- 1Peking University People’s Hospital, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University, Beijing, China
- 2Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT), as one of the life-saving treatments for severe aplastic anemia (SAA), is widely used because of its great donor availability. Over decades, granulocyte colony-stimulating factor (G-CSF)/antithymocyte globulin (ATG)-based protocol (the so-called Beijing Protocol) has achieved favorable engraftment and survival outcomes. In this study, we modified the conventional Beijing Protocol: the full-dose Cyclophosphamide (Cy) (200 mg/kg in total) was divided into 42.75 mg/kg Cy on day -5 to day -2 and Low dose post-transplant Cy (PTCy) (14.5 mg/kg on days +3 and +4), hoping to reduce the incidence of severe acute graft-versus-host disease (aGVHD) and to guarantee successful and stable engraftment. Here we retrospectively reported and analyzed the data of first 17 patients with SAA who had received haplo-HSCT using this novel regimen between August 2020 and August 2022. The median follow-up was 522 days (range, 138-859 days). No patient developed primary graft failure. Four (23.5%) patients developed grade II bladder toxicity, two (11.8%) patients developed grade II cardiotoxicity. All patients achieved neutrophil and platelet engraftment at median times of 12 days (range, 11–20 days) and14 days (range, 8-36 days). During our follow-up, no patients developed grade III-IV aGVHD. The cumulative incidence of grade II and grade I aGVHD at 100 days was 23.5% (95% CI, 6.8%-49.9%) and 47.1% (95% CI, 23.0%-72.2%). Three patients (17.6%) developed chronic GVHD of skin, mouth, and eyes and all of which were mild. All patients are alive by the end of the follow-up, with a failure-free survival of 100%, which was defined as survival without treatment failures, such as death, graft failure, or relapse rate. The rate of cytomegalovirus (CMV) reactivation was 82.4% (95% CI, 64.3%-100%). The rate of Epstein-Barr virus (EBV) reactivation was 17.6% (95% CI, 3.8%-43.4%). No CMV disease and post-transplantation lymphoproliferative disorder (PTLD) occurred among these patients. In conclusion, the encouraging results of prolonged survival outcomes and reduced incidence of GVHD suggest promising effect of this novel regimen in haplo-HSCT for patients with SAA. Larger-sample prospective clinical trials are needed to confirm the effectiveness of this regimen.
1 Introduction
Patients suffering from severe acquired aplastic anemia (SAA) are at a high risk of death without prompt and appropriate treatment (1, 2). Because of its great donor availability, haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has drawn increasing interest as a curative option for SAA (3–5). However, it remains currently relegated to rather late in the therapeutic algorithm, owing to concerns of life-threatening morbidity and transplant-related mortality (TRM) (6, 7).
Graft failure (GF) and severe graft-versus-host disease (GVHD) are two primary obstacles to successful haplo-HSCT (8, 9). Over decades, granulocyte colony-stimulating factor (G-CSF)/antithymocyte globulin (ATG)-based protocol (the Beijing Protocol (10)) has achieved favorable engraftment and survival outcomes both as an upfront and salvage treatment in SAA patients, which is comparable to those of transplantation from matched donors (11, 12). In the Beijing Protocol, all patients received conditioning regimens of busulfan (Bu), Cy and ATG. The infused graft consisted of G-CSF-stimulated bone marrow (BM) and peripheral blood stem cells (PBSCs). All patients were treated with cyclosporine A (CsA), mycophenolate mofetil (MMF) and short-term methotrexate (MTX) for GVHD prophylaxis. Whereas the relatively high incidence of acute GVHD (aGVHD), 29-33% for grade II-IV and 9-10% for grade III-IV aGVHD, hinders this platform from the more universal application (11–13). Apart from the Beijing Protocol, high-dose post-transplant cyclophosphamide (HD-PTCy, typically 50 mg/kg on days +3 and +4) has been successfully used in haplo-HSCT for SAA, and an inspiring decrease in aGVHD has been consecutively reported (5, 14–18). The European Society for Blood and Bone Marrow Transplantation (EBMT) Severe Aplastic Anemia Working Party reported the outcomes of HD-PTCy-based haplo-HSCT in a multicenter study (19). The incidence of grade II-III aGVHD was 23%, and there was no gradeIV aGVHD. It is particularly worrying that only 67% of patients achieved primary engraftment by day +28. Therefore, it is reasonable to modify the current two transplant protocols to exploit their strength and avoid unfavorable conditions.
Our team has demonstrated that combining low-dose PTCy of 14.5 mg/kg on days +3 and +4 (LD-PTCy) with ATG significantly reduced GVHD without compromising the potency of graft function in hematologic malignancies (20, 21). But it is undetermined if a similar procedure is feasible for patients with SAA. In this study, we modified the conventional Beijing Protocol (the transplantation procedure of which has been described in previous studies (10, 11)): the full-dose Cy (200 mg/kg in total) was divided into 42.75 mg/kg Cy on day -5 to day -2 and LD-PTCy (14.5 mg/kg on days +3 and +4), hoping to reduce the incidence of severe aGVHD and to guarantee successful and stable engraftment. Here we retrospectively reported and analyzed the clinical outcomes of 17 patients who received this modified protocol.
2 Methods
2.1 Patients
The effectiveness and safety of low-dose PTCy protocol for haplo-HSCT of hematological malignancies based on the Beijing protocol has been proved both in animal models and clinical trial in our center (20, 21). Starting from August 2020, we began to apply this protocol in haplo-HSCT for severe aplastic anemia. Between 1 August 2020 and 31 August 2022, 17 patients diagnosed with SAA accepting haplo-HSCT under the PTCy regimen at Peking University People’s Hospital Xizhimen Campus were enrolled in this study. The last follow-up date for all surviving patients was December 31, 2022 (Figure 1). This retrospective study summarized the data of the first 17 patients. Patients met the following criteria: (1) diagnosed with SAA or vSAA, as defined by the International Aplastic Anemia Study Group; (2) under 40 years old; HCT-CI ≤ 3; (3) can tolerate the toxicity of Cy 200mg/kg (total dose) and receive haplo-HSCT with the shift low-dose PTCy regimen; (4) no uncontrolled infectious diseases and liver, lung, renal and heart diseases; (5) lack of available HLA-identical, related sibling or unrelated donor. The protocol was approved by the ethics committee at Peking University People’s Hospital (PKUPH), and the study followed the Helsinki Declaration.
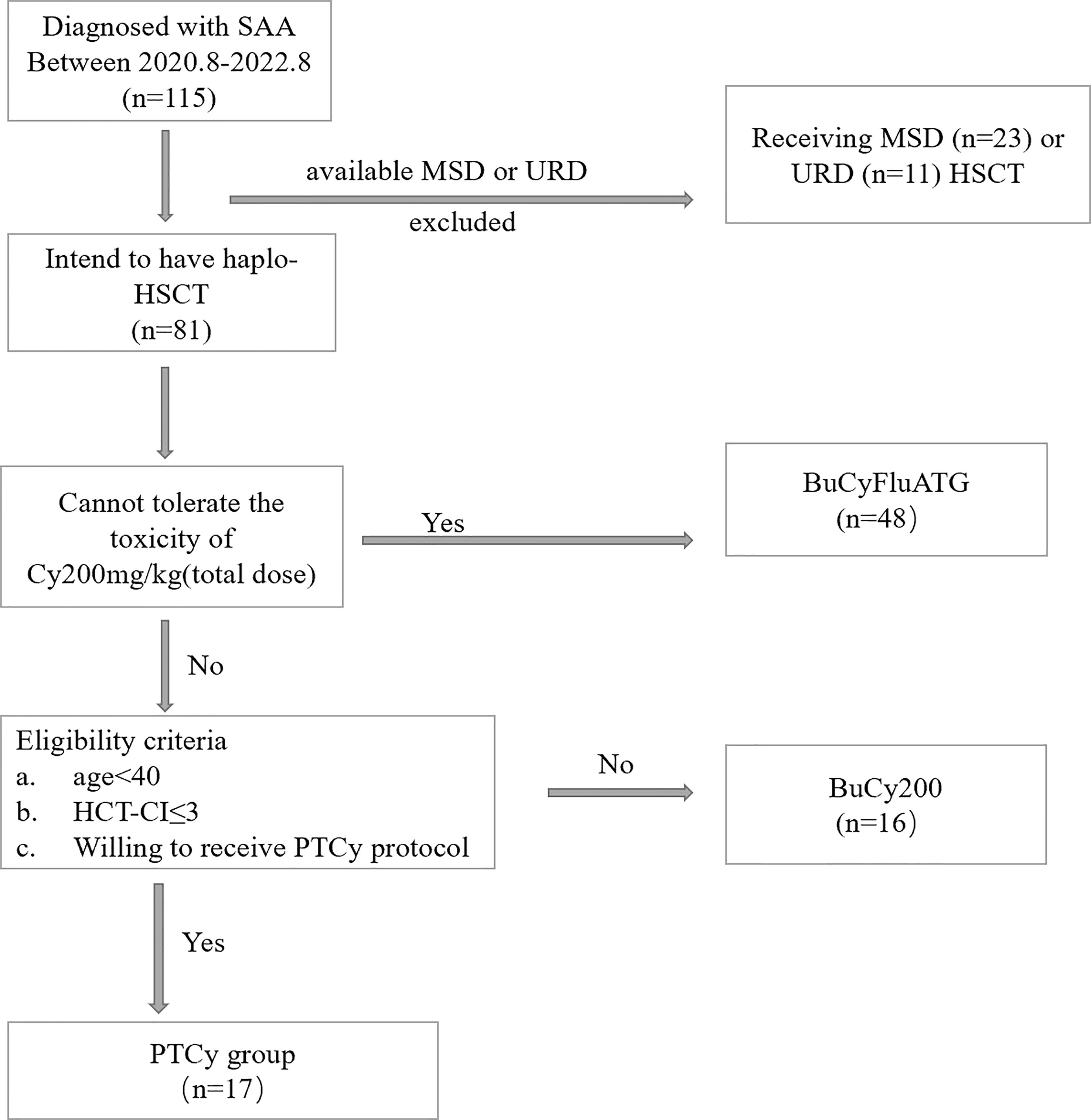
Figure 1 The Diagram of Patient Inclusion. BuCyFluATG regimen: Bu, 3.2mg/kg/d on days -8 and -7; Cy, 25 mg/kg/d on days -5 to -2; Flu, 30 mg/m2 daily on days -6 to -2, and ATG, 2.5 mg/kg daily on days -5 to -2; BuCy200 regimen: Bu, 3.2 mg/kg/d on days -8 and -7; Cy, 50 mg/kg/d, on days -5 to -2; ATG, 2.5 mg/kg/d, on days -5 to -2.
2.2 Definitions
Neutrophil engraftment was defined as the first of three consecutive days when the neutrophil count was ≥0.5 × 109/L, while platelet engraftment was defined as the first occurrence of seven consecutive days with a platelet count ≥20 × 109/L without transfusion. Patients with low donor chimerism who did not exhibit engraftment by day +28 were classified as having primary GF. Acute GVHD and cGVHD were diagnosed and graded according to international criteria (22, 23). Prognostic assessment included evaluation of overall survival (OS), failure-free survival (FFS), GVHD-free or failure-free survival (GFFS). OS was defined as the time from HSCT to death or the last follow-up for any reason. FFS was defined as survival without treatment failures, such as death, graft failure, or relapse. GFFS was defined as survival without grade III–IV aGVHD, moderate-to-severe cGVHD, or treatment failure including death, primary or secondary GF, and relapse. CMV viremia is defined as DNA monitoring greater than >1000 copies/ml. Regimen-related toxicity was defined and graded according to the Bearman criteria (24).
2.3 Transplantation procedures
The conditioning regimen, illustrated in Figure 2, consisted of four intravenous (i.v.) doses of 0.8 mg/kg busulfan (BU) on days -8 and -7, one intravenous dose of 42.75 mg/kg cyclophosphamide (CY) from days -5 to -2, and a four-day intravenous treatment of 2.5 mg/kg rabbit ATG (from SangStat) from day -5 to day -2. For GvHD prophylaxis, aside from PTCy which was administered at 14.5mg/kg and ATG mentioned above, all patients received CsA, MMF, and short-term MTX. CsA was administered intravenously (1.5mg/kg, q12h) from day -9 and switched to oral administration once bowel function returned to normal. MMF was given orally (0.5g q12h in adults or 0.25 g q12h in pediatric patients) from day -9, tapered in half on day +30, and discontinued on day +60. MTX was administered intravenously at a dose of 15mg/m2 on day +1, followed by doses of 10mg/m2 on days +3, +6, and +11.
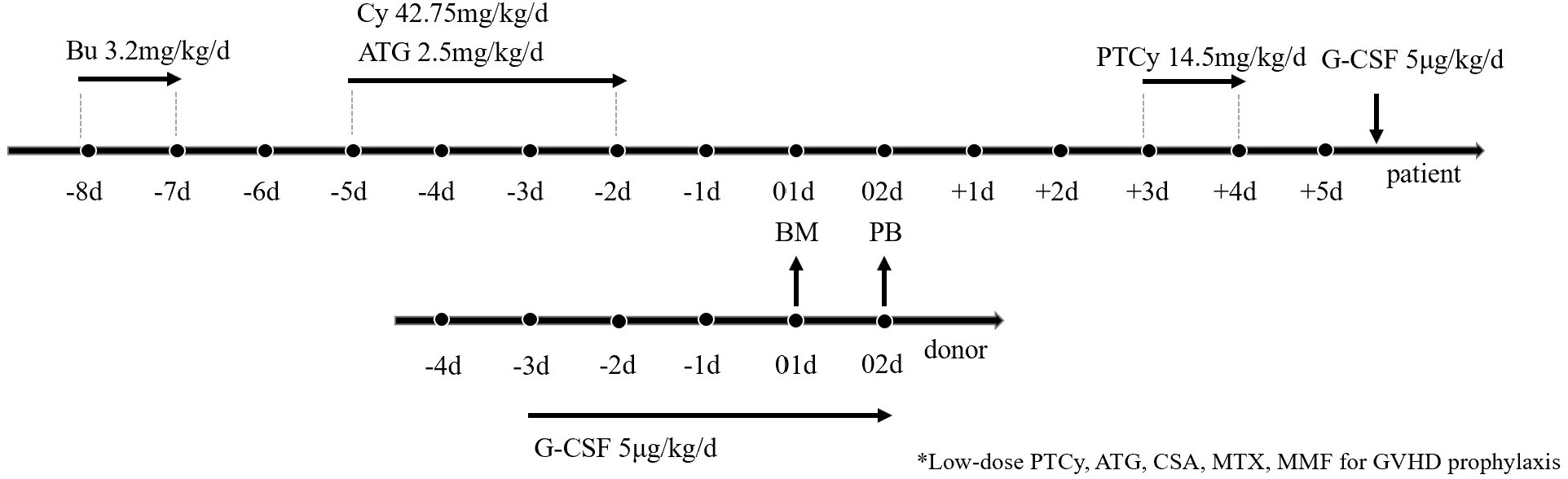
Figure 2 Transplantation Protocol of G-CSF/ATG/Low-dose PTCy regimen. Busulfan at 3.2 mg/kg was administered intravenously on days -7 to -6, followed by ATG at 2.5 mg/kg was administered intravenously on days -5 to -2, along with cyclophosphamide at 42.75 mg/kg intravenously on days -5 to -2. low-dose cyclophosphamide was administered from day 3 to day 4 at 14.5 mg/kg. For GvHD prophylaxis, aside from PTCy administered at 14.5mg/kg and ATG mentioned above, all patients received CsA, MMF, and short-term MTX. Donor stem cells were mobilized with subcutaneous G-CSF injection at a dosage of 5 μg/kg/day from day -3 until the last day of collection. Bone marrow (BM) grafts on day +1 and peripheral blood stem cells (PBSC) on day +2 were collected from donors.
BM grafts were collected on day 01, and peripheral blood stem cells (PBSC) were collected on day 02 from donors who received granulocyte colony-stimulating factor (G-CSF, 5μg/kg/d). BM cells were harvested to achieve a target volume of 10–12 mL/kg of donor weight or a target mononuclear cell (MNC) count of 2-4×108/kg of recipient weight. On the fifth day of mobilization, PBSCs were collected with a COBE Blood Cell Separator (Spectra LRS; COBE BCT Inc., Lakewood, CO, USA) at a rate of 80 mL/min from a total blood volume of 10–12 L. The target MNC from BM and PB was 6-8×108/kg of recipient weight. An additional collection of PBSCs was required the next day if the cell numbers from the previous 2 days were insufficient. Prophylactic therapy with ganciclovir (5mg/kg intravenously, twice daily, from days -8 to -2) was administered to all patients who underwent haploidentical transplantation. Preemptive therapy with ganciclovir (5mg/kg intravenously, twice daily) was started upon diagnosis of CMV viremia, and if the viremia became refractory, treatment was continued with a combination of foscarnet (60 mg/kg intravenously, twice daily) and immunoglobulin.
2.4 Statistical analysis
The main characteristics of patients were reported as descriptive statistics according to the available information. Continuous variables were presented as median and range. Survival analysis was conducted using the Kaplan-Meier method and long-rank test. For dichotomous variables, X² test and Fisher’s exact tests were used, while t-test was used for continuous variables. All p-values were two-tailed, and statistical significance was set at p<0.05. Statistical analysis was performed using SPSS 26.0 (SPSS, Inc., Chicago, IL, NY) and R 3.5.1 (http://www.r-project.org).
3 Results
3.1 Patients characteristics
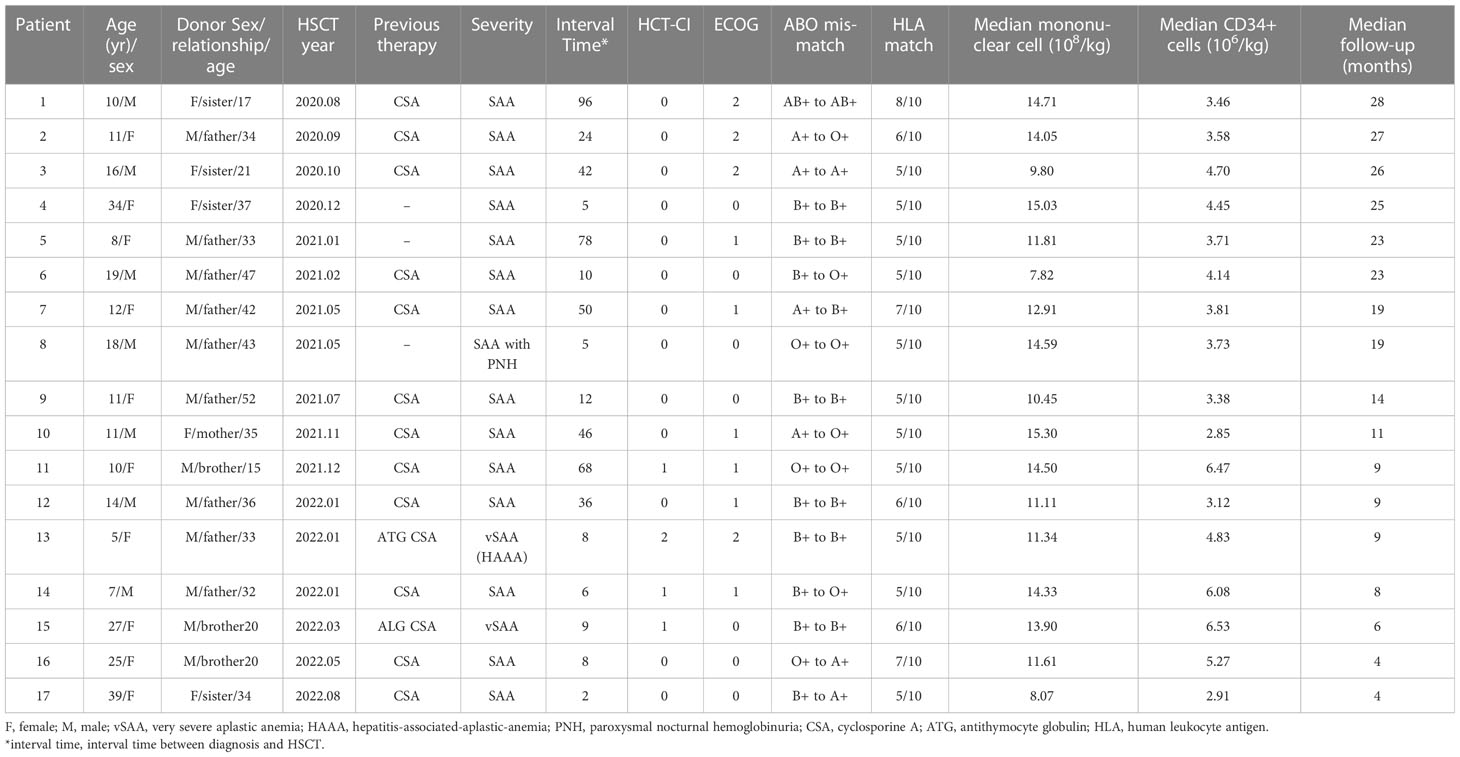
A total of 17 patients received haplo-HSCT using the low-dose PTCy regimen and their characteristics are summarized in Table 1. The median follow-up was 522 days (range, 138-859 days). Seven patients were males (41.18%) and the median age of these patients was 12 (range, 5-39) years old. Two (11.76%) patients had no response to previous IST treatment, including ATG and CSA before HST; the remaining patients failed to respond to CsA ± stanozolol or other promoting-hematopoiesis drugs. The median age of the donors was 34 years (15 to 47 years), and 70.6% (12 of 17) of the donors were male. All donors were related to the patients, most commonly parents (58.8%). The blood types of 10 (58.82%) patients were matched between the donors and recipients, while those of 7 patients were mismatched.
3.2 Engraftment
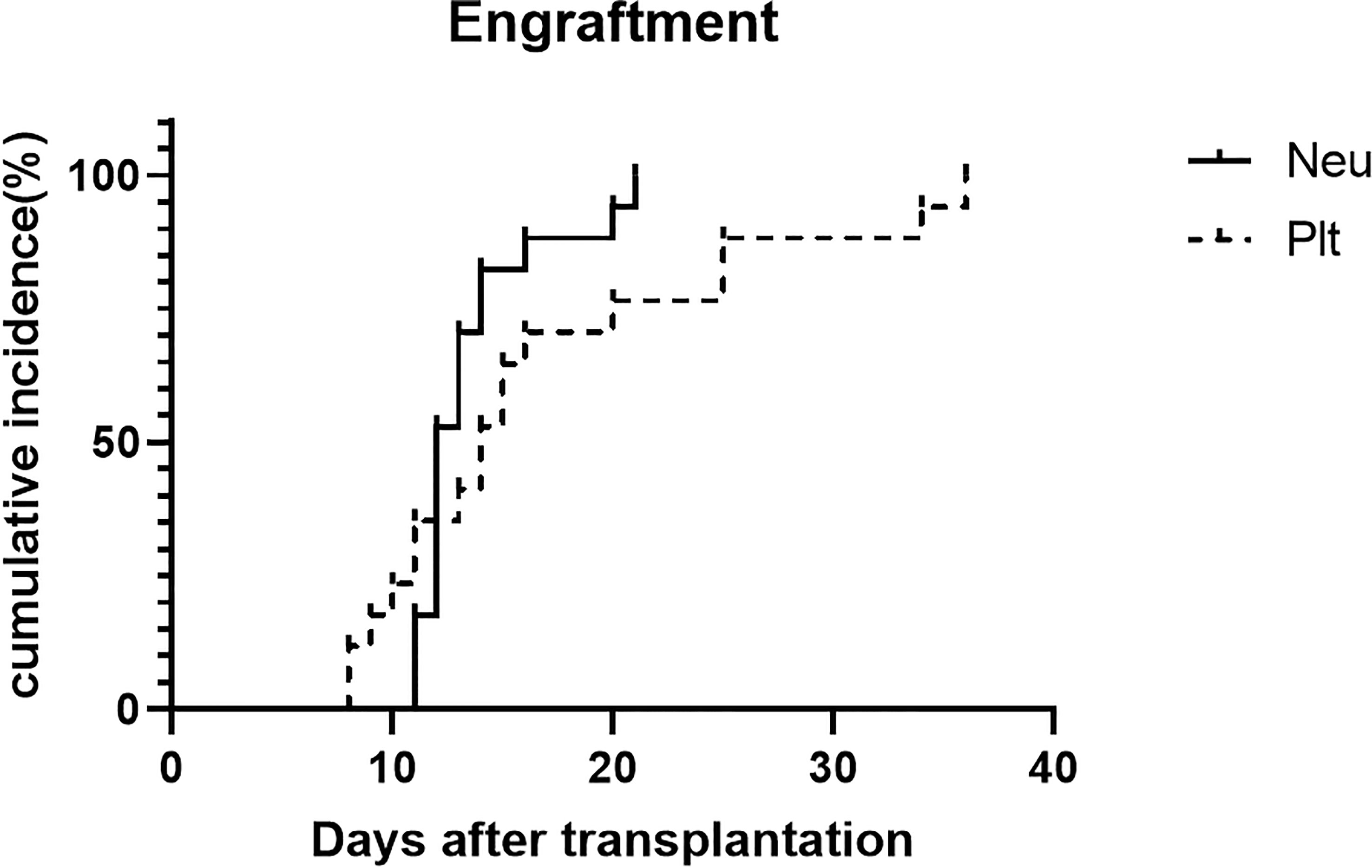
The median mononuclear cells infused in the HSCT were 12.91 (range, 7.82-15.3) ×108/kg. The median CD34+cell count in grafts was 3.38×106/kg (range, 0.46 to 6.53×106/kg). Myeloid recovery and full donor chimerism were achieved in 17 patients after HSCT without primary graft failure. The median time for neutrophil engraftment was 12 days (range, 11–20 days). Platelet engraftment was achieved in all patients at median times of 14 days (range, 8-36 days). The incidence of engraftment is shown in Figure 3.
3.3 GVHD
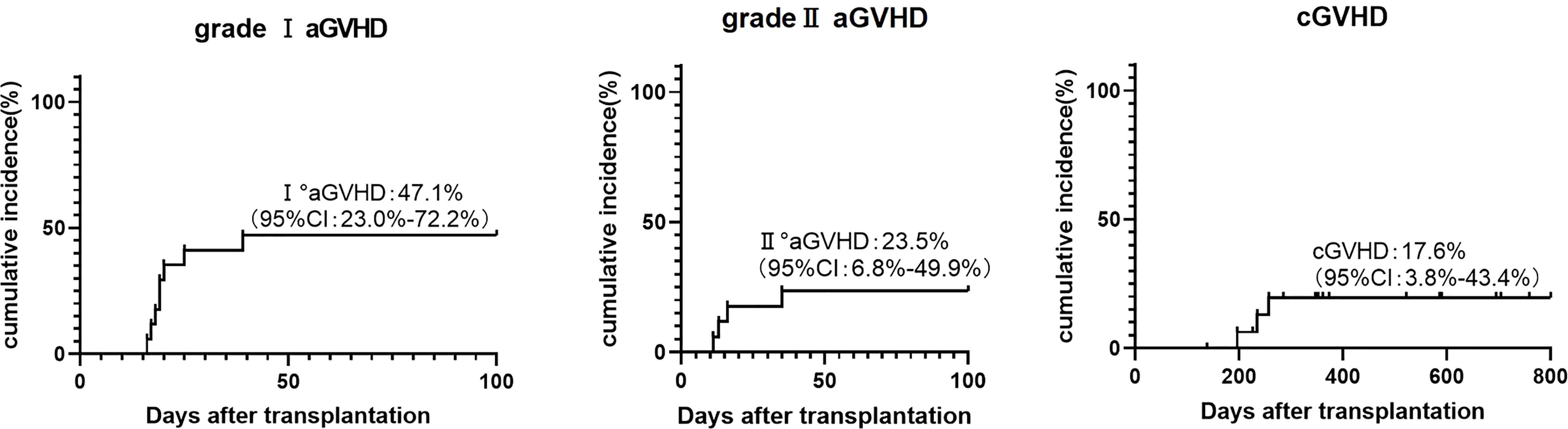
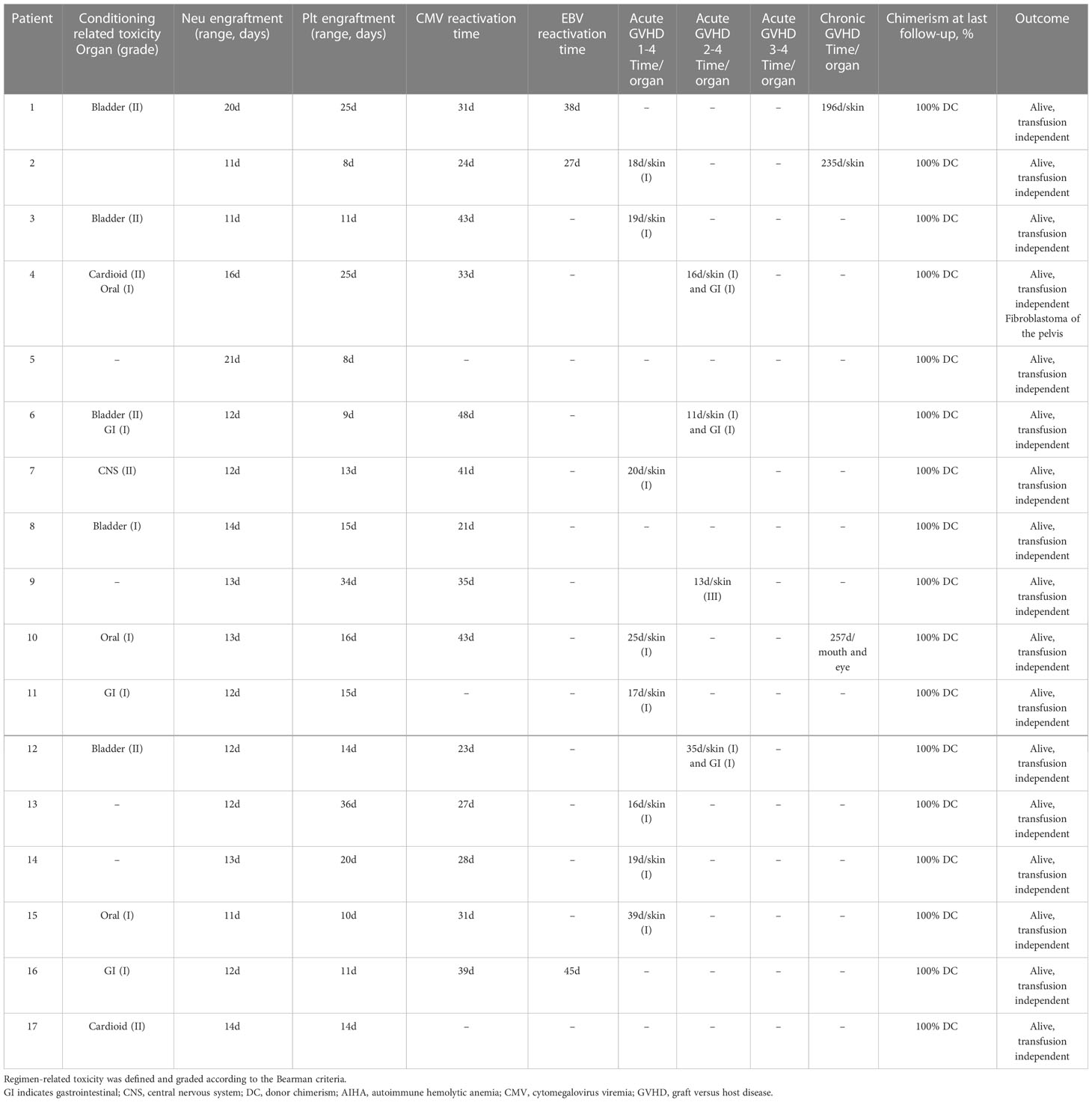
During our follow-up, no patients developed grade III-IV aGVHD. There were four cases of grade II aGVHD, including three cases of skin and gastrointestinal and one case of skin. The cumulative incidence of grade II and grade I aGVHD at 100 days was 23.5% (95% CI, 6.8%-49.9%) and 47.1% (95% CI, 23.0%-72.2%). Three patients (17.623.5%) developed chronic GVHD of skin, mouth, and eyes and all of which were mild (Figure 4). Two of them were diagnosed with skin-related cGVHD on the day+196 and day+235, respectively, presenting as flat mossy changes and skin sclerosis, which quickly recovered under methylprednisolone therapy. Another patient had symptoms in both mouth and eyes (Table 2).
3.4 Regimen-related toxicity and virus reactivation
All patients received the low-dose PTCy conditioning regimen as mentioned above (Figure 1). All of the regimen-related toxicity were mild or moderate. In all, 12 (71.1%) patients exhibited different degrees of toxicity (Table 2). Four (23.5%) patients developed grade II bladder toxicity, two (11.8%) patients developed grade II cardiotoxicity, and the other patients exhibited grade I-II regimen toxicity affecting organs like gastrointestinal tract, central nervous system, bladder, and oral. All toxicities happened are controlled during appropriate treatment and no death was caused by the conditioning regimen.
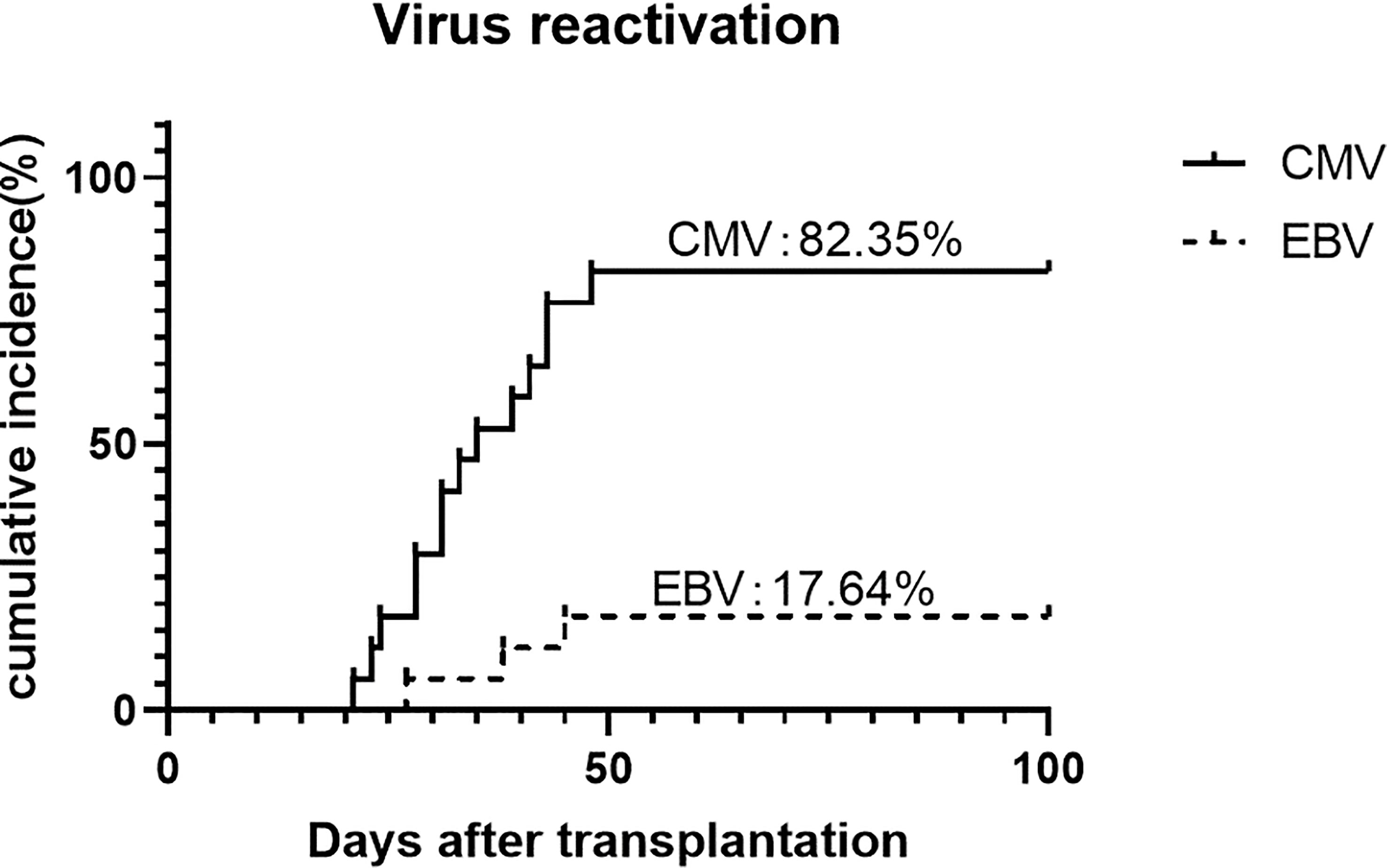
CMV reactivation was discovered in 82.4% (95% CI, 64.3%-100%) of the patients, with a median time of 35 days (range, 21-48 days). No CMV disease occurred during the follow-up. Three (17.6%) patients were discovered to have EBV reactivation and no post-transplantation lymphoproliferative disorder happened (Figure 5).
3.5 Survival and outcomes
The median follow-up was 522 days (range, 138-859 days). All patients survived and had no primary graft failure during our follow-up. The 2-year overall survival (OS) of these patients was 100%. Since there was neither death, graft failure, relapse, nor grade III-IV acute GVHD or moderate-to-severe chronic GVHD, the 2-year FFS and GRFS of these patients were also 100%. By the end of the follow-up, all patients were transfusion-independent at the end of the follow-up.
4 Discussion
The ideal conditioning regimen in SAA specifically should bring sustained engraftment, minimal regimen related toxicity, and a lack of GVHD. Both G-CSF/ATG-based and PTCy-based protocols have shown merits and demerits in haplo-HSCT for SAA (8, 15). Thus, our goal is to maximize the probability of engraftment and minimize GVHD. Based on a modified G-CSF/ATG/LD-PTCy protocol, our encouraging results of 100% primary engraftment, mild toxicity, reduced GVHD, and 100% probabilities of OS and GFFS have been achieved in haplo-HSCT for SAA patients.
During the early stage, GF is the primary concern after haplo-HSCT (25). With the Beijing protocol, the incidence of primary GF is less than 1-2% (11–13). In addition, fast and stable engraftment has been observed according to previous reports on G-CSF/ATG-based platforms (26). While the conventional HD-PTCy-based platforms usually include non-myeloablative regimens and de facto lead to a prolonged time to engraftment, as well as a higher risk of GF. Clay et al. (n = 8) and Esteves et al. (n = 16) have applied the HD-PTCy-based protocol with Flu 150 mg/m2, Cy 29 mg/kg, and total body irradiation (TBI) 2-6 Gy. Two primary GF (25%) and two secondary GF (12.5%), respectively, were documented in their studies (5, 14). The recent EBMT study analyzed multicentric data of thirty-three patients with SAA undergoing HD-PTCy-based haplo-HSCT from 2011 to 2017 (19). Notably, the incidence of myeloid engraftment is relatively low [67% (95% CI 51–83) at day +28 and 79% (95% CI 65–93) at day +100]. Therefore, it is reasonable to apply a more intensified conditioning when considering haplo-HSCT combining PTCy and G-CSF/ATG platform for SAA patients. More recently, Dezern et al. tried to add low-dose ATG (4.5 mg/kg) to the conventional HD-PTCy-based protocol in haplo-HSCT for SAA patients (16). A very concerning risk of GF (3 in an initial 7 patients) was noted in the early attempt, and they, therefore, increased the dose of TBI to 4Gy to ensure successful engraftment. Similar results have been revalidated in a prospective trial, in which 16% of patients developed GF and required a second transplant (27). On the other hand, several centers in China have administrated intravenous Bu with in-depth ablation of recipient-originated BM and hence sustained engraftment to replace TBI in conventional HD-PTCy-based protocol (28, 29). In the present study, we used a modified RIC regimen of Bu 3.2 mg/kg for 2 days and Cy 42.75 mg/kg for 4 days to shift the balance toward sustained engraftment. We demonstrated for the first time that the use of RIC regimen in the G-CSF/ATG/LD-PTCy platform enabled favorable engraftment with an inspiring short time to engraftment and no primary GF in haplo-HSCT for SAA patients. In line with our previous finding, no patient developed mixed chimerism and secondary GF (26, 30).
The other major problem for haploidentical transplantation in SAA patients is GVHD since there is no need for a graft-versus-tumor effect in SAA and it significantly contributes to TRM and inferior long-term survival. Huang et al. led two large multicenter studies and reported the incidences of grade II-IV aGVHD were 30.3% and 33.7%, grade III-IV aGVHD 7.9% and 10.1%, and cGVHD 22.4% and 30.6%, respectively, in SAA patients receiving upfront and salvage haplo-HSCT based on the G-CSF/ATG platform (11, 12). Of note, extended evidence reveals a very low rate of GVHD with the PTCy protocol (31, 32). The cumulative incidences were 12-26% for grade II-IV aGVHD and 0-28% for cGVHD, respectively (5, 28). In general, G-CSF/ATG can mitigate GVHD by eliminating alloreactive T cells and facilitating immune tolerance, while HD-PTCy can reduce alloreactive T cell proliferation, impair the function of surviving alloreactive T cells, and lead to preferential recovery of regulatory T cells (33, 34). Utilizing pre-transplant ATG and HD-PTCy, Dezern et al. have shown a rate of 11% for grade II-IV aGVHD and 8% for 2-year cGVHD. In addition, the rates of grade II-IV aGVHD and 1-year cGVHD were informed to be 16% and 26%, respectively, when giving the same protocol to patients with relapsed or refractory SAA (16). Recently, Wang and Chang et al. demonstrated that LD-PTCy can enhance the preventive effect of G-CSF/ATG on GVHD, and reduced incidences of aGVHD and cGVHD without compromising graft function and anti-tumor effect have been prospectively confirmed in a group of patients with high GVHD risk (21). Consistent with previous experience, we found 23.5% of patients developed grade II aGVHD and the rate of cGVHD was 17.6%. Inspiringly, no patients developed severe aGVHD or moderate-to-severe cGVHD in this small cohort. Our result again proves that LD-PTCy, in concert with ATG, is sufficient to induce immune tolerance. In this way it could further mitigates GVHD and paves the way for using G-CSF/ATG/LD-PTCy protocol in the SAA population.
In this study, we retained the standard dose of ATG from Beijing protocol to provide adequate immunosuppression. We excluded radiation from the regimen to avoid post-transplantation malignancies and growth impairment. Additionally, we shifted low-dose PTCy in the regimen to reduce incidence of GVHD and avoid delayed immune reconstruction. Consequently, the sustained engraftment and low incidences of GVHD have brought encouraging survival. Previously, the OS was 78-90% and GFFS was 60-70% for patients with SAA after haplo-HSCT. Of note, no TRM, treatment failure, or severe GVHD were documented in our study, and therefore the probabilities of OS and GFFS were 100%. Furthermore, with the well-controlled cGVHD and sustained transfusion independence, one can expect a satisfying quality of life following haplo-HSCT (35, 36).
With the intensive immunosuppression, we also paid close attention to the viral infection. We found that the incidence of CMV viremia was relatively high, which was 83.3% in our study. In contrast, it was 19-23% according to Dezern et al. utilizing ATG 4.5mg/kg and HD-PTCy (16). For the record, most of the Chinese population is born CMV seropositive and therefore at a high risk of post-transplant CMV reactivation. The rates of CMV reactivation were 52-80% and 75-82% in the G-CSF/ATG setting and the HD-PTCy setting, respectively (36–38). Li et al. reported a cumulative incidence of CMV reactivation of 53.54% when applying ATG 6 mg/kg plus HD-PTCy protocol, which is similar to the design of Dezern’s. Though the profile of immune reconstitution was absent, we assumed that adding LD-PTCy to the standard dose ATG of 10 mg/kg further delayed immune reconstitution and was partly responsible for increased CMV reactivation. Despite that the rate of CMV reactivation was high, one should note that no CMV disease or CMV-related death occurred. The use of novel agents such as letermovir as CMV prophylaxis may be beneficial in the G-CSF/ATG/LD-PTCy setting (39). In addition, it is possible to extend the modification by reducing ATG dose, given the rationale for ATG de-escalation presented in a prospective study including SAA patients. On the contrary, the rate of EBV reactivation in our study was acceptable compared to previous studies, and no EBV-associated PTLD occurred. Given that PTCy may deplete EBV-harboring lymphocytes, as is done by prophylactic rituximab, the result makes sense.
It is also essential to find this modified regimen was well tolerated. Cardiotoxicity and hemorrhagic cystitis associated with high-dose cyclophosphamide have been extensively reported (40, 41). In this study, no fatal cardiotoxicity or other RRT was observed, although high-dose cyclophosphamide (cumulative dose of 200/kg) was retained in the conditioning and many patients were heavily transfused before transplantation. The rate of hemorrhagic cystitis was also acceptable with no severe hemorrhagic cystitis occurring.
This study has some limitations. Initially, with the small sample size, it takes caution to interpret our results. Long-term follow-up is needed to confirm the encouraging transplant outcomes. As shown in the current cohort, however, G-CSF/ATG/LD-PTCy protocol did lower the incidence of GVHD without imperiling potent engraftment in haplo-HSCT for SAA patients, which can hopefully further improve the therapeutic effect and expand the donor pool. Thus, a prospective clinical trial is about to conduct. Moreover, information on immune reconstitution, as well as further modification such as ATG dose reduction to avoid transplant-related morbidity, are needed.
In conclusion, our data revealed that it is feasible and effective to use the G-CSF/ATG/LD-PTCy protocol in haplo-HSCT for SAA. With RIC preparative regimen and the use of LD-PTCy, the incidence of severe GVHD was reduced and successful and stable engraftment was achieved.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the protocol was approved by the ethics committee at Peking University People’s Hospital (PKUPH), and the study followed the Helsinki Declaration. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
LX and XH designed the study. The data analysis and manuscript development were led by XM and ZX. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2022YFA1103300), the National Natural Science Foundation of China (No.81670167).
Acknowledgments
We extended our gratitude to all colleagues who contributed to this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brodsky RA, Jones RJ. Aplastic anaemia. Lancet (2005) 365:1647–56. doi: 10.1016/S0140-6736(05)66515-4
3. Bacigalupo A. How I treat acquired aplastic anemia. Blood (2017) 129:1428–36. doi: 10.1182/blood-2016-08-693481
4. Passweg JR, Pérez WS, Eapen M, Camitta BM, Gluckman E, Hinterberger W, et al. Bone marrow transplants from mismatched related and unrelated donors for severe aplastic anemia. Bone Marrow Transp (2006) 37:641–9. doi: 10.1038/sj.bmt.1705299
5. Clay J, Kulasekararaj AG, Potter V, Grimaldi F, McLornan D, Raj K, et al. Nonmyeloablative peripheral blood haploidentical stem cell transplantation for refractory severe aplastic anemia. Biol Blood Marrow Transp (2014) 20:1711–6. doi: 10.1016/j.bbmt.2014.06.028
6. Lu D-P, Dong L, Wu T, Huang X-J, Zhang M-J, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood (2006) 107:3065–73. doi: 10.1182/blood-2005-05-2146
7. Lu Y, Sun R-J, Zhao Y-L, Xiong M, Cao X-Y, Zhang J-P, et al. Unmanipulated haploidentical hematopoietic stem cell transplantation achieved outcomes comparable with matched unrelated donor transplantation in young acquired severe aplastic anemia. Biol Blood Marrow Transplant (2018) 24:1881–7. doi: 10.1016/j.bbmt.2018.05.015
8. Xu L-P, Wang S-Q, Ma Y-R, Gao S-J, Cheng Y-F, Zhang Y-Y, et al. Who is the best haploidentical donor for acquired severe aplastic anemia? experience from a multicenter study. J Hematol Oncol (2019) 12:87. doi: 10.1186/s13045-019-0775-9
9. Xu Z-L, Huang X-J. Optimizing outcomes for haploidentical hematopoietic stem cell transplantation in severe aplastic anemia with intensive GVHD prophylaxis: a review of current findings. Expert Rev Hematol (2021) 14:449–55. doi: 10.1080/17474086.2021.1923475
10. Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH, et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant (2012) 47:1507–12. doi: 10.1038/bmt.2012.79
11. Xu L-P, Wang S-Q, Wu D-P, Wang J-M, Gao S-J, Jiang M, et al. Haplo-identical transplantation for acquired severe aplastic anaemia in a multicentre prospective study. Br J Haematol (2016) 175:265–74. doi: 10.1111/bjh.14225
12. Xu L-P, Jin S, Wang S-Q, Xia L-H, Bai H, Gao S-J, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol (2017) 10:25. doi: 10.1186/s13045-017-0398-y
13. Ma Y-R, Wang W-J, Cheng Y-F, Zhang Y-Y, Mo X-D, Han T-T, et al. Impact of ABO incompatibility on outcomes after haploidentical hematopoietic stem cell transplantation for severe aplastic anemia. Bone Marrow Transplant (2020) 55:1068–75. doi: 10.1038/s41409-020-0779-7
14. Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V, et al. Haploidentical BMT and post-transplant cy for severe aplastic anemia: a multicenter retrospective study. Bone Marrow Transplant (2015) 50:685–9. doi: 10.1038/bmt.2015.20
15. Dezern AE, Luznik L, Fuchs EJ, Jones RJ, Brodsky RA. Post-transplantation cyclophosphamide for GVHD prophylaxis in severe aplastic anemia. Bone Marrow Transp (2011) 46:1012–3. doi: 10.1038/bmt.2010.213
16. DeZern AE, Zahurak M, Symons H, Cooke K, Jones RJ, Brodsky RA. Alternative donor transplantation with high-dose post-transplantation cyclophosphamide for refractory severe aplastic anemia. Biol Blood Marrow Transplant (2017) 23:498–504. doi: 10.1016/j.bbmt.2016.12.628
17. Klein OR, Chen AR, Gamper C, Loeb D, Zambidis E, Llosa N, et al. Alternative-donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for nonmalignant disorders. Biol Blood Marrow Transp (2016) 22:895–901. doi: 10.1016/j.bbmt.2016.02.001
18. DeZern AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, et al. Haploidentical BMT for severe aplastic anemia with intensive GVHD prophylaxis including posttransplant cyclophosphamide. Blood Adv (2020) 4:1770–9. doi: 10.1182/bloodadvances.2020001729
19. Prata PH, Eikema D-J, Afansyev B, Bosman P, Smiers F, Diez-Martin JL, et al. Haploidentical transplantation and posttransplant cyclophosphamide for treating aplastic anemia patients: a report from the EBMT severe aplastic anemia working party. Bone Marrow Transplant (2020) 55:1050–8. doi: 10.1038/s41409-019-0773-0
20. Wang Y, Chang Y-J, Chen L, Xu L-P, Bian Z-L, Zhang X-H, et al. Low-dose post-transplant cyclophosphamide can mitigate GVHD and enhance the G-CSF/ATG induced GVHD protective activity and improve haploidentical transplant outcomes. Oncoimmunology (2017) 6:e1356152. doi: 10.1080/2162402X.2017.1356152
21. Wang Y, Wu D-P, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol (2019) 12:88. doi: 10.1186/s13045-019-0781-y
22. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-Host disease: i. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant (2015) 21(3):389–401.e1. doi: 10.1016/j.bbmt.2014.12.001
23. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant (1995) 15:825–8.
24. Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol (1988) 6:1562–8. doi: 10.1200/JCO.1988.6.10.1562
25. Ozdemir ZN, Civriz Bozdağ S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci (2018) 57:163–7. doi: 10.1016/j.transci.2018.04.014
26. Xu Z-L, Cheng Y-F, Zhang Y-Y, Mo X-D, Han T-T, Wang F-R, et al. The incidence, clinical outcome, and protective factors of mixed chimerism following hematopoietic stem cell transplantation for severe aplastic anemia. Clin Transplant (2021) 35:e14160. doi: 10.1111/ctr.14160
27. DeZern AE, Eapen M, Wu J, Talano J-A, Solh M, Dávila Saldaña BJ, et al. Haploidentical bone marrow transplantation in patients with relapsed or refractory severe aplastic anaemia in the USA (BMT CTN 1502): a multicentre, single-arm, phase 2 trial. Lancet Haematol (2022) 9:e660–9. doi: 10.1016/S2352-3026(22)00206-X
28. Xu L, Fu B, Wang W, Xu Y, Wu D, Wang S, et al. Haploidentical hematopoietic cell transplantation for severe acquired aplastic anemia: a case-control study of post-transplant cyclophosphamide included regimen vs. anti-thymocyte globulin & colony-stimulating factor-based regimen. Sci China Life Sci (2020) 63:940–2. doi: 10.1007/s11427-019-9585-x
29. Yang K, Gong S, Jiang T, Liang X, Hu J, Zhu P, et al. Haploidentical peripheral stem cell transplantation for young patients with severe aplastic anemia using post-transplantation cyclophosphamide and methotrexate. Transplant Cell Ther (2021) 27:429.e421–429.e427. doi: 10.1016/j.jtct.2021.02.014
30. Xu L-P, Xu Z-L, Zhang Y-Y, Cheng Y-F, Mo X-D, Han T-T, et al. Bulsufan decreases the incidence of mixed chimaerism in HLA-matched donor transplantation for severe aplastic anaemia. Bone Marrow Transp (2022) 57:1204–6. doi: 10.1038/s41409-022-01682-x
31. Hashem H, Najjar R, Abu-Shanap M, Khattab E, Rihani R, Tbakhi A, et al. Haploidentical hematopoietic cell transplantation using post-transplant cyclophosphamide for children with non-malignant diseases. J Clin Immunol (2021) 41:1754–61. doi: 10.1007/s10875-021-01113-4
32. Arcuri LJ, Nabhan SK, Loth G, Atta EH, Oliveira M, Nichele S, et al. A case series of post-transplantation cyclophosphamide in unrelated donor hematopoietic cell transplantation for aplastic anemia. Biol Blood Marrow Transplant (2020) 26:e222–6. doi: 10.1016/j.bbmt.2020.05.023
33. Nishihori T, Al-Kadhimi Z, Hamadani M, Kharfan-Dabaja MA. Antithymocyte globulin in allogeneic hematopoietic cell transplantation: benefits and limitations. Immunotherapy (2016) 8:435–47. doi: 10.2217/imt.15.128
34. Nunes NS, Kanakry CG. Mechanisms of graft-versus-Host disease prevention by post-transplantation cyclophosphamide: an evolving understanding. Front Immunol (2019) 10:2668. doi: 10.3389/fimmu.2019.02668
35. Xu L-P, Xu Z-L, Wang S-Q, Wu D-P, Gao S-J, Yang J-M, et al. Long-term follow-up of haploidentical transplantation in relapsed/refractory severe aplastic anemia: a multicenter prospective study. Sci Bull (Beijing) (2022) 67:963–70. doi: 10.1016/j.scib.2022.01.024
36. Li Y, Wang N, Li L, Cao Y, Xu J, Wang J, et al. Haploidentical transplantation with modified post-transplantation cyclophosphamide for patients with primary aplastic anemia: a multicenter experience. Transplant Cell Ther (2021) 27:331.e331–331.e337. doi: 10.1016/j.jtct.2021.01.018
37. Zhang Y-Y, Mo W-J, Zuo Y-Y, Zhou M, Zhang X-H, Wang Y, et al. Comparable survival outcome between transplantation from haploidentical donor and matched related donor or unrelated donor for severe aplastic anemia patients aged 40 years and older: a retrospective multicenter cohort study. Clin Transplant (2020) 34:e13810. doi: 10.1111/ctr.13810
38. Zu Y, Zhou J, Fu Y, Fang B, Liu X, Zhang Y, et al. Feasibility of reduced-dose posttransplant cyclophosphamide and cotransplantation of peripheral blood stem cells and umbilical cord-derived mesenchymal stem cells for SAA. Sci Rep (2021) 11:253. doi: 10.1038/s41598-020-80531-7
39. Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. New Engl J Med (2017) 377:2433–44. doi: 10.1056/NEJMoa1706640
40. Marumo A, Omori I, Tara S, Otsuka Y, Konuma R, Adachi H, et al. Cyclophosphamide-induced cardiotoxicity at conditioning for allogeneic hematopoietic stem cell transplantation would occur among the patients treated with 120 mg/kg or less. Asia Pac J Clin Oncol (2022) 18:e507–14. doi: 10.1111/ajco.13674
Keywords: post-transplant cyclophosphamide, GvHD prophylaxis, haploidentical hematopoietic stem cell transplant, severe aplastic anemia, bone marrow transplantation
Citation: Ma X, Xu Z, Han T, Zhang Y, Han W, Fu H, Zhang X, Lin F, Huang X and Xu L (2023) Low-dose post-transplant cyclophosphamide with G-CSF/ATG based haploidentical protocol provides favorable outcomes for SAA patients. Front. Immunol. 14:1173320. doi: 10.3389/fimmu.2023.1173320
Received: 24 February 2023; Accepted: 19 April 2023;
Published: 10 May 2023.
Edited by:
Christopher G. Kanakry, National Cancer Institute (NIH), United StatesReviewed by:
Roberto Crocchiolo, Niguarda Ca’ Granda Hospital, ItalyPhilippe Lewalle, Université libre de Bruxelles, Belgium
Copyright © 2023 Ma, Xu, Han, Zhang, Han, Fu, Zhang, Lin, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanping Xu, lpxu_0415@sina.com
†These authors have contributed equally to this work and share first authorship
 Xiaodi Ma
Xiaodi Ma Zhengli Xu
Zhengli Xu Tingting Han
Tingting Han Yuanyuan Zhang1
Yuanyuan Zhang1 Xiaohui Zhang
Xiaohui Zhang Fan Lin
Fan Lin Xiaojun Huang
Xiaojun Huang Lanping Xu
Lanping Xu