- 1Department of Psychiatry and Behavioral Medicine, Medical College of Wisconsin, Milwaukee, WI, United States
- 2Department of Medicine, Medical College of Wisconsin, Milwaukee, WI, United States
- 3Department of Microbiology & Immunology, Medical College of Wisconsin, Milwaukee, WI, United States
- 4Department of Pediatrics, Division of Hematology/Oncology, University of Washington School of Medicine, Seattle, WA, United States
- 5Palliative Care and Resilience Program, Center for Clinical and Translational Research, Seattle Children’s Research Institute, Seattle, WA, United States
- 6Department of Psychiatry, Harvard Medical School/Massachusetts General Hospital, Boston, MA, United States
- 7Department of Pediatrics, University of Minnesota Medical School, Minneapolis, MN, United States
- 8College of Nursing, University of Wisconsin – Milwaukee, Milwaukee, WI, United States
- 9Froedtert Hospital, Froedtert & The Medical College of Wisconsin, Milwaukee, WI, United States
- 10Department of Psychosocial Oncology and Palliative Care, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, United States
- 11Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 12Department of Adult Health and Nursing Systems, School of Nursing, Virginia Commonwealth University, Richmond, VA, United States
- 13Department of Nursing, University of Florida, Gainesville, FL, United States
- 14Cancer Population Science, University of Florida Health Cancer Center, University of Florida, Gainesville, FL, United States
- 15Department of Psychiatry, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
A growing body of literature has emphasized the importance of biobehavioral processes – defined as the interaction of behavior, psychology, socioenvironmental factors, and biological processes – for clinical outcomes among transplantation and cellular therapy (TCT) patients. TCT recipients are especially vulnerable to distress associated with pandemic conditions and represent a notably immunocompromised group at greater risk for SARS-CoV-2 infection with substantially worse outcomes. The summation of both the immunologic and psychologic vulnerability of TCT patients renders them particularly susceptible to adverse biobehavioral sequelae associated with the Covid-19 pandemic. Stress and adverse psychosocial factors alter neural and endocrine pathways through sympathetic nervous system and hypothalamic-pituitary-adrenal axis signaling that ultimately affect gene regulation in immune cells. Reciprocally, global inflammation and immune dysregulation related to TCT contribute to dysregulation of neuroendocrine and central nervous system function, resulting in the symptom profile of depression, fatigue, sleep disturbance, and cognitive dysfunction. In this article, we draw upon literature on immunology, psychology, neuroscience, hematology and oncology, Covid-19 pathophysiology, and TCT processes to discuss how they may intersect to influence TCT outcomes, with the goal of providing an overview of the significance of biobehavioral factors in understanding the relationship between Covid-19 and TCT, now and for the future. We discuss the roles of depression, anxiety, fatigue, sleep, social isolation and loneliness, and neurocognitive impairment, as well as specific implications for sub-populations of interest, including pediatrics, caregivers, and TCT donors. Finally, we address protective psychological processes that may optimize biobehavioral outcomes affected by Covid-19.
Intersecting Adversities: Transplantation and Cellular Therapy and the Covid-19 Pandemic
The Covid-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has claimed the lives of over 5 ½ million people worldwide as of January 28th, 2022 (1, 2). Resulting public health measures have significantly altered the social environment, increasing social isolation and feelings of loneliness (2). The pandemic has also precipitated economic hardship and financial strife (3). These social stressors have adversely affected mental health, particularly within socially and physically vulnerable populations (4).
Transplantation and cellular therapy (TCT) recipients comprise an immunocompromised group at greater risk for Covid-19 infection and poorer outcomes, including increased risk for mortality (5). They are already vulnerable due to their intense treatment regimens accompanied by prolonged hospitalizations, high risk of medical complications, and immediate and long-term medical comorbidities (6, 7). TCT recipients also have heightened risk for clinically significant distress and poor psychological function due to pandemic conditions (2, 8, 9).
A growing body of literature documents the importance of biobehavioral processes – defined as the interaction of behavior, psychology, socioenvironmental factors, and biological processes – for clinical outcomes among TCT patients (10, 11). The summation of both the immunologic and psychological vulnerability of TCT patients renders them particularly susceptible to adverse biobehavioral sequelae associated with the conditions of the current global pandemic, potentially affecting clinical and patient-reported outcomes.
Biobehavioral Processes in TCT
Cancer development and progression, as well as TCT treatment, alter innate and adaptive immune function (12). The significant stress associated with a cancer diagnosis, treatment, and accompanying life disruption can also adversely affect immune function (13, 14). Specifically, stress and adverse psychosocial factors alter neural and endocrine pathways that ultimately affect gene regulation in immune cells (15–19). This signaling is primarily mediated through the sympathetic nervous system (SNS) (20, 21) and hypothalamic-pituitary-adrenal (HPA) axis (22). These biobehavioral signals affect the tumor microenvironment through mechanisms including the promotion of tumor-cell growth and spread, angiogenesis, and alterations in antibody and cytokine production profiles and cell trafficking (22). Inflammation due to tissue damage from intensive conditioning therapy and the occurrence of infections contributes to dysregulation of neuroendocrine and CNS function, resulting in the symptom profile of depression, fatigue, sleep disturbance, and cognitive dysfunction (12, 22). In addition, inflammation plays a central role in the pathogenesis of graft-versus-host disease, a debilitating syndrome that attacks organs following allogeneic HCT resulting in a cytokine storm of inflammatory activity, as well as the CRS common following CAR T cell therapy, further exacerbating neuropsychiatric symptoms (23, 24). Gene expression profiling studies of people exposed to chronic threat have identified a “conserved transcriptional response to adversity” (CTRA) in circulating immune cells (25). CTRA expression is mediated by SNS activity and associated with adverse TCT outcomes (26) and may represent a pathway by which pandemic-associated stress influences clinical TCT outcomes.
Psychosocial Effects of Covid-19 for TCT Patients
TCT patients are already at an increased risk for adverse psychological effects, including depression, anxiety, and persistent distress (13, 27). The Covid-19 pandemic has exacerbated stress, with cancer patients reporting increased depression, anxiety, and distress related to their physical health since the onset of the pandemic (8, 9, 28–32). For TCT patients, care disruptions and delays can be particularly stressful (10, 33). Because of the increased risk of severe Covid-19, many TCT patients also have heightened fear of contracting the SARS-CoV-2 virus (34, 35). The pandemic-induced limitations on travel creates additional distress for patients who must travel long distances to receive care at the few TCT centers in the U.S. and around the world (36). Social isolation and quarantine requirements during post-TCT recovery already cause distress for TCT recipients (37), which is worsened by more extensive isolation related to Covid-19 precautions (7). The widespread economic hardship associated with the Covid-19 pandemic can also exacerbate the adverse financial impact of TCT (38–40).
Biological Effects of Covid-19 Disease Processes Relevant to TCT
SARS-CoV-2 targets the angiotensin-converting enzyme 2 expressed by airway epithelial cells, alveolar epithelial cells, vascular endothelial cells, and macrophages in the lung (41–44). The virus then penetrates the host cell membrane wherein viral RNA is released and gains access to the cellular machinery necessary for self-replication and release, spreading infection and damaging host cells (41, 42). The body’s initial innate immune response is characterized by the inflammatory actions of alveolar macrophages and recruitment of T lymphocytes, monocytes, and neutrophils. In a healthy immune response, this inflammation is carefully regulated and works in tandem with the body’s slower adaptive immune mechanisms—characterized by the action of CD4+ and CD8+ T cells and the release of antibodies by B cells—to eliminate infected cells and neutralize the virus. However, most patients with severe Covid-19 infection instead experience a dysfunctional immune response, in which unchecked inflammation continues and becomes widespread, driving systemic cytokine storm and eventually resulting in pulmonary edema, pneumonia, possible microthrombus formation, and multi-organ damage (42–44). TCT patients are especially vulnerable to severe infection and associated complications.
Further, SARS-CoV-2 infection impacts the central nervous system (CNS) directly through several mechanisms. Viral penetration triggers a neuroinflammatory reaction that leads to microglial activation, triggering a demyelinating process that acts as a primary etiology for encephalopathy (45). In preclinical models, coronavirus particles are capable of invading cells of the cortex, hypothalamus, thalamus, amygdala, basal ganglia, pyriform cortex, and brain stem while proliferating in the limbic structures, which can ultimately lead to behavioral changes (46).
Summary
In this article, we expand upon the above overviews to examine intersections of inflammation, immunology, aging, psychology, infection, neuroscience, cancer, viruses, stress, Covid-19 pathophysiology, and TCT processes that can influence TCT outcomes. The major relationships and pathways described are summarized in Figure 1. The goal is to provide an overview of the significance of biobehavioral factors in understanding the relationship between Covid-19 and TCT. Specific symptoms and states that will be discussed include depression, anxiety, fatigue, sleep, social isolation and loneliness, and neurocognitive impairment (NI). We will also discuss specific implications for sub-populations of interest within the TCT context, including pediatrics, caregivers, and donors. Finally, we address protective psychological processes that may optimize biobehavioral outcomes affected by Covid-19.
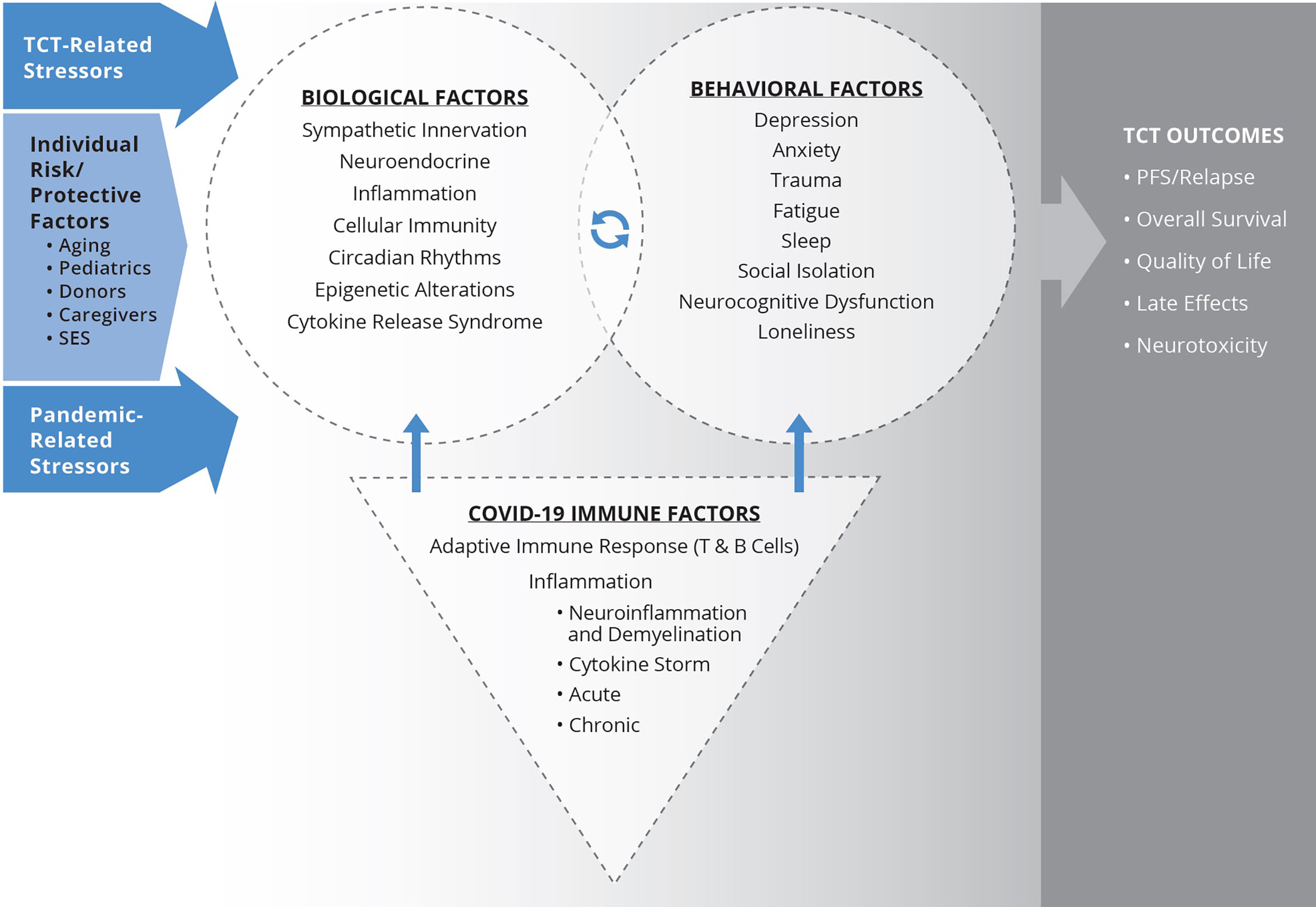
Figure 1 Biobehavioral model illustrating Covid-19 impact for TCT patients. Biological processes affected by TCT, including inflammation and disrupted circadian rhythms, alter central nervous system pathways that evoke behavioral symptoms. Stress-related behavioral factors activate HPA and SNS axes and are associated with downstream immunologic and genomic alterations. The products of these pathways, as well as direct sympathetic innervation of the bone marrow microenvironment, can modulate immune recovery and inflammation, potentially influencing clinical outcomes following TCT. Behavioral factors also directly relate to quality of life and late effects. Stress and adversity associated with the Covid-19 pandemic can exacerbate biological and behavioral impacts of TCT and biobehavioral mechanisms associated with poorer clinical outcomes. Similarly, infection with SARS-CoV-2 can also adversely affect biobehavioral factors and relationships. The paper further considers the intersection of Covid-19 impacts and individual differences in risk and protective factors, including age, SES, caregiver status, and donor characteristics relevant to biobehavioral processes and TCT outcomes.
Stress-Related Biobehavioral Factors
Depression and Anxiety
Patients receiving TCT are at risk for short and long-term neuropsychiatric toxicities, including anxiety, depression, and trauma-related stress symptoms. These symptoms may be a consequence of underlying disease, the treatment itself, and the complex psychosocial and environmental factors that are interwoven into the cellular therapy experience and may also be exacerbated by the Covid-19 pandemic. The prevalence of psychological symptoms in hematopoietic cell transplant (HCT) patients is high compared to the general population, typically with a peak in the peri-transplant period and improvement over time (47). In a study of 90 HCT patients, nearly 30% met criteria for PTSD and 43% reported clinically significant depression symptoms at 6 months post-transplant (48). In survivors of pediatric HCT, the prevalence of clinically significant anxiety is estimated to be between 16-37%, which is higher than age-matched controls and siblings (49, 50). Less is known about prevalence of these symptoms in patients receiving chimeric antigen receptor (CAR) T cell therapy, but early data suggest nearly 50% of patients experience at least one clinically meaningful negative neuropsychiatric outcome (51).
Psychosocial Effects of Covid-19
The impact of pandemic-related personal and societal disruptions on cellular therapy patients’ mental health is still being investigated. Covid-19 survivors have consistently reported anxiety, depression, and trauma-related symptoms following their diagnosis (52). A recent study compared patient-reported depression, anxiety, and PTSD symptoms between HCT recipients who received treatment just prior to (March 2019-January 2020) and during (March 2020-January 2021) the Covid-19 pandemic (7). This study did not find any differences in psychological symptoms between the two cohorts. Covid-19-era patients described negative impacts of the pandemic on their HCT recovery, including increased isolation during their hospitalization and heightened distress about being infected by SARS-CoV-2. They also articulated positive downstream effects of the pandemic, including increased access to their support network through adaptation of social activities to virtual platforms and comfort in mandated public health safety measures (7).
Biological Effects of Covid-19
Symptoms of depression, anxiety, and traumatic stress all have well-documented neuroimmune correlates relevant to cellular therapy patients (12, 33, 53). SNS-mediated beta-adrenergic signaling (activated during ‘fight-or-flight’ states such as anxiety or the hypervigilance of PTSD) influences stem cell migration, homing, and proliferation (54, 55). In CAR T cell therapy, cytokine release syndrome (CRS) and related neurotoxicity syndromes lead to profound peripheral and sometimes central inflammation, with demonstrated downstream neuropsychiatric sequelae (51, 56). There is overlap in the pathophysiology of neuroimmune-mediated symptomatology between HCT and CAR T cell therapy and Covid-19 disease. Thus, it is important to consider the interrelated psychoneuroimmune effects of a SARS-CoV-2 infection in patients with baseline aberrant immune repertoires. The SARS-CoV-2 virus can elicit an extreme, life-threatening acute inflammatory response, but even mild infections can result in significant neuroinflammation and myelin loss (57). The resulting symptoms, which can persist beyond the acute infection period as a post-Covid condition (including “Long Covid”) share some features with neurotoxicity syndromes or chemotherapy-associated neuropsychiatric dysfunction (57). Close attention to new or worsening neuropsychiatric symptoms in cellular therapy patients following a Covid-19 diagnosis is warranted.
Summary
Pandemic-related individual and societal disruptions have had almost universally negative effects on global mental health, but we are still learning about specific effects on mood symptoms in patients receiving HCT or CAR T cell therapy. There also may be some systemic adaptations to the pandemic that have the potential to positively impact patients’ experience and mood.
Fatigue and Sleep
Fatigue and sleep disruption are some of the most prevalent and distressing symptoms experienced by TCT recipients before, during, and after treatment (58–60). Fatigue tends to be ubiquitous during hospitalization for HCT but may gradually remit for patients during the 100-day post-transplant recovery period. Although fatigue often improves over time, evidence suggests that as many as 35-64% of HCT recipients go on to experience persistent fatigue during long-term post-transplant recovery (61–63). A similar pattern exists for sleep problems, with over 50% of patients experiencing sleep disruption prior to transplant and up to 43% following discharge home (27, 59, 64). In addition, early work characterizing patient-reported outcomes among patients receiving CAR T cell therapy suggests that fatigue and sleep disruption do not improve even by six months following treatment (60).
Persistent fatigue and sleep disruption not only disrupt engagement in everyday routines, but have been linked to self-reported cognitive problems (51, 65, 66), diminished social adjustment and engagement (51, 67, 68), unemployment status (69, 70), and impaired mood in TCT patients (27, 71). There is also evidence to suggest that fatigue and sleep disruption may predict clinical outcomes following HCT, with findings from a recent study revealing that high pre-HCT sleep disruption predicted an increased risk of disease recurrence and poorer survival, and that greater post-HCT fatigue interference was also associated with poorer survival (72).
The contributors to fatigue and sleep disruption among TCT recipients are multifactorial, with demographic, biological, medical, psychosocial, and behavioral influences (10, 11, 53, 73, 74). While evidence is preliminary, the Covid-19 pandemic is likely to influence the symptoms experienced by TCT patients across these multifactorial domains.
Psychosocial Effects of Covid-19
The protracted recovery period following TCT, in which patients are immunosuppressed and endure protective isolation, imposes drastic and prolonged lifestyle adaptations (e.g., social isolation, activity reduction) that may exacerbate fatigue and sleep disruption long-term. These adaptations are amplified in the context of the Covid-19 pandemic. In addition, Covid-19 related stressors are associated with a heightened and prolonged state of psychological stress that can initiate or contribute to symptoms of fatigue and sleep disruption (31, 75). However, a recent report revealed no differences in pre-HCT fatigue before or during the Covid-19 pandemic (7). Findings highlight the importance of capturing the symptom experience across the TCT trajectory as well as the value in understanding sources of both vulnerability and resiliency from the pandemic and effects on biobehavioral symptoms.
Biological Effects of Covid-19
Both psychological stress and viral infection can disrupt the HPA system and affect fatigue and sleep disruption directly through alternations in cortisol or indirectly via modulation of immune pathways (2, 10, 76). Further, HPA axis dysregulation and inflammatory processes can disrupt circadian rhythms, which has been linked to poor QOL among HCT recipients (77) and heightened fatigue and sleep disruption among other cancer populations (78). As noted previously, inflammation is common in the TCT setting (23) and occurs in response to infection with the novel SARS-CoV-2 infection (5). This inflammatory cascade can affect CNS circuitry leading to behavioral responses associated with the withdrawal and conservation of energy, including fatigue and sleep disturbance (79–81). In HCT patients, fatigue and sleep disruption are both associated with higher circulating levels of interleukin (IL)-6, particularly in earlier post-transplant recovery (62, 82–85). There is evidence of a similar hyperinflammatory state occurring amongst a subgroup of patients with severe SARS-CoV-2 infection that may share underlying pathophysiology and subsequent biobehavioral symptom profiles (86, 87). It remains unclear whether this mechanism influences the prolonged biobehavioral symptoms exhibited in “post-Covid-19 syndrome” (88).
Summary
A common framework used to understand fatigue and insomnia is a biopsychosocial model of predisposing, precipitating, and perpetuating factors (89–91). Predisposing factors refer to characteristics that increase an individual’s vulnerability to symptom development. Precipitating factors refer to triggers leading to the onset of fatigue or insomnia episodes. Finally, perpetuating factors are features that contribute to the maintenance or exacerbation of symptoms over time. With the added complications of the Covid-19 pandemic, consistent use of this framework to guide research efforts may prove useful for advancing the biobehavioral science of fatigue and sleep disruption in the TCT context.
Social Isolation and Loneliness
Social isolation and loneliness have been increasingly recognized as a public health priority due to their effects on mental and physical health (92) and have been a significant concern during the Covid-19 pandemic. Although social isolation and loneliness are sometimes correlated, social scientists make conceptual and empirical distinctions between the two constructs (93). Social isolation is a lack of (or infrequent) social contact that may occur when an individual lives alone or has few social ties, whereas loneliness is a subjective feeling of social disconnection that results from a discrepancy between an individual’s actual and desired social relationships (94). To date, more than a dozen studies have investigated loneliness in cancer patients and survivors, including TCT recipients, whereas social isolation has received little empirical attention in these populations. A meta-analysis conducted prior to the pandemic suggested that 32-47% of cancer survivors experience loneliness (95). In a study of HCT recipients, 39% of the sample and 70% of the subset of patients with elevated psychological distress reported feeling lonely (96).
Psychosocial Effects of Covid-19
Due to immunocompromised status and increased risk for severe complications from Covid-19 (97–99), cancer patients have been particularly advised to physically isolate from others during the pandemic. The degree of physical isolation required during the pandemic is in clear contrast to the typical recommendation for cancer patients to engage their social and support networks, including family members and other social ties, community organizations, and places of worship (100, 101). For TCT recipients, the routine physical isolation that occurs during hospitalization and the early recovery period may persist longer and be even more restrictive during Covid-19 due to no-visitor hospital policies, contributing to social isolation and loneliness (102). Indeed, several studies conducted during the pandemic estimate the prevalence of loneliness in cancer patients and survivors is 48-53% (103–107), an increase from pre-pandemic estimates. In addition, a handful of longitudinal studies found that loneliness in cancer patients increased during the pandemic and was associated with poorer mental health (28, 108–110). This research identified active treatment, younger age, non-white, unmarried/unpartnered, living alone, and having a pre-existing mental health condition as risk factors for loneliness during the pandemic (104, 106, 107). Although one qualitative study suggested that HCT recipients experienced decreased social connection and support during the pandemic (36), empirical data on loneliness among TCT patients specifically are not yet available. However, one study reported that blood cancer survivors were more likely to report being lonely and had a larger increase in depression symptoms during the pandemic as compared to other cancer populations (28).
A meta-analysis also found that social isolation and loneliness were associated with a 29% and 26% increased risk of all-cause mortality, respectively, which is comparable in magnitude to well-established risk factors such as obesity and smoking (111). Among cancer patients and survivors, loneliness and has also been associated with higher pain, fatigue, anxiety, and depression, as well as poorer cognitive function and health-related QOL (112–116). In two studies with breast cancer survivors, social isolation (i.e., few intimate contacts and group memberships) was associated with increased risk for cancer recurrence, breast cancer mortality, and all-cause mortality, and survivors who were isolated were more likely to smoke and engage in less physical activity than those who were socially integrated (117, 118). To date, however, the potential impact of social isolation and loneliness, including during the Covid-19 pandemic, on transplant-related and other health outcomes among TCT recipients has not been investigated, representing an important research direction.
Biological Effects of Covid-19
Social isolation and loneliness are forms of chronic stress that also initiate a stress-signaling cascade characterized by increased SNS and HPA axis activation that can affect the expression of immune response genes, including those involved in inflammation and the anti-viral response (119). In non-cancer populations, loneliness has been associated with peripheral and transcriptomic indicators of SNS and HPA axis activity, inflammation, and antiviral immunity, including elevated urinary norepinephrine levels, altered diurnal cortisol patterns (e.g., cortisol awakening response), increased peripheral markers of inflammation (e.g., IL-6), and elevated inflammatory and reduced glucocorticoid receptor and antiviral gene expression (for review, see Cacioppo and Cacioppo, 2018) (120). Inflammation reciprocally feeds back to the brain to further influence feelings of loneliness (121, 122). Other research has found that social isolation and loneliness are associated with lower influenza antibody levels following vaccination (123) and greater symptoms of infection following exposure to the common cold virus (124, 125), with potentially important implications for Covid-19 vaccination response and risk for infection among immunocompromised TCT recipients.
In a study with breast cancer survivors, lonely patients showed greater synthesis of IL-6 and IL-1b in peripheral blood mononuclear cells stimulated with lipopolysaccharide following an acute stressor in the laboratory compared to those who were less lonely (115). Another study with colorectal cancer patients found that those who were lonely had greater expression of vascular endothelial growth factor in tumor tissues following surgery; however, self-reported loneliness did not show an association (126). Finally, research with breast and ovarian cancer survivors suggests that women reporting lower levels of emotional closeness in their relationships, which is conceptually similar to loneliness, had pro-metastatic molecular profiles (e.g., gene expression patterns consistent with epithelial-mesenchymal transition) in primary tumors and exosomes (127–129).
Summary
Together, these findings suggest that social isolation and loneliness may affect key stress response and immune pathways, including those related to cancer progression. Given that social isolation and loneliness may be amplified as a result of the Covid-19 pandemic, it will be important for researchers and clinicians working with TCT populations to consider these potential risk factors that may interact with TCT treatments to influence transplant-related and other behavioral and patient-reported outcomes (130).
Neurocognitive Impairment
NI, also referred to as neurocognitive dysfunction or cognitive dysfunction, is a complex issue with varying manifestations affecting multiple domains of cognition, including memory, attention, concentration, executive function, processing speed, and learning (131). Risk factors for NI include underlying disease burden, radiation and chemotherapy, and immunologic therapies (132, 133). NI is a top concern for TCT patients and caregivers, with 40- 60% of adult HCT survivors reporting NI five or more years post HCT (74, 134–136) (137, 138). Early data from CAR T cell therapy recipients demonstrate that approximately 37.5% reported NI at 1 to 5 years post-therapy, including problems with memory, word finding, and concentration (51). NI has also been observed in 15-80% of sampled Covid-19 participants across several studies (139). Risk factors for NI in Covid-19 patients are not yet known, although there is some evidence that those with more severe or complicated infections are at greater risk (140).
Psychosocial Effects of Covid-19
Psychological stress is associated with impaired cognitive function, presenting a point of vulnerability for biobehavioral complications of Covid-19 in TCT recipients. Among cancer patients, 91.5% of those classified as experiencing extremely high stress had NI, as compared to an incidence of 75% among cancer patients more broadly (31). Stressors associated with the Covid-19 pandemic may also exacerbate NI in the TCT population, although this is yet to be formally investigated. Early data among CAR T cell recipients shows that blood kynurenine concentrations – a molecular marker linking inflammation and the brain – are increased for those reporting more depression (141). In turn, increased kynurenine and its metabolites were further predictive of greater neurotoxicity (141), suggesting one plausible biologic mechanism linking CNS function to NI that could be exacerbated by pandemic conditions.
Biological Effects of Covid-19
Inflammation, HPA axis dysfunction, and altered monoamine neurotransmission may contribute to NI following treatment (142–155). Among HCT patients, increases in circulating markers of inflammation, including IL-6 and soluble tumor necrosis factor (TNF) receptor II, are associated with worsening cognitive function from pre-HCT to 90 days following HCT, while decreases in C-reactive protein are associated with better cognitive performance (133).
The two most common toxicities associated with CAR T cell therapy are CRS and immune effector cell-associated neurotoxic syndrome (ICANS), both of which are unique to CAR T vs. HCT and are associated with neurocognitive sequela (51). Inflammatory cytokines, including interferon gamma, IL-6, IL-8, IL-10, and monocyte chemoattractant protein-1, are elevated in individuals progressing to severe CRS (143). ICANS typically - though not always - presents in conjunction with CRS, suggesting overlapping but not identical pathology (144).
Given that inflammation is a significant component of Covid-19 pathophysiology and directly contributes to NI, it is plausible that infection with SARS-CoV-2 may additively or synergistically contribute to NI among TCT recipients, although there is not yet research addressing this. In particular, the cytokine storm associated with Covid-19 may contribute to worse NI among TCT recipients. This effect may be compounded for CAR T cell recipients who concurrently experience CRS or ICANS, though this remains to be investigated. Given that TCT patients become more severely ill if infected with SARS-CoV-2, they may also be more likely to experience adverse neuropsychiatric sequelae and NI based on what is known about NI outcomes with severe or complicated Covid-19.
Summary
Data on the pathophysiology of Covid-19, TCT therapies, the neuropsychiatric sequelae of SARS-CoV-2 infection and pandemic restrictions, and the overlapping biologic etiologies therein strongly support the need for further investigation of the impact of Covid-19 on NI in the TCT population.
Subpopulations of Interest Within the Context of TCT
Pediatrics
Cellular therapies, which both create and harness extreme immune dysregulation, have revolutionized the care of many types of pediatric cancer (53, 145, 146). Young patients receiving TCT in the era of Covid-19 are positioned to experience the convergence of profound social, neuropsychiatric, and immune disturbances in a distinct way. The Covid-19 pandemic has undeniably affected children and adolescents differently than older adults. This applies to both the pathophysiology of SARS-CoV-2 infection in younger persons, as well as the psychosocial implications of the large-scale societal disruptions. Not only do children and adolescents undergoing TCT face the same Covid-19-related risks as other children, but they must also cope with the immense burdens of cancer and treatment.
Healthy children and adolescents typically experience less severe disease after infection with SARS-CoV-2 compared to adults (147). However, children and adolescents with cancer may be at increased risk of poor outcomes. In a global cohort of pediatric patients with cancer or HCT, severe or critical illness occurred in nearly 20% of patients, and 4% died from Covid-19-related complications (148). Indirect complications of Covid-19 in pediatric TCT patients mirror that of adults, including delayed diagnoses, treatment interruptions, loss of caregivers, and postponement or even withdrawal of cellular therapies due to strained healthcare resources (149, 150). Most pediatric cellular therapy patients are treated as part of a clinical trial, and thus pandemic-related disruptions in research infrastructure can significantly impact the clinical care of these patients (151).
At the pathophysiologic level, there are commonalities between some of the unique manifestations of Covid-19 in children and the sequelae of cellular therapy. For example, multisystem inflammatory syndrome in children is a rare but serious entity characterized by hyperinflammation and end-organ dysfunction (152). Dangerous over-activation of the immune system is also a hallmark of CRS and ICANS following CAR T cells (56, 153). Given the known associations between severe inflammation and adverse neuropsychiatric outcomes (56, 79), close evaluation of these patients is warranted. For children and adolescents, there are likely significant implications of experiencing these inflammatory insults during critical developmental periods. Indeed, there are reports of prolonged neurocognitive deficits and adverse QOL in pediatric Covid-19 survivors (154, 155). There is a scarcity of data regarding severe Covid-19 in pediatric patients with baseline dysregulated immune systems, and the long-term neuropsychiatric sequelae of severe inflammation associated with SARS-CoV-2 infection in young cellular therapy patients should be an area of active research focus.
Not only can the inflammatory response drive poor neuropsychiatric outcomes, but behavioral and socioenvironmental factors can also influence the immune system (11, 53, 156). Extreme isolation, interruption of school and peer supports, health risks, exacerbation of economic hardships, compounded family stress, and death of friends and family have culminated in a youth mental health crisis during the pandemic (157, 158). Young patients with cancer are experiencing alarmingly high rates of anxiety and depression, with 60-80% of patients reporting worsening of symptoms since the onset of the pandemic (105, 159). Cellular therapy patients may be at even higher risk of poor mental health outcomes. These types of physical and existential threats have been associated with downstream immune effector cell function, which have clear implications for cellular therapy patients (160–162). Thus, the overlay of pandemic-related distress onto cancer-related psychological and emotional challenges at critical developmental periods for young patients may have implications at the biologic level.
Summary
The Covid-19 pandemic has introduced a plethora of new stressors that are unique to young cellular therapy patients. Important questions remain regarding pediatric-specific immune responses to SARS-CoV-2 infection, especially for patients who may have abnormal immune repertoires. Applying a biobehavioral lens to research and clinical care as the pandemic continues to unfold may offer valuable insights and tools in the care of this particularly vulnerable TCT subgroup during this unprecedented time and as we prepare for the future (53, 163).
Caregivers
Family caregivers play a critical role in the well-being of patients undergoing TCT. Caregiver involvement has been associated with lower patient distress and even improved patient survival (164–166). Caregiver distress is also associated with poorer patient response to treatment (167), and caregiver well-being is associated with better patient QOL (168). Therefore, the biological and psychological effects of pandemic-related stress for caregivers has the potential to translate to downstream adverse TCT patient outcomes (167, 169–173). The Covid-19 pandemic undoubtedly imposes additional stress on TCT caregivers; however, little research has directly explored this (174–176). Meanwhile, the current body of TCT caregiver biobehavioral research (170, 177–181) – albeit limited – helps offer insight into how the unique stressors of a global pandemic likely have consequences not only for caregivers, but potentially implications for patient outcomes as well.
Psychosocial Effects of Covid-19
Prolonged stress contributes to adverse psychological outcomes for TCT caregivers, including anxiety and depression, family strain, and spiritual and existential distress (172, 173, 182, 183) (184–186). TCT caregivers show even higher levels of stress than do dementia caregivers, with 30% of HCT caregivers meeting criteria for clinically significant psychological distress (187–189).
The Covid-19 pandemic adds to the caregiving burden and increases distress for TCT caregivers. Caregivers have less access to support during the pandemic, thereby increasing their caregiving responsibilities and burden (188). Since the pandemic started, caregivers report more anxiety, depression, fatigue, and sleep disturbance in addition to lower social participation and poorer financial well-being than non-caregivers (190). Adults caring for a parent with hematologic cancer reported distress related to reduced in-person health care, increased uncertainty, and social isolation during pandemic conditions (175). Parents supporting their children through HCT during Covid-19 shared that the HCT experience uniquely prepared them to cope with the pandemic; however, it also diminished their access to coping resources, reduced their social support, and amplified their germ-related fears (174). Further, parents of children with hematologic cancers reported feeling more vulnerable during the pandemic (176). Notably, increased isolation to protect their loved one from Covid-19 may contribute to TCT caregiver loneliness (191).
Biological Effects of Covid-19
While the biological effects of Covid-19 on caregivers have not been specifically studied, stress-related dysregulation of the HPA axis has been observed among TCT caregivers and may be exacerbated by Covid-19 related stressors (170, 180, 181, 189, 192, 193). Caregiver stress is also associated with enhanced expression of the proinflammatory CTRA gene profile (26, 138, 177). In a study of caregivers of patients with colorectal cancer, social isolation and lack of social support were associated with increased CTRA gene expression, which has implications for social isolation experienced during the Covid-19 pandemic (177).
Summary
The prolonged stress of Covid-19 likely contributes directly to adverse psychological and biological sequelae of TCT caregivers, as well as indirectly to TCT recipient outcomes, through the manifestation of caregiver stress and social isolation. Further research is needed to evaluate the intersection of burdens of TCT caregiving and pandemic conditions with the goal of optimizing caregiver and patient outcomes.
Donors
The impact of Covid-19 on the clinical and physiologic status of hematopoietic cell donors and the downstream effects on HCT recipients has not been well-described to date. However, there are several plausible impacts that warrant further evaluation. First, socioeconomic hardships associated with Covid-19 may affect the ability of individuals to volunteer as donors because of financial, work, or childcare constraints, although this has not yet been confirmed. Even when willing to donate, finding times for confirmatory testing and donation pose greater challenges and barriers than prior to the Covid-19 pandemic.
Beyond the logistical donor issues, the downstream effects of pandemic-related stress alter the bone marrow microenvironment where hematopoietic stem cells are produced, affecting the donor stem cell product with short- and long-term consequences for the HCT recipient. HCT recipients who received fully-matched peripheral blood stem cells from donors with greater socioeconomic disadvantage were observed to experience inferior overall survival (OS), disease-free survival (DFS), and transplant-related mortality (TRM) (137). These findings were independent of donor or recipient race and of recipient SES. Findings highlight the adverse effects of socioeconomic stress down to the level of the hematopoietic cell, and even after the cells engraft in a new host. The biologic underpinnings of these associations are attributed to a pro-inflammatory state induced by chronic stress (194, 195). However, another study found that donor inflammatory cytokine and adipokine levels (IL-6, IL-1b, TNF-a, leptin, adiponectin, and ST2) were not associated with recipient outcomes (196). Novel work is investigating associations between donor CTRA gene profile and recipient outcomes (197); however, results of those analyses are not yet available (137).
As the Covid-19 pandemic persists, further attention to its impact on HCT donors is needed. The ramifications of donor availability or alterations in hematopoietic cell function and health secondary to pandemic stressors may have broad-reaching implications for HCT outcomes.
Biobehavioral Factors That Confer Resilience
While the focus thus far has been on processes of stress and impairment, it is also important to identify biobehavioral processes that mitigate stress and optimize adjustment and recovery. Responses to adversity can be characterized by survival with impairment, a pattern of persistently compromised functioning, which is distinguished from resilience, defined as a return to normal or baseline functioning, which is then further distinguished from thriving, described as exceeding one’s original level of functioning (198, 199). Identifying factors that confer psychological and biological recovery and resilience to TCT and Covid-19-related impacts has translational relevance for the development of effective interventions.
Psychosocial Factors That Confer Resilience
At the time of writing, we are not aware of published data regarding resilience factors in the context of intersecting stressors associated with TCT and the Covid-19 pandemic. The TCT literature, however, highlights the salubrious influence of supportive social relationships, which are associated with more optimal psychological and physical function (200, 201). A recent study of cancer survivors suggested that older age may mitigate the impact of Covid-19 related stressors, with older adults redeploying and repurposing coping previously used for cancer and other health concerns (202). A recent study found that successfully navigating a cancer diagnosis and treatment may have prepared cancer survivors for the existential distress of the pandemic and conferred additional resilience (203).
How TCT patients approach both external stressors and their internal responses to stress can also affect outcomes. HCT patients who respond to difficult thoughts and emotions mindfully, without judgement or reactivity, and those patients who actively cultivate a sense of meaning and purpose, show better psychological adjustment and physical function (204, 205). Coping approaches that involve active engagement with stressors (e.g., planning, problem-solving) and internal experience (e.g., emotional processing) also facilitate better psychological function and fewer physical symptoms among HCT patients (200, 201). Consistent with these findings, a large study of adults in the US and Canada found that active, approach-oriented coping strategies in response to Covid-19 pandemic stressors were associated with more optimal mood and QOL, whereas coping strategies characterized by avoidance (of the stressor itself and one’s emotions and thoughts) were associated with greater mood disturbance and poorer QOL (154). Cognitive behavioral and mindfulness-based interventions that help TCT patients to engage with stressors and emotion may be particularly fruitful in facilitating recovery and well-being for those facing the dual challenges of TCT and the Covid-19 pandemic.
Biological Factors That Confer Resilience
Several key mechanisms of biological resilience to stress have been well documented and may be of particular relevance for patients facing intersecting stressors associated with TCT and Covid-19. First, the HPA axis plays an important role in resilience to stress (206). Postsynaptic receptors of the hippocampus, such as G-protein coupled GABAB receptors, are important in stress regulation and confer resilience to stress-induced anhedonia and social avoidance. The vagus nerve is also important in autonomic control of cardiac activity and plays a vital role in mind-body interactions that involve the immune and endocrine systems as well as neurocognitive effects. In addition, the ability to maintain heart rate variability (HRV) during stressful events is associated with a faster and more robust recovery of immune function and optimizes endocrine and cardiovascular responses to stress (207) (208)., Finally, gene expression variations and chromatin alterations in the ventral tegmental area (midbrain) and nucleus accumbens (forebrain) have been observed in resilient animals (209, 210); identification of such ‘resilience genes’ may facilitate targets for pharmacologic and behavioral interventions.
Biomarker discovery and risk stratification have also yielded insights into biological factors that confer resilience to stress and psychiatric disorders. Studies have only shown weak to moderate associations of genes related to the HPA axis or serotonergic systems with resilient phenotypes. Neuropeptide Y regulates HPA axis activity and has been shown to reduce anxiety following intranasal delivery (211, 212). Epigenetic alterations (DNA methylation, histone modifications, non-coding RNAs) may also influence recovery and resilience (213), with differential DNA methylation patterns observed in resilient individuals versus those who developed PTSD following a traumatic event (214). These patterns were related to the HPA axis, inflammatory pathways, and methylation function (214–216).
Taken together, this growing body of work suggests novel therapeutic targets that have potential to mitigate stress associated with the Covid-19 pandemic and optimize the biological and psychological recovery from TCT. With respect to pharmacologic approaches, propranolol, a non-selective beta antagonist, has the potential to enhance biological resilience under conditions of stress and adversity; a recent study found that propranolol reduces CTRA gene expression and inhibits cellular and molecular pathways associated with adverse outcomes in HCT recipients (217). Behavioral interventions including physical activity, slow breathing, and meditation also improve vagal function and facilitate recovery from stress through immunologic, endocrine, and psychologic mechanisms (218). These efficient and cost-effective interventions can be delivered in the context of the demands and limitations associated with the pandemic and TCT and may confer long-lasting resilience throughout the recovery period, though the specific impacts have yet to be directly investigated.
Summary and Future Directions
The Covid-19 pandemic has dramatically changed not only the current world conditions, but the clinical practice of TCT. The intersection of behavioral, psychological, socioenvironmental, and biological processes associated with both Covid-19 and TCT will likely have far-reaching implications on clinical and patient-reported outcomes for the foreseeable future. Here we have reviewed and integrated literature supporting how both the immunologic and psychologic vulnerability of TCT patients renders them particularly susceptible to adverse biobehavioral sequelae associated with Covid-19 infection, restrictions, and stressors.
Psychosocial factors associated with Covid-19 pandemic conditions activate intersecting psychobiological processes that lead to alterations in neural and endocrine pathways, predominately through SNS and HPA axis signaling. Dysregulation of these signaling pathways adversely affect cellular and immune function, increase inflammation, and are associated with worse cancer and TCT outcomes. Responses to both the stressor and infectivity of Covid-19 share overlapping pathophysiology modalities, particularly with inflammation. Rigorous translational investigation needs to focus on further delineating the mechanisms and clinical outcomes of the biobehavioral overlap to inform risk-stratification and targeted interventions. Patients’ emotional responses to stressors associated with Covid-19 should be directly addressed, and pharmacologic and behavioral interventions targeting end organ biologic effects secondary to biobehavioral processes should also continue to be investigated with the goal of mitigating adverse cancer outcomes.
As recently described in a publication from the American Society for Transplantation and Cellular Therapy Biobehavioral Research Special Interest Group (53), it is essential for the biobehavioral research community to unite on initiatives and priorities to move the science forward in a deliberate fashion for optimal outcomes. By integrating knowledge from prior biobehavioral work, we can test informed hypotheses regarding psychological and immunological responses to Covid-19 among TCT patients, thus promoting post-pandemic recovery and preparedness for future pandemics or other significant stressors.
Author Contributions
All authors substantially contributed to the conception and design of the work; participated in drafting and revising the work; have provided approval for publication of the content; and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Steering Committee members of the American Society for Transplantation and Cellular Therapy Biobehavioral Research Special Interest Group for their review and approval of this manuscript.
References
2. Hornick MG, Olson ME, Jadhav AL. SARS-CoV-2 Psychiatric Sequelae: A Review of Neuroendocrine Mechanisms and Therapeutic Strategies. Int J Neuropsychopharmacol (2021) 25(1):1–12. doi: 10.1093/ijnp/pyab069
3. Pak A, Adegboye OA, Adekunle AI, Rahman KM, McBryde ES, Eisen DP. Economic Consequences of the COVID-19 Outbreak: The Need for Epidemic Preparedness. Front Public Health (2020) 8:241. doi: 10.3389/fpubh.2020.00241
4. Xiong JQ, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, et al. Impact of COVID-19 Pandemic on Mental Health in the General Population: A Systematic Review. J Affect Disord (2020) 277:55–64. doi: 10.1016/j.jad.2020.08.001
5. Sharma A, Bhatt NS, St Martin A, Abid MB, Bloomquist J, Chemaly RF, et al. Clinical Characteristics and Outcomes of COVID-19 in Haematopoietic Stem-Cell Transplantation Recipients: An Observational Cohort Study. Lancet Haematology (2021) 8(3):e185–93. doi: 10.1016/S2352-3026(20)30429-4
6. Duell T, Van Lint MT, Ljungman P, Tichelli A, Socie G, Apperley JF, et al. Health and Functional Status of Long-Term Survivors of Bone Marrow Transplantation. Ann Internal Med (1997) 126(3):184–92. doi: 10.7326/0003-4819-126-3-199702010-00002
7. Amonoo HL, Topping CEW, Clay MA, Reynolds MJ, Rice J, Harnedy LE, et al. Distress in a Pandemic: Association of the Coronavirus Disease-2019 Pandemic With Distress and Quality of Life in Hematopoietic Stem Cell Transplantation. Transplant Cell Ther (2021) 27(12):1015.e1–e7. doi: 10.1016/j.jtct.2021.09.001
8. Bäuerle A, Musche V, Schmidt K, Schweda A, Fink M, Weismüller B, et al. Mental Health Burden of German Cancer Patients Before and After the Outbreak of COVID-19: Predictors of Mental Health Impairment. Int J Environ Res Public Health (2021) 18(5):2318 .doi: 10.3390/ijerph18052318
9. Romito F, Dellino M, Loseto G, Opinto G, Silvestris E, Cormio C, et al. Psychological Distress in Outpatients With Lymphoma During the COVID-19 Pandemic. Front Oncol (2020) 10:1270. doi: 10.3389/fonc.2020.01270
10. Knight JM, Lyness JM, Sahler OJZ, Liesveld JL, Moynihan JA. Psychosocial Factors and Hematopoietic Stem Cell Transplantation: Potential Biobehavioral Pathways. Psychoneuroendocrinology (2013) 38(11):2383–93. doi: 10.1016/j.psyneuen.2013.06.016
11. Costanzo ES, Juckett MB, Coe CL. Biobehavioral Influences on Recovery Following Hematopoietic Stem Cell Transplantation. Brain Behavior Immun (2013) 30:S68–74. doi: 10.1016/j.bbi.2012.07.005
12. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-Immune Mechanisms of Behavioral Comorbidities in Patients With Cancer. J Clin Oncol (2008) 26(6):971–82. doi: 10.1200/JCO.2007.10.7805
13. McQuellon RP, Duckworth KE, Campbell CR, Russell GB, Miskewicz KR, Alsobrooks A, et al. Fear of Cancer Recurrence, Distress, Depressive Symptoms, and Quality of Life in Hematopoietic Stem Cell Transplantation Patients. J Psychosocial Oncol Res Practice (2019) 1(2):e12. doi: 10.1097/or9.0000000000000012
14. Syrjala KL, Sutton SK, Jim HS, Knight JM, Wood WA, Lee SJ, et al. Cancer and Treatment Distress Psychometric Evaluation Over Time: A BMT CTN 0902 Secondary Analysis. Cancer (2017) 123(8):1416–23. doi: 10.1002/cncr.30454
15. Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic Status, C-Reactive Protein, Immune Factors, and Responses to Acute Mental Stress. Brain Behavior Immunity (2003) 17(4):286–95. doi: 10.1016/S0889-1591(03)00058-8
16. Steptoe A, Marmot M. The Role of Psychobiological Pathways in Socio-Economic Inequalities in Cardiovascular Disease Risk. Eur Heart J (2002) 23(1):13. doi: 10.1053/euhj.2001.2611
17. Fagundes CP, Glaser R, Hwang BS, Malarkey WB, Kiecolt-Glaser JK. Depressive Symptoms Enhance Stress-Induced Inflammatory Responses. Brain Behavior Immunity (2013) 31:172–6. doi: 10.1016/j.bbi.2012.05.006
18. Irwin MR, Cole S, Olmstead R, Breen EC, Cho JJ, Moieni M, et al. Moderators for Depressed Mood and Systemic and Transcriptional Inflammatory Responses: A Randomized Controlled Trial of Endotoxin. Neuropsychopharmacology (2019) 44(3):635–41. doi: 10.1038/s41386-018-0259-6
19. Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Arch Internal Med (2006) 166(16):1756–62. doi: 10.1001/archinte.166.16.1756
20. Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JCY, et al. Chronic Stress Enhances Progression of Acute Lymphoblastic Leukemia via Beta-Adrenergic Signaling. Brain Behav Immunity. (2012) 26(4):635–41. doi: 10.1016/j.bbi.2012.01.013
21. Knight JM, Costanzo ES, Singh S, Yin Z, Szabo A, Pawar DS, et al. The IL-6 Antagonist Tocilizumab is Associated With Worse Depression and Related Symptoms in the Medically Ill. Trans Psychiatry (2021) 11(1):58. doi: 10.1038/s41398-020-01164-y
22. Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The Influence of Bio-Behavioural Factors on Tumour Biology: Pathways and Mechanisms. Nat Rev Cancer (2006) 6(3):240–8. doi: 10.1038/nrc1820
23. Copelan EA. Hematopoietic Stem-Cell Transplantation. New Engl J Med (2006) 354(17):1813–26. doi: 10.1056/NEJMra052638
24. Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T Cell Immunotherapy. Am J Hematology (2019) 94(S1):S42–9. doi: 10.1002/ajh.25445
25. Cole SW. The Conserved Transcriptional Response to Adversity. Curr Opin Behav Sci (2019) 28:31–7. doi: 10.1016/j.cobeha.2019.01.008
26. Knight JM, Rizzo JD, Logan BR, Wang T, Arevalo JMG, Ma J, et al. Low Socioeconomic Status, Adverse Gene Expression Profiles, and Clinical Outcomes in Hematopoietic Stem Cell Transplant Recipients. Clin Cancer Res (2016) 22(1):69–78. doi: 10.1158/1078-0432.ccr-15-1344
27. Nelson AM, Jim HSL, Small BJ, Nishihori T, Gonzalez BD, Cessna JM, et al. Sleep Disruption Among Cancer Patients Following Autologous Hematopoietic Cell Transplantation. Bone Marrow Transplant (2018) 53(3):307–14. doi: 10.1038/s41409-017-0022-3
28. Gallagher S, Bennett KM, Roper L. Loneliness and Depression in Patients With Cancer During COVID-19. J Psychosoc Oncol (2021) 39(3):445–51. doi: 10.1080/07347332.2020.1853653
29. Iob E, Frank P, Steptoe A, Fancourt D. Levels of Severity of Depressive Symptoms Among at-Risk Groups in the UK During the COVID-19 Pandemic. JAMA Network Open (2020) 3(10):e2026064. doi: 10.1001/jamanetworkopen.2020.26064
30. Jakovljevic K, Kober KM, Block A, Cooper BA, Paul SM, Hammer MJ, et al. Higher Levels of Stress are Associated With a Significant Symptom Burden in Oncology Outpatients Receiving Chemotherapy. J Pain Symptom Manage (2021) 61(1):24–31.e4. doi: 10.1016/j.jpainsymman.2020.07.019
31. Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno H, Chang S, et al. Stress and Symptom Burden in Oncology Patients During the COVID-19 Pandemic. J Pain Symptom Manage (2020) 60(5):e25–34. doi: 10.1016/j.jpainsymman.2020.08.037
32. Zheng X, Tao G, Huang P, He F, Shao X, Xu Y, et al. Self-Reported Depression of Cancer Patients Under 2019 Novel Coronavirus Pandemic. (2020). doi: 10.2139/ssrn.3555252
33. Wang W, Cao X. Beta-Adrenergic Signaling in Tumor Immunology and Immunotherapy. Crit Rev Immunol (2019) 39(2):93–103. doi: 10.1615/CritRevImmunol.2019031188
34. Greco R, Alexander T, Burman J, Del Papa N, de Vries-Bouwstra J, Farge D, et al. Hematopoietic Stem Cell Transplantation for Autoimmune Diseases in the Time of COVID-19: EBMT Guidelines and Recommendations. Bone Marrow Transplant (2021) 2021:1–16. doi: 10.1038/s41409-021-01326-6
35. Sahu KK, Siddiqui AD. A Review on Recipients of Hematopoietic Stem Cell Transplantation Patients With COVID-19 Infection. Ther Adv Infect Disease. (2021) 8:20499361211013252. doi: 10.1177/20499361211013252
36. Amonoo HL, Longley RM, El-Jawahri A. The COVID-19 Pandemic: Unmasking Challenges and Vulnerability in the HSCT Population. J Psychosocial Oncol (2021) 39(3):309–12. doi: 10.1080/07347332.2020.1868647
37. Amonoo HL, Fenech A, Greer JA, Temel JS, Huffman JC, El-Jawahri A. Does Desire to Pursue Pleasurable Activities Matter? The Impact of Pretransplantation Anhedonia on Quality of Life and Fatigue in Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26(8):1477–81. doi: 10.1016/j.bbmt.2020.04.014
38. Bona K, London WB, Guo D, Abel G, Lehmann L, Wolfe J. Prevalence and Impact of Financial Hardship Among New England Pediatric Stem Cell Transplantation Families. Biol Blood Marrow Transplant (2015) 21(2):312–8. doi: 10.1016/j.bbmt.2014.10.016
39. Thom B, Benedict C, Friedman DN, Watson SE, Zeitler MS, Chino F. Economic Distress, Financial Toxicity, and Medical Cost-Coping in Young Adult Cancer Survivors During the COVID-19 Pandemic: Findings From an Online Sample. Cancer (2021) 127(23):4481–91. doi: 10.1002/cncr.33823
40. Cusatis R T-NYI, Piehowski C, Akinola I, Crawford E, Craig J, Thiengmeny A, et al. Longitudinal Financial Impact on Patients Receiving Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: A Mixed Methods Pilot Study. Blood (2021). doi: 10.1182/blood-2021-146032
41. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat Rev Immunol (2020) 20(6):363–74. doi: 10.1038/s41577-020-0311-8
42. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19) A Review. JAMA (2020) 324(8):782–93. doi: 10.1001/jama.2020.12839
43. Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: Current State of the Science. Immunity (2020) 52(6):910–41. doi: 10.1016/j.immuni.2020.05.002
44. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 Pathophysiology: A Review. Clin Immunol (2020) 215:108427. doi: 10.1016/j.clim.2020.108427
45. Jasti M, Nalleballe K, Dandu V, Onteddu S. A Review of Pathophysiology and Neuropsychiatric Manifestations of COVID-19. J Neurology (2021) 268(6):2007–12. doi: 10.1007/s00415-020-09950-w
46. Banerjee D, Viswanath B. Neuropsychiatric Manifestations of COVID-19 and Possible Pathogenic Mechanisms: Insights From Other Coronaviruses. Asian J Psychiatry (2020) 54:102350. doi: 10.1016/j.ajp.2020.102350
47. McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of Life Measurement in Bone Marrow Transplantation: Development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) Scale. Bone Marrow Transplant (1997) 19(4):357–68. doi: 10.1038/sj.bmt.1700672
48. El-Jawahri AR, Vandusen HB, Traeger LN, Fishbein JN, Keenan T, Gallagher ER, et al. Quality of Life and Mood Predict Posttraumatic Stress Disorder After Hematopoietic Stem Cell Transplantation. Cancer (2016) 122(5):806–12. doi: 10.1002/cncr.29818
49. Packman W, Weber S, Wallace J, Bugescu N. Psychological Effects of Hematopoietic SCT on Pediatric Patients, Siblings and Parents: A Review. Bone Marrow Transplant (2010) 45(7):1134–46. doi: 10.1038/bmt.2010.74
50. Di Giuseppe G, Thacker N, Schechter T, Pole JD. Anxiety, Depression, and Mental Health-Related Quality of Life in Survivors of Pediatric Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review. Bone Marrow Transplant (2020) 55(7):1240–54. doi: 10.1038/s41409-020-0782-z
51. Ruark J, Mullane E, Cleary N, Cordeiro A, Bezerra ED, Wu V, et al. Patient-Reported Neuropsychiatric Outcomes of Long-Term Survivors After Chimeric Antigen Receptor T Cell Therapy. Biol Blood Marrow Transplant (2020) 26(1):34–43. doi: 10.1016/j.bbmt.2019.09.037
52. Kyzar EJ, Purpura LJ, Shah J, Cantos A, Nordvig AS, Yin MT. Anxiety, Depression, Insomnia, and Trauma-Related Symptoms Following COVID-19 Infection at Long-Term Follow-Up. Brain Behav Immun Health Oct (2021) 16:100315. doi: 10.1016/j.bbih.2021.100315
53. Kelly DL, Syrjala K, Taylor M, Rentscher KE, Hashmi S, Wood WA, et al. Biobehavioral Research and Hematopoietic Stem Cell Transplantation: Expert Review From the Biobehavioral Research Special Interest Group of the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther (2021) 27(9):747–57. doi: 10.1016/j.jtct.2021.06.007
54. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic Nervous System Regulation of the Tumour Microenvironment. Nat Rev Cancer (2015) 15(9):563–72. doi: 10.1038/nrc3978
55. Katayama Y, Battista M, Kao WM, et al. Signals From the Sympathetic Nervous System Regulate Hematopoietic Stem Cell Egress From Bone Marrow. Cell (2006) 124(2):407–21. doi: 10.1016/j.cell.2005.10.041
56. Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T Cell-Associated Neurotoxicity. Front Immunol (2020) 11:577027. doi: 10.3389/fimmu.2020.577027
57. Fernández-Castañeda A, Lu P, Geraghty AC, Song E, Lee M-H, Wood J, et al. Mild Respiratory SARS-CoV-2 Infection can Cause Multi-Lineage Cellular Dysregulation and Myelin Loss in the Brain. bioRxiv (2022). doi: 10.1101/2022.01.07.475453
58. Bevans MF, Mitchell SA, Marden S. The Symptom Experience in the First 100 Days Following Allogeneic Hematopoietic Stem Cell Transplantation (HSCT). Supportive Care Cancer (2008) 16(11):1243–54. doi: 10.1007/s00520-008-0420-6
59. Jim HS, Evans B, Jeong JM, Gonzalez BD, Johnston L, Nelson AM, et al. Sleep Disruption in Hematopoietic Cell Transplantation Recipients: Prevalence, Severity, and Clinical Management. Biol Blood Marrow Transplant (2014) 20(10):1465–84. doi: 10.1016/j.bbmt.2014.04.010
60. Cheng R, Scippa K, Locke FL, Snider JT, Jim H. Patient Perspectives on Health-Related Quality of Life in Diffuse Large B-Cell Lymphoma Treated With Car T-Cell Therapy: A Qualitative Study. Oncol Ther (2021) 1–19. doi: 10.1007/s40487-021-00174-0
61. Gielissen M, Schattenberg A, Verhagen C, Rinkes M, Bremmers M, Bleijenberg G. Experience of Severe Fatigue in Long-Term Survivors of Stem Cell Transplantation. Bone Marrow Transplant (2007) 39(10):595–603. doi: 10.1038/sj.bmt.1705624
62. Costanzo ES, Knight JM, Coe CL, Hematti P, Juckett MB. Prevalence and Biobehavioral Predictors of Early and Long-Term Fatigue Following Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2020) 26(3):S68. doi: 10.1016/j.bbmt.2019.12.237
63. Jim HS, Sutton SK, Jacobsen PB, Martin PJ, Flowers ME, Lee SJ. Risk Factors for Depression and Fatigue Among Survivors of Hematopoietic Cell Transplantation. Cancer (2016) 122(8):1290–7. doi: 10.1002/cncr.29877
64. Rischer J, Scherwath A, Zander A, Koch U, Schulz-Kindermann F. Sleep Disturbances and Emotional Distress in the Acute Course of Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2009) 44(2):121–8. doi: 10.1038/bmt.2008.430
65. Ghazikhanian SE, Dorfman CS, Somers TJ, O'Sullivan ML, Fisher HM, Edmond SN, et al. Cognitive Problems Following Hematopoietic Stem Cell Transplant: Relationships With Sleep, Depression and Fatigue. Bone Marrow Transplant (2017) 52(2):279–84. doi: 10.1038/bmt.2016.248
66. Jacobs SR, Small BJ, Booth-Jones M, Jacobsen PB, Fields KK. Changes in Cognitive Functioning in the Year After Hematopoietic Stem Cell Transplantation. Cancer (2007) 110(7):1560–7. doi: 10.1002/cncr.22962
67. Walsh CA, Yi JC, Rosenberg AR, Crouch MLV, Leisenring WM, Syrjala KL. Factors Associated With Social Functioning Among Long-Term Cancer Survivors Treated With Hematopoietic Stem Cell Transplantation as Adolescents or Young Adults. Psycho-Oncology (2020) 29(10):1579–86. doi: 10.1002/pon.5460
68. Park J, Wehrlen L, Mitchell SA, Yang L, Bevans MF. Fatigue Predicts Impaired Social Adjustment in Survivors of Allogeneic Hematopoietic Cell Transplantation (HCT). Supportive Care Cancer (2019) 27(4):1355–63. doi: 10.1007/s00520-018-4411-y
69. Tichelli A, Gerull S, Holbro A, Buser A, Nair G, Medinger M, et al. Inability to Work and Need for Disability Pension Among Long-Term Survivors of Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2017) 52(10):1436–42. doi: 10.1038/bmt.2017.115
70. Morrison EJ, Ehlers SL, Bronars CA, Patten CA, Brockman TA, Cerhan JR, et al. Employment Status as an Indicator of Recovery and Function One Year After Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2016) 22(9):1690–5. doi: 10.1016/j.bbmt.2016.05.013
71. Hacker ED, Fink AM, Peters T, Park C, Fantuzzi G, Rondelli D. Persistent Fatigue in Hematopoietic Stem Cell Transplantation Survivors. Cancer Nursing (2017) 40(3):174. doi: 10.1097/NCC.0000000000000405
72. Rentscher KE, Carroll JE, Juckett MB, Coe CL, Broman AT, Rathouz PJ, et al. Sleep Disruption, Fatigue, and Depression as Predictors of 6-Year Clinical Outcomes Following Allogeneic Hematopoietic Cell Transplantation. JNCI: J Natl Cancer Institute (2021) 113(10):1405–14. doi: 10.1093/jnci/djab032
73. Bower JE. The Role of Neuro-Immune Interactions in Cancer-Related Fatigue: Biobehavioral Risk Factors and Mechanisms. Cancer (2019) 125(3):353–64. doi: 10.1002/cncr.31790
74. Irwin MR. Why Sleep is Important for Health: A Psychoneuroimmunology Perspective. Annu Rev Psychol (2015) 66:143–72. doi: 10.1146/annurev-psych-010213-115205
75. Park CL, Russell BS, Fendrich M, Finkelstein-Fox L, Hutchison M, Becker J. Americans’ COVID-19 Stress, Coping, and Adherence to CDC Guidelines. J Gen Internal Med (2020) 35(8):2296–303. doi: 10.1007/s11606-020-05898-9
76. Raony Í, de Figueiredo CS, Pandolfo P, Giestal-de-Araujo E, Oliveira-Silva Bomfim P, Savino W. Psycho-Neuroendocrine-Immune Interactions in COVID-19: Potential Impacts on Mental Health. Front Immunol (2020) 11:1170. doi: 10.3389/fimmu.2020.01170
77. Hoogland AI, Bulls HW, Gonzalez BD, Small BJ, Liu L, Pidala J, et al. Circadian Rhythmicity as a Predictor of Quality of Life in Allogeneic Hematopoietic Cell Transplant Patients. J Pain Symptom Manage (2019) 57(5):952–60.e1. doi: 10.1016/j.jpainsymman.2019.01.015
78. Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal Cortisol Rhythm and Fatigue in Breast Cancer Survivors. Psychoneuroendocrinology (2005) 30(1):92–100. doi: 10.1016/j.psyneuen.2004.06.003
79. Miller AH, Raison CL. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat Rev Immunol (2016) 16(1):22–34. doi: 10.1038/nri.2015.5
80. Rademacher L, Lasselin J, Karshikoff B, Hundt JE, Engler H, Lange T. The Different Faces of Sickness. Front Psychiatry (2021) 12. doi: 10.3389/978-2-88971-354-7
81. Lasselin J. Back to the Future of Psychoneuroimmunology: Studying Inflammation-Induced Sickness Behavior. Brain Behavior Immunity-Health (2021) 18:100379. doi: 10.1016/j.bbih.2021.100379
82. Smeland KB, Loge JH, Aass HC, Aspelin T, Bersvendsen H, Bolstad N, et al. Chronic Fatigue is Highly Prevalent in Survivors of Autologous Stem Cell Transplantation and Associated With IL-6, Neuroticism, Cardiorespiratory Fitness, and Obesity. Bone Marrow Transplant (2019) 54(4):607–10. doi: 10.1038/s41409-018-0342-y
83. Wang XS, Shi Q, Shah ND, Heijnen CJ, Cohen EN, Reuben JM, et al. Inflammatory Markers and Development of Symptom Burden in Patients With Multiple Myeloma During Autologous Stem Cell Transplantation. Clin Cancer Res (2014) 20(5):1366–74. doi: 10.1158/1078-0432.CCR-13-2442
84. Wang XS, Shi Q, Williams LA, Cleeland CS, Mobley GM, Reuben JM, et al. Serum Interleukin-6 Predicts the Development of Multiple Symptoms at Nadir of Allogeneic Hematopoietic Stem Cell Transplantation. Cancer (2008) 113(8):2102–9. doi: 10.1002/cncr.23820
85. Nelson AM, Coe CL, Juckett MB, Rumble ME, Rathouz PJ, Hematti P, et al. Sleep Quality Following Hematopoietic Stem Cell Transplantation: Longitudinal Trajectories and Biobehavioral Correlates. Bone Marrow transplant (2014) 49(11):1405–11. doi: 10.1038/bmt.2014.179
86. Mehta P, Fajgenbaum DC. Is Severe COVID-19 a Cytokine Storm Syndrome: A Hyperinflammatory Debate. Curr Opin Rheumatol (2021) 33(5):419. doi: 10.1097/BOR.0000000000000822
87. Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A, et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist (2020) 26(5-6):402–14. doi: 10.1177/1073858420941476
88. Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, et al. Psychiatric and Neuropsychiatric Presentations Associated With Severe Coronavirus Infections: A Systematic Review and Meta-Analysis With Comparison to the COVID-19 Pandemic. Lancet Psychiatry (2020) 7(7):611–27. doi: 10.1016/S2215-0366(20)30203-0
89. Spielman AJ, Caruso LS, Glovinsky PB. A Behavioral Perspective on Insomnia Treatment. Psychiatr Clinics North America (1987) 10(4):541–53. doi: 10.1016/S0193-953X(18)30532-X
90. Suvinen TI, Reade PC, Kemppainen P, Könönen M, Dworkin SF. Review of Aetiological Concepts of Temporomandibular Pain Disorders: Towards a Biopsychosocial Model for Integration of Physical Disorder Factors With Psychological and Psychosocial Illness Impact Factors. Eur J Pain (2005) 9(6):613–33. doi: 10.1016/j.ejpain.2005.01.012
91. Goedendorp MM, Gielissen MF, Verhagen CA, Bleijenberg G. Development of Fatigue in Cancer Survivors: A Prospective Follow-Up Study From Diagnosis Into the Year After Treatment. J Pain Symptom Management (2013) 45(2):213–22. doi: 10.1016/j.jpainsymman.2012.02.009
92. Holt-Lunstad J, Robles TF, Sbarra DA. Advancing Social Connection as a Public Health Priority in the United States. Am Psychol (2017) 72(6):517–30. doi: 10.1037/amp0000103
93. Holt-Lunstad J, Smith TB. Loneliness and Social Isolation as Risk Factors for CVD: Implications for Evidence-Based Patient Care and Scientific Inquiry. Heart (2016) 102(13):987–9. doi: 10.1136/heartjnl-2015-309242
94. Peplau LAPD. Loneliness : A Sourcebook of Current Theory, Research, and Therapy. (1982) Hoboken, NJ: John Wiley & Sons Inc
95. Deckx L, van den Akker M, Buntinx F. Risk Factors for Loneliness in Patients With Cancer: A Systematic Literature Review and Meta-Analysis. Eur J Oncol Nursing (2014) 18(5):466–77. doi: 10.1016/j.ejon.2014.05.002
96. Rusiewicz A, DuHamel KN, Burkhalter J, Ostroff J, Winkel G, Scigliano E, et al. Psychological Distress in Long-Term Survivors of Hematopoietic Stem Cell Transplantation. Psycho-Oncology (2008) 17(4):329–37. doi: 10.1002/pon.1221
97. Liang WH, Guan WJ, Chen RC, Wang W, Li JF, Xu K, et al. Cancer Patients in SARS-CoV-2 Infection: A Nationwide Analysis in China. Lancet Oncol (2020) 21(3):335–7. doi: 10.1016/s1470-2045(20)30096-6
98. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical Characteristics of COVID-19-Infected Cancer Patients: A Retrospective Case Study in Three Hospitals Within Wuhan, China. Ann Oncol (2020) 31(7):894–901. doi: 10.1016/j.annonc.2020.03.296
99. Zheng ZH, Peng F, Xu BY, Zhao JJ, Liu HH, Peng JH, et al. Risk Factors of Critical & Mortal COVID-19 Cases: A Systematic Literature Review and Meta-Analysis. J Infection (2020) 81(2):E16–25. doi: 10.1016/j.jinf.2020.04.021
100. Curigliano G, Cardoso MJ, Poortmans P, Gentilini O, Pravettoni G, Mazzocco K, et al. Recommendations for Triage, Prioritization and Treatment of Breast Cancer Patients During the COVID-19 Pandemic. Breast (2020) 52:8–16. doi: 10.1016/j.breast.2020.04.006
101. Nekhlyudov L, Duijts S, Hudson SV, Jones JM, Keogh J, Love B, et al. Addressing the Needs of Cancer Survivors During the COVID-19 Pandemic. J Cancer Survivorship (2020) 14(5):601–6. doi: 10.1007/s11764-020-00884-w
102. Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, et al. The Challenge of COVID-19 and Hematopoietic Cell Transplantation; EBMT Recommendations for Management of Hematopoietic Cell Transplant Recipients, Their Donors, and Patients Undergoing CAR T-Cell Therapy. Bone Marrow Transplantation (2020) 55(11):2071–6. doi: 10.1038/s41409-020-0919-0
103. Bargon CA, Batenburg MCT, van Stam LE, van der Molen DRM, van Dam IE, van der Leij F, et al. Impact of the COVID-19 Pandemic on Patient-Reported Outcomes of Breast Cancer Patients and Survivors. Jnci Cancer Spectr (2021) 5(1):pkaa104. doi: 10.1093/jncics/pkaa104
104. Howden K, Yan AP, Glidden C, Romanescu RG, Scott I, Deleemans JM, et al. Loneliness Among Adolescents and Young Adults With Cancer During the COVID-19 Pandemic: A Cross-Sectional Survey. Supportive Care Cancer (2022) 30:2215–24. doi: 10.1007/s00520-021-06628-5
105. Kosir U, Loades M, Wild J, Wiedemann M, Krajnc A, Roskar S, et al. The Impact of COVID-19 on the Cancer Care of Adolescents and Young Adults and Their Well-Being: Results From an Online Survey Conducted in the Early Stages of the Pandemic. Cancer (2020) 126(19):4414–22. doi: 10.1002/cncr.33098
106. Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno HT, Chang SM, et al. Loneliness and Symptom Burden in Oncology Patients During the COVID-19 Pandemic. Cancer (2021) 127(17):3246–53. doi: 10.1002/cncr.33603
107. Younger E, Smrke A, Lidington E, Farag S, Ingley K, Chopra N, et al. Health-Related Quality of Life and Experiences of Sarcoma Patients During the COVID-19 Pandemic. Cancers (2020) 12(8):2288. doi: 10.3390/cancers12082288
108. Rentscher KE, Zhao XT, Small BJ, Cohen HJ, Dilawari AA, Patel SK, et al. Loneliness and Mental Health During the COVID-19 Pandemic in Older Breast Cancer Survivors and Noncancer Controls. Cancer (2021) 127(19):3671–9. doi: 10.1002/cncr.33687
109. Schellekens MPJ, van der Lee ML. Loneliness and Belonging: Exploring Experiences With the COVID-19 Pandemic in Psycho-Oncology. Psycho-Oncology (2020) 29(9):1399–401. doi: 10.1002/pon.5459
110. Swainston J, Chapman B, Grunfeld EA, Derakshan N. COVID-19 Lockdown and Its Adverse Impact on Psychological Health in Breast Cancer. Front Psychol (2020) 11:2033. doi: 10.3389/fpsyg.2020.02033
111. Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and Social Isolation as Risk Factors for Mortality: A Meta-Analytic Review. Perspect Psychol Scie (2015) 10(2):227–37. doi: 10.1177/1745691614568352
112. Ernst J, Mehnert A, Dietz A, Hornemann B, Esser P. Perceived Stigmatization and its Impact on Quality of Life - Results From a Large Register-Based Study Including Breast, Colon, Prostate and Lung Cancer Patients. BMC Cancer (2017) 17:741. doi: 10.1186/s12885-017-3742-2
113. Hill EM, Hamm A. Intolerance of Uncertainty, Social Support, and Loneliness in Relation to Anxiety and Depressive Symptoms Among Women Diagnosed With Ovarian Cancer. Psycho-Oncology (2019) 28(3):553–60. doi: 10.1002/pon.4975
114. Jaremka LM, Andridge RR, Fagundes CP, Alfano CM, Povoski SP, Lipari AM, et al. Pain, Depression, and Fatigue: Loneliness as a Longitudinal Risk Factor. Health Psychol (2014) 33(9):948–57. doi: 10.1037/a0034012
115. Jaremka LM, Fagundes CP, Peng J, Bennett JM, Glaser R, Malarkey WB, et al. Loneliness Promotes Inflammation During Acute Stress. Psychol Scie (2013) 24(7):1089–97. doi: 10.1177/0956797612464059
116. Boer H, Elving W, Seydel E. Psychosocial Factors and Mental Health in Cancer Patients: Opportunities for Health Promotion. Psychology Health Med (1998) 3(1):71–9. doi: 10.1080/13548509808400591
117. Kroenke CH, Michael YL, Poole EM, Kwan ML, Nechuta S, Leas E, et al. Postdiagnosis Social Networks and Breast Cancer Mortality in the After Breast Cancer Pooling Project. Cancer (2017) 123(7):1228–37. doi: 10.1002/cncr.30440
118. Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social Networks, Social Support, and Survival After Breast Cancer Diagnosis. J Clin Oncol (2006) 24(7):1105–11. doi: 10.1200/jco.2005.04.2846
119. Irwin MR, Cole SW. Reciprocal Regulation of the Neural and Innate Immune Systems. Nat Rev Immunol (2011) 11(9):625–32. doi: 10.1038/nri3042
120. Cacioppo JT, Cacioppo S. Loneliness in the Modern Age: An Evolutionary Theory of Loneliness (ETL). In: Olson JM, editor. Advances in Experimental Social Psychology, vol. Vol 58 Lake Charles, LA:American Pres (2018). p. 127–97.
121. Cole SW, Capitanio JP, Chun K, Arevalo JMG, Ma J, Cacioppo JT. Myeloid Differentiation Architecture of Leukocyte Transcriptome Dynamics in Perceived Social Isolation. Proc Natl Acad Sci USA (2015) 112(49):15142–7. doi: 10.1073/pnas.1514249112
122. Moieni M, Eisenberger NI. Effects of Inflammation on Social Processes and Implications for Health. Ann New York Acad Sci (2018) 1428(1):5–13. doi: 10.1111/nyas.13864
123. Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, Social Network Size, and Immune Response to Influenza Vaccination in College Freshmen. Health Psychol (2005) 24(3):297–306. doi: 10.1037/0278-6133.24.3.297
124. LeRoy AS, Murdock KW, Jaremka LM, Loya A, Fagundes CP. Loneliness Predicts Self-Reported Cold Symptoms After a Viral Challenge. Health Psychol (2017) 36(5):512–20. doi: 10.1037/hea0000467
125. Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social Ties and Susceptibility to the Common Cold. JAMA (1997) 277(24):1940–4. doi: 10.1001/jama.1997.03540480040036
126. Nausheen B, Carr NJ, Peveler RC, Moss-Morris R, Verrill C, Robbins E, et al. Relationship Between Loneliness and Proangiogenic Cytokines in Newly Diagnosed Tumors of Colon and Rectum. Psychosomatic Med (2010) 72(9):912–6. doi: 10.1097/PSY.0b013e3181f0bc1c
127. Bower JE, Shiao SL, Sullivan P, Lamkin DM, Atienza R, Mercado F, et al. Prometastatic Molecular Profiles in Breast Tumors From Socially Isolated Women. Jnci Cancer Spectr (2018) 2(3):pky029. doi: 10.1093/jncics/pky029
128. Lutgendorf SK, Penedo F, Goodheart MJ, Dahmoush L, Arevalo JMG, Thaker PH, et al. Epithelial-Mesenchymal Transition Polarization in Ovarian Carcinomas From Patients With High Social Isolation. Cancer (2020) 126(19):4407–13. doi: 10.1002/cncr.33060
129. Lutgendorf SK, Thaker PH, Arevalo JM, Goodheart MJ, Slavich GM, Sood AK, et al. Biobehavioral Modulation of the Exosome Transcriptome in Ovarian Carcinoma. Cancer (2018) 124(3):580–6. doi: 10.1002/cncr.31078
130. Ferrer RA, Acevedo AM, Agurs-Collins TD. COVID-19 and Social Distancing Efforts-Implications for Cancer Control. JAMA Oncol (2021) 7(4):503–4. doi: 10.1001/jamaoncol.2020.6786
131. Kelly DL, Buchbinder D, Duarte RF, Auletta JJ, Bhatt N, Byrne M, et al. Neurocognitive Dysfunction in Hematopoietic Cell Transplant Recipients: Expert Review From the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Complications and Quality of Life Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplantation (2018) 24(2):228–41. doi: 10.1016/j.bbmt.2017.09.004
132. Beitinjaneh A, McKinney AM, Cao Q, Weisdorf DJ. Toxic Leukoencephalopathy Following Fludarabine-Associated Hematopoietic Cell Transplantation. Biol Blood Marrow Transplantation (2011) 17(3):300–8. doi: 10.1016/j.bbmt.2010.04.003
133. Hoogland AI, Nelson AM, Gonzalez BD, Small BJ, Breen EC, Sutton SK, et al. Worsening Cognitive Performance is Associated With Increases in Systemic Inflammation Following Hematopoietic Cell Transplantation. Brain Behavior Immun (2019) 80:308–14. doi: 10.1016/j.bbi.2019.04.008
134. Syrjala KL, Artherholt SB, Kurland BF, Langer SL, Roth-Roemer S, Elrod JB, et al. Prospective Neurocognitive Function Over 5 Years After Allogeneic Hematopoietic Cell Transplantation for Cancer Survivors Compared With Matched Controls at 5 Years. J Clin Oncol (2011) 29(17):2397. doi: 10.1200/JCO.2010.33.9119
135. Barata A, Hoogland A, Hyland K, Kommalapati A, Jayani R, Irizarry-Arroyo NE, et al. Subjective Cognition in Chimeric Antigen Receptor T-Cell Therapy Recipients. Am Soc Clin Oncol (2020), 7020. doi: 10.1200/JCO.2020.38.15_suppl.7020
136. Wood EH. Blood and Marrow Transplant Information Network. J Consumer Health Internet (2005) 9(4):55–9. doi: 10.1300/J381v09n04_06
137. Turcotte LM, Wang T, Beyer KM, Cole SW, Spellman SR, Allbee-Johnson M, et al. Donor Socioeconomic Status As a Predictor of Altered Immune Function and Treatment Response Following Hematopoietic Cell Transplantation for Hematologic Malignancy. Blood (2021) 138(Supplement 1):843–3. doi: 10.1182/blood-2021-146668
138. Antoni MH, Bouchard LC, Jacobs JM, Lechner SC, Jutagir DR, Gudenkauf LM, et al. Stress Management, Leukocyte Transcriptional Changes and Breast Cancer Recurrence in a Randomized Trial: An Exploratory Analysis. Psychoneuroendocrinology (2016) 74:269–77. doi: 10.1016/j.psyneuen.2016.09.012
139. Daroische R, Hemminghyth MS, Eilertsen TH, Breitve MH, Chwiszczuk LJ. Cognitive Impairment After COVID-19—a Review on Objective Test Data. Front Neurology (2021) 1238. doi: 10.3389/fneur.2021.699582
140. Vanderlind WM, Rabinovitz BB, Miao IY, Oberlin LE, Bueno-Castellano C, Fridman C, et al. A Systematic Review of Neuropsychological and Psychiatric Sequalae of COVID-19: Implications for Treatment. Curr Opin Psychiatry (2021) 34(4):420. doi: 10.1097/YCO.0000000000000713
141. Knight JM, Szabo A, Arapi IRW, Emmrich A, Hackett E, et al. Patient-Reported Outcomes, Neurotoxicity, and Kynurenine Concentrations With Bispecific LV20.19 CAR T Cell Therapy. Commun Med (2022) 2(1):1–10. doi: 10.1038/s43856-022-00116-5
142. Lyon DE, Cohen R, Chen H, Kelly DL, McCain NL, Starkweather A, et al. Relationship of Systemic Cytokine Concentrations to Cognitive Function Over Two Years in Women With Early Stage Breast Cancer. J Neuroimmunol (2016) 301:74–82. doi: 10.1016/j.jneuroim.2016.11.002
143. Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome After Chimeric Antigen Receptor T-Cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov (2016) 6(6):664–79. doi: 10.1158/2159-8290.CD-16-0040
144. Siegler EL, Kenderian SS. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights Into Mechanisms and Novel Therapies. Front Immunol (2020) 11:1973. doi: 10.3389/fimmu.2020.01973
145. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med (2014) 371(16):1507–17. doi: 10.1056/NEJMoa1407222
146. Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K, et al. High Success Rate of Hematopoietic Cell Transplantation Regardless of Donor Source in Children With Very High-Risk Leukemia. Blood (2011) 118(2):223–30. doi: 10.1182/blood-2011-01-333070
147. Delahoy MJ, Ujamaa D, Whitaker M, O'Halloran A, Anglin O, Burns E, et al. Hospitalizations Associated With COVID-19 Among Children and Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep (2021) 70(36):1255–60. doi: 10.15585/mmwr.mm7036e2
148. Mukkada S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, et al. Global Characteristics and Outcomes of SARS-CoV-2 Infection in Children and Adolescents With Cancer (GRCCC): A Cohort Study. Lancet Oncol (2021) 22(10):1416–26. doi: 10.1016/S1470-2045(21)00454-X
149. Madhusoodhan PP, Pierro J, Musante J, Kothari P, Gampel B, Appel B, et al. Characterization of COVID-19 Disease in Pediatric Oncology Patients: The New York-New Jersey Regional Experience. Pediatr Blood Cancer (2021) 68(3):e28843. doi: 10.1002/pbc.28843
150. Parasole R, Stellato P, Conter V, De Matteo A, D'Amato L, Colombini A, et al. Collateral Effects of COVID-19 Pandemic in Pediatric Hematooncology: Fatalities Caused by Diagnostic Delay. Pediatr Blood Cancer (2020) 67(8):e28482. doi: 10.1002/pbc.28482
151. Auletta JJ, Adamson PC, Agin JE, Kearns P, Kennedy S, Kieran MW, et al. Pediatric Cancer Research: Surviving COVID-19. Pediatr Blood Cancer (2020) 67(9):e28435. doi: 10.1002/pbc.28435
152. Kaushik A, Gupta S, Sood M, Sharma S, Verma S. A Systematic Review of Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 Infection. Pediatr Infect Dis J (2020) 39(11):e340–6. doi: 10.1097/INF.0000000000002888
153. Kotch C, Barrett D, Teachey DT. Tocilizumab for the Treatment of Chimeric Antigen Receptor T Cell-Induced Cytokine Release Syndrome. Expert Rev Clin Immunol (2019) 15(8):813–22. doi: 10.1080/1744666X.2019.1629904
154. Ciazynska M, Pabianek M, Szczepaniak K, Ulanska M, Skibinska M, Owczarek W, et al. Quality of Life of Cancer Patients During Coronavirus Disease (COVID-19) Pandemic. Psychooncology (2020) 29(9):1377–9. doi: 10.1002/pon.5434
155. Schober ME, Robertson CL, Wainwright MS, Roa JD, Fink EL. COVID-19 and the Pediatric Nervous System: Global Collaboration to Meet a Global Need. Neurocrit Care (2021) 35(2):283–90. doi: 10.1007/s12028-021-01269-2
156. Cole SW. New Challenges in Psycho-Oncology: Neural Regulation of the Cancer Genome. Psychooncology (2018) 27(10):2305–9. doi: 10.1002/pon.4838
157. Liang L, Ren H, Cao R, Hu Y, Qin Z, Li C, et al. The Effect of COVID-19 on Youth Mental Health. Psychiatr Q (2020) 91(3):841–52. doi: 10.1007/s11126-020-09744-3
158. Berken JA, Heard-Garris N, Wakschlag LS. Guardians at the Gate: Early Adversity, Neurocognitive Development, and the Role of the Pediatrician in the Era of COVID-19. Front Pediatr (2021) 9:665335. doi: 10.3389/fped.2021.665335
159. Jacobson C, Miller N, Mulholland R, Baker L, Glazer D, Betts E, et al. Psychological Distress and Resilience in a Multicentre Sample of Adolescents and Young Adults With Cancer During the COVID-19 Pandemic. Clin Child Psychol Psychiatry (2021) 27(1):201–13. doi: 10.1177/13591045211056923
160. Haykin H, Rolls A. The Neuroimmune Response During Stress: A Physiological Perspective. Immunity (2021) 54(9):1933–47. doi: 10.1016/j.immuni.2021.08.023
161. Cole SW, Sood AK. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin Cancer Res (2012) 18(5):1201–6. doi: 10.1158/1078-0432.Ccr-11-0641
162. Knight JM, Rizzo JD, Wang T, He N, Logan BR, Spellman SR, et al. Molecular Correlates of Socioeconomic Status and Clinical Outcomes Following Hematopoietic Cell Transplantation for Leukemia. JNCI Cancer Spectr (2019) 3(4):pkz073. doi: 10.1093/jncics/pkz073
163. Taylor MR, Knight JM, Rosenberg AR. The Biology of Stress in Cancer: Applying the Biobehavioral Framework to Adolescent and Young Adult Oncology Research. Brain Behav Immun Health (2021) 17:100321. doi: 10.1016/j.bbih.2021.100321
164. Beattie S, Lebel S, Tay J. The Influence of Social Support on Hematopoietic Stem Cell Transplantation Survival: A Systematic Review of Literature. PLoS One (2013) 8(4):e61586. doi: 10.1371/journal.pone.0061586
165. Rini C, Redd WH, Austin J, Mosher CE, Meschian YM, Isola L, et al. Effectiveness of Partner Social Support Predicts Enduring Psychological Distress After Hematopoietic Stem Cell Transplantation. J Consulting Clin Psychol (2011) 79(1):64–74. doi: 10.1037/a0022199
166. Foster LW, McLellan L, Rybicki L, Dabney J, Copelan E, Bolwell B. Validating the Positive Impact of in-Hospital Lay Care-Partner Support on Patient Survival in Allogeneic BMT: A Prospective Study. Bone Marrow Transplantation (2013) 48(5):671–7. doi: 10.1038/bmt.2012.208
167. Barata A, Hoogland AI, Hyland KA, Otto AK, Kommalapati A, Jayani RV, et al. Quality of Life in Caregivers of Patients Receiving Chimeric Antigen Receptor T-Cell Therapy. Psycho-Oncology (2021) 30(8):1294–301. doi: 10.1002/pon.5674
168. Sannes TS, Simoneau TL, Mikulich-Gilbertson SK, Natvig CL, Brewer BW, Kilbourn K, et al. Distress and Quality of Life in Patient and Caregiver Dyads Facing Stem Cell Transplant: Identifying Overlap and Unique Contributions. Supportive Care Cancer (2019) 27(6):2329–37. doi: 10.1007/s00520-018-4496-3
169. Akgul N, Ozdemir L. Caregiver Burden Among Primary Caregivers of Patients Undergoing Peripheral Blood Stem Cell Transplantation: A Cross Sectional Study. Eur J Oncol Nursing (2014) 18(4):372–7. doi: 10.1016/j.ejon.2014.03.013
170. Bevans MF, Ross A, Wehrlen L, Klagholz SD, Yang L, Childs R, et al. Documenting Stress in Caregivers of Transplantation Patients: Initial Evidence of HPA Dysregulation. Stress-the Int J Biol Stress (2016) 19(2):175–84. doi: 10.3109/10253890.2016.1146670
171. Cooke L, Grant M, Eldredge DH, Maziarz RT, Nail LM. Informal Caregiving in Hematopoietic Blood and Marrow Transplant Patients. Eur J Oncol Nursing (2011) 15(5):500–7. doi: 10.1016/j.ejon.2011.01.007
172. Jim HSL, Quinn GP, Barata A, Cases M, Cessna J, Gonzalez B, et al. Caregivers' Quality of Life After Blood and Marrow Transplantation: A Qualitative Study. Bone Marrow Transplantation (2014) 49(9):1234–6. doi: 10.1038/bmt.2014.118
173. Polomeni A, Lapusan S, Bompoint C, Rubio MT, Mohty M. The Impact of Allogeneic-Hematopoietic Stem Cell Transplantation on Patients' and Close Relatives' Quality of Life and Relationships. Eur J Oncol Nursing (2016) 21:248–56. doi: 10.1016/j.ejon.2015.10.011
174. Chardon ML, Klages KL, Joffe NE, Pai ALH. Family Adjustment to Pediatric Hematopoietic Stem Cell Transplant During COVID-19. J Pediatr Psychol (2021) 46(10):1172–81. doi: 10.1093/jpepsy/jsab092
175. Fisher CL, Wright KB, Hampton CN, Vasquez TS, Kastrinos A, Applebaum AJ, et al. Blood Cancer Caregiving During COVID-19: Understanding Caregivers' Needs. Trans Behav Med (2021) 11(5):1187–97. doi: 10.1093/tbm/ibab021
176. Steinberg DM, Andresen JA, Pahl DA, Licursi M, Rosenthal SL. "I've Weathered Really Horrible Storms Long Before This Horizontal Ellipsis ": The Experiences of Parents Caring for Children With Hematological and Oncological Conditions During the Early Months of the COVID-19 Pandemic in the US. J Clin Psychol Med Settings (2021) 28(4):720–7. doi: 10.1007/s10880-020-09760-2
177. Kim Y, Cole SW, Carver CS, Antoni MH, Penedo FJ. Only the Lonely: Expression of Proinflammatory Genes Through Family Cancer Caregiving Experiences. Psychosomatic Med (2021) 83(2):149–56. doi: 10.1097/psy.0000000000000897
178. Laudenslager ML, Simoneau TL, Kilbourn K, Natvig C, Philips S, Spradley J, et al. A Randomized Control Trial of a Psychosocial Intervention for Caregivers of Allogeneic Hematopoietic Stem Cell Transplant Patients: Effects on Distress. Bone Marrow Transplantation (2015) 50(8):1110–8. doi: 10.1038/bmt.2015.104
179. Laudenslager ML, Simoneau TL, Mikulich-Gilbertson SK, Natvig C, Brewer BW, Sannes TS, et al. A Randomized Control Trial of Stress Management for Caregivers of Stem Cell Transplant Patients: Effect on Patient Quality of Life and Caregiver Distress. Psycho-Oncology (2019) 28(8):1614–23. doi: 10.1002/pon.5126
180. Laudenslager ML. "Anatomy of an Illness": Control From a Caregiver's Perspective. Brain Behav Immunity (2014) 36:1–8. doi: 10.1016/j.bbi.2013.08.012
181. Sannes TS, Mikulich-Gilbertson SK, Natvig CL, Laudenslager ML. Intraindividual Cortisol Variability and Psychological Functioning in Caregivers of Hematopoietic Stem Cell Transplant Patients. Psychosomatic Med (2016) 78(2):242–7. doi: 10.1097/psy.0000000000000272
182. Applebaum AJ, Bevans M, Son T, Evans K, Hernandez M, Giralt S, et al. A Scoping Review of Caregiver Burden During Allogeneic HSCT: Lessons Learned and Future Directions. Bone Marrow Transplantation (2016) 51(11):1416–22. doi: 10.1038/bmt.2016.164
183. Chaar D, Shin JY, Mazzoli A, Vue R, Kedroske J, Chappell G, et al. A Mobile Health App (Roadmap 2.0) for Patients Undergoing Hematopoietic Stem Cell Transplant: Qualitative Study on Family Caregivers' Perspectives and Design Considerations. JMIR Mhealth Uhealth (2019) 7(10):e15775. doi: 10.2196/15775
184. Beattie S, Lebel S. The Experience of Caregivers of Hematological Cancer Patients Undergoing a Hematopoietic Stem Cell Transplant: A Comprehensive Literature Review. Psycho-Oncology (2011) 20(11):1137–50. doi: 10.1002/pon.1962
185. Sabo B, McLeod D, Couban S. The Experience of Caring for a Spouse Undergoing Hematopoietic Stem Cell Transplantation Opening Pandora's Box. Cancer Nursing (2013) 36(1):29–40. doi: 10.1097/NCC.0b013e31824fe223
186. Bishop MM, Beaumont JL, Hahn EA, Cella D, Andrykowski MA, Brady MJ, et al. Late Effects of Cancer and Hematopoietic Stem-Cell Transplantation on Spouses or Partners Compared With Survivors and Survivor-Matched Controls. J Clin Oncol (2007) 25(11):1403–11. doi: 10.1200/jco.2006.07.5705
187. Posluszny DM, Bovbjerg DH, Syrjala KL, Agha M, Dew MA. Correlates of Anxiety and Depression Symptoms Among Patients and Their Family Caregivers Prior to Allogeneic Hematopoietic Cell Transplant for Hematological Malignancies. Supportive Care Cancer (2019) 27(2):591–600. doi: 10.1007/s00520-018-4346-3
188. Irani E, Niyomyart A, Hickman RL. Family Caregivers' Experiences and Changes in Caregiving Tasks During the COVID-19 Pandemic. Clin Nurs Res (2021) 30(7):1088–97. doi: 10.1177/10547738211014211
189. Laudenslager ML, Simoneau TL, Philips S, Benitez P, Natvig C, Cole S. A Randomized Controlled Pilot Study of Inflammatory Gene Expression in Response to a Stress Management Intervention for Stem Cell Transplant Caregivers. J Behav Med (2016) 39(2):346–54. doi: 10.1007/s10865-015-9709-3
190. Beach SR, Schulz R, Donovan H, Rosland A-M. Family Caregiving During the COVID-19 Pandemic. Gerontologist (2021) 61(5):650–60. doi: 10.1093/geront/gnab049
191. McAndrew NS, Guttormson JL, Erickson J, Patel JJ. Experiences of Family Caregivers of Critically Ill Hematopoietic Stem Cell Transplant Patients. Am J Respir Crit Care Med (2021) 203(9):A3187–A. doi: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A3187
192. Irwin MR. Inflammation at the Intersection of Behavior and Somatic Symptoms. Psychiatr Clinics North Am (2011) 34(3):605. doi: 10.1016/j.psc.2011.05.005
193. Yucel E, Zhang SY, Panjabi S. Health-Related and Economic Burden Among Family Caregivers of Patients With Acute Myeloid Leukemia or Hematological Malignancies. Adv Ther (2021) 38(10):5002–24. doi: 10.1007/s12325-021-01872-x
194. Muscatell KA, Brosso SN, Humphreys KL. Socioeconomic Status and Inflammation: A Meta-Analysis. Mol Psychiatry (2020) 25(9):2189–99. doi: 10.1038/s41380-018-0259-2
195. Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of Socioeconomic Status With Inflammation Markers in Black and White Men and Women in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Soc Sci Med (2009) 69(3):451–9. doi: 10.1016/j.socscimed.2009.05.018
196. Turcotte LM, Wang T, Hemmer MT, Spellman SR, Arora M, Yingst A, et al. Proinflammatory Cytokine and Adipokine Levels in Adult Unrelated Marrow Donors Are Not Associated With Hematopoietic Cell Transplantation Outcomes. Biol Blood Marrow Transplant (2019) 25(1):12–8. doi: 10.1016/j.bbmt.2018.08.011
197. Turcotte LM, Verneris MR. Is It Better to Be Rich or Relaxed? Sociobiology Meets Bone Marrow Transplant. Clin Cancer Res (2016) 22(1):6–8. doi: 10.1158/1078-0432.CCR-15-2112
198. O'Leary VE, Ickovics JR. Resilience and Thriving in Response to Challenge: An Opportunity for a Paradigm Shift in Women's Health. Women's Health (Hillsdale NJ) (1995) 1(2):121–42.
199. Carver CS. Resilience and Thriving: Issues, Models, and Linkages. J Soc Issues (1998) 54(2):245–66. doi: 10.1111/j.1540-4560.1998.tb01217.x
200. Wells KJ, Booth-Jones M, Jacobsen PB. Do Coping and Social Support Predict Depression and Anxiety in Patients Undergoing Hematopoietic Stem Cell Transplantation? J Psychosoc Oncol (2009) 27(3):297–315. doi: 10.1080/07347330902978947
201. Schwartz JR, Thomas EB, Juckett MB, Costanzo ES. Predictors of Posttraumatic Growth Among Hematopoietic Cell Transplant Recipients. Psychooncology (2022) 1–9. doi: 10.1002/pon.5892
202. Galica J, Liu Z, Kain D, Merchant S, Booth C, Koven R, et al. Coping During COVID-19: A Mixed Methods Study of Older Cancer Survivors. Supportive Care Cancer (2021) 29(6):3389–98. doi: 10.1007/s00520-020-05929-5
203. McAndrew NS, Strong Y, Morris KJ, Sannes TS, Pirl WF, Cole S, et al. Impact of the COVID-19 Pandemic on Cancer Patients and Psycho-Oncology Providers: Perspectives, Observations, and Experiences of the American Psychosocial Oncology Society Membership. Psycho-Oncology (2022) 31(6):1031–40. doi: 10.1002/pon.5894
204. Larson AG, Morris KJ, Juckett MB, Coe CL, Broman AT, Costanzo ES. Mindfulness, Experiential Avoidance, and Recovery From Hematopoietic Stem Cell Transplantation. Ann Behav Med (2019) 53(10):886–95. doi: 10.1093/abm/kay097
205. Leeson LA, Nelson AM, Rathouz PJ, Juckett MB, Coe CL, Caes EW, et al. Spirituality and the Recovery of Quality of Life Following Hematopoietic Stem Cell Transplantation. Health Psychol (2015) 34(9):920. doi: 10.1037/hea0000196
206. Gaffey AE, Bergeman C, Clark LA, Wirth MM. Aging and the HPA Axis: Stress and Resilience in Older Adults. Neurosci Biobehav Rev (2016) 68:928–45. doi: 10.1016/j.neubiorev.2016.05.036
207. Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current Directions in the Auricular Vagus Nerve Stimulation I–a Physiological Perspective. Front Neurosci (2019) 13:854. doi: 10.3389/fnins.2019.00854
208. Dedoncker J, Vanderhasselt M-A, Ottaviani C, Slavich GM. Mental Health During the COVID-19 Pandemic and Beyond: The Importance of the Vagus Nerve for Biopsychosocial Resilience. Neurosci Biobehav Rev (2021) 125:1–10. doi: 10.1016/j.neubiorev.2021.02.010
209. Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, et al. Imipramine Treatment and Resiliency Exhibit Similar Chromatin Regulation in the Mouse Nucleus Accumbens in Depression Models. J Neurosci (2009) 29(24):7820–32. doi: 10.1523/JNEUROSCI.0932-09.2009
210. Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell (2007) 131(2):391–404. doi: 10.1016/j.cell.2007.09.018
211. Sayed S, Van Dam NT, Horn SR, Kautz MM, Parides M, Costi S, et al. A Randomized Dose-Ranging Study of Neuropeptide Y in Patients With Posttraumatic Stress Disorder. Int J Neuropsychopharmacol (2018) 21(1):3–11. doi: 10.1093/ijnp/pyx109
212. DePierro J, Lepow L, Feder A, Yehuda R. Translating Molecular and Neuroendocrine Findings in Posttraumatic Stress Disorder and Resilience to Novel Therapies. Biol Psychiatry (2019) 86(6):454–63. doi: 10.1016/j.biopsych.2019.07.009
213. Schiele M, Domschke K. Epigenetics at the Crossroads Between Genes, Environment and Resilience in Anxiety Disorders. Genes Brain Behavior (2018) 17(3):e12423. doi: 10.1111/gbb.12423
214. Smeeth D, Beck S, Karam EG, Pluess M. The Role of Epigenetics in Psychological Resilience. Lancet Psychiatry (2021) 8(7):620–9. doi: 10.1016/S2215-0366(20)30515-0
215. Mehta D, Miller O, Bruenig D, David G, Shakespeare-Finch J. A Systematic Review of DNA Methylation and Gene Expression Studies in Posttraumatic Stress Disorder, Posttraumatic Growth, and Resilience. J Traumatic Stress (2020) 33(2):171–80. doi: 10.1002/jts.22472
216. Sipahi L, Wildman DE, Aiello AE, Koenen KC, Galea S, Abbas A, et al. Longitudinal Epigenetic Variation of DNA Methyltransferase Genes is Associated With Vulnerability to Post-Traumatic Stress Disorder. Psychol Med (2014) 44(15):3165–79. doi: 10.1017/S0033291714000968
217. Knight JM, Rizzo JD, Hari P, Pasquini MC, Giles KE, D’Souza A, et al. Propranolol Inhibits Molecular Risk Markers in HCT Recipients: A Phase 2 Randomized Controlled Biomarker Trial. Blood Advances (2020) 4(3):467–76. doi: 10.1182/bloodadvances.2019000765
Keywords: transplantation and cellular therapy, biobehavioral, stress, Covid-19, outcomes
Citation: Knight JM, Taylor MR, Rentscher KE, Henley EC, Uttley HA, Nelson AM, Turcotte LM, McAndrew NS, Amonoo HL, Mohanraj L, Kelly DL and Costanzo ES (2022) Biobehavioral Implications of Covid-19 for Transplantation and Cellular Therapy Recipients. Front. Immunol. 13:877558. doi: 10.3389/fimmu.2022.877558
Received: 16 February 2022; Accepted: 01 June 2022;
Published: 05 July 2022.
Edited by:
Christian Chabannon, Aix-Marseille Université, FranceReviewed by:
Monica Thakar, Fred Hutchinson Cancer Research Center, United StatesRoberto Cacciola, Policlinico Tor Vergata, Italy
Copyright © 2022 Knight, Taylor, Rentscher, Henley, Uttley, Nelson, Turcotte, McAndrew, Amonoo, Mohanraj, Kelly and Costanzo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer M. Knight, jmknight@mcw.edu
 Jennifer M. Knight
Jennifer M. Knight Mallory R. Taylor
Mallory R. Taylor Kelly E. Rentscher1
Kelly E. Rentscher1 Ashley M. Nelson
Ashley M. Nelson