- 1Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, West China Hospital, Sichuan University, Chengdu, China
Background: Immune-mediated necrotizing myopathy (IMNM) is characterized by markedly elevated creatinine kinase and histologically scattered necrotic muscle fibers and generally associated with autoantibodies against signal recognition particle (SRP) or 3-hydroxy-3-methylglutaryl-coA-reductase (HMGCR). Poor clinical response to conventional therapies and relapses commonly occur in severe cases. Anti-B-cell therapies have been used in refractory/relapsing cases.
Methods: The characteristics of a patient with IMNM associated with anti-SRP antibodies including physical examination, laboratory tests, and disease activity assessment were evaluated. Conventional therapy, belimumab treatment schedule, and follow-up data were recorded. Medical records of IMNM patients treated in our department from September 2014 to June 2021 were reviewed to evaluate the efficacy and safety of anti-B-cell therapy for anti-SRP IMNM. A literature review of patients with anti-SRP IMNM treated with anti-B-cell therapies was performed.
Results: We describe a case of a 47-year-old woman with IMNM associated with anti-SRP antibodies who relapsed twice after conventional therapy but showed good response and tolerance to belimumab at 28 weeks follow-up. In this review, three patients from our department were treated with rituximab. Two of the three patients rapidly improved after treatment. Twenty patients and five retrospective studies were included in the literature review. All patients were administered rituximab as an anti-B-cell drug.
Conclusion: Despite a lack of rigorous clinical trials, considerable experience demonstrated that anti-B-cell therapy might be effective for patients with IMNM associated with anti-SRP antibodies. Belimumab in association with steroids might be an encouraging option for refractory/relapsing cases.
Introduction
Immune-mediated necrotizing myopathy (IMNM), also known as necrotizing autoimmune myopathy, is characterized by markedly elevated creatinine kinase and histologically scattered necrotic muscle fibers and generally associated with autoantibodies against signal recognition particle (SRP) or 3-hydroxy-3-methylglutaryl-coA-reductase (HMGCR) (1). Poor clinical response to conventional therapies and relapses commonly occur in severe cases.
In previous reports, anti-B-cell therapy, especially rituximab (RTX), an anti-monoclonal CD20 antibody, has been used in IMNM (2–12). In some cases, patients benefited from RTX, while in other cases, patients showed poor response or died from complications, such as infection (2–12).
Belimumab is a human monoclonal antibody targeting B-cell-activating factor (BAFF). Belimumab has been used in several rheumatoid diseases, including systemic lupus erythematosus, Sjogren’s syndrome, systemic sclerosis, and antiphospholipid syndrome (13–16).
In this study, we report a case of a patient with anti-SRP IMNM who relapsed twice after conventional therapy but showed a good response and tolerance to belimumab. We also reviewed patients with anti-SRP IMNM who received anti-B-cell therapy in our department and in the literature.
Patients and Methods
Case Record
Patient characteristics, including medical history, physical examination, laboratory tests, and radiological examinations, were recorded. Disease activity was assessed using the Myositis Disease Activity Assessment Visual Analogue Scale (MYOACT), Myositis Intention-to-Treat Activity Index (MITAX), 36-item Short Form Health Survey Physical Component Score (SF-36 PCS), 36-item Short Form Health Survey Mental Component Score (SF-36 MCS), and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Conventional therapy, belimumab treatment schedule, and follow-up data were recorded.
Retrospective Review of Patients With Anti-SRP IMNM Treated With Anti-B-Cell Therapies at a Single Center
We retrospectively reviewed all the medical records of patients in our institution between September 2014 and June 2021. Patients treated with anti-B-cell therapy for anti-SRP IMNM were included. All the subjects meet the 119th ENMC or 224th ENMC classification criteria for IMNM (1, 17).
Clinical characteristics, treatment schedules, and follow-up data were recorded.
Refractory was defined as disease worsening after treatment with high-dose glucocorticoids (equivalent of prednisone 1.0 mg/kg/day for at least 1 month) and at least one immunosuppressant (including methotrexate, azathioprine, and mycophenolate mofetil) or intravenous immunoglobulin.
A Literature Review of Patients With Anti-SRP IMNM Treated With Anti-B-Cell Therapy
We searched in PubMed, Web of Science, Embase, and Cochrane for all cases of anti-SRP IMNM treated with anti-B-cell therapy, until June 2021. All items of anti-B-cell agents that have been presented in Cochrane were included in the study; these include the following: RTX, rituxan, mabthera, ofatumumab, GA101, ofatumumab, inotuzumab, SM03, epratuzumab, belimumab, LY2127399, imalumab, VAY736, tabalumab, AMBER, isatuximab, SAR650984, daratumumab, dara, or MOR202. The disease was searched with “exp Neuromuscular Disease/or (neuromuscular disease or neuromuscular disorder or muscular disease or muscular disorder or muscle disease).tw. or exp Muscular Disease/or exp Myositis/or (myotoni dystroph, myotoni disorder, muscular dystroph, myopath, myotonia congenita, or paramyotonia congenita).tw. or (periodic paralysis or central core disease or mitochondrial cytopath).mp. or glycogen storage disease, glycogen storage disorder, fatty oxidation disorder, inflammatory myopathy, polymyositis, dermatomyositis, inclusion body myositis, or endocrine myopathy).mp.” and “anti-srp.mp. or anti-signal recognition particle. or signal recognition particle.mp.”
Inclusion criteria are as follows: (1) adults >18 years of age, (2) following the 119th ENMC or 224th ENMC classification criteria for IMNM, and (3) the patient tested positive for anti-SRP antibodies. Patients with other myopathy diseases were excluded from the study.
Results
Belimumab Treatment in a Patient With Relapsing Anti-SRP IMNM
A 47-year-old woman presented with upper and lower extremity weakness. Elevated creatinine kinase (CK) and positive antinuclear antibodies (ANA) and anti-SRP antibodies were identified. Other autoantibodies, including anti-Sm, anti-RNP, anti-SSA/Ro, anti-SSB/La, anti-topoisomerase 1, anti-hystidyl-tRNA synthetase, anti-ribosomal P, and anti-chromatin, were negative. A muscle biopsy showed scattered necrotic muscle fibers. The patient was diagnosed with immune-mediated necrotizing myopathy and she began to receive prednisone at a dose of 50 mg/day and methotrexate at a dose of 15 mg once weekly. The patient responded well to the treatment, and the dose of prednisone was gradually tapered to 10 mg/day in 1 year.
Seventeen months later, muscle weakness recurred and creatinine kinase increased again. The patient was administered cyclosporine 75 mg twice daily, combined with methotrexate and prednisone. Creatinine kinase decreased but did not return to the normal range, and muscle weakness persisted. The patient was hospitalized for a second relapse of the disease 7 months later. Belimumab was added at a dose of 10 mg/kg once every 2 weeks for 6 weeks, followed by 10 mg/kg once a month. Meanwhile, the dose of prednisone was changed to 60 mg once a day as well as methotrexate at a dose of 12.5 mg once a week. The patient showed a good response and tolerance to this combination therapy, and no adverse effects were noted with the use of belimumab. All scores, including MYOACT, MITAX, SF-36 PCS, SF-36 MCS, and FACIT-F, improved after belimumab therapy (Table 1). Twenty-three weeks later, the CK level of the patient decreased to normal and was maintained while the dose of prednisone was gradually tapered to 12.5 mg once a day. The anti-SRP antibody test results were negative.
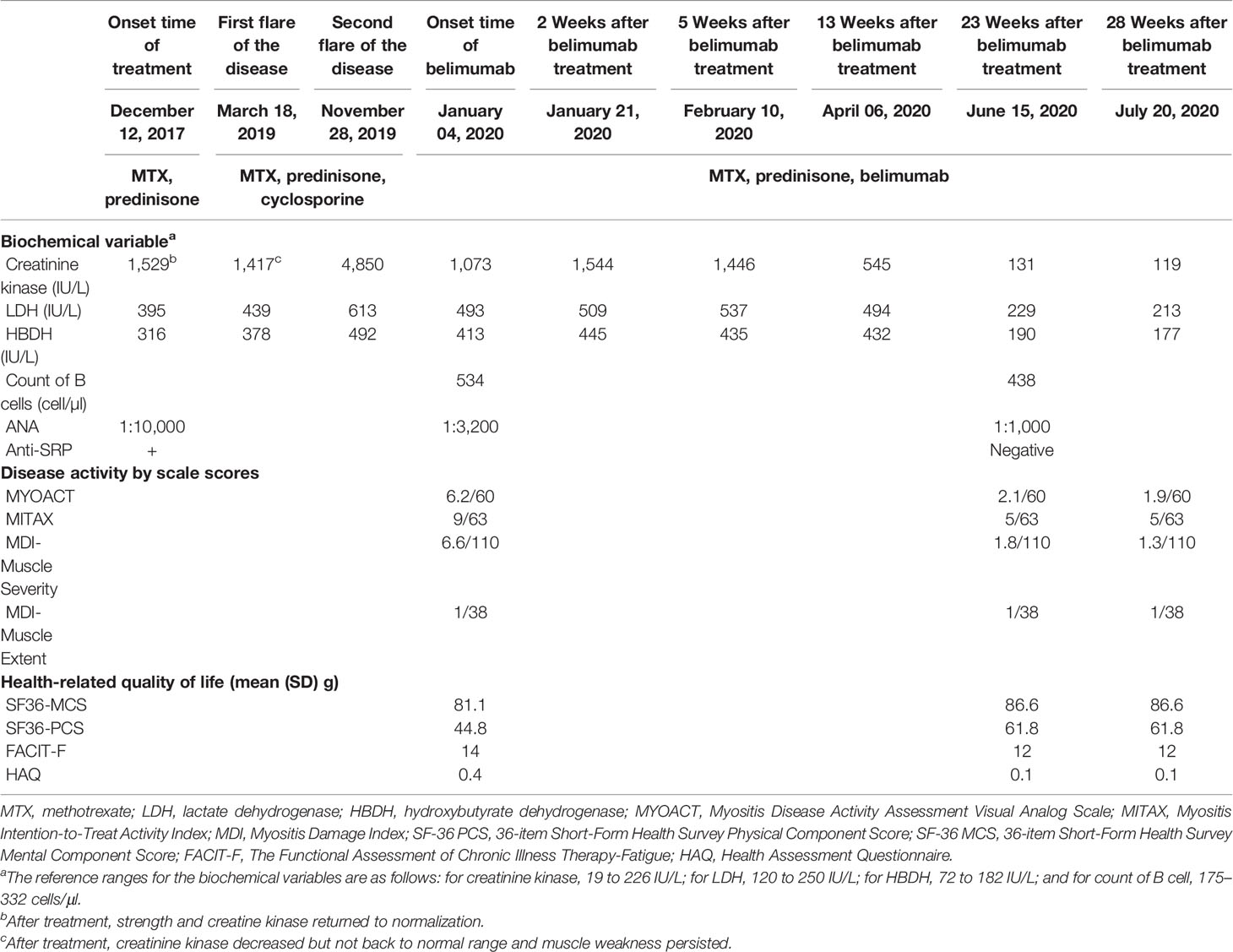
Table 1 Biochemical variables and scale scores in response to different methods of immunosuppression.
Retrospective Review of Patients With Anti-SRP IMNM Treated With Anti-B-Cell Therapies in a Single Center
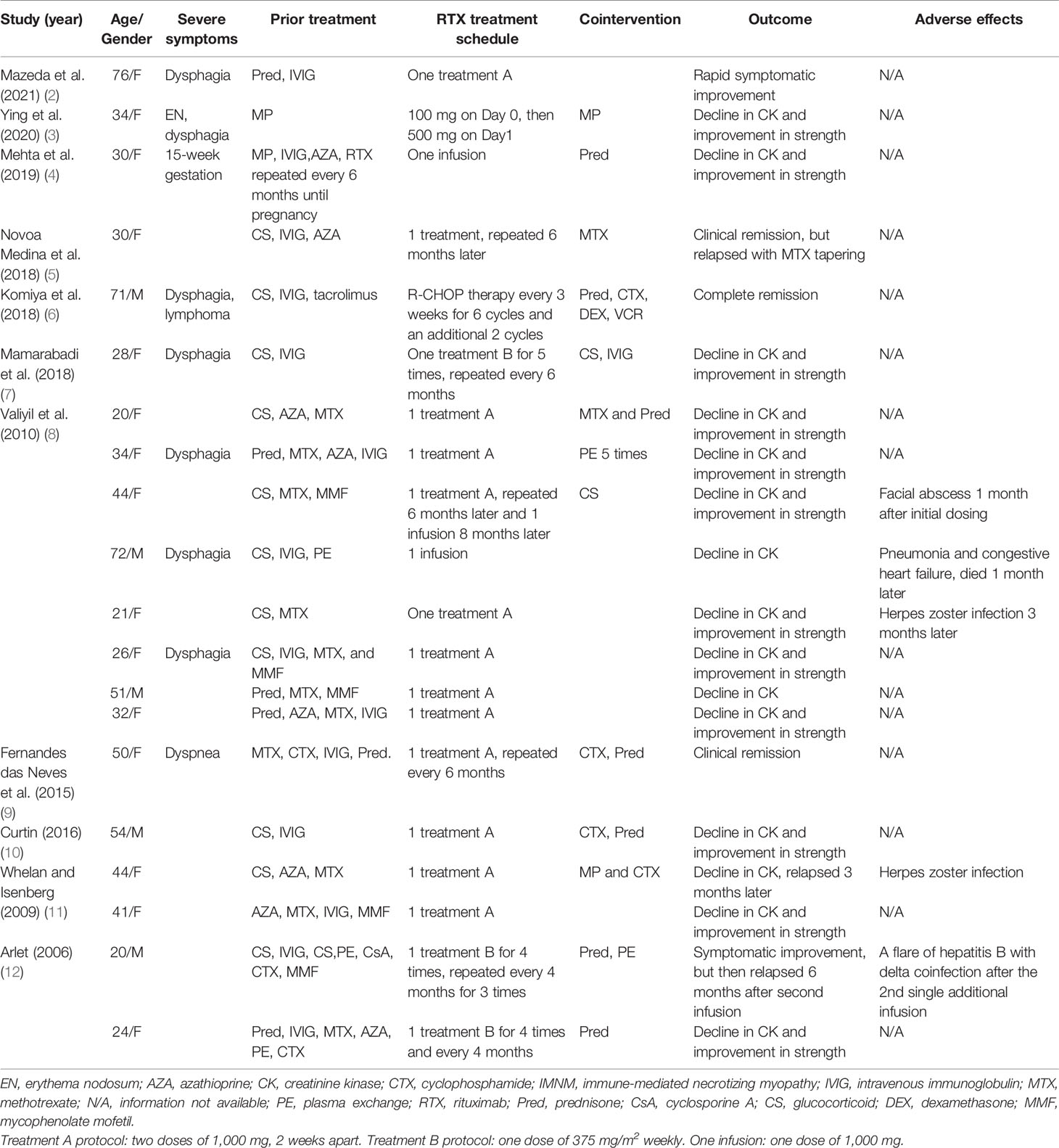
A total of 112 patients with anti-SRP IMNM who visited our department between September 2014 and June 2021 were reviewed. Only three patients were treated with RTX (anti-B-cell therapy). The first patient was a refractory case and received RTX six times (a dose of 500 mg/week for 2 weeks, then repeated 1 month later and 500 mg, two times, 1 year apart). The symptoms persisted. The other two patients responded to RTX; however, herpes zoster developed in the third patient after the first infusion and the treatment was discontinued. Patients’ characteristics, laboratory data, treatment schedules, and outcomes are presented in Table 2.
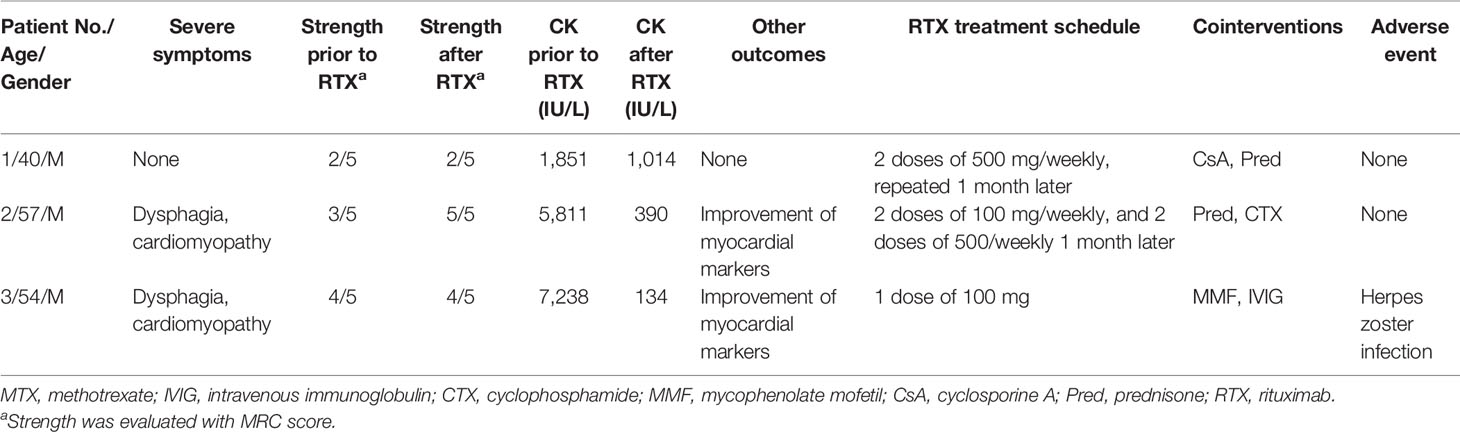
Table 2 Retrospective review of patients with anti-SRP IMNM treated with anti-B-cell therapies in a single center.
A Literature Review of Patients With Anti-SRP IMNM Treated With Anti-B-Cell Therapy
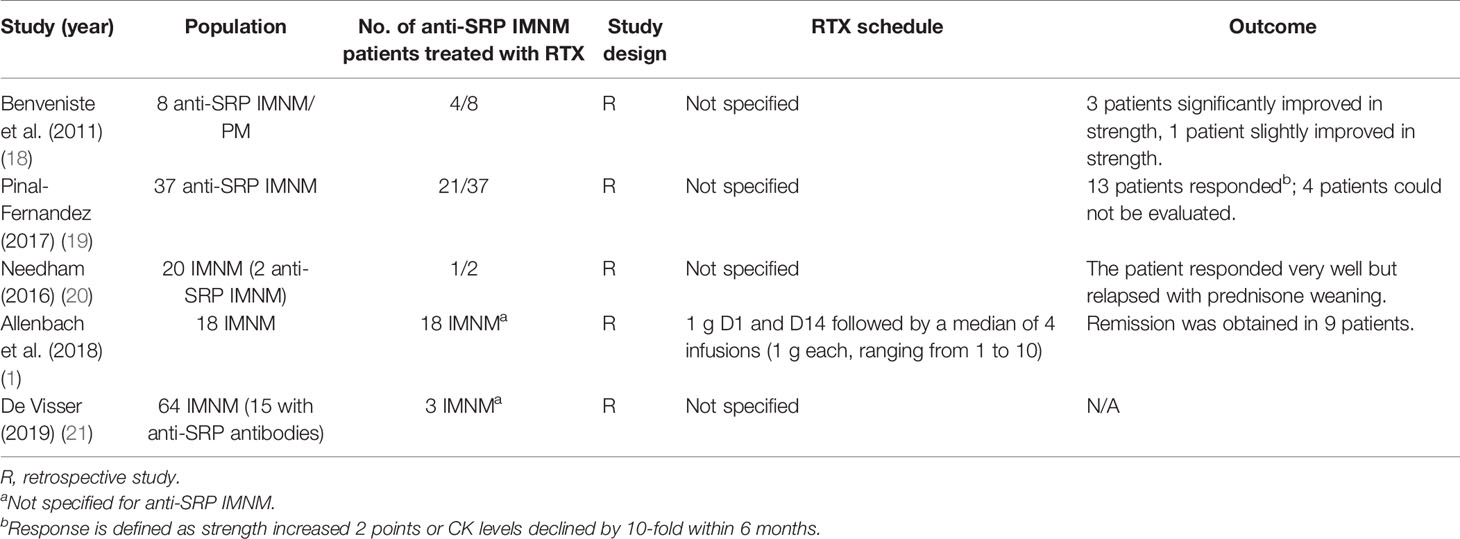
A total of 124 articles were identified from the database. After excluding articles that are not written in English or patients who matched the exclusion criteria, 15 articles were finally selected. Twenty patients with anti-SRP IMNM and RTX treatment from case reports and case series were reviewed, and five retrospective studies were included. Details are summarized in Tables 3, 4.
Discussion
To our knowledge, this is the first case of belimumab in anti-SRP IMNM. The patient showed good response and tolerance to belimumab.
There are still no randomized trials or large enough case series to make formal recommendations for IMNM treatment. Based on the European Neuromuscular Center workshop, corticosteroids are considered the first-line treatment (1). High-dose corticosteroids should be used immediately upon diagnosis. For patients with an incomplete response to corticosteroid monotherapy or multisystem involvement, second-line treatments are warranted; these include methotrexate, azathioprine, and mycophenolate mofetil. In some cases, cyclosporine and tacrolimus may be used as adjuncts. In addition to conventional immunosuppression, IVIg is considered an effective treatment for initial therapy, especially in anti-HMGCR myopathy (1).
In our study, we reviewed all B-cell therapies for IMNM, and only RTX was identified to be effective (1–12, 18–21). B-cell depletion therapy with RTX in anti-SRP IMNM is commonly effective. As our study showed, all patients from case reports and case series showed a decline in CK, while three patients relapsed during tapering. In addition, five patients developed infections after RTX therapy, and one patient died from pneumonia and congestive heart failure. From the reported literature, it was gathered that most patients responded; in one study, however, only half of patients achieved remission (1). The author of the study supposed that a low ratio of remission might be related to the delay in RTX use. Despite a lack of rigorous clinical trials, considerable experience has demonstrated that anti-B-cell therapy might be effective for patients with IMNM.
Although belimumab has never been reported for use in IMNM treatment, the important role of BAFF or B-lymphocyte stimulator in the pathogenesis of idiopathic inflammatory myopathies (IIM) has been demonstrated in previous studies (22–24). In a study by Yuan, 10 of 29 patients with refractory anti-SRP IMNM showed positive BAFF in necrotic tissue regenerated muscle fibers and individual lymphocytes, while BAFF receptor was found in 24 of 29 patients. Moreover, refractory patients with anti-SRP IMNM had more BAFF receptors than nonrefractory patients. These findings suggest that BAFF and its receptors may participate in muscle fiber injury (22).
The efficacy and safety of belimumab in other autoimmune diseases have been evaluated in randomized clinical trials. The BLISS trial, a randomized, double-blind, placebo-controlled trial, demonstrated the efficacy of belimumab in SLE (13). The BLISS-LN study, a multicenter, randomized, double-blind trial included 448 patients with lupus nephritis. At week 104, primary responses occurred more often in the belimumab group than in the placebo group. Infection and infestation occurred in 15 of 224 patients in the belimumab group and 18 of 224 patients, while the number of infection-associated deaths were equal to the two groups (three patients in each group) (25). In a bicentric prospective 1-year open-label trial on Sjogren’s syndrome, patients achieved improvement in several aspects, including disease activity index, dryness, fatigue, and VAS scores. Only one of 30 patients suffered from a severe adverse event (pneumococcus meningitis) (14). A multicenter, double-blind, placebo-controlled trial on the efficacy and safety of belimumab in IIMs is ongoing by Northwell Health (NCT02347891).
Consistent with previous reports, not only improvement of disease activity but also a decline in anti-SRP antibodies was observed in our case. Some evidence has demonstrated that anti-SRP antibodies may participate in the pathogenesis of IMNM by triggering an immune reaction, resulting in the release of myotoxic cytokines (8, 22, 26). In vitro, positive SRP was found on the plasma membrane of cultured myoblast cells stained with anti-SRP serum (27). In animal models, muscle weakness was observed in C57/Bl6 or Rag2-deficient or complement 3-deficient mice after passive IgG transfer from patients with anti-SRP IMNM (28). Moreover, SRP protein was identified in the muscle of anti-SRP IMNM patients via colabeling with the transsarcolemmal protein dysferlin and sarcoplasmic neural cell adhesion molecule, respectively, and further cellular experiments demonstrated exposed SRP protein localized at the surface of myotubes (29).
There are some limitations to this case: since this is the first case describing belimumab in IMNM, more cases and studies are needed to confirm the effects. Based on this case, we observed that belimumab was effective in IMNM associated with anti-SRP antibodies and suggest that belimumab might be option for severe cases.
Conclusion
Belimumab improved the clinical condition of our patient without any severe adverse events. In the review of the records of our center and literature, B-cell therapy with RTX benefited some patients with anti-SRP IMNM, but at the same time, increased the risk of infection. In conclusion, the present study demonstrates that belimumab in association with steroids might be an encouraging option for refractory/relapsing cases.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of West China Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors contributed to one or more of the following aspects of the manuscript: conception, acquisition of data, drafting, and revising the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Clinical Research Incubation Project, West China Hospital, Sichuan University (2019HXFH038).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IMNM, immune-mediated necrotizing myopathy; SRP, signal recognition particle; HMGCR, 3-hydroxy-3-methylglutaryl-coA-reductase; RTX, rituximab; BAFF, B-cell-activating factor; IIM, idiopathic inflammatory myopathies.
References
1. Allenbach Y, Mammen AL, Benveniste O, Stenzel W. Immune-Mediated Necrotizing Myopathies Working Group ENMC International Workshop. 224th ENMC International Workshop: Clinico-Sero-Pathological Classification of Immune-Mediated Necrotizing Myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord (2018) 28(1):87–99. doi: 10.1016/j.nmd.2017.09.016
2. Mazeda C, Cunha R, Ferreira PG, Barcelos A, Aguiar R. Myopathy Associated With Anti-Signal Recognition Particle Autoantibodies With Pulmonary Involvement and Response to RTX. Rheumatol Int (2021) 04:1–5. doi: 10.1007/s00296-021-04904-5
3. Ying S, Li S, Tang S, Sun Q, Fang D, Li Y, et al. Immune-Mediated Necrotizing Myopathy Initially Presenting as Erythema Nodosum. J Inflamm Res (2020) 13:471–6. doi: 10.2147/JIR.S270114
4. Mehta P, Dorsey-Campbell R, Dassan P, Nelson-Piercy C, Viegas S. Difficult Case: RTX in Anti-SRP Antibody Myositis in Pregnancy. Pract Neurol (2019) 19(5):1–3. doi: 10.1136/practneurol-2018-002168
5. Nóvoa Medina FJ, Gutiérrez Martínez J, González González Y, Romero Díaz B, Machín García S, Rosas Romero A. Rituximab Therapy in Necrotizing Autoimmune Myopathy Associated With Anti-SRP Antibody: A Clinical Case Review. Reumatol Clin (Engl Ed) (2018) 14(6):379–81. doi: 10.1016/j.reuma.2017.02.009
6. Komiya H, Hagihara M, Tanaka K, Tada M, Joki H, Koyano S, et al. Case of Immune-Mediated Necrotizing Myopathy Associated With Anti-Signal Recognition Particle Autoantibodies: Dramatic Improvement After Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone Therapy for Intravascular Large B-Cell Lymphoma. Clin Exp Neuroimmunol (2018) 9(3):177–81. doi: 10.1111/cen3.12469
7. Mamarabadi M, Baisre A, Leitch M, Hsu V, Kanduri JS, Chen S. Case of Anti-Single Recognition Particle-Mediated Necrotizing Myopathy After Influenza Vaccination. J Clin Neuromuscul Dis (2018) 19(4):211–6. doi: 10.1097/CND.0000000000000208
8. Valiyil R, Casciola-Rosen L, Hong G, Mammen A, Christopher-Stine L. Rituximab Therapy for Myopathy Associated With Anti-Signal Recognition Particle Autoantibodies: A Case Series. Arthritis Care Res (Hoboken) (2010) 62(9):1328–34. doi: 10.1002/acr.20219
9. Fernandes das Neves M, Caetano J, Oliveira S, Delgado Alves J. Immune-Mediated Necrotising Myopathy Associated With Antibodies to the Signal Recognition Particle Treated With a Combination of Rituximab and Cyclophosphamide. BMJ Case Rep (2015) 3:1–4. doi: 10.1136/bcr-2014-206250
10. Curtin D, Costigan D, McCarthy C, Jansen M, Farrell M, Reid V, et al. Novel Antibody Associations in Immune-Mediated Necrotising Myopathy Without Inflammation. Ir J Med Sci (2016) 185(4):1–3. doi: 10.1007/s11845-014-1207-z
11. Whelan BR, Isenberg DA. Poor Response of Anti-SRP-Positive Idiopathic Immune Myositis to B-Cell Depletion. Rheumatology (Oxf Engl) (2009) 48(5):594–5. doi: 10.1093/rheumatology/kep027
12. Arlet JB, Dimitri D, Pagnoux C, Boyer O, Maisonobe T, Authier FJ, et al. Marked Efficacy of a Therapeutic Strategy Associating Prednisone and Plasma Exchange Followed by RTX in Two Patients With Refractory Myopathy Associated With Antibodies to the Signal Recognition Particle (SRP). Neuromuscul Disord (2006) 16(5):334–6. doi: 10.1016/j.nmd.2006.03.002
13. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, et al. A Phase III, Randomized, Placebo-Controlled Study of Belimumab, a Monoclonal Antibody That Inhibits B Lymphocyte Stimulator, in Patients With Systemic Lupus Erythematosus. Arthritis Rheum (2011) 63(12):3918–30. doi: 10.1002/art.30613
14. Mariette X, Seror R, Quartuccio L, Baron G, Salvin S, Fabris M, et al. Efficacy and Safety of Belimumab in Primary Sjögren’s Syndrome: Results of the BELISS Open-Label Phase II Study. Ann Rheum Dis (2015) 74(3):526–31. doi: 10.1136/annrheumdis-2013-203991
15. Gordon JK, Martyanov V, Franks JM, Bernstein EJ, Szymonifka J, Magro C, et al. Belimumab for the Treatment of Early Diffuse Systemic Sclerosis: Results of a Randomized, Double-Blind, Placebo-Controlled, Pilot Trial. Arthritis Rheumatol (2018) 70(2):308–16. doi: 10.1002/art.40358
16. Sciascia S, Rubini E, Radin M, Cecchi I, Rossi D, Roccatello D. Anticardiolipin and Anti-Beta 2 Glycoprotein-I Antibodies Disappearance in Patients With Systemic Lupus Erythematosus and Antiphospholipid Syndrome While on Belimumab. Ann Rheum Dis (2018) 77(11):1–2. doi: 10.1136/annrheumdis-2018-213496
17. Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC International Workshop: Trial Design in Adult Idiopathic Inflammatory Myopathies, With the Exception of Inclusion Body Myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul Disord (2004) 14(5):337–45. doi: 10.1016/j.nmd.2004.02.006
18. Benveniste O, Drouot L, Jouen F, Charuel JL, Bloch-Queyrat C, Behin A, et al. Correlation of Anti-Signal Recognition Particle Autoantibody Levels With Creatine Kinase Activity in Patients With Necrotizing Myopathy. Arthritis Rheum (2011) 63(7):1961–71. doi: 10.1002/art.30344
19. Pinal-Fernandez I, Parks C, Werner JL, Albayda J, Paik J, Danoff SK, et al. Longitudinal Course of Disease in a Large Cohort of Myositis Patients With Autoantibodies Recognizing the Signal Recognition Particle. Arthritis Care Res (Hoboken) (2017) 69(2):1–27. doi: 10.1002/acr.22920
20. Ashton C, Junckerstorff R, Bundell C, Hollingsworth P, Needham M. Treatment and Outcomes in Necrotising Autoimmune Myopathy: An Australian Perspective. Neuromuscul Disord (2016) 26(11):734–40. doi: 10.1016/j.nmd.2016.08.013
21. Lim J, Rietveld A, De Bleecker JL, Badrising UA, Saris CGJ, van der Kooi AJ, et al. Seronegative Patients Form a Distinctive Subgroup of Immune-Mediated Necrotizing Myopathy. Neurol Neuroimmunol Neuroinflamm (2019) 6(1):1–6. doi: 10.1212/NXI.0000000000000513
22. Zhao Y, Zhang W, Liu Y, Wang Z, Yuan Y. Factors Associated With Refractory Autoimmune Necrotizing Myopathy With Anti-Signal Recognition Particle Antibodies. Orphanet J Rare Dis (2020) 15(1):181. doi: 10.1186/s13023-020-01431-7
23. Kryštůfková O, Barbasso Helmers S, Venalis P, Malmström V, Lindroos E, Vencovský J, et al. Expression of BAFF Receptors in Muscle Tissue of Myositis Patients With Anti-Jo-1 or Anti-Ro52/anti-Ro60 Autoantibodies. Arthritis Res Ther (2014) 16(5):1–9. doi: 10.1186/s13075-014-0454-8
24. Peng QL, Shu XM, Wang DX, Wang Y, Lu X, Wang GC. B-Cell Activating Factor as a Serological Biomarker for Polymyositis and Dermatomyositis. biomark Med (2014) 8(3):395–403. doi: 10.2217/bmm.13.124
25. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N Engl J Med (2020) 383(12):1117–28. doi: 10.1056/NEJMoa2001180
26. Suzuki S, Nishikawa A, Kuwana M, Nishimura H, Watanabe Y, Nakahara J, et al. Inflammatory Myopathy With Anti-Signal Recognition Particle Antibodies: Case Series of 100 Patients. Orphanet J Rare Dis (2015) 13(10):1–9. doi: 10.1186/s13023-015-0277-y
27. Rojana-udomsart A, Mitrpant C, Bundell C, Price L, Luo YB, Fabian V, et al. Complement-Mediated Muscle Cell Lysis: A Possible Mechanism of Myonecrosis in Anti-SRP Associated Necrotizing Myopathy (ASANM). J Neuroimmunol (2013) 15(264):65–70. doi: 10.1016/j.jneuroim.2013.08.008
28. Bergua C, Chiavelli H, Allenbach Y, Arouche-Delaperche L, Arnoult C, Bourdenet G, et al. In Vivo Pathogenicity of IgG From Patients With Anti-SRP or Anti-HMGCR Autoantibodies in Immune-Mediated Necrotising Myopathy. Ann Rheum Dis (2019) 78(1):1–13. doi: 10.1136/annrheumdis-2018-213518
Keywords: immune-mediated necrotizing myopathy, SRP antibody, refractory IMNM, belimumab, BAFF, rituximab
Citation: Cui B-B, Tian Y-R, Ma X-Y, Yin G and Xie Q (2021) Belimumab for Immune-Mediated Necrotizing Myopathy Associated With Anti-SRP Antibodies: A Case Report and Retrospective Review of Patients Treated With Anti-B-Cell Therapy in a Single Center and Literature. Front. Immunol. 12:777502. doi: 10.3389/fimmu.2021.777502
Received: 15 September 2021; Accepted: 08 November 2021;
Published: 02 December 2021.
Edited by:
Savino Sciascia, University of Turin, ItalyReviewed by:
Eleni Tiniakou, Johns Hopkins University, United StatesGillian Sandra Butler-Browne, Center of Research in Myology, France
Olivier Boyer, Université de Rouen, France
Copyright © 2021 Cui, Tian, Ma, Yin and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qibing Xie, qibingxie@126.com
 Bei-Bei Cui1
Bei-Bei Cui1 Geng Yin
Geng Yin Qibing Xie
Qibing Xie