- Department of Urology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Objective: Considering the striking evidence revealed by immunotherapy in advanced or metastatic bladder cancer, investigators have explored neoadjuvant immunotherapy and chemoimmunotherapy in muscle-invasive bladder cancer (MIBC). Currently, there have been a large number of studies reporting varied efficacy and safety of these approaches. Herein, we pooled the available evidence in terms of oncological outcomes (pathological complete response [pCR] and pathological partial response [pPR]) and safety outcomes (immune-related adverse events [irAEs], treatment-related adverse events [TRAEs]), through a systematic review and meta-analysis.
Method: We searched PubMed, Embase, Cochrane Library, and American Society of Clinical Oncology meeting abstracts to identify relevant studies up to June 2022. Studies were included if they evaluated the neoadjuvant immunotherapy or chemoimmunotherapy in MIBC and reported at least the pCR.
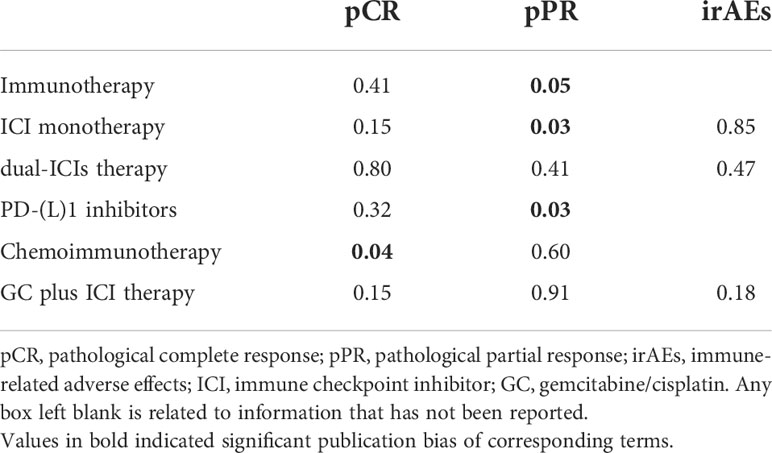
Results: A total of 22 records involving 843 patients were included. For pCR of immunotherapy, the pooled rate of immune checkpoint inhibitor (ICI) monotherapy and dual-ICIs therapy was 24% (95% confidence interval [CI]: 15.3% - 32.8%) and 32.1% (95%CI: 20.6% - 43.7%), respectively. For pCR of chemoimmunotherapy, the overall pooled rate was 42.6% (95% CI: 34.9% - 50.2%). Subgroup of gemcitabine/cisplatin (GC) plus ICI had a pCR rate of 41.7% (95%CI: 35.8% - 47.5%). In terms of safety, the pooled rate of Grade≥3 irAEs was 11.7% (95% CI: 6.5%-16.9%). In subgroup analysis, the Grade≥3 irAEs rate of ICI monotherapy, dual-ICIs therapy, and GC plus ICI therapy was 7.4% (95% CI: 4.3%-10.5%), 30.3% (95% CI: 15.3%-45.3%), and 14.5% (95% CI: 3.5% - 25.4%), respectively. Besides, the pooled Grade≥3 TRAEs rate for chemoimmunotherapy was 32.4% (95% CI: 13.1% - 51.6%).
Conclusion: Neoadjuvant immunotherapy and chemoimmunotherapy were effective and safe in the treatment of MIBC. Compared to ICI monotherapy, dual-ICIs therapy or chemoimmunotherapy can improve the response rate, while increasing the morbidity of Grade≥ 3 irAEs or Grade≥ 3 TRAEs.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD4202233771.
Introduction
Bladder cancer is one of the most common urological malignancies worldwide, with newly diagnosed around 500 000 cases and 200 000 deaths each year (1). Based on the involvement of the bladder muscle or not, bladder cancer can be generally classified into muscle-invasive (MIBC) and non-muscle-invasive bladder cancer (NMIBC), each with distinct biological features and prognosis. NMIBC accounts for approximately 70% of newly diagnosed cases and denotes a favorable prognosis, with 5-year overall survival (OS) rate approaching 90% (2). The remaining 20% and 10% of cases present with muscle-invasive features and advanced stage, and suffer from poor prognosis, with the 5-year OS rate decreasing to 60% and less than 30%, respectively (3). Besides, the dissemination features dramatically increase the metastatic risk, resulting in a 5-year OS rate plunging to 6% (2). Therefore, effective management of MIBC at an early stage can attenuate the risk of local or distant spread and benefit survival.
Neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) represents the standard of care (SoC) for cisplatin-eligible MIBC, remarkably improving the OS. To be specific, for MIBC patients achieving a pathological partial response (pPR) at the time of RC, the 5-year OS rate can approach 90% (2). However, this attractive therapeutic strategy fails to meet the needs of cisplatin-ineligible MIBC, as defined by a previous study (4). Furthermore, a partial of cisplatin-eligible MIBC patients may suffer from severe treatment-related adverse effects and have to discontinue the treatment protocol.
Thanks to the in-depth knowledge of molecular mechanisms of immune checkpoints in the resistance to anti-tumor immunity, immune checkpoint inhibitors (ICIs) have reshaped the treatment paradigm and revolutionized the prognosis of several cancers including melanoma, renal cell carcinoma, and non–small cell lung cancer. A growing number of studies have explored the feasibility and safety of neoadjuvant ICI therapy in MIBC, considering their efficacy in the advanced stage or metastatic setting (5). In the PURE-01 study, preoperative three cycles of pembrolizumab contributed to a 37% pathological complete response (pCR) rate and 55% pPR rate (5). Inspired by these preliminary findings, investigators further explored the feasibility and safety of dual-ICIs and chemoimmunotherapy strategies, in an attempt to amplify the efficacy and promote the prognosis. Van Dijk et al. reported a 45.8% pCR rate in the NABUCCO cohort I study in which three cycles of ipilimumab plus nivolumab were given to stage III MIBC (6). The pCR rate climbed to 51.3% in the BLASST-1 study investigating gemcitabine/cisplatin (GC) plus nivolumab (7).
Herein, we conducted a systemic review on the recent progress of neoadjuvant immunotherapy and chemoimmunotherapy in stage II-III MIBC. Although data on the role of immunotherapy plus targeted therapy for MIBC is limited, a review of current preliminary results has been performed.
Methods and materials
We performed the study according to the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (8). PROSPERO registration number: CRD42022337714.
Literature research
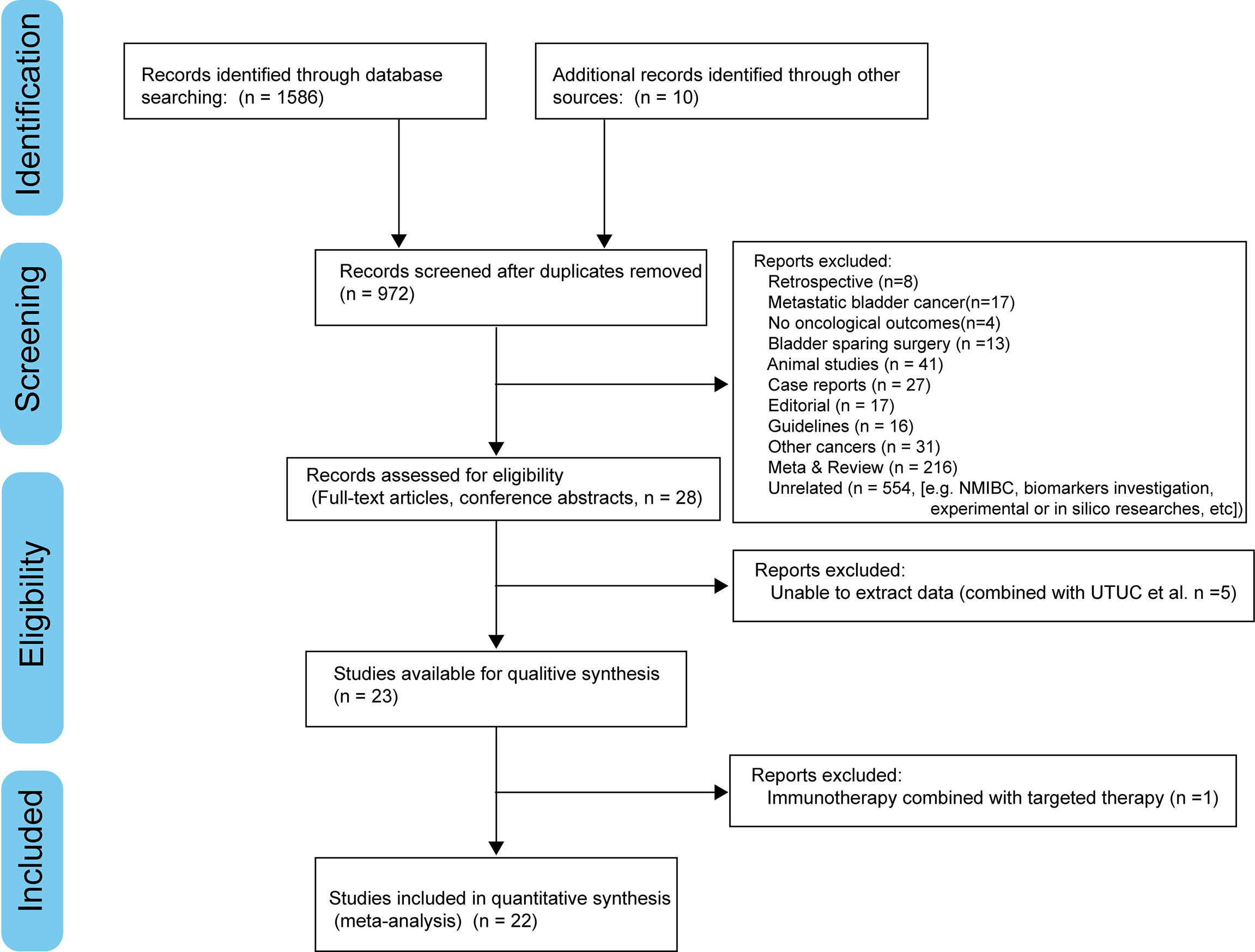
We searched PubMed, Embase, Cochrane Library, and American Society of Clinical Oncology (ASCO) meeting abstracts to identify relevant studies up to June 2022. Figure 1 demonstrates the workflow of literature identification.
The search algorithm was formulated using the following Boolean strategy: (“immunotherapy” OR “nivolumab” OR “ipilimumab” OR “atezolizumab” OR “pembrolizumab” OR “durvalumab” OR “avelumab” OR “tremelimumab” OR “immune checkpoint inhibitor” OR “chemotherapy” OR “gemcitabine” OR “cisplatin”) AND (“neoadjuvant” OR “preoperative”) AND (“urothelial carcinoma” OR “bladder cancer” OR “bladder carcinoma”).
Two authors (H.L.C. and W.J.Y.) independently reviewed the search results and any discrepancy was resolved by consulting with a third author (Z.G.J).
Inclusion and exclusion criteria
The inclusion criteria were listed as follows:
(1) Patients with MIBC (stage II/III);
(2) Patients received at least one type of anti–PD-(L)1 or anti-CTLA4 in the neoadjuvant setting, combined with chemotherapy or not;
(3) Patients underwent RC;
(4) Oncological endpoints were reported: pCR and pPR. The pCR was defined as pathological staging = ypT0N0M0 and the pPR was defined as pathological staging ≤ ypT1N0M0 including ypT0-1N0M0 and ypTisN0M0, consistent with previous studies (9).
(1) Eligible study type: single-arm, RCTs, and non-randomized controlled studies (non-RCTs).
The exclusion criteria were listed as follows:
(1) Patients with other cancers or metastatic bladder cancer;
(2) Patients underwent bladder sparing surgery;
(3) No oncological outcomes were reported;
(4) Non-eligible study type: retrospective, animal, review, meta-analysis, case reports, editorials, guidelines.
Data extraction
Two authors (H.L.C. and W.J.Y.) independently extracted the data and any discrepancy was resolved by consulting with a third author (Z.G.J).
Three types of data were extracted:
(5) Patients: age, cisplatin eligibility, performance status (PS) score, tumor stage, number at recruitment, and RC;
(6) Therapeutic strategy: type of immunotherapy/chemoimmunotherapy, regimen, surgical timeframe (from the last dose to RC), non-response rate (PD and stable disease [SD]), and follow-up period;
(7) Oncological and survival outcomes: pCR, pPR, recurrence-free survival (RFS), and OS;
(8) Safety outcomes: immune-related adverse events (irAEs), treatment-related adverse events (TRAEs), surgical complications, steroid requirement, and death;
(9) Study: first author, trail name/ID, trial phase, study duration, and study design.
We also contacted the corresponding authors via e-mail if the above-mentioned data was not available.
Quality assessment
The modified Jadad scale was used for RCTs (10) and the methodological index for non-randomized studies (MINORS) was used for non-RCTs or single-arm studies (11).
Statistical analysis
All included studies were prospective trials. Considering unpredictable withdrawal from the original well-designed protocol, not all enrolled patients underwent RC after neoadjuvant therapies. Therefore, we performed per protocol (PP) analysis in the pooled oncological outcomes. R version 4.2.0 (The R Foundation for Statistical Computing, MO, USA) and the “meta” package were utilized in the analysis (12). Owing to significant heterogeneity across these studies, we utilized the random effects model to pool these results. Publication bias was assessed by Egger’s regression test (13). Influence analysis was performed to evaluate the impact of each study on the overall pooled result.
Results
Records screening results and characteristics
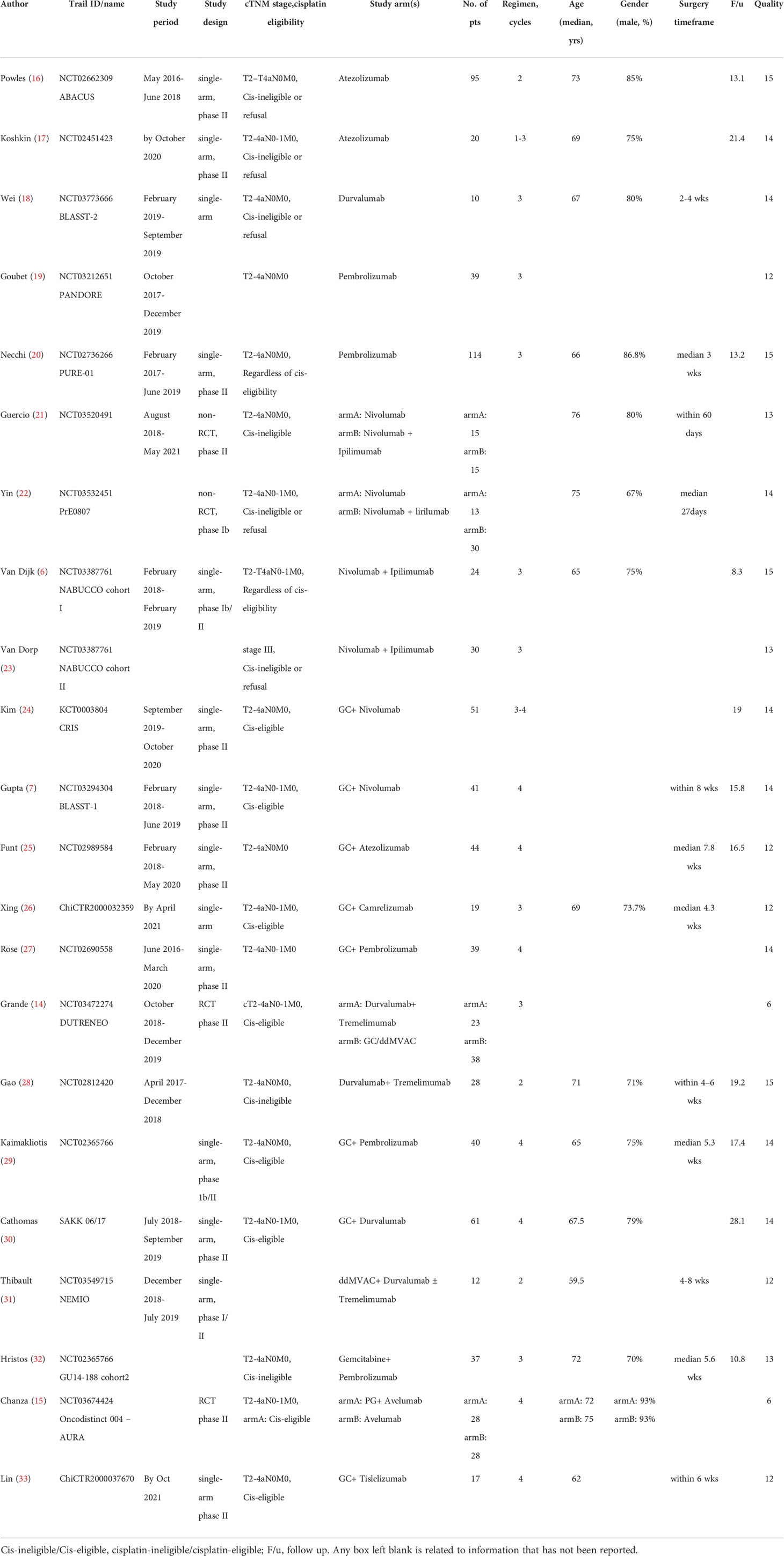
After screening, 22 records (24 cohorts) involving 843 patients, were included in this analysis. Most studies were phase II single-arm trials and carried out within the last six years. Two RCTs scored 6 points by the modified Jadad scale and were regarded to be high-quality (14, 15). The other single-arm designed studies were assessed as acceptable for the meta-analysis, with scores ranging from 12 to 15 points by the MINORS index. The clinical tumor stage was assessed as stage II-III. 13 records involving 542 patients reported the proportion of TNM stage in detail: 65.7% of cT2, 33.4% of cT3-4a, and 2.0% of cN1. Neoadjuvant ICI monotherapy was explored in eight cohorts (two pembrolizumab, two atezolizumab, two nivolumab, and one durvalumab, and one avelumab), dual-ICIs therapy in five cohorts (three ipilimumab plus nivolumab and two durvalumab plus tremelimumab) and chemoimmunotherapy in 11 cohorts (eight gemcitabine/cisplatin [GC] plus ICI, one dose-dense course of methotrexate, vinblastine, doxorubicin, and cisplatin [ddMVAC] plus ICIs, one gemcitabine plus ICI, and one paclitaxel/gemcitabine [PG] plus ICI). Table 1 demonstrated the characteristics in detail.
Oncological outcomes
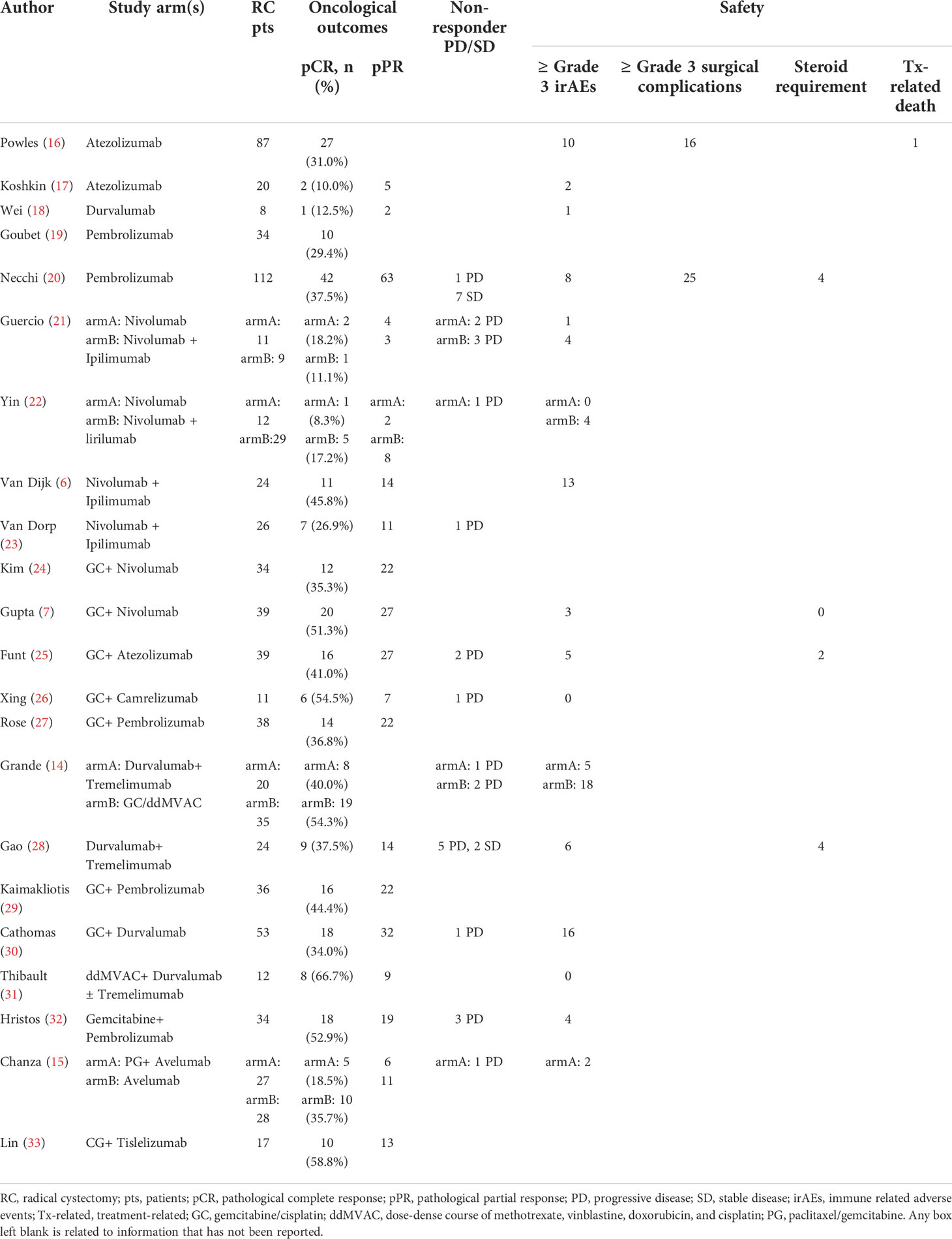
Table 2 presented the oncological outcomes in detail.
pCR
For neoadjuvant immunotherapy, 12 records (eight ICI monotherapy and five dual-ICIs therapy cohorts) involving 415/454 (91.4%) RC patients were included in this analysis. Within the 12 studies, there were four PD-L1 cohorts (16–18, 34), two PD-1 cohorts (19, 20), two CTLA-4 cohorts (21, 22), three CTLA-4 plus PD-1 cohorts (6, 21, 23), and two CTLA-4 plus PD-L1 cohorts (14, 28). The overall pooled pCR rate was 26.8% (95% confidence interval [CI]: 19.8% - 33.7%, Figure 2A). In subgroup analysis, patients of ICI monotherapy and dual-ICIs therapy had a pooled pCR rate of 24% (95% CI: 15.3% - 32.8%, Figure 2B) and 32.1% (95%CI: 20.6% - 43.7%, Figure 2C), respectively. Considering the significant heterogeneity in the pooled results, we further analyzed the pooled pCR rate of PD-(L)1 monotherapy and also observed significant heterogeneity (I2 = 65%, P < 0.01, Figure 2D), which may be explained by the variations of the therapeutic strategies such as treatment cycles and dosage.
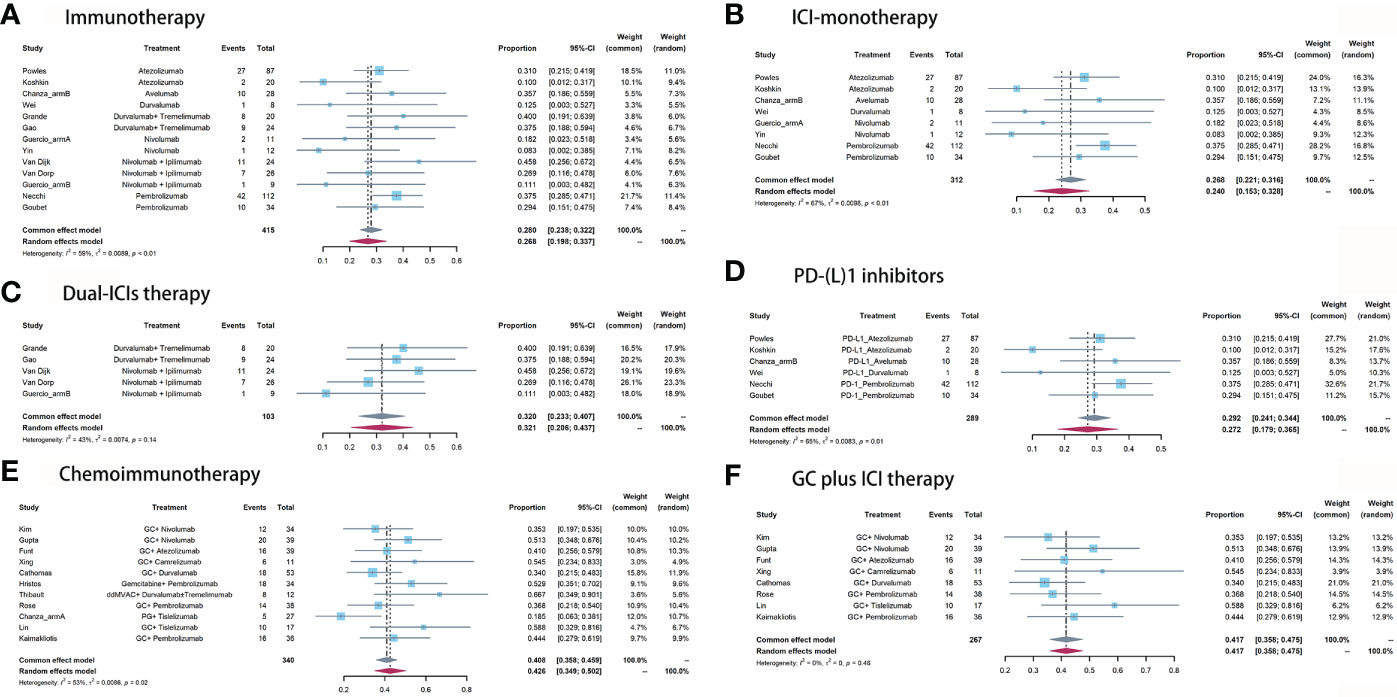
Figure 2 Pooled pCR of (A) immunotherapy, (B) ICI monotherapy, (C) dual-ICIs therapy, (D) PD-(L)1 inhibitors, (E) chemoimmunotherapy, and (F) GC plus ICI therapy.
For neoadjuvant chemoimmunotherapy, 11 records (over 70% explored the GC plus ICI therapy) involving 340/389 (87.4%) RC patients were included in this analysis. The overall pooled pCR rate was 42.6% (95% CI: 34.9% - 50.2%, Figure 2E). The heterogeneity was significant. Therefore, we performed a subgroup analysis based on the eight studies that investigated GC plus ICI therapy. Results suggested the pooled pCR rate of 41.7% (95%CI: 35.8% - 47.5%) and no significant heterogeneity (I2 = 0%, P = 0.46, Figure 2F).
pPR
Nine records (ten cohorts) involving 274 RC patients investigating immunotherapy and 11 studies involving 340 RC patients investigating chemoimmunotherapy were included in this analysis.
For immunotherapy, the overall pooled pPR rate was 40.9% (95% CI: 31%-50.8%, Figure 3A). In subgroup analysis, the pooled pPR rate of ICI monotherapy was 35.1% (95% CI: 21.5% - 48.7%, Figure 3B). Although these ICIs belong to PD-(L)1 inhibitor, significant heterogeneity was also observed (I2 = 74%, P < 0.01). The pooled pPR rate of dual-ICIs therapy was 50.4% (95% CI: 39.9% - 61.0%, Figure 3C).
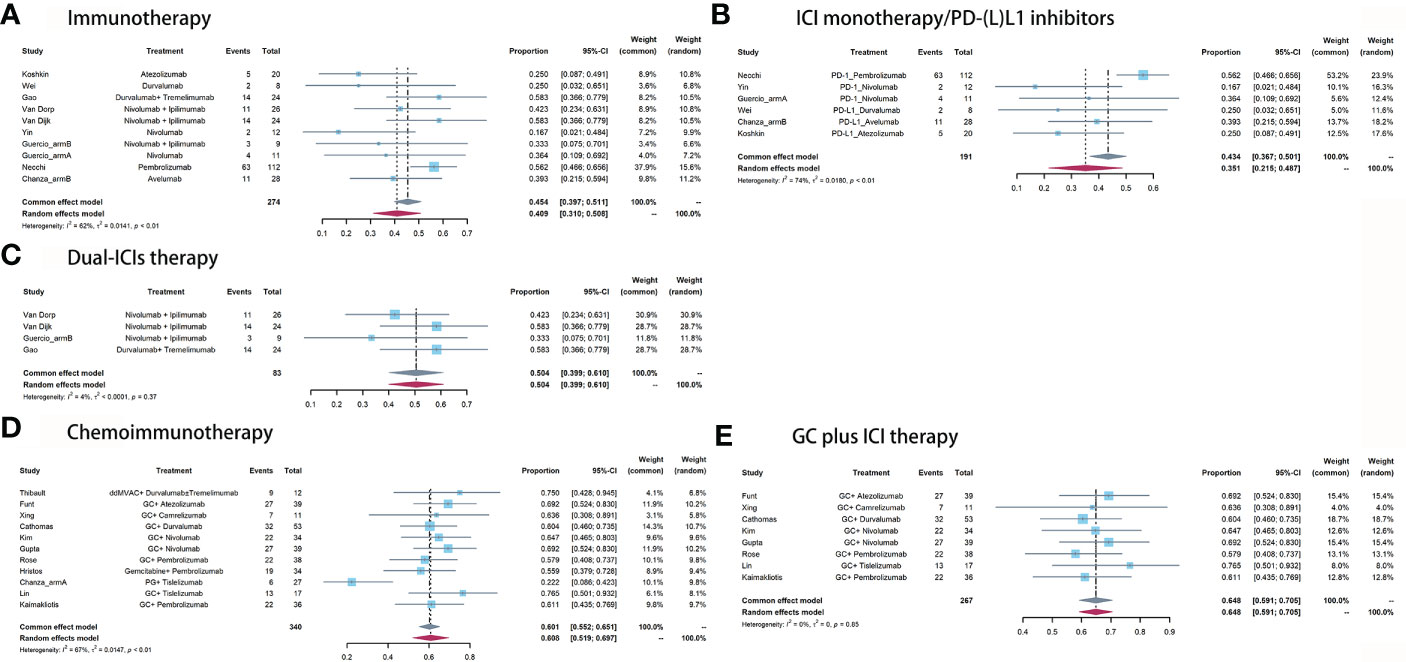
Figure 3 Pooled pPR of (A) immunotherapy, (B) ICI monotherapy/PD-(L)1 inhibitors, (C) dual-ICIs therapy, (D) chemoimmunotherapy, and (E) GC plus ICI therapy.
For chemoimmunotherapy, the overall pooled pPR rate was 60.8% (95% CI: 51.9%-69.7%, Figure 3D). In subgroup of GC plus ICI therapy, the pooled pPR rate was 64.8% (95% CI: 59.1%-70.5%) and no significant heterogeneity was observed (I2 = 0, P = 0.85, Figure 3E).
Safety outcomes
The non-responder proportion was 7.7% (11/142), 4.2% (8/189), and 12.5% (12/96) in ICI monotherapy, dual-ICIs therapy, and chemoimmunotherapy, respectively (Table 2). The higher rate in dual-ICIs therapy may be explained by the hyperprogression (35).
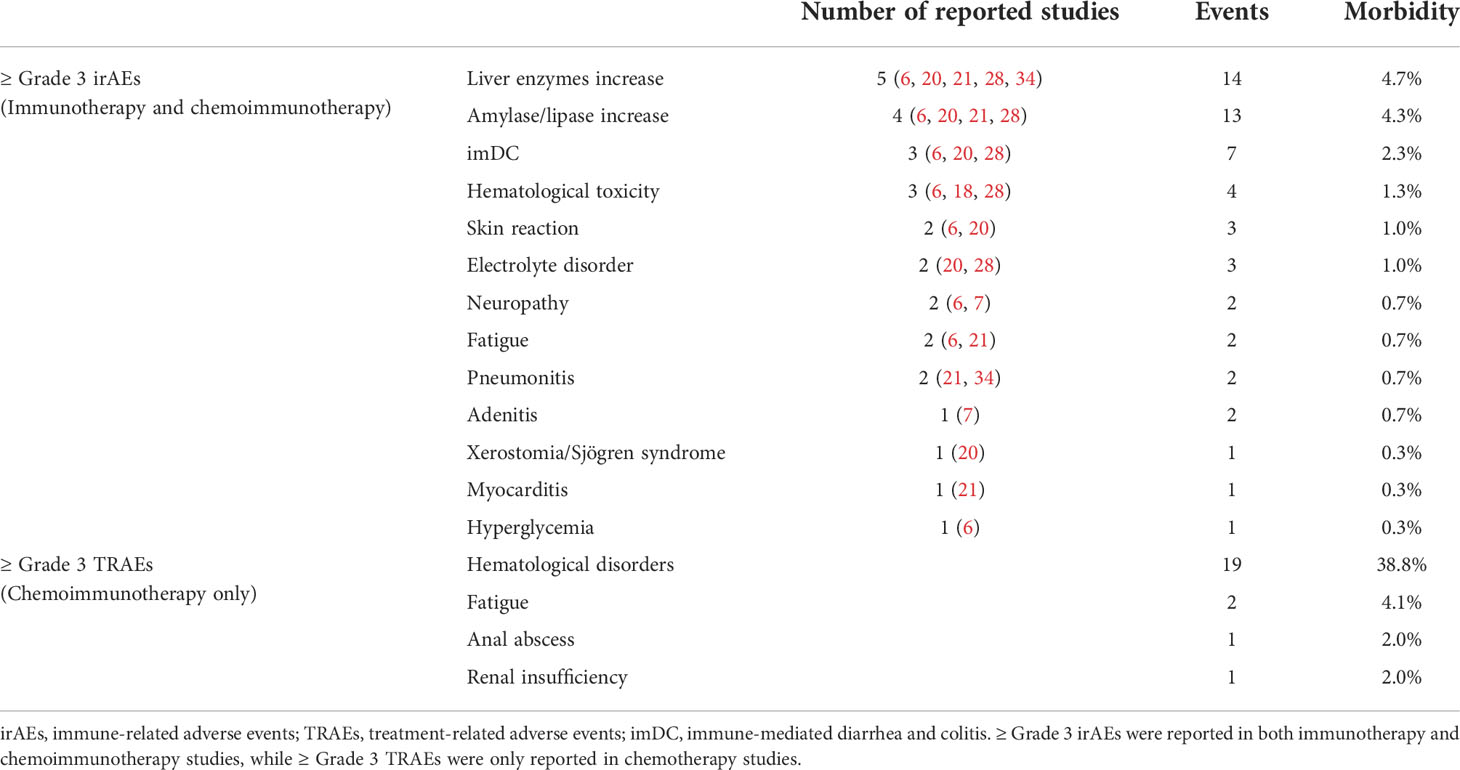
Across these included studies, Grade≥3 irAEs (17 cohorts) were more widely reported than either all grade irAEs (four cohorts) or TRAEs (five cohorts). The Grade≥3 irAEs morbidity ranged from 0 (22) to 54.2% (6). Nine of the 16 studies reported the Grade≥3 irAEs in detail. The top three most common Grade≥3 irAEs were hepatitis (4.7%), pancreatitis (4.3%), and immune-mediated diarrhea and colitis (imDC, 2.3%, Table 3). The pooled morbidity of Grade≥3 and all grade irAEs was 11.7% (95% CI: 6.5%-16.9%, Figure 4A) and 75.6% (95% CI: 55.4%-95.8%, Figure 4E), respectively. In subgroup analysis, the pooled Grade≥3 irAEs rate for ICI monotherapy, dual-ICIs therapy, and GC plus ICI therapy was 7.4% (95% CI: 4.3%-10.5%, Figure 4B), 30.3% (95% CI: 15.3%-45.3%, Figure 4C), and 14.5% (95% CI: 3.5% - 25.4%, Figure 4D), respectively.
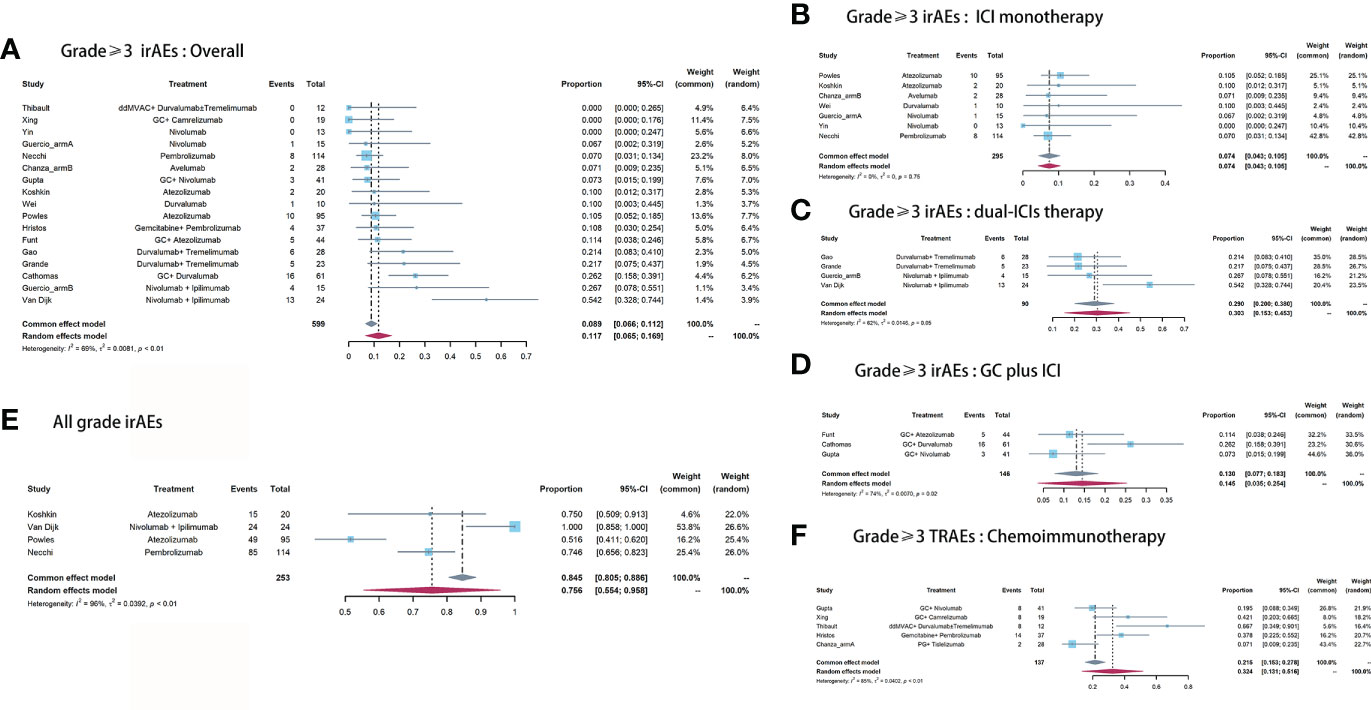
Figure 4 Pooled Grade≥ 3 irAEs rate of (A) overall, (B) ICI monotherapy, (C) dual-ICIs therapy, (D) GC plus ICI therapy. (E) Pooled all grade irAEs rate. (F) Pooled Grade≥ 3 TRAEs rate of chemoimmunotherapy.
To control the irAEs, the systemic steroid was required for two, four, and four patients in the studies by Funt et al. Necchi et al., and Gao et al, respectively (20, 25, 28). Only the ABACUS study reported one treatment-related death (16).
To assess the cumulative toxicity of chemotherapy, we further extracted the Grade≥ 3 TRAEs in patients receiving chemoimmunotherapy. The pooled morbidity was 32.4% (95% CI: 13.1% - 51.6%, Figure 4F). The hematological disorder was the most common Grade≥ 3 TRAEs, with morbidity of 38.8%. The detailed Grade≥ 3 irAEs and TRAEs were demonstrated in Table 3.
Three studies reported surgery-related complications (16, 20, 28). The postoperative all grade and Grade≥3 complication rate was 57.8% and 18.4%, respectively.
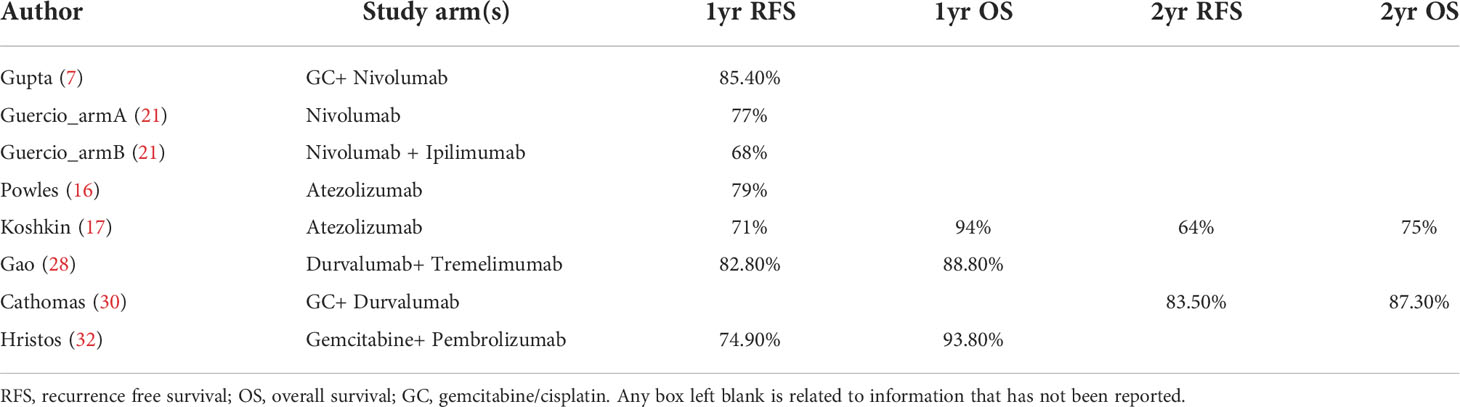
Survival outcomes
No long-term survival data was available and no adequate time-to-event data existed for pooled analysis. Table 4 demonstrated the survival data in detail. The two most reported survival outcomes were 1yr or 2yr OS rate and RFS rate. 1yr RFS rate varied from 68% (21) to 84.4% (7). 1yr OS rate varied from 88.8% (28) to 94% (17). Only two studies reported a 2yr OS rate and RFS rate (17, 30).
Publication bias and influence analysis
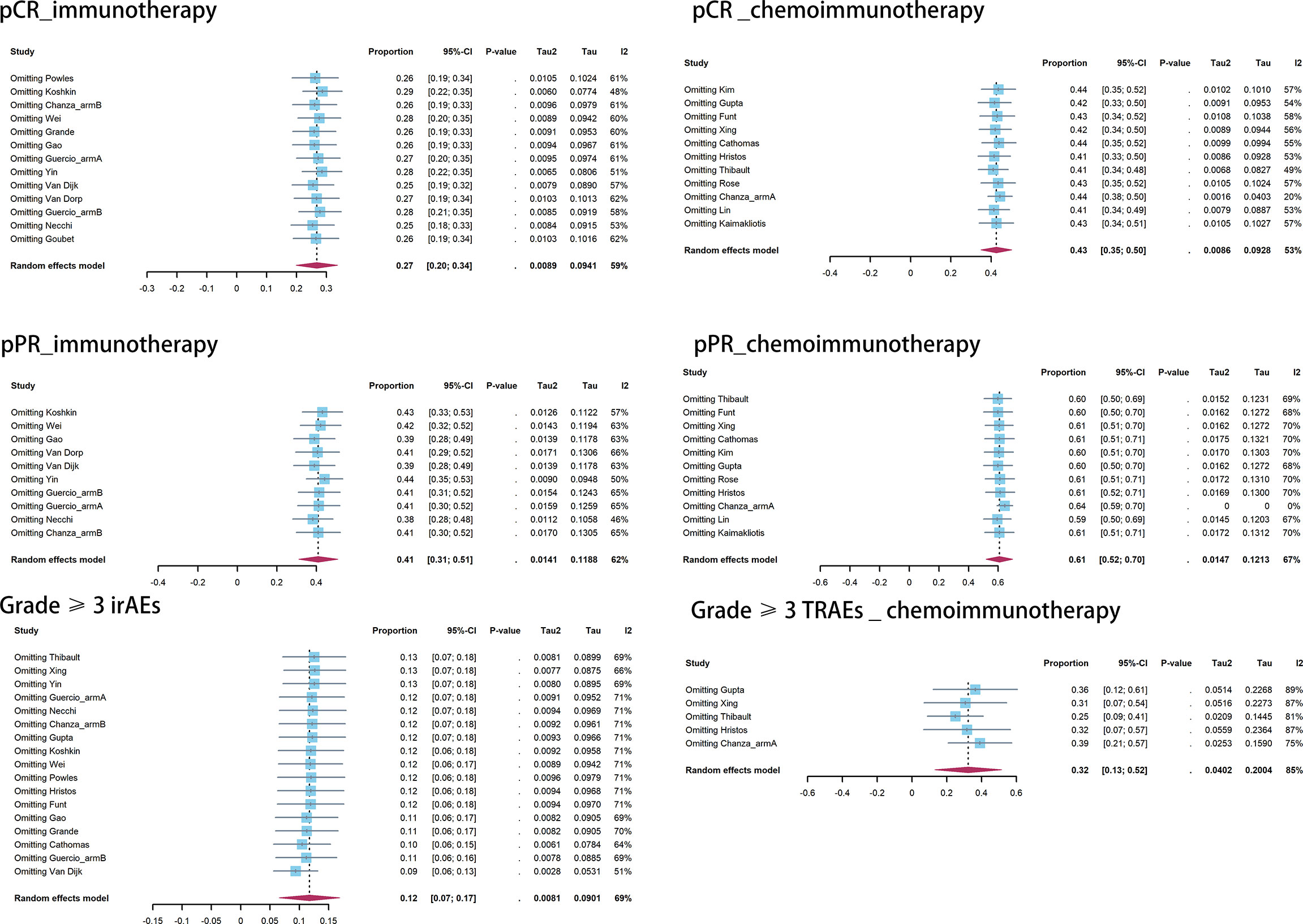
Publication bias was observed in the pooled pCR of chemoimmunotherapy and the pooled pPR of immunotherapy (Table 5). Influence analysis for Grade≥ 3 irAEs and Grade≥ 3 TRAEs revealed the most influential study (if omitted from the analysis, Figure 5).
Discussion
Given the remarkable evidence revealed by the IMvigor 210 (36) and CheckMate 275 studies (37), there has been growing interest in investigating ICIs in the neoadjuvant treatment for patients with MIBC. To date, three main types of immunological therapies have been widely evaluated in the neoadjuvant setting: ICI monotherapy, dual-ICIs therapy, and chemoimmunotherapy (dominated by GC plus ICI therapy).
Role of ICI immunotherapy
For cisplatin-ineligible patients, ICIs treatment has shaped the therapeutic paradigm. Compared to ICI monotherapy, dual-ICIs therapy (combined inhibition of PD-1/PD-L1 and CTLA-4) can enhance the therapeutic efficacy. In our study, the pooled pCR of dual-ICIs therapy was slightly higher than that of ICI monotherapy (32.1% vs 24%). The superiority of dual-ICIs therapy to ICI monotherapy was more evident in terms of the pooled pPR (50.4% vs 35.1%). In one recent meta-analysis involving 16 studies of 988 participants (38), Jiang et al. comprehensively integrated the evidence of neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer (NSCLC) and reported that the pooled pCR (ypT0N0M0) for ICI monotherapy and dual-ICIs therapy was 9.9% (95% CI: 5.7% - 15.3%) and 28.6% (13.8% - 50.7%), respectively.
Blocking PD-(L)1 and CTLA-4 simultaneously can destroy the immune suppression induced by tumor cells and enhance tumor rejection via effective T cells infiltration, activation, survival, and proliferation (39). Several studies have widely investigated the efficacy of nivolumab and ipilimumab in the treatment of melanoma and lung cancer in comparison to monotherapy. These findings suggested a significantly higher response rate and more favorable prognosis of dual-ICIs therapy, compared to ICI monotherapy (40, 41). Based on the remarkable findings uncovered by studies on melanoma and lung cancer, there have been a large number of studies exploring combined PD-(L)1 and CTLA-4 inhibition in other tumor types including MIBC, and PD-(L)1 inhibitors in combination with other immune checkpoints inhibitors. For example, Yin et al. in a prospective non-randomized study involving 43 MIBC patients, explored the efficacy between nivolumab monotherapy and combination immunotherapy of nivolumab and KIR inhibitor lirilumab. They found that the pCR of combination immunotherapy was 18%, higher than that of monotherapy (8%) (22). Small sample size may be responsible for the underestimated results. Collectively, patients receiving neoadjuvant dual-ICIs therapy can obtain more clinical benefits.
On the other hand, higher rates of pCR and pPR were associated with more frequent Grade≥ 3 irAEs. Patients who received ICI monotherapy reported a Grade≥ 3 irAEs morbidity of 7.4%, while those who received dual-ICIs therapy had a rate of 30.3%. One meta-analysis involving 2410 patients with advanced solid cancers, also reported a significantly higher irAEs rate induced by dual-ICIs therapy, compared to ICI monotherapy (42). Therefore, oncologists have to weigh the advantages and disadvantages of dual-ICIs therapy for cisplatin-ineligible patients. Future well-designed RCTs are needed to draw more robust conclusions in this concern.
Role of chemoimmunotherapy
For cancers in the advanced or metastatic setting, chemoimmunotherapy has demonstrated a more favorable OS and PFS, compared to chemotherapy alone. The IMvigor130 study explored survival differences between atezolizumab plus chemotherapy (n=451) and chemotherapy alone (n=400). Results revealed an 18% PFS and a 17% OS benefit for those who received chemoimmunotherapy combinations compared with those who received chemotherapy alone (43). More significant findings have been reported in the JAVELIN Bladder 100 study (44). In the phase III RCT trial, 700 patients were allocated to maintenance avelumab plus best supportive care and best supportive care alone in a 1:1 ratio. Patients receiving combinatory treatment achieved a 31% OS and 38% PFS benefit. Intriguing results promoted investigators to evaluate the efficacy of chemoimmunotherapy in the neoadjuvant setting.
In our study, the pooled pCR and pPR for chemoimmunotherapy was 42.6% and 60.8%, respectively, comparable to that of NAC (45). Considering the establishment of effective immune memory, immunotherapy was featured for the long-lasting anti-tumor efficacy, compared to chemotherapy. Compared to patients receiving traditional NAC, whether patients receiving chemoimmunotherapy had a more favorable prognosis or not remains unclear, since these enrolled patients are under active long-term follow-up in terms of survival. Promisingly, several studies have uncovered that achieving pCR in patients receiving NAC was associated with a favorable prognosis (46, 47), suggesting the expectation of clinical benefits of neoadjuvant chemoimmunotherapy in survival outcomes.
In comparison to ICI immunotherapy, chemoimmunotherapy had a comparable pCR to dual-ICIs therapy and a higher pCR to ICI monotherapy, consistent with the findings uncovered by Jiang et al. in their study of NSCLC. Compared to inhibition of a single immune checkpoint, the addition of chemotherapy can destroy the tumor cells and release large amounts of tumor antigen at the time of high tumor burden (preoperative setting). Therefore, the efficacy of immunotherapy can be promoted (48). When interpreting these results, we must keep in mind that the participants’ selection bias may skew the results. Most participants receiving ICI immunotherapy are cisplatin-ineligible and have worse physic conditions compared to participants receiving chemoimmunotherapy, as impaired physic conditions (evaluated by ECOG PS score) correlated with limited response to immunotherapy and unfavorable prognosis (49).
In terms of safety, the Grade≥ 3 irAEs morbidity was much lower than that of dual-ICIs therapy. However, the Grade≥ 3 TRAEs rate of chemoimmunotherapy was 32.4% (ranging from 7.1% to 66.7%), comparable to that of NAC in MIBC (50).
Neoadjuvant ICI combined with targeted therapy: the rational and current progress
Targeted therapy is designed to interfere with critical biological pathways (such as RTK/RAS/MAP-Kinase pathway and I3K/Akt signaling) and block dysregulated proteins (such as EGFR and BRAF) which play essential roles in tumor formation, growth, and invasion (51). Although the impressive anti-tumor effects of various targeted agents in selected patients have been well-documented, the rapid emergence of resistance has become a major concern due to, for example, the restoration of the pathways or biological functions by activation of upstream or downstream effectors (52). Therefore, a novel treatment strategy is important to prolong the anti-tumor effect and amplify the prognostic benefits. Preliminary findings have addressed the potent anti-tumor immunity by ICIs in intractable cancers including metastatic melanoma and metastatic urothelial carcinoma (53, 54). More importantly, ICI therapy demonstrated long-term benefits due to restored host anti-tumor response and sustained immune memory. The weaknesses of targeted therapy and strengths of ICIs have inspired the idea of combinatorial therapy that can benefit patients by its synergistic effect:
(1) The immunosuppressive tumor microenvironment (TME) is a hallmark of most malignancies and hinders anti-tumor immunity. Targeted therapy can induce rapid destruction of this feature and enhance the cytotoxicity of immunotherapy (55);
(2) The tumor antigen released by targeted therapy can act as tumor vaccinations to consolidate the host immune response and anti-tumor effects (56);
(3) By inhibiting the regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) and improving the effects of antigen-presenting DC, targeted therapy can restore the activity of effector T cells (57).
Currently, there was only one study by Rodriguez-Moreno and colleagues (58) evaluating the combinatorial therapy (durvalumab in 1500 mg q4wks and Olaparib in 500 mg bid orally, followed by RC within 8-10 wks) for MIBC in the neoadjuvant setting. After 28 patients with cT2-T4a MIBC enrolled, 12 of them underwent RC and 44.5% achieved a pCR rate of 44.5%. In terms of safety, one surgery-related death was reported. Although over 90% of patients presented with any grade of AEs, only 8.3% suffered from Grade 3-4 AEs. Besides, no Grade≥3 Olaparib-related AEs were observed. Limited by the single-arm design and sample size, robust conclusions were hard to be addressed.
Strengths and limitations
Although there have been several published reviews/meta-analyses addressing this concern, our study has some strengths. First, considering the rapid development of immunotherapy, we performed the literature research up to June 2022 after the 2022 ASCO Annual Meeting to search for the latest eligible records. Second, we performed strict literature identification and only included the updated results of one trail to avoid duplicate records. Third, given the discrepancies in included studies, we pooled the oncological outcomes by PP analysis, which can guide the counsel of patients. Fourth, we performed subgroup analysis according to the types of treatment regimens, which can represent the current progress of each approach to some extent.
There were some limitations in our study. First, although subgroup analysis was performed, significant heterogeneity still presented in the majority of outcomes of our study, which may be caused by the variations in dosage and cycles of regimens and the included population bias (varied eligibility to cisplatin and physic performance). Second, most included trials were single-arm designed, with small sample size, which prevented drawing robust recommendations. Third, the lack of long-term survival data made it hard to know the lasting benefits of immunotherapy in the neoadjuvant setting.
Conclusions
The three main immunotherapeutic approaches, including ICI monotherapy, dual-ICIs therapy, and chemoimmunotherapy (dominated by GC plus ICI therapy), were effective and safe in the treatment of MIBC in the neoadjuvant setting. Compared to ICI monotherapy, dual-ICIs therapy and chemoimmunotherapy had higher pCR and pPR. On the other hand, the morbidity of Grade≥ 3 irAEs or Grade≥ 3 TRAEs of dual-ICIs therapy and chemoimmunotherapy was higher than that of ICI monotherapy. Current single-arm designed studies with missing long-term prognostic data, however, prevented us from drawing reliable recommendations. Oncologists must weigh the pros and cons before deciding on one specific therapeutic strategy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Study design and registration: HLC. and XQX. Search strategy design: HLC. and YJL. literature search: HLC. and ZHJ. Study selection: HLC. and WJY. Data collection and extraction: HLC. and WJY. Data analysis and writing: HLC. Arrangement and supervision: ZGJ. All authors contributed to the article and approved the submitted version
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lenis AT, Lec PM, Chamie K. Bladder cancer: A review. JAMA (2020) 324(19):1980–91. doi: 10.1001/jama.2020.17598
2. Funt SA, Rosenberg JE. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol (2017) 14(4):221–34. doi: 10.1038/nrclinonc.2016.188
3. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
4. Galsky MD, Hahn NM, Rosenberg JE, Sonpavde G, Oh WK, Dreicer R, et al. Defining “cisplatin ineligible” patients with metastatic bladder cancer. J Clin Oncol (2011) 29(7_suppl):238. doi: 10.1200/jco.2011.29.7_suppl.238
5. Hahn NM, Necchi A, Loriot Y, Powles T, Plimack ER, Sonpavde G, et al. Role of checkpoint inhibition in localized bladder cancer. Eur Urol Oncol (2018) 1(3):190–8. doi: 10.1016/j.euo.2018.05.002
6. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med (2020) 26(12):1839–44. doi: 10.1038/s41591-020-1085-z
7. Gupta S, Gibb E, Sonpavde GP, Gupta S, Maughan BL, Agarwal N, et al. Biomarker analysis and updated clinical follow-up from BLASST-1 (Bladder cancer signal seeking trial) of nivolumab, gemcitabine, and cisplatin in patients with muscle-invasive bladder cancer (MIBC) undergoing cystectomy. J Clin Oncol (2022) 40(6 SUPPL):528. doi: 10.1200/JCO.2022.40.6_suppl.528
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev (2021) 88:105906. doi: 10.1186/s13643-021-01626-4
9. Peyrottes A, Ouzaid I, Califano G, Hermieu JF, Xylinas E. Neoadjuvant immunotherapy for muscle-invasive bladder cancer. Medicina (Kaunas) (2021) 57(8):769. doi: 10.3390/medicina57080769
10. Bañares R, Albillos An, Rincón D, Alonso S, González M, Ruiz-del-Arbol L, et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology (2002) 35(3):609–15. doi: 10.1053/jhep.2002.31354
11. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
12. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with r: a practical tutorial. Evid-Based Ment Health (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117
13. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics (2018) 74(3):785–94. doi: 10.1111/biom.12817
14. Grande E, Guerrero F, Puente J, Galante I, Duran I, Dominguez M, et al. DUTRENEO trial: a randomized phase II trial of DUrvalumab and TREmelimumab versus chemotherapy as a NEOadjuvant approach to muscle-invasive urothelial bladder cancer (MIBC) patients (pts) prospectively selected by an interferon (INF)-gamma immune signature. J Clin Oncol (2020) 38(15):5012–5012. doi: 10.1200/JCO.2020.38.15_suppl.5012
15. Martinez Chanza N, Carnot A, Barthélémy P, Casert V, Staudacher L, Van Den Brande J, et al. Avelumab as the basis of neoadjuvant regimen in platinum-eligible and-ineligible patients with nonmetastatic muscle-invasive bladder cancer: AURA (Oncodistinct-004) trial. Am Soc Clin Oncology; (2022) 40(16_suppl):4517. doi: 10.1200/JCO.2022.40.16_suppl.4517
16. Powles T, Rodriguez-Vida A, Duran I, Crabb SJ, van der Heijden MS, Pous AF, et al. A phase II study investigating the safety and efficacy of neoadjuvant atezolizumab in muscle invasive bladder cancer (ABACUS). J Clin Oncol (2018) 36(15_suppl):4506–4506. doi: 10.1200/JCO.2018.36.15-suppl.4506
17. Koshkin VS, Natesan D, Zhang L, Oh DY, Porten SP, Meng M, et al. Phase II trial of escalating doses of neoadjuvant atezolizumab for patients with non-metastatic urothelial carcinoma ineligible for cisplatin-based neoadjuvant chemotherapy. J Clin Oncol (2021) 39(6 SUPPL):442–442. doi: 10.1200/JCO.2021.39.6-suppl.442
18. Wei XX, McGregor BA, Lee RJ, Gao X, Kilbridge KL, Preston MA, et al. Durvalumab as neoadjuvant therapy for muscle-invasive bladder cancer: Preliminary results from the bladder cancer signal seeking trial (BLASST)-2. J Clin Oncol (2020) 38(6):507–507. doi: 10.1200/JCO.2020.38.6_suppl.507
19. Goubet A-G, Silva CAC, Melo LLD, Gazzano M, Lebacle C, Thibault C, et al. Bacteria-specific CXCL13-producing follicular helper T cells are putative prognostic markers to neoadjuvant PD-1 blockade in muscle-invasive urothelial carcinoma. J Clin Oncol (2022) 40(6_suppl):535. doi: 10.1200/JCO.2022.40.6_suppl.535
20. Necchi A, Raggi D, Gallina A, Madison R, Colecchia M, Lucianò R, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol (2020) 77(4):439–46. doi: 10.1016/j.eururo.2019.10.026
21. Guercio BJ, Pietzak EJ, Brown S, Chen JF, Peters V, Regazzi AM, et al. Neoadjuvant nivolumab (N) +/-ipilimumab (I) in cisplatin-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). J Clin Oncol (2022) 40(6 SUPPL):498–498. doi: 10.1200/JCO.2022.40.6_suppl.498
22. Yin PGJ, Koshkin VS, Cole S, Jain RK, Dreicer R, Cetnar JP, et al. PrE0807: A phase ib feasibility trial of neoadjuvant nivolumab (N) without or with lirilumab (L) in cisplatin-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). J Clin Oncol (2021) 39(15 SUPPL):4518–4518. doi: 10.1200/JCO.2021.39.15-suppl.4518
23. Van Dorp J, Suelmann BBM, Mehra N, Van Montfoort M, Van Dijk N, Hendricksen K, et al. LBA31 high- vs low-dose pre-operative ipilimumab and nivolumab in locoregionally advanced urothelial cancer (NABUCCO cohort 2). Ann Oncol (2021) 32:S1305–S6. doi: 10.1016/j.annonc.2021.08.2107
24. Kim H, Kim R, Hong J, Kwon GY, Kim CK, Park W, et al. A prospective phase II trial of neoadjuvant nivolumab plus gemcitabine/cisplatin chemotherapy in muscle-invasive urothelial carcinoma of bladder. J Clin Oncol (2022) 40(6_suppl):494. doi: 10.1200/JCO.2022.40.6_suppl.494
25. Funt SA, Lattanzi M, Whiting K, Al-Ahmadie HA, Quinlan C, Teo MY, et al. Neoadjuvant atezolizumab (A) with gemcitabine and cisplatin (GC) in patients (pts) with muscle invasive bladder cancer (MIBC): A multicenter, single-arm, phase 2 trial. J Clin Oncol (2021) 39(15 SUPPL):4517–4517. doi: 10.1200/JCO.2021.39.15-suppl.4517
26. Xing N, Han S, Jiang J, Xu W, Shi B, Ping H, et al. 703P camrelizumab in combination with gemcitabine plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Ann Oncol (2021) 32:S714. doi: 10.1016/j.annonc.2021.08.099
27. Rose TL, Harrison MR, Deal AM, Ramalingam S, Whang YE, Brower B, et al. Phase II study of gemcitabine and split-dose cisplatin plus pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder cancer. J Clin Oncol (2021) 39(28):3140–8. doi: 10.1200/JCO.2021.39.6_suppl.396
28. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med (2020) 26(12):1845–51. doi: 10.1038/s41591-020-1086-y
29. Kaimakliotis* H, Albany C, Hoffman-Censits J, Trabulsi E, Kelly WK, Picus J, et al. PD52-03 a multicenter phase 1B/2 study of neoadjuvant pembrolizumab and cisplatin chemotherapy for muscle invasive urothelial cancer. J Urol (2019) 201(Supplement 4):e924–e5. doi: 10.1097/01.JU.0000556959.45525.89
30. Cathomas R, Petrausch U, Hayoz S, Schneider M, Schardt JA, Seiler R, et al. Perioperative chemoimmunotherapy with durvalumab (Durva) in combination with cisplatin/gemcitabine (Cis/Gem) for operable muscle-invasive urothelial carcinoma (MIUC): Preplanned interim analysis of a single-arm phase II trial (SAKK 06/17). J Clin Oncol (2020) 38(6):499–499. doi: 10.1200/JCO.2020.38.6_suppl.499
31. Thibault C, Elaidi R, Pouessel D, Flechon A, Borchiellini D, Barthelemy P, et al. NEMIO: A randomized phase I-II trial evaluating efficacy and safety of dose dense MVAC (ddMVAC) plus durvalumab plus/-tremelimumab as neoadjuvant treatment in patients with bladder muscle-invasive urothelial carcinoma. Ann OF Oncol (2020) 31:S723. doi: 10.1016/j.annonc.2020.08.1179
32. Kaimakliotis HZ, Adra N, Kelly WK, Trabulsi EJ, Lauer RC, Picus J, et al. Phase II neoadjuvant (N-) gemcitabine (G) and pembrolizumab (P) for locally advanced urothelial cancer (laUC): Interim results from the cisplatin (C)-ineligible cohort of GU14-188. J Clin Oncol (2020) 38(15_suppl):5019. doi: 10.1200/JCO.2020.38.15_suppl.5019
33. Lin T, Li K, Fan J, Wang S, Yu D, Xu T, et al. Interim results from a multicenter clinical study of tislelizumab combined with gemcitabine and cisplatin as neoadjuvant therapy for patients with cT2-T4aN0M0 MIBC. Am Soc Clin Oncology; (2022) 40(16_suppl):4580–4580. doi: 10.1200/JCO.2022.40.16_suppl.4580
34. Barthélémy P, Casert V, Staudacher L, Van Den Brande J, Sautois B, Vanhaudenarde V, et al. Avelumab as the basis of neoadjuvant regimen in platinum-eligible and -ineligible patients with nonmetastatic muscle-invasive bladder cancer: AURA (Oncodistinct-004) trial. J Clin Oncol (2022) suppl 16:4517–4517. doi: 10.1200/JCO.2022.40.16_suppl.4517. Nieves Martinez Chanza AC.
35. Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok J. Combination immunotherapy: a road map. J ImmunoTher Cancer (2017) 5(1):1–15. doi: 10.1186/s40425-017-0218-5
36. Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet (2017) 389(10064):67–76. doi: 10.1016/S0140-6736(16)32455-2
37. Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol (2017) 18(3):312–22. doi: 10.1016/s1470-2045(17)30065-7
38. Jiang J, Wang Y, Gao Y, Sugimura H, Minervini F, Uchino J, et al. Neoadjuvant immunotherapy or chemoimmunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res (2022) 11(2):277–94. doi: 10.21037/tlcr-22-75
39. Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA (2010) 107(9):4275–80. doi: 10.1073/pnas.0915174107
40. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med (2015) 372(21):2006–17. doi: 10.1056/NEJMoa1414428
41. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
42. Ma X, Zhang Y, Wang S, Wei H, Yu J. Immune checkpoint inhibitor (ICI) combination therapy compared to monotherapy in advanced solid cancer: A systematic review. J Cancer (2021) 12(5):1318–33. doi: 10.7150/jca.49174
43. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10236):1547–57. doi: 10.1016/S0140-6736(20)30230-0
44. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN bladder 100 phase III interim analysis. J Clin Oncol (2020) 38(18_suppl):LBA1–LBA. doi: 10.1200/JCO.2020.38.18_suppl.LBA1
45. Nguyen T-T, Huillard O, Dabi Y, Anract J, Sibony M, Zerbib M, et al. Neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer and its impact on surgical morbidity and oncological outcomes: A real-world experience. Front surgery (2018) 5:58. doi: 10.3389/fsurg.2018.00058
46. Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysispCR and association with clinical outcomes in breast cancer. Clin Cancer Res (2020) 26(12):2838–48. doi: 10.1158/1078-0432.CCR-19-3492
47. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Vavassori I, Barni S. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol (2014) 65(2):350–7. doi: 10.1016/j.eururo.2013.06.049
48. Ramakrishnan R, Gabrilovich D. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Immunotherapy (2013) 62(3):405–10. doi: 10.1007/s00262-012-1390-6
49. Tomasik B, Bieńkowski M, Braun M, Popat S, Dziadziuszko R. Effectiveness and safety of immunotherapy in NSCLC patients with ECOG PS score≥ 2–systematic review and meta-analysis. Lung Cancer (2021) 158:97–106. doi: 10.1016/j.lungcan.2021.06.004
50. Salminen AP, Montoya Perez I, Klén R, Ettala OO, Syvänen KT, Elo LL, et al. Adverse events during neoadjuvant chemotherapy for muscle invasive bladder cancer. Bladder Cancer (2019) 5(4):273–9. doi: 10.3233/BLC-190246
52. Sabnis AJ, Bivona TG. Principles of resistance to targeted cancer therapy: Lessons from basic and translational cancer biology. Trends Mol Med (2019) 25(3):185–97. doi: 10.1016/j.molmed.2018.12.009
53. Safi M, Al-Azab M, Jin C, Trapani D, Baldi S, Adlat S, et al. Age-based disparities in metastatic melanoma patients treated in the immune checkpoint inhibitors (ICI) versus non-ICI era: A population-based study. Front Immunol (2021) 12:609728. doi: 10.3389/fimmu.2021.609728
54. Mori K, Pradere B, Moschini M, Mostafaei H, Laukhtina E, Schuettfort VM, et al. First-line immune-checkpoint inhibitor combination therapy for chemotherapy-eligible patients with metastatic urothelial carcinoma: A systematic review and meta-analysis. Eur J Cancer (2021) 151:35–48. doi: 10.1016/j.ejca.2021.03.049
55. Allegrezza MJ, Conejo-Garcia JR. Targeted therapy and immunosuppression in the tumor microenvironment. Trends Cancer (2017) 3(1):19–27. doi: 10.1016/j.trecan.2016.11.009
56. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer (2012) 12(4):237–51. doi: 10.1038/nrc3237
57. Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res (2009) 15(6):2148–57. doi: 10.1158/1078-0432.CCR-08-1332
58. Rodriguez-Moreno JF, De Velasco G, Fernandez IB, Alvarez-Fernandez C, Fernandez R, Vazquez-Estevez S, et al. Impact of the combination of durvalumab (MEDI4736) plus olaparib (AZD2281) administered prior to surgery in the molecular profile of resectable urothelial bladder cancer: NEODURVARIB trial. J Clin Oncol (2020) 38(6):TPS503–TPS503. doi: 10.1200/JCO.2020.38.6_suppl.542
Keywords: neoadjuvant, immunotherapy, chemoimmunotherapy, muscle-invasive bladder cancer (MIBC), meta-analysis
Citation: Chen H, Yang W, Xue X, Li Y, Jin Z and Ji Z (2022) Neoadjuvant immunotherapy and chemoimmunotherapy for stage II-III muscle invasive bladder cancer. Front. Immunol. 13:986359. doi: 10.3389/fimmu.2022.986359
Received: 05 July 2022; Accepted: 28 July 2022;
Published: 17 August 2022.
Edited by:
Yann-Alexandre Vano, APHP-Paris Centre, FranceReviewed by:
Geraldine Pignot, Institut Paoli-Calmettes (IPC), FranceNadine Houede, Centre Hospitalier Universitaire de Nîmes, France
Copyright © 2022 Chen, Yang, Xue, Li, Jin and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Ji, jzgjxmc@163.com
 Hualin Chen
Hualin Chen Wenjie Yang
Wenjie Yang Xiaoqiang Xue
Xiaoqiang Xue Yingjie Li
Yingjie Li Zhaoheng Jin
Zhaoheng Jin Zhigang Ji
Zhigang Ji