- 1Department of Respiratory and Critical Care Medicine, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 2Beijing Institute of Hepatology, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 3Beijing Engineering Research Center for Precision Medicine and Transformation of Hepatitis and Liver Cancer, Beijing, China
- 4Fourth Department of Liver Disease (Difficult & Complicated Liver Diseases and Artificial Liver Center), Beijing You’an Hospital, Capital Medical University, Beijing, China
- 5Department of Nephrology, Shanxi Provincial People's Hospital, The Affiliated People's Hospital of Shanxi Medical University, Shanxi, China
- 6Department of Traditional Chinese Medicine, Qinhuangdao Shanhaiguan People's Hospital, Hebei, China
Background: Acute-on-Chronic Liver Failure (ACLF) patients experience systemic inflammation as well as immune dysfunction and exhaustion. The phenotype and functionality of monocyte-derived dendritic cells in ACLF patients with different clinical parameters have not been elucidated.
Methods: This study included 37 cases of ACLF, 20 cases of Chronic Hepatitis B (CHB) patients, and 12 healthy controls. Demographic and laboratory parameters were collected from the enrolled patients. Peripheral blood samples were obtained from the participants. Monocyte-derived dendritic cells were induced and cultured, followed by co-culturing with T cells from the patients. Cell surface markers and intracellular markers were analyzed using flow cytometry. The relationship between these markers and clinical parameters was compared.
Results: Our study found that ACLF patients had lower expression levels of HLA-DR, CD86, and CD54 on monocyte-derived dendritic cells compared to both CHB patients and healthy controls. IL-4, GM-CSF, and alcohol were found to promote the expression of HLA-DR, CD86, and CD54 on monocyte-derived dendritic cells. In ACLF patients, higher levels of procalcitonin (PCT), lower levels of albumin, decreased prothrombin activity and deceased patients were associated with lower expression of HLA-DR, CD86, and CD54 on monocyte-derived dendritic cells. Peripheral blood mononuclear cells (PBMCs), after removing adherent cells, were co-cultured with monocyte-derived DC. Our study revealed that patients with infection and low albumin levels exhibited a decreased proportion of T cell subsets within PBMCs. Additionally, these patients’ T cells showed lower levels of Ki-67 and interferon-gamma (IFN-γ) production.
Conclusion: ACLF patients exhibit varying clinical states, with differences in the phenotype and the ability of monocyte-derived dendritic cells to stimulate T cells. Alcohol can stimulate the maturation of monocyte-derived dendritic cells.
Introduction
Acute-on-chronic liver failure (ACLF) is a severe form of acute decompensated liver cirrhosis, characterized by the failure of one or more major organ systems (liver, kidney, circulation/respiration/brain, and coagulation), as well as systemic inflammation that may be caused by acute precipitating factors (either intrahepatic or extrahepatic stimuli, or both) (1, 2). In Europe, alcohol-related factors are the primary contributors to ACLF, while in Asia, approximately 70% of ACLF cases are caused by Hepatitis B Virus (HBV) (3, 4). Despite advances in antiviral medications and life support technologies, the mortality rate associated with ACLF remains high (5, 6).
Until now, there are still many unknowns regarding the pathophysiology of ACLF (7). Currently, we know that systemic inflammation plays a crucial role in driving ACLF (8, 9). However, our understanding of how systemic inflammation develops and its impact on organ function is limited. Additionally, another observed immune dysfunction in ACLF is immune cell paralysis, which can lead to bacterial infections and worsen the overall condition (10, 11). Evidence of excessive inflammatory response includes elevated levels of C-reactive protein and white blood cells in the plasma, increased levels of inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokines (IL-10 and IL-1ra) in the circulatory system, as well as activation of macrophages (8). Evidence of immune dysfunction includes impaired neutrophil function in ACLF patients (12), decreased expression of HLA-DR in monocytes (mHLA-DR) (13), low levels of TNF-α production in response to LPS stimulation (14), and recent studies have also found a reduction in Natural Killer (NK) cells and their association with poor prognosis in ACLF patients (6).
Monocyte-derived dendritic cells (MoDCs) play a critical role in the immune system (15, 16). They serve as important antigen-presenting cells, capable of capturing foreign antigens and activating T cells, thereby triggering an immune response. Additionally, MoDCs produce various cytokines and chemokines that regulate the activity and interaction of immune cells (17). Monocytes and DC cells play important roles in the development of ACLF (18). Studies have shown that ACLF patients experience a decrease in HLA-DR expression on peripheral blood monocytes as the disease worsens, and severe cases exhibit reduced secretion of pro-inflammatory cytokines IL-1β and TNF-α (19). Additionally, research suggests that DCs migrate from the blood to the liver in ACLF patients, where they suppress the secretion of IFN-γ by CD8 T cells, thereby reducing liver damage (16). However, the exact role of MoDCs in ACLF development remains unclear, especially regarding the status of MoDCs in ACLF patients with different clinical conditions.
To address this question, we compared the phenotype of MoDCs between patients with acute-on-chronic liver failure (ACLF), chronic hepatitis B (CHB), and healthy controls. We then categorized ACLF patients based on infection, albumin levels, prothrombin activity, and outcome status. We further compared the functional properties of MoDCs and their ability to promote T cell responses in ACLF patients with different clinical profiles.
Materials and methods
Study participants
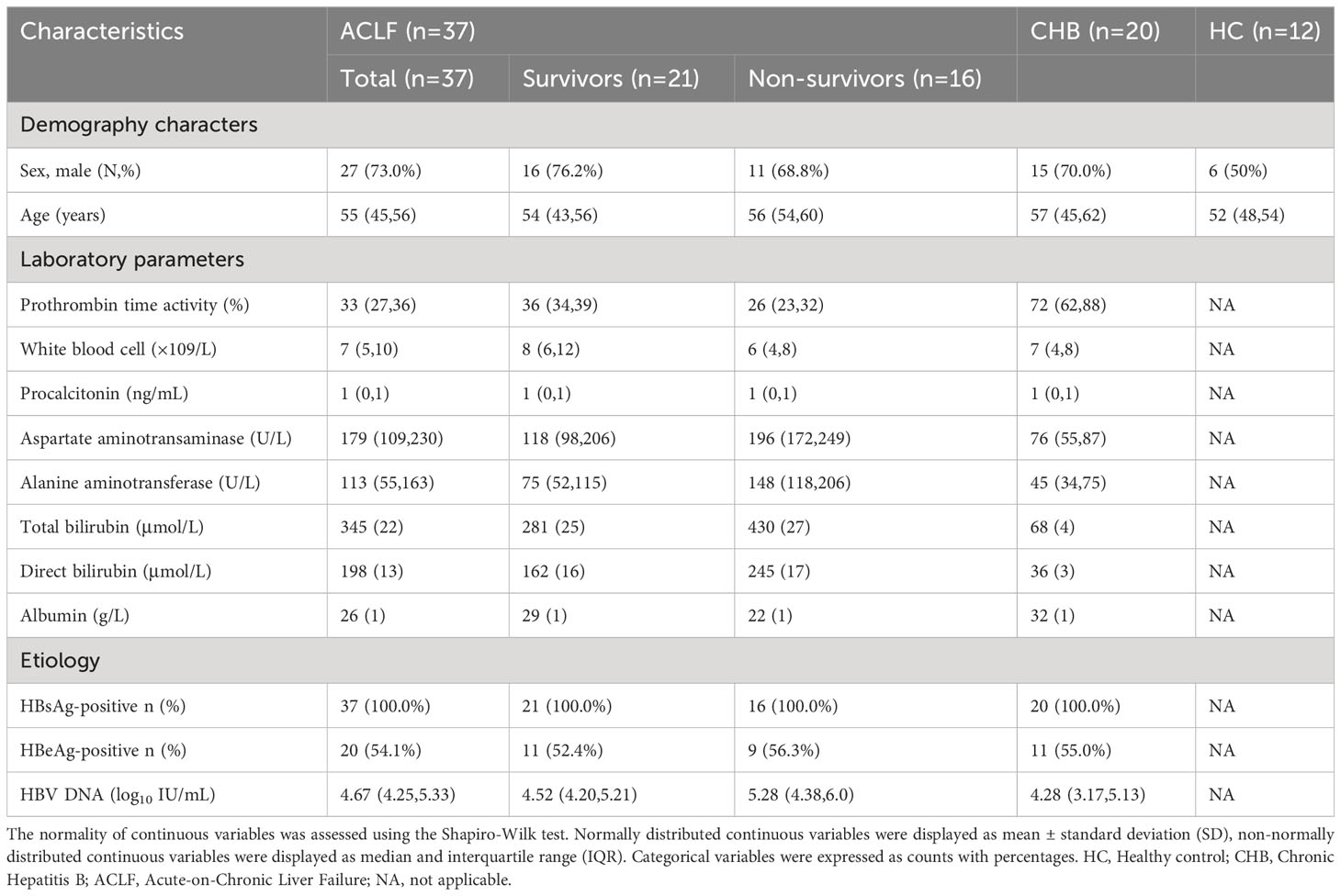
This study enrolled a total of 37 patients with ACLF who were treated at Beijing Youan Hospital, Capital Medical University, from March 2022 to October 2022. Additionally, 20 patients with Chronic Hepatitis B (CHB) and 12 healthy individuals were included as control groups. The ACLF patients were monitored until either their death or discharge from the hospital. The diagnosis of ACLF was based on the criteria established by the Asian Pacific Association for the Study of the Liver, which defines it as an acute liver injury characterized by jaundice (serum bilirubin ≥ 85.5 μmol/L [5 mg/dL]) and impaired coagulation (prothrombin activity < 40% or international normalized ratio ≥ 1.5), accompanied by clinical manifestations of ascites and/or encephalopathy within four weeks in patients with previously diagnosed or undiagnosed chronic liver disease/cirrhosis (3). The diagnosis of CHB followed the guidelines outlined in the Chronic Hepatitis B Prevention and Treatment Guidelines (2019 edition) (4). Individuals with concurrent HIV infection, hepatitis C virus (HCV) or hepatitis G virus (HGV) infections, autoimmune liver diseases, malignancies, age below 18 or above 80 years, and pregnant women were excluded from the study. Due to the significant impacts of immune modulators and steroids on the immune system, the patients included in our study did not receive these medications, or the blood samples were collected prior to the administration of these medications. The study collected clinical characteristics and recorded 28-day mortality data. Demographic information and baseline laboratory parameters, including gender, age, and various laboratory values such as prothrombin time activity, white blood cell count, hemoglobin, calcitonin, alanine aminotransferase, aspartate aminotransferase, platelet count, total bilirubin, direct bilirubin, and albumin, and HBsAg status, HBeAg status, and HBV DNA levels were documented for the enrolled patients (Table 1). The development of acute injury in HBV-ACLF and the treatment history of antiviral therapy are presented in Tables S1, S2, respectively. The study received approval from the Ethics Committee of Beijing Youan Hospital, Capital Medical University, and informed consent was obtained from patients or their family members in cases the patients themselves were unable to sign the consent form before participating in the study.
Blood sample processing and cell culture
Under sterile conditions, 10 mL of peripheral whole blood sample was collected using purple-capped anticoagulant tubes. Peripheral blood mononuclear cells (PBMCs) were separated from the blood sample using Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. In brief, whole blood was mixed with RPMI 1640 medium (Gibco, cat. no.: C11875500CP) and lymphocyte separation solution (STEMCELL, cat. no.: 7851) in a 1:1:1 ratio, transferred to Leucosep centrifuge tubes (Greiner Bio-One, Germany), and centrifuged at 2000 rpm for 10 minutes at 21°C without braking. The white cell pellet containing PBMCs was collected, and the cells were washed twice with 10 mL of fresh RPMI-1640. Cell counting was performed, and the cells were resuspended at a density of 2×10^6 cells/mL in serum-free culture medium and transferred to a 6-well culture plate with 2 mL of serum-free culture medium. The culture plate was then incubated at 37°C for 2 hours. After incubation, monocytes adhered to the culture plate while lymphocytes remained in suspension. The supernatant containing lymphocytes was collected and discarded, and the cell suspension was frozen and stored using RPMI-1640 (10%) + FBS (80%) (Gibco, cat. no.: 10099141) + dimethyl sulfoxide (DMSO; 10%) (Sigma, USA, cat. no.: D2650) as the freezing medium. The cells were divided into small aliquots and stored at -80°C for future use.
The immature MoDCs from monocytes were generated using a previously established method (20). Monocytes were cultured in 6-well cell culture plates using VIVO medium (Lonza, cat. no.: 04-418Q) for 6 days. The medium was supplemented with 10% fetal bovine serum (FBS), IL-4 (Peprotech, cat. no.: AF-200-04-20UG) at a concentration of 50 ng/mL, and GM-CSF (Peprotech, cat. no.: AF-300-03-20UG). After washing the immature dendritic cells, they were further cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin-glutamine solution (PSG) in a 96-well plate. On the seventh day, the cells were stimulated again using a combination of IL-4 and GM-CSF or cytokines with alcohol (30 μl/ml). On the eighth day, the cells were collected and washed twice with PBS. Cell aggregates were removed using a cell filter. The dendritic cells were co-cultured with lymphocytes at a ratio of 1:10, and autologous lymphocyte suspension and IL-2 (Peprotech, cat. no.: AF-200-02) at a concentration of 50 U/L were added. The co-culture was maintained for a total of 4 days.
Flow cytometry
For cell surface and intracellular staining, standard protocols were followed using monoclonal antibodies. Cells and antibodies were co-incubated on ice in the dark for 30 minutes. For intracellular staining of cytokines, cells were permeabilized using the Cytofix/Cytoperm Kit (BD Biosciences, San Jose, CA, USA) for 30 minutes on ice, followed by an additional 30-minute incubation with antibodies in the dark at 4°C. The following antibodies were used: Anti-HLA-DR-APC (BD, cat. no.: 339194), anti-human CD54-APC (BD, cat. no.: 561899), anti-human CD86-FITC (BD, cat. no.: 560958), anti-human CD3-Percp (BD, cat. no.: 552851), anti-Human CD4-PE (BD, cat. no.: 557344), anti-Human CD8-FITC (BD, cat. no.: 555366), anti-human Ki-67-APC (BioLegend, cat. no.: 350514), and anti-IFN-gamma-APC (BD, cat. no.: IC285A-100). Imaging flow cytometry was performed using the ImageStreamX MarkII quantitative imaging flow cytometer, while conventional flow cytometry was performed using the BD FACS Calibur flow cytometer.
Statistical analyses
Data analysis and graphing were performed using GraphPad Prism (Version 9.0, La Jolla, CA, USA). The data were presented as mean with standard deviation (SD) and analyzed using two-way analysis of variance (ANOVA). Significance levels were denoted as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Result
The proportion of mature moDCs in patients with acute-on-chronic liver failure is lower compared to those with chronic hepatitis B and healthy controls
In order to investigate the changes in monocyte-derived dendritic cells in patients with ACLF, we compared the monocyte-derived dendritic cells from peripheral blood among three groups: healthy controls, CHB patients, and ACLF patients. A total of 37 ACLF cases, 20 CHB cases, and 12 healthy individuals were included in the study, and the necessary characteristics are presented in Table 1. Human leukocyte antigen-DR (HLA-DR), along with CD86 and CD54, are important surface markers for dendritic cell maturation. Our study found that the expression of HLA-DR, CD86, and CD54 in ACLF patients was lower compared to both CHB patients and healthy controls. IL-4 and GM-CSF can stimulate dendritic cell maturation, and our previous research has shown that alcohol can also induce DC maturation (Figures S1, S2). After adding these stimulatory factors, the expression of HLA-DR, CD86, and CD54 on DCs increased, but the ACLF group still exhibited lower expression compared to the CHB and HC groups (Figure 1 and Figure S3).

Figure 1 Summary data of HLA-DR, CD86, CD54 expression on moDCs from HC, CHB and ACLF with or without stimulation. moDCs: Monocyte-derived dendritic cells; HC: Healthy control; CHB: Chronic Hepatitis B; ACLF: Acute-on-Chronic Liver Failure; HLA-DR: human leukocyte antigen-DR; GM-CSF: Granulocyte-Macrophage Colony-Stimulating Factor; SD: Standard Deviation. Data analyzed by two-way analysis of variance (ANOVA), displayed as Mean with SD. *P <0.05, **P <0.01, ***P <0.001 and ****P <0.0001.
The proportion of mature moDCs in patients with acute-on-chronic liver failure varies based on different clinical parameters
In order to compare the functional differences of moDCs in ACLF patients based on different clinical parameters, we grouped the patients according to their levels of procalcitonin (PCT), albumin, Prothrombin activity (PTA), and whether they had deceased. The study revealed a correlation between the maturity of moDCs and these clinical indicators. Patients with high PCT levels, low albumin levels, low coagulation factor activity, and deceased patients exhibited lower expression of HLA-DR, CD86, and CD54 on moDCs. We also observed that alcohol can stimulate the maturation of moDCs. However, even after alcohol stimulation, patients with high PCT levels, low albumin levels, low PTA, and deceased patients still showed lower expression of HLA-DR, CD86, and CD54 (Figure 2 and Figures S4-S7).
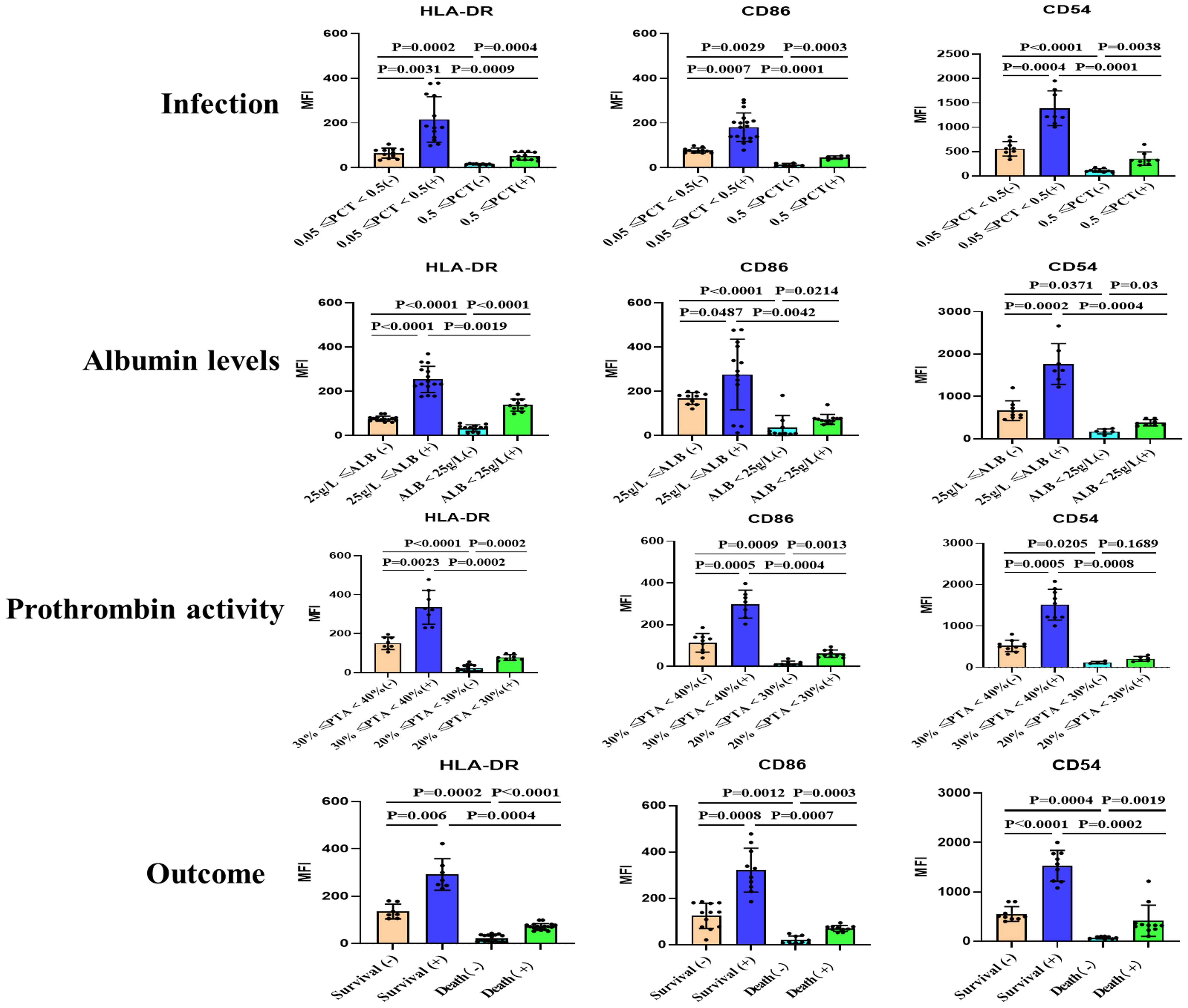
Figure 2 Summary data on the surface expression of HLA-DR, CD86, and CD54 on monocyte-derived dendritic cells (moDCs) from patients with acute-on-chronic liver failure (ACLF) with or without stimulation. Grouping was performed based on infection, Albumin levels, Prothrombin activity and Outcome. (+): Stimulated with alcohol. Representative data was determined as mean fluorescence intensity (MFI). moDCs: Monocyte-derived dendritic cells; ACLF: Acute-on-Chronic Liver Failure; HLA-DR: human leukocyte antigen-DR; PCT: Procalcitonin; SD: Standard Deviation. Data analyzed by two-way analysis of variance (ANOVA), displayed as Mean with SD.
The subsets of T cells, their proliferation, and interferon-γ production after co-culture with monocyte-derived dendritic cells are associated with the grouping based on clinical parameters
To compare the stimulatory capacity of monocyte-derived dendritic cells (moDCs) on T cells among patients with different clinical parameters, we grouped the patients based on their levels of procalcitonin (PCT) and albumin. Peripheral blood monocytes, after removing adherent cells, were co-cultured with monocyte-derived DC. Our study found that patients with infection and low albumin levels exhibited a decreased proportion of T cell subsets within PBMCs. Additionally, these patients’ T cells showed lower levels of Ki-67 (Figure 3 and Figure S8) and interferon-γ production (Figure 4 and Figure S9). Alcohol stimulation can enhance the stimulatory effect of monocyte-derived DC on T cells, thereby increasing T cell proliferation and interferon-γ production. However, compared to the non-infection group and non-low albumin group, the infection group and low albumin group still demonstrated lower levels of Ki-67 and interferon-γ production in T cells.
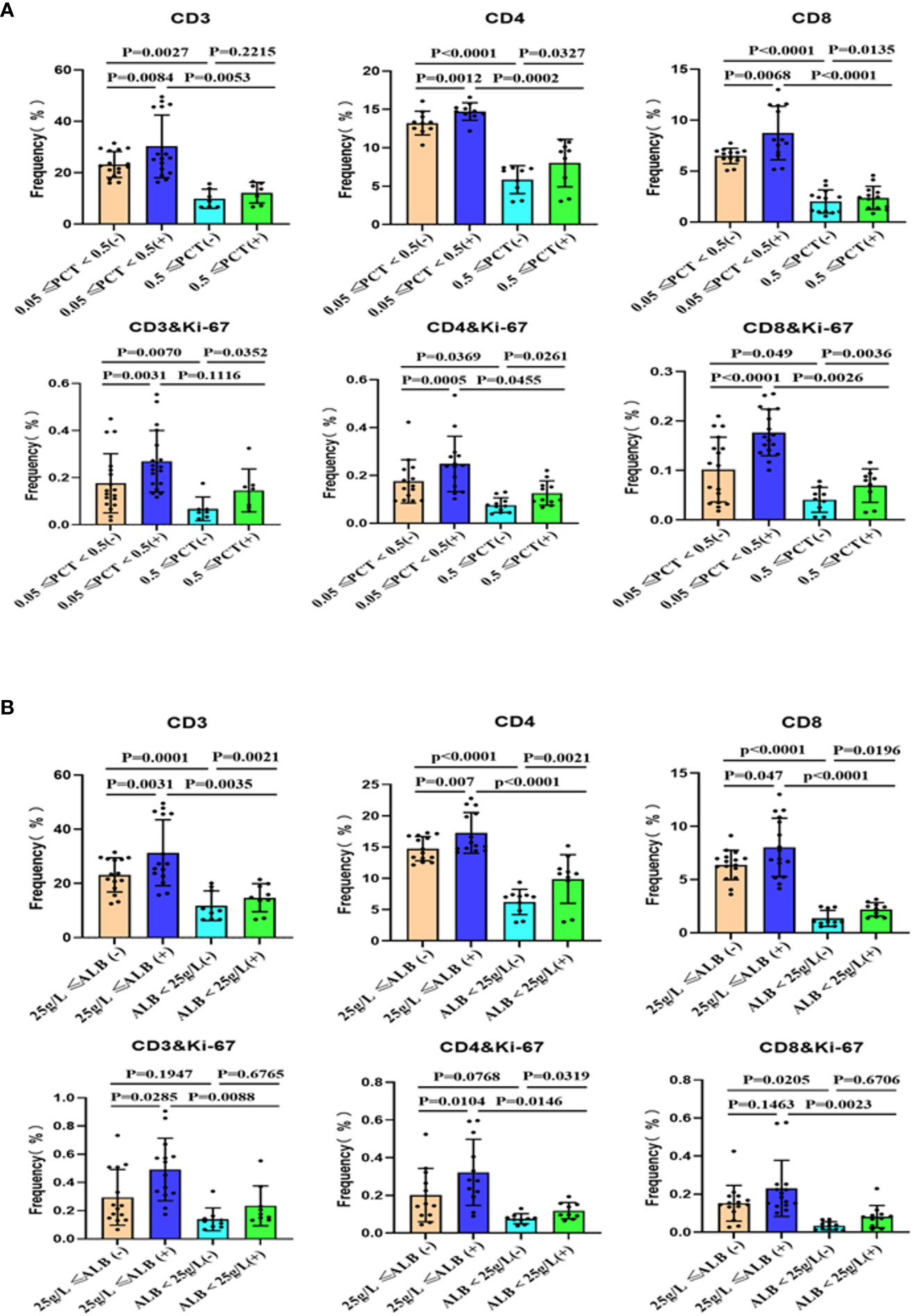
Figure 3 Subsets of peripheral blood T cells and their proliferation after co-culture with moDCs from ACLF with or without stimulation. Summary of the data for the groups based on infection (A) and albumin levels (B). (+): Stimulated with alcohol. moDCs: Monocyte-derived dendritic cells; ACLF: Acute-on-Chronic Liver Failure; T: T lymphocytes; PCT: Procalcitonin; SD: Standard Deviation. Data analyzed by two-way analysis of variance (ANOVA), displayed as Mean with SD.

Figure 4 Subsets of peripheral blood T cells and their interferon-γ production after co-culture with mo-DCs from ACLF with or without stimulation. Summary of the data for the groups based on infection (A) and albumin levels (B). (+): Stimulated with alcohol. mo-DCs: Monocyte-derived dendritic cells; ACLF: Acute-on-Chronic Liver Failure; T: T lymphocytes; PCT: Procalcitonin; SD: Standard Deviation. Data analyzed by two-way analysis of variance (ANOVA), displayed as Mean with SD.
Discussion
Dendritic cells (DCs) are an important cell type in the immune system, responsible for antigen presentation and promoting immune responses by activating specific T cells (15). Dendritic cells possess highly differentiated cell surface molecules, including HLA-DR, CD86, and CD54, which play important roles in the process of dendritic cell activation of T cells (21–25). This study found that patients with ACLF exhibited significantly reduced expression of surface markers HLA-DR, CD86, and CD54 compared to patients with CHB or healthy individuals. We categorized the patients based on their infection status, albumin levels, prothrombin activity, and outcomes, and found that ACLF patients with infections, low albumin levels, low prothrombin activity, and poor prognosis had significantly decreased surface marker expression on monocyte-derived dendritic cells. When co-cultured with autologous T cells, dendritic cells from these patients showed a significant impairment in their ability to stimulate T cell proliferation and INF-γ production. Additionally, our research revealed that alcohol can effectively promote the maturation of monocyte-derived dendritic cells, thereby enhancing T cell proliferation and INF-γ production.
Immunological dysfunction and exhaustion are important characteristics of ACLF (18). Research has indicated that monocytes in ACLF patients undergo exhaustion due to bioenergetic failure (26). Furthermore, research has demonstrated that monocytes and dendritic cells are impaired in severe alcoholic hepatitis. This immune dysfunction is associated with a higher risk of infection and mortality (27). Another study has shown a correlation between impaired TLR3 signaling in MoDCs and disease severity in ACLF patients. In addition, there was a significant correlation observed between the expression of TLR3 signaling components (TLR3/IFN-β) in individual ACLF patients and markers of disease severity (total bilirubin and prothrombin activity) (28). However, there is still a lack of research on the clinical characteristics of ACLF patients and the features of monocyte-derived dendritic cells.
Prothrombin activity is an important measure of disease severity in ACLF patients and is included in the diagnostic criteria for ACLF. It is also associated with patient prognosis (1, 3). Infection may serve as a triggering factor for ACLF and can contribute to poor patient outcomes (29). Procalcitonin is a significant diagnostic marker for bacterial infections in clinical practice (30, 31). Albumin is commonly used to assess the nutritional status of patients (32, 33). Our study found that these clinical parameters, including mortality, are associated with the functional status of monocyte-derived dendritic cells in ACLF patients.
For ACLF patients, infection can be a contributing factor to disease progression and is commonly associated with complications and poor prognosis (11, 29, 34, 35). Studies have indicated that patients with chronic hepatitis C virus (HCV) infection exhibit decreased levels of DC cells in peripheral blood and reduced release of IFN-alpha compared to healthy individuals (36). The diminished function of DCs may promote susceptibility to infection, and severe infections can lead to excessive activation of immune function, resulting in immune exhaustion and further disease aggravation (18).
Furthermore, research has shown that during the progression of ACLF, the immune response shifts from an initial severe pro-inflammatory phase to an anti-inflammatory phase. The pro-inflammatory cytokines IL-1β and TNF-α secreted by monocytes are increased in the early stage and decreased in the later stage (19). In summary, the interaction between infection and reduced DC function is likely a mutually reinforcing process.
The level of albumin is an important indicator of the clinical nutritional status of patients (1). Currently, specific data on ACLF is lacking, but it can be inferred that malnutrition and muscle wasting are common in most ACLF patients. Studies focused on decompensated cirrhosis patients have shown a high prevalence of malnutrition and muscle wasting, ranging from 22% to 62% reported incidence (37). Extensive data has demonstrated that malnutrition is an independent risk factor for bacterial infection, further decompensation, and mortality in decompensated cirrhosis patients (38, 39). Therefore, special attention needs to be given to the nutritional status of ACLF patients (40). However, there is a lack of research on the relationship between nutritional status and immune status in these patients. Our study, for the first time, suggests a potential association between albumin levels and the maturation of monocyte-derived dendritic cells in patients, and the underlying mechanisms require further investigation.
Prothrombin activity is one of the indicators used to assess clotting function (1). In patients with acute-on-chronic liver failure, there is a correlation between prothrombin activity, infection, and poor prognosis (41–43). While prothrombin activity is primarily used to assess clotting function, there is extensive interaction between the clotting system and the immune system (44). A study on HBV-ACLF revealed a correlation between white blood cell count, lymphocytes, and peripheral T lymphocytes with prothrombin activity (PTA) (45). Another study indicated a negative correlation between plasma sCD163 levels and prothrombin activity (46). There exists a complex interrelationship between prothrombin activity and immune function. Further research is needed to delve into the interactions between the clotting system and the immune system in order to better understand their roles in disease development and treatment.
Research on the impact of alcohol on dendritic cell (DC) function has been conducted. It has been found that chronic alcohol-induced liver disease can inhibit DC function (47). Additionally, studies have indicated that alcohol can directly interfere with DC function, suppressing their ability to combat pathogens. This may result in a weakened immune system and increased susceptibility to infections (48). Both in vivo and in vitro studies have explored the effects of ethanol on DC function, revealing its influence on the generation, expression of co-stimulatory molecules, and functionality of both plasmacytoid and myeloid dendritic cell subsets (49). However, our research indicates that alcohol can stimulate the maturation of monocyte-derived dendritic cells. We speculate that the reasons for contradictory research outcomes may be related to factors such as cell sources and the timing of stimulation. Prolonged stimulation, which can lead to functional exhaustion of dendritic cells and subsequent functional suppression, may explain the opposite findings. Our study involved a short-term stimulation. The underlying mechanisms require further investigation.
In summary, this study suggests that even among ACLF patients, who have varying degrees of disease severity, the immune cell function, particularly that of monocyte-derived dendritic cells, may differ. Therefore, it may be necessary to consider different treatment approaches for different patient subtypes.
Our study also has several limitations. Firstly, the sample size was relatively small, and further subgrouping of ACLF patients based on the etiology of their disease was not performed. Secondly, detailed information regarding the clinical treatment of patients was not recorded. Thirdly, we did not extract monocytes specifically from PBMCs of patients and then induce and culture them. Fourthly, further subtyping of dendritic cells was not performed. Fifthly, the specific mechanisms by which alcohol stimulates dendritic cells were not investigated, and this is an area for future research.
Conclusion
ACLF patients exhibit varying clinical states, with differences in the phenotype and the ability of monocyte-derived dendritic cells to stimulate T cells. Alcohol can stimulate the maturation of monocyte-derived dendritic cells.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethical Committee of Beijing Youan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZW: Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Writing - original draft, Writing - review & editing. HongbS: Conceptualization, Supervision, Validation, Writing - original draft, Writing - review & editing, Funding acquisition. LZ: Data curation, Formal Analysis, Supervision, Writing - original draft. HonglS: Data curation, Supervision, Writing - original draft. XM: Data curation, Formal Analysis, Supervision, Writing - original draft. LC: Data curation, Formal Analysis, Supervision, Writing - original draft. YC: Conceptualization, Supervision, Writing - review & editing. YM: Conceptualization, Supervision, Writing - review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Beijing Nova Program (Grant No. 20220484201), Beijing Natural Science Foundation (Grant No. M22030) and Beijing municipal medical research institute public welfare development and reform pilot project (Grant No. jingyiyan2021-10).
Acknowledgments
The authors would like to thank Beijing Youan Hospital for allowing us to access the computerized health records.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1290445/full#supplementary-material
Supplementary Table 1 | Acute insults in patients with HBV-ACLF.
Supplementary Table 2 | History of antiviral treatment in patients with HBV-ACLF.
Supplementary Figure 1 | Imaging cytometry of moDCs from HC, CHB, ACLF with or without stimulation.
Supplementary Figure 2 | Imaging cytometry of mo-DCs without stimulation or with alcohol stimulation.
Supplementary Figure 3 | Representative dot plots of HLA-DR, CD86, CD54 expression on mo-DCs from HC, CHB and ACLF with or without stimulation.
Supplementary Figure 4–7 | Representative data depicting the surface expression of HLA-DR, CD86, and CD54 on monocyte-derived dendritic cells (moDCs) from patients with acute-on-chronic liver failure (ACLF), with or without stimulation. Grouping was performed based on infection (Figure S4), albumin levels (Figure S5), prothrombin activity (Figure S6), and outcome (Figure S7).
Supplementary Figure 8 | Subsets of peripheral blood T cells and their proliferation after co-culture with moDCs from ACLF with or without stimulation.
Supplementary Figure 9 | Subsets of peripheral blood T cells and their interferon-γ production after co-culture with mo-DCs from ACLF with or without stimulation.
Abbreviations
moDCs, Monocyte-derived dendritic cells; ACLF, Acute-on-Chronic Liver Failure; CHB, Chronic Hepatitis B; HLA-DR, human leukocyte antigen-DR; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; PCT, Procalcitonin; PBMCs, Peripheral blood mononuclear cells; PTA, Prothrombin activity, IFN-γ, interferon-gamma; HBV, Hepatitis B Virus; NK cells, Natural Killer cells; HCV, hepatitis C virus; HGV, hepatitis G virus.
References
1. European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol (2023) 79(2):461–91. doi: 10.1016/j.jhep.2023.04.021
2. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology (2013) 144(7):1426–37, 1437.e1-9. doi: 10.1053/j.gastro.2013.02.042
3. Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int (2019) 13(4):353–90. doi: 10.1007/s12072-019-09946-3
4. Guideline for diagnosis and treatment of liver failure. Zhonghua Gan Zang Bing Za Zhi (2019) 27(1):18–26. doi: 10.3760/cma.j.issn.1007-3418.2019.01.006
5. Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut (2018) 67(12):2181–91. doi: 10.1136/gutjnl-2017-314641
6. Li HJ, Yang N, Mu X, Tang L, Wang SS, Zhou CB, et al. Reduction of natural killer cells is associated with poor outcomes in patients with hepatitis B virus-related acute-on-chronic liver failure. Hepatol Int (2022) 16(6):1398–411. doi: 10.1007/s12072-022-10386-9
7. Tyc O, Shi Y, Fan Y-C, Trebicka J, Xiang X. Editorial: Acute-on-chronic liver failure: systemic inflammation and immunosuppression. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1260749
8. Arroyo V, Angeli P, Moreau R, Jalan R, Claria J, Trebicka J, et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol (2021) 74(3):670–85. doi: 10.1016/j.jhep.2020.11.048
9. Bernsmeier C, Pop OT, Singanayagam A, Triantafyllou E, Patel VC, Weston CJ, et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology (2015) 148(3):603–15.e14. doi: 10.1053/j.gastro.2014.11.045
10. Martin-Mateos R, Alvarez-Mon M, Albillos A. Dysfunctional immune response in acute-on-chronic liver failure: it takes two to tango. Front Immunol (2019) 10:973. doi: 10.3389/fimmu.2019.00973
11. Schierwagen R, Gu W, Brieger A, Brune B, Ciesek S, Dikic I, et al. Pathogenetic mechanisms and therapeutic approaches of acute-to-chronic liver failure. Am J Physiol Cell Physiol (2023) 325(1):C129–40. doi: 10.1152/ajpcell.00101.2023
12. Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology (2007) 46(3):831–40. doi: 10.1002/hep.21737
13. Berry PA, Antoniades CG, Carey I, McPhail MJ, Hussain MJ, Davies ET, et al. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis. Intensive Care Med (2011) 37(3):453–60. doi: 10.1007/s00134-010-2099-7
14. Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, Vidacek D, Siewert E, et al. Patients with acute on chronic liver failure display "sepsis-like" immune paralysis. J Hepatol (2005) 42(2):195–201. doi: 10.1016/j.jhep.2004.10.019
15. Palucka K, Banchereau J. Dendritic cells: A link between innate and adaptive immunity. J Clin Immunol (1999) 19(1):12–25. doi: 10.1023/A:1020558317162
16. Zhang Z, Zou ZS, Fu JL, Cai L, Jin L, Liu YJ, et al. Severe dendritic cell perturbation is actively involved in the pathogenesis of acute-on-chronic hepatitis B liver failure. J Hepatol (2008) 49(3):396–406. doi: 10.1016/j.jhep.2008.05.017
17. Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol (2006) 6(6):476–83. doi: 10.1038/nri1845
18. Qiang R, Liu XZ, Xu JC. The immune pathogenesis of acute-on-chronic liver failure and the danger hypothesis. Front Immunol (2022) 13:935160. doi: 10.3389/fimmu.2022.935160
19. Tan W, Xia J, Dan Y, Li M, Lin S, Pan X, et al. Genome-wide association study identi fi es HLA-DR variants conferring risk of HBV-related acute-on-chronic liver failure. Gut (2018) 67(4):757–66. doi: 10.1136/gutjnl-2016313035
20. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med (1994) 179(4):1109–18. doi: 10.1084/jem.179.4.1109
21. Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol (2008) 8(9):675–84. doi: 10.1038/nri2379
22. Sousa MG, Ghosn EE, Nascimento RC, Bomfim GF, Noal V, Santiago K, et al. Monocyte-derived dendritic cells from patients with severe forms of chromoblastomycosis induce CD4+ T cell activation in vitro. Clin Exp Immunol (2009) 156(1):117–25. doi: 10.1111/j.1365-2249.2008.03870.x
23. Kamide Y, Utsugi M, Dobashi K, Ono A, Ishizuka T, Hisada T, et al. Intracellular glutathione redox status in human dendritic cells regulates IL-27 production and T-cell polarization. Allergy (2011) 66(9):1183–92. doi: 10.1111/j.1398-9995.2011.02611.x
24. Kubo S, Yamaoka K, Kondo M, Yamagata K, Zhao J, Iwata S, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis (2014) 73(12):2192–8. doi: 10.1136/annrheumdis-2013-203756
25. Zhao L, Yang X. Cross talk between natural killer T and dendritic cells and its impact on T cell responses in infections. Front Immunol (2022) 13:837767. doi: 10.3389/fimmu.2022.837767
26. Maheshwari D, Kumar D, Jagdish RK, Nautiyal N, Hidam A, Kumari R, et al. Bioenergetic failure drives functional exhaustion of monocytes in acute-on-chronic liver failure. Front Immunol (2022) 13:856587. doi: 10.3389/fimmu.2022.856587
27. Weichselbaum L, Azouz A, Smolen KK, Das J, Splittgerber M, Lepida A, et al. Epigenetic basis for monocyte dysfunction in patients with severe alcoholic hepatitis. J Hepatol (2020) 73(2):303–14. doi: 10.1016/j.jhep.2020.02.017
28. Li N, Li Q, Qian Z, Zhang Y, Chen M, Shi G. Impaired TLR3/IFN-beta signaling in monocyte-derived dendritic cells from patients with acute-on-chronic hepatitis B liver failure: relevance to the severity of liver damage. Biochem Biophys Res Commun (2009) 390(3):630–5. doi: 10.1016/j.bbrc.2009.10.018
29. Simone Incicco S, Angeli P, Piano S. Bacterial infections in acute on chronic liver failure. Clin Liver Dis (2023) 27(3):703–16. doi: 10.1016/j.cld.2023.03.013
30. Trippella G, Galli L, De Martino M, Lisi C, Chiappini E. Procalcitonin performance in detecting serious and invasive bacterial infections in children with fever without apparent source: a systematic review and meta-analysis. Expert Rev Anti Infect Ther (2017) 15(11):1041–57. doi: 10.1080/14787210.2017.1400907
31. Omer I, Abuthiyab N, Al Zaid N, Alkanani R, Abualnaja R, Khan G, et al. Procalcitonin as a tool to antimicrobial stewardship in COVID-19 patients with superimposed bacterial infections: A systematic review. J Inflammation Res (2022) 15:6055–64. doi: 10.2147/JIR.S377644
32. Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol (2010) 21(2):223–30. doi: 10.1681/ASN.2009020213
33. Qi C, Wang L, Duan G. Nutritional and inflammatory immune-based hemoglobin, albumin, lymphocyte, and platelet (HALP) score as a prognostic biomarker in patients with high-risk neuroblastoma. Asian J Surg (2023) 46(2023):4068–9. doi: 10.1016/j.asjsur.2023.04.054
34. Li J, Liang X, Jiang J, Yang L, Xin J, Shi D, et al. PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut (2022) 71(1):163–75. doi: 10.1136/gutjnl-2020-323395
35. Zhang Q, Shi B, Wu L. Characteristics and risk factors of infections in patients with HBV-related acute-on-chronic liver failure: a retrospective study. PeerJ (2022) 10:e13519. doi: 10.7717/peerj.13519
36. Goutagny N, Vieux C, Decullier E, Ligeoix B, Epstein A, Trepo C, et al. Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J Infect Dis (2004) 189(9):1646–55. doi: 10.1086/383248
37. Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol (2021) 75 Suppl 1(Suppl 1):S147–62. doi: 10.1016/j.jhep.2021.01.025
38. Bischoff SC, Bernal W, Dasarathy S, Merli M, Plank LD, Schutz T, et al. [ESPEN Practical Guideline: clinical nutrition in liver disease]. Nutr Hosp (2022) 39(2):434–72. doi: 10.20960/nh.03856
39. European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol (2019) 70(1):172–93. doi: 10.1016/j.jhep.2018.06.024
40. Reuter B, Shaw J, Hanson J, Tate V, Acharya C, Bajaj JS. Nutritional assessment in inpatients with cirrhosis can be improved after training and is associated with lower readmissions. Liver Transpl (2019) 25(12):1790–9. doi: 10.1002/lt.25602
41. Xu MM, Kong M, Yu PF, Cao YY, Liu F, Zhu B, et al. Clinical course and outcome patterns of acute-on-chronic liver failure: A multicenter retrospective cohort study. J Clin Transl Hepatol (2021) 9(5):626–34. doi: 10.14218/JCTH.2020.00179
42. Li X, Wang L, He N, Zhang Y, Pang J, Wang H, et al. The relationship between zinc deficiency and infectious complications in patients with hepatitis B virus-related acute-on-chronic liver failure. Gastroenterol Rep (Oxf) (2022) 10:goac066. doi: 10.1093/gastro/goac066
43. Yang J, Xue R, Wu J, Jia L, Li J, Yu H, et al. Development and validation of a nomogram for 90-day outcome in patients with hepatitis B virus-related acute-on-chronic liver failure. J Clin Transl Hepatol (2022) 10(3):458–66. doi: 10.14218/JCTH.2021.00202
44. Teodoro AGF, Rodrigues WF, Farnesi-de-Assunção TS, Borges AVBe, Obata MMS, Neto JRdC, et al. Inflammatory response and activation of coagulation after COVID-19 infection. Viruses (2023) 15(4):938. doi: 10.3390/v15040938
45. Wang F, Sun W, Xiao Q, Liang C, Jiang S, Lian Y, et al. Peripheral T lymphocytes predict the severity and prognosis in patients with HBV-related acute-on-chronic liver failure. Med (Baltimore) (2021) 100(5):e24075. doi: 10.1097/MD.0000000000024075
46. Ye H, Wang LY, Zhao J, Wang K. Increased CD163 expression is associated with acute-on-chronic hepatitis B liver failure. World J Gastroenterol (2013) 19(18):2818–25. doi: 10.3748/wjg.v19.i18.2818
47. Zhang H, Zhang F, Zhu Z, Luong D, Meadows GG. Chronic alcohol consumption enhances iNKT cell maturation and activation. Toxicol Appl Pharmacol (2015) 282(2):139–50. doi: 10.1016/j.taap.2014.11.013
48. Rendon JL, Janda BA, Bianco ME, Choudhry MA. Ethanol exposure suppresses bone marrow-derived dendritic cell inflammatory responses independent of TLR4 expression. J Interferon Cytokine Res (2012) 32(9):416–25. doi: 10.1089/jir.2012.0005
Keywords: monocyte-derived dendritic cell, phenotype, T cell stimulatory function, acute-on-chronic liver failure, clinical parameters
Citation: Wu Z, Shi H, Zhang L, Shi H, Miao X, Chen L, Chen Y and Ma Y (2023) Comparative analysis of monocyte-derived dendritic cell phenotype and T cell stimulatory function in patients with acute-on-chronic liver failure with different clinical parameters. Front. Immunol. 14:1290445. doi: 10.3389/fimmu.2023.1290445
Received: 07 September 2023; Accepted: 15 November 2023;
Published: 04 December 2023.
Edited by:
Mary A. Markiewicz, University of Kansas Medical Center, United StatesReviewed by:
Matthew D. Taylor, Feinstein Institute for Medical Research, United StatesSachiyo Yoshio, National Center For Global Health and Medicine, Japan
Copyright © 2023 Wu, Shi, Zhang, Shi, Miao, Chen, Chen and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingmin Ma, ma.yingmin@163.com; Yu Chen, chybeyond1071@ccmu.edu.cn
†These authors have contributed equally to this work
 Zhipeng Wu
Zhipeng Wu Hongbo Shi
Hongbo Shi Lei Zhang5,6
Lei Zhang5,6 Yu Chen
Yu Chen Yingmin Ma
Yingmin Ma