- 1Division of Allergy- Immunology- Rheumatology, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 2Faculty of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 3Division of Rheumatology, Allergy, and Immunology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 4School of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Objective: To compare changes in bone mineral density (BMD) in rheumatoid arthritis (RA) patients receiving three-year conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD), tumor necrosis factor-α inhibitors (TNFi), and abatacept.
Methods: Patients with RA were recruited from September 2014 to February 2021. Dual-energy X-ray absorptiometry was used to measure BMD at the femoral neck (FN), total hip (TH), and lumbar spine (L1-4) at enrollment and three years later. Changes in the BMD of each regimen group were analyzed. Multiple ordinary least squares regression was used with the dependent variables to develop a model to predict the change in BMD.
Results: A total of 752 participants were enrolled and 485 completed the three-year follow-up period. Of these, 375 (Group I), 84 (Group II), and 26 (Group III) participants received csDMARDs, TNFi, and abatacept therapy, respectively. Considering both type of therapy and completion of the follow-up period, participants were divided into groups A (csDMARDs, n = 104), B (TNFi, n = 52), and C (abatacept, n = 26). Compared to baseline, BMD decreased significantly at FN (p = 0.003) and L1-4 (p = 0.002) in Group A and at L1-4 (p = 0.005) in Group B, but remained stable at all sites in Group C. In terms of regression-adjusted percent change in BMD, there was a significant difference seen at all measured sites between group C compared to both groups A and B (+0.8%, -2.7%, -1.8% at FN; +0.5%, -1.1%, -1.0% at TH; +0.8%, -2.0%, -3.5% at L1-4, respectively; all p < 0.05). Anti-osteoporosis therapy had a BMD-preserving effect in RA.
Conclusion: Compared with csDMARDs and TNFi, abatacept may have a better BMD-preserving effect in RA. Anti-osteoporosis therapy can prevent systemic bone loss irrespective of RA therapy.
Introduction
Rheumatoid arthritis (RA) is one of the most common forms of chronic inflammatory arthritis. This symmetrical polyarthritis mainly affects middle-aged females and leads to progressive joint destruction and loss of function (1). Osteoporosis is characterized by low bone mass, leading to bone fragility as well as a consequent increase in fracture risk (2). It is well known that patients with RA have an increased risk of developing osteoporosis. It has been reported that annual bone loss is greater in patients with active RA than in healthy patients (3). Compared with the general population, a two-fold increase in the frequency of osteoporosis in the spine was observed in RA patients (4). In addition, a meta-analysis revealed that the relative risk for bone fracture was higher among patients with RA than among those without RA (risk ratio 2.25) (5).
In recent years, a greater understanding of immunopathology has facilitated the development of biologic disease-modifying antirheumatic drugs (bDMARDs), which target specific components of the immune response and improve the clinical outcomes of RA (1). Inhibition of pro-inflammatory cytokines, such as tumor necrosis factor-α inhibitor (TNFi), seems to be effective in reducing disease activity and inhibiting bone loss in patients with RA (6–9). CTLA-4 Ig (Abatacept) is a fusion protein that regulates the T-cell co-stimulatory signal and is effective in attenuating disease activity and reducing joint damage in RA patients who have an inadequate response to methotrexate (10, 11). Previous research has demonstrated that abatacept can increase BMD at the femoral neck (FN) and is superior to that of other biologics in patients with RA (12).
In our previous investigation, we demonstrated that three-year biological/targeted synthetic DMARD (b/tsDMARD) treatment can prevent bone loss in RA patients and conventional synthetic DMARD (csDMARD) does not (13). However, the long-term effect in preserving BMD in patients with RA treated with TNFi or abatacept is unknown. The primary aim was to explore the BMD changes in patients with RA treated with csDMARD, TNFi, and abatacept via a three-year, real-world, observational, cohort study. The secondary aim was to investigate the synergistic effect of anti-osteoporosis therapy (AOT) on BMD in patients with RA receiving different therapies.
Methods
Study Population
This was a multi-center, three-year, real-world, observational cohort study. This study was approved by the Institutional Ethics Committee of Chang Gung Memorial Hospital, Kaohsiung (CGMHK) (approval number: 104-3530B, 106–0047 C) and Taipei Veterans General Hospital (TVGH) (approval number: 2018-04-006BC, 2020-09-013CC) and was conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants. The enrollment criteria included patients with RA who fulfilled the 1987 American College of Rheumatology (ACR) revised criteria (14) or the 2010 ACR/European League Against Rheumatism (EULAR) classification criteria (15), visited the rheumatology clinic at these two medical centers since September 2014, and received csDMARD, TNFi, or abatacept following the National Institute for Health and Care Excellence guidelines during the three-year observation period.
Bone Mineral Density
The BMD at the FN, hip (total) (TH), and lumbar vertebra 1–4 (L1–4) of each participant were measured using dual-energy X-ray absorptiometry scanners (CGMHK, Delphi A; Hologic Corp., Waltham, MA, USA; TVGH, QDR 4500A; Hologic Inc., Waltham, MA, USA) upon enrollment and three years later.
Clinical and laboratory assays of the RA patients were recorded upon enrollment, including age, sex, body height, body weight, body mass index (BMI), and the presence of rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (ACPA). RA disease activity was measured using C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and Disease Activity Score in 28 joints based on ESR (DAS28-ESR) (16). Information on current medications at the time of enrollment was collected. In addition, risk factors for fragility fractures based on the FRAX tool were recorded. Considering all of these, the 10-year probabilities of major and hip fractures were calculated and recorded. Prescription of oral systemic glucocorticoids was recorded at baseline and during the study period, and was converted to a prednisolone equivalent dose. Baseline exposure was defined as current glucocorticoid usage of > 3 months before enrollment, noting and calculating the mean daily dose within the last three months. The mean daily dose of glucocorticoids during the observation period was determined by this equation: cumulative dose of glucocorticoid prescribed ÷ cumulative dispensing days during the three-year observation period.
Statistical Analysis
Independent Student’s t-test was used to compare numerical data that exhibited normal distribution and Mann–Whitney U test was used for data that showed otherwise. Categorical variables were evaluated with chi-square test or Fisher’s exact test. Change in BMD of each participant from baseline was calculated using paired t-test. A one-way ANOVA or Kruskal-Wallis test was used to determine the significant difference between the three treatment groups in terms of parametric data. Multiple ordinary least squares (OLS) regression was used to assess the independent effects of drug treatment on the three dependent variables, controlling for age, gender, BMI (> or ≦ 24), disease duration, ACPA positivity, and baseline DAS28-ESR (> or ≦ 3.2), based on which the predicted value of the changes in BMD was calculated. Data are presented as mean ± SD or median (interquartile range, IQR) for normal and non-normal distribution datasets. The p-value was two-tailed and interpreted as significant when the value was < 0.05. Statistical analyses were performed using SPSS version 22.0 (IBM SPSS Statistics for Windows, IBM, Armonk, New York, USA).
Results
Patients
A total of 752 participants were registered from September 2014 to February 2021, but only 485 participants completed the three-year follow-up period by the end of February 2021. The disposition of the participants is illustrated in Figure 1; 188 patients lost to follow-up or followed less than 3 years since enrollment, while 79 patients received TNFi or abatacept less than 1 year or switched to an another biologic agent with a different mechanism of action during observation period.
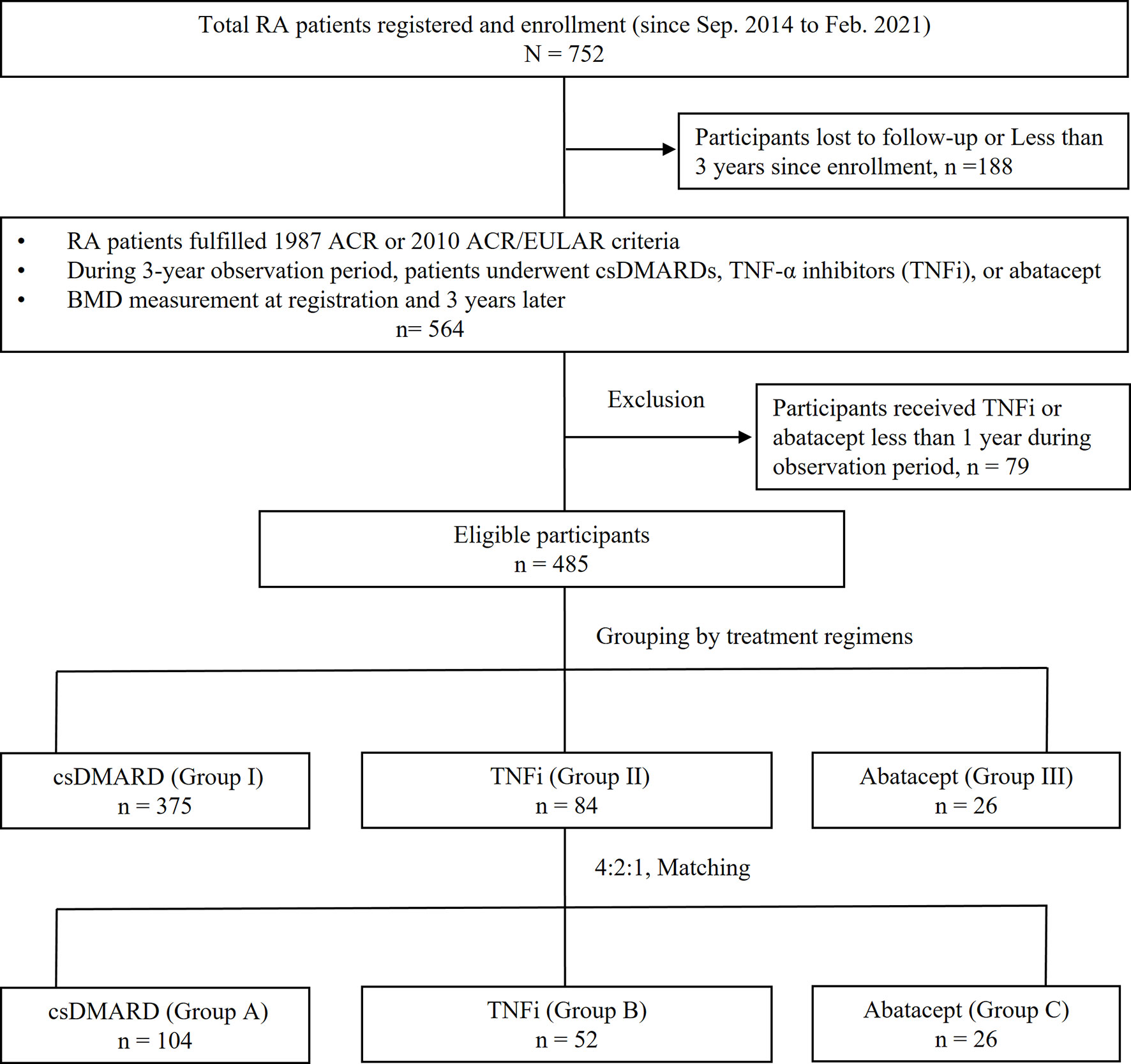
Figure 1 Disposition of participants and grouping. RA, rheumatoid arthritis; ACR, American College of Rheumatology; EULAR, European League Against Rheumatism collaborative initiative; conventional synthetic DMARD (csDMARD), including methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, cyclosporine; TNFi, including etanercept, adalimumab, golimumab; BMD, bone mineral density.
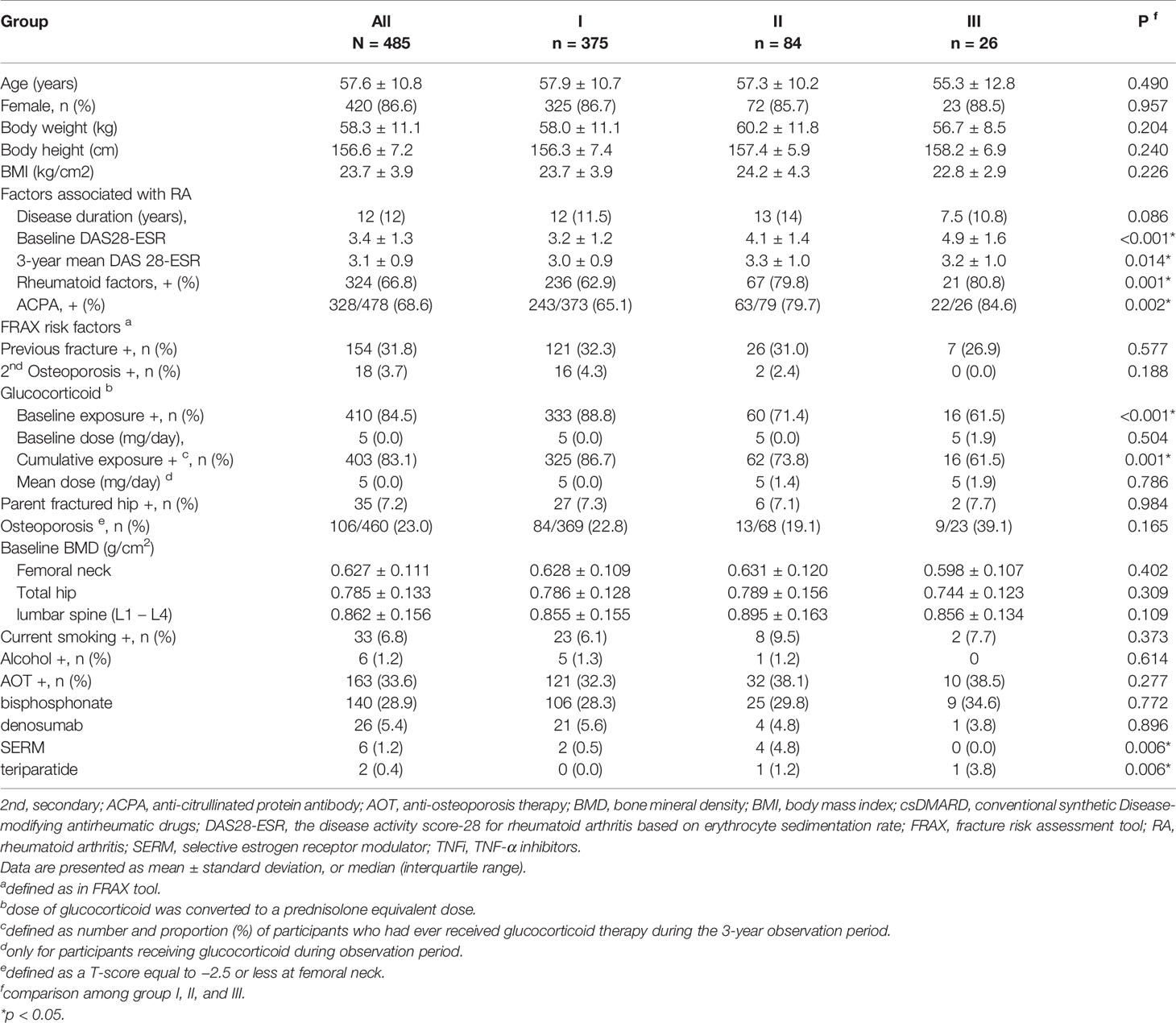
The demographics and clinical characteristics of the enrolled patients are shown in Table 1. Eligible participants were grouped into group I (n = 375, csDMARD), II (n = 84, TNFi, including etanercept, adalimumab, and golimumab), and III (n = 26, abatacept) or by regimens used during the observation period. Mean age and sex were not significantly different between the groups. The characteristics of RA disease entities were not obviously different among groups except for baseline disease activity (DAS28-ESR) (p < 0.001), three-year mean DAS28-ESR (p = 0.014), rate of positive RF (p = 0.001), rate of positive ACPA (p = 0.002), baseline glucocorticoid exposure (p < 0.001), and rate of cumulative exposure to glucocorticoids (p = 0.001). In addition, baseline BMD and risk factors for fragility fractures in the FRAX tool were comparable among the groups.
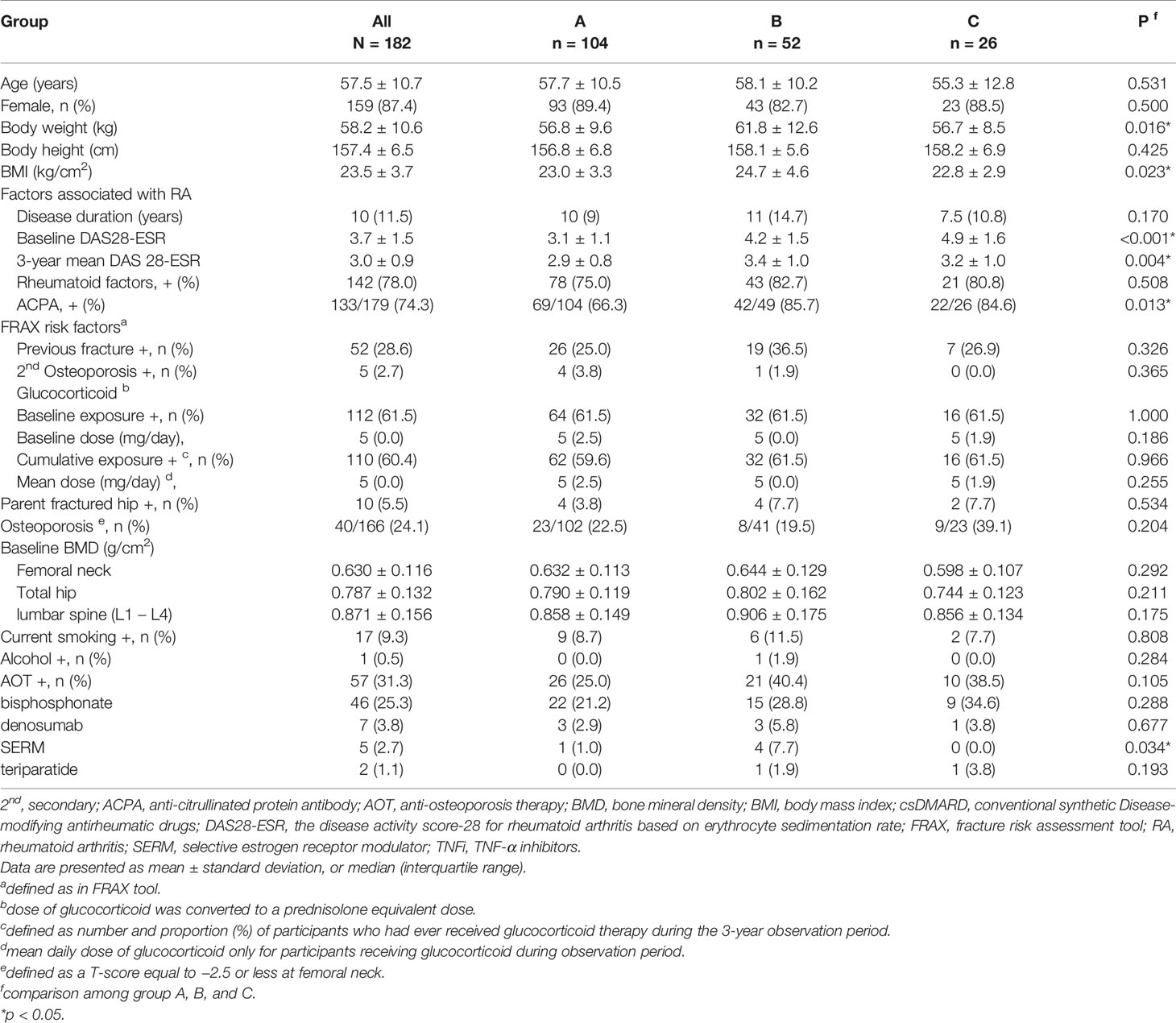
Matching the rate of glucocorticoid use across groups I to III to a ratio of 4:2:1, the groups were subdivided into groups A (n = 104, csDMARD), B (n = 52, TNFi), and C (n = 26, abatacept), respectively. The demographic and clinical characteristics of the participants are shown in Table 2. Mean age and sex were not significantly different between the groups. Body mass index was significantly different (p = 0.023). The characteristics of RA disease entities were not obviously different among groups except for baseline disease activity (DAS28-ESR) (p < 0.001), three-year mean DAS28-ESR (p = 0.004), and rate of positive ACPA (p = 0.013). The baseline BMD and risk factors for fragility fracture in the FRAX tool were comparable among groups after matching. Fifty-seven patients received AOT; 44, 5, 4, and 1 patient treated with bisphosphonate, denosumab, selective estrogen receptor modulators (SERM), and teriparatide alone, while one patient in group B received denosumab and teriparatide, another one patient in group B received bisphosphonate and SERM, and one patient in group C received bisphosphonate and denosumab during observation period. There was no significant difference in the percentage of patients on bisphosphonate, denosumab, and teriparatide between different groups, while more patients in group B received SERM when compared to those in other groups.
Comparison of BMD Changes With Baseline After Matching
Comparing baseline values of all participants, BMD at FN and L1-4 significantly decreased in group A (p = 0.003 and 0.002, respectively) (Table 3 and Figure 2A). Although BMD of L1-4 significantly decreased in group B (p = 0.005), there were no significant changes seen at the three measured sites in group C. Changes in BMD in participants who received AOT are shown in Table 3 and Figures 2B, C. BMD at FN, TH, and L1-4 in participants who received AOT remained stable in the three groups. An exemption to this was BMD at FN, which significantly increased compared to baseline in group C (p = 0.012). Participants in group A and without AOT showed significant declines in BMD at FN, TH, and L1-4 (p = 0.003, 0.027, and < 0.001, respectively) (Figure 2C). BMD of group B participants without AOT revealed a significant decline at–L1-4 (p = 0.010). BMD of group C participants without AOT remained stable at FN, TH, and L1-4 (p = 0.530, p = 0.888, and p = 0.741, respectively (Figure 2C).
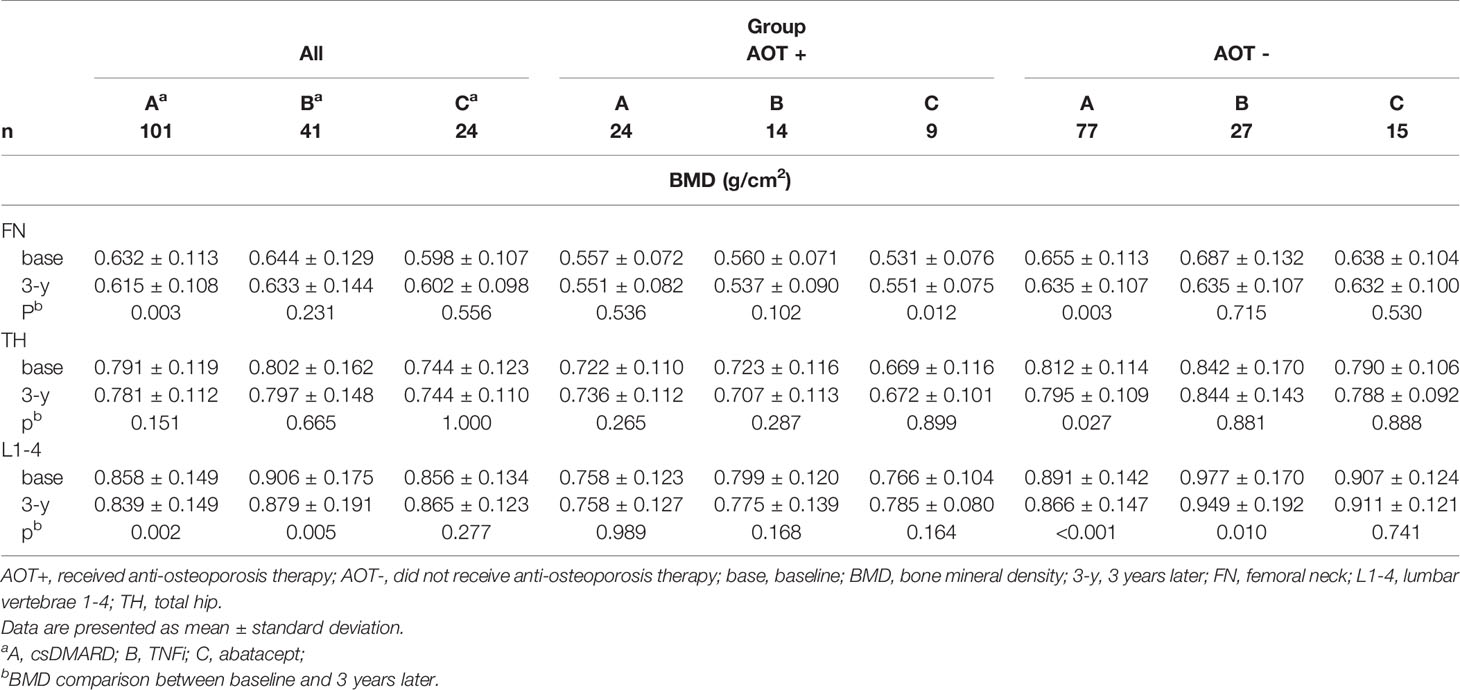
Table 3 Comparison of BMD between baseline and 3 years later in each treatment group, after matching.
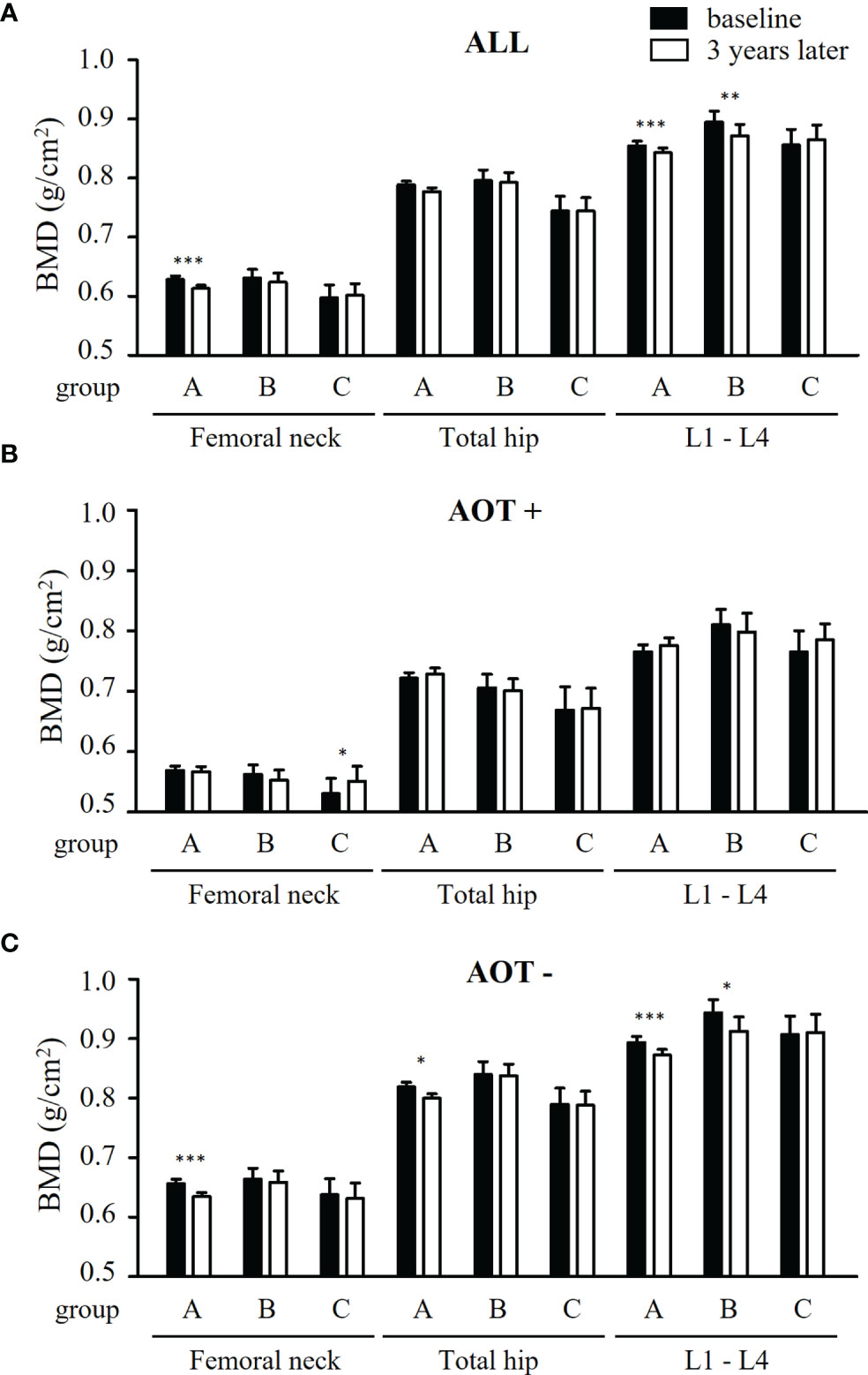
Figure 2 Comparison of BMD at baseline and 3 years later in all patients and patients with and without anti-osteoporosis therapy, after matching. (A) Differences of bone mineral density (BMD) at femoral neck, total hip, and lumbar spine 1-4 (L1-4) between baseline (black bars) and 3 years later (white bars) a in patients receiving conventional synthetic disease-modifying antirheumatic drugs (group A), TNF-α inhibitors (group B), or abatacept (group C). (B, C) Differences of BMD at three measured sites between baseline and 3 years later in group A - C, combined with anti-osteoporosis therapy (AOT) (B) or not (C). *p < 0.05; **p < 0.01; ***p < 0.005.
Differences in Percent Change of BMD Among Groups After Matching
After three years, percent changes in BMD (ΔBMD%) at FN and TH were not significantly different among groups except at L1-4 (p = 0.026) after matching in all participant groups (Table 4 and Figure 3A). ΔBMD% at L1-4 in group C was significantly different from that in group A (median [interquartile range], +3.2 [7.6] % vs. -2.0 [7.7] %, p = 0.007) and group B (+3.2 [7.6] % vs. -2.5 [8.6] %, p = 0.002), respectively. ΔBMD% at FN, TH, and L1-4 were not obviously different among participants with AOT (p = 0.083, 0.356, 0.232, respectively) or without (p = 0.246, 0.478, 0.068, respectively) (Table 4). However, ΔBMD% at FN (+4.1 [7.0] % vs. -4.0 [16.9] %, p = 0.033) and L1-4 (+3.2 [6.6] % vs. -4.3 [11.7] %, p = 0.020) in group C were significantly different compared to group B participants with AOT. Meanwhile, ΔBMD% at L1-4 in group C was significantly different from group A (+2.8 [8.1] % vs. -2.4 [7.3] %, p = 0.033) or group B (+2.8 [8.1] % vs. -1.8 [9.1] %, p = 0.034) in participants without AOT.
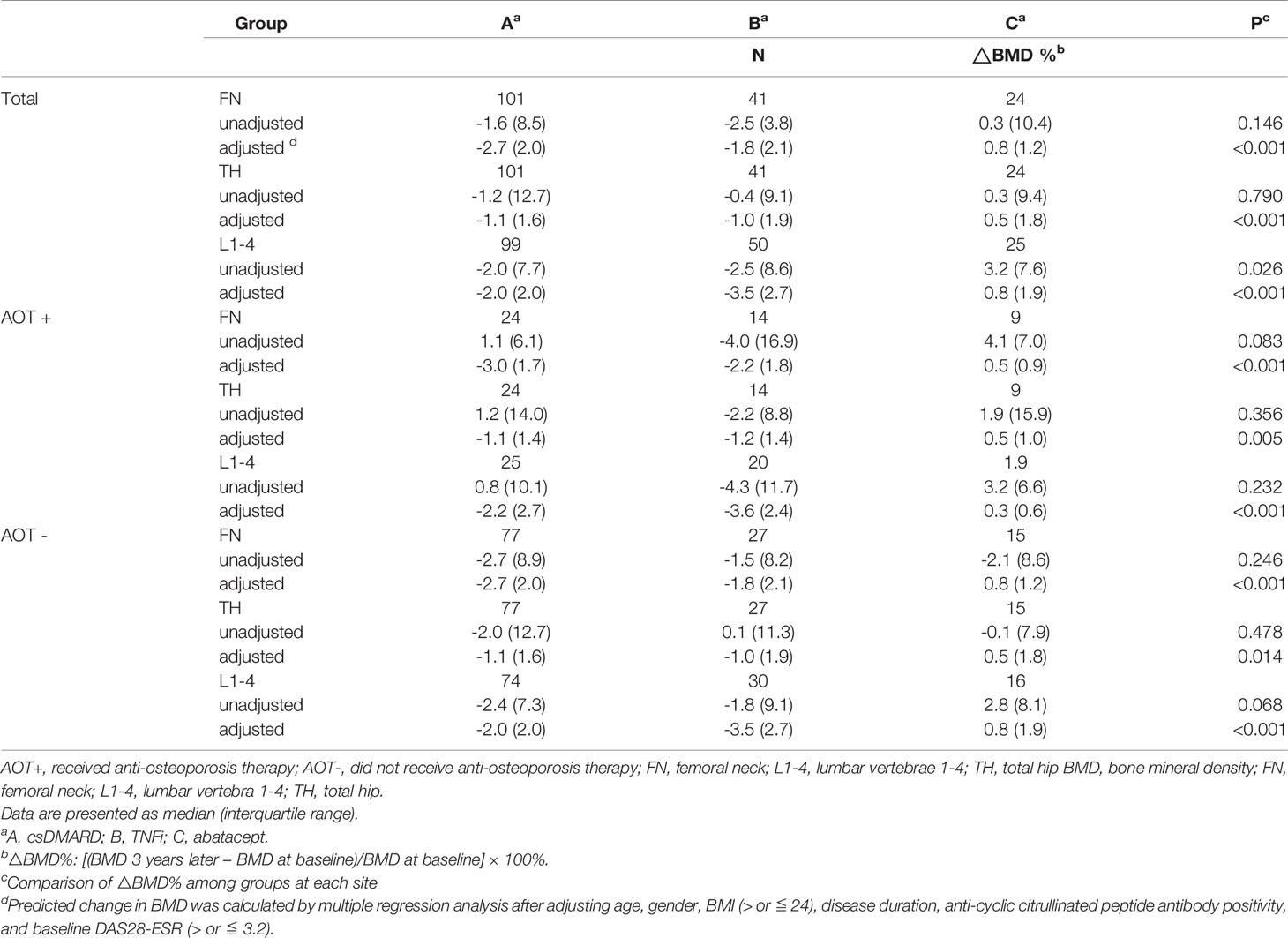
Table 4 Regression-adjusted percentage change in BMD from baseline in each treatment group, after matching.
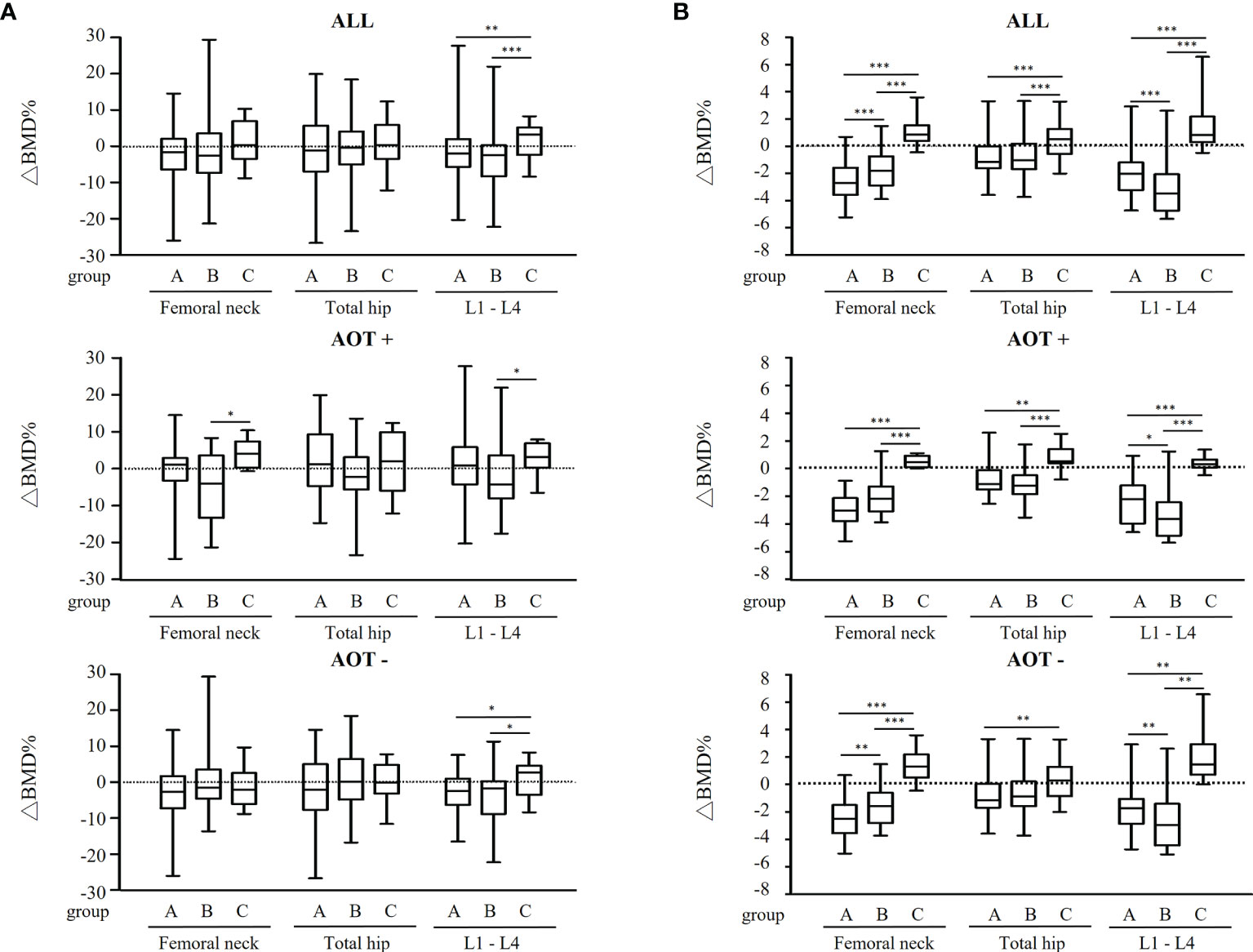
Figure 3 Regression-adjusted percentage change in BMD from baseline in each treatment group, after matching in all participants and participants with or without anti-osteoporosis therapy. Unadjusted (A) and regression-adjusted (B) percentage of change in BMD at femoral neck, total hip, and lumbar spine 1-4 (L1-4) after 3 years in patients receiving conventional synthetic disease-modifying antirheumatic drugs (group A), TNF-α inhibitors (group B), or abatacept (group C), combined with anti-osteoporosis therapy (AOT) or not. Box-and-whisker plots showed the median, interquartile range, and extreme values. *p < 0.05; **p < 0.01; ***p < 0.005.
Next, predicted change in BMD was calculated by the multiple regression analysis after adjusting age, gender, BMI, disease duration, ACPA positivity, and baseline DAS28-ESR (Figure 3B and Table 4). A decline in BMD in group A and B (-2.7 [2.0] % and -1.8 [2.1] % at FN, -1.1 [1.6] % and -1.0 [1.9] % at TH, -2.0 [2.0] % and -3.5 [2.7] % at L1-4, respectively), but BMD in group C remained (+0.8 [1.2] %, +0.5 [1.8] %, and +0.8 [1.9] % at FN, TH, and L1-4, respectively) when compared to 3 years earlier. Regardless of AOT, regression-adjusted ΔBMD% at all measured sites in group C was significantly different from that in group A and B (all p < 0.05), except regression-adjusted ΔBMD% at TH were not significantly different among group B and C participants without AOT (p = 0.088). Regression-adjusted ΔBMD% at FN and L1-4 in group B was significantly different from that in group A (both p < 0.05), while the regression-adjusted ΔBMD% at FN became less statistically different among participants with AOT (p = 0.053).
Discussion
Current investigation demonstrated that AOT can prevent bone loss irrespective of regimen used, while in patients without AOT, participants who received csDMARD had the most obvious bone loss at all sites. Participants who received abatacept therapy demonstrated stable BMD at all sites, irrespective taking AOT therapy or not. This investigation revealed that abatacept may have a better systemic bone loss protection effect in RA patients than the other two regimens.
The b/tsDMARD not only demonstrated better control of disease activity, but also showed a better bony erosion protection effect than csDMARD in RA patients (17–26). Our previous investigation revealed that long-term b/tsDMARD therapy demonstrated a potentially beneficial effect on the protection of systemic bone loss (13). However, previous investigations regarding the effect of biologics on the prevention of systemic bone loss in RA have only focused on changes in bone turnover markers (18–21), over a short-term observation period (25, 26), and with a lack of an adequate control group (25, 27). In addition, the long-term effects of bDMARDs with different mechanisms of action on systemic bone loss in patients with RA remain obscure.
CTLA-4 is a negative regulator that inhibits antigen-specific immune responses after T-cell activation by interfering with the interaction of CD28 on T-cells and CD80/86 on antigen-presenting cells, including dendritic cells, macrophages, and B-cells (28, 29). Axmann et al. found that CTLA-4 directly binds to osteoclast precursor cells and dose-dependently inhibits receptor activator of nuclear factor-κB ligand (RANKL)-mediated osteoclastogenesis in an animal model (30). However, in human studies, abatacept demonstrated a controversial effect on the protective effect of bone loss in RA patients (12, 31).
Pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1, play a key role in the pathogenesis of RA and have been approved as treatment targets (7). The interaction between inflammation and osteoporosis has been previously described (32). In synovial fibroblasts, TNF-α upregulates Dickkopf-1 (DKK1), a negative regulator of Wnt signaling, leading to suppression of bone formation (8). In addition, TNF-α directly inhibits osteoblast differentiation and function (9). Therefore, the inhibition of pro-inflammatory cytokines seems to be an effective bone-protecting principle in patients with RA. Indeed, previous studies have demonstrated that TNFi is potentially beneficial for bone loss (17–26). However, the results of these studies were based on their influence on bone turnover markers (18–21). Furthermore, a significant increase in serum parathyroid hormone was found in RA patients undergoing TNFi treatment, which might promote bone resorption and blunt the anti-osteoporotic effect of TNFi (33). Taking together, whether or not there is a substantial improvement in BMD in RA patients treated with TNFi remains controversial (22–24).
As TNFi and abatacept were the first two regimens launched and are the most commonly prescribed biologics in Taiwan, we compared the protective effect to systemic bone loss of these two biologics with csDMARD. The current investigation revealed that abatacept therapy arrested systemic bone loss at FN, TH, and L1-4 in a three-year follow-up period. In addition, it seems that abatacept exhibited bone loss protective effect whether on AOT or not in RA patients. Furthermore, in terms of percent change of BMD, compared with TNFi, abatacept demonstrated a superior effect at FN and L1-4 and at L1-4 in patients who were taking AOT or not taking AOT, respectively.
It remains unclear why abatacept had a more favorable effect on BMD than csDMARD or TNFi. Our previous investigation demonstrated that adequate control of disease activity can protect bone loss in RA patients (34). Meanwhile, it has been demonstrated that positive ACPA status is associated with a differential treatment response to abatacept, but not TNFi (35). Furthermore, abatacept treatment showed differential efficacy in RA patients with higher ACPA titers (36). Previous studies reported that RA patients positive for ACPA had a lower BMD and a higher 10-year probability of fracture as evaluated by FRAX® (37, 38). Patients with RA receiving abatacept therapy demonstrated a decline in ACPA titers or ACPA seronegative conversion effects (39). In addition, compared with group I or II, the rate of ACPA positivity was significantly higher in the abatacept group (group III) (p = 0.002) in our cohort. Based on the aforementioned findings, we hypothesize that adequate control of disease activity (DAS28-ESR) between baseline and during the observation period (4.9 ± 1.6 and 3.2 ± 1.0, p < 0.001) in the abatacept group and higher rate of ACPA in group III could partly explain the discrepancy in the effect on BMD among the groups. It is also well known that low BMI is one of risk factors of systemic bone loss or osteoporosis (40). In current investigation, abatacept group, although had lower BMI, increased more BMD than other groups after 3 years. After adjusting age, gender, BMI, disease duration, ACPA positivity, and baseline DAS28-ESR, predicted change in BMD at all measured sites in group C remained better than in other groups, suggesting that the better effect of abatacept on prevention of systemic bone loss in RA when compared to other comparison regimens.
As the current study is a real-world investigation, we did not exclude participants who received AOT during the observation period to elucidate the interaction of DMARD therapy and AOT in terms of bone protective effects. Participants who did not receive AOT had significant bone loss at all sites in group A and at the lumbar vertebrae in group B. AOT had a protective effect against bone loss in all groups at all sites. These results suggest that AOT plays the most important role in bone loss protection in RA patients receiving either csDMARD or biologics.
A strength of the current investigation is that it is a longitudinal, real-world, observational, registry, cohort study. We measured and recorded the characteristics of the disease entity at baseline and serial disease activity, which could potentially influence BMD changes during the study period. In addition, most previous studies were single-arm studies without an adequate control group (25, 27). Our initial investigation revealed a significant difference in the rate of glucocorticoid use among groups. As glucocorticoid use is a well-known risk factor for bone loss, we performed a 4:2:1 matching for glucocorticoid use to exclude the confounding effect of glucocorticoids, which had not been done in previous investigations. Furthermore, to adjust confounders of osteoporosis, multiple regression analysis was used to develop a model to predict the change in BMD. Finally, this study is the first investigation to explore the long-term systemic bone loss protective effect among DMARDs with different mechanisms of action to elucidate the effects of biologics on RA patients.
The current study has some limitations. As it was a real-world study, we did not exclude participants who had already received biologics before enrollment. Hence, we could not exclude the residual effect of previous medications on BMD after enrollment. However, we excluded participants with biologic switching during the observation period to avoid additional confounding effects. In addition, we did not compare the bone loss protective effect between abatacept and biologics other than TNFi, eg. tocilizumab, rituximab. So far, we could not know which biologics is the best one to prevent bone loss in RA patients. Fracture prevention is a hard outcome to measure in osteoporosis studies, and we could not compare the effect of fracture prevention among the treatment groups owing to the short duration and relatively small sample size of our study.
Conclusion
RA patients receiving long-term abatacept illustrated a better bone loss protective effect than patients receiving csDMARD or TNFi. Anti-osteoporosis therapy has a vital protective effect on bone loss irrespective of regimens for RA therapy used. Further studies are needed to clarify whether abatacept or other biologics could prevent fragility fractures in patients with RA.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Chang Gung Memorial Hospital, Kaohsiung and Taipei Veterans General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study concept: M-HC and T-TC. Study design: M-HC, S-FY, and T-TC. Data analysis: M-HC, S-FY, T-TC. M-HC, S-FY, J-FC, W-SC, T-LL, C-TC, C-YH, H-ML, Y-CC, C-YT, and T-TC were responsible for interpretation of the data and for drafting and revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants CMRPG8F1111 and CMRPG8K0441 from Chang Gung Memorial Hospital (https://www.cgmh.org.tw/), which sponsored the cost of data collection, gathering, processing, and publication. The funder had no role in the study design, data analysis, decision to publish, nor manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACPA, anti-cyclic citrullinated peptide antibodies; ACR, American College of Rheumatology; AOT, anti-osteoporosis therapy; bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; CRP, C-reactive protein; csDMARD, conventional synthetic DMARD; DAS28-ESR, Disease Activity Score in 28 joints based on erythrocyte sedimentation rate; DKK1, Dickkopf-1; EULAR, European League Against Rheumatism; FN, femoral neck; FRAX, fracture risk assessment tool; L1–4, lumbar vertebra; RA, rheumatoid arthritis; RANKL, receptor activator of nuclear factor-κB ligand; RF, rheumatoid factor; TH, total hip; TNFi, tumor necrosis factor-α inhibitor; tsDMARD, targeted synthetic DMARD.
References
1. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid Arthritis. Nat Rev Dis Primers (2018) 4:18001. doi: 10.1038/nrdp.2018.1
2. Delmas PD. Treatment of Postmenopausal Osteoporosis. Lancet (2002) 359:2018–26. doi: 10.1016/S0140-6736(02)08827-X
3. Gough AK, Lilley J, Eyre S, Holder RL, Emery P. Generalised Bone Loss in Patients With Early Rheumatoid Arthritis. Lancet (1994) 344:23–7. doi: 10.1016/S0140-6736(94)91049-9
4. Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK. Bone Mineral Density and Frequency of Osteoporosis in Female Patients With Rheumatoid Arthritis: Results From 394 Patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum (2000) 43:522–30. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y
5. Xue AL, Wu SY, Jiang L, Feng AM, Guo HF, Zhao P. Bone Fracture Risk in Patients With Rheumatoid Arthritis: A Meta-Analysis. Med (Baltimore) (2017) 96:e6983. doi: 10.1097/MD.0000000000006983
6. Zhao B. TNF And Bone Remodeling. Curr Osteoporos Rep (2017) 15:126–34. doi: 10.1007/s11914-017-0358-z
7. Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, et al. Biologics for Rheumatoid Arthritis: An Overview of Cochrane Reviews. Sao Paulo Med J (2010) 128:309–10. doi: 10.1590/S1516-31802010000500013
8. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a Master Regulator of Joint Remodeling. Nat Med (2007) 13:156–63. doi: 10.1038/nm1538
9. Karmakar S, Kay J, Gravallese EM. Bone Damage in Rheumatoid Arthritis: Mechanistic Insights and Approaches to Prevention. Rheum Dis Clin North Am (2010) 36:385–404. doi: 10.1016/j.rdc.2010.03.003
10. Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Effects of Abatacept in Patients With Methotrexate-Resistant Active Rheumatoid Arthritis: A Randomized Trial. Ann Intern Med (2006) 144:865–76. doi: 10.7326/0003-4819-144-12-200606200-00003
11. Kremer JM, Genant HK, Moreland LW, Russell AS, Emery P, Abud-Mendoza C, et al. Results of a Two-Year Followup Study of Patients With Rheumatoid Arthritis Who Received a Combination of Abatacept and Methotrexate. Arthritis Rheum (2008) 58:953–63. doi: 10.1002/art.23397
12. Tada M, Inui K, Sugioka Y, Mamoto K, Okano T, Koike T. Abatacept Might Increase Bone Mineral Density at Femoral Neck for Patients With Rheumatoid Arthritis in Clinical Practice: AIRTIGHT Study. Rheumatol Int (2018) 38:777–84. doi: 10.1007/s00296-017-3922-z
13. Chen JF, Hsu CY, Yu SF, Ko CH, Chiu WC, Lai HM, et al. The Impact of Long-Term Biologics/Target Therapy on Bone Mineral Density in Rheumatoid Arthritis: A Propensity Score-Matched Analysis. Rheumatol (Oxford) (2020) 59:2471–80. doi: 10.1093/rheumatology/kez655
14. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum (1988) 31:315–24. doi: 10.1002/art.1780310302
15. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum (2010) 62:2569–81. doi: 10.1002/art.27584
16. Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified Disease Activity Scores That Include Twenty-Eight-Joint Counts. Development and Validation in a Prospective Longitudinal Study of Patients With Rheumatoid Arthritis. Arthritis Rheum (1995) 38:44–8. doi: 10.1002/art.1780380107
17. Dimitroulas T, Nikas SN, Trontzas P, Kitas GD. Biologic Therapies and Systemic Bone Loss in Rheumatoid Arthritis. Autoimmun Rev (2013) 12:958–66. doi: 10.1016/j.autrev.2013.03.015
18. Vis M, Wolbink GJ, Lodder MC, Kostense PJ, van de Stadt RJ, de Koning MH, et al. Early Changes in Bone Metabolism in Rheumatoid Arthritis Patients Treated With Infliximab. Arthritis Rheum (2003) 48:2996–7. doi: 10.1002/art.11292
19. Chopin F, Garnero P, le Henanff A, Debiais F, Daragon A, Roux C, et al. Long-Term Effects of Infliximab on Bone and Cartilage Turnover Markers in Patients With Rheumatoid Arthritis. Ann Rheum Dis (2008) 67:353–7. doi: 10.1136/ard.2007.076604
20. Ziolkowska M, Kurowska M, Radzikowska A, Luszczykiewicz G, Wiland P, Dziewczopolski W, et al. High Levels of Osteoprotegerin and Soluble Receptor Activator of Nuclear Factor Kappa B Ligand in Serum of Rheumatoid Arthritis Patients and Their Normalization After Anti-Tumor Necrosis Factor Alpha Treatment. Arthritis Rheum (2002) 46:1744–53. doi: 10.1002/art.10388
21. Yasunori K, Masaaki T, Tetsuyuki N, Hayato K, Akira N. Reduction of Urinary Levels of Pyridinoline and Deoxypyridinoline and Serum Levels of Soluble Receptor Activator of NF-kappaB Ligand by Etanercept in Patients With Rheumatoid Arthritis. Clin Rheumatol (2008) 27:1093–101. doi: 10.1007/s10067-008-0870-8
22. Vis M, Havaardsholm EA, Haugeberg G, Uhlig T, Voskuyl AE, van de Stadt RJ, et al. Evaluation of Bone Mineral Density, Bone Metabolism, Osteoprotegerin and Receptor Activator of the NFkappaB Ligand Serum Levels During Treatment With Infliximab in Patients With Rheumatoid Arthritis. Ann Rheum Dis (2006) 65:1495–9. doi: 10.1136/ard.2005.044198
23. Marotte H, Pallot-Prades B, Grange L, Gaudin P, Alexandre C, Miossec P. A 1-Year Case-Control Study in Patients With Rheumatoid Arthritis Indicates Prevention of Loss of Bone Mineral Density in Both Responders and Nonresponders to Infliximab. Arthritis Res Ther (2007) 9:R61. doi: 10.1186/ar2219
24. Wijbrandts CA, Klaasen R, Dijkgraaf MG, Gerlag DM, van Eck-Smit BL, Tak PP. Bone Mineral Density in Rheumatoid Arthritis Patients 1 Year After Adalimumab Therapy: Arrest of Bone Loss. Ann Rheum Dis (2009) 68:373–6. doi: 10.1136/ard.2008.091611
25. Lange U, Teichmann J, Muller-Ladner U, Strunk J. Increase in Bone Mineral Density of Patients With Rheumatoid Arthritis Treated With Anti-TNF-Alpha Antibody: A Prospective Open-Label Pilot Study. Rheumatol (Oxford) (2005) 44:1546–8. doi: 10.1093/rheumatology/kei082
26. Vis M, Voskuyl AE, Wolbink GJ, Dijkmans BA, Lems WF, Group OS. Bone Mineral Density in Patients With Rheumatoid Arthritis Treated With Infliximab. Ann Rheum Dis (2005) 64:336–7. doi: 10.1136/ard.2003.017780
27. Eekman DA, Vis M, Bultink IE, Kuik DJ, Voskuyl AE, Dijkmans BA, et al. Stable Bone Mineral Density in Lumbar Spine and Hip in Contrast to Bone Loss in the Hands During Long-Term Treatment With Infliximab in Patients With Rheumatoid Arthritis. Ann Rheum Dis (2011) 70:389–90. doi: 10.1136/ard.2009.127787
28. Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) Bind With Similar Avidities But Distinct Kinetics to CD28 and CTLA-4 Receptors. Immunity (1994) 1:793–801. doi: 10.1016/S1074-7613(94)80021-9
29. Greene JL, Leytze GM, Emswiler J, Peach R, Bajorath J, Cosand W, et al. Covalent Dimerization of CD28/CTLA-4 and Oligomerization of CD80/CD86 Regulate T Cell Costimulatory Interactions. J Biol Chem (1996) 271:26762–71. doi: 10.1074/jbc.271.43.26762
30. Axmann R, Herman S, Zaiss M, Franz S, Polzer K, Zwerina J, et al. CTLA-4 Directly Inhibits Osteoclast Formation. Ann Rheum Dis (2008) 67:1603–9. doi: 10.1136/ard.2007.080713
31. Suzuki T, Nakamura Y, Kato H. Effects of Denosumab on Bone Metabolism and Bone Mineral Density With Anti-TNF Inhibitors, Tocilizumab, or Abatacept in Osteoporosis With Rheumatoid Arthritis. Ther Clin Risk Manag (2018) 14:453–59. doi: 10.2147/TCRM.S156350
32. Jones D, Glimcher LH, Aliprantis AO. Osteoimmunology at the Nexus of Arthritis, Osteoporosis, Cancer, and Infection. J Clin Invest (2011) 121:2534–42. doi: 10.1172/JCI46262
33. Adami G, Orsolini G, Adami S, Viapiana O, Idolazzi L, Gatti D, et al. Effects of TNF Inhibitors on Parathyroid Hormone and Wnt Signaling Antagonists in Rheumatoid Arthritis. Calcif Tissue Int (2016) 99:360–4. doi: 10.1007/s00223-016-0161-3
34. Hsu CY, Chen JF, Su YJ, Chen YC, Lai HM, Yu SF, et al. Time-Averaged Disease Activity of Rheumatoid Arthritis Associated With Long-Term Bone Mineral Density Changes. Ther Adv Chronic Dis (2020) 11:2040622320981517. doi: 10.1177/2040622320981517
35. Harrold LR, Litman HJ, Connolly SE, Kelly S, Hua W, Alemao E, et al. Effect of Anticitrullinated Protein Antibody Status on Response to Abatacept or Antitumor Necrosis Factor-Alpha Therapy in Patients With Rheumatoid Arthritis: A US National Observational Study. J Rheumatol (2018) 45:32–9. doi: 10.3899/jrheum.170007
36. Sokolove J, Schiff M, Fleischmann R, Weinblatt ME, Connolly SE, Johnsen A, et al. Impact of Baseline Anti-Cyclic Citrullinated Peptide-2 Antibody Concentration on Efficacy Outcomes Following Treatment With Subcutaneous Abatacept or Adalimumab: 2-Year Results From the AMPLE Trial. Ann Rheum Dis (2016) 75:709–14. doi: 10.1136/annrheumdis-2015-207942
37. Guler H, Turhanoglu AD, Ozer B, Ozer C, Balci A. The Relationship Between Anti-Cyclic Citrullinated Peptide and Bone Mineral Density and Radiographic Damage in Patients With Rheumatoid Arthritis. Scand J Rheumatol (2008) 37:337–42. doi: 10.1080/03009740801998812
38. Cheng TT, Yu SF, Su FM, Chen YC, Su BY, Chiu WC, et al. Anti-CCP-Positive Patients With RA Have a Higher 10-Year Probability of Fracture Evaluated by FRAX(R): A Registry Study of RA With Osteoporosis/Fracture. Arthritis Res Ther (2018) 20:16. doi: 10.1186/s13075-018-1515-1
39. Jansen D, Emery P, Smolen JS, Westhovens R, Le Bars M, Connolly SE, et al. Conversion to Seronegative Status After Abatacept Treatment in Patients With Early and Poor Prognostic Rheumatoid Arthritis is Associated With Better Radiographic Outcomes and Sustained Remission: Post Hoc Analysis of the AGREE Study. RMD Open (2018) 4:e000564. doi: 10.1136/rmdopen-2017-000564
Keywords: rheumatoid arthritis, osteoporosis, bone mineral density, tumor necrosis factor-α inhibitors, abatacept
Citation: Chen M-H, Yu S-F, Chen J-F, Chen W-S, Liou T-L, Chou C-T, Hsu C-Y, Lai H-M, Chen Y-C, Tsai C-Y and Cheng T-T (2021) Different Effects of Biologics on Systemic Bone Loss Protection in Rheumatoid Arthritis: An Interim Analysis of a Three-Year Longitudinal Cohort Study. Front. Immunol. 12:783030. doi: 10.3389/fimmu.2021.783030
Received: 25 September 2021; Accepted: 03 December 2021;
Published: 20 December 2021.
Edited by:
Maria I. Bokarewa, University of Gothenburg, SwedenReviewed by:
Francesca Romana Spinelli, Sapienza University of Rome, ItalyOmbretta Viapiana, University of Verona, Italy
Copyright © 2021 Chen, Yu, Chen, Chen, Liou, Chou, Hsu, Lai, Chen, Tsai and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tien-Tsai Cheng, tiantsai0919@gmail.com
†These authors share first authorship
 Ming-Han Chen
Ming-Han Chen Shan-Fu Yu
Shan-Fu Yu Jia-Feng Chen3,4
Jia-Feng Chen3,4 Wei-Sheng Chen
Wei-Sheng Chen Chang-Youh Tsai
Chang-Youh Tsai Tien-Tsai Cheng
Tien-Tsai Cheng