Age-related changes in sperm traits and evidence for aging costs of sperm production in a sexually promiscuous passerine
- 1Department of Zoology, Faculty of Science, Charles University, Prague, Czechia
- 2Institute of Vertebrate Biology, The Czech Academy of Sciences, Brno, Czechia
- 3Department of Botany and Zoology, Faculty of Science, Masaryk University, Brno, Czechia
In many animal species, organismal performance declines with age in a process known as aging or senescence. Senescence typically leads to a deterioration of physiological functionality and can impact the development of primary sexual phenotypes. Sperm production is a complex and costly process that is sensitive to changes in individual physiological state, yet remarkably little is known about age-related changes in sperm performance and aging costs of sperm production. Here we use a non-linear generalized additive mixed models (GAMM) modelling to evaluate age-related changes in postcopulatory sexual traits in the European barn swallow (Hirundo rustica rustica), a relatively short lived sexually promiscuous passerine species, where male extra-pair fertilization success has been shown to increase with age. We confirmed a positive relationship between sperm midpiece length and sperm velocity in this species. Within-male changes in sperm morphology and sperm velocity were in general absent, with only sperm length decreasing linearly with increasing age, although this change was negligible compared to the overall variation in sperm size among males. In contrast, the cloacal protuberance (CP) size changed nonlinearly with age, with an initial increase between the first and third year of life followed by a plateau. The results further indicate the existence of a trade-off between investments in sperm production and survival as males with large CP tended to have a reduced lifespan. This seems consistent with the idea of expensive sperm production and survival aging costs associated with investments in post-copulatory traits in this sexually promiscuous species.
Introduction
Aging leads to a deterioration of physiological functionality and reduced individual fitness and reproductive performance (Partridge and Barton, 1993). A central idea of the contemporary theory of aging is the declining force of natural selection with increasing age (Medawar, 1952; see also the antagonistic pleiotropy theory; Williams, 1957). Because individuals typically have limited resources they invest in growth, reproduction and somatic maintenance (Kirkwood and Rose, 1991), this potentially leads to an evolutionary trade-off between reproductive effort early in adulthood and aging, i.e., there is a trade-off between allocation of resources to reproduction versus somatic maintenance (disposable soma theory of aging, Kirkwood, 1977). So far, evidence for these allocation trade-offs is mostly available for females (Lemaître et al., 2015; Lemaître and Gaillard, 2017). In males, the existing studies evaluating potential trade-offs between reproduction and aging mostly focused on the investment in secondary sexual traits (e.g., Evans, 2003; Robinson et al., 2006), while less attention is currently paid to postcopulatory traits, such as sperm production, performance and morphology reviewed in Lemaître and Gaillard (2017) and Lemaître et al. (2020). This is despite the fact that in sexually promiscuous species, sperm quality and numbers typically determine male fitness reviewed in Snook (2005) and sperm production is presumably an energetically demanding process (Hayward and Gillooly, 2011). Thus, resource allocation to postcopulatory traits may play an important role in early vs. late life investment trade-offs, thereby determining male life-history strategies (Lemaître et al., 2020).
There is considerable variability in the rate of aging among unrelated phylogenetic lineages (Jones et al., 2014), among closely related species (Fletcher and Selman, 2015), or within species among individuals (Lecomte et al., 2010). Aging is typically associated with changes in physiological and behavioral traits, such as foraging efficiency, immune system efficiency, health or individual condition (Reimers et al., 2005; Catry et al., 2006; Angelier et al., 2007). Similarly, individual reproductive performance may deteriorate with increasing age (reproductive senescence; e.g., Reid et al., 2003; Radwan et al., 2005; but see Bouwman et al., 2007). However, given the assumed trade-off between reproduction and somatic maintenance, it has also been hypothesized that individual strategies to optimize lifetime fitness may involve a change of life history allocation based on life expectancy, such that early in life individuals would invest primarily in survival, whereas towards the end of life they would invest more in reproduction at the cost of self-maintenance (Jones et al., 2014). This “terminal investment” hypothesis (Clutton-Brock, 1984) does not predict a deterioration of sperm traits with age but rather an increase of sperm quality and sperm production at terminal age.
Sperm phenotype is an important predictor of male reproductive success in sexually promiscuous species. The length of sperm (and its components) has been shown to play a role in determining sperm fertilization capacity across vertebrate taxa (LaMunyon and Ward, 1998; Knief et al., 2017). Sperm morphology, especially flagellum or midpiece length, can affect sperm velocity, another relevant factor affecting male fertilization success (Firman and Simmons, 2010; Knief et al., 2017; reviewed in Gomendio and Roldan, 2008). Sperm morphometry, and to a lesser extent sperm velocity, is genetically determined (Birkhead et al., 2005; Mossman et al., 2009), but there is evidence of plasticity caused by various factors, such as dietary quality (Støstad et al., 2019), redox state (Tomášek et al., 2017), stage of the breeding season (Edme et al., 2019), social environment (Immler et al., 2010), or immune reaction and health status (Losdat et al., 2011; Svobodová et al., 2018). These observations imply that, similar to precopulatory sexual traits (Andersson 1994), sperm traits may be condition dependent. Moreover, increased sperm production (and large ejaculate volumes) may allow males to transfer more sperm or increase the copulation frequency compared to other males, thus outcompeting males with low sperm production (Schulte-Hostedde and Millar, 2004; Boschetto et al., 2011); however, sperm production seems to be costly, as evidenced by the fact that males adjust ejaculate quality and quantity to the level of sperm competition (e.g., Ramm and Stockley, 2009) and that sperm production is often restricted to short periods of the year when sexually receptive females are present in the population (Calhim and Birkhead, 2007; Lüpold et al., 2012).
If sperm production is costly, it could be assumed that sperm quantity and performance change with age (Lemaître et al., 2020). However, the results of the few studies analyzing the correlation between age and sperm traits are inconsistent. There could be no age-related pattern in sperm sizes (Girndt et al., 2019) or older males produce longer sperm (Gasparini et al., 2010; Langen et al., 2017) or sperm with reduced velocity (Pasqualotto et al., 2005; Gasparini et al., 2010; Meunier et al., 2022). Similarly, old males could produce either higher (Schiavone et al., 2012; Jin et al., 2016) or lower (Pasqualotto et al., 2005; Langen et al., 2017; Meunier et al., 2022) sperm numbers compared to young males. Unfortunately, most available studies are cross-sectional and distinguish only between two age categories (young and old males). Only a few studies, mostly on farm animals, evaluated within-male changes in sperm traits (Dean et al., 2010; Waheed et al., 2015) but focused on a restricted part of individual life cycle (see also Vega-Trejo et al., 2019 for a similar approach in fish). Exceptions are studies on domestic fowl (Gallus gallus; Cornwallis et al., 2014) and houbara bustards (Chlamydotis undulata; Preston et al., 2011), which showed that the association between age and sperm phenotypes may not always be so straightforward and sperm quality may change nonlinearly with male age. Both studies showed a decline in sperm quality traits with progressing age. In contrast, sperm traits did not decrease with male age in a longitudinal study on ants (Metzler et al., 2018).
In sexually promiscuous songbird species (Passeriformes), old males tend to be more successful than young males in both protecting the within-pair paternity (Bowers et al., 2015; Hsu et al., 2017) and obtaining extra-pair mates (Lifjeld et al., 2011). Although age-related changes in sperm quality and sperm production have been hypothesized to partly account for this phenomenon (e.g., Laskemoen et al., 2010; Lifjeld et al., 2022), data on age-related changes in sperm traits are typically not available for species studied with regard to extrapair fertilization success of young and old males (but see DuVal, 2012; Sardell and DuVal, 2014), and the evidence for age-related changes in sperm morphology and velocity is inconsistent (Laskemoen et al., 2010; Sætre et al., 2018; Edme et al., 2019; Lifjeld et al., 2022). On the other hand, old males may have larger testes than young males, which might indicate intensified sperm production (Laskemoen et al., 2008; Lifjeld et al., 2022). Along with the seasonal increase in testis mass, male passerines produce a pronounced swelling of the cloaca, known as cloacal protuberance (hereafter CP; Wolfson, 1952). The cloacal protuberance is a sperm storage organ the size of which has been shown to positively correlate with testis mass (Birkhead et al., 1993; Laskemoen et al., 2008), the level of sperm competition (Birkhead and Møller, 1998), reflects ejaculate volumes at within-species level (Laskemoen et al., 2010; Girndt et al., 2019) and tends to be positively associated with male fertilization success (Laskemoen et al., 2008, 2010). As with sperm traits, there is contradictory evidence for an association between CP sizes and male age, with some cross-sectional studies reporting no effect (Girndt et al., 2019) or a positive association between CP and male age (Laskemoen et al., 2008).
To date, most available evidence for age-related changes in sperm traits and sperm production available is based on cross-sectional studies (see above) but longitudinal data are urgently needed to assess within-male age-related patterns in postcopulatory traits, as well as to reveal hidden survival and aging costs of potentially expensive and energetically demanding sperm production reviewed in Lemaître et al. (2020). Here we assess age related changes in sperm morphology traits, velocity, and CP sizes in longitudinally observed European barn swallow (Hirundo rustica rustica) males (Michálková et al., 2019; Kauzálová et al., 2022) using generalized additive mixed models (Wood, 2017; Cooper et al., 2021) to construct aging trajectories of each trait across individual lifespans. The studied population of barn swallows is characterized by moderate to high levels of sperm competition, with older males more successful in gaining extra-pair fertilizations than younger males (Michálková et al., 2019). Such pattern could reflect no age-related deterioration of sperm traits in barn swallows, increased investments in sperm production in old males, their increased attractiveness to females (Hsu et al., 2015) but also selective disappearance of low-quality individuals from the population (van de Pol and Verhulst, 2006). In addition, longitudinal data allowed, for the first time in a free-living bird species, to assess the potential survival and aging costs of sperm production.
Methods
Study area and general field procedure
Free-living barn swallows were captured with mist nest approximately every 3 weeks from beginning of May to the end of July at four localities in South Bohemia, in the protected landscape area of Třeboňsko - Hamr in Lužnice (49°3′24.217”N, 14°46′9.361″E), Šaloun in Lomnice nad Lužnicí (49°04′07.6”N, 14°42′37.7″E), Břilice (49°01′13.4”N 14°44′17.5″E), and Stará Hlína (49°02′21.2”N 14°49′06.5″E) in the breeding seasons of 2010–2021. All birds were marked with an aluminum National Museum of Prague ring and an individual combination of color rings. The age of birds that were ringed as a nestling at our study area (intensive ringing of the population started in 2008) was known. Birds that were captured un-ringed were assumed as 1-year old that hatched outside our observed populations. This approach has been used in previous studies (Costanzo et al., 2017; Kauzálová et al., 2022) and is possible because of high breeding philopatry and fidelity of barn swallows. Adult birds that did not return to the breeding locality were presumed dead (Costanzo et al., 2017; Kauzálová et al., 2022). Only resident birds (repeatedly occurring on breeding grounds) with a known year of birth and death were included in the analysis.
To obtain CP sizes, we measured the height of male cloaca and its maximum dimension along the two perpendicular axes (d1 – transverse and d2 – longitudinal, see also Laskemoen et al., 2010). The size of CP was calculated by the formula of an ellipsoidal cylinder: height × π × 0.5d1 × 0.5d2 (Laskemoen et al., 2008, 2010). CP sized were obtained immediately before sperm collection (see below) to avoid size changes after cloacal massage. Barn swallows usually produce sperm throughout the entire breeding season (April to August). The dataset only includes sexually active males that provided a good quality ejaculate sample and were sampled at the peak of the breeding season (early May to second half of July). Using this dataset, we tested the effect of sampling date (linear and second-order polynomial) on CP size and found no relationship (Supplementary Table S1). All measurements were performed blindly with respect to knowledge of individual age.
Sperm morphology
Sample of male ejaculate for sperm morphology measurement was taken from each male using a non-invasive method of cloacal massage (Wolfson, 1952; Albrecht et al., 2013). The sample was placed in a 5% formalin solution until the smears were prepared. Using an automatic pipette, 7 μL of each sample were transferred to a slide and allowed to air-dry before rinsing with distilled water to remove impurities. The slides were viewed at 400× magnification using a light microscope (BX51, Olympus, Japan), digital camera (DP71, Olympus, Japan) and imaging software (QuickPHOTO Industrial, Olympus Japan). Passerine sperms have a spiral conformation, the head is helical and the midpiece is distinctly elongated along the flagellum (Humphreys, 1972). Any sperm that did not conform to the characteristic helical conformation was marked as abnormal and was not included in the analysis. However, the number of abnormal sperm was very low (typically <1% sperm cells in the ejaculate). Using QuickPHOTO Industrial software, images of 10 spermatozoa with regular morphology from each ejaculate were taken. Sperm components (head length, midpiece length, and tail length) were measured to obtain an average value of sperm morphology traits for each ejaculate, which was used in further analyses. Total sperm length was calculated as the sum of these three sperm components, while flagellum length was calculated as the sum of the midpiece and tail length. All sperm morphological traits were significantly but rather weakly correlated with each other (N = 921 ejaculates, all r < 0.50; Supplementary Figure S1; Supplementary Table S2), except for midpiece and tail (r = −0.73) and total sperm length and flagellum (r = 0.97). Tail is the remaining part of the flagellum where the midpiece does not extend, and, in contrast to the midpiece (Knief et al., 2017), its biological relevance is unclear. Therefore, we did not use this trait in further analyses of age-related changes in sperm morphological traits. We also did not analyze age-related changes in flagellum length, as this was strongly correlated with total sperm length (above).
Sperm velocity
Samples for sperm velocity analysis were immediately transferred to Dulbecco’s Modified Eagle Medium (Invitrogen) prewarmed at 40°C. Then we transferred a small amount of sample onto a prewarmed 4-chambered microscope Leja slide (20 μL deep, Leja, Netherlands). Sperm performance was recorded at 100× magnification using microscope CX41 (Olympus, Japan) fitted with the thermo plate (MATS-U55S Tokai Hit, Olympus), phase contrast, digital camera UI-1540-C (Olympus) and Olympus software QuickPHOTO Industrial. Sperm performance was recorded at multiple locations on the slide to record a sufficient number of sperms.
The recordings were analyzed using the CEROS computer-assisted sperm analysis system (Hamilton Thorne, Inc., United States). In the statistical analysis, the curvilinear velocity (VCL) value was used. This value characterized sperm speed over the entire trajectory by determining speed at each point of the pathway and it is an appropriate measure of swimming speed because of the absence of directional cues (Kleven et al., 2009; Laskemoen et al., 2010). Only progressive tracks were included in analysis (i.e., static and slow tracks were eliminated to remove the potential effect of drift in the chamber; Kleven et al., 2009). Moreover, cases where multiple sperm pathways merged were eliminated and samples containing less than 20 motile sperms were excluded from analyses. Sperm velocity measurements were performed blindly with respect to knowledge of the individual age.
Statistical analysis
Statistical analysis was performed using R 4.2.0 (R Core Team, 2020). Repeatability was estimated for males with more than one observation in life using the package rptR (the number of bootstraps was set to 1.000, Stoffel et al., 2017). Coefficient of variation (CV) was calculated as SD/mean × 100. Associations between sperm morphology and velocity were analyzed using lmer models in the lme4 (Bates et al., 2014) and lmerTest packages (Kuznetsova et al., 2017) with male identity and capture year as a random effect. To explain relationships between selected variables and age we used GAMM in the mgcv package with implemented nonparametric smoothing functions because of the possibility that aging trajectories may not follow parametric functions. GAMM are extended versions of generalized linear mixed models which include nonparametric terms and they are also not limited by parametric functions (Wood, 2017). The effect of age was modelled using penalized thin plate regression splines which estimated relationships between age and variables with penalized additive smoothing functions determined by restricted maximum likelihood. The penalized additive value of the number of smoothing functions is the effective number of degrees of freedom (EDF). An effective number of degrees of freedom value 1 indicates that the relationship is linear, a higher value determines the polynomial of the corresponding order (2 quadratic, 3 cubic, etc.). Only one measurement in each year for each male was used in the models. All models also included lifespan as one of the explanatory variables and male identity and capture year as random effects. The use of male identity as random affect allowed us to control for within-individual between-season variation in reproductive traits. Including both age and lifespan in the model allows us to identify within-male changes in sperm traits and sperm production and test selective disappearance of individuals in relation to postcopulatory traits investments. For example, in the presence of selective survival of males with better sperm performance, analyses would give false-positive age-related trends for these traits only because poorer-quality males disappeared (van de Pol and Verhulst, 2006). In case nonlinearity was only weakly supported for a given trait, we also fitted a linear mixed model using lmer in the lmerTest package. Since our dataset contained only one male older than 6 years (lifespan 8 years, i.e., one data point for age 7 and 8 years, respectively), the effect of age on sperm traits was also tested after removing this longest living potentially highly influential male and the results are presented simultaneously. To evaluate effect size of age in GAM models, we report the proportion of deviance explained by the variable. The value was calculated as the difference between the proportion of deviance explained by the model that did and did not include age among predictors (Wood, 2017). The significance level was 0.05 for all analyses. Means are reported with their associated standard error (SE).
Results
Sperm traits, cloacal protuberance sizes, and their within-male repeatability between seasons
An overview of the phenotypic variation in sperm traits and CP sizes along with sample sizes is provided in Table 1. Repeated measurements (at least two per male in different years) of sperm morphology, sperm velocity and CP sizes were available for 225 males (592 observations), 179 males (453 observations) and 217 males (568 observations), respectively. The within-male between-season repeatability was significant in all sperm traits and ranged between 0.24 and 0.80 (Table 2). While sperm morphology traits exhibited a remarkably high within-male repeatability, sperm velocity was a more variable trait, and the CP size exhibited the highest variability between seasons.
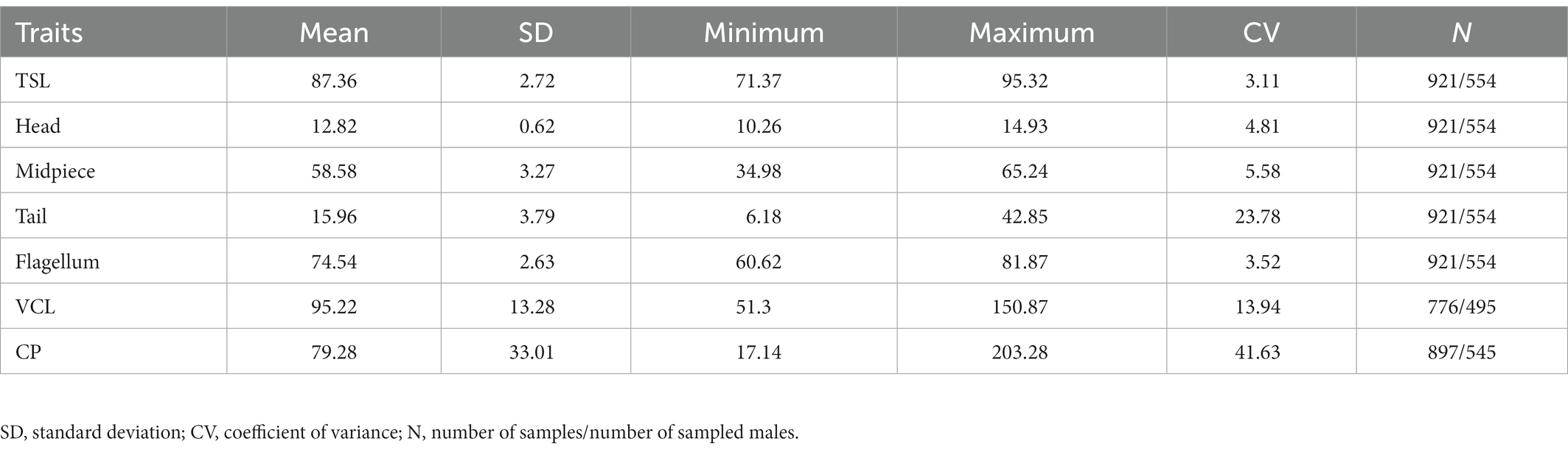
Table 1. Descriptive statistics of total sperm length (TSL), head, midpiece, tail and flagellum length, velocity (VCL) and cloacal protuberance (CP) size.
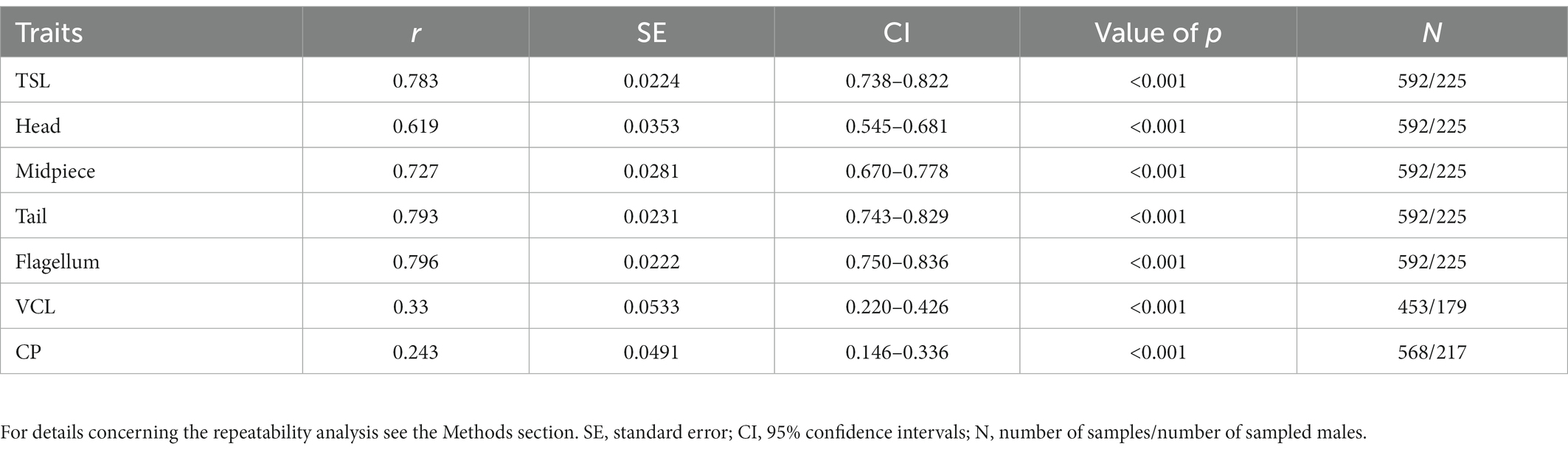
Table 2. Within-male between-season repeatability (r) of selected sperm traits (total length – TSL, head, midpiece, tail and flagellum), sperm velocity (VCL) and cloacal protuberance (CP) size for males sampled in multiple seasons.
Sperm morphology and velocity
First, we evaluated the association between sperm morphology traits and velocity (Supplementary Table S3). Velocity was not associated with total sperm length (estimate: −0.180 ± 0.189, p = 0.342), head length (estimate: −1.155 ± 0.806, p = 0.153) and flagellum length (estimate: −0.126 ± 0.195, p = 0.520). Velocity was positively correlated with sperm midpiece length (estimate: 0.514 ± 0.154, p < 0.001) and negatively with tail length (estimate: −0.440 ± 0.134, p = 0.001).
Age-related changes in sperm traits and cloacal protuberance size
Spline and parametric effects are shown in Table 3. Detailed results for GAMM for each trait (including R2, relevant test statistics and the variance explained by random effects), and proportion of deviance explained by the variable are available in Supplementary Tables S4–S8. There was a tendency for total sperm length to decrease linearly with age (EDF = 1.488, F = 2.427, p = 0.063). Linear mixed model of the same data also revealed a decrease of sperm length with age with a linear trend best supported (estimate: −0.127 ± 0.058, p = 0.030; compared to the second-order non-linear model: Chi2 = 1.741, ∆df = 1, p = 0.187; Figure 1A). Results remained unchanged when the exceptionally long-lived male was removed from the analysis (Supplementary Table S9; Supplementary Figure S2). Proportion of deviance explained by age in both models was only 0.2%. Head (EDF = 1.001, F = 2.061, p = 0.152) and midpiece (EDF = 1.214, F = 0.060, p = 0.910) showed no age-related trend. Results remained unchanged when the exceptionally long-lived male was removed from the analysis (Supplementary Tables S10, S11).
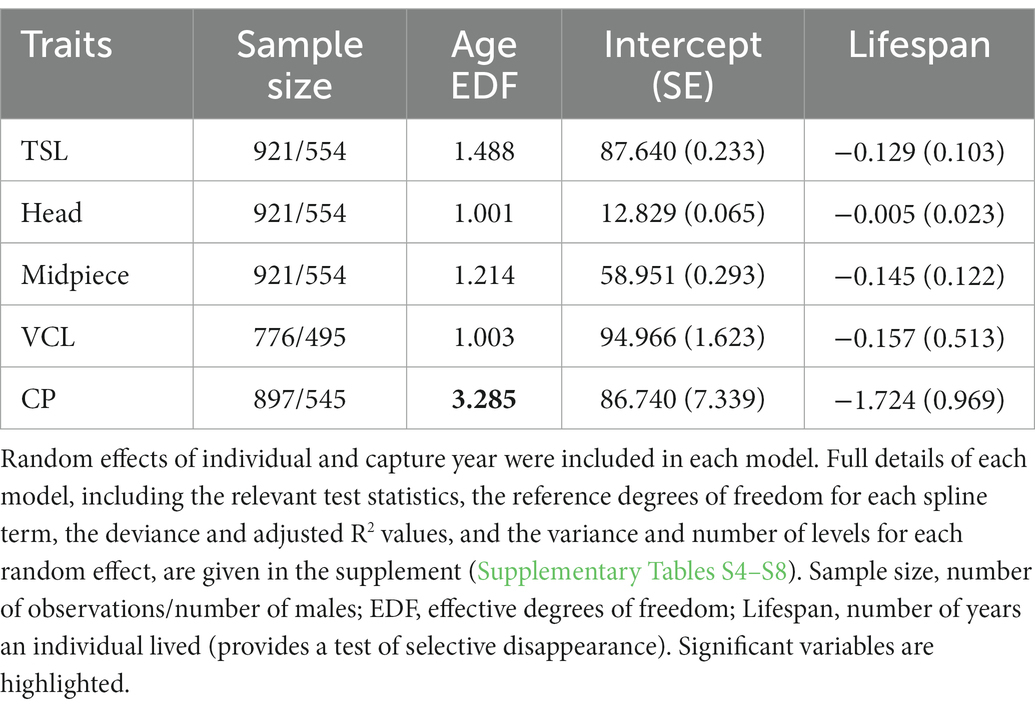
Table 3. Spline and parametric effects describing age-related changes in sperm morphometry (total length – TSL, head and midpiece), sperm velocity (VCL), and cloacal protuberance (CP) size using generalized additive mixed models.
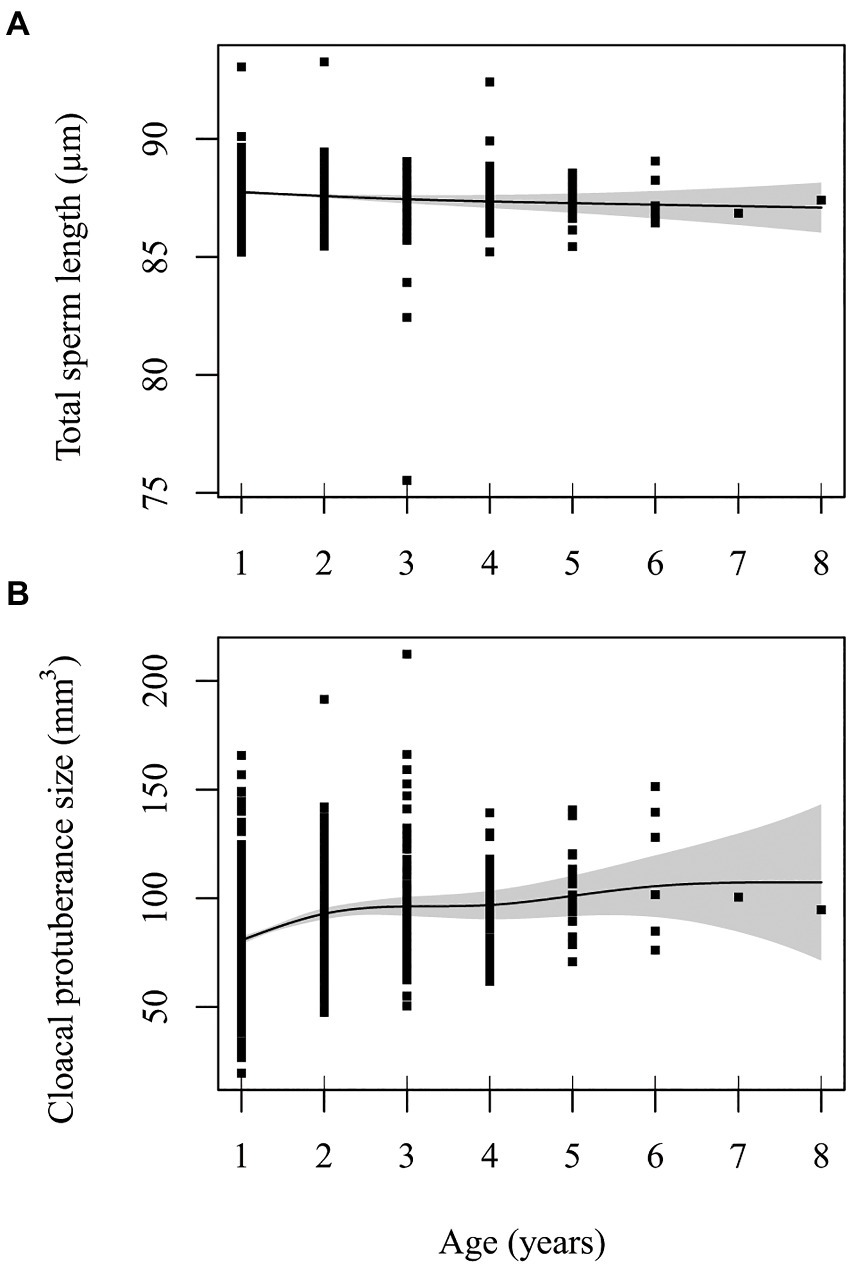
Figure 1. Effect of male age (years) on (A) total sperm length (μm) and (B) cloacal protuberance size (mm3). Line is predicted value from GAMM with 95% confidence intervals. Points are partial residuals from model that included lifespan as a covariate, along with random effects of ring number (ID) and capture year.
Sperm velocity showed no change with male age (EDF = 1.003, F = 0.004, p = 0.967). Results were similar when the exceptionally long-lived male was removed from the analysis (Supplementary Table S12).
The CP size changed nonlinearly with increasing age (EDF = 3.285, F = 10.416, p < 0.001 for the whole dataset; EDF = 2.851, F = 13.241, p < 0.001 when the exceptionally long-lived male was removed; Supplementary Table S13). In early life, the CP increases in size and remains stable after the third year of life (Figure 1B; Supplementary Figure S3, respectively). Proportion of deviance explained by age in model was 5.9%, respectively 5.8% when the exceptionally long-lived male was removed. At the same time, CP size tended to be negatively correlated with male lifespan (estimate: −1.724 ± 0.969, p = 0.076 for the whole dataset and − 2.138 ± 1.022, p = 0.037 when the exceptionally long-lived male was removed from the analysis).
Discussion
In this study we used longitudinal data to analyze within-male age-related changes in selected sperm traits and in sperm production (the CP sizes) in a small sexually promiscuous passerine. Previous studies on free-living vertebrates have typically used a cross-sectional approach to study age-related changes in sexual traits potentially involved in post-copulatory mate choice. Moreover, at least in promiscuous passerine species, age was usually assessed at a very coarse scale, distinguishing only two age categories, i.e., young males and old males, making it impossible to analyze changes in sexual traits with age or potential survival and aging costs of sperm production.
Our results indicate that sperm traits exhibit moderate to high within-individual repeatability between seasons. Specifically, morphological sperm traits showed higher repeatability compared to sperm velocity and CP sizes. Lower repeatability of velocity compared to sperm morphology was recorded also in other passerines (e.g., Birkhead et al., 2005; Mossman et al., 2009; Opatová et al., 2016; Sætre et al., 2018). This may indicate that while sperm morphology is under significant genetic control (Birkhead et al., 2005; Knief et al., 2017) and less prone to be affected by environment (e.g., reduced phenotypic plasticity, see also Tomášek et al., 2017), sperm velocity and sperm production may be susceptible to adverse environmental effects, may reflect individual condition (e.g., Simmons and Emlen, 2006) and be influenced by aging processes. Despite differences in within-individual repeatability of sperm morphology traits and sperm velocity, our results suggest a positive association between sperm velocity and midpiece length (and a negative between tail length and sperm velocity). Sperm cells with a longer midpiece-mitochondria (and hence a shorter remaining tail part) are probably able to produce larger amounts of ATP and more energy (Vladić et al., 2002; Rowe et al., 2013) to swim faster (Firman and Simmons, 2010). The positive effect of midpiece length and relative midpiece length on sperm velocity seems to be a general phenomenon in passerine birds (e.g., Laskemoen et al., 2010; Knief et al., 2017; Tomášek et al., 2017; but see Cramer et al., 2015).
No age-related changes in the length of the head and midpiece of sperm were detected in barn swallows, consistent with the relatively high repeatability of morphological traits between seasons (see above). There was evidence for an age-related change in total sperm length, with sperm becoming shorter with progressing age. However, age explained only 0.2% of the deviance in sperm length (Supplementary Table S4), and the within-male sperm shortening (~ 0.13 μm/year) was negligible compared to the inter-male variability in sperm lengths (71 to 95 μm; Table 1). Our results based on a longitudinal approach cannot be directly compared with existing published cross-sectional data. Probably the only available longitudinal study mapping changes in sperm size with age is on ants where age had no effect on sperm size (Metzler et al., 2018). However, cross-sectional studies mostly found no changes in sperm morphology with age of males in various passerine species (Laskemoen et al., 2008; Sætre et al., 2018; Edme et al., 2019; Girndt et al., 2019) including North American subspecies of barn swallows (Lifjeld et al., 2022). On the contrary, some studies, albeit using a limited dataset, found sperm of older males longer than in young males (Cramer et al., 2020). In general, sperm morphology seems to be a relatively stable trait within the life of the male, which is in agreement with the relatively high observed heritability of sperm morphology traits in passerine species (Birkhead et al., 2005; Mossman et al., 2009; Edme et al., 2019). Although some environmental stressors may influence sperm morphology, e.g., oxidative stress (Tomášek et al., 2017), heat stress (Armengol et al., 2015) or pesticides (Urióstegui-Acosta et al., 2014), these stressors may not be important in wild populations or the susceptibility of individuals to these stressors may not be age-related.
Although sperm velocity is a trait exhibiting reduced year to year consistency compared to sperm morphological traits and may be influenced by individual condition or redox state (Tomášek et al., 2017), the lack of association between male age and sperm velocity may reflect an intimate association between sperm velocity and midpiece size, the trait that is also unaffected by age in our population of barn swallows. In cross-sectional studies, sperm velocity did not change with male age in other passerines (Laskemoen et al., 2010; Sætre et al., 2018) but sperm of old males tended to swim faster than sperm of young males in North American subspecies of barn swallows (Lifjeld et al., 2022). However, cross-sectional and longitudinal studies may provide contrasting results as shown in feral fowl (Gallus g. domesticus) where no within-male age related change in velocity was found in a longitudinal study, while a cross-sectional approach revealed that older males had reduced sperm velocity compared to young males (Dean et al., 2010).
We found evidence for a previously unknown age-related pattern in CP dynamics in barn swallow males – within individuals, CP sizes increased with age, reaching a plateau when at the age of 3 years, and then remaining stable in size until the male disappeared from the population. The proportion of deviance explained by the age in the model was 5.9% (Supplementary Table S8), suggesting that the effect of age on CP size is not strong, but neither it is negligible. Age related changes in CP sizes in passerines have been documented in several previous cross-sectional studies. In a typical scenario, CP size is larger in older males than young males (Bouwman et al., 2007; Laskemoen et al., 2008; but see Girndt et al., 2019). Age related changes in CP sizes may reflect age related dynamics in testes mass – in North American subspecies of barn swallows testes size increased with age in a cross-sectional study (Lifjeld et al., 2022). CP sizes may reflect ejaculate sizes and investments in sperm production (Laskemoen et al., 2010; Girndt et al., 2019). Thus, an enlargement of CP size with age could explain high extrapair fertilization success of old males in our study population (Michálková et al., 2019).
Lifespan of males was included in all models to evaluate possible selective disappearance (van de Pol and Verhulst, 2006) in relation to postcopulatory traits. Overall, CP size tended to be negatively associated with lifespan and individuals with enlarged CPs were prone to disappear disproportionally from the population, indicating that the production of large ejaculates is costly. It was believed that sperm production is a relatively cheap process (in contrast to egg production; Parker, 1970, 1982). However, comparing energy expenditure on gamete production between the sexes is problematic (Hayward and Gillooly, 2011). Our results indicate an existence of a trade-off between reproduction and survival, and aging costs of sperm production (sensu Lemaître et al., 2020). Consistent with costly sperm production is the reduction in testicular size during nutritional stress (Perry and Rowe, 2010), outside the breeding season (Calhim and Birkhead, 2007) or a trade-off between ejaculate quality and either immunity (Simmons, 2012) or pre-copulatory sexual traits (Simmons and Emlen, 2006; Preston et al., 2011). Similar to our study, males in domestic fowl with higher sperm production lived shorter lives (Cornwallis et al., 2014). In contrast, we did not find a relationship between lifespan and sperm morphology traits or sperm velocity. This may indicate producing large sperm quantities, rather than maintaining stable sperm length or sperm velocity, is a costly life-history trait in birds, a taxon where sperm numbers may be a major factor determining male fertilization success (Immler et al., 2011).
To conclude, our results indicate that the production of highly motile sperm with optimal morphology is likely cheaper than the production of large sperm quantities, as only CP sizes were associated with lifespan and underwent a complex dynamic with male age. In general, old males may be more attractive to females as extra-pair partners (but see Girndt et al., 2018), may have easier access to extra-pair copulations than young males (Hsu et al., 2015), and the production of large ejaculates (allowing more copulations) may be rewarding even at the expense of reduced survival. Further studies should aim to reveal the complex relationship between CP sizes, male survival, and extra-pair paternity component of male life-time fitness in sexually promiscuous free-living songbird populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee, The Czech Academy of Science; Animal Care and Use Committee, Charles University in Prague.
Author contributions
TA conceived the study. KM, OT, and TA designed the study. KM, OT, VJ, MŠ, LP, JA, and TA collected the data. KM and JA analyzed sperm data. KM, OT, LP, and TA performed statistical analyses. KM and TA drafted the first version of the manuscript. All authors contributed to the final version of the manuscript.
Funding
This study was funded by the Czech Science Foundation (GAČR), projects 19-22538S, 21-22160S, and 20-06110Y. KM was funded by the Charles University (GAUK) project 1308120.
Acknowledgments
We are grateful to all students who helped with sample collection in the field.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1105596/full#supplementary-material
References
Albrecht, T., Kleven, O., Kreisinger, J., Laskemoen, T., Omotoriogun, T. C., Ottosson, U., et al. (2013). Sperm competition in tropical versus temperate zone birds. Proc. R. Soc. B Biol. Sci. 280:2434. doi: 10.1098/rspb.2012.2434
Angelier, F., Weimerskirch, H., Dano, S., and Chastel, O. (2007). Age, experience and reproductive performance in a long-lived bird: a hormonal perspective. Behav. Ecol. Sociobiol. 61, 611–621. doi: 10.1007/s00265-006-0290-1
Armengol, M. F. L., Sabino, G. A., Forquera, J. C., de la Casa, A., and Aisen, E. G. (2015). Sperm head ellipticity as a heat stress indicator in Australian merino rams (Ovis aries) in northern Patagonia, Argentina. Theriogenology 83, 553–559.e2. doi: 10.1016/j.theriogenology.2014.10.020
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2014). Fitting linear mixed-effects models using lme4. arXiv preprint. doi: 10.48550/arXiv.1406.5823
Birkhead, T. R., Briskie, J. V., and Møller, A. P. (1993). Male sperm reserves and copulation frequency in birds. Behav. Ecol. Sociobiol. 32, 85–93. doi: 10.1007/BF00164040
Birkhead, T. R., and Møller, A. P. (1998). Sperm competition and sexual selection - 1st edition. 1998th Edn Academic Press Available at: https://www.elsevier.com/books/sperm-competition-and-sexual-selection/birkhead/978-0-12-100543-6.
Birkhead, T. R., Pellatt, E. J., Brekke, P., Yeates, R., and Castillo-Juarez, H. (2005). Genetic effects on sperm design in the zebra finch. Nature 434, 383–387. doi: 10.1038/nature03374
Boschetto, C., Gasparini, C., and Pilastro, A. (2011). Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. doi: 10.1007/s00265-010-1085-y
Bouwman, K. M., van Dijk, R. E., Wijmenga, J. J., and Komdeur, J. (2007). Older male reed buntings are more successful at gaining extrapair fertilizations. Anim. Behav. 73, 15–27. doi: 10.1016/j.anbehav.2006.01.031
Bowers, E. K., Forsman, A. M., Masters, B. S., Johnson, B. G. P., Johnson, L. S., Sakaluk, S. K., et al. (2015). Increased extra-pair paternity in broods of aging males and enhanced recruitment of extra-pair young in a migratory bird. Evolution 69, 2533–2541. doi: 10.1111/evo.12746
Calhim, S., and Birkhead, T. R. (2007). Testes size in birds: quality versus quantity—assumptions, errors, and estimates. Behav. Ecol. 18, 271–275. doi: 10.1093/beheco/arl076
Catry, P., Phillips, R. A., Phalan, B., and Croxall, J. P. (2006). Senescence effects in an extremely long-lived bird: the grey-headed albatross Thalassarche chrysostoma. Proc. R. Soc. B Biol. Sci. 273, 1625–1630. doi: 10.1098/rspb.2006.3482
Clutton-Brock, T. H. (1984). Reproductive effort and terminal Investment in Iteroparous animals. Am. Nat. 123, 212–229. doi: 10.1086/284198
Cooper, E. B., Bonnet, T., Osmond, H. L., Cockburn, A., and Kruuk, L. E. B. (2021). Aging and senescence across reproductive traits and survival in superb fairy-wrens (Malurus cyaneus). Am. Nat. 197, 111–127. doi: 10.1086/711755
Cornwallis, C. K., Dean, R., and Pizzari, T. (2014). Sex-specific patterns of aging in sexual ornaments and gametes. Am. Nat. 184, E66–E78. doi: 10.1086/677385
Costanzo, A., Ambrosini, R., Caprioli, M., Gatti, E., Parolini, M., Canova, L., et al. (2017). Lifetime reproductive success, selection on lifespan, and multiple sexual ornaments in male European barn swallows. Evolution 71, 2457–2468. doi: 10.1111/evo.13312
Cramer, E., Krauss, N., Rowlison, T., and Comizzoli, P. (2020). Sperm morphology and male age in black-throated blue warblers, an ecological model system. Animals 10:1175. doi: 10.3390/ani10071175
Cramer, E. R. A., Laskemoen, T., Stensrud, E., Rowe, M., Haas, F., Lifjeld, J. T., et al. (2015). Morphology-function relationships and repeatability in the sperm of passer sparrows: sparrow sperm morphology and function. J. Morphol. 276, 370–377. doi: 10.1002/jmor.20346
Dean, R., Cornwallis, C. K., Løvlie, H., Worley, K., Richardson, D. S., and Pizzari, T. (2010). Male reproductive senescence causes potential for sexual conflict over mating. Curr. Biol. 20, 1192–1196. doi: 10.1016/j.cub.2010.04.059
DuVal, E. H. (2012). Variation in annual and lifetime reproductive success of lance-tailed manakins: alpha experience mitigates effects of senescence on siring success. Proc. R. Soc. B Biol. Sci. 279, 1551–1559. doi: 10.1098/rspb.2011.1840
Edme, A., Zobač, P., Korsten, P., Albrecht, T., Schmoll, T., and Krist, M. (2019). Moderate heritability and low evolvability of sperm morphology in a species with high risk of sperm competition, the collared flycatcher Ficedula albicollis. J. Evol. Biol. 2, 205–217. doi: 10.1111/jeb.13404
Evans, M. R. (2003). Survival of male scarlet-tufted malachite sunbirds (Nectarinia johnstoni) on Mount Kenya and the influence of ornamentation. Biol. J. Linn. Soc. 80, 125–133. doi: 10.1046/j.1095-8312.2003.00224.x
Firman, R. C., and Simmons, L. W. (2010). Sperm midpiece length predicts sperm swimming velocity in house mice. Biol. Lett. 6, 513–516. doi: 10.1098/rsbl.2009.1027
Fletcher, Q. E., and Selman, C. (2015). Aging in the wild: insights from free-living and non-model organisms. Exp. Gerontol. 71, 1–3. doi: 10.1016/j.exger.2015.09.015
Gasparini, C., Marino, I. A. M., Boschetto, C., and Pilastro, A. (2010). Effect of male age on sperm traits and sperm competition success in the guppy (Poecilia reticulata). J. Evol. Biol. 23, 124–135. doi: 10.1111/j.1420-9101.2009.01889.x
Girndt, A., Chng, C. W. T., Burke, T., and Schroeder, J. (2018). Male age is associated with extra-pair paternity, but not with extra-pair mating behavior. Sci. Rep. 8:8378. doi: 10.1038/s41598-018-26649-1
Girndt, A., Cockburn, G., Sánchez-Tójar, A., Hertel, M., Burke, T., and Schroeder, J. (2019). Male age and its association with reproductive traits in captive and wild house sparrows. J. Evol. Biol. 32, 1432–1443. doi: 10.1111/jeb.13542
Gomendio, M., and Roldan, E. R. S. (2008). Implications of diversity in sperm size and function for sperm competition and fertility. Int. J. Dev. Biol. 52, 439–447. doi: 10.1387/ijdb.082595mg
Hayward, A., and Gillooly, J. F. (2011). The cost of sex: quantifying energetic Investment in Gamete Production by males and females. PLoS One 6:e16557. doi: 10.1371/journal.pone.0016557
Hsu, Y.-H., Schroeder, J., Winney, I., Burke, T., and Nakagawa, S. (2015). Are extra-pair males different from cuckolded males? A case study and a meta-analytic examination. Mol. Ecol. 24, 1558–1571. doi: 10.1111/mec.13124
Hsu, Y.-H., Simons, M. J. P., Schroeder, J., Girndt, A., Winney, I. S., Burke, T., et al. (2017). Age-dependent trajectories differ between within-pair and extra-pair paternity success. J. Evol. Biol. 30, 951–959. doi: 10.1111/jeb.13058
Humphreys, P. N. (1972). BRIEF OBSERVATIONS ON THE SEMEN AND SPERMATOZOA OF CERTAIN PASSERINE AND NON-PASSERINE BIRDS. Reproduction 29, 327–336. doi: 10.1530/jrf.0.0290327
Immler, S., Pitnick, S., Parker, G. A., Durrant, K. L., Lüpold, S., Calhim, S., et al. (2011). Resolving variation in the reproductive tradeoff between sperm size and number. Proc. Natl. Acad. Sci. 108, 5325–5330. doi: 10.1073/pnas.1009059108
Immler, S., Pryke, S. R., Birkhead, T. R., and Griffith, S. C. (2010). Pronounced within-individual plasticity in sperm morphometry across social environments. Evolution 64, 1634–1643. doi: 10.1111/j.1558-5646.2009.00924.x
Jin, L., Mi, Z. P., and Liao, W. B. (2016). Altitudinal variation in male reproductive investment in a polyandrous frog species (Hyla gongshanensis jingdongensis). Anim. Biol. 66, 289–303. doi: 10.1163/15707563-00002505
Jones, O. R., Scheuerlein, A., Salguero-Gómez, R., Camarda, C. G., Schaible, R., Casper, B. B., et al. (2014). Diversity of aging across the tree of life. Nature 505, 169–173. doi: 10.1038/nature12789
Kauzálová, T., Tomášek, O., Mulder, E., Verhulst, S., and Albrecht, T. (2022). Telomere length is highly repeatable and shorter in individuals with more elaborate sexual ornamentation in a short-lived passerine. Mol. Ecol. 31, 6172–6183. doi: 10.1111/mec.16397
Kirkwood, T. B. L., and Rose, M. R. (1991). Evolution of senescence: late survival sacrificed for reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 332, 15–24. doi: 10.1098/rstb.1991.0028
Kleven, O., Fossøy, F., Laskemoen, T., Robertson, R. J., Rudolfsen, G., and Lifjeld, J. T. (2009). Comparative evidence for the evolution of sperm swimming speed by sperm competition and female sperm storage duration in passerine birds. Evolution 63, 2466–2473. doi: 10.1111/j.1558-5646.2009.00725.x
Knief, U., Forstmeier, W., Pei, Y., Ihle, M., Wang, D., Martin, K., et al. (2017). A sex-chromosome inversion causes strong overdominance for sperm traits that affect siring success. Nat. Ecol. Evol. 1, 1177–1184. doi: 10.1038/s41559-017-0236-1
Kuznetsova, A., Brockhoff, P. B., and Christensen, R. H. B. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. doi: 10.18637/jss.v082.i13
LaMunyon, C. W., and Ward, S. (1998). Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc. R. Soc. Lond. Ser. B Biol. Sci. 265, 1997–2002. doi: 10.1098/rspb.1998.0531
Langen, K., Bakker, T. C. M., Baldauf, S. A., Shrestha, J., and Thünken, T. (2017). Effects of aging and inbreeding on the reproductive traits in a cichlid fish I: the male perspective. Biol. J. Linn. Soc. 120, 752–761. doi: 10.1093/biolinnean/blw002
Laskemoen, T., Fossøy, F., Rudolfsen, G., and Lifjeld, J. T. (2008). Age-related variation in primary sexual characters in a passerine with male age-related fertilization success, the bluethroat Luscinia svecica. J. Avian Biol. 39, 322–328. doi: 10.1111/j.0908-8857.2008.04178.x
Laskemoen, T., Kleven, O., Fossøy, F., Robertson, R. J., Rudolfsen, G., and Lifjeld, J. T. (2010). Sperm quantity and quality effects on fertilization success in a highly promiscuous passerine, the tree swallow Tachycineta bicolor. Behav. Ecol. Sociobiol. 64, 1473–1483. doi: 10.1007/s00265-010-0962-8
Lecomte, V. J., Sorci, G., Cornet, S., Jaeger, A., Faivre, B., Arnoux, E., et al. (2010). Patterns of aging in the long-lived wandering albatross. Proc. Natl. Acad. Sci. 107, 6370–6375. doi: 10.1073/pnas.0911181107
Lemaître, J.-F., Berger, V., Bonenfant, C., Douhard, M., Gamelon, M., Plard, F., et al. (2015). Early-late life trade-offs and the evolution of aging in the wild. Proc. R. Soc. B Biol. Sci. 282:209. doi: 10.1098/rspb.2015.0209
Lemaître, J.-F., and Gaillard, J.-M. (2017). Reproductive senescence: new perspectives in the wild. Biol. Rev. 92, 2182–2199. doi: 10.1111/brv.12328
Lemaître, J., Gaillard, J., and Ramm, S. A. (2020). The hidden aging costs of sperm competition. Ecol. Lett. 23, 1573–1588. doi: 10.1111/ele.13593
Lifjeld, J. T., Kleven, O., Fossøy, F., Jacobsen, F., Laskemoen, T., Rudolfsen, G., et al. (2022). When older males sire more offspring—increased attractiveness or higher fertility? Behav. Ecol. Sociobiol. 76:61. doi: 10.1007/s00265-022-03170-0
Lifjeld, J. T., Kleven, O., Jacobsen, F., McGraw, K. J., Safran, R. J., and Robertson, R. J. (2011). Age before beauty? Relationships between fertilization success and age-dependent ornaments in barn swallows. Behav. Ecol. Sociobiol. 65, 1687–1697. doi: 10.1007/s00265-011-1176-4
Losdat, S., Richner, H., Blount, J. D., and Helfenstein, F. (2011). Immune activation reduces sperm quality in the great tit. PLoS One 6:e22221. doi: 10.1371/journal.pone.0022221
Lüpold, S., Birkhead, T. R., and Westneat, D. F. (2012). Seasonal variation in ejaculate traits of male red-winged blackbirds (Agelaius phoeniceus). Behav. Ecol. Sociobiol. 66, 1607–1617. doi: 10.1007/s00265-012-1415-3
Metzler, S., Schrempf, A., and Heinze, J. (2018). Individual- and ejaculate-specific sperm traits in ant males. J. Insect Physiol. 107, 284–290. doi: 10.1016/j.jinsphys.2017.12.003
Meunier, L., Sorci, G., Abi Hussein, H., Hingrat, Y., Rehspringer, N., Saint-Jalme, M., et al. (2022). Pre-but not post-meiotic senescence affects sperm quality and reproductive success in the north African houbara bustard. Front. Ecol. Evol. 10:7184. doi: 10.3389/fevo.2022.977184
Michálková, R., Tomášek, O., Adámková, M., Kreisinger, J., and Albrecht, T. (2019). Extra-pair paternity patterns in European barn swallows Hirundo rustica are best explained by male and female age rather than male ornamentation. Behav. Ecol. Sociobiol. 73:119. doi: 10.1007/s00265-019-2725-5
Mossman, J., Slate, J., Humphries, S., and Birkhead, T. (2009). Sperm morphology and velocity are genetically codetermined in the zebra finch. Evolution 63, 2730–2737. doi: 10.1111/j.1558-5646.2009.00753.x
Opatová, P., Ihle, M., Albrechtová, J., Tomášek, O., Kempenaers, B., Forstmeier, W., et al. (2016). Inbreeding depression of sperm traits in the zebra finch Taeniopygia guttata. Ecol. Evol. 6, 295–304. doi: 10.1002/ece3.1868
Parker, G. A. (1970). Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567. doi: 10.1111/j.1469-185X.1970.tb01176.x
Parker, G. A. (1982). Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 96, 281–294. doi: 10.1016/0022-5193(82)90225-9
Partridge, L., and Barton, N. H. (1993). Optimally, mutation and the evolution of aging. Nature 362, 305–311. doi: 10.1038/362305a0
Pasqualotto, F. F., Sobreiro, B. P., Hallak, J., Pasqualotto, E. B., and Lucon, A. M. (2005). Sperm concentration and normal sperm morphology decrease and follicle-stimulating hormone level increases with age. BJU Int. 96, 1087–1091. doi: 10.1111/j.1464-410X.2005.05806.x
Perry, J. C., and Rowe, L. (2010). Condition-dependent ejaculate size and composition in a ladybird beetle. Proc. R. Soc. B Biol. Sci. 277, 3639–3647. doi: 10.1098/rspb.2010.0810
Preston, B. T., Jalme, M. S., Hingrat, Y., Lacroix, F., and Sorci, G. (2011). Sexually extravagant males age more rapidly: sexually extravagant males age rapidly. Ecol. Lett. 14, 1017–1024. doi: 10.1111/j.1461-0248.2011.01668.x
R Core Team (2020). R: A language and environment for statistical computing The R Development Core Team https://www.rproject.org/.
Radwan, J., Michalczyk, Ł., and Prokop, Z. (2005). Age dependence of male mating ability and sperm competition success in the bulb mite. Anim. Behav. 69, 1101–1105. doi: 10.1016/j.anbehav.2004.09.006
Ramm, S. A., and Stockley, P. (2009). Adaptive plasticity of mammalian sperm production in response to social experience. Proc. R. Soc. B Biol. Sci. 276, 745–751. doi: 10.1098/rspb.2008.1296
Reid, J. M., Bignal, E. M., Bignal, S., McCracken, D. I., and Monaghan, P. (2003). Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: patterns and processes in a natural population. J. Anim. Ecol. 72, 765–776. doi: 10.1046/j.1365-2656.2003.00750.x
Reimers, E., Holmengen, N., and Mysterud, A. (2005). Life-history variation of wild reindeer (Rangifer tarandus) in the highly productive north Ottadalen region, Norway. J. Zool. 265, 53–62. doi: 10.1017/S0952836904006041
Robinson, M. R., Pilkington, J. G., Clutton-Brock, T. H., Pemberton, J. M., and Kruuk, L. E. B. (2006). Live fast, die young: trade-offs between fitness components and sexually antagonistic selection on weaponry in Soay sheep. Evolution 60, 2168–2181. doi: 10.1111/j.0014-3820.2006.tb01854.x
Rowe, M., Laskemoen, T., Johnsen, A., and Lifjeld, J. T. (2013). Evolution of sperm structure and energetics in passerine birds. Proc. R. Soc. B Biol. Sci. 280:2616. doi: 10.1098/rspb.2012.2616
Sætre, C. L. C., Johnsen, A., Stensrud, E., and Cramer, E. R. A. (2018). Sperm morphology, sperm motility and paternity success in the bluethroat (Luscinia svecica). PLoS One 13:e0192644. doi: 10.1371/journal.pone.0192644
Sardell, R. J., and DuVal, E. H. (2014). Small and variable sperm sizes suggest low sperm competition despite multiple paternity in a lekking suboscine bird. Auk 131, 660–671. doi: 10.1642/AUK-14-38.1
Schiavone, R., Zilli, L., Storelli, C., and Vilella, S. (2012). Changes in hormonal profile, gonads and sperm quality of Argyrosomus regius (Pisces, Scianidae) during the first sexual differentiation and maturation. Theriogenology 77, 888–898. doi: 10.1016/j.theriogenology.2011.09.014
Schulte-Hostedde, A. I., and Millar, J. S. (2004). Intraspecific variation of testis size and sperm length in the yellow-pine chipmunk (Tamias amoenus): implications for sperm competition and reproductive success. Behav. Ecol. Sociobiol. 55, 272–277. doi: 10.1007/s00265-003-0707-z
Simmons, L. W. (2012). Resource allocation trade-off between sperm quality and immunity in the field cricket, Teleogryllus oceanicus. Behav. Ecol. 23, 168–173. doi: 10.1093/beheco/arr170
Simmons, L. W., and Emlen, D. J. (2006). Evolutionary trade-off between weapons and testes. Proc. Natl. Acad. Sci. 103, 16346–16351. doi: 10.1073/pnas.0603474103
Snook, R. R. (2005). Sperm in competition: not playing by the numbers. Trends Ecol. Evol. 20, 46–53. doi: 10.1016/j.tree.2004.10.011
Stoffel, M. A., Nakagawa, S., and Schielzeth, H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Støstad, H. N., Rowe, M., Johnsen, A., Tomášek, O., Albrecht, T., and Lifjeld, J. T. (2019). Sperm head abnormalities are associated with excessive omega-6 fatty acids in two finch species feeding on sunflower seeds. J. Avian Biol. 50:2056. doi: 10.1111/jav.02056
Svobodová, J., Bauerová, P., Eliáš, J., Velová, H., Vinkler, M., and Albrecht, T. (2018). Sperm variation in great tit males (Parus major) is linked to a haematological health-related trait, but not ornamentation. J. Ornithol. 159, 815–822. doi: 10.1007/s10336-018-1559-7
Tomášek, O., Albrechtová, J., Němcová, M., Opatová, P., and Albrecht, T. (2017). Trade-off between carotenoid-based sexual ornamentation and sperm resistance to oxidative challenge. Proc. R. Soc. B Biol. Sci. 284:20162444. doi: 10.1098/rspb.2016.2444
Urióstegui-Acosta, M., Hernández-Ochoa, I., Sánchez-Gutiérrez, M., Piña-Guzmán, B., Rafael-Vázquez, L., Solís-Heredia, M. J., et al. (2014). Methamidophos alters sperm function and DNA at different stages of spermatogenesis in mice. Toxicol. Appl. Pharmacol. 279, 391–400. doi: 10.1016/j.taap.2014.06.017
van de Pol, M., and Verhulst, S. (2006). Age-dependent traits: a new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773. doi: 10.1086/503331
Vega-Trejo, R., Fox, R. J., Iglesias-Carrasco, M., Head, M. L., and Jennions, M. D. (2019). The effects of male age, sperm age and mating history on ejaculate senescence. Funct. Ecol. 33, 1267–1279. doi: 10.1111/1365-2435.13305
Vladić, T. V., Afzelius, B. A., and Bronnikov, G. E. (2002). Sperm quality as reflected through morphology in Salmon alternative life Histories1. Biol. Reprod. 66, 98–105. doi: 10.1095/biolreprod66.1.98
Waheed, M. M., Ghoneim, I. M., and Abdou, M. S. S. (2015). Morphometric characteristics of spermatozoa in the Arabian horse with regard to season, age, sperm concentration, and fertility. J. Equine Vet. 35, 244–249. doi: 10.1016/j.jevs.2015.01.005
Williams, G. C. (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411. doi: 10.2307/2406060
Wolfson, A. (1952). The Cloacal protuberance: a means for determining breeding condition in live male passerines. Bird-Banding 23, 159–165. doi: 10.2307/4510381
Keywords: senescence, post-copulatory sexual selection, life-history trade-offs, sperm morphology, sperm velocity, cloacal protuberance, Hirundo rustica
Citation: Míčková K, Tomášek O, Jelínek V, Šulc M, Pazdera L, Albrechtová J and Albrecht T (2023) Age-related changes in sperm traits and evidence for aging costs of sperm production in a sexually promiscuous passerine. Front. Ecol. Evol. 11:1105596. doi: 10.3389/fevo.2023.1105596
Edited by:
Jean-Francois Lemaitre, UMR5558 Biométrie et Biologie Evolutive (LBBE), FranceReviewed by:
Claudia Fricke, Martin Luther University of Halle-Wittenberg, GermanyCarl Soulsbury, University of Lincoln, United Kingdom
Copyright © 2023 Míčková, Tomášek, Jelínek, Šulc, Pazdera, Albrechtová and Albrecht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristýna Míčková, ✉ mickovkr@natur.cuni.cz; Tomáš Albrecht, ✉ albrecht@ivb.cz
 Kristýna Míčková
Kristýna Míčková Oldřich Tomášek
Oldřich Tomášek Václav Jelínek2
Václav Jelínek2  Tomáš Albrecht
Tomáš Albrecht