- 1Key Lab of Animal Epidemiology and Zoonoses of Ministry of Agriculture and Rural Affairs, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2Tianjin Key Laboratory of Agricultural Animal Breeding and Healthy Husbandry, College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin, China
- 3Department of Biology, Institute for Functional Microbial Genomics, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany
Chlamydia psittaci (C. psittaci) is an obligate intracellular, gram-negative bacterium, and mainly causes systemic disease in psittacine birds, domestic poultry, and wild fowl. The pathogen is threating to human beings due to closely contacted to employees in poultry industry. The polymorphic membrane proteins (Pmps) enriched in C. psittaci includes six subtypes (A, B/C, D, E/F, G/I and H). Compared to that of the 1 pmpG gene in Chlamydia trachomatis (C. trachomatis), the diverse pmpG gene-coding proteins of C. psittaci remain elusive. In the present study, polymorphic membrane protein 17G (Pmp17G) of C. psittaci mediated adhesion to different host cells. More importantly, expression of Pmp17G in C. trachomatis upregulated infections to host cells. Afterwards, crosstalk between Pmp17G and EGFR was screened and identified by MALDI-MS and Co-IP. Subsequently, EGFR overexpression in CHO-K1 cells and EGFR knockout in HeLa 229 cells were assessed to determine whether Pmp17G directly correlated with EGFR during Chlamydial adhesion. Finally, the EGFR phosphorylation was recognized by Grb2, triggering chlamydial invasion. Based on above evidence, Pmp17G possesses adhesive property that serves as an adhesin and activate intracellular bacterial internalization by recognizing EGFR during C. psittaci infection
Introduction
Chlamydia psittaci (C. psittaci) is an important zoonotic agent with a wide host spectrum (1). It causes systemic infection, especially in birds, which leads to profound economic losses in poultry annually (2). For reporting cases of C. psittaci infection, a national surveillance program in the United States (U.S.) has been operational since 1998. However, no surveillance or case reporting system has been implemented for identifying human chlamydiosis in China. In recent years, case of human psittacosis has increased gradually, and 15 workers were reported to be infected in a U.S. poultry slaughterhouse in 2018, indicating a high risk of zoonotic transmission of Psittacosis (3). In recent years, human psittacosis has increased gradually in China due to implementation of next generation genomic sequencing. Infected hosts (both animal and human) show a diversity of clinical signs, from asymptomatic disease for a large portion of infected organisms to multiple organ failure, sepsis, and death (4). However, the mechanism by which the target hosts is infected and tissue tropism exhibited by C. psittaci upon infection remain unknown.
Attachment and invasion to host cells are milestones in Chlamydia infection and growth. Chlamydia has evolved capabilities to invade host cells via receptors and multiple pathways, similar to Legionella pneumophila (5), Brucella (6) and other intracellular pathogens (7). Multiple outer membrane molecules on Chlamydia have been identified and shown to be associated with adhesion, such as major outer membrane protein (MOMP) (8, 9), lipopolysaccharide (LPS) (10), CT017(Ctad1) (11), and outer membrane complex protein B (OmcB) (12–14). More importantly, polymorphic membrane proteins (Pmps) are dominant adhesins that elicit chlamydial infection (15–17). In addition, Pmps are immunodominant and located on the elementary bodies (EBs) surface of Chlamydia trachomatis (C. trachomatis) and Chlamydia pneumoniae (C. pneumoniae) (18–21). However, the role of Pmps in C. psittaci has been rarely studied, and Pmps interaction mechanism with host cells remains unclear. Regarding diverse pmpG genes and transcription across species, only one pmpG gene has been identified in C. trachomatis genome, and the pmpG gene encoding amino acid sequences in different C. trachomatis serovars is highly conserved. For instance, the identity of PmpG in serovar E and L2 is 98%. However, 37%, 28%, and 33% similarity has been found among Pmp7G, Pmp17G and Pmp21G in C. psittaci. The virulence and pathogenicity are similar to those of C. psittaci 6BC and more homologous pmpG genes have evolved, as indicated by the numbers of functional sequences. Interestingly, the highly homologous pmpG genes of the C. psittaci Cal10 strain are expressed during the late life cycle, while low levels of homologous pmpG genes, identified by a relatively low level of transcripts, are found in the whole cycle (22). These results indicate that pmpG genes play different roles during the life cycle and that highly homologous PmpG proteins may be involved in the assembly of EBs and critical for bacterial adhesion to host cells. According to a recent report, Pmp17G is associated with host adaptations as an adhesin (16). However, the mechanism of Pmp17G-mediated attachment of bacteria to host cells remains unclear. Therefore, we hypothesize that Pmp17G in C. psittaci 6BC is recognized and interacts with potential receptor(s), thereby mediating tissue tropism, multi-host infection and diverse pathogenicity. PmpG-mediated adhesion to and invasion into host cells by recognizing specific receptors is urgently needed during C. psittaci infection.
Materials and Methods
Cell Lines and Bacterial Strains
HeLa 229 cells (No. TCHu 20) and Vero cells (No. GNO10) were purchased from the National Collection of Authenticated Cell Cultures (NCACC; Beijing, China). HEp-2 cells (ATCC, No. CCL-23) and DF-1 cells (ATCC, No. CRL-12203) were maintained in 8% foetal bovine serum (FBS; Gibco, Beijing, China)/Dulbecco’s modified Eagle’s medium (DMEM) (Solarbio Life Science Ltd., Beijing, China), and Chinese hamster ovary (CHO-K1; No. SCSP-507) cells were purchased from NCACC and cultured in Ham’s F-12K (Invitrogen, Beijing, China) supplemented with 10% FBS. All cell lines were cultured at 37°C with a 5% CO2 flow. C. psittaci 6BC (GenBank: CP002549.1) was kindly donated by Professor Yimo Wu (University of South China, Hunan, China) and cultivated, purified and titrated as previously described (23). C. trachomatis L2 (Institute for Functional Microbial Genomics, Heinrich-Heine-Universität, Düsseldorf, Germany) were propagated in HEp-2 cells as described (15).
Antibodies and Reagents
The following antibodies were used in this study: anti-GAPDH (MA5-15738), anti-6×His (MA1-21315), anti-HA (26183) and anti-flag (MA1-91878) mouse monoclonal antibodies (mAbs) and goat anti-mouse IgG H+L (HRP) secondary antibody (31430), which were commercially obtained from Thermo Fisher Scientific (Beijing, China), and anti-EGFR (sc-373746), and anti-phospho-EGFR (1068) (sc-81488) and anti-Grb2 (sc-8034) mAbs, which were purchased from Santa Cruz Biotechnology (Santa Cruz, Shanghai, China). The remaining anti-EGFR rabbit mAbs (4267; CST, Beijing, China) and goat anti-rabbit IgG H&L (HRP) secondary antibody (ab6721; Abcam, Beijing, China) were also commercially available products. Anti-GroEL rabbit pAbs, anti-MOMP mouse mAbs and anti-S1 rabbit pAbs were from Heinrich-Heine-Universität and described previously (11).Anti-MOMP mouse mAbs were prepared by immunizing mice with recombinant MOMP from C. psittaci 6BC in our laboratory as described previously (19). Anti-Pmp17G rabbit polyclonal antibodies (pAbs) were prepared in rabbits inoculated with recombinant Pmp17G protein that was expressed and purified from E. coli BL21 cells (TransGen Biotech Ltd., Beijing, China). The antibodies were purified using HiTrap™ rProtein AF (GE, Beijing, China). The specificities of the MOMP monoclonal antibodies and polyclonal antibodies against Pmp17G were identified with ELISAs as described previously (19).
Plasmid Construction and Protein Preparation
The pmp17G gene was amplified from EBs of C. psittaci 6BC and then subcloned into a pKM255 shuttle vector(Heinrich-Heine-Universität, Düsseldorf, Germany), a pET28a prokaryotic vector or a pCMV-C-HA eukaryotic vector (Beyotime Biotech Ltd., Shanghai, China), respectively. Targeted ectodomain of Pmp17G protein was listed in Figure S2. cDNA samples of EGFR from HeLa 229 cells were amplified by RT-PCR and then subcloned into a pCMV-C-flag vector (Beyotime Biotech Ltd, Shanghai, China), named pCMV-EGFR-N-flag. Four restriction enzymes and T4 DNA ligase were obtained from a commercial company (New England Biolabs, Beijing, China). PCR was performed using Q5 High-Fidelity DNA polymerase (New England Biolabs, Beijing, China). PCR primers were listed in Table S1. Both recombinant Pmp17G protein and MOMP protein were induced by isopropyl-β-D-thiogalactopyranoside (IPTG; Beyotime Biotech Ltd., Shanghai, China) in 500 ml of cultured E. coli BL21 cells. After purification with Ni-NTA Resin(88221, Thermo Fisher Scientific, Beijing, China), the recombinant MOMP protein and Pmp17G were confirmed by SDS-PAGE and Western blots as described previously (19).
Adhesion Assays With the Pmp17G Protein
First, HeLa 229, Vero and DF-1 cells were grown in 24-well plates for 24 h. Then, the culture media were removed, and the cells were washed 3 times with cold PBS and then incubated with 200 µl of Pmp17G (200 µg/ml) to cover the surface of the cells. Subsequently, the cultures were incubated at 37°C with 5% CO2 flow and monitored at 15, 30, 60, 90, and 120 min. Finally, the cell cultures were washed 5 times with PBS, and then, the cells were lysed in 50 µl of RIPA buffer with phenylmethylsulfonyl fluoride (PMSF; Beyotime Biotech Ltd, Shanghai, China). The lysed solutions were centrifuged at 12,000 g for 10 min, and then, the supernatants were boiled with loading buffer. Finally, 10 µl of the cell lysate was identified by SDS-PAGE and Western blots.
Expression of C. psittaci-Specific Pmp17G in C. trachomatis L2 (C. trachomatis L2 (pKM255::pmp17G)
Chlamydial transformation was performed as described previously (24). Briefly, the pKM255::pmp17G plasmid (Institute for Functional Microbial Genomics, Heinrich-Heine-Universität, Düsseldorf, Germany), was extracted from a Dam- and Dcm-methylase-deficient strain of E. coli (GM-48), using a plasmid mega kit (Qiagen, Hilden, Germany). Penicillin (1µg/µl) was used for the selection of transformed C. trachomatis L2. A mixture of C. trachomatis L2 EBs and pKM255::Pmp17G was incubated in calcium chloride buffer (10 mM Tris, 50 mM calcium chloride, pH 7.4) for 30 min at room temperature. Subsequently, C. trachomatis L2 was mixed with pKM255::pmp17G and incubated with HEp-2 cells in calcium chloride buffer for 1 h at room temperature. The total calcium chloride mixture was discarded and replaced with fresh 10% FBS/DMEM containing 2 µg/ml cycloheximide. After 24 hpi, penicillin (1 µg/µl) was added and further incubated for 36 h at 37°C under 5% CO2. Afterwards, the infected epithelial cells were scraped and lysed with glass beads for next passages untill inclusions were observed obviously. Positive expression of Pmp17G in C. trachomatis L2 was identified to bind anti-flag antibody by immunofluorescence (IF). Furthermore, location of the Pmp17G was analyzed using different detergent extractions as described previously (11).
Co-IP of Overexpressed Proteins and Surface Complexes
For overexpressing proteins, HeLa 229 cells were transfected with plasmids containing the EGFR gene and/or pmp17G gene using Lipofectamine™ 3000 (Invitrogen, Beijing, China) according to the manufacturer’s protocol. As for immunoprecipitation(IP) of surface complexes, HeLa 229 cells were grown to 90% confluency, and the cell cultures were washed with PBS and incubated with 200 µg/ml purified Pmp17G in FBS-free DMEM at 37°C for 2 h. Twenty-four hours after transfection or 2 h after incubation, cell cultures were rinsed 3 times with PBS and then lysed in IP lysis buffer (containing 1% NP-40, 1% Triton X-100, 20 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1 mM Na3VO4, 5 mM NaF, and 2 mM EDTA) and protease inhibitor cocktail (Beyotime Biotech Ltd, Shanghai, China) at 4°C for 30 min. Then, cell lysates were precleared with standard IgG (rabbit sera) (Solarbio Life Science, Beijing, China) together with protein A/G PLUS-agarose (Santa Cruz, Shanghai, China) at 4°C for 30 min. Then, the precleared cell lysates were used for IP reactions. Co-IP reactions were carried out by mixing anti-EGFR mAbs or anti-Pmp17G pAbs at 4°C for 6 h and then washing the lysates 3 times with IP buffer. Finally, the target proteins were identified by Western blots using HA-tagged or flag-tagged antibodies.
Blocking Infection With Pmp17G or Anti-Pmp17G Antibody
HeLa 229 cells were incubated with Pmp17G (200 µg/ml) at 37°C for 2 h. Afterwards, the unbound proteins were discarded after washing 5 times with PBS, and the cells were infected with C. psittaci 6BC at an MOI of 5 in medium containing 8% FBS/DMEM and 2 µg/ml cycloheximide (Sigma-Aldrich, Shanghai, China) and then incubated at 37°C for 36-48 h. To confirm blocking infection, C. psittaci 6BC EBs were diluted with SPG solution (containing 0.25 M sucrose, 10 mM sodium phosphate, and 5 mM L-glutamic acid, pH 7.2-7.5) to a final concentration of 1.0×106 inclusion-forming units (IFUs)/ml. The diluted EBs were incubated with purified anti-Pmp17G pAbs at 50 µg/ml, and the cultured EBs were allowed to react at room temperature for 2 h using a shaking machine. Later, HeLa 229 cells were infected with the above cultures at 37°C for 36-48 h. IFUs were quantified using a monoclonal antibody against MOMP and IF microscopy. The adhesion studies and internalization assays were carried out using Pmp17G-coated fluorescent beads as described previously (25).
Effect of EGFR Regulation on C. psittaci Infection
To identify the effect of EGFR on C. psittaci 6BC infection, HeLa 229 cells were pretreated with different concentrations of cetuximab (Sigma-Aldrich, Shanghai, China), an anti-EGFR antibody, at 37°C for 2 h. Then, the unbound antibodies were removed from the reaction followed by washing 5 times with PBS, and finally, cell cultures were infected with C. psittaci 6BC at an MOI of 5 and incubated at 37°C for 36-48 h. In another experiment, EGFR in HeLa 229 cells was knocked down via transfection with EGFR siRNA (Santa Cruz, Shanghai, China) according to the manufacturer’s protocol, and the knockdown efficiency was confirmed by Western blots. Then, the transfected HeLa 229 cells were exposed to C. psittaci 6BC at an MOI of 5 at 37°C for 36-48 h. To construct overexpressed EGFR in CHO-K1 cells, the cells were transfected with pCMV-EGFR-N-flag at 37°C for 24 h. Subsequently, the overexpressed cells were infected at an MOI of 5 at 37°C for 36-48 h. IFUs were determined as described above.
Effect of Pmp17G Recognition of EGFR on the Phosphorylation of Try1068 and Grb2
HeLa 229 cells were cultivated with 8% FBS at 37°C for 24 h and then replaced with FBS-free medium for 12 h. Subsequently, cell cultures were stimulated with different concentrations of Pmp17G (from 25 to 500 µg/ml) or C. psittaci 6BC (MOI from 0.1 to 100) at 37°C for 2 h. Then, the cell cultures were washed 5 times with cold PBS, and the cells were lysed in 50 µl of IP lysis buffer with protease and phosphatase inhibitor cocktail. The lysed solutions were centrifuged at 12,000 g for 10 min, and then, the supernatants were boiled with 1x loading buffer for 10 min. Later, 10 µl of the cell lysates were identified by SDS-PAGE and Western blots. The supernatants were tested for EGFR, pEGFR1068, and Grb2 expression using anti-EGFR, anti-pEGFR1068, anti-Grb2 and anti-GAPDH antibodies, respectively. The optimal concentrations of Pmp17G and EBs of C. psittaci 6BC were applied in a time-dependent manner. HeLa cells were infected with 200 µg/ml Pmp17G or C. psittaci 6BC at an MOI of 5 for different durations (from 0, 5, 10, 15, 30 to 60 min). Then, the cell cultures were treated as described above. To carry out Co-IP with EGFR at Try1068 and Grb2, HeLa cells were infected with 200 µg/ml Pmp17G or C. psittaci 6BC at an MOI of 5 for 30 min. The cell cultures were treated IP lysis buffer containing a protease and phosphatase inhibitor cocktail. Finally, the supernatants were subjected to IP with anti-EGFR antibodies as explained above.
Statistical Analysis
Statistical analyses were carried out by analysis of variance (ANOVA). Multiple comparisons and differences were analyzed using the least-significant difference (LSD) method. A p-value (two-tailed) of 0.05 or less was considered to indicate significance. Analyses were carried out using GraphPad Prism software (GraphPad Software Inc., CA, USA) and Image J (National Institutes of Health, Maryland, USA).
Results
Pmp17G Triggered Adhesion to Multi-Host Cells, and Recombinant Pmp17G Blocked C. psittaci Infection
In the present study, strong Pmp17G adhesion was found to HeLa 229 cells, and secondary higher attaching capability was observed in Vero cells than DF-1 cells did. Regarding the time course, early adhesion was observed at 15 min, and an increasing attachment was correlated with prolonged incubation and the strongest signal was detected at 120 min, while secondary adhesion occurred at 60 min in Vero cells and 120 min in DF-1 cells, respectively (Figure 1A). Subsequently, 3 different host cells were incubated with Pmp17G, and the relative C. psittaci infection of HeLa 229 cells, DF-1 cells and Vero cells was reduced to 72.15%, 70.04% and 76.97%, respectively, which was higher than that of the heparin-positive group. However, no difference was found among the 3 host cells prior to treatment with recombinant Pmp17G (Figure 1B), indicating that Pmp17G might attach to multiple host cells and involve in tissue tropism, contributing to the wide spectrum of C. psittaci infection in comparison with C. trachomatis (15) and C. pneumoniae (17).
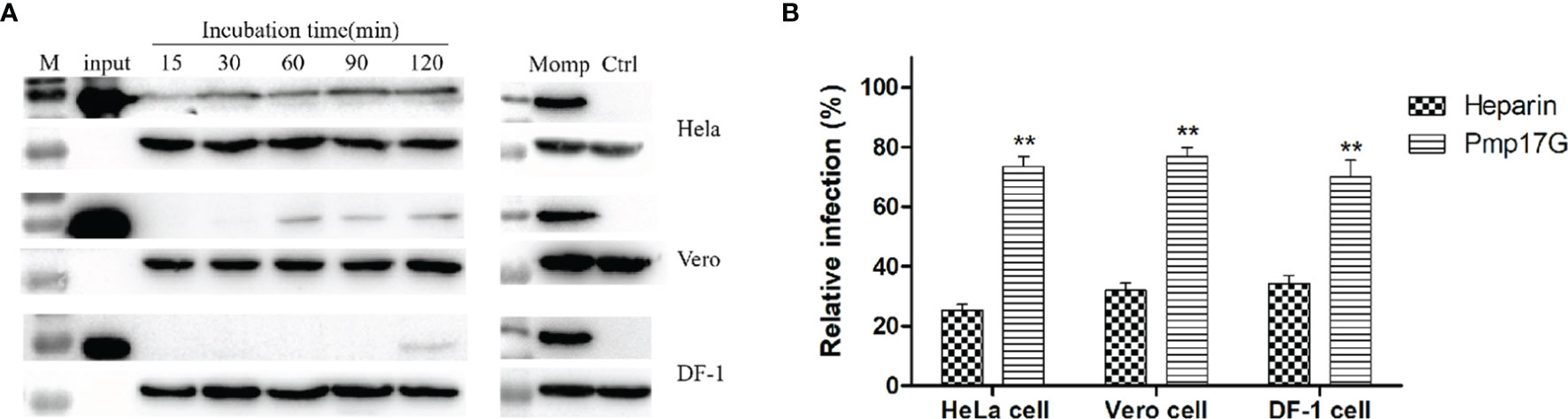
Figure 1 Pmp17G mediated adhesion to host cells, and recombinant Pmp17G blocked C. psittaci infection. (A) HeLa 229 cells, Vero cells and DF-1 cells were incubated with 200 μg/ml Pmp17G, while inactivated EBs at an MOI of 5 were used as a positive control for different durations. Positive bands were reacted with anti-His mAbs or anti-MOMP antibody, and GAPDH was the loading control. (B) HeLa 229 cells, Vero cells and DF-1 cells were pretreated with 200 μg/ml of Pmp17G and 500 μg/ml heparin, as the positive control for 2 h, and then exposed to C. psittaci 6BC at an MOI of 5 for 36-48 h Inclusion-forming units (IFUs) were quantified by indirect immunofluorescence (IIF). Relative Infection (%)=(IFUs of the treated group/IFUs of the PBS group)×100. Statistical analysis was performed by one-way ANOVA, and the data from 3 independent experiments were expressed as the means ± standard deviations (SD). Asterisks indicate statistical significance: **p < 0.01.
Ectopic Expression of C. psittaci-Specific Pmp17G Enhanced C. trachomatis Infection
Regarding the only one pmpG gene of C. trachomatis, the role of ectopic expression of C. psittaci-specific Pmp17G in C. trachomatis will be a fundamental model to elucidate the functions of the duplication of pmpGs in C. psittaci. Interestingly, expression of C. psittaci-specific Pmp17G in C. trachomatis L2 was observed using indirect immunofluorescence (IF) at 24-hour post infection (hpi) (Figure 2A). Moreover, locations of C. psittaci-specific PmpG17 were found both on outer membrane and cytoplasm of EBs by Western blots. Meanwhile, GroEL was located both on outer membrane surface and cytoplasm, MOMP as outer membrane protein and S1 protein located in cytoplasm of C. trachomatis L2 were used as the control groups (Figure 2B). To warrant above observation, Hep-2 cells were treated with Pmp17G or Ctad1 and infected with ectopic expression of C. trachomatis L2 (pKM255::pmp17G).Interestingly, Pmp17G displayed strong blocking infection compared to Ctad1 post infection of C. trachomatis L2 (pKM255::pmp17G) (p<0.05) (Figure 2C).
Figure 2 Expression of C.psittaci-specific PmpG17 upregulated C. trachomatis infection. (A) Identification of C. psittaci-specific PmpG17 in C. trachomatis L2 strain by indirect immunofluorescence at 24 hpi. (B) Expressions of C psittaci-specific PmpG17 were located both on outer membrane and cytoplasm of elementary bodies by Western blots. GroEL, a heat-shock protein located both on outer membrane surface and cytoplasm, MOMP, an outer membrane protein, and S1 protein, an intracellular protein of C. trachomatis L2 as the control group. Elementary bodies of C. trachomatis L2 (pMK255::pmp17G) were treated with PBS, 1% Triton-X 100, 2% Sarkosyl or N-40 with DTT, respectively. Pellet (P) and supernatant (S) fractions were prepared by centrifugation and incubated with anti-S1, anti-MOMP, anti-GroEL1 and anti-flag antibodies, respectively. (C) HEp-2 cells were preincubated with 200 μg/ml of recombinant Pmp17G or Ctad1 as a positive control. Afterwards, above cell cultures were inoculated with C. trachomatis L2 (pMK255::pmp17G) at an MOI of 5 for 24 hours. IFUs were quantified as above described. Relative infection (%)=(IFUs of the treated group/IFUs of the PBS group)×100. Statistical analysis was performed by one-way ANOVA, and the data from 3 independent experiments were expressed as the means ± SD. Asterisks indicate statistical significance: *p < 0.05.
Pmp17G Directly Binded to and Interacted With the Cellular Transmembrane Protein
Pmp17G was incubated with HeLa 229 cells for 2 h, and the attached fractions in the pull-down elution using Ni-NTA were identified and analyzed by MALDI-MS (Figure S1). Although no difference was found in the SDS-PAGE assay with Coomassie staining (Figure S1A), an increasing intensity was observed in the Pmp17G-linked DTSSP compared to Pmp17G alone (Figure S1B), and higher intensity of Pmp17G-related EGFR was found in the Pmp17-linked group compared to the Pmp17G alone group (Figure S1C). To confirm that Pmp17G directly interacted with EGFR in host cells, Co-immunoprecipitation (Co-IP) assays were carried out with anti-His mAbs or anti-EGFR mAbs, and Pmp17 binding to EGFR was confirmed by Western blots (Figures 3A, B). Then, overexpression of Pmp17G and EGFR was used to validate the interaction. Finally, positive band of Pmp17G-recognized EGFR was identified with anti-HA antibody (Figure 3C) and anti-flag antibody (Figure 3D), indicating that EGFR is a receptor of Pmp17G.
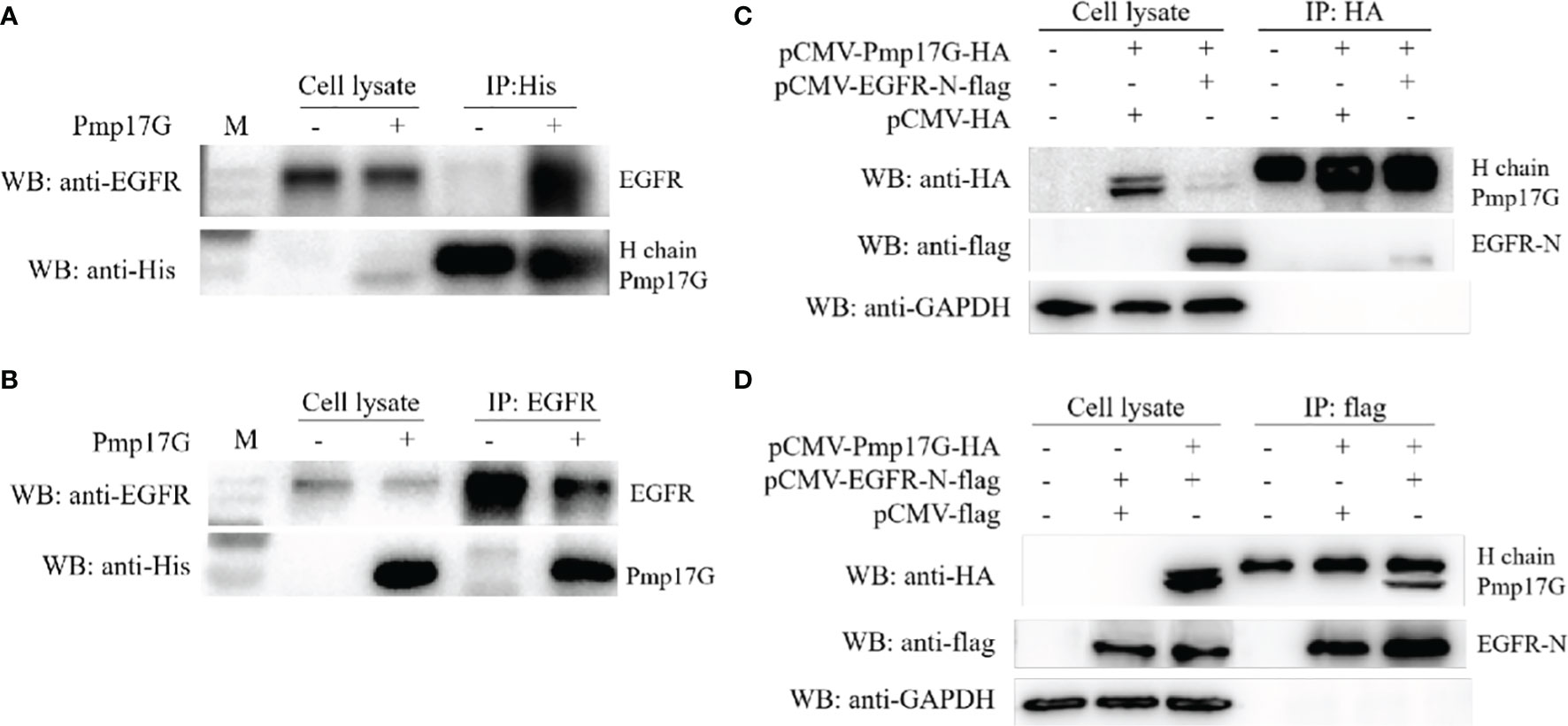
Figure 3 Crosstalk of Pmp17G with EGFR. (A) Pmp17G was incubated with HeLa 229 cells for 2 h, immunoprecipitated with anti-His mAbs, and then reacted with anti-EGFR mAbs and anti-His mAbs. (B) After incubation with HeLa 229 cells, IP was assayed with anti-EGFR mAbs, and then, Pmp17G-recognized EGFR was identified with anti-His mAbs and anti-EGFR mAbs using Western blots. (C) Co-expression of Pmp17G and EGFR in HeLa 229 cells. HeLa 229 cells were transfected with pCMV-Pmp17G-HA and pCMV-EGFR-N-flag plasmids, reacted with anti-HA antibody for IP, and then incubated with anti-flag and anti-EGFR mAbs by Western blots. (D) Co-expression of Pmp17G and EGFR in HeLa 229 cells was evaluated by Co-IP. HeLa 229 cells were transfected with pCMV-Pmp17G-HA and pCMV-EGFR-N-flag plasmids, immunoprecipitated with anti-flag antibody, and then identified with anti-flag and anti-EGFR mAbs using Western blots.
EGFR Was a Receptor During C. psittaci Infection
To further confirm the roles of EGFR during C. psittaci infection, serial doses of cetuximab were used to block EGFR in host cells. After inoculation with C. psittaci 6BC, 20 μg/ml cetuximab was optimized for blocking Chlamydia infection, and a dose-dependent effect was found in a neutralization test (Figure 4A). After treatment with EGFR siRNA, the relative infection was reduced dramatically compared to that of the control siRNA and the mock groups (Figure 4B). Moreover, the relative infection was increased significantly in the EGFR overexpression group compared to that in the CHO-K1 cell lines or CHO-K1 cells transfected with the control vector (p<0.01) (Figure 4C), indicating that EGFR plays a major role in early C. psittaci infection.
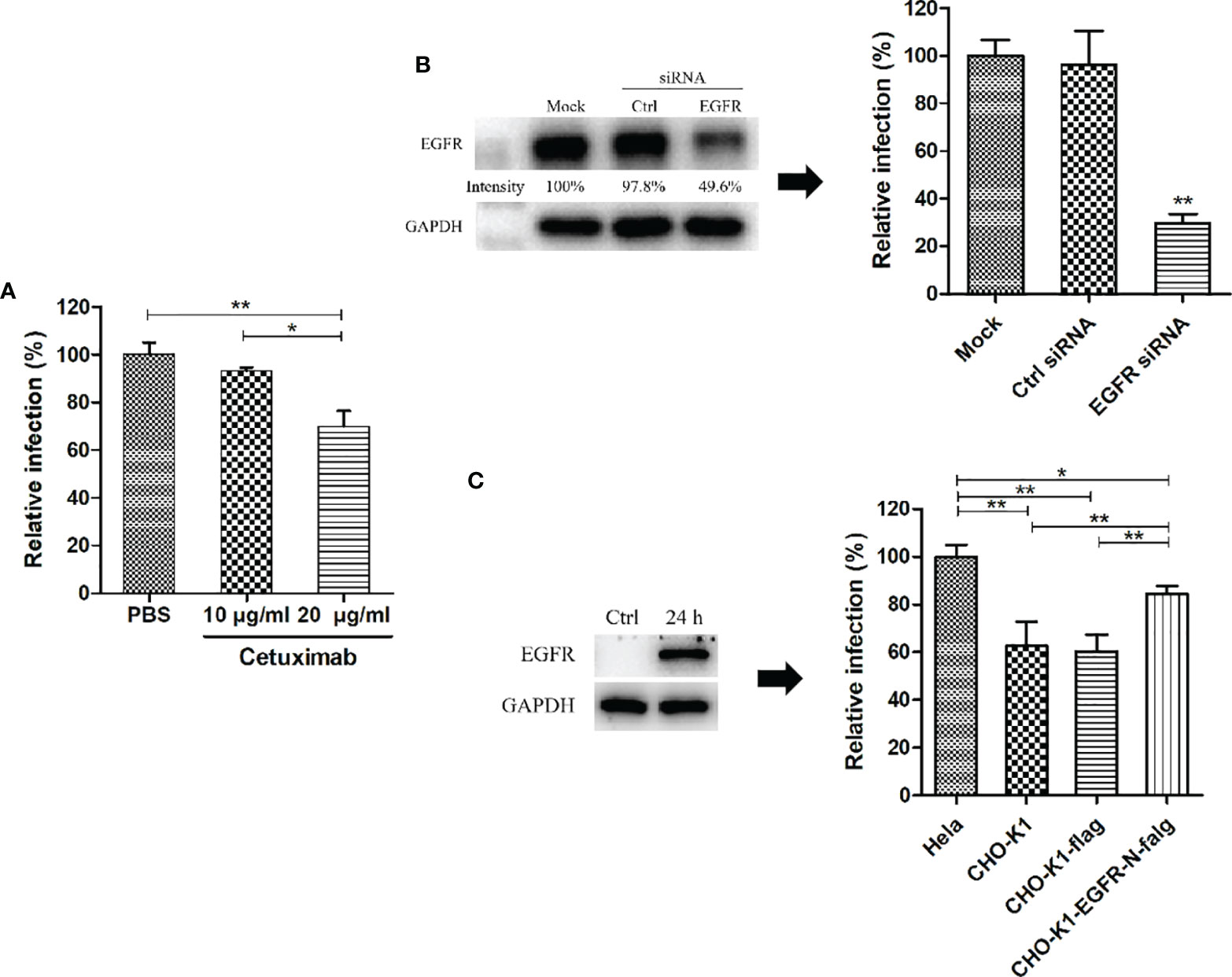
Figure 4 EGFR was essential for C. psittaci infection. (A) HeLa 229 cells were treated with cetuximab (10 μg/ml and 20 μg/ml) for 2 h and then inoculated with C. psittaci 6BC (MOI=5). Relative infection was calculated on the basis of inclusion bodies, and Relative infection (%)=(IFUs of the treated group/IFUs of the control group) ×100. (B) HeLa cells were transfected with EGFR siRNA (siEGFR) or control siRNA (Ctrl). Twenty-four hours after the 2nd treatment with siRNA, the cell cultures were exposed to C psittaci 6BC (MOI=5) and, concurrently lysed to determine efficiency of siRNA by Western blots. The intensity of the bands was quantified using ImageJ. GAPDH was used as the loading group. Intensity was determined as follows: Intensity(%)=(EGFR/GAPDH) ×100. In addition, inclusion bodies were counted and relative infection was determined as follows: Relative infection (%)=(IFUs of the siRNA-treated group/IFUs of the mock group) ×100. (C) CHO-K1 cells were transfected with exotic EGFR-flag, and ectopic expression of EGFR was analysed by Western blots. Twenty-four hours post-transfection, cell cultures were infected with C. psittaci 6BC (MOI=5). At 36-48 hpi, inclusions were measured by IIF. Relative infection was calculated using the formula: Relative infection(%)= (IFUs of the CHO-K1 group/IFUs of the HeLa 229 group) × 100. Statistical analysis was performed by one-way ANOVA, and the data were expressed as the means ± SD. Asterisks indicate statistical significance: *p < 0.05, and **p < 0.01.
Pmp17G-Mediated Adhesion and Invasion During C. psittaci Infection
After C. psittaci 6BC was pretreated with anti-Pmp17G antibody or anti-MOMP antibody, a significant difference was found among the IgG (mouse) group, IgG(rabbit) group, the MOMP antibody-treated group and the Pmp17G antibody-treated group, suggesting that the Pmp17G antibody reduced dramatically the extent of the Chlamydia infection (Figure 5A). To monitor Pmp17G-mediated adhesive capability, an increase in the FITC-A subset was observed in the Pmp17G-coated group compared to the positive MOMP-coated group (p<0.05) or the negative BSA-coated group (p<0.01). To elucidate the role of EGFR, HeLa 229 cells were pretreated with cetuximab and then incubated with Pmp17G-coated green fluorescent beads. Bead adhesion to host cells was inhibited significantly in the cetuximab/Pmp17G group compared to the Pmp17G group (p<0.05) (Figure 5B). More interestingly, only Pmp17G-coated beads exhibited intracellular localization and 20.7% internalized beads were determined while BSA-labelled beads presented solely on cellular surfaces (Figures 5C, D), suggesting that Pmp17G is not only an adhesin but also an invasin during C.psittaci infection.
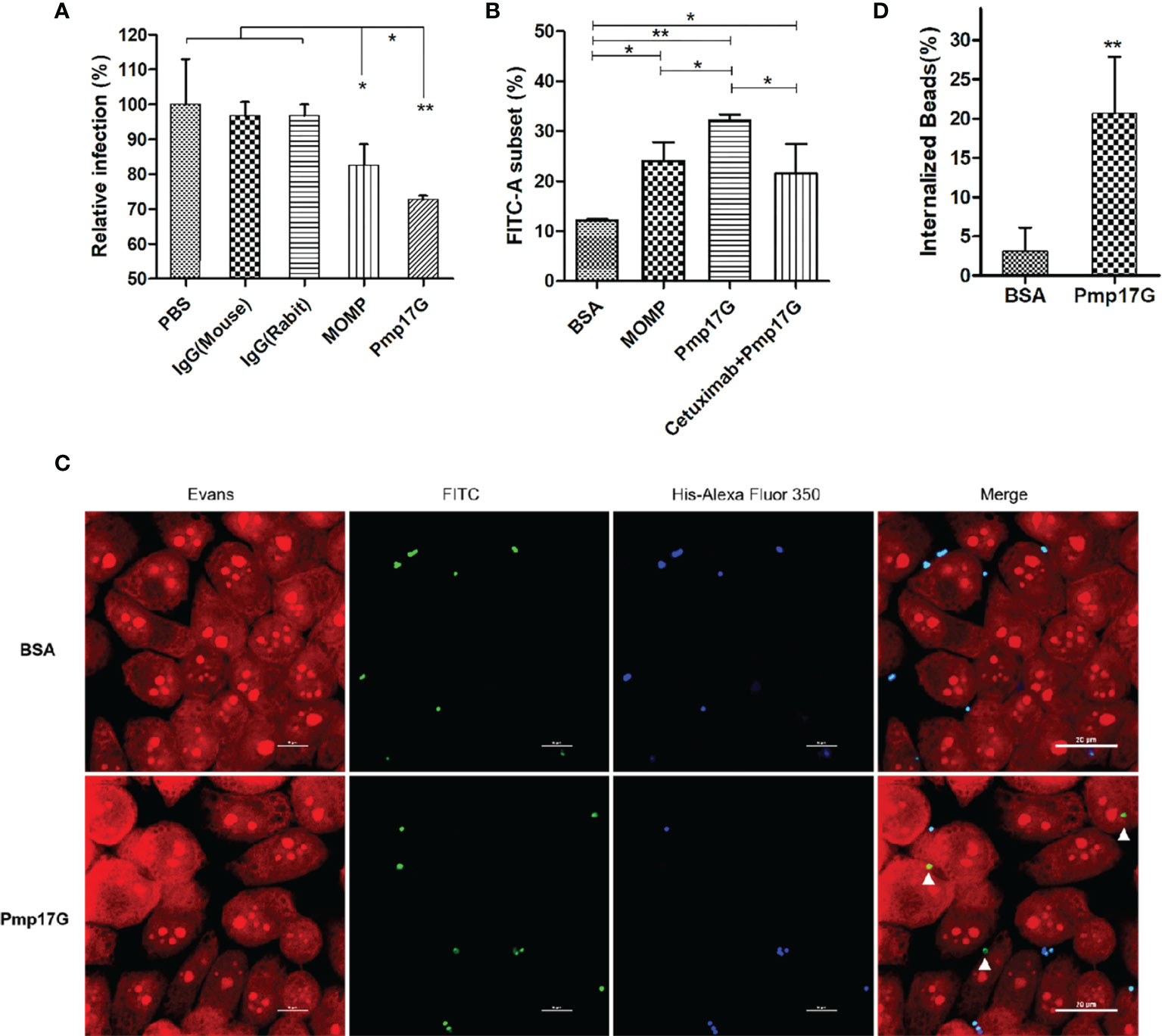
Figure 5 Pmp17G contributed to adhesion and invasion during C. psittaci infection. (A) C. psittaci 6BC was treated with anti-Pmp17G antibody or anti-MOMP antibody while IgG(mouse), IgG(rabbit) and PBS were control groups. Then, HeLa 229 cells were infected with these preparations for 36-48 h Relative infection (%)=(IFUs of the antibody-treated group/IFUs of control group) ×100. (B) Pmp17G adhesive capability to HeLa 229 cells. HeLa 229 cells were incubated with BSA-, MOMP- or Pmp17G-coated green fluorescent beads for 2 h at 37°C. Then, cell cultures were treated with trypsin (non-EDTA) for 3 min and washed 3 times with PBS, and the cells were resuspended in PBS. Adhesion of FITC-A subsets was measured using flow cytometry and proportion of FITC-A subsets was determined by dividing the number of FITC-labelled cells by the total number of cells. Three replicates of each measurement were performed. (C) Pmp17G invasion capability into HeLa 229 cells. HeLa 229 cells were incubated with BSA-, or Pmp17G-coated green fluorescent beads for 6 h at 37°C. Subsequently, the cell cultures were washed 5 times with PBS and fixed with 0.4% paraformaldehyde. Later, the extracellular binding beads were stained with 6×His-tag antibody (blue). Internalized beads displayed green. Cell structures were stained with Evans blue (red). (D) Internalized bead(%)=(Numbers of internalized beads/all the binding beads) ×100. Statistical analysis was performed by one-way ANOVA, and the data were expressed as the means ± SD. Asterisks indicate statistical significance: *p < 0.05, and **p < 0.01.
Pmp17G Activated EGFR Phosphorylation and Grb2 Recruitment
After HeLa 229 cells were inoculated with different doses of C. psittaci 6BC, phosphorylation of EGFR at site 1068 was activated in a dose-dependent manner with C. psittaci inoculation ranging from 0.1 to 1.0 MOI, while a gradual reduction in EGFR phosphorylation was observed with C. psittaci 6BC inoculation ranged from 5.0 to 100.0 MOI. Compared to pY1068-EGFR, no difference of EGFR expression was found after infection with different doses of C. psittaci. In contrast, Grb2 expression was downregulated in a dose-dependent manner (Figure 6A). Upon Pmp17G treatment, the expression of EGFR, pY1068-EGFR and Grb2 displayed similar trends (Figure 6B). The time-dependent effect was assessed, and the results showed that increased EGFR phosphorylation was observed with prolonged time, while a negative correlation was found between time points and Grb2 expression after exposure to EBs (Figure 6C). Upon Pmp17G treatment, EGFR phosphorylation increased gradually with prolonged time. However, the expression of EGFR and Grb2 displayed no difference after Pmp17G treatment (Figure 6D). To determine whether Grb2 was translocated from the cellular reticulum to EGFR phosphorylation at site 1068 in the plasma membrane, we carried out Co-IP to determine whether EGFR binds to Grb2. Subsequently, positive bands were observed in both the C. psittaci group and the Pmp17G treated group (Figure 6E), indicating that EGFR phosphorylation might recruit Grb2 and trigger C. psittaci internalization.
Figure 6 Pmp17G stimulated the phosphorylation of EGFR and downregulated Grb2 expression. (A) HeLa 229 cells were infected with serial doses of C. psittaci (MOI from 0.1 to 100), and the expression of EGFR, pY1068-EGFR and Grb2 was identified using Western blots. (B) HeLa 229 cells were treated with serial doses of Pmp17G (from 10 μg/ml to 500 μg/ml), and the expression of these proteins was determined using Western blots. (C) HeLa 229 cells were infected with C. psittaci (MOI=1.0), and the expression of the aforementioned proteins was identified at different time points (at 5, 15, 30, 60, 90, 120, and 180 min) by Western blots. (D) HeLa 229 cells were treated with 200 μg/ml of Pmp17G, and the expression levels of these proteins were determined at different time points. GAPDH was used as the loading control. (E) Grb2 recognized pY1068-EGFR by Co-IP assay. HeLa cells were treated with C. psittaci 6BC (MOI=1.0) or 200 μg/ml of Pmp17G, and IP was performed with anti-EGFR mAbs for 0.5 h at 4°C. Then, IP compounds were incubated with anti-EGFR, anti-pY1068-EGFR or anti-Grb2 antibodies, respectively and identified by Western blot assay. β-Tubulin was used as the loading control.
Discussion
Compared to C. trachomatis, C. pneumoniae and Chlamydia muridarum (C. muridarum), the pathogen C. psittaci infects diverse hosts, causing mild symptoms to severe pathogenicity and even mortality. Attachment and invasion to host cells are two main steps of Chlamydia infection. Chlamydia has a powerful ability to infect host cells by attaching or binding receptors. Pmps are enriched in the outer membrane protein complex of Chlamydia species. However, the role of PmpG-mediated tissue tropism has been poorly elucidated. In the current study, Pmp17G was found to mediate adhesive properties as an adhesin in a dependent manner by binding EGFR. More importantly, Pmp17G activated intracellular invasion in an EGFR-dependent manner during C. psittaci infection. Therefore, Pmp17G acts as both an adhesin and invasin during C. psittaci infection.
As an adhesin of C. psittaci, Pmp17G is able to bind to multiple host cells and promotes chlamydial adhesion in early infection. The typical time-dependent pattern was observed in HeLa 229 cells and Vero cells, with identifiable adhesion at 120 min after treatment with Pmp17G. More importantly, expression of Pmp17G enhanced C.trachomatis infection, implying that Pmp17G might contribute to infection of the multiple hosts and tissue tropism of C. psittaci. Our data corroborate a recent report that Pmp17G plays a role as an adhesin in early Chlamydial infection (15–17). However, our data are inconsistent with reports of high adhesive capacity of avian cells (DF-1 cells) and low adhesion to mammalian cells (McCoy cells) (16). However, a blocking assay indicated that there was no significant difference in relative infection among HeLa 229 cells, Vero cells and DF-1 cells, with infection in all these cells reduced from 23.03% to 29.96%. Pmps-mediated adhesion is associated with conserved 4-peptide motifs (14, 19) and oligomers (26, 27). The numbers of motifs are crucial for the adhesive capability of Pmps to certain cells, but no correlation was found between motif-mediated adhesion and inhibited infectivity in our study. Although only 3 motifs are present in recombinant Pmp17G, high adherence to HeLa 229 cells was observed in the study.
Activation of EGFR is required for the attachment and development of Chlamydia (25, 28, 29). Pmp17G binding to EGFR was identified by pull-down assay and mass spectrometry. The interaction of Pmp17G and EGFR was also determined by Co-IP. Adhesion was inhibited by cetuximab, an EGFR-specific antibody. Moreover, C. psittaci infection was reduced significantly either using cetuximab or EGFR knockdown in HeLa 229 cells. In contrast, Chlamydia infection was recovered by the overexpression of EGFR in CHO-K1 cell lines. Therefore, Pmp17G binding to EGFR is the first step for intracellular adhesion. The role of EGFR in C. psittaci infection revealed in our study is consistent with that described in previous reports of C. pneumoniae attachment via Pmp21-recognized EGFR (25) and C. trachomatis infection (28, 29). As an adhesion component, Pmp21 was the first identified Pmps component that interacted with EGFR during C. pneumoniae infection (25). In our study, the diversity of C. psittaci adhesion to host cells might be associated with Pmp17G-mediated EGFR affinity due to EGFR enrichment in the placenta and kidney of human tissues (30). However, EGFR abundance is unclear among HeLa 229 cells, Vero cells and DF-1 cell.The further investigation will be elucidated diverse affinity to host cells.
Additionally, Pmp17G recognition of EGFR facilitates bacterial invasion. Both Pmp17G and C. psittaci EBs are able to induce phosphorylation at the 1068 site of EGFR, which is consistent with previous reports of C. pneumoniae EBs/Pmp21 (14). Regarding EGFR phosphorylation, Pmp17G induces weaker activation than whole C. psittaci EBs. Once EGFR is activated, Grb2 is rapidly recruited to the plasma membrane, where it binds to phosphorylated EGFR and triggers bacterial invasion by forming the EGFR-Grb2 complex, in which Grb2 plays a role in the macropinocytic internalization pathway of EGFR in activated cells (31). Our data are consistent with a previous report that Pmp21 binding to EGFR resulted in the recruitment of both Grb2 and c-Cbl, as well as activation of downstream ERK1/2 signalling (25). In another previous report, the EGFR and TGF-β signalling pathways were found to cooperate to optimize inclusion development, cytoskeletal remodelling, and induction of the pathogenic epithelial-mesenchymal transition (EMT) during C. trachomatis infection (28). Functional analysis of phosphoproteome and transcriptome data confirmed the involvement of these pathways in the EMT during infection, a phenotype that was confirmed in infected cells, along with the essential roles of ERK1/2, ETS1, and ERF activation in C. trachomatis replication (32). However, effect of Pmp17G on inclusion development, cytoskeletal remodelling and EMT stabilization by EGFR phosphorylation remains elusive, and further work is required to discover the potential mechanism.
Taken together, our results show that C. psittaci-specific Pmp17G possesses adhesive properties, enabling it to serve as an adhesin to host cells. It also activates chlamydial invasion in EGFR-dependent manner, activating EGFR through Tyr1068 phosphorylation and forming the EGFR-Grb2 complex, contributing to intracellular attachment and internalization during C. psittaci infection. Pmp17G is a critical biomarker due to its roles as an adhesin and invasion. However, further investigation is urgently needed to identify the target mechanism for combatting Chlamydia infection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization: CH and JH. Methodology: XL and ZZ. Investigation: XL, ZZ, and YW. Visualization: XL and ZZ. Supervision: CH and JHH Writing-original draft: XL, ZZ. Writing—review and editing: CH. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (grant 31672517) and Taishan Scholar Project of Shandong province (grant ts201511084).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Yongzheng Wu at Unit of Innate Defense & Inflammation, Institut Pasteur, Paris, France for great helpful comments. Dr. Guanggang Qu and Miss Lei Cui are acknowledged for the preparation of recombinant proteins and antibodies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.818487/full#supplementary-material
References
1. Radomski N, Einenkel R, Müller A, Knittler MR. Chlamydia-Host Cell Interaction Not Only From a Bird’s Eye View: Some Lessons From Chlamydia Psittaci. FEBS Lett (2016) 590:3920–40. doi: 10.1002/1873-3468.12295
2. Gitsels A, Van Lent S, Sanders N, Vanrompay D. Chlamydia: What Is on the Outside Does Matter. Crit Rev Microbiol (2020) 46:100–19. doi: 10.1080/1040841X.2020.1730300
3. Tolba HMN, Abou Elez RMM, Elsohaby I. Risk Factors Associated With Chlamydia Psittaci Infections in Psittacine Birds and Bird Handlers. J Appl Microbiol (2019) 126:402–10. doi: 10.1111/jam.14136
4. Laroucau K, Aaziz R, Meurice L, Servas V, Chossat I, Royer H, et al. Outbreak of Psittacosis in a Group of Women Exposed to Chlamydia Psittaci-Infected Chickens. Euro Surveillance (2015) 20(24):21155. doi: 10.2807/1560-7917.ES2015.20.24.21155
5. Stein MP, Müller MP, Wandinger-Ness A. Bacterial Pathogens Commandeer Rab GTPases to Establish Intracellular Niches. Traffic (Copenhagen Denmark) (2012) 13:1565–88. doi: 10.1111/tra.12000
6. Czibener C, Merwaiss F, Guaimas F, Del Giudice MG, Serantes DA, Spera JM, et al. BigA Is a Novel Adhesin of Brucella That Mediates Adhesion to Epithelial Cells. Cell Microbiol (2016) 18:500–13. doi: 10.1111/cmi.12526
7. Henderson IR, Lam AC. Polymorphic Proteins of Chlamydia Spp.–Autotransporters Beyond the Proteobacteria. Trends Microbiol (2001) 9:573–8. doi: 10.1016/S0966-842X(01)02234-X
8. Mehlitz A, Rudel T. Modulation of Host Signaling and Cellular Responses by Chlamydia. Cell Commun Signaling: CCS (2013) 11:90. doi: 10.1186/1478-811X-11-90
9. Su H, Raymond L, Rockey DD, Fischer E, Hackstadt T, Caldwell HD. A Recombinant Chlamydia Trachomatis Major Outer Membrane Protein Binds to Heparan Sulfate Receptors on Epithelial Cells. Proc Natl Acad Sci United States America (1996) 93:11143–8. doi: 10.1073/pnas.93.20.11143
10. Ajonuma LC, Fok KL, Ho LS, Chan PK, Chow PH, Tsang LL, et al. CFTR Is Required for Cellular Entry and Internalization of Chlamydia Trachomatis. Cell Biol Int (2010) 34:593–600. doi: 10.1042/CBI20090227
11. Stallmann S, Hegemann JH. The Chlamydia Trachomatis Ctad1 Invasin Exploits the Human Integrin β1 Receptor for Host Cell Entry. Cell Microbiol (2016) 18:761–75. doi: 10.1111/cmi.12549
12. Fadel, Eley A. Chlamydia Trachomatis OmcB Protein Is a Surface-Exposed Glycosaminoglycan-Dependent Adhesin. J Med Microbiol (2007) 56:15–22. doi: 10.1099/jmm.0.46801-0
13. Moelleken K, Hegemann JH. The Chlamydia Outer Membrane Protein OmcB Is Required for Adhesion and Exhibits Biovar-Specific Differences in Glycosaminoglycan Binding. Mol Microbiol (2008) 67:403–19. doi: 10.1111/j.1365-2958.2007.06050.x
14. Puolakkainen M, Kuo CC, Campbell LA. Chlamydia Pneumoniae Uses the Mannose 6-Phosphate/Insulin-Like Growth Factor 2 Receptor for Infection of Endothelial Cells. Infect Immun (2005) 73:4620–5. doi: 10.1128/IAI.73.8.4620-4625.2005
15. Becker E, Hegemann JH. All Subtypes of the Pmp Adhesin Family Are Implicated in Chlamydial Virulence and Show Species-Specific Function. MicrobiologyOpen (2014) 3:544–56. doi: 10.1002/mbo3.186
16. Favaroni A, Trinks A, Weber M, Hegemann JH, Schnee C. Pmp Repertoires Influence the Different Infectious Potential of Avian and Mammalian Chlamydia Psittaci Strains. Front Microbiol (2021) 12:656209. doi: 10.3389/fmicb.2021.656209
17. Mölleken K, Schmidt E, Hegemann JH. Members of the Pmp Protein Family of Chlamydia Pneumoniae Mediate Adhesion to Human Cells via Short Repetitive Peptide Motifs. Mol Microbiol (2010) 78:1004–17. doi: 10.1111/j.1365-2958.2010.07386.x
18. Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia RC, Tan C, et al. Chlamydia Trachomatis Polymorphic Membrane Protein D Is a Species-Common Pan-Neutralizing Antigen. Proc Natl Acad Sci USA (2006) 103:1894–9. doi: 10.1073/pnas.0508983103
19. Cui L, Qu G, Chen Y, Wu Y, Wang C, Cheng H, et al. Polymorphic Membrane Protein 20G: A Promising Diagnostic Biomarker for Specific Detection of Chlamydia Psittaci Infection. Microb Pathogene (2021) 155:104882. doi: 10.1016/j.micpath.2021.104882
20. de la Maza LM, Zhong G, Brunham RC. Update on Chlamydia Trachomatis Vaccinology. Clin Vaccine Immunol: CVI (2017) 24(4):e00543-16. doi: 10.1016/j.arr.2018.11.003
21. Tan C, Hsia RC, Shou H, Haggerty CL, Ness RB, Gaydos CA, et al. Chlamydia Trachomatis-Infected Patients Display Variable Antibody Profiles Against the Nine-Member Polymorphic Membrane Protein Family. Infect Immun (2009) 77:3218–26. doi: 10.1128/IAI.01566-08
22. Voigt A, Schöfl G, Saluz HP. The Chlamydia Psittaci Genome: A Comparative Analysis of Intracellular Pathogens. PloS One (2012) 7:e35097. doi: 10.1371/journal.pone.0035097
23. Chu J, Li X, Qu G, Wang Y, Li Q, Guo Y, et al. Chlamydia Psittaci PmpD-N Exacerbated Chicken Macrophage Function by Triggering Th2 Polarization and the TLR2/MyD88/NF-κb Signaling Pathway. Int J Mol Sci (2020) 21(6):2003. doi: 10.3390/ijms21062003
24. Wang Y, Cutcliffe LT, Skilton RJ, Ramsey KH, Thomson NR, Clarke IN. The Genetic Basis of Plasmid Tropism Between Chlamydia Trachomatis and Chlamydia Muridarum. Pathog Dis (2014) 72:19–23. doi: 10.1111/2049-632X.12175
25. Mölleken K, Becker E, Hegemann JH. The Chlamydia Pneumoniae Invasin Protein Pmp21 Recruits the EGF Receptor for Host Cell Entry. PloS Pathog (2013) 9:e1003325. doi: 10.1371/journal.ppat.1003325
26. Luczak SE, Smits SH, Decker C, Nagel-Steger L, Schmitt L, Hegemann JH. The Chlamydia Pneumoniae Adhesin Pmp21 Forms Oligomers With Adhesive Properties. J Biol Chem (2016) 291:22806–18. doi: 10.1074/jbc.M116.728915
27. Paes W, Dowle A, Coldwell J, Leech A, Ganderton T, Brzozowski A. The Chlamydia Trachomatis PmpD Adhesin Forms Higher Order Structures Through Disulphide-Mediated Covalent Interactions. PloS One (2018) 13:e0198662. doi: 10.1371/journal.pone.0198662
28. Igietseme JU, Partin J, George Z, Omosun Y, Goldstein J, Joseph K, et al. Epidermal Growth Factor Receptor and Transforming Growth Factor β Signaling Pathways Cooperate To Mediate Chlamydia Pathogenesis. Infect Immun (2020) 88(4):e00819-19. doi: 10.1128/IAI.00819-19
29. Patel AL, Chen X, Wood ST, Stuart ES, Arcaro KF, Molina DP, et al. Activation of Epidermal Growth Factor Receptor Is Required for Chlamydia Trachomatis Development. BMC Microbiol (2014) 14:277. doi: 10.1186/s12866-014-0277-4
30. Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol Cell Proteom: MCP (2014) 13:397–406. doi: 10.1074/mcp.M113.035600
31. Yamazaki T, Zaal K, Hailey D, Presley J, Lippincott-Schwartz J, Samelson LE. Role of Grb2 in EGF-Stimulated EGFR Internalization. J Cell Sci (2002) 115:1791–802. doi: 10.1242/jcs.115.9.1791
Keywords: C. psittaci, Pmp17G, EGFR, adhesion, invasion
Citation: Li X, Zuo Z, Wang Y, Hegemann JH and He C (2022) Polymorphic Membrane Protein 17G of Chlamydia psittaci Mediated the Binding and Invasion of Bacteria to Host Cells by Interacting and Activating EGFR of the Host. Front. Immunol. 12:818487. doi: 10.3389/fimmu.2021.818487
Received: 19 November 2021; Accepted: 24 December 2021;
Published: 31 January 2022.
Edited by:
Gee W. Lau, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Catherine Mary O’Connell, University of North Carolina at Chapel Hill, United StatesJieh-Juen Yu, University of Texas at San Antonio, United States
Copyright © 2022 Li, Zuo, Wang, Hegemann and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng He, Chenghe@cau.edu.cn
†These authors have contributed equally to this work and share first authorship
 Xiaohui Li
Xiaohui Li Zonghui Zuo
Zonghui Zuo Yihui Wang
Yihui Wang Johannes H. Hegemann
Johannes H. Hegemann Cheng He
Cheng He