- 1Department of Pediatrics, Division of Pediatric Nephrology, University of California at San Diego and Rady Children’s Hospital, San Diego, CA, United States
- 2Department of Pediatrics, Division of Allergy and Immunology, University of California at San Diego and Rady Children’s Hospital, San Diego, CA, United States
- 3Department of Pediatrics, Division of Pediatric Infectious Diseases, University of Washington and Seattle Children's Hospital, Seattle, WA, United States
Balancing enough immunosuppression to prevent allograft rejection and yet maintaining an intact immune system to respond to vaccinations, eliminate invading pathogens or cancer cells is an ongoing challenge to transplant physicians. Antibody mediated allograft rejection remains problematic in kidney transplantation and is the most common cause of graft loss despite current immunosuppressive therapies. The goal of immunosuppressive therapies is to prevent graft rejection; however, they prevent optimal vaccine responses as well. At the center of acute and chronic antibody mediated rejection and vaccine responses is the B lymphocyte. This review will highlight the role of B cells in alloimmune responses including the dependency on T cells for antibody production. We will discuss the need to improve vaccination rates in transplant recipients and present data on B cell populations and SARS-CoV-2 vaccine response rates in pediatric kidney transplant recipients.
Introduction
The role of pre-existing humoral antibodies against donor cells mediating immediate graft loss or hyperacute rejection was identified early in the history of transplantation (1). Genotyping human leukocyte antigen (HLA) class I and class II alleles in donors and recipients and careful testing of recipients’ serum for anti-donor HLA antibodies before transplantation to avoid transplanting donors to recipients with preformed antibodies have essentially eliminated hyperacute rejection (2). However, development of anti-T cell responses and de novo anti-donor antibodies to allografts remains problematic. Acute allograft rejection is strongly related to the development of biopsy-proven chronic allograft dysfunction and subsequent graft loss (3). Preventing T cell mediated, and antibody-mediated destruction of allografts is the goal of induction and maintenance immunosuppression. However, the intensity of immunosuppression is tempered by the risks of overwhelming infections and post-transplant lymphoproliferative disease.
The basics: T cell activation
The immune system is comprised to two components—innate and adaptive immunity. The innate system involves natural killer (NK) cells, macrophages, and dendritic cells (DC), among others, that express receptors for a broad range of pathogen- or danger-associated molecular patterns (PAMPs and DAMPs) (4). Once thought to be minimally involved in transplant rejection, it is now recognized that innate system activation can set the stage for an adaptive alloimmune response. This is particularly relevant in that ischemia reperfusion injury (IRI) is thought to incite an innate response that can prime later allograft rejection (5). While this is an area of active study, a detailed discussion of the innate immune system involvement in allograft rejection is beyond the scope of this review.
In light of this, the adaptive immune response is the most well characterized aspect of transplant rejection. Adaptive immune responses are classified as cellular or humoral. Cellular responses are mediated by T lymphocytes while humoral responses are characterized by the production of antibodies by B lymphocytes and plasma cells. Both responses are intertwined and overlap. CD4 T cells provide critical help to B cells to produce antibodies and B cells can play a role in T cell activation via antigen presentation, co-stimulation and cytokine secretion. In addition, both T cells and B cells have regulatory functions. The interaction between dendritic cells, naïve T cells, and B cells is critical for the initiation and differentiation of the immune response resulting in elimination of the invading pathogen and induction of a life-long memory response (6).
Naïve lymphocytes circulate between the blood, lymph nodes and spleen. Within secondary lymphoid organs, naïve T and B cells are physically separated (7). Dendritic cells reside within the T cell areas of secondary lymphoid tissue and are the most potent antigen presenting cells (8). They extend their dendrites and are constantly contacting and scanning the surrounding T cells in search of those that are specific for the antigens they have processed and presented on the surface as peptide/major histocompatibility complexes (9). The 3-signal model of T-cell activation in the development of an adaptive immune response applies to allograft rejection (10–13).
Alloantigen recognition by T cells (signal 1) can occur through three different pathways: the direct pathway, the indirect pathway or the semidirect pathway (14–21). The direct pathway occurs when the T-cell receptor on the surface of the T cell interacts with intact allogeneic HLA molecules on the surface of donor antigen-presenting cells (APCs) that have migrated out of the graft to secondary lymphoid tissue. The indirect pathway occurs when donor HLA proteins or other antigens are processed, and peptides are loaded onto recipient MHC complexes and presented on the surface of recipient APCs. The semidirect pathway occurs when recipient APCs acquire and present intact donor-derived HLA molecules. Although it is typically presumed that early acute graft rejection is mediated by the direct pathway while the indirect pathway is responsible for later chronic allograft rejection, this is likely an oversimplification as the indirect pathway has a nuanced involvement in both processes (14).
The second costimulatory signal between APCs and T cell is crucial for productive T cell activation (22). The most extensively studied of the costimulatory signals is CD28 (23). Binding of CD28 to its ligands (B7-1, CD80 and B7-2, CD86) on APCs promotes optimal T-cell receptor (TCR) signaling events that trigger IL-2 production, clonal expansion and generation of effector and memory T cells (24). Inflammation initiates a cascade of events that results in dendritic cell maturation as well as tight and prolonged interactions between antigen bearing DCs and antigen specific T cells whereby costimulatory signals are provided to the T cell (signal 2) resulting in T cell activation and clonal expansion (25). Many other costimulatory molecules have been shown to play a role in T cell activation and there are ongoing efforts underway to block costimulation with the hope of inducing long lasting tolerance (26).
After activation, CD4 T cells interact with CD8 T cells to provide help and the necessary proinflammatory cytokines (signal 3) to promote differentiation into effector and memory cytotoxic T cells and graft rejection (13). Activated CD4 and CD8 T cells alter their cell surface phenotype to express different cell surface molecules enabling cells to migrate from secondary lymphoid organs into peripheral tissues such as the allograft, inducing graft destruction (27). Acute T cell mediated rejection, according to the Banff classification of kidney transplant biopsies, is diagnosed with the presence of interstitial lymphocytic inflammation involving >25% of non-sclerotic cortical parenchyma and tubulitis involving one or more tubules (28).
B cell activation
Although T cells are sufficient to mediate graft destruction, they play a key role in activating B cells. With the help of CD4 T lymphocytes, B cells are able to generate long-lasting, high-affinity IgG antibodies (29). Within secondary lymphoid tissue, activated antigen specific CD4 T cells migrate to the edges of the T cell zones to interact with antigen specific B cells (30, 31). This interaction is critical for B cell expansion, isotype switching and antibody production (32). When present on the surface of B lymphocytes, the immunoglobulin serves as the antigen receptor for B cells. B cells that bind antigen via their B cell receptor upregulate chemokine receptor 7 (CCR7) and Epstein-Barr virus–induced receptor 2 (EBI2) levels on their cell surface and are able to migrate to the B-T cell border (33). The B cells internalize the protein antigen and process it into peptides loaded on class II major histocompatibility complex (MHC) molecules. It is at the T-B cell border where they encounter cognate T cells that provide the necessary costimulatory help. B cells characterized by the expression of the B cell lymphoma 6 (BCL6) transcription factor migrate to the germinal centers and undergo clonal expansion, somatic hypermutation and immunoglobulin isotype class switch recombination, resulting in affinity selection to generate a highly specific anti-donor response (34). The germinal center reaction results in the generation of memory B cells and long-lived plasma cells, both of which allow for a long-term donor-specific humoral immune response (35).
B cell memory
Most memory B cells bind their specific antigen with a higher affinity than their naïve counterparts (36). Upon antigen re-challenge, memory B cells undergo activation, clonal expansion and germinal center formation much faster and to a more enhanced degree compared to naïve B cells. The result is a rapidly generated high affinity and specific antibody. The longevity of memory B cells is dictated by the BCR signaling and thus will influence the secondary response (37–39). The mechanism underlying control of memory B cell longevity is not well elucidated. A diversity of the BCR occurs as a result of somatic hypermutation (SHM) and class-switch recombination. By uncoupling these two processes, it has been demonstrated that class switching to IgG1 favors the formation of plasma cells while SHM can reduce the longevity of memory B cells (37). Similarly, it has also been suggested that B cell memory relies on varying numbers of isotype switched immunoglobulins and that antigen specific memory B cell longevity may also be a result of genetic predisposition (38, 39). Studies to identify antigen specific B cells in circulation of transplant recipients would be a valuable tool to determine who is at risk for chronic antibody-mediated rejection especially in those cases where donor specific antibodies (DSAs) are not present in circulation (40).
Long lasting plasma cells are also part of the memory response and they persist for several years or even lifelong in the bone marrow (41). They constitutively secrete antibody even in the absence of antigen (42). When directed against the allograft, these secondary responses are problematic. The reactivation of memory B cell responses and generation of long-lived antibody producing plasma cells cause a long-lasting humoral anti-donor immune response resulting in continuous inflammatory damage to the graft that ultimately leads to graft loss. Current immunosuppressive medications are T cell centric and focus on preventing T cell activation. They are very effective at preventing naïve responses but less effective in blunting a memory immune response. By preventing T cell activation, B cell responses and antibody production are thwarted in most cases. However, T cell independent B cell responses have the capability to occur despite adequate maintenance immunosuppression (43). This suggests that some B cell mediated allograft injury and DSA formation can occur in the absence of T cell help.
Antibody mediated allograft rejection
The hallmark of acute and chronic antibody mediated rejection (AMR) is presence of circulating DSAs, evidence for antibody binding to the vascular endothelium inducing complement activation (e.g., C4d deposition) and microvascular inflammation (44, 45). Antibody producing cells can be short lived while circulating in the blood and long lived when found in bone marrow, secondary lymphoid tissue and peripheral tissues such as an allograft (46). Studies are currently underway to phenotype the various DSA producing cell populations. DSAs can cause a progressive ischemic insult to the allograft eventually resulting in graft loss (45). Five isotypes of antibodies (IgM, IgD, IgG, IgA, and IgE) exist in circulation and are classified according to the constant regions of the heavy chain. The constant domains make up the Fc fragment, which mediates effector functions via Fc receptors and complement activation. The variable domains of the antibodies determine antigen specificity and antigen binding. The antibodies that are involved in allograft rejection are usually IgG (47). There are four subclasses of IgG antibodies (IgG1, IgG2, IgG3, and IgG4) with IgG1 being the predominant subclass in circulation. Each isotype varies in its half-life, ability to cross placenta, the degree to which it can neutralize pathogens, and activate macrophages or complement. The nature of the antigen and the cytokine environment during activation results in the different isotypes. Various subclasses of donor specific anti-HLA antibodies have been described in transplant recipients (23, 24, 48) and appear to have the same distribution in the plasma as the general immunoglobulin population.
DSAs can be directed again HLA and non-HLA molecules and have been reported to be associated with poor renal allograft outcomes (49–51). The presence of circulating DSA is a biomarker for T cell activation and previous acute or chronic cellular rejection (52–55). DSAs bind to endothelium of the allograft and have the potential to cause microvascular inflammation, ischemia and graft damage (i.e., glomerulitis, and peritubular capillaritis) (28, 56).
DSA can be of the immunoglobulin subclasses that can fix complement and those that cannot. The binding of DSA to the endothelial cell surface activates the classical complement pathway by engaging the C1 complex. The membrane attack complex is generated and the coagulation cascade is initiated resulting in thrombosis, fibrinoid necrosis, ischemia and loss of graft function (47). As a covalently bound degradation product of the classical complement pathway, C4d deposits detected on the endothelium serve as a marker of AMR (57). In particular, it has been shown that C1q binding DSA portend a higher risk of adverse graft outcomes compared to non-C1q binding DSA (55). Alternatively, circulating DSA that do not fix complement promote graft damage by engaging the Fcγ receptors on natural killer cells, macrophages and neutrophils (58) resulting in the release of growth factors, endothelial and smooth muscle cell proliferation or platelet activation (59, 60).
Chronic AMR has emerged as a leading cause of kidney allograft loss (61, 62). Chronic AMR appears to be less responsive to current immunosuppression compared to T cell mediated rejection (63). As a result, there is intense interest in understanding the detailed mechanisms of B cell memory generation, antibody production and plasma cell persistence. Therapies for AMR include antibody removal with plasmapheresis, B-cell depletion with agents such as rituximab, or targeting memory B cells with proteasome inhibitors such as bortezomib.
While de novo generation of DSAs and subsequent AMR can be devastating to a kidney allograft, the immunologic process of antibody production directed against pathogens is protective and can be lifesaving. Vaccination is a means by which both cellular and humoral memory responses can be induced to protect against life threatening pathogens. We measure vaccine responses with serologic testing however, that does not take into consideration the essential cellular components i.e., T and B lymphocytes mentioned above. While immunosuppressive therapies can successfully prevent graft rejection, they are problematic in that they prevent optimal vaccine responses. Ultimately, the goal is to fully vaccinate transplant recipients prior to transplantation in order to optimize a response before exposure to an immunosuppressive regimen. In this regard, the coronavirus disease of 2019 (COVID-19) pandemic has posed novel challenges to the transplant community.
Vaccinations in transplant recipients
The introduction of routine childhood immunizations has saved lives (64). Many vaccine preventable diseases have either been irradicated or reduced in frequency in the general population due to national vaccine efforts. In the US, the proportion of unvaccinated children remains low (< 1%), and thus the benefits of herd immunity can protect pediatric solid organ transplant (SOT) recipients (65). The American Society of Transplantation (AST) recommends that pediatric SOT candidates be immunized prior to transplantation (66). Although it is better to vaccinate patients prior to transplant, vaccination rates in adult kidney transplant recipients remain low (67). There is no national policy to mandate pre-transplant vaccinations and as a result some patients are inadequately vaccinated prior to transplant (68). In some circumstances, patients under transplant evaluation may be receiving immunosuppressive therapies that could alter antibody responses. In those cases, it may be warranted to delay vaccination for weeks or even months depending on the immunosuppressive therapies for safety and efficacy reasons. Although vaccine rates among UNOS kidney transplant centers are improving (69) continued efforts to implement quality improvement measures must be ongoing.
All kidney transplant recipients should receive non-live vaccines based on the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (70). This is especially true for those vaccines involved in cancer prevention (eg HPV). Transplant recipients should not receive live vaccines due to the risk of developing disease from the vaccine strain (66, 70, 71). Those on the transplant waitlist who receive a live virus vaccine should wait a minimum of 4 weeks before receiving a transplant. It is generally recommended to wait for 3–6 months after transplantation and until maintenance immunosuppression levels are achieved before starting vaccination in order to maximize the chances of an adequate immune response (66, 70, 71). For the same concern, vaccinations should be withheld from SOT recipients during intensified immunosuppression, such as 2–6 months after treatment of acute rejection episodes (71). However, in the setting of a global pandemic, such as with COVID-19, vaccination, albeit suboptimal, offers the possibility of decreasing mortality and morbidity of viral infection.
SARS CoV-2 mRNA vaccination
Balancing enough immunosuppression to block the alloimmune response while permitting antigen specific immune responses to vaccines is a tightrope walk. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination is quite effective in a healthy general population however, lower rates of seroconversion ranging between 30-56% are seen in adult and pediatric kidney transplant recipients (72–77). The mechanisms by which immunosuppression might alter lymphocyte function in this population remains unclear. An additional wrinkle in the plot is the risk of inducing an acute rejection episode due to the heterogeneity of the immune response and possible cross reactivity (78, 79).
We looked at our single center retrospective cohort of pediatric and adolescent kidney transplant recipients (KTR) who received a two or three dose series of an mRNA SARS-CoV-2 vaccine. Forty-three pediatric and adolescent KTR received 2-doses of an mRNA SARS-CoV-2 vaccine and 30 received a third dose. Forty (93%) received BNT162b2 (Pfizer-BioNTech), two (5%) received mRNA-1273 (Moderna) vaccine and one received a mixed vaccine series. SARS-CoV-2 spike protein antibody levels measured 4-8 weeks after their second vaccine dose and again after the third vaccine dose. We found that 56% of pediatric kidney transplant recipients seroconverted following a 2-dose series. Seroconversion increased to 85% in those who received a third dose. In the 16 patients who did not seroconvert after a two-dose series, 12 (75%) seroconverted following the third dose (Figure 1). We did not observe any post-vaccination rejection episodes (80).
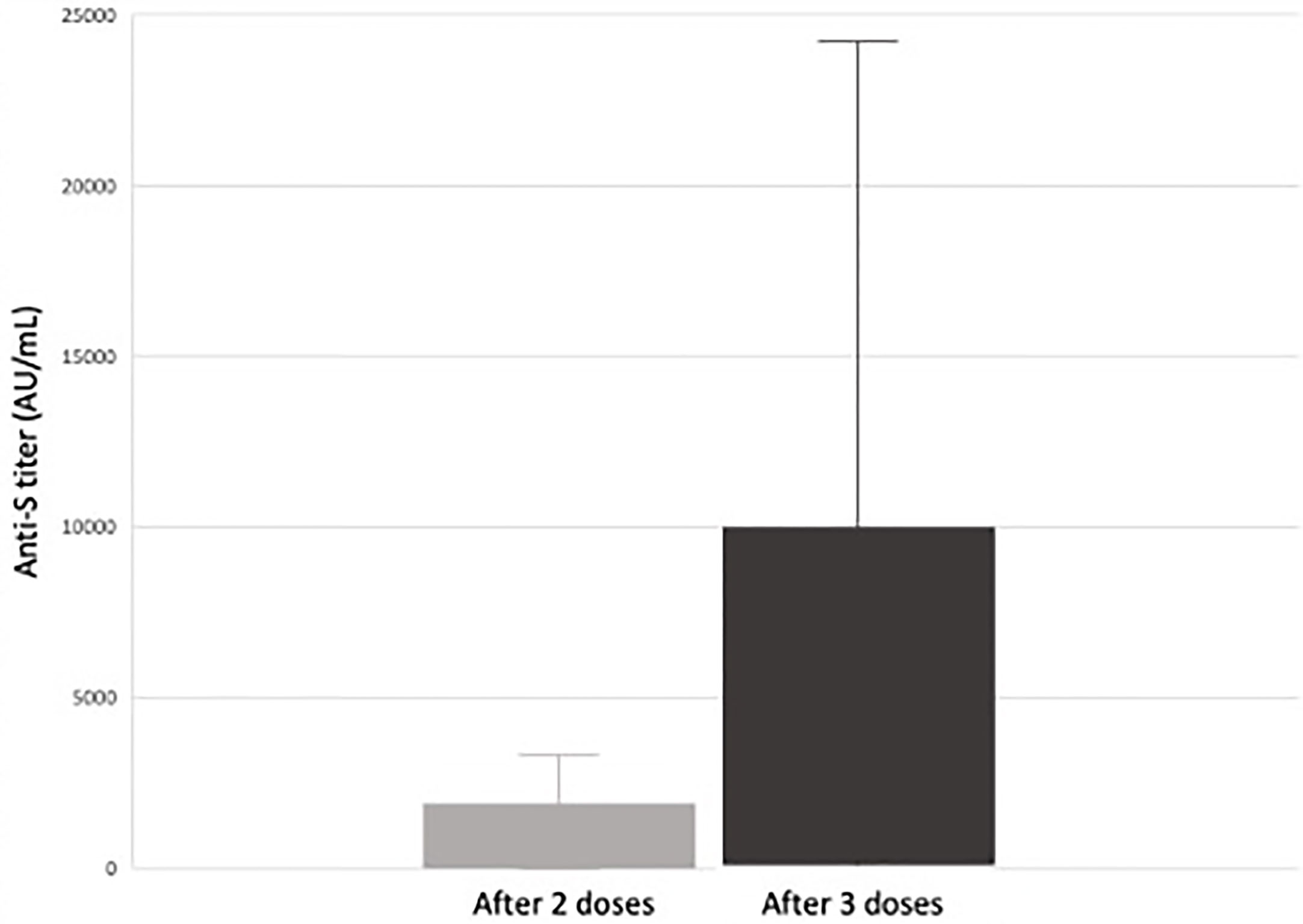
Figure 1 Median response and range of anti-S antibody titers (AU/mL) in response to SARS-CoV-2 vaccination in pediatric and adolescent kidney transplant recipients (80). Of the 26 pediatric and adolescent kidney transplant recipients who received a third mRNA SARS-CoV-2 vaccine dose, 22 (85%) seroconverted (defined as an anti-spike protein antibody titer >50 AU/mL). There was a significant increase in antibody titers between dose 2 and 3 from a median of 66 AU/mL after two doses to 881 AU/mL after three doses. In the 16 subjects responding only after a third dose, there was a significant increase in anti-S titer from 9.4 AU/mL to 682 AU/mL (p < 0.01) versus an increase from 4.9 AU/mL to 7.4 AU/mL (p = 0.3) in those who did not (80).
In a subset of those enrolled in a prospective study to examine the effects of immunosuppression on B-cell populations and infections, T and B lymphocyte subsets, immunoglobulin levels (IgA, IgG, IgM, and IgE), and vaccine titers (pneumococcal, tetanus, diphtheria, pertussis, and hepatitis B), and immunosuppressive medication doses (tacrolimus, mycophenolate mofetil, azathioprine, and prednisone) were evaluated prior to vaccination and at 6-month intervals.
No significant difference in immunoglobulin levels, T cell populations, or vaccine titers was observed. There was a trend toward lack of seroconversion with higher doses of mycophenolate mofetil (MMF), at 91 mg/m2/day median difference (p=0.06). All patients on azathioprine instead of MMF seroconverted. Those who did not seroconvert had lower hemoglobin levels (β=-1.30, p=0.009) and lower platelet count (β=-56.00, p=0.057). Non-responders to the vaccine showed a trend toward increased naïve B cell percentage (β= 12.50, p=0.11) and decreased total memory B cell percentage (β=-12.54, p=0.080). Increasing MMF dosage was associated with an increase in naïve B-cell percentage (β=0.016, p=0.0032) decrease in total memory B cell percentage (β=-0.016, p=0.0034) (Figure 2), and decreased in IgG level (β=-0.35, p=0.012).
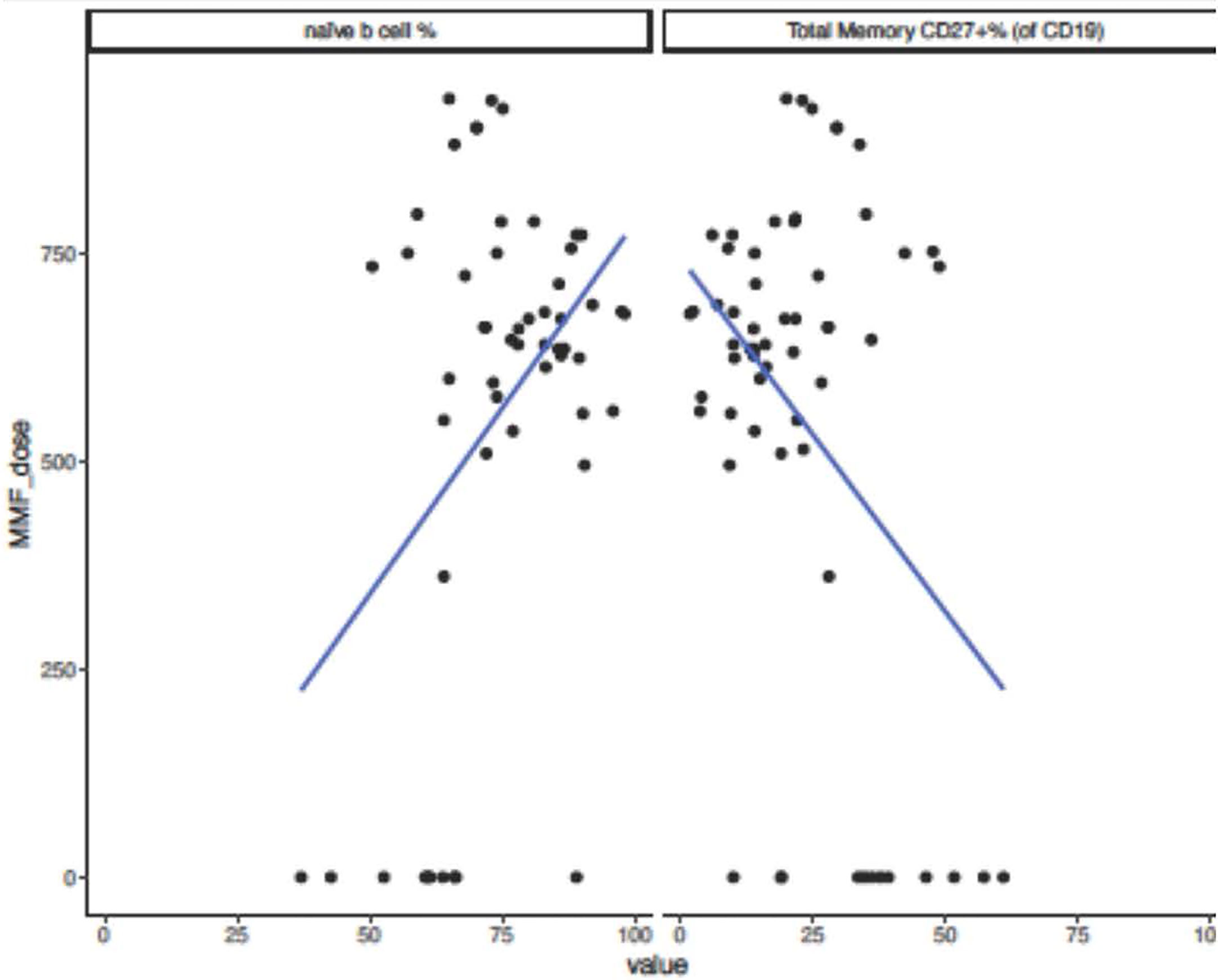
Figure 2 Naïve and Memory B-cell percentages and MMF dose in pediatric and adolescent kidney transplant recipients after SARS-CoV-2 mRNA vaccination. Subjects who did not seroconvert following SARS-CoV-2 mRNA vaccination showed a trend toward decreased total memory B-cell percentage (β=-12.54, p=0.080) and increased naïve B-cell percentage (β= 12.50, p=0.11). When analyzing effect of increasing MMF dose on immune parameters and increase in MMF dosing of 1 unit correlated to an increase in naïve B-cell percentage (β=0.016, p=0.0032) and decrease in total memory B-cell percentage (β=-0.016, p=0.0034).
Disruption in B cell population is likely due to immunosuppression and associated with the use of MMF. Non-responders showed non-significant trends toward high MMF dosage, increased naïve B-cell percentage, and decreased total memory B cell percentage. Trend toward decreased hemoglobin levels and normal red blood cell mean cell volume (MCV) supports that anemia could be due to bone marrow suppression caused by MMF. Future studies will need to investigate whether the defect is at the level of T cell activation or T cell help. Work in adult kidney transplant recipients has demonstrated additional vaccine doses can induce a functional maturation of vaccine-reactive T cells with significantly higher frequencies of IL-2 and IL-4 secreting and polyfunctional T cells being seen after a third dose (81).
Despite immunosuppression, the underlying immunologic machinery has the potential to “break through” after repeated exposure and stimuli. Additional SARS-CoV-2 mRNA vaccine doses are safe in kidney transplant recipients but may be necessary to overcome the iatrogenic suppression of T-cell proliferation and disruption of B-cell populations to optimize a humoral response. However, the risk of causing enough inflammation to promote acute rejection of the allograft remains a potential concern that merits ongoing observation.
Author contributions
CC manuscript writing, data collection and analysis, LL data collection, CA statistical analysis, BG study design and analysis, EI manuscript writing, study design and analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet (1966) 2(7465):662–5. doi: 10.1016/S0140-6736(66)92829-7
2. Singal DP, Mickey MR, Mittal KK, Terasaki PI. Serotyping for homotransplantation. XVII. preliminary studies of HL-a subunits and alleles. Transplantation (1968) 6(8):904–12. doi: 10.1097/00007890-196811000-00005
3. Basadonna GP, Matas AJ, Gillingham KJ, Payne WD, Dunn DL, Sutherland DE, et al. Early versus late acute renal allograft rejection: impact on chronic rejection. Transplantation (1993) 55(5):993–5. doi: 10.1097/00007890-199305000-00007
4. Murphy SP, Porrett PM, Turka LA. Innate immunity in transplant tolerance and rejection. Immunol Rev (2011) 241(1):39–48. doi: 10.1111/j.1600-065X.2011.01009.x
5. Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transpl (2011) 11(8):1563–9. doi: 10.1111/j.1600-6143.2011.03579.x
6. Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol (2001) 19:23–45. doi: 10.1146/annurev.immunol.19.1.23
7. Picker LJ. Mechanisms of lymphocyte homing. Curr Opin Immunol (1992) 4(3):277–86. doi: 10.1016/0952-7915(92)90077-R
8. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature (1998) 392(6673):245–52. doi: 10.1038/32588
9. Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev (1997) 156:25–37. doi: 10.1111/j.1600-065X.1997.tb00956.x
10. Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol (2010) 22(3):333–40. doi: 10.1016/j.coi.2010.02.013
11. Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-Cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature (1998) 393(6684):480–3. doi: 10.1038/31002
12. Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol (2001) 2(5):415–22. doi: 10.1038/87720
13. Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T cell-dependent conditioning of dendritic cells to produce IL-12 results in CD8-mediated graft rejection and avoidance of tolerance. J Immunol (2005) 174(11):6909–17. doi: 10.4049/jimmunol.174.11.6909
14. Siu JHY, Surendrakumar V, Richards JA, Pettigrew GJ. T Cell allorecognition pathways in solid organ transplantation. Front Immunol (2018) 9:2548. doi: 10.3389/fimmu.2018.02548
15. Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, et al. A novel pathway of alloantigen presentation by dendritic cells. J Immunol (2004) 173(8):4828–37. doi: 10.4049/jimmunol.173.8.4828
16. Womer KL, Sayegh MH, Auchincloss H. Involvement of the direct and indirect pathways of allorecognition in tolerance induction. Philos Trans R Soc Lond B Biol Sci (2001) 356(1409):639–47. doi: 10.1098/rstb.2001.0843
17. Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol (2003) 3(2):147–58. doi: 10.1038/nri1002
18. Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, et al. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol (2007) 8(4):388–97. doi: 10.1038/ni1446
19. Reiser JB, Darnault C, Guimezanes A, Grégoire C, Mosser T, Schmitt-Verhulst AM, et al. Crystal structure of a T cell receptor bound to an allogeneic MHC molecule. Nat Immunol (2000) 1(4):291–7. doi: 10.1038/79728
20. Bolton EM, Bradley JA, Pettigrew GJ. Indirect allorecognition: not simple but effective. Transplantation (2008) 85(5):667–9. doi: 10.1097/TP.0b013e3181664db3
21. Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol (2002) 3(9):844–51. doi: 10.1038/ni831
22. Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev (2009) 229(1):271–93. doi: 10.1111/j.1600-065X.2009.00781.x
23. Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science (1990) 248(4961):1349–56. doi: 10.1126/science.2113314
24. Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol (1991) 147(8):2461–6.
25. Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med (1997) 185(12):2133–41. doi: 10.1084/jem.185.12.2133
26. Schroder PM, Fitch ZW, Schmitz R, Choi AY, Kwun J, Knechtle SJ. The past, present, and future of costimulation blockade in organ transplantation. Curr Opin Organ Transpl (2019) 24(4):391–401. doi: 10.1097/MOT.0000000000000656
27. Yopp AC, Fu S, Honig SM, Randolph GJ, Ding Y, Krieger NR, et al. FTY720-enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol (2004) 173(2):855–65. doi: 10.4049/jimmunol.173.2.855
28. Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. The banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transpl (2020) 20(9):2318–31. doi: 10.1111/ajt.15898
29. Ohm B, Jungraithmayr W. B cell immunity in lung transplant rejection - effector mechanisms and therapeutic implications. Front Immunol (2022) 13:845867. doi: 10.3389/fimmu.2022.845867
30. Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific b and T lymphocyte interactions in the lymph node. Science (1998) 281(5373):96–9. doi: 10.1126/science.281.5373.96
31. Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral b cell fate. Immunity (1995) 3(6):691–701. doi: 10.1016/1074-7613(95)90059-4
32. Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, et al. The fate of self-reactive b cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J Exp Med (1996) 183(5):2313–28. doi: 10.1084/jem.183.5.2313
33. Gatto D, Wood K, Brink R. EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct b cell migration and organization in follicles and the germinal center. J Immunol (2011) 187(9):4621–8. doi: 10.4049/jimmunol.1101542
34. Shlomchik MJ, Weisel F. Germinal center selection and the development of memory b and plasma cells. Immunol Rev (2012) 247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x
35. Chong AS, Sciammas R. Memory b cells in transplantation. Transplantation (2015) 99(1):21–8. doi: 10.1097/TP.0000000000000545
36. Cancro MP, Tomayko MM. Memory b cells and plasma cells: The differentiative continuum of humoral immunity. Immunol Rev (2021) 303(1):72–82. doi: 10.1111/imr.13016
37. Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent roles of switching and hypermutation in the development and persistence of b lymphocyte memory. Immunity (2016) 44(4):769–81. doi: 10.1016/j.immuni.2016.01.011
38. Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different b cell populations mediate early and late memory during an endogenous immune response. Science (2011) 331(6021):1203–7. doi: 10.1126/science.1201730
39. Pape KA, Maul RW, Dileepan T, Paustian AS, Gearhart PJ, Jenkins MK. Naive b cells with high-avidity germline-encoded antigen receptors produce persistent IgM. Immunity (2018) 48(6):1135–43.e4. doi: 10.1016/j.immuni.2018.04.019
40. Eyer K, Doineau RCL, Castrillon CE, Briseño-Roa L, Menrath V, Mottet G, et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat Biotechnol (2017) 35(10):977–82. doi: 10.1038/nbt.3964
41. Lightman SM, Utley A, Lee KP. Survival of long-lived plasma cells (LLPC): Piecing together the puzzle. Front Immunol (2019) 10:965. doi: 10.3389/fimmu.2019.00965
42. Manz RA, Löhning M, Cassese G, Thiel A, Radbruch A. Survival of long-lived plasma cells is independent of antigen. Int Immunol (1998) 10(11):1703–11. doi: 10.1093/intimm/10.11.1703
43. Struijk GH, Minnee RC, Koch SD, Zwinderman AH, van Donselaar-van der Pant KA, Idu MM, et al. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int (2010) 78(9):934–40. doi: 10.1038/ki.2010.269
44. Idica A, Everly MJ. Donor-specific HLA antibodies: A review of data published in 2016. Clin Transpl (2016) 32:13–22.
45. Smith RN, Colvin RB. Chronic alloantibody mediated rejection. Semin Immunol (2012) 24(2):115–21. doi: 10.1016/j.smim.2011.09.002
46. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol (2015) 15(3):160–71. doi: 10.1038/nri3795
47. Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transpl (2014) 19(1):33–40. doi: 10.1097/MOT.0000000000000040
48. Lobashevsky A, Rosner K, Goggins W, Higgins N. Subtypes of immunoglobulin (Ig)-G antibodies against donor class II HLA and cross-match results in three kidney transplant candidates. Transpl Immunol (2010) 23(1-2):81–5. doi: 10.1016/j.trim.2010.03.003
49. Porcheray F, DeVito J, Yeap BY, Xue L, Dargon I, Paine R, et al. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation (2010) 89(10):1239–46. doi: 10.1097/TP.0b013e3181d72091
50. Dinavahi R, George A, Tretin A, Akalin E, Ames S, Bromberg JS, et al. Antibodies reactive to non-HLA antigens in transplant glomerulopathy. J Am Soc Nephrol (2011) 22(6):1168–78. doi: 10.1681/ASN.2010111183
51. Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med (2005) 352(6):558–69. doi: 10.1056/NEJMoa035717
52. Nath DS, Angaswamy N, Basha HI, Phelan D, Moazami N, Ewald GA, et al. Donor-specific antibodies to human leukocyte antigens are associated with and precede antibodies to major histocompatibility complex class I-related chain a in antibody-mediated rejection and cardiac allograft vasculopathy after human cardiac transplantation. Hum Immunol (2010) 71(12):1191–6. doi: 10.1016/j.humimm.2010.09.012
53. Sis B, Campbell PM, Mueller T, Hunter C, Cockfield SM, Cruz J, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transpl (2007) 7(7):1743–52. doi: 10.1111/j.1600-6143.2007.01836.x
54. Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol (2012) 8(6):348–57. doi: 10.1038/nrneph.2012.81
55. Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med (2013) 369(13):1215–26. doi: 10.1056/NEJMoa1302506
56. Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol (2001) 12(3):574–82. doi: 10.1681/ASN.V123574
57. Worthington JE, McEwen A, McWilliam LJ, Picton ML, Martin S. Association between C4d staining in renal transplant biopsies, production of donor-specific HLA antibodies, and graft outcome. Transplantation (2007) 83(4):398–403. doi: 10.1097/01.tp.0000251430.11723.b6
58. Lee CY, Lotfi-Emran S, Erdinc M, Murata K, Velidedeoglu E, Fox-Talbot K, et al. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation (2007) 84(10):1324–34. doi: 10.1097/01.tp.0000287457.54761.53
59. Kuo HH, Morrell CN, Baldwin WM. Alloantibody induced platelet responses in transplants: potent mediators in small packages. Hum Immunol (2012) 73(12):1233–8. doi: 10.1016/j.humimm.2012.06.011
60. Trayssac M, Nègre-Salvayre A, Thomsen M. Mechanisms of human smooth muscle cell proliferation and transplant vasculopathy induced by HLA class I antibodies: in vitro and in vivo studies. Hum Immunol (2012) 73(12):1253–60. doi: 10.1016/j.humimm.2012.06.012
61. Loupy A, Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med (2018) 379(12):1150–60. doi: 10.1056/NEJMra1802677
62. Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol (2015) 26(7):1711–20. doi: 10.1681/ASN.2014060588
63. Böhmig GA, Eskandary F, Doberer K, Halloran PF. The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl Int (2019) 32(8):775–88. doi: 10.1111/tri.13436
64. Ventola CL. Immunization in the united states: Recommendations, barriers, and measures to improve compliance: Part 1: Childhood vaccinations. P T (2016) 41(7):426–36.
65. Fox TG, Nailescu C. Vaccinations in pediatric kidney transplant recipients. Pediatr Nephrol (2019) 34(4):579–91. doi: 10.1007/s00467-018-3953-z
66. Danziger-Isakov L, Kumar D, Practice AIDCo. Vaccination in solid organ transplantation. Am J Transpl (2013) 13 Suppl 4:311–7. doi: 10.1111/ajt.12122
67. Lee DH, Boyle SM, Malat G, Sharma A, Bias T, Doyle AM. Low rates of vaccination in listed kidney transplant candidates. Transpl Infect Dis (2016) 18(1):155–9. doi: 10.1111/tid.12473
68. Ladd JM, Karkazis K, Magnus D. Parental refusal of vaccination and transplantation listing decisions: a nationwide survey. Pediatr Transpl (2013) 17(3):244–50. doi: 10.1111/petr.12046
69. Chon WJ, Kadambi PV, Harland RC, Thistlethwaite JR, West BL, Udani S, et al. Changing attitudes toward influenza vaccination in U.S. kidney transplant programs over the past decade. Clin J Am Soc Nephrol (2010) 5(9):1637–41. doi: 10.2215/CJN.00150110
70. Group KDIGOKTW. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transpl (2009) 9 Suppl 3:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x
71. Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis (2014) 58(3):309–18. doi: 10.1093/cid/cit816
72. Georgery H, Devresse A, Yombi JC, Belkhir L, De Greef J, Darius T, et al. Disappointing immunization rate after 2 doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation (2021) 105(12):e283–e4. doi: 10.1097/TP.0000000000003861
73. Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int (2021) 99(6):1498–500. doi: 10.1016/j.kint.2021.04.005
74. Masset C, Kerleau C, Garandeau C, Ville S, Cantarovich D, Hourmant M, et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int (2021) 100(5):1132–5. doi: 10.1016/j.kint.2021.08.017
75. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA (2021) 325(21):2204–6. doi: 10.1001/jama.2021.7489
76. Cucchiari D, Egri N, Bodro M, Herrera S, Del Risco-Zevallos J, Casals-Urquiza J, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transpl (2021) 21(8):2727–39. doi: 10.1111/ajt.16701
77. Crane C, Phebus E, Ingulli E. Immunologic response of mRNA SARS-CoV-2 vaccination in adolescent kidney transplant recipients. Pediatr Nephrol (2022) 37(2):449–53. doi: 10.1007/s00467-021-05256-9
78. Vnučák M, Graňák K, Beliančinová M, Jeseňák M, Macháleková KK, Benko J, et al. Acute kidney rejection after anti-SARS-CoV-2 virus-vectored vaccine-case report. NPJ Vaccines (2022) 7(1):30. doi: 10.1038/s41541-022-00445-5
79. Sarwar R, Adeyi OA, Lake J, Lim N. Acute cellular rejection in liver transplant recipients following vaccination against COVID-19: A case series. Liver Transpl (2022) 28(8):1388–92. doi: 10.1002/lt.26446
80. Crane C, Phebus E, Ingulli E. Antibody response to 2 and 3 dose SARS-CoV-2 mRNA vaccination in pediatric kidney transplant recipients. Pediatr Nephrol (2022) 1–4. doi: 10.1007/s00467-022-05661-8
Keywords: B cells, antibody mediated rejection, vaccinations, kidney transplant recipients, SARS CoV2 mRNA
Citation: Crane C, Loop L, Anterasian C, Geng B and Ingulli E (2022) Balancing B cell responses to the allograft: implications for vaccination. Front. Immunol. 13:948379. doi: 10.3389/fimmu.2022.948379
Received: 19 May 2022; Accepted: 06 July 2022;
Published: 27 July 2022.
Edited by:
Marilia Cascalho, University of Michigan, United StatesReviewed by:
Srinivasa Reddy Bonam, University of Texas Medical Branch at Galveston, United StatesCopyright © 2022 Crane, Loop, Anterasian, Geng and Ingulli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth Ingulli, eingulli@health.ucsd.edu
†ORCID: Clarkson Crane, orcid.org/0000-0002-2985-8559
Elizabeth Ingulli, orcid.org/0000-0003-2591-8945
 Clarkson Crane
Clarkson Crane Lauren Loop
Lauren Loop Christine Anterasian2,3
Christine Anterasian2,3 Elizabeth Ingulli
Elizabeth Ingulli