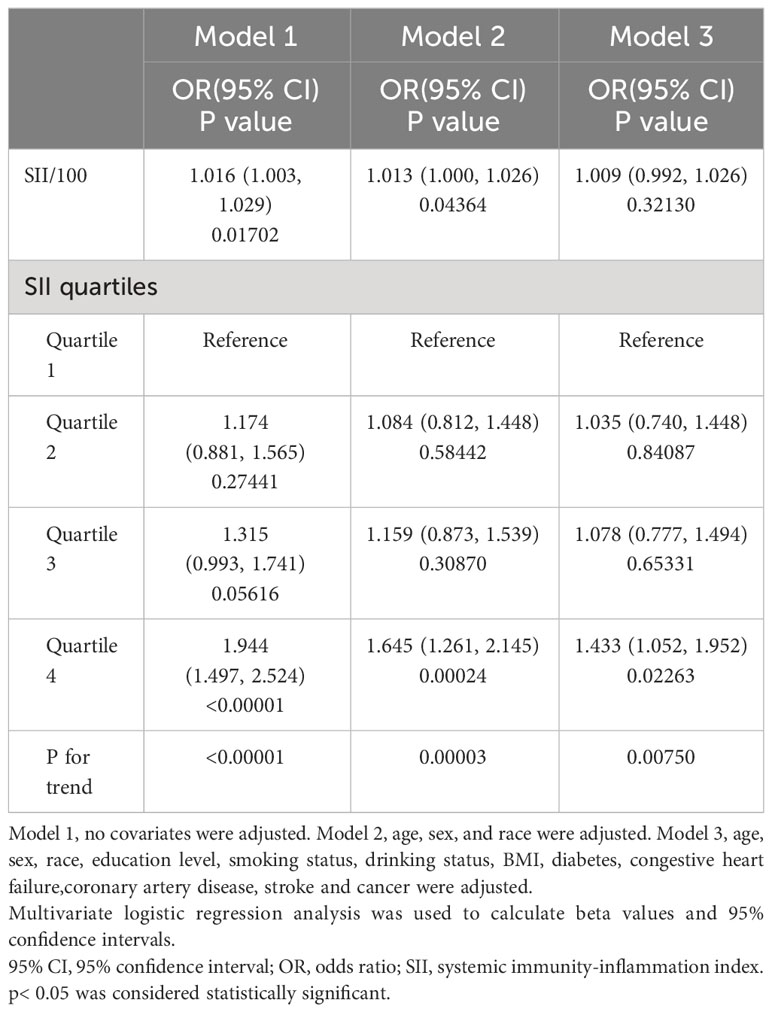
Our findings suggest that the higher the SII, the greater the likelihood of developing psoriasis. This association remained significant in both our crude model (OR=1.016; 95% CI, 1.003–1.029, p<0.05) and minimally adjusted model (OR=1.013; 95% CI, 1.000–1.026, p<0.05). However, after adjusting all covariates, this significant positive correlation became insignificant in model 3 (OR=1.009; 95% CI, 0.992–1.026,p>0.05). We further transformed SII for sensitivity analysis from continuous variables to categorical variables (quartiles). Participants in the highest quartile (OR=1.645; 95% CI, 1.261–2.145, p<0.05) of SII had a statistically significant 64.5% increased risk of developing psoriasis when contrasted with those situated in the lowest SII quartile. Participants belonging to SII quartile 2 and 3 displayed an increased risk of psoriasis in comparison to the lowest quartile; however, this association did not attain statistical significance (Table 2).
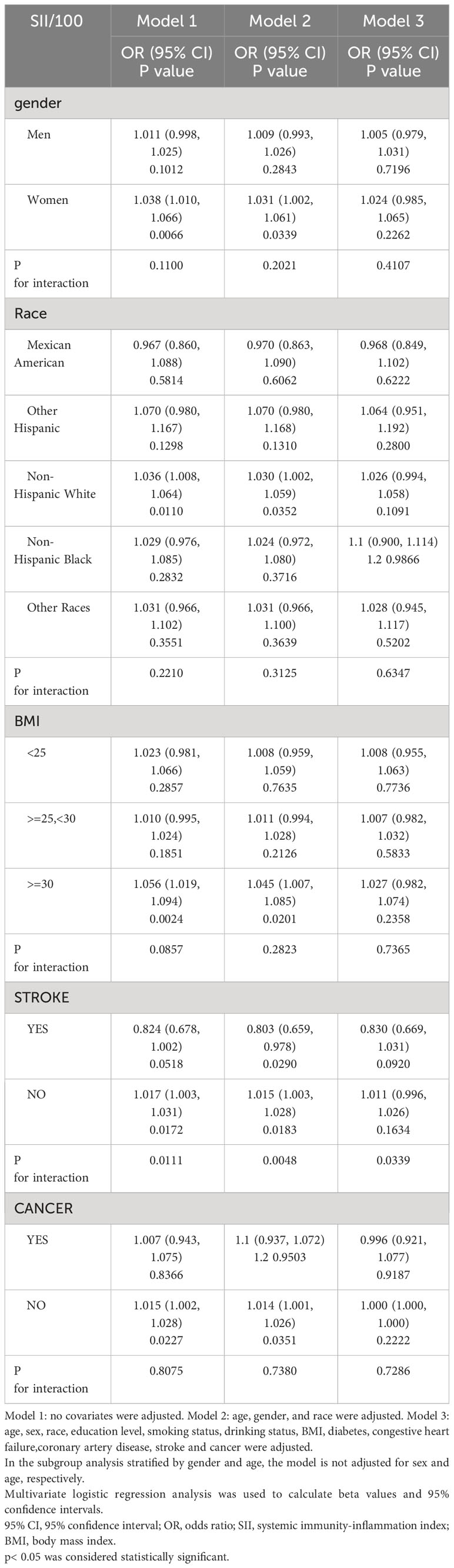
Further subgroup analysis revealed inconsistent associations between SII and psoriasis, as shown in Table 3. In subgroup analyses by gender stratification, our results revealed an independent and significantly positive association between SII and psoriasis exclusively among women (OR = 1.031; 95% CI, 1.002−1.061, p<0.05) but not statistically significant in all models for men. When examining subgroups by degree of body mass index, we observed a robust positive correlation between SII and the obese group (≥ 30 kg/m2) in both the unadjusted and partially adjusted models. A significant relationship between SII with psoriasis was detected in non-cancer subjects (OR = 1.014, 95% CI 1.001−1.026, p<0.05). The interaction test indicated that no significant dependence was observed for the positive correlations between sex, age, body mass index, and cancer with SII and psoriasis (p for interaction >0.05).Only in the stroke stratification the association between SII and psoriasis was significantly different.
Because people with SII greater than 2000 are more discrete, data with SII greater than 2000 are removed from the curve fitting. We fitted smoothed curves to characterize the nonlinear relationship between SII and psoriasis (Figure 2). We found two consecutive inverted U-shaped relationships between SII and psoriasis, with the most significant inflection point of 797.067.
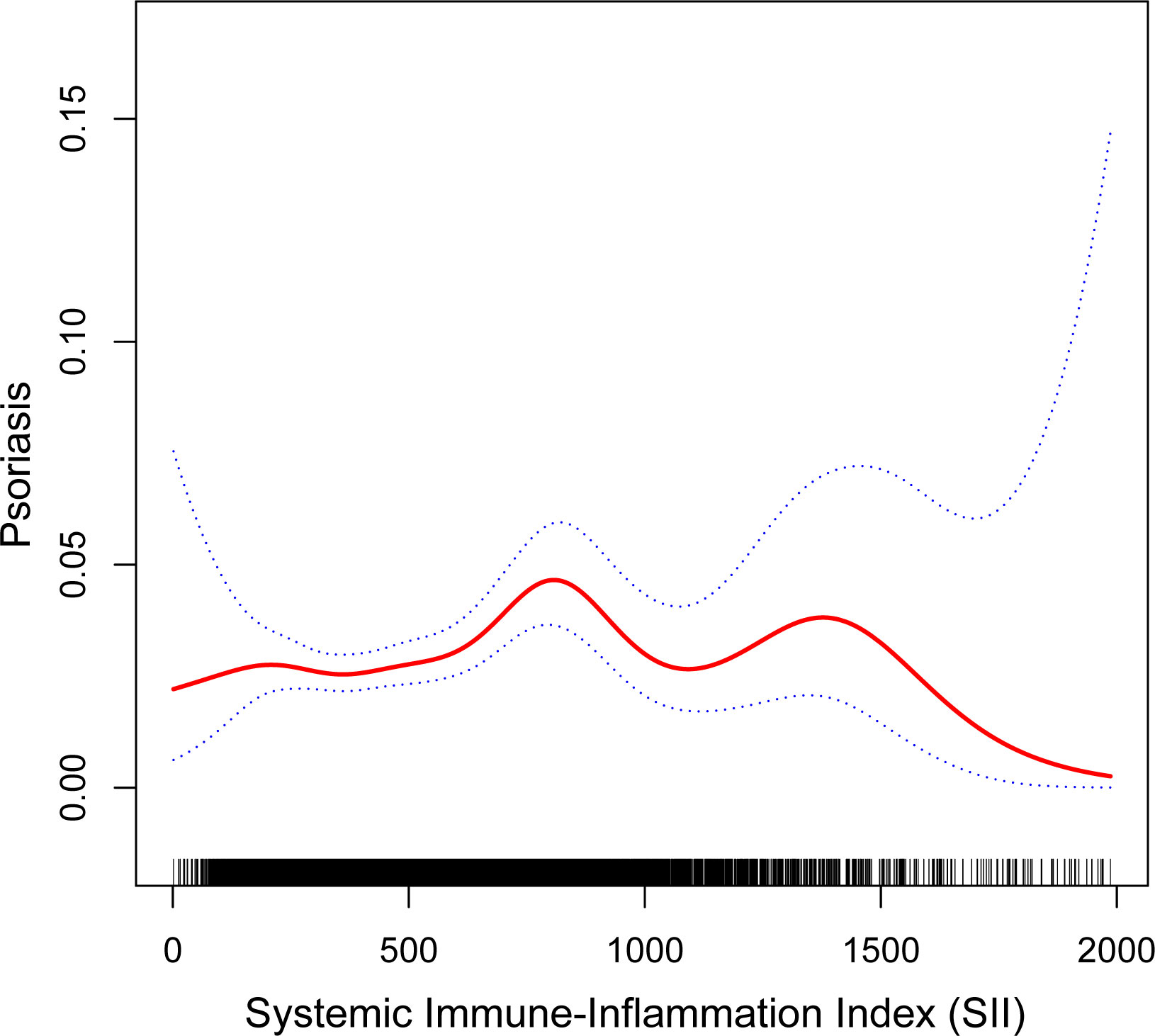
Figure 2 The nonlinear associations between SII and psoriasis. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit.
4 Discussion
The study sample utilized in our research is representative of the American population at a national level. We observed that the higher the SII participants with the greater the chance of psoriasis. Prominently, two consecutive inverted U-shaped relationships between SII and psoriasis was revealed for the first time.The risk was highest when SII was 797.067. Interestingly, the significant positive correlation between SII and psoriasis was only observed among non-Hispanic whites. Additionally, gender or weight status may have played a moderating role in the association between psoriasis and SII. There was an association in women, but not in men. The association was observed only in the obese group. In fact, Model 3 was built based on a comprehensive adjustment for all possible relevant variables, which included various factors that may influence inflammatory status, such as age, BMI, co-morbidities, and so on. If adjustments for these variables are able to attenuate or eliminate differences in SII among psoriasis patients, then the results may become less significant.
As far as we know, it is the first NHANES-based investigation of the relationship between SII and psoriasis. The link here reported regarding SII and psoriasis is similar to those previously reported, with one study from Turkey (30) showing that SII of psoriasis patients was considerably greater than that of control group and patients with moderate/severe psoriasis had higher SII than with mild psoriasis. More recently, Sugimoto et al. also demonstrated a positive correlation between SII and PASI scores and found that patients with higher SII scores had lower persistence of treatment with conventional systemic medications (31). Dincer Rota et al. reported that significantly elevated SII values were observed in both the subgroup with PASI ≥ 4.5 and among patients experiencing nail and genital involvement (32). Moreover, it has been discovered that the mean SII was lower in the patients receiving biologic treatment compared to the untreated patients (33, 34). According to an observational retrospective study, SII was positively correlated with disease severity and was an independent prognostic factor for mild and moderate psoriasis (35).
Our findings are closely related to the evidence that psoriasis is a systemic inflammatory disease (36–40). Recent research has revealed that the pathogenesis of psoriasis and its complications are related to systemic inflammatory pathways.Observations from the 18F-fluorodeoxyglucose positron emission tomography/computed Tomography study also confirmed the hypothesis that psoriasis is a systemic inflammatory disease (41). In multiple studies, in addition to marked increases in systemic arterial and subcutaneous inflammation, subclinical inflammation was also observed in the liver, joints, and tendons for individuals with moderate-to-severe psoriasis, and in the aorta of patients with mild psoriasis (42). Furthermore, insulin resistance may be more helpful in predicting subclinical atherosclerosis than traditional cardiovascular disease risk factors, and femoral artery ultrasonography better detects subclinical atherosclerosis in patients with moderate-to-severe psoriasis (43, 44). Serum levels of several proinflammatory cytokines, including TNF-α,IFN-γ,IL-6,IL-8,IL-12,IL-17A and IL-18 were elevated in individuals diagnosed with psoriasis compared to healthy controls, suggesting that psoriasis develops systemically (45).
Furthermore, the positive correlation between SII and psoriasis exhibits significant variations in relation to stroke occurrence. Extensive research has demonstrated that individuals with psoriasis face an elevated risk for cerebrovascular disease(stroke)which is attributed to the severity of their psoriasis condition (46–49). In addition, the incidence of ischemic heart disease and cerebrovascular disease increased in patients with psoriasis combined with depression (50). Numerous studies have sought to establish a causal link between psoriasis and cardiovascular disease. Cardiovascular risk factors such as dyslipidemia, diabetes mellitus, hypertension, metabolic syndrome and obesity have also increased in psoriasis patients (51). Atherosclerosis is the main pathologic change that precedes myocardial infarction and stroke. Notably, individuals with psoriasis have been found to have higher arterial stiffness than healthy controls, with arterial stiffness positively correlating with psoriasis duration (52, 53). Multiple studies measured by imaging techniques have reported a higher prevalence and greater severity of coronary artery calcification and atherosclerosis among individuals diagnosed with psoriasis in comparison to their healthy counterparts (54–56). The association between psoriasis and cardiometabolic disorders can be attributed to various mechanisms, involving the secretion of adipokines, oxidative stress, insulin resistance, angiogenesis, microparticles, hypercoagulability and common inflammatory pathways (51). Thus, the administration of effective systemic anti-inflammatory drugs may prove beneficial in reducing cardiovascular risk in psoriasis patients (57).
Platelet, neutrophil, and lymphocyte counts constitute SII. The pathogenic role of neutrophils, the most abundant innate immune cells, is associated with chronic inflammatory and autoimmune diseases (58). The abundant presence of neutrophils in psoriatic lesions is a distinctive histopathological characteristic of psoriasis (59). Recent reports have proposed that neutrophils play a role in psoriasis pathophysiology through processes such as respiratory burst, degranulation, and the formation of neutrophil extracellular traps, influencing psoriatic immunity and clinical outcomes (60). In addition, patients with psoriasis continue to consume platelets due to an enhanced coagulation response (61). Platelets play an important role in cardiovascular disease as mediators of hemostasis and thrombotic inflammation (62). Numerous studies have reported elevated levels of platelet activation biomarkers in individuals with psoriasis when compared to healthy controls. These biomarkers include platelet-derived microparticles (PDMPs) and mean platelet volume (MPV). Interestingly, a positive correlation has been observed between the levels of these biomarkers and PASI scores (63). The core pathogenesis of psoriasis involves aberrant function of multiple T-cell subsets, including regulatory T cells (Tregs), T helper (Th)1 cells, Th2 cells, Th17 cells, and Th22 cells, accompanied by aberrant release of associated cytokines, such as IFN-γ, tumor necrosis factor (TNF)-α, and members of the IL-23 and IL-17 families (45).
In recent years, with the advent of biologics, the effectiveness and safety of psoriasis treatments have greatly improved. A major challenge and limitation is to find better predictors of treatment response to determine which patients are more likely to benefit from specific biologics. SII is positively correlated with PASI scores (30–32), and the correlation with treatment response (31, 33, 34) has been confirmed by some studies. In addition, correlations have been found between SII and metabolic syndrome (64), cardiovascular disease (65), and depression (66) in psoriasis comorbidities. Taken together, SII appears to be a possible biomarker associated with psoriasis disease activity, treatment response, and the development of comorbidities, thus helping patients to develop more personalized and effective treatment strategies.
Our study has several limitations worth noting. Firstly, due to the cross-sectional design of our research, we cannot establish causality. Second, relying on self-reported questionnaire data without clinical assessment by healthcare professionals to determine the presence or absence of psoriasis may lead to misdiagnosis or underestimation of psoriasis cases, especially mild or atypical psoriasis cases. Recall bias may also affect the validity of study results. Moreover, the NHANES database does not differentiate between the various types of psoriasis and fails to obtain information on which period of disease progression (progressive/quiescent/degenerative) a psoriasis patient is in, whereas different types of psoriasis at different stages may interact biologically with SII in different ways. As it is well known that the inflammatory response is a dynamic process, high (or low) values of SII observed at a single time point may not fully reflect the complexity and dynamics of systemic inflammation. However, the interaction between inflammation and psoriasis is complex. Although we controlled for some confounders, other confounders, such as duration of psoriasis, types of psoriasis complications not included, use of biologics, and history of long-term use of anti-inflammatory medications to treat complications, may still have had an impact on the study results. Because NHANES did not document these factors, we were unable to include them in our analysis. Therefore, our results may not fully reflect the complete picture.
5 Conclusion
Our results establish a connection between elevated SII levels and psoriasis. SII may be a simple, practical, and easily accessible tool for monitoring disease activity and treatment efficacy in patients with psoriasis. To validate our findings, additional extensive prospective studies are warranted.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: www.cdc.gov/nchs/nhanes/.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
XZ: Data curation, Writing – original draft, Writing – review & editing. JL: Writing – original draft. XL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Taiyuan Science and Technology Project Program (No.202213), Shanxi Provincial Medical Key Scientific Research Project (No.2020XM20) and Central Guided Local Science and Technology Development Special Funds Program (No.YDZX20191400004470).
Acknowledgments
We would like to thank all participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in A dults in the United States. JAMA Dermatol. (2021) 157:940–6. doi: 10.1001/jamadermatol.2021.2007
2. Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. (2021) 397:754–66. doi: 10.1016/S0140-6736(21)00184-7
3. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. (2019) 20:1475. doi: 10.3390/ijms20061475
4. Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. (2019) 20:4347. doi: 10.3390/ijms20184347
5. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. (2021) 397:1301–15. doi: 10.1016/S0140-6736(20)32549-6
6. Petit RG, Cano A, Ortiz A, Espina M, Prat J, Muñoz M, et al. Psoriasis: from pathogenesis to pharmacological and nano-technological-based therapeutics. Int J Mol Sci. (2021) 22:4983. doi: 10.3390/ijms22094983
7. Singh R, Koppu S, Perche PO, Feldman SR. The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int J Mol Sci. (2021) 22:12793. doi: 10.3390/ijms222312793
8. Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. (2018) 9:579. doi: 10.3389/fimmu.2018.00579
9. Tashiro T, Sawada Y. Psoriasis and systemic inflammatory disorders. Int J Mol Sci. (2022) 23:4457. doi: 10.3390/ijms23084457
10. Patrick MT, Li Q, Wasikowski R, Mehta N, Gudjonsson JE, Elder JT, et al. Shared genetic risk factors and causal association between psoriasis and coronary artery disease. Nat Commun. (2022) 13:6565. doi: 10.1038/s41467-022-34323-4
11. Saalbach A, Kunz M. Impact of chronic inflammation in psoriasis on bone metabolism. Front Immunol. (2022) 13:925503. doi: 10.3389/fimmu.2022.925503
12. Herbert D, Franz S, Popkova Y, Anderegg U, Schiller J, Schwede K, et al. High-fat diet exacerbates early psoriatic skin inflammation independent of obesity: saturated fatty acids as key players. J Invest Dermatol. (2018) 138:1999–2009. doi: 10.1016/j.jid.2018.03.1522
13. Barros G, Duran P, Vera I, Bermúdez V. Exploring the links between obesity and psoriasis: A comprehensive review. Int J Mol Sci. (2022) 23:7499. doi: 10.3390/ijms23147499
14. Gjersvik P. Biologics: targeting systemic inflammation in psoriasis. Br J Dermatol. (2018) 179:247–8. doi: 10.1111/bjd.16775
15. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. (2020) 182:840–8. doi: 10.1111/bjd.18245
16. Gao W, Wang Z, Li W, Li Y, Liu M. Biomarkers and biologics related with psoriasis and psoriatic arthritis. Int Immunopharmacol. (2023) 122:110646. doi: 10.1016/j.intimp.2023.110646
17. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA. (2020) 323:1945–60. doi: 10.1001/jama.2020.4006
18. Asahina A, Kubo N, Umezawa Y, Honda H, Yanaba K, Nakagawa H. Neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and mean platelet volume in Japanese patients with psoriasis and psoriatic arthritis: Response to therapy with biologics. J Dermatol. (2017) 44:1112–21. doi: 10.1111/1346-8138.13875
19. Paliogiannis P, Satta R, Deligia G, Farina G, Bassu S, Mangoni AA, et al. Associations between the neutrophil-to-lymphocyte and the platelet-to-lymphocyte ratios and the presence and severity of psoriasis: a systematic review and meta-analysis. Clin Exp Med. (2019) 19:37–45. doi: 10.1007/s10238-018-0538-x
20. Liu YY, Ruan GT, Ge YZ, Li QQ, Zhang Q, Zhang X, et al. Systemic inflammation with sarcopenia predicts survival in patients with gastric cancer. J Cancer Res Clin Oncol. (2022) 148:3925–6. doi: 10.1007/s00432-022-03925-2
21. He K, Si L, Pan X, Sun L, Wang Y, Lu J, et al. Preoperative systemic immuneinflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol. (2022) 12:829689. doi: 10.3389/fonc.2022.829689
22. Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. (2019) 33:e22964. doi: 10.1002/jcla.22964
23. Huang W, Luo J, Wen J, Jiang M. The relationship between systemic immune inflammatory index and prognosis of patients with non-small cell lung cancer: A meta-analysis and systematic review. Front Surg. (2022) 9:898304. doi: 10.3389/fsurg.2022.898304
24. Jomrich G, Paireder M, Kristo I, Baierl A, Ilhan-Mutlu A, Preusser M, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. (2021) 273:532–41. doi: 10.1097/SLA.0000000000003370
25. Han R, Tian Z, Jiang Y, Guan G, Sun X, Yu Y, et al. Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery. Gland Surg. (2022) 11:576–87. doi: 10.21037/gs-22-117
26. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23:6261–72. doi: 10.3748/wjg.v23.i34.6261
27. Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. (2020) 50:e13230. doi: 10.1111/eci.13230
28. Qin Z, Li H, Wang L, Geng J, Yang Q, Su B, et al. Systemic immune-inflammation index is associated with increased urinary albumin excretion: A population-based study. Front Immunol. (2022) 13:863640. doi: 10.3389/fimmu.2022.863640
29. Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
30. Yorulmaz A, Hayran Y, Akpinar U, Yalcin B. Systemic immune-inflammation index (SII) predicts increased severity in psoriasis and psoriatic arthritis. Curr Health Sci J. (2020) 46:352–7. doi: 10.12865/CHSJ.46.04.05
31. Sugimoto E, Matsuda H, Shibata S, Mizuno Y, Koyama A, Li L, et al. Impact of pretreatment systemic inflammatory markers on treatment persistence with biologics and conventional systemic therapy: A retrospective study of patients with psoriasis vulgaris and psoriatic arthritis. J Clin Med. (2023) 12:3046. doi: 10.3390/jcm12083046
32. Dincer Rota D, Tanacan E. The utility of systemic-immune inflammation index for predicting the disease activation in patients with psoriasis. Int J Clin Pract. (2021) 75:e14101. doi: 10.1111/ijcp.14101
33. Kvist-Hansen A, Kaiser H, Krakauer M, Gørtz PM, Wang X, Becker C, et al. Neutrophil-to-lymphocyte ratio and the systemic immune-inflammation index as potential biomarkers of effective treatment and subclinical atherosclerotic cardiovascular disease in patients with psoriasis. J Eur Acad Dermatol Venereol. (2023) 37:e586–9. doi: 10.1111/jdv.18860
34. Albayrak H. Neutrophil-to-lymphocyte ratio, neutrophil-to-monocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index in psoriasis patients: response to treatment with biological drugs. J Clin Med. (2023) 12:5452. doi: 10.3390/jcm12175452
35. Tiucă OM, Morariu SH, Mariean CR, Tiucă RA, Nicolescu AC, Cotoi OS. Impact of blood-count-derived inflammatory markers in psoriatic disease progression. Life (Basel). (2024) 14:114. doi: 10.3390/life14010114
36. Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. (2014) 32:227–55. doi: 10.1146/annurev-immunol-032713-120225
37. Grän F, Kerstan A, Serfling E, Goebeler M, Muhammad K. Current developments in the immunology of psoriasis. Yale J Biol Med. (2020) 93:97–110.
38. Ayala-Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl). (2016) 6:7–32. doi: 10.2147/PTT.S64950
39. Benhadou F, Mintoff D, Del Marmol V. Psoriasis:Keratinocytes or immune cells-which is the trigger? Dermatology. (2019) 235:91–100. doi: 10.1159/000495291
40. Girolomoni G, Griffiths CE, Krueger J, Nestle FO, Nicolas JF, Prinz JC, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: A hypothesis paper. J Dermatolog Treat. (2015) 26:103–12. doi: 10.3109/09546634.2014.880396
41. Kim BS, Lee WK, Pak K, Han J, Kim GW, Kim HS, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: Feasibility study using 18F-fluorodeoxyglucose PET/CT. J Am Acad Dermatol. (2019) 80:1322–31. doi: 10.1016/j.jaad.2018.03.011
42. Mehta NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, Raper A. et al Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. (2011) 147:1031–9. doi: 10.1001/archdermatol.2011.119
43. Gonzalez-Cantero A, Gonzalez-Cantero J, Sanchez-Moya AI, Perez-Hortet C, Arias-Santiago S, Schoendorff-Ortega C, et al. Subclinical atherosclerosis in psoriasis. Usefulness of femoral artery ultrasound for the diagnosis, and analysis of its relationship with insulin resistance. PloS One. (2019) 14:e0211808. doi: 10.1371/journal.pone.0211808
44. González-Cantero A, Gonzalez-Cantero J, Sanchez-Moya AI, Perez-Hortet C, Arias-Santiago S, Martin-Rodriguez JL, et al. Femoral artery ultrasound for improving the detection of atherosclerosis in psoriasis. J Am Acad Dermatol. (2019) 80:784–6. doi: 10.1016/j.jaad.2018.07.007
45. Hu P, Wang M, Gao H, Zheng A, Li J, Mu D, et al. The role of helper T cells in psoriasis. Front Immunol. (2021) 12:788940. doi: 10.3389/fimmu.2021.788940
46. Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. (2008) 159:895–902. doi: 10.1111/bjd.2008.159.issue-4
47. Li WQ, Han JL, Manson JE, Rimm EB, Rexrode KM, Curhan GC, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. (2012) 166:811–8. doi: 10.1111/bjd.2012.166.issue-4
48. Chiang CH, Huang CC, Chan WL, Huang PH, Chen YC, Chen TJ, et al. Psoriasis and increased risk of ischemic stroke in Taiwan: a nationwide study. J Dermatol. (2012) 39:279–81. doi: 10.1111/j.1346-8138.2011.01401.x
49. Siegel D, Devaraj S, Mitra A, Raychaudhuri SP, Raychaudhuri SK, Jialal I. Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol. (2013) 44:194–204. doi: 10.1007/s12016-012-8308-0
50. Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Association between depression and risk of atrial fibrillation and stroke in patients with psoriasis: a Danish nationwide cohort study. Br J Dermatol. (2015) 173:471–9. doi: 10.1111/bjd.13778
51. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. (2017) 18:2211. doi: 10.3390/ijms18102211
52. Gisondi P, Fantin F, Del Giglio M, Valbusa F, Marino F, Zamboni M, et al. Chronic plaque psoriasis is associated with increased arterial stiffness. Dermatology. (2009) 218:110–3. doi: 10.1159/000182256
53. Choi BG, Kim MJ, Yang HS, Lee YW, Choe YB, Ahn KJ. Assessment of arterial stiffness in Korean patients with psoriasis by cardio-ankle vascular index. Angiology. (2017) 68:608–13. doi: 10.1177/0003319716652284
54. Bissonnette R, Cademartiti F, Maffei E, Tardif JC. Increase in coronary atherosclerosis severity and the prevalence of coronary artery mixed plaques in patients with psoriasis. Br J Dermatol. (2017) 176:800–2. doi: 10.1111/bjd.14797
55. Zeb I, Budoff M. Coronary artery calcium screening: does it perform better than other cardiovascular risk stratification tools? Int J Mol Sci. (2015) 16:6606–20. doi: 10.3390/ijms16036606
56. Eckert J, Schmidt M, Magedanz A, Voigtländer T, Schmermund A. Coronary CT angiography in managing atherosclerosis. Int J Mol Sci. (2015) 16:3740–56. doi: 10.3390/ijms16023740
57. Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:480–9. doi: 10.1136/annrheumdis-2014-206624
58. Liew PX, Kubes P. The neutrophil's role during health and disease. Physiol Rev. (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
59. Murphy M, Kerr P, Grant-Kels JM. The histopathologic spectrum of psoriasis. Clin Dermatol. (2007) 25:524–8. doi: 10.1016/j.clindermatol.2007.08.005
60. Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in psoriasis. Front Immunol. (2019) 10:2376. doi: 10.3389/fimmu.2019.02376
61. Visser MJE, Tarr G, Pretorius E. Thrombosis in psoriasis: cutaneous cytokine production as a potential driving force of haemostatic dysregulation and subsequent cardiovascular risk. Front Immunol. (2021) 12:688861. doi: 10.3389/fimmu.2021.688861
62. Herster F, Karbach S, Chatterjee M, Weber ANR. Platelets: underestimated regulators of autoinflammation in psoriasis. J Invest Dermatol. (2021) 141:1395–403. doi: 10.1016/j.jid.2020.12.025
63. Jiang Z, Jiang X, Chen A, He W. Platelet activation: a promoter for psoriasis and its comorbidity, cardiovascular disease. Front Immunol. (2023) 14:1238647. doi: 10.3389/fimmu.2023.1238647
64. Zhao Y, Shao W, Zhu Q, Zhang R, Sun T, Wang B, et al. Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and Nutrition Examination Survey 2011-2016. J Transl Med. (2023) 21:691. doi: 10.1186/s12967-023-04491-y
65. Ye Z, Hu T, Wang J, Xiao R, Liao X, Liu M, et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: A systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:933913. doi: 10.3389/fcvm.2022.933913
Keywords: systemic immune-inflammation index, psoriasis, PASI, NHANES, stroke
Citation: Zhao X, Li J and Li X (2024) Association between systemic immune-inflammation index and psoriasis: a population-based study. Front. Immunol. 15:1305701. doi: 10.3389/fimmu.2024.1305701
Received: 02 October 2023; Accepted: 21 February 2024;
Published: 05 March 2024.
Edited by:
Matias Ostrowski, University of Buenos Aires, ArgentinaReviewed by:
Antonio Costanzo, Humanitas Research Hospital, ItalySerena Bergamo, ULSS2 Marca Trevigiana, Italy
Copyright © 2024 Zhao, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinhua Li, tylixinhua@sina.com
 Xiya Zhao
Xiya Zhao Junqin Li2,3
Junqin Li2,3