Postharvest strategies for preventing flower wilting and leaf yellowing in cut Ranunculus flowers
- 1Department of Agricultural and Environmental Sciences, Università degli Studi di Milano, Milano, Italy
- 2Department of Agricultural, Forest and Food Sciences, DISAFA, Vegetable Crops and Medicinal and Aromatic Plants, VEGMAP, University of Torino, Grugliasco, Italy
- 3Florcoop Sanremo Società Cooperativa Agricola (S.C.A.), Taggia, Italy
Introduction: Appropriate postharvest treatment, as well as adequate conditions of storage, can be adopted to elongate the lifespan of cut flowers. Thidiazuron (TDZ), a substituted phenylurea, and 1-methylcycloproene (1-MCP), a non-toxic inhibitor of ethylene perception, are nowadays substances commonly used to prevent early damage caused by senescence and to delay chlorophyll degradation. Ranunculus asiaticus L. is cultivated for cut flower production and is highly sensitive to ethylene and leaf yellowing. In this study, the effect of different pulse-tratment in prolonging cut ranunculus vase life was analyzed.
Methods: TDZ 10 µM, 1-MCP 500 ppb, and a combination of both were applied for 24 hours after harvest. The effect of the treatments was evaluated by performing non-destructive (% loss of fresh weight, chlorophyll a fluorescence, in vivo chlorophyll content, and Nitrogen Flavonol Index – NFI) and destructive (chlorophyll, carotenoids, anthocyanins concentration, and phenolic index) analyses at 0, 1, 12, and 14 days from treatments.
Results and Discussion: Flower wilting was delayed by 4 days in 1-MCP + TDZ 10 µM treatments, which also reduced weight loss and chlorophyll degradation compared to controls. The effectiveness of these compounds in preventing senescence has been confirmed by the decreased biosynthesis of phenolic compounds.
1 Introduction
The global floricultural industry is nowadays a multi-billion-dollar enterprise in constant growth and with international dimensions (Naing et al., 2022). Consumers are becoming more interested in flower quality and longevity (Darras, 2021); however, during long trade transportation, qualitative characteristics can be compromised by eventual ethylene exposure, causing early petal and bud abscission. Therefore, appropriate control of storage conditions (temperature, humidity, and atmospheric conditions) (Fernandes et al., 2020) and postharvest treatments, which include different chemical or organic substances, can be adopted (van Doorn et al., 2011; da Costa et al., 2015; da Costa et al., 2021; Sun et al., 2022) to meet consumers’ preference for prolonged flower longevity and freshness (Naing et al., 2022). The inhibition of ethylene perception in flowers can represent a powerful instrument to prolong their longevity. Silver thiosulfate (STS) was one of the first chemical compounds utilized for this purpose. However, the release of silver ions also represents a serious concern to the environment and public health because of their toxicity (van Doorn et al., 2011; Naing et al., 2022). 1-methylcyclopropene (1-MCP – chemical formula C4H6) was discovered in 1995 and it is a non-toxic gaseous compound which inhibits ethylene action by binding itself to hormone receptors in an irreversible manner: the ability of 1-MCP to bind receptors is ten times greater compared to ethylene (Blankenship and Dole, 2003; Ferrante and Francini, 2006; Naing et al., 2022). It has been reported that the vase life of various cut flowers such as carnation (Dianthus cariophyllus L.) (Ichimura and Shimizu, 2002), stock (Matthiola incana) (Çelikel and Reid, 2002), Consolida ajacis (Santos et al., 2005), chrysanthemum (Chrisantemum morifolium Ram.) (Hassan and Gerzson, 2002), snapdragon (Anthirrinum majus L.) (Heffron and Korban, 2022), and Delphinium hybrid (cv. Bellamosum) (Ichimura and Shimizu, 2002) can be extended by exposure to 1-MCP (Rani and Singh, 2014). Moreover, treatments with this compound elongate the vase life of sweet pea (Ichimura and Shimizu, 2002) and rose (Chamoni et al., 2005) and prevent bud and floral abscission of Phalaenopsis cultivars (Sun et al., 2009). Vase life can be also defined by leaf senescence. It has been demonstrated that treatments with cytokinins and gibberellins inhibit chlorophyll degradation and leaf senescence (Janowska and Andrzejak, 2022). Thidiazuron (TDZ, N-phenyl-N’-1,2,3-thiadiazol-5-ylurea) is a herbicide and defoliant compound of substituted phenylurea with high cytokinin-like activity (Macnish et al., 2010; Ferrante et al., 2012). Different studies on ruscus, Alstroemeria, and other cut flowers sensitive to leaf yellowing demonstrated the effectiveness of TDZ treatment in preventing leaf senescence (Ferrante et al., 2002b; Ferrante et al., 2003; Mutui et al., 2005; Bulgari et al., 2015).
Ranunculus asiaticus L. is a geophyte with a vegetative phase during cool winters and a quiescence phase in hot summer periods. It is the only Ranunculus species cultivated for ornamental scope, especially in the Mediterranean area, where Italy is the largest producer (300-350 ha, with almost half of them, 132 million stems, produced in Sanremo, Italy). Ranunculus represents 0.4% of the total sales of cut flowers and data from Royal FloraHolland, collected during the period January 2015 - May 2017, shows that Ranunculus stems come primarily from the Netherlands, Israel, and Italy (Beruto et al., 2018). Flowers belonging to the family of Ranuncolaceae appear to be highly ethylene-sensitive (Evans et al., 2002; Mensuali Sodi et al., 2002; Scariot et al., 2009) and, considering the importance of ranunculus for the Italian flower market, it appears of extreme importance to find new treatments which can elongate the vase life of cut stems, retarding the appearance of senescence symptoms. In this work, cut ranunculus flowers were treated with TDZ, 1-MCP, and with a combination of both to evaluate their effectiveness in prolongating the flowers’ vase life.
2 Materials and methods
2.1 Plant material
Cut ranunculus flowers (Ranunculus asiaticus L., “Venere rosa”) were provided by Floorcoop Sanremo (Taggia, IM, Italy) and then they were wet transported to the University of Milan and treated in the 24 hours (h) after harvest as described below.
2.2 Treatments and evaluation of stem quality
Forty stems, chosen for uniform growth and stage of development—the “open flower” stage according to Shahri and Tahir (2011)—were divided into four groups and pulse-treated for 24 h in a controlled environment (temperature 20° C, relative humidity 60-70%, and light intensity 10-15 μmol m-2 s-1 PPFD for 12 h per day using white fluorescent tube lamps) as follow: distilled water (control), TDZ 10 µM, 1-MCP 500 ppb m-3, and 1-MCP + TDZ 10 µM. TDZ was dissolved in 0.1 mM KOH and then pH adjusted with 36% HCl. Flowers of each condition were separated into three different glass bottles containing 750 mL of solution (distilled water or TDZ 10 µM). 1-MCP application was performed by fumigation in an airtight container, dissolving 1.6 g of powder in 250 mL of distilled water. Concentrations were chosen based on previous experiments performed by Ferrante et al. (2012). At the end of this period, each stem was transferred to a 50 mL tube, rinsed daily with distilled water during the entire experiment, and flowers were kept in the same controlled environment explained previously.
The quality of each flower was assessed by visually determining the presence or absence of senescence symptoms every day. The time duration between the start of the experiment and the day of the appearance of leaf yellowing, flower senescence, and the first petal loss was recorded for every replicate of each treatment (n = 10).
2.3 Non-destructive analyses
2.3.1 Loss of fresh weight
The fresh weight (FW) of the stems (n = 10) for each condition was measured after 1, 7, 12, and 14 days from the treatments, and the percentage of FW loss was estimated as follows:
where “t1” refers to the FW measured after 24 h from treatment, and “tx” to all the other time points.
2.3.2 In vivo chlorophyll content, Nitrogen Flavonol Index, and chlorophyll a fluorescence
In vivo content of chlorophyll and Nitrogen-Flavonol Index (NFI)—a good indicator of the nutritional status of plants—were measured using a multi-pigment meter MPM-100 (ADC BioSCientific Ltd.), randomly choosing ten leaves for each condition (n = 10). Chlorophyll a fluorescence was measured using a hand-portable fluorimeter (Handy-PEA, Hansatech Instruments), randomly choosing three leaves for each condition (n = 3). Before measurements, the leaves were dark-adapted with leaf clips (diameter 4 mm) for 30–40 min, then they were exposed to a saturating light (3000 µmol m−2 s −1) provided by an array of three high-intensity light-emitting diodes for 1 s. Information about the structural and functional status of the photosynthetic apparatus was provided by the parameters measured: the maximum quantum efficiency of photosystem II (FV/FM) and the area (value proportional to the number of electrons trasferred by the reaction centers to the quinones - QA - during photosynthesis).
Both analyses were performed at 0, 1, 7, 12, and 14 days after treatments.
2.4 Destructive analyses
In order to perform the destructive analyses, three leaves were randomly selected for each treatment from among the ten replicates in all the time points considered to obtain plant materials. The selected stems were weighed at the beginning and after leaf detachment to obtain accurate FW and loss of FW measurements.
2.4.1 Chlorophyll and carotenoids concentration
Chlorophylls and carotenoids were extracted from leaf tissues using 5 mL of 99.9% (v/v) methanol. Three leaf disc samples (5 mm diameter, 30 mg FW), for each treatment and time were kept in a dark room for 24 h at 4°C. After that, absorbance readings were measured with a spectrophotometer (Thermo Italy) at 665.2 and 652.4 nm for chlorophylls, and 470 nm for total carotenoids, and pigments levels were calculated by Lichtenthaler’s formula. The results were expressed as µg of pigments g-1 FW (Lichtenthaler, 1987). Samplings were conducted at 0, 1, 7, 12, and 14 days after treatment.
2.4.2 Phenolic index and anthocyanins concentration
Phenolic index and total anthocyanin were determined from leaf disc samples (5 mm diameter, 30 mg FW). Three leaf samples for each treatment and time were transferred to a tube containing 3 mL of methanol acidified with hydrochloric acid (1% v/v) and were kept in a dark room for 24 h at 4°C. Absorbance readings were measured with a spectrophotometer at 320 nm for total phenols (Ke and Saltveit, 1989), and at 535 nm for anthocyanin determination (Klein and Hagen, 1961). Phenolic index was expressed as ABS320 nm g−1 FW. Anthocyanins concentration was expressed in mg cyanidin-3-glucoside equivalents 100 g-1 FW using a molar extinction coefficient (ϵ) of 29,600 L M−1 cm−1. Samplings were conducted at 0, 1, 7, 12, and 14 days after treatment.
2.5 Statistical analysis
Data are reported as the means of replicates. Statistics were performed using GraphPad Prism version 8 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com). A two-way ANOVA was performed, followed by the Tukey post-hoc test (p< 0.05), considering the variables of time, treatment, and their interaction. Additional information is reported in the figure legends.
3 Results
3.1 Loss of FW and quality of the stems
All the treatments showed an increase in FW losses as vase life went on. Only the flowers treated with TDZ 10 µM had no significant differences at 12 and 14 days after treatment. Among the different treatments, it was possible to observe how the treated cut flowers showed, in general, lower FW losses compared with the control group after 7 and 12 days (Figure 1). In particular, flowers treated with TDZ 10 µM and 1-MCP + TDZ 10 µM showed the best results. The interaction between time and treatment was not significative.
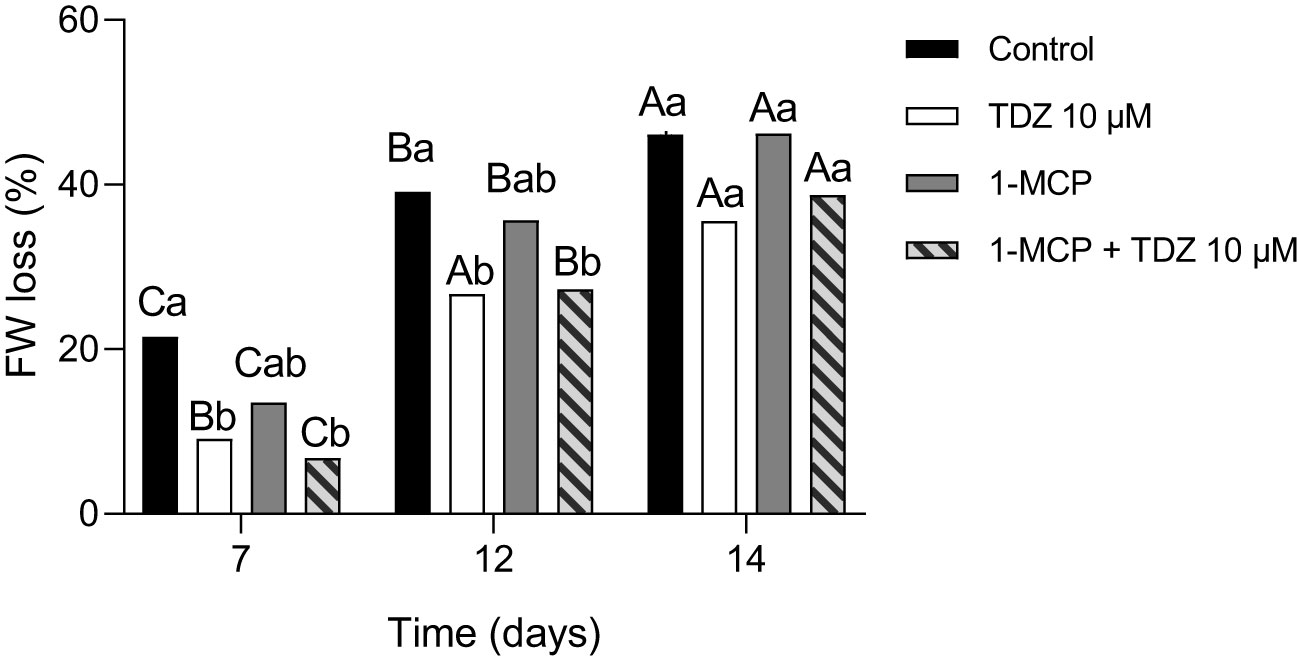
Figure 1 Percentage of fresh weight losses in the different treatments at 7, 12, and 14 days. The interaction of “time x treatment” was not significative. Various lowercase letters indicate significant differences among treatments at the same time after two-way ANOVA (p< 0.05) (n = 10); various uppercase letters various uppercase letters indicate significant differences between the time points for each treatment after two-way ANOVA (p< 0.05) (n = 10).
Cut flower quality was assessed, evaluating the time duration between the beginning of vase life and the appearance of the first symptoms of senescence, represented by leaf yellowing, flower wilting, and petal fall (Figures 2A, B). Considering leaf yellowing, no significant differences were observed between the control and 1-MCP treatment groups, in which leaf yellowing occurred after 5-6 days. However, both the treatments containing TDZ delayed this phenomenon by 3 days (Figure 2B). All treatments delayed flower senescence by 3 days (Figure 2A), while loss of petals was delayed by 3 days in treatment with only TDZ and 4 days in the other two treatments, compared to the control group (Table 1).

Figure 2 Quality of the stems (A) and leaf yellowing (B) in the different treatments after 7 days from the application of TDZ 10 µM, 1-MCP, and 1-MCP + TDZ 10 µM.
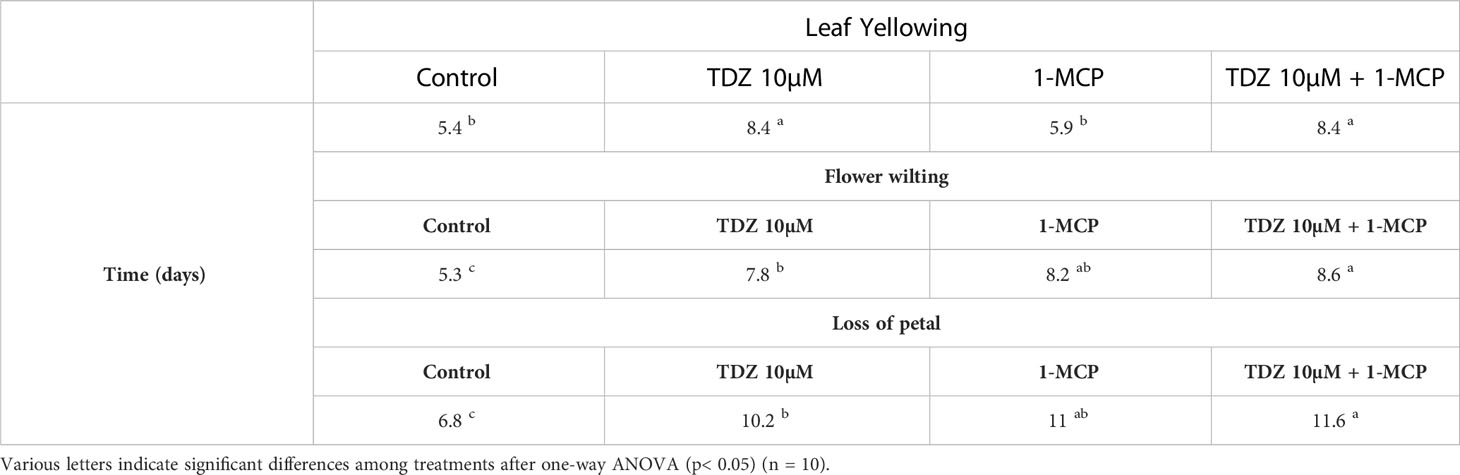
Table 1 Days (expressed as mean value) before the appearance of the main symptoms considered in the evaluation of stem quality in the different treatments (leaf yellowing, flower wilting, and loss of petal).
3.2 In vivo chlorophyll content, NFI and chlorophyll a fluorescence
In the cut flowers sector, non-destructive measurements allow the early detection of stress or senescence. In the present experiment, non-destructive analyses always showed a significative interaction of “time x treatment” (p< 0.05), with the exception for NFI values.
In vivo chlorophyll concentrations were higher in TDZ and 1-MCP + TDZ 10 µM at day 7 compared with the controls. After this time point, the values tended to decrease in all the treatments, less evidently in TDZ 10 µM stems. At 14 days, 1-MCP showed even lower levels of chlorophyll, compared to controls (Figure 3A). In treatment with TDZ 10 µM, higher values of NFI at all time points confirmed a better status of the leaves compared to the control and 1-MCP groups (Figure 3B). While NFI values decreased over time in 1-MCP + TDZ 10 µM treatments, at 12 and 14 days the leaves showed intermediate values of between the TDZ 10 µM and control groups.

Figure 3 In vivo chlorophyll content (A) and NFI (B) in the different treatments after 0, 1, 7, 12, and 14 days from treatment. The interaction of “time x treatment” was significative only for chlorophyll content (A); various letters indicate significant differences after two-way ANOVA (p< 0.05) (n = 10). In the NFI graph (B), various lowercase letters indicate significant differences among treatments at the same time after two-way ANOVA (p< 0.05) (n = 10); various uppercase letters indicate significant differences between the time points for each treatment after two-way ANOVA (p< 0.05) (n = 10).
Chlorophyll a fluorescence was measured at different time points to check the health status of the cut flowers. Among the different parameters, Fv/Fm and area were considered for evaluating the effects of the treatments. The maximum quantum efficiency of photosystem II (Fv/Fm), which has an optimal value of around 0.83 in unstressed plants (Maxwell and Johnson, 2000), was not different from controls at 0 and 1 days. Cut flowers treated with TDZ or 1-MCP + TDZ 10 µM showed a retention of the PSII functionality until day 12 (Figure 4A). In 1-MCP, the lowest results were recorded at 12 and 14 days after treatment. Area (Figure 4B) tends to decrease as environmental stresses or plant senescence occur (Kalaji and Guo, 2008). As the process of senescence went on, cut flowers of both treatments which included TDZ 10 µM showed higher values compared to the control and 1-MCP groups.
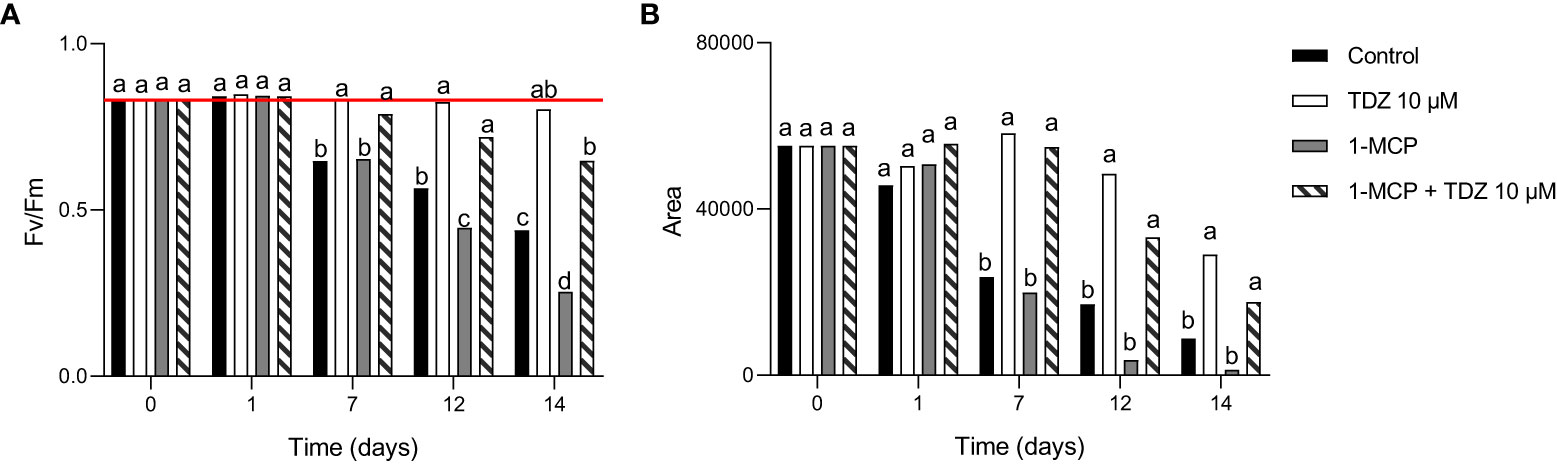
Figure 4 FV/FM ratio (A) and Area under the chlorophyll a fluorescence induction curve (B) parameters in different treatments after 0, 1, 7, 12, and 14 days from the treatment. The red line (A) indicates the optimal threshold of 0.83. The interaction of “time x treatment” was always significative. Various letters indicate significant differences after two-way ANOVA (p< 0.05) (n = 3).
3.3 Destructive analyses on leaves of cut flowers
Destructive analyses showed a significative interaction of “time x treatment” (p< 0.05).
The total chlorophyll concentration in the flowers at harvest was 1.5-1.7 µg/mg FW. Control flowers showed lower concentrations of total chlorophyll starting from 7 days after treatment. According to the different leaf yellowing observed, 1-MCP demonstrated a similar behavior to the controls, while 1-MCP + TDZ 10 µM and, particularly, TDZ 10 µM showed higher concentrations of chlorophyll with values of 0.9 µg/mg FW at the end of the experiment (Figure 5A). A similar trend was observed for leaf carotenoids concentration (Figure 5B). After 12 and 14 days, the carotenoids increased in the TDZ and 1-MCP + TDZ treatments, with values of 0.20-0.25 µg/mg FW and 0.14-0.16 µg/mg FW, respectively, that were higher than the controls.
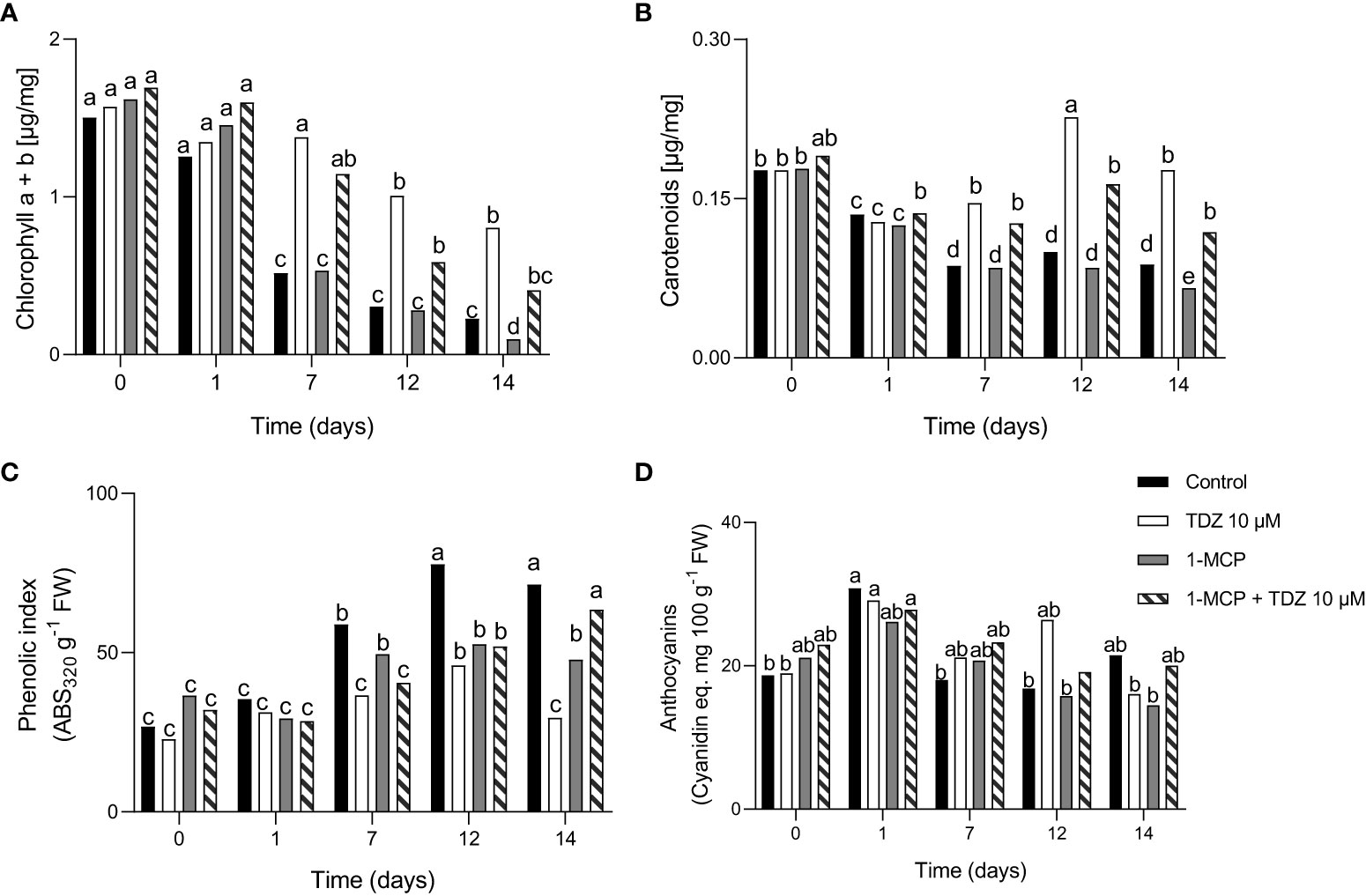
Figure 5 The concentration of chlorophyll a+b (A), carotenoids (B), phenolic index (C), and anthocyanins (D) in leaves of the different treatments after 0, 1, 7, 12, and 14 days from treatments. The interaction of “time x treatment” was always significative. Various letters indicate significant differences after two-way ANOVA (p< 0.05) (n = 10). All the values are expressed on the basis of FW.
Anthocyanins concentration did not show significant differences among the treatments except after 12 days. At this time point, the TDZ showed a higher value of anthocyanins (Figure 5D). The concentration of phenolic compounds increased during the experimental period in control flowers (Figure 5C). Treatment with TDZ did not show an increase in phenolic compounds. In the other treatments the phenolic index increased in time, but less evidently compared to control up to 12 days.
4 Discussion
The quality of cut flowers mainly depends on their visual appearance and the longevity of petals and leaves. The cut flower industry is persistently looking for new strategies to enhance postharvest handling practices. Delaying the process of flower and leaf senescence appears to be of pivotal importance, because the vase life is a critical factor in determining commercial and consumer appreciation (Horibe, 2020). The optimization of storage conditions, along with postharvest treatments, are crucial for extending the storage and the vase life of cut flowers. Among the possible strategies, the application of different chemical and organic compounds can help in enhancing the vase life of different species (da Costa et al., 2021).
1-MCP is a non-toxic ethylene action inhibitor that protects cut flowers from ethylene produced by flowers or present in the environment as a pollutant. It has been largely used to delay senescence processes in fruit, leafy vegetables, and cut flowers, and more than 40 countries have currently approved its use (Grozeff et al., 2010; Dias et al., 2021).
TDZ is a substitute of phenyl urea with high cytokinin-like activity, and its action can be observed in both light and dark conditions. The biosynthesis of cytokinins occurs in roots; therefore, the harvest and detachment of cut flowers from their roots cause the loss of the supply of these plant hormones (Kakimoto, 2003; Sakakibara, 2021; Janowska and Andrzejak, 2022): the reduction of endogenous cytokinins associated with dark conditions postharvest accelerate leaf senescence induction (Eason, 2006; Hönig et al., 2018; Guo et al., 2021). Moreover, TDZ application has shown capability in increasing the level of carotenoids, which can be considered the molecules used for counteracting the senescence process of cut flowers, protecting the flower from excessive damage caused by reactive oxygen species (Ferrante et al., 2002a; Ferrante et al., 2003; Sankhala et al., 2003; Sankhala et al., 2005; Macnish et al., 2010; Hönig et al., 2018). The effectiveness of these compounds has been largely studied, yet little is known about the possible positive effect of the combination of TDZ and 1-MCP.
In this work, pulse applications of TDZ alone or in combination with 1-MCP were studied to preserve the quality and prolong the vase life of cut ranunculus flowers. Our results suggested that all tested treatments strongly inhibited petal abscission and flower senescence, delaying flower wilting by 3 days in TDZ 10 µM and 4 days in both 1-MCP and 1-MCP + TDZ 10 µM treatments, compared to controls. The effectiveness of these compounds compared to controls in preventing senescence has been confirmed by the decreased biosynthesis of phenolic compounds in treated flowers. These secondary metabolites are involved in a wide range of biological functions. They are highly accumulated during vase life to counteract the stress generated by flower or leaf senescence (Cavaiuolo et al., 2013). On the other hand, the 1-MCP treatment did not have an effect in preventing leaf yellowing, which occurred after 5 days in both treatments and in controls. The TDZ 10 µM treatment seems to preserve the health status of flowers, as evidenced by the analysis of the Fv/Fm index and by a greater content of chlorophylls, as both in vivo and destructive analyses showed. These results suggest a better performance of the photosynthetic apparatus in the TDZ 10 µM thesis, followed by 1-MCP + TDZ 10 µM. A high concentration of carotenoids, with higher levels in TDZ 10 µM treatment followed by 1-MCP + TDZ 10 µM treatment, reveals the important contribution of these pigments in preserving the quality of flowers, acting as photo-protectors and antioxidants able to reduce the oxidation of other molecules (Maoka, 2020; Pérez-Gálvez et al., 2020). The cut flowers treated with TDZ 10 µM and 1-MCP + TDZ 10 µM showed the best results also in terms of less weight loss over the entire period of observation. TDZ is effective at very low concentrations; therefore, it can be a valid postharvest treatment for preserving flowers sensitive to leaf yellowing.
5 Conclusion
In contrast to a previous study on stock flowers (Ferrante et al., 2012), our findings showed how the combination of 1-MCP + TDZ 10 µM could represent an efficient postharvest treatment to extend the cut ranunculus vase life, delaying both flower and leaf senescence. In further works, it could be interesting to perform deeper analyses on the senescence process, taking into account the color of flower, the level of total sugars, the ethylene biosynthesis, the leaf gas exchanges, and the activities of senescence associated enzymes. Expanding our knowledge on the interaction between 1-MCP and TDZ can provide further insights into the mechanisms that contribute to the combined beneficial effect observed. These valuable insights can then be utilized as practical tools for implementing postharvest guidelines, enabling us to effectively maintain and enhance the quality of cut flowers throughout the entire supply chain. In fact, delaying the appearance of senescence phenomena, even for few days, could be a significant result for the cut flowers market.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VC: Analytical determinations and writing. RB: Training methods. FF: experimental set up. DG; Experimental set up and analysis. SV: non-destructive analysis. AF Supervision, Editing manuscript. All authors contributed to the article and approved the submitted version.
Funding
Research activities were funded by Psr 2014-2020 Liguria Region action 16.1 “Ottimizzazione della programmazione della fioritura e della conservazione postraccolta di alcune specie da fiore reciso di interesse per la Riviera Ligure di Ponente” – Ottiprogram.
Conflict of interest
The authors AF, RB, FF declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beruto M., Rabaglio M., Viglione S., Van Labeke M.-C., Dhooge E. (2018). “Ranunculus,” in Ornamental crops. Ed. Huylenbroeck J. V. (Belle, Belgium: Springer), 649–672.
Blankenship S. M., Dole J. M. (2003). 1-methylcyclopropene: a review. Postharvest Biol. Technol. 28, 1–25. doi: 10.1016/S0925-5214(02)00246-6
Bulgari R., Negri M., Ferrante A. (2015). Evaluation of postharvest storage and treatments in cut ruscus foliage. Adv. Hortic. Sci. 29, 103–108. doi: 10.13128/ahs-22687
Cavaiuolo M., Cocetta G., Ferrante A. (2013). The antioxidants changes in ornamental flowers during development and senescence. Antioxidants 2, 132–155. doi: 10.3390/antiox2030132
Çelikel F. G., Reid M. S. (2002). Postharvest handling of stock (Matthiola incana). HortScience 37, 144–147. doi: 10.21273/HORTSCI.37.1.144
Chamoni E., Kalighi A., Joyce D. C., Irving D. E., Zamani Z. A., Mostofi Y., et al. (2005). Ethylene and anti-ethylene treatment effects on cut “First-red” rose. J. Appl. Horticolture 7, 3–7. doi: 10.37855/jah.2005.v07i01.01
da Costa L. C., de Araujo F. F., Ribeiro W. S., de Sousa Santos M. N., Finger F. L. (2021). Postharvest physiology of cut flowers. Ornamental Horticulture 27, 374–385. doi: 10.1590/2447-536x.v27i3.2372
da Costa L. C., Ribeiro R. R., Carneiro W. S., Barbosa J. A., Finger F. L. (2015). Postharvest longevity of Heliconia wagneriana. Acta Horticolturae 1060, 193–199. doi: 10.17660/ActaHortic.2015.1060.28
Darras A. (2021). Overview of the dynamic role of specialty cut flowers in the international cut flower market. Horticulturae 7, 51–60. doi: 10.3390/horticulturae7030051
Dias C., Ribeiro T., Rodrigues A. C., Ferrante A., Vasconcelos M. W., Pintado M. (2021). Improving the ripening process after 1-MCP application: implications and strategies. Trends Food Sci. Technol. 113, 382–396. doi: 10.1016/j.tifs.2021.05.012
Eason J. R. (2006). Molecular and genetic aspects of flower senescence. Stewart Postharvest Rev. 2, 1–7. doi: 10.2212/spr.2006.2.6
Evans A. C., Burge G. K., Littlejohn R. P., Douglas M. H., Bicknell R. A., Lill R. E. (2002). Mount cook lily (Ranunculus lyallii) - a potential cut flower? N Z J. Crop Hortic. Sci. 30, 69–78. doi: 10.1080/01140671.2002.9514200
Fernandes L., Casal S., Pereira J. A., Saraiva J. A., Ramalhosa E. (2020). An overview on the market of edible flowers. Food Rev. Int. 36, 258–275. doi: 10.1080/87559129.2019.1639727
Ferrante A., Francini A. (2006). “Ethylene and leaf senescence,” in Ethylene action in plants. Ed. Khan N. A. (Berlin, Germany: Springer), 51–67.
Ferrante A., Hunter D. A., Hackett W. P., Reid M. S. (2002a). Thidiazuron – a potent inhibitor of leaf senescence in alstroemeria. Postharvest Biol. Technol. 25, 333–338. doi: 10.1016/S0925-5214(01)00195-8
Ferrante A., Serra G., Tognoni F. (2002b). Effects of ethylene and cytokinins on vase life of cut Eucalyptus parvifolia cambage branches. Plant Growth Regul. 38, 119–125. doi: 10.1023/A:1021212601768
Ferrante A., Tognoni F., Mensuali-Sodi A., Serra G. (2003). Treatment with thidiazuron for preventing leaf yellowing in cut tulips and chrysanthemum. Acta Horticolturae 624, 357–363. doi: 10.17660/ActaHortic.2003.624.49
Ferrante A., Trivellini A., Mensuali Sodi A. (2012). Interaction of 1-methylcyclopropene and thidiazuron on cut stock flowers vase life. Open Horticulture J. 5, 1–5. doi: 10.2174/1874840601205010001
Grozeff G. G., Micieli M. E., Gómez F., Fernández L., Guiamet J. J., Chaves A. R., et al. (2010). 1-methylcyclopropene extends postharvest life of spinach leaves. Postharvest Biol. Technol. 55, 182–185. doi: 10.1016/j.postharvbio.2009.10.004
Guo Y., Ren G., Zhang K., Li Z., Miao Y., Guo H. (2021). Leaf senescence: progression, regulation, and application. Mol. Horticulture 1, 5. doi: 10.1186/s43897-021-00006-9
Hassan F. A. S., Gerzson L. (2002). Effect of 1-MCP (1-methylcyclopropene) on the vase life of Chrysanthemum and carnation cut flowers. Int. J. Hortic. Sci. 8, 29–32. doi: 10.31421/IJHS/8/3-4/357
Heffron L. M., Korban S. S. (2022). Evaluation of ethylene mutant snapdragon lines for rooting, gravitropism, 1-MCP, ethylene, and vase-life responses. Sci. Hortic. 304, 111274. doi: 10.1016/j.scienta.2022.111274
Hönig M., Plíhalová L., Husičková A., Nisler J., Doležal K. (2018). Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 19, 4045. doi: 10.3390/ijms19124045
Horibe T. (2020). Use of light stimuli as a postharvest technology for cut flowers. Front. Plant Sci. 11, 10–13. doi: 10.3389/fpls.2020.573490
Ichimura K., Shimizu H. (2002). Effect of 1-methylcyclopropene (1-MCP) on the vase life of cut carnation, Delphinium and sweet pea flowers. Bull. Natl. Inst. Flor. Sci. 2, 1–8.
Janowska B., Andrzejak R. (2022). The role of cytokinins and gibberellins on post-harvest longevity of florists’ greens. Agriculture 12, 1–14. doi: 10.3390/agriculture12091375
Kakimoto T. (2003). Biosynthesis of cytokinins. J. Plant Res. 116, 233–239. doi: 10.1007/s10265-003-0095-5
Kalaji H., Guo P. (2008). “Chlorophyll fluorescence: a useful tool in barley plant breeding programs,” in Photochemistry research progress. Eds. Sanchez A., Gutierrez S. J. (Portland, OR, USA: Nova Science), 447–471.
Ke D., Saltveit M. E. (1989). Wound-induced ethylene production, phenolic metabolism and susceptibility to russet spotting in iceberg lettuce. Physiol. Plant 76, 412–418. doi: 10.1111/j.1399-3054.1989.tb06212.x
Klein A. O., Hagen C. W. (1961). Anthocyanin production in detached petals of impatiens balsamina l. Plant Physiol. 36, 1–9. doi: 10.1104/pp.36.1.1
Lichtenthaler H. K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382. doi: 10.1016/0076-6879(87)48036-1
Macnish A. J., Jiang C. Z., Reid M. S. (2010). Treatment with thidiazuron improves opening and vase life of iris flowers. Postharvest Biol. Technol. 56, 77–84. doi: 10.1016/j.postharvbio.2009.11.011
Maoka T. (2020). Carotenoids as natural functional pigments. J. Natural Medicines 74 (1), 1–16. doi: 10.1007/s11418-019-01364-x
Maxwell K., Johnson G. N. (2000). Chlorophyll fluorescence–a practical guide. J. Exp. Bot. 51, 659–668. doi: 10.1093/jexbot/51.345.659
Mensuali Sodi A., Ferrante A., Serra G., Rognoni F. (2002). Ethylene involvement in ranunculus asiaticus l. longevity. Proc. VIth Giornate Scientifiche Spoleto 2, 419–420.
Mutui T. M., Mibus H., Serek M. (2005). Effects of thidiazuron, ethylene, abscisic acid and dark storage on leaf yellowing and rooting of Pelargonium cuttings. J. Hortic. Sci. Biotechnol. 80, 543–550. doi: 10.1080/14620316.2005.11511975
Naing A. H., Win N. M., Kyu S. Y., Kang I.-K., Kim C. K. (2022). Current progress in application of 1-methylcyclopropene to improve postharvest quality of cut flowers. Hortic. Plant J. 8, 1–13. doi: 10.1016/j.hpj.2021.11.014
Pérez-Gálvez A., Viera I., Roca M. (2020). Carotenoids and chlorophylls as antioxidants. Antioxidants 9 (6), 505. doi: 10.3390/antiox9060505
Rani P., Singh N. (2014). Senescence and postharvest studies of cut flowers: a critical review. Pertanika J. Trop. Agric. Sci. 37, 159–201.
Sakakibara H. (2021). Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J. 105, 421–430. doi: 10.1111/tpj.15011
Sankhala N., Mackay W. A., Davis T. D. (2003). Reduction of flower abscission and leaf senescence in cut phlox inflorescence by thidiazuron. Acta Horticolturae 628, 837–841. doi: 10.17660/ActaHortic.2003.628.106
Sankhala N., Mackay W. A., Davis T. D. (2005). Effect of thidiazuron on senescence of flowers in cut inflorescences of Lupinus densiflorus benth. Acta Horticolturae 669, 239–243. doi: 10.17660/ActaHortic.2005.669.31
Santos V. R., Finger F. L., Barbosa J. G., Barros R. S. (2005). Influence of ethylene and 1-MCP on senescence and longevity of Consolida ajacis inflorescences. Bragantia 64, 33–38. doi: 10.1590/S0006-87052005000100004
Scariot V., Larcher F., Caser M., Costa E., Beruto M., Devecchi M. (2009). Flower longevity in ten cultivars of cut Ranunculus asiaticus l. as affected by ethylene and ethylene inhibitors. Eur. J. Hortic. Sci. 74, 137–142.
Shahri W., Tahir I. (2011). Flower development and senescence in ranunculus asiaticus l. J. Fruit Ornamental Plant Res. 19, 123–131.
Sun Y., Christensen B., Liu F., Wang H., Müller R. (2009). Effects of ethylene and 1-MCP (1-methylcyclopropene) on bud and flower drop in mini-Phalaenopsis cultivars. Plant Growth Regul. 59, 83–91. doi: 10.1007/s10725-009-9391-y
Sun J., Guo H., Tao J. (2022). Effects of harvest stage, storage, and preservation technology on postharvest ornamental value of cut peony (Paeonia lactiflora) flowers. Agronomy 12 (2), 230. doi: 10.3390/agronomy12020230
Keywords: senescence, ethylene, Ranunculus asiaticus L., 1-methylcyclopropene, thidiazuron, vase life
Citation: Cavallaro V, Bulgari R, Florio FE, Restuccia P, Vinci G, Guffanti D, Vignati S and Ferrante A (2023) Postharvest strategies for preventing flower wilting and leaf yellowing in cut Ranunculus flowers. Front. Hortic. 2:1183754. doi: 10.3389/fhort.2023.1183754
Received: 10 March 2023; Accepted: 05 June 2023;
Published: 19 June 2023.
Edited by:
Anastasios Darras, University of Peloponnese, GreeceReviewed by:
Pavlos Tsouvaltzis, Aristotle University of Thessaloniki, GreeceMichele Valquíria Reis, Universidade Federal de Lavras, Brazil
Stefanos Hatzilazarou, Aristotle University of Thessaloniki, Greece
Copyright © 2023 Cavallaro, Bulgari, Florio, Restuccia, Vinci, Guffanti, Vignati and Ferrante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Ferrante, antonio.ferrante@unimi.it
 Viviana Cavallaro
Viviana Cavallaro Roberta Bulgari
Roberta Bulgari Francesco Elia Florio
Francesco Elia Florio Pasquale Restuccia3
Pasquale Restuccia3  Davide Guffanti
Davide Guffanti Antonio Ferrante
Antonio Ferrante