Forest Fragments, Lemur Communities and Local Perception of Nature in a Protected Area of Northwestern Madagascar
- 1Eco-Anthropologie CNRS/MNHN/Univ de Paris, UMR 7206, Muséum National d’Histoire Naturelle, Brunoy, France
- 2Environnement Ville Société, UMR 5600, Université Jean Moulin Lyon 3, Lyon, France
- 3Département d’Ecologie et Biologie Végétales, Université d’Antananarivo, Antananarivo, Madagascar
- 4Département Diversité Biologique et Culturelle, Université de Mahajanga, Mahajanga, Madagascar
- 5Département de Biologie Animale et d’Ecologie, Université de Mahajanga, Mahajanga, Madagascar
- 6Mecadev CNRS/MNHN, UMR 7179, Muséum National d’Histoire Naturelle, Brunoy, France
Biological conservation projects conducted in inhabited areas are often based on the combination of ecological diagnostics and study of practices and use of the environment by local communities. They less frequently integrate the influence of the perception and representation of nature on these practices, while these should also be taken into account in the initiation of sustainable conservation actions. We carried out a long-term study combining biological and social science approaches in North-western Madagascar in the Antrema protected area (with dry forest/savannah/coastal ecosystems), including an analysis of the use and perception of nature by its inhabitants. Together with the study of tree diversity, forest structure and biomass in 7 forest fragments, we estimated population densities of whole communities of diurnal and nocturnal lemurs, one of which is considered sacred. We interviewed local resource users from several villages using classical methods of social anthropology supplemented with perception tests derived from sensory evaluation methods. The structure of forest fragments as well as their basal area and richness in tree species varied with human pressure on specific plants (timber extraction) or with historical changes in pasture management (forest regrowth). Lemurs were generally abundant, with a high total biomass compared to other dry forests. Although the inhabitants of Antrema (Sakalava, Tsimihety, and Betsileo) still strongly adhered to local use rights and shared deeply rooted knowledge about the forest, the use and perception of nature (e.g., regarding the sacred lemur Propithecus coronatus) have changed since the Antrema protection project in 2000. The results suggest that local communities tend to integrate traditional rules about nature with international environmental regulation, perhaps a sign of a new ecological awareness. However, in the new management mode accompanying this transition, it can also be a means of local empowerment that takes advantage of a program supporting pro-environmental management of the Antrema area.
Introduction
In Madagascar, human activity related to logging, slash-and-burn agriculture, and intense harvest of forest products are important causes of deforestation (Vielledent et al., 2018). The impact can be all the stronger as the natural regeneration of forests in Madagascar appears slowed down by the slow growth rate of trees, the washing of soils due to erosion, and relatively inefficient pioneer plant species and secondary vegetation (Koechlin et al., 1974; Ganzhorn, 1988; Wright, 1999; Leigh et al., 2007). Together with illegal hunting and organized timber trade, deforestation and forest fragmentation have resulted in the loss of large mammals, including primates and less emblematic animals (Schwitzer et al., 2014; Eppley et al., 2020). However, more positive perspectives for maintaining biodiversity can be expected in some places (Cormier-Salem, 2006; Aubertin and Rodary, 2009; Méral et al., 2009), for instance where human conceptions about nature are favorable to biological conservation and deserve to be supported at national and international level beyond traditional representations. For example, in Antrema, a costal forest-savanna anthropo-ecosystem in the northwest of the island mainly populated by Sakalavas, ancestral community rules have been the central starting point for initiating consolidated protection operations. There, a consensus was achieved in 2000 between local communities, the University of Antananarivo and the National Museum of Natural History of Paris to promote sustainable management of the area through a formal contract. Traditional protection ranged from protection of the sacred elements of nature, whether individual trees or lemur species, to more complex ecological units such as forest patches and lakes (Harpet et al., 2008). Concretely, by its initial designation as the “multi-use forest station of Antrema,” the project aimed to promote further these rules to increase pro-environmental behaviors and ecological awareness with regard to agro-pastoral practices. The logging pressure should therefore be moderate and framed by rules enacted in the conservation plan to allow fishermen and other community members to run their way of life. Local communities in counterpart would receive financial support for the development of infrastructures (a school, wells, bridges,…) and for reducing local poverty (Gauthier et al., 2001). They would also receive assistance in terms of human resources and expertise for habitat restoration where natural disturbances occurred (like cyclones) and, more recently, for the development of ecotourism using qualified and trained local guides. The administrative status of the multi-use forest station changed in 2015 to that of a New Protected Area (category VI of IUCN) recognized by the Malagasy government, following the establishment of a development and management plan. Areas were delimited for conservation or for agriculture, and the mode of governance was defined with the objective of transferring management to local populations (Reniala, 2013).1 Inclusive governance was chosen to maximize chances of success (as per Andersson and Agrawal, 2011; Wright et al., 2016). It should be noted that forest policies developed since 1990 in Madagascar have considered local human communities and management transfer as a major component for successful project outcomes (Levrel, 2008; Razafindrabe, 2015).
However, in Antrema, not all villages and individuals may have perceived their role or benefit as equitable in these new principles of governance. The history of local communities indeed obeyed more or less different political and economic logics before the advent of this conservation-oriented development aid, and then followed their own dynamics (Harpet, in press). In addition, while these new rules have been established with the support of a representative council made up of the Sakalava (the major ethnic group in the area) village committees, they were placed under the spiritual power of the Prince, the guarantor of protection of symbolic and natural identity values over the area (Harpet et al., 2008; Gauthier, 2013). The question of sustainability arose in a context of varying benefit expectations depending on the villages, partly linked to their level of deference to the authority of the Prince and his privileged relationship with nature: would the co-management of the Antrema protected area with local communities resist increased economic and social pressures? These pressures concerned, and still concern, timber trade, coal mining, the commodification of other local natural resources, and the internal social dynamics including changes in intergenerational relationships and perception of nature. All these aspects were linked to a great extent to the geographical proximity of a large city (Mahajanga), facilitating economic and cultural evolution (in particular through the development of telecommunications and digital technology). Evaluating the efficiency of conservation plans based on management transfer is a difficult task as many criteria should be met to avoid opportunist behaviors in favor of collective, well-understood positive actions (e.g., Razafindrabe, 2015). In addition, as pointed by Blanc-Pamart and Ramiarantsoa (2008), the issue of a possible misunderstanding in the respective roles of the contracting stakeholders may arise in the formalization of these partnerships. There are many reasons why criteria are not fully met, but one is that the meaning of the objects and planned actions described in contractual documents may refer to different perceptions of nature, interpretations and ultimately distinct ontologies (in our case, animistic vs. naturalistic sensu Descola, 2005).
The aim of this paper is to analyze the current situation from a biological and anthropological perspective in some forest areas of the reserve. In particular, we sought to understand whether conservation actions can still be based on the representation of nature including lemurs, an emblematic fauna in conservation ecology.
We undertook a biological characterization of dry semi-deciduous forest fragments and forest/savanna ecotone, analyzing the structure of primate communities within these fragments, and examining the use and representation of the nature by villages within the protected area. With regard to the animal taxa chosen for our investigation, we focused on lemurs which are all threatened, though to a different extent according to IUCN. The respectful relation between humans and a species of lemur (Propithecus coronatus) in the Sakalava society of Antrema has been a crucial anchor in the decision to start a sustainable management plan (Gauthier, 2013). Indeed, in Antrema, ten villages are under the traditional Sakalava authority, embodied by the Prince, who has the power to heal with plants, a knowledge that he received from the Sifaka (i.e., Propithecus coronatus). This representation has traditionally motivated conservation activities by community members not only for ensuring the well-being of the sifaka, but also to promote the ecological health of its forest habitat. It is therefore probable that all the lemurs present in Antrema and the other creatures living there have benefited from the privileged relationship of village inhabitants with this species.
Materials and Methods
Study Area and Study Site
The Antrema peninsula is located in the Boeny region, on the west bank of the Betsiboka river near the town of Mahajunga. The NPA (New Protected Area; S 15°42′–15° 50′, E 46° 00′–46° 15′) covers 12,270 ha including 1,000 ha of marine area (Reniala, 2013). Annual rainfall averages around 1,500 mm and a clear dry season of 6–7 months is observed from April to October (Pichon, 2012; Rasoamanantenaniaina, 2016). Twelve villages and hamlets are established, making about 1,000 inhabitants (vs. 806 in 2006; Navarro, 2008), mainly Sakalava fishermen and their family but also Tsimihety and Betsileo who settled in Antrema. The Sakalava people indeed constitute the most frequently encountered “ethnic” group on the northwest coast of Madagascar, from Diego Suarez (North) to Tulear (South). Originally nomads and zebu breeders, they gradually settled down during their transhumance in the nineteenth and twentieth centuries to become farmers and fishermen depending on the environmental resources available in the area (Fauroux, 1997). From an eco-geographic point of view, the NPA of Antrema belongs to the Western region (Hochreutiner, 1955). It is included in the western low altitude eco-floristic zone (Koechlin et al., 1974; Faramalala and Rajeriarison, 1999). Forest vegetation cover is strongly determined by soil conditions. Thus, 5 plant formations occur within the NPA, depending on the peculiarities of the soil. Dense dry deciduous and semi-deciduous forests are found on ferruginous or sandy soils. Shrub thickets are found on sandy soils while mangroves are found on halomorphic coastal soils, the characteristic species of which is the mangrove tree [Ceriops tagal (Perr.) C.B.Rob., Rhizophora mucronata Lam.]. Savannas are made up of grasses and woody plants with the Bismarkia nobilis palm (Arecaceae). Finally, swamp formations are characterized by the following dominant species: Raphia farinifera (Gaertn.) Hyl. (Arecaceae) and Ravenala madagascariensis Sonn. (Strelitziaceae). According to the most recent inventory used to confirm the Site Bioculturel d’Antrema as a NPA (Goodman et al., 2019), the NPA includes 220 plant species divided into 170 genera and 72 families. The study site is therefore characterized by a very high plant diversity at different taxonomic levels.
Data Collection
The global design of the study was as follows: The selection of sites was made on the basis of a preliminary localization by satellite images (Google earth images), combined with an on-site exploration of the fragments, and taking into account the proximity of the villages to the forests of interest. After GPS positioning of the areas to be studied, the counting of nocturnal and diurnal lemurs along transects was coupled with an analysis of the diversity, biomass and structure of the vegetation, with standing taxonomic identification and herbaria sampling. This standardized design applied to the plant analysis offered a global overview of the specific richness and diversity of the various fragments.
The study took place during two wet seasons in 2012 and 2014, with different sampling effort according to objectives, human resources available, and methods applied. During these two study periods, a cumulative sampling effort of 10 months was carried out for the forest inventory and the structural analysis of the vegetation. A shorter period of about 4 months was devoted to assessing lemur densities, and 6 months analyzing human activities linked to nature and representation of nature by members of the Antrema community. Although our article brings together ethnographic data from 2012 and 2014 (3 months in 2012 and 2014, respectively), it follows more intermittent research that we have conducted since 2008 and which, together, has allowed us to deepen our understanding of the human situation in Antrema.
•Human perception and use of nature: In 2012, 49 semi-structured interviews (Sauvayre, 2013; Abric, 2016) were carried in several villages and hamlets (Antrema andafiroa, Antrema aranta, Mangararabo, Ankoririka, Ambalarano, Masokoamena, Kapahazo, Ampapamena, Beankama, Bevoravo, Bako; Figure 1). The sample included, in terms of profession/activity, housewives, fishermen, farmers, cattle breeders, students, committee members. All the interviews were individual interviews conducted in the local dialect (Sakalava) with simultaneous translation provided either by the reserve guides or by Malagasy students who participated in the research. In parallel we implemented a specific methodological tool on 26 individuals out of this panel, balanced between men and women, to draw up an inventory of the perceptions and uses of some plants and animals representative of the territory studied. This methodological tool is derived from biophysical studies aimed at objectifying the individual sensory preferences associated with the perception of a stimulus (e.g., likes and dislikes; Simmen et al., 2004). Briefly, it consists of presenting each person tested (in a single, individualized session) a set of photos of the flora and the fauna, which he or she then arranges along a scale marked by a series of labels read aloud in vernacular language. The 7-point scale allows the participant to position the animals or plants from those who are least appreciated, or that he may even wish to disappear, to those he likes the most (Supplementary Table 3). Unknown species have a separate label (even though, in general, plants and animals used for testing were common). Note that the translation was carried out by carefully taking into account the difficulties of the language and the heterogeneity of the audience questioned. Seventeen animal species (including insects, lizards, fossa, lemurs,…) and 31 plant species (trees, palms, lianas, aquatic plants, monocotyledone) were tested. Pictures of plants and animals—a list of which is provided in Supplementary Table 4—were taken and printed in situ by CMH, the designer of the method. In a few cases (the aye aye, Daubentonia madagascariensis, and the fossa, Cryptoprocta ferox) the photos were taken from naturalist books. Although fun for participants whatever their age (15–87-year old), sex, place of residence, or profession, the experiment was designed to avoid too long a test period. The test was always combined with simultaneous interviews to make the perception scores intelligible. This broad taxonomic approach of plants and animals (Harpet et al., 2014) was preferred over a specific focus on a few plant or animal taxa (like lemurs) to broaden our analysis of the overall perception and representation of living beings by community members.
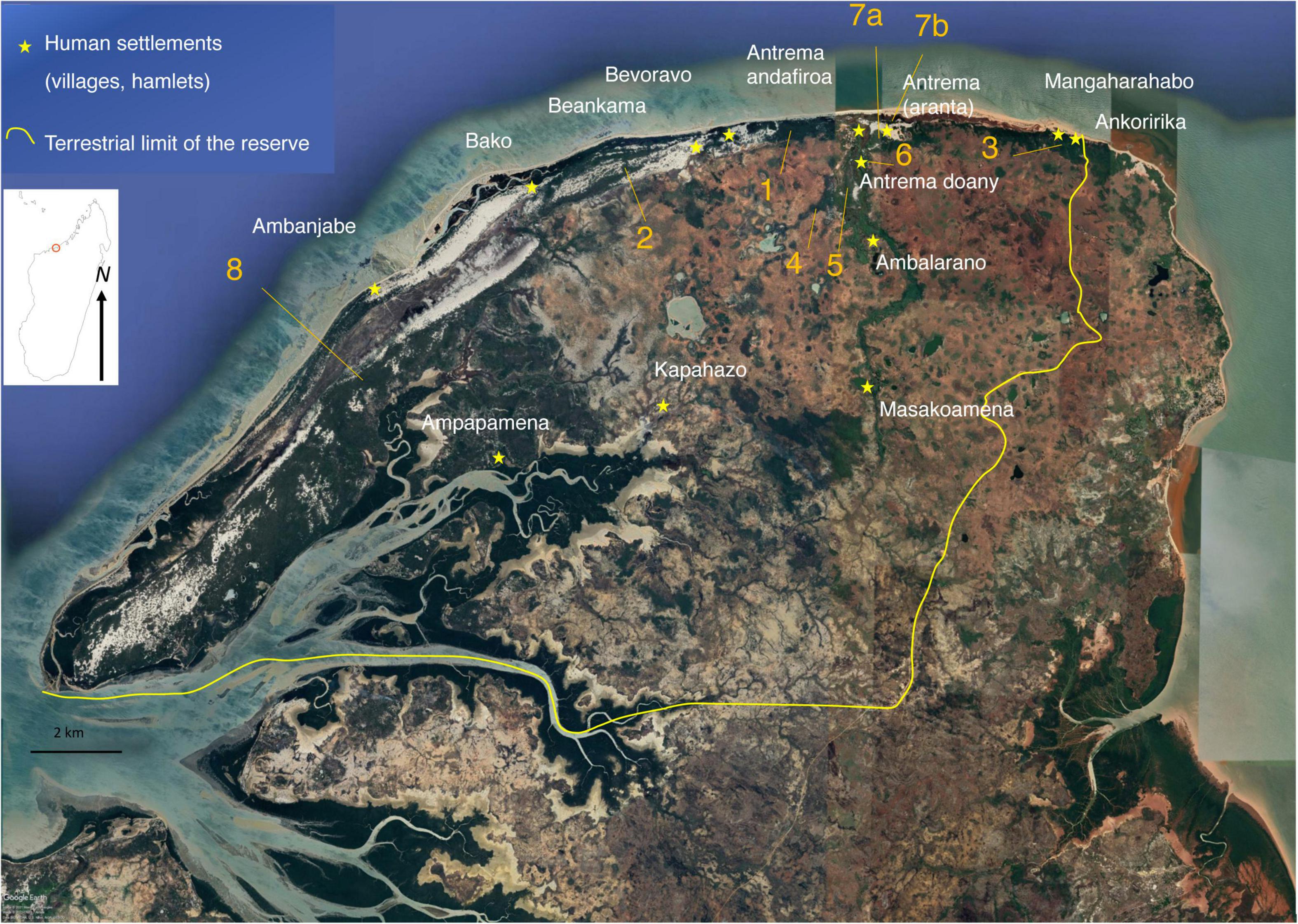
Figure 1. Location of villages, lemur surveys and forest sites prospected in Antrema. The inset map shows Madagascar and the study area (open circle). Note that the following forest sites (identified and located by their number) are sometimes named after the nearest village: 1, Badrala; 2, Beankama/Bako; 3, Ankoririka/Mangararabo; 4, Matsaboriandolo 1–2; 5, Matsaboriandolo 3; 6, Antrema doany; 7a, Antrema aranta; 7b, Antrema aranta (dune/mangrove); 8, Ambanjabe. Map data: Google, CNES/Airbus, Maxar Technologies, Data SIO, NOAA, U.S. Navy, NGA, GEBCO.
In 2014, we carried out a more in-depth ethnographic analysis of 5 villages (or hamlets) around the forest of Badrala and Beankama/Bako, chosen in relation to their increasing daily exploitation. The villages were those of Antrema (Antrema andafiroa, Antrema aranta), Bevoravo, Beankama, and Bako. Antrema doany, the prince’s isolated residence (the Ampanjaka), was also included in these investigations. Fifty semi-structured interviews focusing on knowledge and uses of the forest were carried out while we accompanied the participants in the forest (participant observation; Blanchet et al., 2013). The sample, partly overlapping that of 2012 which included these villages, consisted in women and men of all ages, including the younger generations (Supplementary Table 2). Its purpose was to highlight the possible evolutions in the knowledge and use of the territory. In addition to this sample, we interviewed a few key informants (open-ended interviews), namely persons who have developed a particular connection to their natural environment. These were the Ampanjaka (the Prince), traditional birth attendants or local forest guards (Vômieran’ala) hired by the NPA to monitor the forest. A cartographic work was also carried out by recording GPS data representing the remarkable places (cemeteries, places of ritual at the foot of a tree) spotted during walks in the forest with the inhabitants, and to delimit portions of forests possibly associated with privileged uses.
•Forest structure. The choice of plot locations was determined according to the types of substrates (e.g., white sand forest, lateritic forest, anthropogenic cultivated), the shape of the formations (tree density, height, etc.), the occurrence of lemurs, and proximity to the villages surveyed. Seven sites were selected, of which 6 were fragmented semi-deciduous forest environments and 1 was the anthropogenic cultivated area of the Prince’s residence (Antrema doany) bordering a raffia-swamp forest (Figure 1). Forest fragments, corresponded to areas of lowland dry forest and shrub. These fragments, varying between 3 and 210 ha, were natural forests with low-level selective logging or secondary forest, all delimited by a savannah matrix or by the sandy coastal fringe.
In these sites, 1–10 0.1-ha plots 20 m by 50 m and 1 0.25-ha plot 50 mby 50 m (Ankoririka) were established to inventory tree species greater than 10 cm in diameter at breast height (dbh; total 24 plots). The plots allowed an evaluation of the tree species richness and floristic composition, as well as of tree basal area and distribution in diameter classes. In parallel, we measured at ground level the incident sunlight intercepted by foliage along a transect line (64–1,024 m long), with measurements spaced every 1 m, to calculate a leaf area index (LAI) for each site (measurements period during the wet months; Cournac et al., 2002). Light measurements were made using a “LAIL” laimeter (Cournac et al., 2002) between noon and 2 p.m. to ensure that the sun was closest to the zenith. Measurements were taken twice every meter under the forest canopy. In parallel, light measurements were made once in direct sunlight in the adjacent open space to allow for the calculation of the LAI (see below). The vegetation surveys were immediately adjacent to the transects used for lemurs counts.
•Lemur communities: The population densities of nocturnal and diurnal lemur species were assessed using line-transect censuses in eight sites including the seven sites studied for vegetation). The eighth site was located along the coastal fringe between mangrove and dry forest on dunes (called 7b in Figure 1). We could not document forest structure there because the mangrove was extremely degraded at the time of the study (with many dead mangroves standing) and the forest strip included a sacred area preventing the quantitative study of the flora. Despite this, the mangrove and its periphery were still heavily used by groups of Propithecus coronatus and nocturnal lemur species (Gauthier et al., 1999, pers. obs.) and it offered the opportunity to count lemurs in good visibility conditions. In addition, we briefly assessed the presence of diurnal lemurs west of the reserve where illegal human activities had been reported. This site (called 8 in Figure 1) was not included in the quantitative analysis of the transects.
Typically, transects were walked during the day or at night by a single trained observer, usually accompanied by a local ranger (especially at night). Given the small size of the forest fragments as well as their variable topography and shape, the transects were placed in such a way that they crossed a large portion of the forest fragments (Beankama, Badrala, Matsaboriandolo, Antrema aranta, Antrema doany), or that they were perpendicular to the forest/dune edge (3 parallel transects 700 and 900 m apart at Ankoririka/Mangararabo; transect location in Supplementary Table 1). For the mangrove census, the transect was parallel to the coastline. For each sighting, we recorded the distance of the first individual seen perpendicular to the transect. Distances were measured with a telemeter, and, for social species, were corrected by adding to the individual seen (usually the closest to the observer) an estimate of group radius (half the mean group spread; Fashing and Cords, 2000; Marshall et al., 2008). The center of the group was positioned visually and its distance from the initial individual seen measured with a telemeter or approximated if the group was far away from the observer. During several close encounters with the observers, we spent some time locating as many individuals as possible in a group. It is sometimes recommended to walk at a constant speed during a census walk to avoid meeting a same group twice. However, there was little risk of redundancy here as the animals generally did not flee away, being relatively accustomed to the presence of non-threatening humans. Transects formed a total of 79 and 50 km, respectively, for daytime and night-time observation. Surveys of the nocturnal, solitary lemurs were made with the help of a frontal beam, taking advantage of the reflective power of the tapetum lucidum of these species. Given the uncertain identification of small nocturnal mouse lemurs at the species level, we will retain the Microcebus sp. nomenclature throughout the paper.
Data Analysis
From the plant surveys, we calculated various diversity indices. Species richness was calculated as the number of species recorded per plot. Species diversity was calculated as the Shannon-Wiener index H’ as follows (Marcon and Morneau, 2010):
where pi is the proportion of species i relative to the total number of species and S is the number of species. From the tree dbh measurements, we calculated the basal area of each plot or set of plots as the sum of the basal area of individual trees. We established the Importance Value Index (IVI; Rasingam and Parthasarathy, 2009; Lü et al., 2010) for each plot, a measure that combines the relative dominance (basal area) with the relative density of each species in a given site. The IVI was calculated as follows:
where ni is the number of stems of species i relative to the total number (N) of stems and gi is the basal area of species i relative to total basal area (G).
In order to calculate the LAI per plot, light measurements made along transects in a given plot were averaged. The LAI was calculated as the average ratio of the light under the canopy to the light in the adjacent open space multiplied by an extinction coefficient that takes into account the angle and orientation of the foliage and the zenith angle of the sun. Measurements are also corrected for attenuation of light in cloudy conditions (Cournac et al., 2002). In order to compare LAI profiles between forest sites, the LAI values were organized by decreasing values in each plot rather than by successive measurements along the transect.
To estimate lemur density (ind. km–2), we used the Kelker method in which the cut-off visibility distance was established for each lemur species in each forest site (Charles-Dominique and Hladik, 1971; Robinette et al., 1974; Marshall et al., 2008). It allowed comparison of lemur densities across all sites surveyed taking into account species and habitat differences in lemur detectability. We did not use the criterion of 50% drop in detection (Kun-Rodrigues et al., 2014), but rather examined the distribution of the histograms (Marshall et al., 2008) and drew a cumulative curve of observations to define the cut-off distance (i.e., the strip width). Specifically, we fitted a linear regression on the data using a stepwise ascending procedure, from the shortest distance to the farthest ones. The distance beyond which the regression adjusted less well to the data is considered the cut-off distance (Supplementary Figure 1). We then calculated population density using only observations inside the strip width as determined above for each species and forest site. Since the shape of the detection curve follows more complex models, we used a second method, where applicable, that more accurately models the distance between the observer and lemurs according to the whole structure of the sighting data (distance sampling software with a half normal/cosine detection function; Buckland et al., 2001; Thomas et al., 2010). As the latter method is not suitable where the number of contacts is excessively low, we limited its use to species and sites for which > 20 independent detection events were obtained, following Peres (1999). However, even with a large number of observations, the standard deviation can be large, so estimates derived from a small number of contacts should be considered the least reliable. We calculated the average encounter rate per kilometer of transect to account for this uncertainty. Lemur biomass (kg. km–2) was estimated from density estimates using published body mass of wild animals (Rasoloarison et al., 2000; Andriaholinirina et al., 2006; Pichon and Simmen, 2015; Gordon et al., 2016).
Statistical tests were performed with R version 4.0.3 (R Core Team, 2020) and RStudio version 1.3.1056 (RStudio Team, 2020), using basic packages and FactoMineR, facto extra, and corrplot packages. A principal component analysis (PCA) was performed on habitat characteristics (forest sites, tree density, leaf area index, …) to look at their relationship with lemur abundance. A hierarchical cluster analysis (with Wards’ method) was performed to reveal clusters among forest sites. We established the relationship between the average appreciation score of the plants tested and the diversity of uses of these same plants by the inhabitants of Antrema interviewed using the Spearman coefficient.
Results
Forest and Shrub Areas
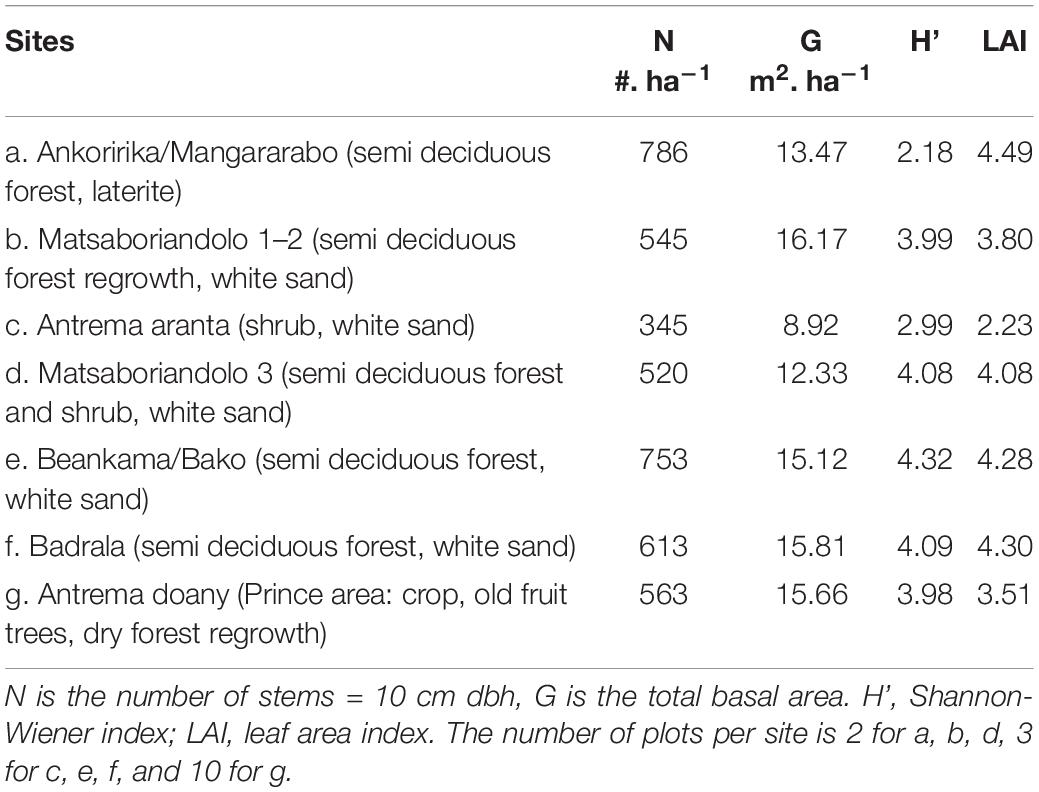
The study sites varied greatly in tree density, species diversity and structure (Table 1). Total tree basal area varied between 8.9 m2. ha–1 (minimum 6.6 m2. ha–1 in a subplot) and 16.2 m2. ha–1 (maximum 24.1 m2. ha–1 in a subplot) in the different forest fragments with the expectedly lowest values in the most bushy area (Antrema aranta “village”; Table 1 and Supplementary Table 5). The sand forests and laterite forests had a comparable tree basal area. Only the bushy area had a much lower basal area, as expected. Disturbances were observed in the plots and took the form of zebu grazing combined with an increasing trunk harvest for domestic use. Wood harvesting was observed near areas with a denser human population. In parallel, the number of trees was highest in plots where many trees had been cut down for local use (Ankoririka, Beankama). There, we observed few large trees but a large number of small ones instead (Supplementary Table 6). The mean LAI was also highest in the sites with greatest tree abundance.
The Prince area, Antrema doany, where large mango trees alternate with clear forest regrowth in old crops (e.g., cashew tree plantations) and open areas (fields and gaps for pasture) was, in terms of basal area and species diversity, comparable to the dry forests studied, reflecting a very heterogeneous habitat The LAI profiles of each area (Figure 2) showed that the forests of Ankoririka and Beankama were the most homogenous, with a relative continuity of the forest cover (as evidenced by the slightly decreasing slope before the inflection point and a low proportion of points after the inflection). In contrast, the shrub area of Antrema aranta was much more open, reflecting a very heterogeneous habitat. The LAI profile of Antrema doany (Figure 2) showed a steadily decreasing slope over the entire range of values, reflecting more punctual, relatively weak disturbances but a strong modification of the forest structure in which forest patches are not maintained. In this comparison, the sample size was uneven across sites (due to varying sizes of forest fragments), but even if we adjust the length of all LAI transects to that of the shortest, the differences in profiles between these sites remain unchanged.
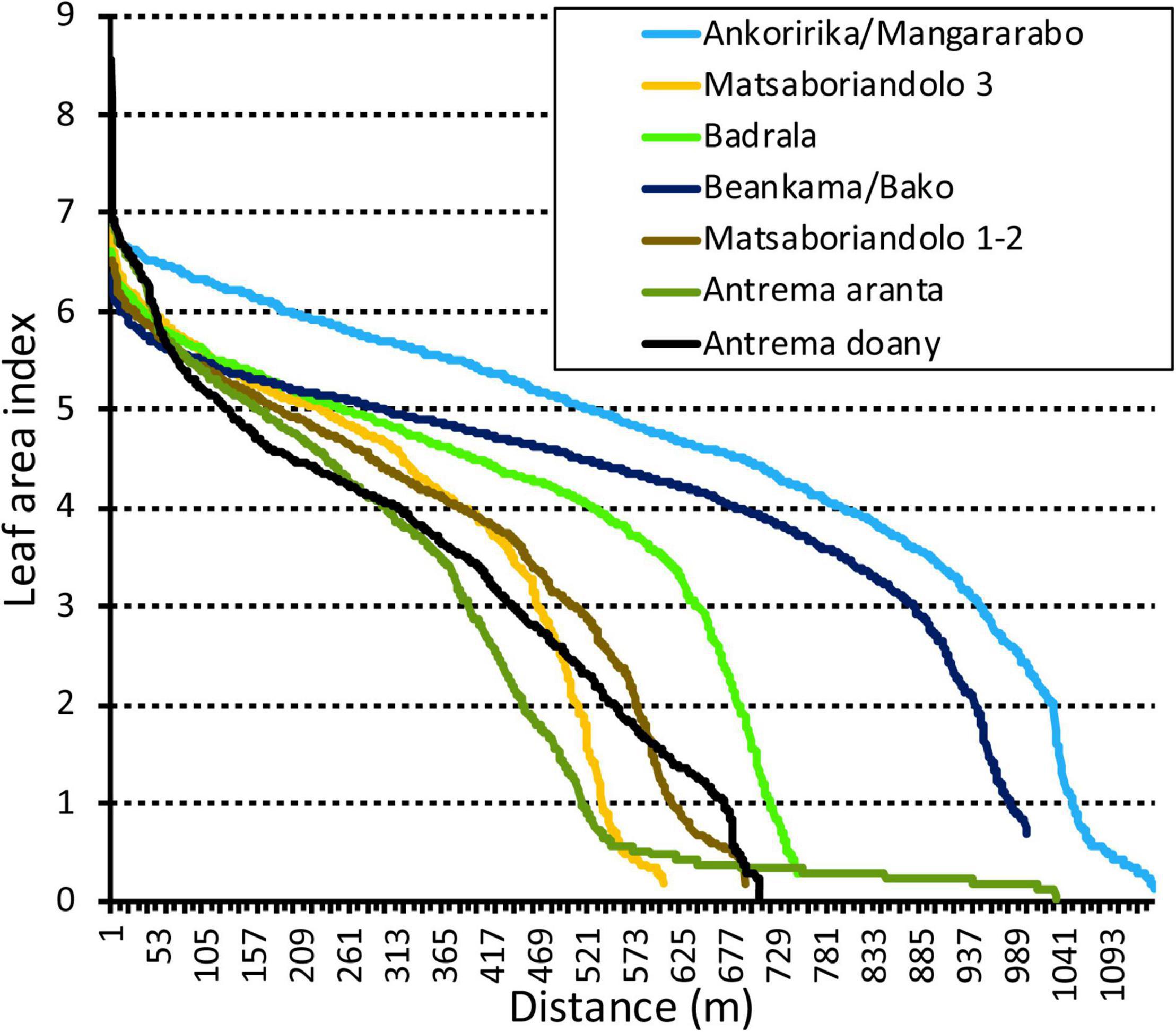
Figure 2. LAI profile in different forest sites. For each profile, LAI values measured every 1 m along a transect are arranged by decreasing values (the x-axis shows the cumulative distance walked in each transect). The steepness and inflexion of the slope indicate the gradient from discontinuous to continuous canopy or disturb vs. undisturbed forest area. For instance, the steep slope of the Antrema doany profile reflects transitions from patches of old mango trees and cultivated fruit tree plots to relatively open fields and gaps while Ankoririka forest shows a more homogenous forest cover.
The diversity of tree species was high in the white sand forest plots compared to the lateritic forest (Table 1).
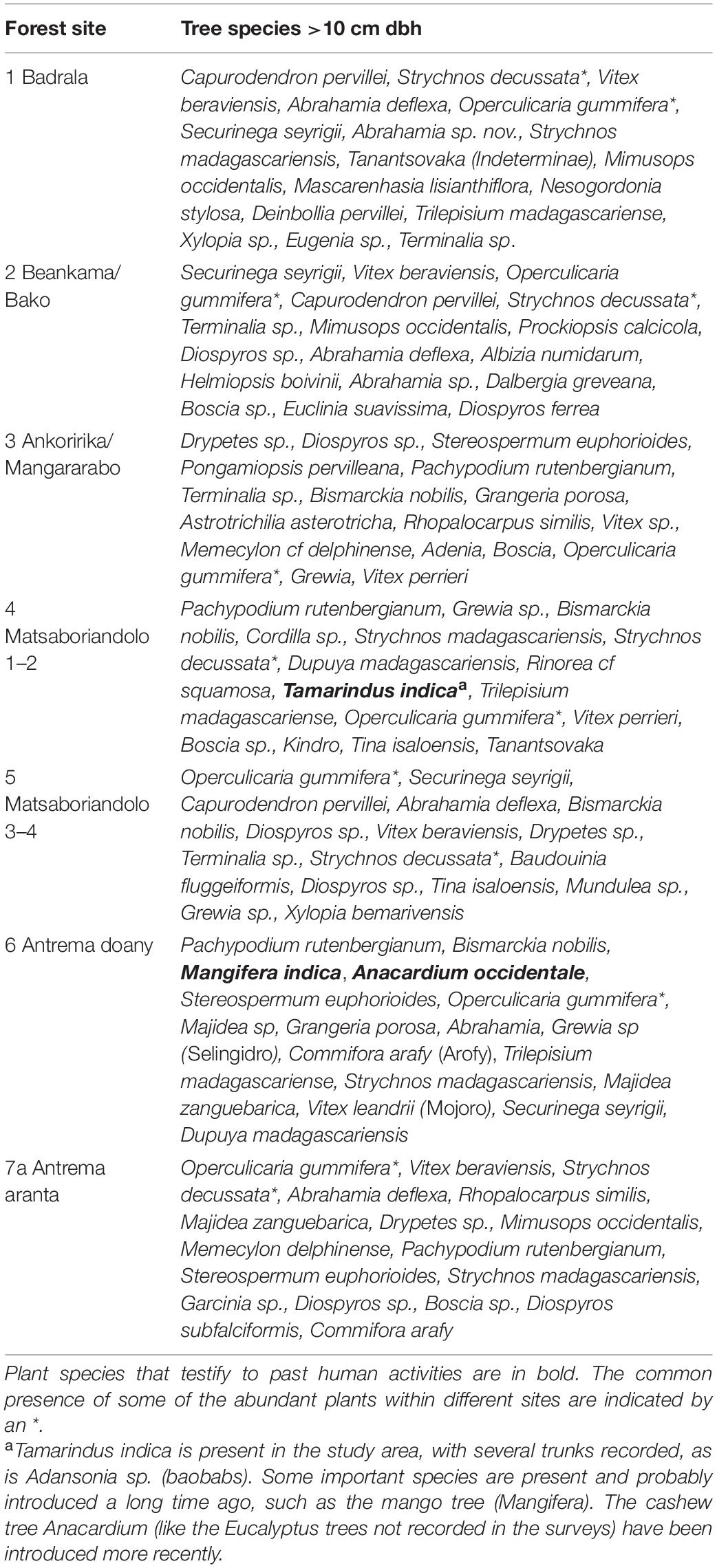
Forest expansion over the savannah was evidenced in Matsaboriandolo 1–2 where Bismarckia palms, usually growing in savannahs, were found inside the forest (but near the edge; Table 2). Tamarind (Tamarindus) trees, a witness of past human activity, also occurred in this forest recruit. The hierarchical classification performed on the forest structural variables revealed 5 clusters among the 7 sites studied (Supplementary Figure 2). This indicated that these areas can be distinguished by their own structural specificity despite the recurrent association of some major plant species (e.g., Strychnos decussata and Operculicarya gummifera; Table 2 and Supplementary Table 7). As expected, Antrema aranta dry thicket differed the most from other sites.
Lemurs and Forest Characteristics
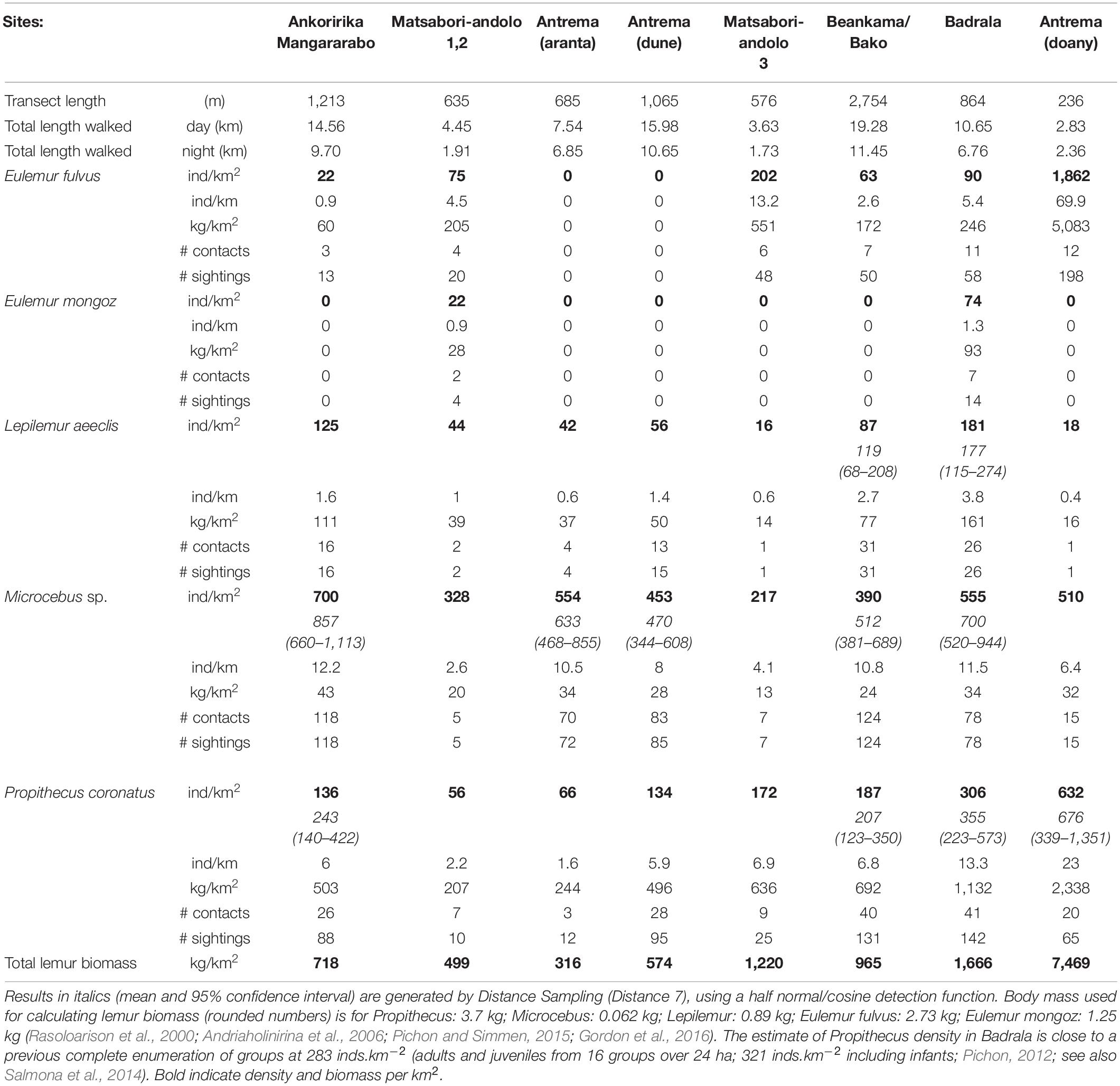
Results of counts and estimates of lemurs sighted during transect walks are presented in Table 3. Mouse lemurs (Microcebus sp.) were very abundant in most sites. The density of predominantly folivorous species, i.e., Lepilemur aeeclis and Propithecus coronatus, was above 100 ind.km–2 in high canopy forests, whether growing on white sand or on a lateritic soil. The density of Lepilemur aeeclis was low in shrub areas, dune forest, and in the wooded agricultural area of the Prince residence (Antrema doany) where it was marginally detected. Propithecus coronatus and Eulemur fulvus on the contrary were disproportionately sighted in the vicinity of the Prince’s residence.
Propithecus coronatus was also regularly seen feeding on green mangoes in Antrema andafiroa village and other anthropogenic areas in Antrema. In Ankoririka, sightings of P. coronatus were concentrated near the edge of the forest where large trees are protected (some are sacred, like baobabs), while others provided these lemurs with sleeping sites.
All lemur species were present in the dry forest regrowth of Matsaboriandolo 1–2, although L. aeeclis, P. coronatus, and Microcebus sp. were only in intermediate densities compared to the other high-canopy forest areas studied. Eulemur mongoz, was rarely observed throughout the study, but was sighted there during the day. The presence of a 6th lemur species could not be ascertained but a solitary animal with slow movements and a morphology close to that of a Cheirogaleus medius was observed twice by NA and BS in two distant closed-canopy forest sites (Matsaboriandolo 3 and Badrala). Interestingly, Sakalava community members use two close vernacular names to describe what they identify as two forms of a small nocturnal lemur-like species (Tsiditsidika and Tsidy).
If we exclude the highly anthropogenic Prince area where densities of Propithecus coronatus and Eulemur fulvus as high as 632 and 1,862 ind.km–2 were recorded, respectively, a PCA performed on site characteristics with population densities of each lemur species as supplementary variables (Figure 3) revealed the following associations: Propithecus coronatus abundance was associated with the closed canopy forests characterized by large total basal area, generally tall trees. The other predominantly folivorous species, Lepilemur aeeclis, was abundant where tree density was high and formed a closed canopy, as indicated by a high LAI, whether they grew on white sand or on lateritic soils. Eulemur fulvus, the most frugivorous species, ranged preferentially in habitats with high plant species diversity. Eulemur mongoz exhibited a pattern similar to that of E. fulvus, but unfortunately the low number of sightings makes this result less certain. We note that pairs of E. mongoz have been repeatedly sighted near villages independently of transect counts, suggesting that this species is able to move away from the forest to come closer to domestic food sources.
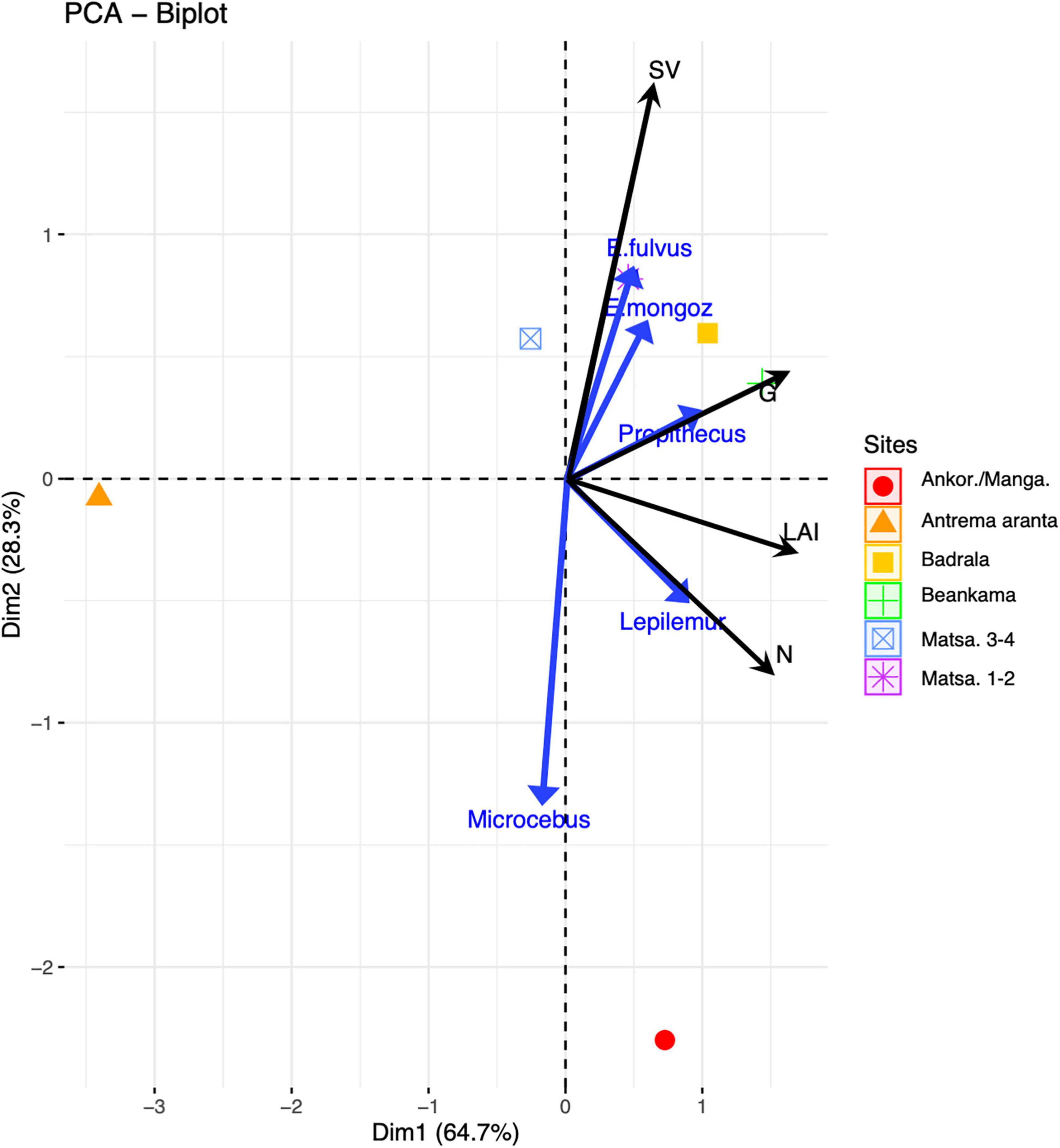
Figure 3. Principal components of forest characteristics (6 sites), with lemur densities as supplementary variables (blue arrows). N, number of trees ≥10 cm dbh per ha; G, total basal area per ha; SV, Shannon-Wiener index; LAI, leaf area index. Forest sites follow the same code as in Table 1 (note that Antrema doany, i.e., the Prince’s area of residence, was not included in this analysis; see Text). Dimension 1 loads positively on LAI, N, and G. Dimension 2 loads positively on SV.
The insectivorous/frugivorous Microcebus sp. was associated with forest fragments characterized by a low plant diversity. This small nocturnal primate was most abundant in the dense forest vegetation composed of trees with moderate girth, like the lateritic forest of Ankoririka/Mangararabo. Microcebus sp., however, was by far the most abundant primate species in all forests studied (Table 3), especially in the bushy area with small trees or shrubs. Several of these preferred habitats provide a wide range of physical supports for moving and searching for invertebrates (the “fine branch” niche; Martin, 1972), or for sheltering from predators, especially aerial ones.
From our estimates of lemur biomass (Table 3), Propithecus coronatus accounted for two thirds (range: 51–86%) of the total lemur biomass on average (excluding the Prince area). As expected, the shrub area harbored less lemur biomass than other forest types. This was mainly due to the infrequent use of such habitats by the large lemurs, presumably in relation to a low canopy, low primary production, and discontinuity of forest cover (as proxied by the LAI profile). The forest on ferralitic soil (Ankoririka/Mangararabo) harbored less lemur biomass than the three white-sand forest sites (Badrala, Beankama, Matsaboriandolo3) despite comparable LAI and tree abundance. The total biomass of lemurs in forest areas showing clear signs of logging (Ankoririka, Beankama) or in forest recruit (Matsaboriandolo 1–2) was lower than in the other forest sites (e.g., Badrala, Matsaboriandolo 3–4).
Local Community: Perception of Animal and Plant Species and Forest Use
The results of the perception test revealed the individual appreciation of the faunistic and floristic elements of the environment (Figure 4). Animals were more frequently judged negatively or insignificant (median score at 30) compared to plants. Animals were disliked or feared because they hurt (caterpillar, millipede) or because they are detrimental to domestic animals (black kite, fossa). Few animals in the sample were highly valued by a majority of people beside Ambassis sp. (Karara) and other fishes, a major consensual food resource with trade interest. Propithecus coronatus was given a median score corresponding to a quite useful or slightly appreciated animal. Although it was “highly appreciated” by some people, this sacred (hasina) primate was considered “neutral or insignificant” by many others (40%), accounting for the large interval between quartiles of the distribution. A same pattern was shared by two other lemurs (Eulemur species) while these species are not founder animals in the tradition anchored around the crowned sifaka. We noted, however, that people rating Propithecus coronatus as neutral or insignificant often indicated that this animal is fady (prohibited). Primates active at night like mouse lemurs and lepilemurs were less appreciated than the previous species but still were positively valued or were eventually considered insignificant. Only two animals were unknown to most people tested: a butterfly species and the aye-aye. It should be noted here that the aye-aye, in the geographical areas where it is present, is considered in Malagasy mythology as an ominous and frightening animal (Harpet, 2011) but this lemur is absent in Antrema.
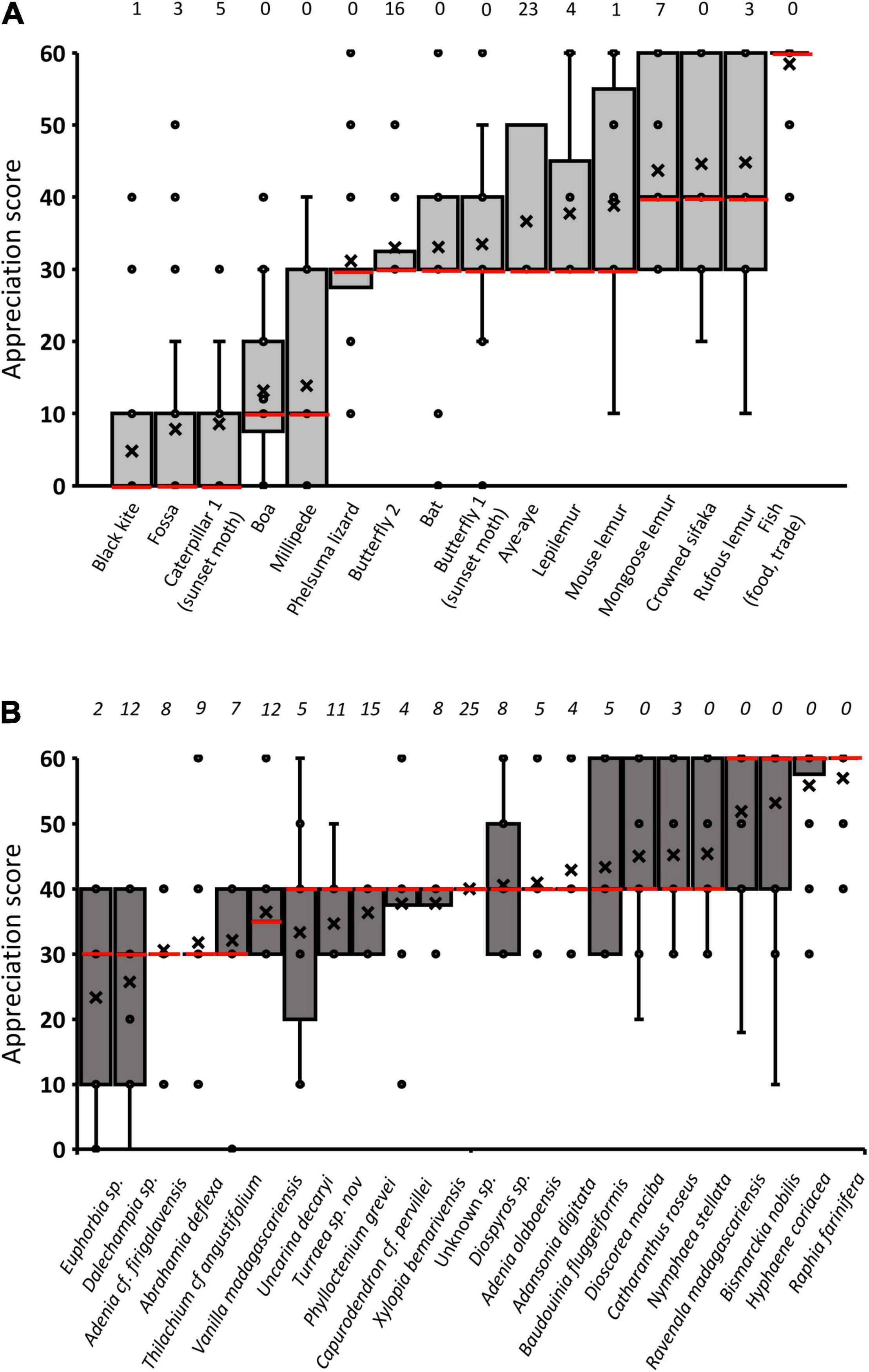
Figure 4. Box plots of the appreciation score for a set of animals (A) and plants (B), assessed using a 7-point labeled scale. The scores vary between 0 (least appreciated, or that one may wish to disappear) and 60 (the most desired), with an intermediate label (30) attributed to insignificant animals or plants. The median is indicated as a red horizontal bar in each box. Other horizontal bars delineate the 25 and 75% quartiles. Means are represented by a cross and outliers by dots. Figures above bars indicate the number of persons in the sample for whom the tested animal or plant is unknown.
Plants were appreciated for various purposes including medicinal properties, construction, symbolic attributes or cosmetic use, with different palm species and ravenalas being most highly valued (Figure 4). Compared with animals, a larger number of plants (mainly forest plants) were unknown to a greater portion of the humans sampled, even when other individuals in the sample assigned a positive value to these species (e.g., the relatively frequent vanilla Vanilla madagascariensis or Turraea, a species with a synchronous spectacular flowering in the understorey).
Most plant parts were used for more than two purposes, but were mainly known in relation to health reasons and as a building material (Figure 5). Plants used as foods (fruits, tubers and roots, stipe of Bismarckia) were slightly less frequently reported and a few species had a magical-religious symbolic dimension. In a correlation analysis, we found that the more the uses reported by a large number of people, the more positive the average appreciation score (Spearman rho = 0.46, p< 0.05). This reflected the relationship between the collective symbolic and material knowledge of the plant, and its overall degree of appreciation. This correlation should not mask the fact that the assessment scores also reflect dimensions that are independent of use. For instance, the latex of the coralliform Euphorbia sp. (“samata”), a plant species with the lowest average rating, known to most people (Figure 4B), is useful for trapping birds and cauterizing wounds, but is also a dangerous cause of blindness according to interviewees. In our sample, however, few plants tested were associated with an ambivalent collective perception (e.g., the lesser-known Dalechampia, which is stinging for some people but medicinal for others).
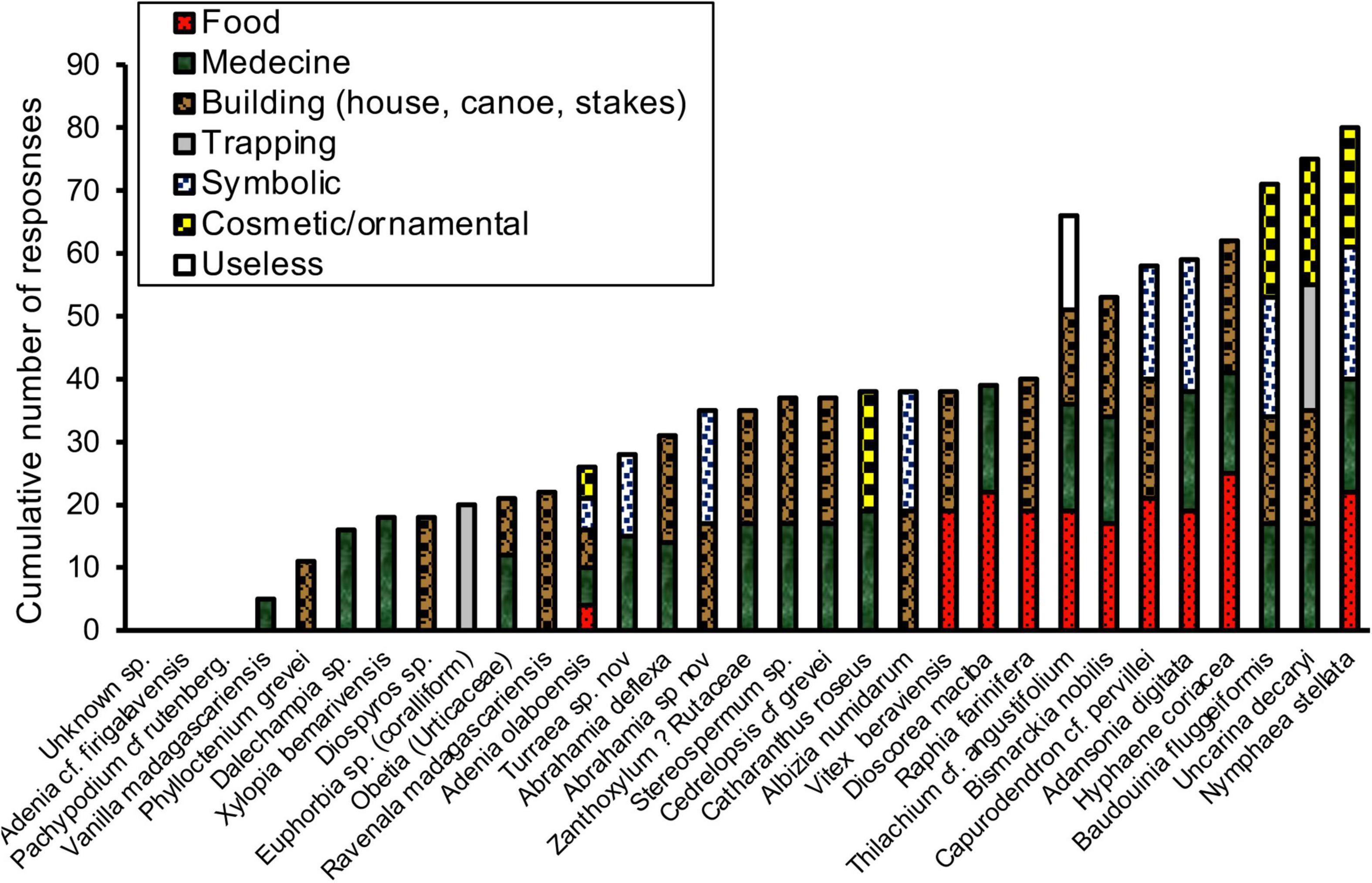
Figure 5. Spontaneous declarative responses with respect to plant use for each plant species presented as a picture in 26 Antrema inhabitants (same panel as in Figure 4). The various uses of the vegetative or reproductive plant parts have been grouped for easier presentation.
All the interviewed members of the community favored the forest area closest to their homes as a space of daily use (collecting dead wood and harvesting forest products), first of all for convenience but also to comply with a form of right of use. Interviews carried out in the villages close to the forests of Badrala and Beankama/Bako (Figure 1) for instance revealed the division of the forest of Beankama/Bako into two relatively equal parts despite the apparent forest cover homogeneity of the area. The use and frequentation of what thus appeared to be three portions of forests from an ethnological point of view, were not the same. It seems that since the creation of the Antrema protected area, a local rule has been established between the inhabitants of Bako and Beankama: each of them only goes beyond the limits of their own forest when necessary, for example when a very important plant species is present in the “Beankama” forest area and cannot be found in “Bako” forest. According to the inhabitants of these two villages, the purpose of forest delimitation, besides user right and convenience, is to facilitate the monitoring of the forest against loggers not belonging to the NPA. The inhabitants of Bako ensure the surveillance of the southern part of the forest and those of Beankama the northern sector against intruders. The inhabitants of more distant villages may sometimes have come to explore these forests, but at the risk of being sanctioned.
The inhabitants nevertheless report the gradual disappearance of several useful tree species in recent years, which can have consequences on the fitness of lemurs. Some large trees (Vitex beraviensis, Securinega seyrigii, Cedrelopsis greveii, Albizia,…) serve as shelter or staple food for lemurs but Vitex beraviensis is used for the construction of canoes while hard woods like Securinega seyrigii and Cedrelopsis greveii are used for building houses. Cedrelopsis grevei is also used for its properties in traditional medicine, two characteristics much appreciated by both the Antrema inhabitants and illegal logging protagonists. Due to the depletion of Cedrelopsis, a logging transfer is exerted toward other tree species, such as Vitex in Badrala and Securinega in Beankama beside other tree species.
Despite this, the Antrema inhabitants declare that “the forest has been present since the dawn of time and always will be, because if it disappears, life will also disappear” which is an unthinkable concept. The vision of an abundant and generous forest rich in natural resources is still present in the local representations, which can be reflected in the generally positive or beneficial perception of forest plants for most of the people interviewed. Even the rating of some plants or animals as “insignificant” on the scale of perception amounts to considering that each species holds a specific place in the order of the world of Antrema inhabitants.
Discussion
In this study, we sought to document the characteristics of forest patches, all subject to human activities, as well as the abundance and habitat preferences of the lemurs present in Antrema. Likewise, we investigated the representation of nature among the local human community, who included various ethnic groups alongside the predominant Sakalavas, to provide elements on the consistency of conservation actions. Beyond the sacred nature of the sifakas and the presence of other endangered lemurs reported during premiminary surveys in Antrema, the high total biomass and abundance of lemurs found in the present study retrospectively confirm the interest of creating a protected area in this region of Boeny (Gauthier, 2013). It seems that the ancestral and recent conservation rules coupled with international environmental regulations enacted to protect the fauna and flora, have been efficient for these coastal forests and forests fragments, at least those located in a large perimeter around the Prince’s residence. For instance, savannah fires are decreasing since the protection of the area in 2000, with one consequence being that grasses (Aristida rufescens, Heteropogon contortus, and Hyparrhenia rufa) consumed by zebus in the savannah gradually disappear and are replaced by the inedible grass Aristida barbicollisa and forest recruits. As a consequence, there is a shift from savannah to closed savannah and regrowth of the forest which provides new habitats for wildlife, including lemurs that were found in significant population densities and biomass in forest recruits.
Ecological Correlates of Lemur Abundance and Primate Biomass
The estimated total biomass of lemurs in the different fragments of medium to high canopy forest in Antrema was in the upper range of biomass recorded in other deciduous forests of Madagascar showing no or little sign of hunting (0.5–1.7 tons.km–2 in Antrema vs. 0.3–0.8 tons.km–2 in 6 western sites or 0.74–1.9 tons.km–2 in the more southern site of Berenty; Ganzhorn, 1992; Simmen et al., 2012). Even in the dry shrub thicket and forest recruit, total lemur biomass (0.3–0.5 tons.km–2) compared well with the biomass found for some tall forests in Madagascar, especially wet forests (e.g., <0.4 tons. km–2 in Analamazaotra and Ranomafana; Ganzhorn, 1992). One would be inclined to conclude that both the abundance of sifaka as well as the high densities found for other species of lemurs (with species variations depending on the habitat) testify to a good community dynamic in these sites. From an ecological perspective, the abundance of lemurs may result from the fact that the dry forests in Antrema are relatively productive. Except in the bushy area and forest regrowth, the mean LAI (a proxy for aboveground biomass) was above 4, a value which falls in the range of 3.6–6 reported for humid forests of Madagascar (Cournac et al., 2003). Similarly, the total tree basal area in these forest fragments was close to or greater than those found in other relatively undisturbed dry forest in Madagascar (e.g., 8–12 m2.ha–1 in Kirindy, unpublished data 2007)—but low compared to rainforests (e.g., 22.4 m2.ha–1 in Manongarivo; D’Amico and Gautier, 2000). In addition, the availability of leaves over a large part of the year (phenological data for Badrala in Pichon et al., 2015) is favorable to maintaining folivorous species. It buffers the effect of the dry season against a possible energy and nutrient imbalance, which may greatly contribute to the survival and reproductive efficiency of Propithecus coronatus, and possibly other seasonal breeding species feeding on leaves like Lepilemur aeeclis.
Along with this global analysis, we found local variations in the structure of the lemur communities in Antrema, the ecological causes of which can be briefly examined. For example, Lepilemur aeeclis, a folivorous primate with a small home range, seems to be sensitive to continuity of forest cover and high tree density. Unlike Propithecus coronatus, another leaf-eating primate common in most forest fragments studied, Lepilemur was not abundant in the wooded agricultural area of the Prince’s residence. This suggests an ecological need for thick forests in Lepilemur aeeclis, perhaps more than in Propithecus. Although the abundance of these two species in Antrema forest fragments was positively correlated (discarding the Prince data), Lepilemur biomass was 8 ±3 times lower than that of sifakas on average. The reasons are not clearly understood but a similar trend in the relative biomass of these species was found in the riverine forest of Anjamena, a moderately disturbed forest close to Antrema area (1995 data; Müller et al., 2000).
The smallest primate in Antrema, Microcebus sp., was abundant in most of the sites studied, suggesting an opportunistic ecological niche as already described for some mouse lemur species (e.g., Microcebus murinus; Andriatsitohaina et al., 2020). It was found in conspicuously high densities (217–700 inds. km–2) compared with other dry forests in Madagascar (with same or different mouse lemur species: <250 inds. km–2, Ganzhorn, 1992; Anjamena: 85 inds. km–2, Müller et al., 2000), but much higher densities of this insectivorous/frugivorous genus can be found elsewhere in Madagascar (Setash et al., 2017).
As for Eulemur fulvus and E. mongoz, both species seemed to be more abundant where plant species diversity was high. However, Eulemur fulvus was also disproportionately sighted in the vicinity of the Prince’s residence, foraging in large, relatively shy groups. Little is known of its ecology in Antrema and its surroundings, but studies of other Eulemur species living similarly in large social units report that they are not very territorial, feed opportunistically on high quality resources, and can travel very long distances in search of fruits (Overdorff, 1993). A similar foraging pattern is possible in Antrema: several groups of E. fulvus were attracted by the same large patches of fruit crop (mango and cashew trees) in the Prince area while being less frequently sighted elsewhere in the forest. Eulemur mongoz was rarely seen during the study, but the mongoose lemur is a pair-living species that exhibits both diurnal and nocturnal activity (Curtis et al., 1999). This behavior, combined with short distances traveled daily, decreases detectability and potentially leads to an underestimation of its density. In addition, we have seen this species marginally near villages, suggesting that it also forages outside forest fragments and probably uses anthropogenic food sources, like Eulemur fulvus.
Finally, a possible case of resistance of lemurs to natural disturbances deserves attention, that of sifakas living in the unusual habitat of the mangrove/coastal fringe facing the village of Antrema aranta (Figure 1). The density of sifakas was remarkable given the impressive destruction of most of the mangroves after the 2005 cyclone blocked the flow of fresh water by accumulating sand at the mouth of the river. The continued presence of groups as well as the fact that some of them had babies in 2012 and 2014 suggested that this population may be on the way of approaching a new demographic balance.
Lemurs and Forest Fragmentation
Lemurs appear presently well protected in areas investigated in our study, but we don’t know the extent to which fragmentation can have an impact in the long term, especially related to the risk of inbreeding. To date, a regional metapopulation study of Propithecus coronatus (Sgarlata et al., 2016) found a high genetic diversity in Antrema for such pattern of forest fragmentation, which reflected efficient gene flow. The absence of large predators like the Fossa (but the presence of the raptor Polyboroides is attested) presumably facilitates individual dispersal between forest fragments (Foltz, 2009). We observed similar movement patterns in several lemurs (Microcebus, Eulemur mongoz) which crossed areas with little or no tree cover. According to the meta-analysis of Andriatsitohaina et al. (2020), mouse lemur species like Microcebus murinus can maintain population connectivity by crossing roads or other open areas, but this requires forest habitats to be spatially close enough to allow individual dispersal. In modeling analyses, Eppley et al. (2020) found that E. fulvus and E. mongoz were little affected by forest fragmentation as long as the proportion of forest and forest cover remained high. However, the author showed that the area of habitats available affected the abundance of E. fulvus, suggesting a threshold of suitable habitats (Eppley et al., 2020) to which we add a criterion of high plant diversity (this study). These ecological requirements were not met throughout Antrema forest sites surveyed, which may partly explain the highly variable densities of E. fulvus, with no sightings in some areas. The forest fragments outside the anthropogenic zone were relatively small and distant one from each other (Figure 1), which could restrict the mobility of a species that depend on temporally and spatially dispersed food like fruits. Contrary to other lemurs, there is evidence that Lepilemur in dry forests are relatively intolerant of forest loss and fragmentation, especially when forest patches are small (Lepilemur edwardsi; Steffens and Lehman, 2018). Despite their small home ranges, small body size and folivorous diet, the minimum area requirements of Lepilemur may be quite high for their size. Finally, edge effects, which are known to influence forest vegetation, appear to have modest and sometimes positive effects on lemur densities in patches of humid forests in the southeast (Lehman et al., 2006). In the dry forests of Antrema, however, it cannot be ruled out that the small size of forest patches and the proximity to the sea have different effects.
Perception of Nature and Social Transitions
The quantitative rating of the elements of flora and fauna documented in our experimental study necessarily offers partial insight into the representation of nature by Antrema inhabitants. The associated interviews nevertheless make it possible to specify the panel of human-nature representations. Despite the socio-economic disparities between community members and multi-ethnic composition of the villages in Antrema (which may affects knowledge and perception of nature), the Sakalava local authority, i.e., the Prince, associated with a representative council of village inhabitants, maintains a global feeling of belonging to the Antrema community. In particular, the Prince, who has the power to communicate with the ancestors thanks to its privileged relationship with the sacred (« hasina ») lemur Propithecus coronatus (Harpet et al., 2008), still federates many people around beliefs and representations of the world through an ontology corresponding globally to an animist vision (Descola, 2005). This animist conception of nature is shared by the entire Sakalava community, including the population converted to Islam.
Maintaining tradition, such as prohibitions and communication with the invisible world through the elements of the forest still contributes greatly to the maintenance of the social order. It gives meaning to life and allows people to find their place in the universe. If familial influences outside Antrema also contribute to the identities of the inhabitants, the forest is a divine creation and is still considered a “bountiful source of resources.” According to the members of the community, since humans do not own the forest, its use requires an attitude of respect toward the elements that compose it and is subject to rules, the transgression of which is likely to attract “misfortune.” This could be reflected in the fact that not only the sacred sifakas, but also other lemurs including nocturnal lemurs, were positively rated by most people in our experiments. However, as indicated by Fauroux (1997), the absolute trust placed in the divine can lead to the development of an attitude relatively close to indifference toward the visible evolutions affecting the forest, for instance due to increased anthropic activities. In our perception tests, an unexpected number of people regarded Propithecus coronatus and other lemurs as insignificant animals, which could reflect a change in the representations of nature, and lemurs in particular.
In fact, our observations suggest a gradual transition/integration of traditional rules with international environmental regulation. This may be a sign of a new ecological consciousness, facilitated by messages conveyed by the NPA management stakeholders on the degradation of forest cover. In particular, some people have acquired the status of guide or forest ranger, training has been given by a variety of conservation actors, including Malagasy researchers, with the aim of transmitting ecological knowledge about the natural environment. The Antrema inhabitants have noticed the disappearance of several tree species, and the demarcation of the Beankama/Bako forest in two parts supervised by two villages could be a sign of the integration of the implicit rules of the rights of use with the ecological awareness of the degradation of the forest and the need to protect it. While community members see illegal logging by intruders from outside Antrema as the cause of the increasing degradation of forest resources—and one of the reasons for forest surveillance—it is nonetheless important not to fall into the simplistic native/migrant opposition. The situation would require an in-depth study on the control of resources in the region, specifically on the power struggles that exist between the different actors of the territory, whether these are different villages, companies, or the forestry administration, traditional and state authorities included. According to this less optimistic vision, the integration of traditions of the local communities in the pro-environmental management of the Antrema NPA, as promoted by the development programs focused on conservation, can be a means of local empowerment, a factor of social tensions (Goedefroit and Revéret, 2007).
Future studies should focus on these new issues, especially in the context of the forthcoming transmission of the ancestral power of the Prince (Ampanjaka) to its successor.
Conclusion
This study highlighted the existence of an abundant lemur community in the forest areas studied. This characteristic could be linked to the high productivity of dry forests locally, but also to the forest structure of the different zones which allowed the specific ecological requirements of lemurs to be met. Lemur species also appear to be currently well protected by traditional rules and new environmental regulations applied to this protected area.
However, these conclusions remain fragile and should be reconsidered at different time scales. For instance the question arises as to whether the abundance of lemurs could result from a crowding effect due to the small size of the forest fragments. While a contraction of the forest could lead to overpopulation, on the contrary we found signs of forest expansion, such as the presence of savannah plant species in several forest plots. Current genetic data on Propithecus coronatus indicate that gene flow is maintained, possibly because this species crosses open areas between fragments. We observed at least two other species of lemurs crossing areas with little or no tree cover. In any case, the viability of lemur populations from density estimates would require ensuring that animal abundance does correspond to healthy populations (Irwin et al., 2010).
On the anthropological effects, the populations of lemurs there, like other forest-dwelling animals still depend heavily on social dynamics and agro-sylvo-pastoral practices. For example, the extraction of timber in the protected areas surveyed in this study could be seen as problematic for the fauna in the mid-term, especially for trees serving as shelter or staple food for lemurs. The Antrema inhabitants themselves reported the recent disappearance of some large tree species in the forests close to their village, indicating that they were increasingly aware of forest degradation. However, the attribution of excessive timber harvesting to intruders as well as the beliefs that the forest cannot disappear does not facilitate the questioning of certain rights of use and hamper the effectiveness of the action and management plan around conservation in Antrema. Moreover, perception tests and associated interviews have shown that even a species traditionally considered sacred (Propithecus coronatus), could now be considered neutral or insignificant by a substantial proportion of people. The potentially changing representation of the sacred (“hasina”) and the forbidden (“fady”) clearly need to be followed over time in relation to the evolution of customary rules developed around the Prince. In this dynamic where the pro-environmental management of the Antrema NPA is based on traditions, the risk of local empowerment and inequitable governance of the territory between villages is possible. In addition, some of the lemur populations were potentially at risk in several forest areas far from the Prince’s residence west of the reserve). These areas, that we could not investigate in details and where charcoal mining and illegal logging are less easy to control, contain the most important dry forest cover of Antrema. Community scientific research efforts should preferably focus on these areas without delay.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study was authorized under permits issued by the Ministère de l’Environnement, de l’Ecologie et des Forêts of Madagascar (N° 264/14/MEEF/SG/DGF/DCB.SAP/SCBSE and 255/15/MEEMF/SG/DGF/DAPT/SCBT). The participants provided their informed consent to participate in this study.
Author Contributions
BS, CH, and BR conceived the study. BS wrote the first draft of the manuscript. BR designed and established the plot network. CH and CP conceived the ethnographic work. C-MH designed the perception test. BS and BR established the lemur counting areas. BR, CH, BS, HLTR, RE, and MC contributed to the discussion and interpretation of data, revised and complemented the manuscript. All authors collected field data and approved the submitted version.
Funding
This research was supported by the Labex BcDIV 2012 (« Diversités biologiques et culturelles : origines, évolution, interactions, devenir »), ATM 2014 (Muséum National d’Histoire Naturelle, Paris). The GEPFE (G. Koppert) provided financial support to the students.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank C.-A. Gauthier and the MEF Madagascar for allowing us to carry out this research in Antrema. We thank Alifah Monaecha for her help in the lemur censuses. We are grateful to L. Tarnaud who contributed to the analysis of lemur densities and to Ravoniarisoa Jolicia Baptistine for her assistance in collecting anthropological data. We thank Vavindraza for her logistic aid, Zo Tahina Nomenjanahary for his botanical assistance in the field and P. Phillipson for his help in the initial identification of the plants sampled. We thank the reviewers for their constructive comments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.772808/full#supplementary-material
Footnotes
References
Andersson, K., and Agrawal, A. (2011). Inequalities, institutions, and forest commons. Global Environ. Change 21, 866–875. doi: 10.1016/j.gloenvcha.2011.03.004
Andriaholinirina, N., Fausser, J.-L., Ross, C., Zinner, D., Thalmann, U., Rabarivola, C., et al. (2006). Molecular phylogeny and taxonomic revision of the sportive lemurs (Lepilemur. Primates). BMC Evol. Biol. 6:17. doi: 10.1186/1471-2148-6-17
Andriatsitohaina, B., Ramsay, M. S., Kiene, F., Lehman, S. M., Rasoloharijaona, S., Rakotondravony, R., et al. (2020). Ecological fragmentation effects in mouse lemurs and small mammals in northwestern Madagascar. Am. J. Primatol. 82:e23059. doi: 10.1002/ajp.23059
Blanchet, A., Ghiglione, R., Massonnat, J., and Trognon, A. (2013). Les Techniques d’Enquête en Sciences Sociales. Paris: Dunod.
Blanc-Pamart, C., and Ramiarantsoa, H. R. (2008). La gestion contractualisée des forêts en pays betsileo et tanala (Madagascar). Cybergeo 426:19323. doi: 10.4000/cybergeo.19323
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L., and Thomas, L. (2001). Introduction to Distance Sampling. Oxford: Oxford University Press.
Charles-Dominique, P., and Hladik, C. M. (1971). Le lépilemur du sud de Madagascar : écologie, alimentation et vie sociale. Rev. Ecol. 25, 3–66.
Cormier-Salem, M.-C. (2006). Vers de nouveaux territoires de la conservation. Ann. Géo. 651, 597–617. doi: 10.3406/geo.2006.21289
Cournac, L., Dubois, M. A., and Riera, B. (2003). “Rapid methods for characterizing forest structure in Madagascar,” in The Natural History of Madagascar, eds S. M. Goodman and J. P. Benstead (Chicago, IL: University of Chicago Press), 96–103.
Cournac, L., Dubois, M. A., Chave, J., and Riera, B. (2002). Fast determination of light availability and leaf area index in tropical forests. J. Trop. Ecol. 18, 295–202. doi: 10.1017/s0266467402002201
Curtis, D. J., Zaramody, A., and Martin, R. D. (1999). Cathemerality in the mongoose lemur, Eulemur mongoz. Am. J. Primatol. 47, 279–298. doi: 10.1002/(SICI)1098-2345(1999)47:4<279::AID-AJP2>3.0.CO;2-U
D’Amico, C., and Gautier, L. (2000). Inventory of a 1-ha lowland rainforest plot in Manongarivo, (NW Madagascar). Candollea 55, 319–340.
Eppley, T. M., Santini, L., Tinsman, J. C., and Donati, G. (2020). Do functional traits offset the effects of fragmentation? The case of large-bodied diurnal lemur species. Am. J. Primatol. 82:e23104. doi: 10.1002/ajp.23104
Faramalala, M. H., and Rajeriarison, C. (1999). Nomenclature des Formations Végétales de Madagascar. Antananarivo: ANGAP.
Fashing, P. J., and Cords, M. (2000). Diurnal primate densities and biomass in the Kakamega Forest: an evaluation of census methods and a comparison with other forests. Am. J. Primatol. 50, 139–152. doi: 10.1002/(SICI)1098-2345(200002)50:2<139::AID-AJP4>3.0.CO;2-N
Fauroux, E. (1997). “Les représentations du monde végétal chez les Sakalava du Menabe” in Milieux et Sociétés dans le Sud-Ouest de Madagascar. ed. J. M. Lebigre (Bordeaux: Cret). 7–26.
Foltz, J. (2009). Structure of a Community of Milne-Edwards sifaka (Propithecus edwardsi) in a Malagasy Fragmented Forest (Demographic, Genetic and Behavioral Approaches). Strasbourg: University of Strasbourg.
Ganzhorn, J. U. (1988). Food partitioning among Malagasy Primates. Oecologia 75, 436–450. doi: 10.1007/BF00376949
Ganzhorn, J. U. (1992). Leaf chemistry and the biomass of folivorous primates in tropical forests. Oecologia 91, 540–547. doi: 10.1007/BF00650329
Gauthier, C.-A. (2013). “Le projet bioculturel d’Antrema à Madagascar : 15 années de valorisation d’un patrimoine naturel et culturel” in Revue Liaison Energie-Francophonie 95, Le Tourisme Durable Pour Valoriser le Patrimoine Naturel et Culturel. eds O. Tebbaa and G. Ruiz (Quebec: IFDD). 71–77.
Gauthier, C.-A., Deniaud, J. L., Rakotomalala, M., Razafindramanana, S., and Renson, G. (1999). Note sur la découverte d’un nouvel habitat occupé par les propithèques couronnés (Propithecus verreauxi coronatus) au nord-ouest de Madagascar. Primatologie 2, 521–527.
Gauthier, C.-A., Huguet, S., Rakotondravony, D., and Roger, E. (2001). Schéma D’aménagement et de Gestion de la Station Forestière d’Antrema (Katsepy). Madagascar: Report for the Ministère des Eaux et Forêt.
Goedefroit, S., and Revéret, J. P. (2007). Etudes Rurales n°178. Quel Développement à Madagascar ?. Paris: Ecoles des Hautes Études en Sciences Sociales.
Goodman, S., Raherilalao, M. J., and Wohlhauser, S. (2019). The Terrestrial Protected Areas of Madagascar: Their History, Description, and Biota. United States: University of Chicago Press.
Gordon, A. D., Johnson, S. E., and Louis, E. E. (2016). Environmental correlates of body mass in true lemurs (Eulemur spp.). Int. J. Primatol. 37, 89–108. doi: 10.1007/s10764-015-9874-9
Harpet, C. (in press). “Entre règles coutumières et règlementations internationales. Regard anthropologique sur la préservation d’une forêt dans le Nord-Ouest Malgache (presqu’île d’Antrema)” in La Protection Juridique des Forêts : perspectives Nationales et Internationales. ed. R. Brett (Paris: Harmattan).
Harpet, C., Combo, A. S., Hladik, C. M., Simmen, B., and Riera, B. (2014). Méthode d’appréciation des perceptions et des représentations locales sur un échantillon ciblé de la faune et de la flore de la côte ouest de Madagascar. Rev. Ecol. 69, 351–355.
Harpet, C., Navarro, L., and Ramanankirahina, R. (2008). Rôle et Implications des croyances et des savoir-faire locaux dans les programmes de conservation : exemple d’un site à lémuriens sacrés au cœur de la Station Forestière à Usages Multiples d’Antrema (pays Sakalava). Rev. Écol. 63, 289–292.
Hochreutiner, B. P. G. (1955). “129e famille – Malvaceae” in Flore de Madagascar et Comores. ed. H. Humbert (Paris: Firmin-Didot).
Irwin, M. T., Junge, R. E., Raharison, J.-L., and Samonds, K. E. (2010). Variation in physiological health of diademed sifakas across intact and fragmented forest at Tsinjoarivo, eastern Madagascar. Am. J. Primatol. 72, 1013–1025. doi: 10.1002/ajp.20847
Koechlin, J., Guillaumet, J.-L., and Morat, P. (1974). Flore et Végétation de Madagascar. New Zealand: Inland Revenue Department.
Kun-Rodrigues, C., Salmona, J., Besolo, A., Rasolondraibe, E., Rabarivola, C., Marques, T. A., et al. (2014). New density estimates of a threatened sifaka species (Propithecus coquereli) in Ankarafantsika National Park. Am. J. Primatol. 76, 515–528. doi: 10.1002/ajp.22243
Lehman, S. M., Rajaonson, A., and Day, S. (2006). Edge effects and their influence on lemur density and distribution in Southeast Madagascar. Am. J. Phys. Anthropol. 129, 232–241. doi: 10.1002/ajpa.20241
Leigh, E. G. Jr., Hladik, A., Hladik, C. M., and Jolly, A. (2007). The biogeography of large islands, or how does the size of the ecological theater affect the evolutionary play. Rev. Ecol. 62, 105–168.
Lü, X.-T., Yin, J.-X., and Tang, J.-W. (2010). Diversity and composition of understory vegetation in he tropical seasonal rain forest of Xishuangbanna, SW China. Rev. Biol. Trop. 59, 455–463.
Marcon, E., and Morneau, F. (2010). Mesures de la Biodiversité, Kourou : uMR EcoFoG. Available online at: www.ecofog.gf, 2010 (revised 2014, https://hal-agroparistech.archives-ouvertes.fr/cel-01205813).
Marshall, A. R., Lovett, J. C., and White, P. C. L. (2008). Selection of line-transct methods for estimating the density of group-living animals: lessons from the primates. Am. J. Primatol. 70, 452–462. doi: 10.1002/ajp.20516
Martin, R. D. (1972). Adaptive radiation and behaviour of the Malgasy lemurs. Proc. Phil. Trans. R. Soc. 264, 295–352. doi: 10.1098/rstb.1972.0013
Méral, P., Froger, G., Froger Andriamahefazafy, F., and Rabearisoa, A. (2009). “Le financement des aires protégées à Madagascar : de nouvelles modalités” in Aires Protégées, Espaces Durables ?. eds C. Aubertin and E. Rodaryn (Marseille: IRD). 135–155.
Müller, P., Velo, A., Raheliarisoa, E.-O., Zaramody, A., and Curtis, D. J. (2000). Surveys of sympatric lemurs at Anjamena, north-west Madagascar. Afr. J. Ecol. 38, 248–257. doi: 10.1046/j.1365-2028.2000.00247.x
Navarro, L. (2008). Ethnoécologie des « Espèces Patrimoniales » de Madagascar : le cas des Baobabs, Lémuriens et Ravenalas Dans la Station Forestière à Usages Multiples d’Antrema. Usages et Perceptions Comme Indicateurs de Patrimonialité et Outils de Conservation. Paris: Muséum national d’histoire naturelle.
Overdorff, D. J. (1993). “Ecological and reproductive correlates to range use in red-bellied lemurs (Eulemur rubriventer) and rufous lemurs (Eulemur fulvus rufus)” in Lemur Social Systems and Their Ecological Basis. eds P. M. Kappeler and J. U. Ganzhorn (New York: Plenum Press), 167–177. doi: 10.1007/978-1-4899-2412-4_12
Peres, C. A. (1999). General guidelines for standardizing line-transect surveys of tropical forest primates. Neotrop. Prim. 7, 11–16.
Pichon, C. (2012). Contraintes Écologiques et Sociales Sur l’acquisition Alimentaire du Propithèque Couronné (Propithecus coronatus) Dans Une Forêt Sèche Semi-Caducifoliée du Nord-Ouest de Madagascar. Paris: Muséum national d’histoire naturelle.
Pichon, C., Hladik, A., Hladik, C. M., Tarnaud, L., Bayart, F., Simmen, B., et al. (2015). Leaf phenological patterns of trees, shrubs and lianas in a dry semi-deciduous forest of northwestern Madagascar: functional types and adaptive significance. Rev. Ecol. 70, 197–212.
Pichon, C., and Simmen, B. (2015). Energy management in crowned sifakas (Propithecus coronatus) and the timing of reproduction in a seasonal environment. Am. J. Phys. Anthropol. 158, 269–278. doi: 10.1002/ajpa.22786
R Core Team (2020). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rasingam, L., and Parthasarathy, N. (2009). Diversity of understory plants in undisturbed and disturbed tropical lowland forests of Little Andaman Island. India. Biodivers. Conserv. 18, 1045–1065.
Rasoamanantenaniaina, C. (2016). Caractérisation Écologique et Estimation des Stocks de Carbone des Forets Sèches du Sud de la Nouvelle aire Protégée d’Antrema (Ambanjabe et Ampampamena). Antananarivo: Université d’Antananarivo.
Rasoloarison, R. M., Goodman, S. M., and Ganzhorn, J. U. (2000). Taxonomic revision of mouse lemurs (Microcebus) in the Western portions of Madagascar. Int. J. Primatol. 21, 963–1019. doi: 10.1023/A:1005511129475
Razafindrabe, A. (2015). Politiques forestières et « bonne gestion » des ressources : le cas de Madagascar. Éthique Publ. 17:2. doi: 10.4000/ethiquepublique.2324
Reniala (2013). Plan D’aménagement et de Gestion de la Nouvelle Aire Protégée D’antrema. Available online at: https://rsis.ramsar.org/RISapp/files/4084726/documents/MG2286_mgt161124.pdf
Robinette, W. L., Loveless, C. M., and Jones, D. A. (1974). Field tests of strip census methods. J. Wildl. Manage. 38, 81–96. doi: 10.2307/3800202
Salmona, S., Rasolondraibe, E., Jan, F., Besolo, A., Rakotoarisoa, H., Meyler, S. V., et al. (2014). Conservation status and abundance of the Crowned Sifaka (Propithecus coronatus). Prim. Conserv. 28, 73–83. doi: 10.1896/052.028.0122
Schwitzer, C., Mittermeier, R. A., Johnson, S. E., Donati, G., Irwin, M., Peacock, H., et al. (2014). Averting lemur extinctions amid Madagascar’s political crisis. Science 343, 842–843. doi: 10.1126/science.1245783
Setash, C. M., Zohdy, S., Gerber, B. D., and Karanewsky, C. J. (2017). A biogeographical perspective on the variation in mouse lemur density throughout Madagascar. Mam. Rev. 47, 212–229. doi: 10.1111/mam.12093
Sgarlata, G. M., Salmona, J., Razanaparany, T. P., Rabarivola, C. J., Jan, F., et al. (2016). Mitochondrial genetic diversity in the Crowned Sifaka (Propithecus coronatus) in a fragmented landscape. Prim. Conserv. 30, 39–57.
Simmen, B., Pasquet, P., and Hladik, C. M. (2004). “Methods for assessing taste abilities and hedonic responses in human and non-human primates” in Researching Food Habits: methods and Problems. eds H. Macbeth and J. MacClancy (Oxford: Berghahn Books). 87–99.
Simmen, B., Tarnaud, L., and Hladik, A. (2012). Leaf nutritional quality as a predictor of primate biomass: further evidence of an ecological anomaly within prosimian communities in Madagascar. J. Trop. Ecol. 28, 141–151. doi: 10.1017/S026646741200003X
Steffens, T. S., and Lehman, S. M. (2018). Lemur species-specific metapopulation responses to habitat loss and fragmentation. PLoS One 13:e0195791. doi: 10.1371/journal.pone.0195791
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., et al. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14. doi: 10.1111/j.1365-2664.2009.01737.x
Vielledent, G., Grinand, C., Rakotomalala, F. A., Ranaivosoa, R., Rakotoarijaona, J. R., Allnutt, T. F., et al. (2018). Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biol. Conserv. 222, 189–197. doi: 10.1016/j.biocon.2018.04.008
Wright, G. D., Andersson, K. P., Gibson, C. C., and Evans, T. P. (2016). Decentralization can help reduce deforestation when user groups engage with local government. Proc. Natl Acad. Sci. 113, 14958–14963. doi: 10.1073/pnas.1610650114
Keywords: dry forest, plant diversity, human-nature coexistence, ecological awareness, community management, flagship species, primate population density
Citation: Simmen B, Harpet C, Hladik A, Edmond R, Pioch C, Combo AS, Andriaholinirina N, Ranarijaona HLT, Randriamanana LME, Chambon M, Li T, Rasoamanantenaniaina C, Randriarisoa AM, Razanajatovo H, Manzi OJL, Hladik C-M and Riera B (2022) Forest Fragments, Lemur Communities and Local Perception of Nature in a Protected Area of Northwestern Madagascar. Front. Ecol. Evol. 10:772808. doi: 10.3389/fevo.2022.772808
Received: 15 September 2021; Accepted: 03 February 2022;
Published: 24 February 2022.
Edited by:
Mauricio Talebi, Federal University of São Paulo, BrazilReviewed by:
Lu Zhang, Sun Yat-sen University, ChinaMitchell Irwin, Northern Illinois University, United States
Copyright © 2022 Simmen, Harpet, Hladik, Edmond, Pioch, Combo, Andriaholinirina, Ranarijaona, Randriamanana, Chambon, Li, Rasoamanantenaniaina, Randriarisoa, Razanajatovo, Manzi, Hladik and Riera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bruno Simmen, bruno.simmen@mnhn.fr
 Bruno Simmen
Bruno Simmen Claire Harpet1,2
Claire Harpet1,2  Hery Lisy Tiana Ranarijaona
Hery Lisy Tiana Ranarijaona Olivier Jean Leonce Manzi
Olivier Jean Leonce Manzi Bernard Riera
Bernard Riera