Short- and Long-Term Social Effects of Parental Sex Roles in Zebra Finches
- 1Department of Ethology, Eötvös Loránd University, Budapest, Hungary
- 2Institute of Animal Welfare and Animal Husbandry, Friedrich-Loeffler-Institut, Celle, Germany
- 3Department of Animal Behaviour, Bielefeld University, Bielefeld, Germany
- 4Behavioural and Physiological Ecology, Groningen Institute for Evolutionary Life Sciences, University of Groningen, Groningen, Netherlands
- 5School of Biological and Marine Sciences, University of Plymouth, Plymouth, United Kingdom
- 6Department of Biology and Biochemistry, University of Bath, Bath, United Kingdom
Parental care is among the most widespread and variable behavioral traits between and within species, associated often both with large fitness costs and benefits. Despite its fitness consequences and evolutionary significance, we know very little about the ontogeny of this behavior, specifically, whether and how social experiences from parents contribute to the development of parental care. Here we used a split-family experimental design to produce uniparentally raised zebra finch nestlings that were provisioned either only by their mother or their father from shortly after hatching until independence. We investigated whether zebra finch nestlings pay attention to who takes care of them (short-term social effects) and whether parental sex roles, i.e., how much each parent provides to offspring, are socially learned and how these early social experiences influence negotiation rules of parental effort as adults (long-term social effects). We found pronounced short-term effects: uniparentally raised young socialized more with their “caring” than with their “non-caring” parent in a two-way choice test and begged more for food from them. When paired as adults based on their caring parent, some combinations of these uniparentally raised finches did not coordinate normally during incubation as first-time parents. By nestling provisioning (and their second breeding) even these pairs assumed normal distribution of parental effort and we therefore conclude that early social experiences influence parental sex roles and coordination, but these can be overridden by own social experiences with the mate when starting to breed.
Introduction
Parental care is among the most beneficial, and, at the same time, among the costliest traits that influence fitness. The large impact on survival and future reproduction on each family member selects for coordination between parental and offspring behaviors, to balance the benefits of offspring needs being satisfied and the costs of parental effort. In species with biparental care (the prevalent type in birds, with ca. 90% of species), both parents contribute to provisioning the offspring (Cockburn, 2006; Royle et al., 2012). Although parents cooperate, sexual conflict (the antagonistic evolutionary interests of the sexes) manifest also in this type of care because each parent gains more if its partner invests to a greater extent into their joint reproduction while decreasing its own share (Arnqvist and Rowe, 2005; Harrison et al., 2009; Royle et al., 2012).
Theoretical models distinguish two possible ways to resolve sexual conflict over care in biparental species. If parental effort has evolved to a fixed best effort in relation to the parental effort of the other sex, and pair members cannot change their effort dynamically based on the effort of their partner, the resolution is reached on an evolutionary time scale (“sealed bid” model; Houston and Davies, 1985). In contrast, if parents dynamically adjust their parental effort as a response to that of their mate, the resolution is reached behaviourally on a real-time scale (“negotiation” models; McNamara et al., 1999, 2003; Johnstone and Hinde, 2006; Lessells and McNamara, 2012). Accumulating empirical evidence supports the second scenario, so that parents use negotiation rules when determining their own level of parental effort and this depends on that of their mate (Harrison et al., 2009; Iserbyt et al., 2017; Savage et al., 2017; Lendvai et al., 2018). Negotiation models imply that parents, by paying close attention to each other, indicate their condition, workload and how they perceive the need of the offspring or the food sources to each other, hence this social information can help pairs to adjust their parental effort in a coordinated manner (Johnstone and Hinde, 2006).
Although theoretical models along with empirical studies have provided a more in-depth view at the selection pressures shaping parental care, we are far from a comprehensive understanding of how parental care patterns evolve and develop. Specifically, we know very little about how parental sex roles and negotiation rules are passed on from one generation to the next. The behavioral flexibility implied in the negotiation models suggests that learning may play an important role for parental care. This is likely to affect also offspring experience, potentially resulting in social inheritance of parental care patterns. Social inheritance can have numerous advantages over genetic inheritance, including faster response and more flexibility to environmental changes (including the social environment; Boyd and Richerson, 1995; Laland, 2004). Furthermore, family life provides ample opportunities for social interactions and observing parents during negotiation. Based on these premises, we investigate here to what extent parental sex roles and negotiation rules are socially learned rather than genetically inherited. We used the monogamous, biparental zebra finch (Taeniopygia guttata) in which parental sex roles differ according to the stage of the breeding cycle. Nest-building is mostly carried out by males, whereas females allocate more of their time to incubation. Post-hatching care (including offspring provisioning and brooding), is shared approximately equally between the parents (Morvai et al., 2016; Krause et al., 2017).
Choosing the zebra finch allowed us to build our study on a growing body of research that uses this species as model to understand how sex roles and sexual conflict are shaped by social experience. First, research into various aspects of sexual imprinting (i.e., the process by which young socially learn about the characteristics of its species and later sexual partners) provided insights into the significance of social learning with regards to sex roles using this small passerine (Immelmann, 1972; Bischof and Clayton, 1991; Vos, 1995; Burley, 2006; Schielzeth et al., 2008). Social learning is well-documented in this species also in other contexts including mate choice copying (i.e., observing and copying the mate preferences of others in the population (Swaddle et al., 2005; Drullion and Dubois, 2008), foraging (Benskin et al., 2002; Katz and Lachlan, 2003; Farine et al., 2015; Templeton et al., 2017), nest building (Guillette et al., 2016), and song learning (Jones et al., 1996; Roper and Zann, 2006; Kniel et al., 2015; Yanagihara and Yazaki-Sugiyama, 2016). Second, the effect of sexual conflict over parental care on offspring fitness have been demonstrated by Royle et al. (2002b). In their experiment, female zebra finch parents were allowed to raise nestlings with and without their partner in two consecutive breedings. They found that females, when they took care for their young uniparentally, provided more per capita care than when they cared together with their mate. Thus, females caring together with their mate allocated less effort into offspring provisioning than they were able to when alone, resulting in lower fitness in biparentally cared as opposed to uniparentally cared nestlings. A recent study revealed that vocal communication may contribute to parental negotiation because zebra finch pairs perform call duets when a foraging partner relieves its incubating partner, and the structure of the duet changes depending on the focal partner's returning time to the nest (Boucaud et al., 2016). The third line of relevant research focused on behavioral synchronization in zebra finch parents. Zebra finch pairs synchronize their provisioning and foraging visits with each other throughout the post-hatching period, and the extent of synchronization correlates with the number of nestlings (Mariette and Griffith, 2012, 2015). Moreover, matching behavior of the partners seems to be important in this species generally, as, for instance, Schuett et al. (2011) found that within-pair similarity of exploratory behavior can affect the fitness of their offspring.
We used a split-family experimental design to investigate how social experiences with parents influence from which parent offspring prefer to solicit care, which parental sex roles they assume as adults and how they coordinate parental care. Zebra finch families were divided to male-only and female-only cared half-broods shortly after hatching. Families were split so that the social structure of the family, as well as acoustic, olfactory and visual contact between parents and all offspring were maintained, although each offspring could receive care from only one of their parents (“caring parent” henceforth). We then tested whether recently fledged (i.e., still dependent), zebra finch nestlings pay attention to who takes care of them (short-term effects of uniparental care). Specifically, the experiment allowed us to answer the following questions: (1.1) do uniparentally raised offspring express preference toward their caring over their non-caring parent? We expected young to socialize more with (i.e., spend more time close to) the parent that provisioned them, and also, to expect (beg for) food from this parent. (1.2) If offspring prefer their caring parent, is this preference generalized to parental sex? We expected offspring to socialize more with and beg food from non-kin parents that are of similar sex to their social parent. (1.3) Are there sex differences in how parental sex roles are socially transferred? Parental sex roles are different and fine-tuning them to environmental changes may be more relevant to one sex than to the other. This would be reflected in offspring sex influencing preference in our experiment.
Once these experimental young fully matured, they were allowed to breed two times with other uniparentally raised birds to test how their own and their partners' social experience affected parental effort and coordination i.e., their share during incubation and offspring provisioning (long-term effects of uniparental care). The long-term experiment addressed the following specific research questions: (2.1) do early social experiences (or the lack of them) influence parental sex roles in uniparentally cared birds? If so, we expected parental effort to change based on the interaction of own sex and uniparental care type received (e.g., increased effort of a male-cared male as opposed to a female-cared male). (2.2) Does the lack of negotiation experiences from parents influence the same behavior (i.e., coordination) as adults? (2.3) If we detect differences in parental coordination, do own breeding experiences shape negotiation rules? If social experiences with the mate when breeding as adults also shape negotiation rules, we expected diminishing differences between our experimental groups from the first to the second breeding. (2.4) If we detect differences in parental coordination, do these influence reproductive success?
Materials and Methods
Study Population and Housing Conditions
Our study was carried out between February 2013 and April 2015 at two locations; first, a study population of 47 breeding pairs (“parental generation” henceforth) was established from the domesticated stock of zebra finches at Bielefeld University, Germany (Forstmeier et al., 2007; Hoffman et al., 2014). Males and females were randomly assigned as pairs to cages (83 x 30 x 40 cm) with a wooden nest box (15 × 15 × 15 cm). Following the experimental manipulation and testing for the short-term social effects (see below), after day 35 post-hatching, offspring were kept in an indoor aviary together with two adult tutors from each sex. Following sexual maturation (zebra finches fully mature by around day 100 post-hatching; Zann, 1996), all offspring (“second generation” henceforth) were transferred to Eötvös Loránd University, Budapest, Hungary, where they were allowed to breed and long-term effects were tested. Zebra finch nestlings in our study population started to hatch on day 13–14 of incubation, and started fledging on day 18–20 post-hatching (both locations; Rehling et al., 2012). Parents continue offspring provisioning for ca. ten days after fledging, when fledged offspring follow their parents and beg food from them, while gradually starting to feed on their own. On day 35 post-hatching we separated the nutritionally independent offspring from their parents and kept them together in indoor aviaries. At both locations, birds were cared for following the same protocol. A 14:10 h light:dark cycle (lights on at 6:00, local time) was maintained. Temperature was kept constant at 20–21°C using air conditioning. Birds were provided with ad libitum access to food and water: the seed mixture consisted of three different types of millet, canary grass and a small portion of Niger seed. Egg-food (Egg food tropical finches, Orlux, Versele-Laga, Belgium) and germinated seeds (home-made from the above seed mixture) were provided daily for additional protein and vitamin (for more details on the diet, see Morvai et al., 2016).
Experimental Design
We used a split-family design to investigate short- and long-term effects of whether nestlings experienced parental care from their mother or their father on their parental preferences as juveniles and on parental sex roles and negotiation as adults. When splitting the family at an early stage of post-hatching development, our aim was to maintain the social structure of the family as close to intact as possible. Broods, nests and cages were split in half, but separated by wire mesh so that nestlings in the adjacent nest boxes could also observe their siblings cared for by their other parent (Figure 1). This manipulation allowed visual, acoustic, and olfactory interactions among all family members, while restricting the care to be received from only one of the parents (i.e., from the caring parent) for any given young. Besides being the most conservative manipulation in our view, we also chose this experimental design because theory as well as empirical research suggest that parental behavior of the remaining parent is different when its mate disappears or when it is present but do not contribute to parental care (cf., McNamara et al., 2003; Lendvai et al., 2009).
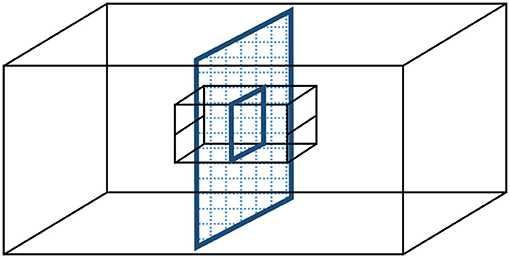
Figure 1. Split-family experimental manipulation to investigate the social effects of uniparental care in zebra finches. Following pair formation and biparental incubation, on day 8 post-hatching, a wire mesh separator was inserted that halved the cage. A parent and half of the brood were placed into each half-cage, and the nest material shared equally between two cardboard nest boxes. The back wall of the nest boxes was removed so that all family members remained in visual, acoustic, and olfactory contact with each other through the wire mesh, while offspring received provisioning from only one of their parents henceforth.
Experimental Protocol—Short-Term Effects of Uniparental Care
Following the establishment of random pairs in unseparated cages, zebra finches received coconut fibers as nest material and nest-building and egg-laying was monitored daily. We considered the reproductive stage as post-hatching from the date when the first egg hatched in a given clutch. On day 8 post-hatching, nestlings were individually marked by cutting their downy feathers on their wings, legs, head and back in a unique combination (Adam et al., 2014). Following body mass measurement by a digital scale (Sartorius PT120, d = 0.01 g), most of the families (n = 39 of 47) were allocated to the manipulated experimental group, so that these families were split into female-only and male-only cared half-broods. The rest of the families (n = 8 of 47) continued as biparental control; in control families, we applied the same protocol as described for the manipulated group but used a wire mesh separator with a hole that did not restrict access of either parents to the nestlings i.e., parents could move freely between the two compartments of the cage. Manipulation of broods (splitting families) were carried out when daily energy demands of young peak on day 8, post-hatching (Lemon, 1993).
When splitting the family, the wooden nest box was replaced by two cardboard nest boxes (each 12 × 12 × 12 cm), attached to the two sides of the wire mesh separator with their entrance facing toward the inside of the cage (Figure 1). The back of these cardboard nest boxes was removed, so that the two nest boxes were separated only by the wire mesh. Following distribution of nest material from the wooden nest box into the two cardboard nest boxes, an equal number of offspring, selected randomly, were placed in each new nest. In case of odd number of nestlings, either the male or the female parent was randomly allocated one extra young. Approximately 75% of the parents from the split families raised successfully their broods (in 19 families both individual parents were successful and 11 male-only caring and 9 female-only caring individuals were successful), so that reproductive success in our experiment was similar to those described previously for captive zebra finches (Griffith et al., 2017). Our study, therefore, included 30 male-only cared half broods (with 44 offspring) and 28 female-only cared half broods (with 45 offspring), in addition to the 8 biparentally cared control broods (with 24 offspring). The number of offspring per nest remained similar between the half-broods cared for by males and females by the start of the preference tests on day 25 post-hatching (mean ± SE no. of fledglings, male-only vs. female-only cared nests: 1.47 ± 0.11 vs. 1.61 ± 0.09; t56 = −0.94; p = 0.352), and these had approximately half of the brood size of the biparentally cared, control nests (3.00 ± 0.46 nestlings).
On day 12 post-hatching, uniparental offspring provisioning was recorded for 3 h (start of recording at 9:00) from outside the cage using digital camcorders fitted with SD cards. The camera view covered the whole cage, so that male-only and female-only care could be quantified. Nestling body mass was measured and nestlings were ringed by a numbered plastic ring for individual identification. On day 16 and 35 post-hatching body mass of nestlings were measured again.
Between day 25 and 27 post-hatching (i.e., when the offspring have already fledged but still depended on parental provisioning), we tested parental preference in a two-way choice apparatus set up in a separate room. The apparatus consisted of three compartments: one stimulus chamber (30 × 40 × 40 cm) on each side of a middle choice chamber (60 × 40 × 40 cm). Stimulus chambers contained one perch each, whereas the choice chamber contained three perches, dividing the choice chamber to three equal zones (left, neutral and right zones, with a perch indicating the center of each zone). To ensure young were hungry and parents were habituated to the choice apparatus, parents were moved to the two side chambers of the apparatus 2 h before the first preference test of the family started and food was removed from the home cages. Offspring were tested individually in a random order; the focal bird was first moved to a small start cage attached to the door of the choice chamber. After ca. 30 s acclimatization, the offspring was released into the choice chamber by remotely operating the door and was then allowed to move freely in the choice chamber for 10 min. After every offspring from a cage were tested in random order, the stimulus birds were swapped to the opposite stimulus chamber and the young were re-tested in the same order as previously to control for possible side effects.
All families (n = 47) involved in the experiment were tested as described above, resulting in parental preference test of 44 male-only, 45 female-only, and 24 biparentally cared offspring. Preference tests were video recorded using digital camcorders for later behavioral coding. The three perches in the choice chamber were also equipped with light barriers, allowing us to monitor parental preference in real-time (time spent close to each parent and number of times they were visited). Since preliminary analysis of the responses of the first 19 families revealed trends in preference toward the caring parent, we carried out further preference tests on two consecutive days, with the subset of the remaining families (n = 28), including 29 male-only, 26 female-only, and 18 biparentally cared offspring. On the day following the parental preference test, offspring were tested again, but this time with two unfamiliar adults (using other parents with recently fledged young i.e., non-kin adults at a similar reproductive stage to that of the genetic parents of the offspring). To account for potential order effects (parental preference test always preceded non-parental preference test), these offspring were tested with their own parents again on the following day (second parental preference test).
Experimental Protocol—Long-Term Effects of Uniparental Care
The sexually mature birds of the second generation were allowed to breed two times. For the first breeding, pairs were formed following a randomized fractional factorial design (i.e., representing all four combinations based on own sex and sex of the caring parent, with control (biparentally cared) birds always paired with other biparentally cared birds). This resulted in the following successful breedings (own sex is given as small letters and the sex of the caring parent as capitals in parentheses, e.g., m(F)/f(M) is a pair in which the male was raised by his mother and the female by her father): n = 5 m(M)/f(M), 5 m(M)/f(F), 6 m(F)/f(M), 3 m(F)/f(F), and 9 m(B)/f(B) (biparental/biparental, control) pairs.
To increase statistical power, after the first successful breeding the focal male or female parent received his/her other potential partner. Pairs were formed by balancing care type to that of their first pair (e.g., a male that received a male-only cared female previously, now was allowed to pair with a female-only cared female). Similarly to their first breeding, biparentally cared control birds were allowed to pair with another biparentally cared bird. The second breeding attempt resulted in n = 5 m(M)/f(M), 2 m(M)/f(F), 2 m(F)/f(M), 5 m(F)/f(F), and 8 m(B)/f(B) families.
Parental behavior of the breeding pairs was monitored using small digital cameras (Mobius Action Cam, JooVuu Store, UK) with wide-angle lenses (116° field of view) that were mounted to the nest boxes. The camera lenses could reach inside the nest through holes cut to the top of the nest boxes, providing a top view of the nest (Morvai et al., 2016, Figure 1). The camera was placed onto the nest box a day before the automated video recording started, so that birds could get used to it. To ensure further that undisturbed parental behavior was recorded, when there was no recording, we replaced the cameras with a same-size dummy camera made of wood. Three hour long video recordings were taken between 10:00 and 13:00 on day 8 of incubation and on day 10, post-hatching. Clutch mass was measured after recording incubation and offspring body mass was measured on day 10, 16, and 35 post-hatching.
Behavioral Coding and Statistical Analyses
Short-Term Effects of Uniparental Care
To assess the preference for socializing with each parent, we coded the time and frequency of visits to all three choice zones (i.e., to the male's zone, to the neutral zone and to the female's zone) from the video recordings of the preference tests using Solomon coder (Péter, 2015). To assess begging preferences of the young, we also coded for how long and how many times they begged for food from the stimulus parent. Data of corresponding zones of swapped trials of a given offspring were summed prior to analysis (Supplementary Table 4).
For each response variable, we calculated the relative response toward the male parent, e.g., for socializing, assessed by the relative time spent with each parent, we calculated the proportion of total time of the two (swapped) trials as:
Time spent in male parent's zone/(time spent in male parent's zone + time spent in female parent's zone)
Statistical analyses were carried out using the R statistical environment (v. 3.5.2; R Core Team, 2015). Short-term effects of uniparental care on parental preference was analyzed in two approaches. First, we used linear mixed models (LMMs, R package “nlme”; Pinheiro et al., 2019) including the parental preference test of all (n = 47) families to investigate the effect of care type (factor with three levels: male-only, female-only or biparental) and offspring sex (male or female) on relative time spent with the father (logit-transformed) and relative time spent begging food from the father (logit-transformed). Second, we analyzed the three repeated preference trials of the subset of the families (n = 28) that were re-tested. These models, in addition to care type and offspring sex, included test repeat (factor with three levels: first parental, non-parental, second parental) as fixed factor. In both of the above analyses, the mixed models included caring parent ID nested in cage ID as random factors. In addition, offspring ID was also included as a nested random term in the analyses of the repeated tests.
Since relative time and relative frequency of visits to the parents (rP = 0.758, n = 111, p < 0.001) and begging from them (rP = 0.944, n = 76, p < 0.001) were highly positively correlated, we show results for relative time only.
In initial models, we tested for the two-way interactions between care type (male-only, female-only, or biparental) and offspring sex, and care type and repeat (only in the models of repeated tests). Furthermore, the possible confounding effects of season (number of days from 25 March i.e., from the start of the experiment), time of day and the exact duration of separation from the parents before the start of the test were analyzed, but since none of these had significant effects, they were excluded from the final models. Stepwise model selection was based on AIC values, and we considered a model to provide a better fit whenever its AIC was lower, and the difference was ≥2. The effects of explanatory variables were analyzed by likelihood ratio tests (LRT); we provide χ2 and the corresponding p-values of LRTs of models with and without the given explanatory variable. In addition, parameter estimates (for LMs and LMMs) and odds ratios [exp(β), for logit-transformed responses in LMMs and for OLRs, see below] with 95% confidence intervals are provided between levels of a given significant fixed effect.
We also investigated whether the strength of preference is predicted by the actual amount of care received from the parents using Pearson's correlation between the relative time offspring spent with the caring parent during the first preference test and nest attendance of the caring parent (proportion of time spent inside the nest) on day 12 post-hatching.
Long-Term Effects of Uniparental Care
Probability to start breeding was analyzed in Cox Proportional Hazards Models (R package “survival,” Therneau, 2015). The models included latencies (in days) until laying eggs that produced young, and occurrence of laying as terminal events, respectively, and care type of the pair received as young (fixed factor with five levels [care type received by the male parent/female parent]: M/M, M/F, F/M, F/F, B/B). Families that did not start laying eggs that produced hatchlings were treated as censored observations (Supplementary Table 5).
From the within-nest box recordings taken on day 8 of incubation and day 10 post-hatching, we coded the following behaviors for each sex separately using Solomon coder: incubation, brooding, being inside the nest and feeding the nestlings (Supplementary Tables 1, 2). Incubation and brooding were defined as when a parent was sitting on the eggs/nestlings or it was in body contact with them (if a parent sat next to its mate on the nest, we considered it as incubating/brooding too, because its body heat likely contributed to warming the eggs/nestlings). Being inside the nest was coded whenever any body part of the bird was visible on the recording, and the bird was doing anything else but incubating the eggs or brooding the nestlings. We defined nest attendance as the sum of incubation (or brooding) and being inside the nest (i.e., whenever the parent is inside or at the nest so that it is visible on the recording). Since nest attendance and incubation (or brooding) were highly correlated (nest attendance vs. incubation: rP = 0.839, n = 28, p < 0.001; nest attendance vs. brooding: rP = 0.858, n = 28, p < 0.001, see also Morvai et al., 2016), we report results for nest attendance only.
From the behavioral codings, we calculated relative male attendance time as the proportion of observation time the male spent inside the nest divided by the sum of the time the male and female parents spent inside the nest during incubation and offspring provisioning, respectively. Relative male feeding visits and feeding time were calculated similarly. Besides individual behaviors, we also calculated joint behaviors (nest attendance and feeding); these represent events when both parents showed a given behavior at the same time.
Social learning of parental sex roles and coordination were analyzed in separate general linear models (LMs) with the above response variables (logit-transformed male parental effort relative to the sum of male and female effort). In each model, we tested for the effect of care type of the pair received as young (fixed factor with five levels [care type received by the male parent/female parent]: M/M, M/F, F/M, F/F, B/B). In addition, initial models of relative male parental effort during offspring provisioning included number of offspring.
The potential consequences of care type on reproductive success were analyzed at multiple levels; clutch size on day 8 of incubation and brood size on day 10 post-hatching were analyzed in separate ordinal logistic regressions (OLRs, R package “ordinal”; Christensen, 2019). With the exception of two nests in which 1 and 3 offspring died, respectively, between day 16 and 35, brood size did not change between day 10 and 35 post-hatching (Supplementary Table 3). Therefore, we report effects on the number of nestlings on day 10 post-hatching only. Average offspring body mass was analyzed in LMMs over the different reproductive stages (day 8 of incubation and day 10, 16, and 35 post-hatching) with cage ID as a random term. The above models also included care type of the pair as an explanatory variable (fixed factor with five levels, see above).
Results
Short-Term Effects of Uniparental Care
Parental preference of the young was influenced by the care type they received. Offspring spent more time socializing with the parent they had received care from (LMM of relative time spent in male's zone, LRT of models with and without care type: = 8.33, p = 0.016). This difference was mainly driven by female-cared offspring spending less time with the male and more time with the female parent than biparentally and male-only cared offspring (B→F: exp(β) = 0.41 [0.18; 0.92]; B→M: exp(β) = 1.04 [0.46; 2.38]; Figure 2A).
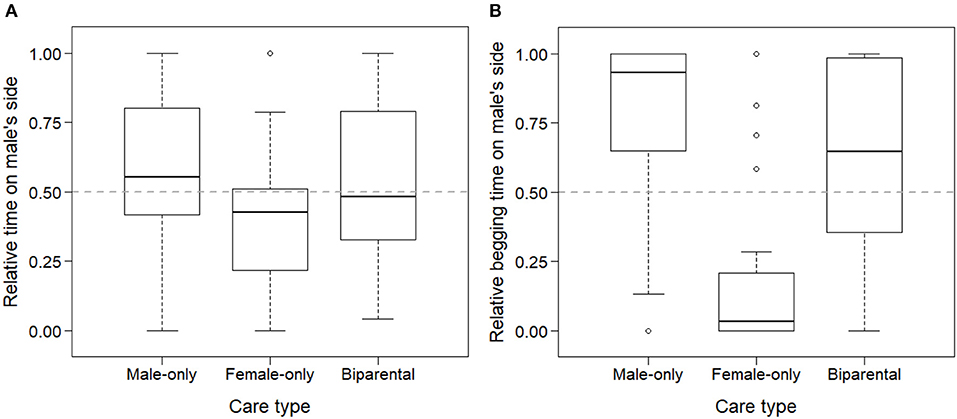
Figure 2. Parental preference of zebra finch offspring based on their social experiences with each of their parents. Offspring spent more time socializing with (A) and begging food from (B) their caring parent in a two-way choice test with their parents used as stimuli. Relative times were calculated as time spent on the male's side in relation to the total time spent on the two parents' sides (male + female).
The actual amount of care that offspring received on day 12 post-hatching did not predict the strength of preference toward the caring parent in the first parental preference test (rP = 0.153, n = 88, p = 0.155).
Begging from each parent was also influenced by early social experiences with the parents. Offspring spent more time with begging food from the parent they received previous provisioning from (LMM of relative time spent begging in male's zone, LRT of care type: = 30.21, p < 0.001). Similarly to socialization, the difference was mainly driven by female-cared offspring spending less time begging from the male and more time begging from the female, than male-only cared and biparentally cared offspring (B→F: exp(β) = 0.07 [0.02; 0.25]; B→M: exp(β) = 2.49 [0.69; 8.94]; Figure 2B).
Offspring sex did not influence socialization with or begging from parents (effect of care type × offspring sex interaction, in both above LMMs: p > 0.51).
Repeated preference tests with non-familiar adults as stimuli in a subset of offspring revealed that the preference is not specific toward the parents, although it is more pronounced toward them (reflected in a two-way interaction between care type and repeat; LMM of relative time in male's zone, LRT of care type x repeat: = 10.36, p = 0.035; LMM of relative begging time from male: = 9.34, p = 0.053; Figure 3). Offspring did not socialize more with their caring parent or with the same-sex non-familiar parent (repeated tests analyzed in separate models, LMMs of relative time in male's zone, LRT of care type, parents used as stimuli: p = 0.111, non-familiar adults used as stimuli: p = 0.984; Figure 3A). However, in both test conditions offspring begged more from their caring parent or from the non-familiar parent of the same sex (LMM of relative begging time from male, parents used as stimuli: = 20.10, p < 0.001; B→F: exp(β) = 0.08 [0.01; 0.49]; B → M: exp(β) = 3.72 [0.70; 19.66]; non-familiar adults used as stimuli: = 7.17, p = 0.028; B→F: exp(β) = 0.56 [0.10; 3.15]; B → M: exp(β) = 5.62 [0.82; 38.38]; Figure 3B). Furthermore, approximately two times more offspring begged from their parents as opposed to the non-familiar adults (78% vs. 40% of the 72 repeatedly tested offspring; = 19.41, p < 0.001).
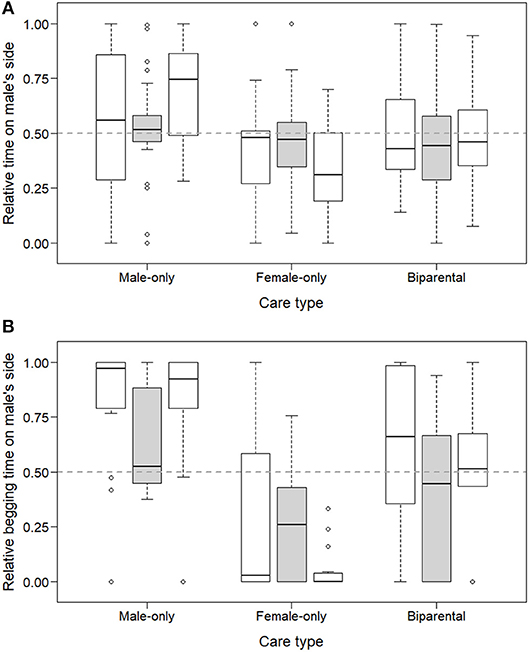
Figure 3. Parental preference of zebra finch offspring when offering them their own parents or non-familiar adults to choose from. Box groups of three represent relative time spent (A) and relative time begging (B) on the male parent's side for each care type received. Within each group, repeated tests of the same offspring are presented i.e., when offering them their own parents (leftmost, empty boxes), non-familiar parents (middle, gray boxes) and again their own parents (rightmost, empty boxes).
Long-Term Effects of Uniparental Care
First Breeding
Probability to start breeding was not different between pairs based on care type received as young (Cox model of latency to start breeding, LRT of care type: = 5.630, P = 0.229).
Relative male parental effort during incubation was different between pairs of parents based on care type received as young (LMs of relative male nest attendance time, LRT of care type: = 10.34, p = 0.035). The differences were due to higher levels of relative male effort when pair members received different uniparental care than when they were cared similarly (B/B→F/F: b = −0.00 [−0.09; 0.09]; B/B→F/M: b = 0.08 [0.01; 0.15]; B/B → M/F: b = 0.08 [0.01; 0.16]; B/B → M/M: b = −0.00 [−0.08; 0.08]; Figure 4).
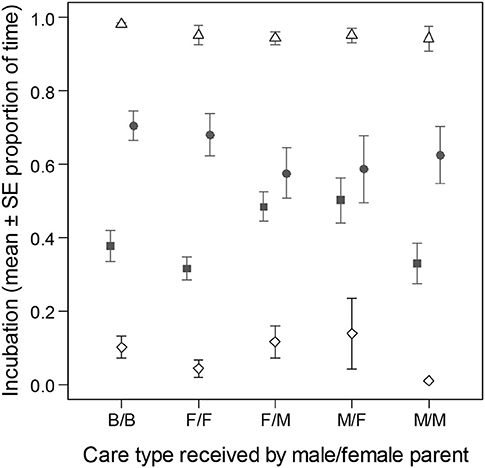
Figure 4. Division of parental effort and coordination in pairs of zebra finch parents during incubation based on their caring parents. The figure shows the proportion of time (mean ± SE) that parents spent inside their nest (i.e., total nest attendance with and without incubation) on day 8 of incubation. Male (filled square), female (filled circle), joint (empty diamond), and total effort (i.e., by at least one of the parents; empty triangle) are presented. Care categories are given in the order of male care/female care (e.g., B/B, biparentally cared male/biparentally cared female; F/M, female-only cared male/male-only cared female).
Relative male parental effort during offspring provisioning was not influenced by care type received as young (LMs of relative male nest attendance time, LRT of care type: = 2.04, p = 0.728; LMs of relative male frequency of feeding, LRT of care type: = 0.21, p = 0.645; LMs of relative male provisioning time, LRT of care type: = 7.80, p = 0.099).
Number of offspring was not different between pairs of parents that received different types of care as young (OLRs of clutch size on day 8 of incubation, and brood size on day 10, post-hatching, effect of care type in both models: p > 0.403). The analyses of offspring body mass, however, revealed differences due to care type (LMMs of offspring body mass, LRT of care type x reproductive stage interaction: = 31.75, p = 0.002; Figure 5). The differences were mainly due to higher body mass of 16 day old nestlings in families with female-cared male and male-cared female parents than in other families. By day 35, however, this difference had disappeared (LMMs of body mass of 35 day old offspring, LRT of care type: p = 0.815).
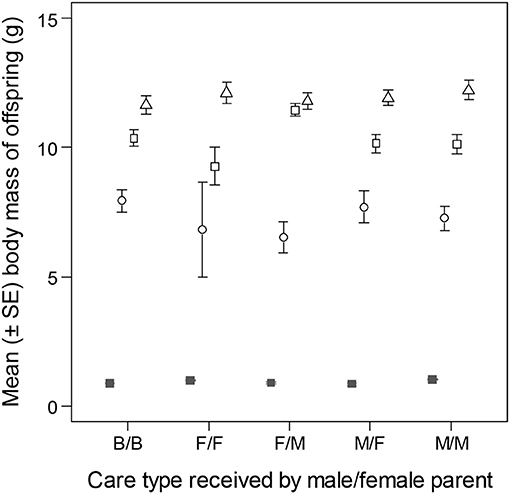
Figure 5. Body mass of zebra finch offspring raised by parents that had received different type of care. Filled squares indicate average egg mass on day 8 of incubation, whereas empty symbols represent average body masses at different reproductive stages post-hatching (circle: day 10; square: day 16; triangle: day 35). Care categories are given in the order of male care/female care (e.g., B/B, biparentally cared male/biparentally cared female; F/M, female-only cared male/male-only cared female).
Second Breeding
Probability to start the second breeding was not different between pairs based on care type received as young (Cox model of latency to start breeding, LRT of care type: = 3.914, P = 0.418).
Unlike in the first breeding attempt, relative male parental effort during incubation was not different between pairs of parents based on care type received as young (LMs of relative male nest attendance time, LRT of care type: = 0.01, p = 0.999).
Relative male parental effort during offspring provisioning was not different between experimental groups either (LMs of relative male nest attendance time, LRT of care type: = 6.41, p = 0.171; LMs of relative male frequency of provisioning, LRT of care type: = 1.45 p = 0.836).
Number and body mass of offspring in the second families of experimental birds were not influenced by care type received as young (OLRs of offspring number in separate models for day 8 of incubation and day 10 post-hatching, LRT of care type: both p > 0.221; LMMs of body mass, LRT of care type × reproductive stage interaction: p = 0.583).
Discussion
Using a split-family experimental design, we investigated in the biparental zebra finch whether early social experiences with each parent would influence offspring behavior toward each parent and, later in life, their own adult parental sex role and negotiation with their pairs. Parental preference tests of uniparentally and biparentally raised control zebra finch young revealed that they pay attention to who takes care of them. Young zebra finches discriminated between their two parents based on their social experiences with each of them, and they might have generalized their expectation to same-sex, non-familiar adults to some extent. When these manipulated birds sexually matured and bred with other manipulated birds for the first time, the typical parental sex roles and coordination of incubation effort was modified in certain experimental groups. In pairs where partners had received care from opposite-sex parents, males spent more time in the nest (both alone and together with the female) compared to pairs in which both partners had received care from the same sex (male or female) or from both parents. At the time of offspring provisioning, however, differences were no longer apparent. Also, when breeding for a second time, parental sex roles and coordination of care seemed normal even already during incubation. Although offspring body mass fluctuated over reproductive stages according to the type of care their parents had received, we found no evidence that our manipulation caused significant changes in terms of number or quality of offspring produced.
Parental preference tests revealed that splitting biparental families, and thereby changing the normal offspring experience of parental sex roles from biparental to uniparental care affects offspring parental preferences. Male-only and female-only cared zebra finch young socialized more with the parent that provisioned them. Change in their parental expectations were clearly demonstrated by their begging behavior during the preference test; beggings were directed mostly toward their caring parent. Since our experimental manipulation allowed visual, olfactory and acoustic interaction among family members, uniparentally raised young could observe their other parent while providing care for their split siblings. Changing parental expectations in our experiment, therefore, suggests that the actual provisioning experiences with individual parents (rather than merely observing their parental effort) are important cues that are taken into account by offspring in future interactions. Paying attention and adjust begging behavior to parental effort can be adaptive because of the high costs and benefits associated with begging (Kilner, 2001; Nettle et al., 2017). Our results are in support of this notion about reconcilable behavior by suggesting that young zebra finches monitor the actual amount of parental effort (including provisioning and brooding) received from each of their parents, individually.
The subsequent preference tests with non-familiar adults provided us with inconclusive results. From the one hand, they suggest that zebra finch young generalize their parental expectations to a certain extent on the basis of parental sex (Jacot et al., 2010; Caspers et al., 2017). A more pronounced preference expressed with parents used as stimuli and more frequent begging from them indicates, on the other hand, that they can discriminate between their parents and other adult conspecifics. Furthermore, based on our experimental design, we cannot exclude two alternative explanations: errors and carry over effects. Firstly, beggings directed toward non-familiar adults might have reflected errors if discrimination of stimulus birds were hindered in our experiment (e.g., because of light conditions or changed acoustic cues in the test situation from parents/non-familiar adults). Secondly, we cannot exclude that we found a carry over effect from the previous parental preference test so that young expected, based on their experiences on the previous day, to encounter with their parents and paid less attention to characteristics of the stimulus birds in the beginning of the test trials. Considering the ecological aspects of the species, zebra finches are colonial breeders and neighbors tend to synchronize their breedings, leading to several families at similar reproductive stages (Zann, 1996; Brandl et al., 2019). Nevertheless, alloparental provisioning has not been reported in this species. Fledglings are about 18–20 days old when they first leave the nest, and they still depend on parental provisioning for at least a week more. The above suggests that parental recognition can be crucial for survival during the first few days in the colony (Zann, 1996), and makes the two alternative explanations (error or carry over effects) more likely than the explanation of generalization.
When uniparentally raised birds bred with each other, long-term effects of early social experiences with parents revealed. Instead of the only effect of the care type received, however, the mechanism seems to be more complex, suggesting a combined effect of early social experiences (when receiving care) and current social experiences (when providing care with the mate). We expected young to modify their own sex roles permanently based on the care type received, so that their parental effort would reflect the combined effect of the sex of their caring parent and that of their own (e.g., we expected male effort to be higher in male-only cared males as opposed to female-only cared males and vice versa). In contrast, we found differences in incubation patterns of first-breeding pairs that are not consistent with this view. When the male and female parent had contrasting social experiences with their parents, relative male effort was higher than in the rest of the experimental groups, including the control. This suggests that similar social experiences of breeding parents are needed for normal coordination of incubation effort to develop. Differences between experimental groups appear to be driven by change in male effort. Indeed, a possible explanation is that our experimental manipulation had a more pronounced effect on one sex (males) than on the other (females). Zebra finch females contribute more to incubation likely because of their brood patch and the more effective heat transfer associated with it (Hill et al., 2014; Morvai et al., 2016). While female effort seems to be more responsive to changes in environmental conditions (such as temperature; Hill et al., 2014), male effort may still be more flexible in terms of negotiation between pair members. Results of the short-term effects (parental preference test), however, are not in line with this explanation by indicating no effect of offspring sex on parental preferences. Furthermore, we note that male and female behaviors are not independent in our experiment, so changes of male incubation effort might have reflected partly (or fully) changes in coordination by females. Further experiments may reveal the exact mechanisms, for instance, by monitoring parental coordination of pairs with only one of the parents raised uniparentally. A recent study suggests that zebra finch pairs use vocal cues to negotiate their incubation efforts, therefore, focusing on within-pair communication may provide insights to coordination between pair members in similar experiments (Boucaud et al., 2016). Manipulating environmental conditions (e.g., by decreasing temperature during breeding) may also facilitate focusing on negotiation, because the significance of parental coordination may increase with deviation from optimal environmental conditions (Vincze et al., 2017).
Another intriguing mechanistic question raised by our results concerns the exact mechanism by which early social experiences resulted in change in adult behavior. Early experiences of the young may be transmitted to adulthood through changes in morphology (including condition) so that pair members that received the same care type might be consequently in a more similar state, allowing also a more efficient coordination between them. Another plausible explanation is that changed parental behavior as a consequence of our manipulation led to divergent, albeit transient, parental behavior of the offspring.
At the time of nestling provisioning (the second reproductive stage that needs extensive coordination in biparental families), uniparentally raised birds showed no direct effect of the experimental treatment. We have two alternative explanations for no apparent change in parenting; first, this reproductive stage involves interaction with the young, and begging of the nestlings is a very strong stimulus for the parents (Godfray, 1991; Royle et al., 2002a). In this reproductive stage, therefore, parental coordination may become less important and give place to each parent coordinating parental care with the offspring instead. In addition, male and female zebra finch parents allocate similar time and effort into brooding and offspring provisioning, suggesting decreased sex-specific task specialization by the post-hatching period (Morvai et al., 2016). Previous negotiation rules over care may therefore change and be replaced by conditional cooperation with alternated nest visits (Iserbyt et al., 2017).
The second alternative explanation for the lack of treatment effect during provisioning is that normal parental coordination has been established by the time of offspring provisioning. Social experiences with the mate (when providing care) may be different to that experienced with the parents (when receiving care as young) with regards to sex roles, and experience with the mate may shape establishing normal parental sex roles and coordination. This explanation assumes a similar, two-stage mechanism to those described for social learning of other traits (e.g., song learning or sexual imprinting; Price, 1979; Bischof, 1994). Early social experiences, then, can either be strengthened as adults (if they were similar to those experienced by most of the other individuals in the population i.e., also by the mate), or in contrast, “corrected” if early experiences were shifted away from the normal behavior. The finding that incubation patterns in the second breeding attempt of the uniparentally raised birds did no longer differ support the latter scenario; social experience with the mate during the first breeding attempt might have helped to establish normal parental sex roles and coordination.
The relatively strong short-term effects compared to the transient long-term effects in our experiment raises the question whether applying the split-family design so that young could observe the other, non-caring parent while provisioning their siblings had different effects when they were young and when they became adults. It is possible that while the effects of such observations have been overridden by own social experiences as young, they manifested and contributed to shaping parental behavior as adults.
Furthermore, we note that a possible reason for the lack of a remarkable effect of our treatment might be the relatively weak sexual conflict in this species, coupled with an expressed synchronization between pair members during parenting. Although a laboratory study (Royle et al., 2002b) found experimental evidence for sexual conflict over parental care (and its consequences) in zebra finches, a number of recent field studies suggest that cooperative synchronization between parents play an important role when dividing labor (Mariette and Griffith, 2012, 2015). We suggest that future studies should involve species with more pronounced sexual conflict and distinguished parental sex roles to account for such possible effects on the extent of social learning of parental sex roles.
In line with the lack of treatment effect on post-hatching parental behavior, we found no persistent consequences on reproductive success either. Although body mass of 16 day old nestlings was different based on care type (and points toward possible unrevealed differences caused by the treatment on parenting), this effect was transient. Number and body mass of offspring were similar over experimental groups by day 35 post-hatching (i.e., by the time offspring became independent). We, however, point out that consequences on reproductive success can take various forms of which we focused only on two aspects, so that differences among experimental groups might have remained unexplored in our study. For instance, finding a suitable mate, pair formation and starting to breed may already involve coordination between parents to various extents. Thus, even a temporarily effect of the early social environment can have lasting consequences in species that forms long-term pair bonds, because of, for instance, missed opportunities to find the best match to mate with. Such an effect of the early rearing environment has been demonstrated with regards to sexual preferences in zebra finches; young that were raised without adult males showed increased preference toward same-sex partners as adults (Adkins-Regan and Krakauer, 2000). Previous experiments suggest a direct link between sexual preferences and the intensity of social experiences with parents during development in the family. When zebra finch young were raised by foster parents (either by non-familiar other zebra finches or birds from a different species), as adults, they showed a stronger sexual preference toward stimulus birds from the species of their foster parent, based on how much they were fed (Bischof and Clayton, 1991; Oetting et al., 1995). We also note, that although our manipulations have been done at early stages of ontogeny (between day 8 and 35 post-hatching), interactions with both sexes might be relevant even at later stages in life (cf. Ten Cate et al., 1984 in which species recognition has been altered in already independent, 30–60 day old zebra finch young).
To facilitate the detection of social learning, we manipulated parental sex roles to the extremes by changing the role models' behavior from biparental to uniparental care. Our results, however, do not point to social learning of parental sex roles per se, rather, social learning of negotiation rules or the cues needed for negotiation such as acoustic cues, for instance (Boucaud et al., 2016). It is usually more adaptive to learn from parents than from other conspecifics, because more reliable information can be assumed based on the fitness incentive in passing on parental knowledge to own offspring. The profound effects of early social experiences from the parents on adult behavior are documented in various species (Lupfer et al., 2003; Griesser et al., 2006). Slagsvold and Wiebe (2007), for instance, cross-fostered clutches between great tits (Parus major) and blue tits (Cyanistes caeruleus) to investigate whether differences in provisioning would influence foraging behavior in adult diet. The manipulation of social experiences resulted in a shifted feeding niche of adults in the direction of the foster species. Moreover, these cross-fostered young, when starting to breed themselves, delivered prey of similar size to that of their social parents (Slagsvold and Wiebe, 2011).
We acknowledge that our sample size in the analysis of the long-term effects were moderate, especially for the second breeding. This then inevitably results in lower statistical power so that only large effects can be detected. We think the lower sample sizes in this analysis was partly due to the effects of no free mate choice in our experiment (pairings were based on uniparental care received as young), and partly due to the lack of breeding experience in our subjects. We argue, however, that our experiment excludes that parental sex roles would develop by strong, deterministic early social experiences from parents, and strongly suggests that although such experiences are important when care is received as young, these translates only to transient effects as adults.
Taken together, our results suggest that parental sex roles and coordination are perceived by zebra finch young and such early social experiences influence their own parental behavior as adults. These effects, however, are not permanent and can be overridden by own social experiences with the mate when starting to breed. A conclusive answer to the question of whether or not young generalize their parental expectations to parental sex, takes further experimenting. Further studies focusing on the exact mechanism of the described parental coordination patterns and an extended survey of the potential fitness consequences are also needed for a comprehensive understanding of the long-term effects of early social experiences with parents.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Ethics Statement
The study was carried out according to the German and Hungarian Laws for the experimentation with animals and with permission of the ELTE MÁB (#02/2014). Breeding and housing of the birds were done under the permission of the Veterinäramt Bielefeld (# 530.421630-1, 18.4.2002). All birds used in the experiments remained for their entire life at the Department of Ethology, Eötvös Loránd University, Budapest. All birds were visually monitored for health status on a daily basis.
Author Contributions
ÁP and TS conceived the study with input from OK and ÁM. NvE and EKr contributed to experimental design and managing the experiments. ÁP, BM, EKi, and TB carried out the experiments and collected data. BM, EKi, TB, and TR coded video recordings. ÁP and BM managed the database, and ÁP analyzed the data. ÁP and BM wrote the manuscript with input from EKr, TR, NvE, JK, TS, ÁM, and OK. All authors contributed to the final paper. ÁP with BM revised the paper.
Funding
This study was supported by the Hungarian Scientific Research Fund (OTKA K109337). ÁP was supported by an Eötvös postdoctoral grant by the Hungarian Scholarship Board, Balassi Institute (32078), the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and the ÚNKP-18-4 New National Excellence Program of the Ministry of Human Capacities, Hungary. This work was completed in the ELTE Institutional Excellence Program (783-3/2018/FEKUTSRAT) supported by the Hungarian Ministry of Human Capacities.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Joanna Rutkowska for her help with acquiring cardbox nest boxes. We thank Elizabeth Adkins-Regan, Simon Charles Griffith and James Luke Savage for their valuable comments on previous versions of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2019.00294/full#supplementary-material
Supplementary Table 1. Long-term social effects dataset, incubation.
Supplementary Table 2. Long-term social effects dataset, offspring provisioning.
Supplementary Table 3. Long-term social effects dataset, reproductive success.
Supplementary Table 4. Short-term social effects dataset, parental preference.
Supplementary Table 5. Long-term effects dataset, probability to start breeding.
References
Adam, I., Scharff, C., and Honarmand, M. (2014). Who is who? Non-invasive methods to individually sex and mark altricial chicks. J. Visual. Exp. 87:e51429. doi: 10.3791/51429
Adkins-Regan, E., and Krakauer, A. (2000). Removal of adult males from the rearing environment increases preference for same-sex partners in the zebra finch. Anim. Behav. 60, 47–53. doi: 10.1006/anbe.2000.1448
Benskin, C. M. H., Mann, N. I., Lachlan, R. F., and Slater, P. J. B. (2002). Social learning directs feeding preferences in the zebra finch, Taeniopygia guttata. Anim. Behav. 64, 823–828. doi: 10.1006/anbe.2002.2005
Bischof, H. J. (1994). Sexual imprinting as a two-stage process, in Causal Mechanisms of Behavioural Development, eds Hogan, J. A., and Bolhuis, J. J. (Cambridge: Cambridge University Press, 82–79.
Bischof, H. J., and Clayton, N. (1991). Stabilization of sexual preferences by sexual experience in male zebra finches Taeniopygia guttata castanotis. Behaviour 118, 144–155. doi: 10.1163/156853991X00256
Boucaud, I. C. A., Mariette, M. M., Villain, A. S., and Vignal, C. (2016). Vocal negotiation over parental care? Acoustic communication at the nest predicts partners' incubation share. Biol. J. Linnean Soc. 117, 322–336. doi: 10.1111/bij.12705
Boyd, R., and Richerson, P. J. (1995). Why does culture increase human adaptability. Ethol. Sociobiol. 16, 125–143. doi: 10.1016/0162-3095(94)00073-G
Brandl, H. B., Griffith, S. C., and Schuett, W. (2019). Wild zebra finches choose neighbours for synchronized breeding. Anim. Behav. 151, 21–28. doi: 10.1016/j.anbehav.2019.03.002
Burley, N. T. (2006). An eye for detail: selective sexual imprinting in zebra finches. Evolution 60, 1076–1085. doi: 10.1554/05-399.1
Caspers, B. A., Hagelin, J. C., Paul, M., Bock, S., Willeke, S., and Krause, E. T. (2017). Zebra finch chicks recognise parental scent, and retain chemosensory knowledge of their genetic mother, even after egg cross-fostering. Sci. Rep. 7:12859. doi: 10.1038/s41598-017-13110-y
Christensen, R. H. B. (2019). ordinal - Regression Models for Ordinal Data. R package version 2019.4–25. Available online at: https://CRAN.R-project.org/package=ordinal (accessed on 30 July, 2019).
Cockburn, A. (2006). Prevalence of different modes of parental care in birds. Proc. Biol. Sci. 273, 1375–1383. doi: 10.1098/rspb.2005.3458
Drullion, D., and Dubois, F. (2008). Mate-choice copying by female zebra finches, Taeniopygia guttata: what happens when model females provide inconsistent information? Behav. Ecol. Sociobiol. 63, 269–276. doi: 10.1007/s00265-008-0658-5
Farine, D. R., Spencer, K. A., and Boogert, N. J. (2015). Early-life stress triggers juvenile zebra finches to switch social learning strategies. Curr. Biol. 25, 2184–2188. doi: 10.1016/j.cub.2015.06.071
Forstmeier, W., Segelbacher, G., Mueller, J. C., and Kempenaers, B. (2007). Genetic variation and differentiation in captive and wild zebra finches (Taeniopygia guttata). Mol. Ecol. 16, 4039–4050. doi: 10.1111/j.1365-294X.2007.03444.x
Godfray, H. C. J. (1991). Signaling of need by offspring to their parents. Nature 352, 328–330. doi: 10.1038/352328a0
Griesser, M., Nystrand, M., and Ekman, J. (2006). Reduced mortality selects for family cohesion in a social species. Proc. Biol. Sci. 273, 1881–1886. doi: 10.1098/rspb.2006.3527
Griffith, S. C., Crino, O. L., Andrew, S. C., Nomano, F. Y., Adkins-Regan, E., Alonso-Alvarez, C., et al. (2017). Variation in reproductive success across captive populations: methodological differences, potential biases and opportunities. Ethology 123, 1–29. doi: 10.1111/eth.12576
Guillette, L. M., Scott, A. C. Y., and Healy, S. D. (2016). Social learning in nest-building birds: a role for familiarity. Proc. R. Soc. B Biol. Sci. 283:20152685. doi: 10.1098/rspb.2015.2685
Harrison, F., Barta, Z., Cuthill, I., and Székely, T. (2009). How is sexual conflict over parental care resolved? A meta-analysis. J. Evol. Biol. 22, 1800–1812. doi: 10.1111/j.1420-9101.2009.01792.x
Hill, D. L., Lindstrom, J., McCafferty, D. J., and Nager, R. G. (2014). Female but not male zebra finches adjust heat output in response to increased incubation demand. J. Exp. Biol. 217(Pt. 8), 1326–1332. doi: 10.1242/jeb.095323
Hoffman, J. I., Krause, E. T., Lehmann, K., and Krüger, O. (2014). MC1R genotype and plumage colouration in the zebra finch (Taeniopygia guttata): population structure generates artefactual associations. PLoS ONE 9:e86519. doi: 10.1371/journal.pone.0086519
Houston, A. I., and Davies, N. B. (1985). The evolution of cooperation and life history in the dunnock Prunella modularis, in Behavioural Ecology, eds Sibley, R. M., and Smith, R. H. (Oxford: Blackwell Scientific Publications, 471–487.
Immelmann, K. (1972). Sexual and other long-term aspects of imprinting in birds and other species. Adv. Study Behav. 4, 147–174. doi: 10.1016/S0065-3454(08)60009-1
Iserbyt, A., Fresneau, N., Kortenhoff, T., Eens, M., and Muller, W. (2017). Decreasing parental task specialization promotes conditional cooperation. Sci. Rep. 7:6565. doi: 10.1038/s41598-017-06667-1
Jacot, A., Reers, H., and Forstmeier, W. (2010). Individual recognition and potential recognition errors in parent-offspring communication. Behav. Ecol. Sociobiol. 64, 1515–1525. doi: 10.1007/s00265-010-0965-5
Johnstone, R. A., and Hinde, C. A. (2006). Negotiation over offspring care—how should parents respond to each other's efforts? Behav. Ecol. 17, 818–827. doi: 10.1093/beheco/arl009
Jones, A. E. X., Cate, C., and Slater, P. J. B. (1996). Early experience and plasticity of song in adult male zebra finches (Taeniopygia guttata). J. Comp. Psychol. 110, 354–369. doi: 10.1037/0735-7036.110.4.354
Katz, M., and Lachlan, R. F. (2003). Social learning of food types in zebra finches (Taenopygia guttata) is directed by demonstrator sex and feeding activity. Anim. Cogn. 6, 11–16. doi: 10.1007/s10071-003-0158-y
Kilner, R. M. (2001). A growth cost of begging in captive canary chicks. Proc. Natl. Acad. Sci. U.S.A. 98, 11394–11398. doi: 10.1073/pnas.191221798
Kniel, N., Dürler, C., Hecht, I., Heinbach, V., Zimmermann, L., and Witte, K. (2015). Novel mate preference through mate-choice copying in zebra finches: sexes differ. Behav. Ecol. 26, 647–655. doi: 10.1093/beheco/aru241
Krause, E. T., Krüger, O., and Pogány, Á. (2017). Zebra finch nestlings, rather than parents, suffer from raising broods under low nutritional conditions. Behav. Ecol. Sociobiol. 71:152. doi: 10.1007/s00265-017-2382-5
Lemon, W. C. (1993). The energetics of lifetime reproductive success in the zebra finch Taeniopygia guttata. Physiol. Zool. 66, 946–963. doi: 10.1086/physzool.66.6.30163748
Lendvai, Á. Z., Akçay, Ç., Stanback, M., Haussmann, M. F., Moore, I. T., and Bonier, F. (2018). Male parental investment reflects the level of partner contributions and brood value in tree swallows. Behav. Ecol. Sociobiol. 72:185. doi: 10.1007/s00265-018-2594-3
Lendvai, Á. Z., Barta, Z., and Chastel, O. (2009). Conflict over parental care in house sparrows: do females use a negotiation rule? Behav. Ecol. 20, 651–656. doi: 10.1093/beheco/arp047
Lessells, C. M., and McNamara, J. M. (2012). Sexual conflict over parental investment in repeated bouts: negotiation reduces overall care. Proc. Biol. Sci. 279, 1506–1514. doi: 10.1098/rspb.2011.1690
Lupfer, G., Frieman, J., and Coonfield, D. (2003). Social transmission of flavor preferences in two species of hamsters (Mesocricetus auratus and Phodopus campbelli). J. Comp. Psychol. 117, 449–455. doi: 10.1037/0735-7036.117.4.449
Mariette, M. M., and Griffith, S. C. (2012). Nest visit synchrony is high and correlates with reproductive success in the wild zebra finch Taeniopygia guttata. J. Avian Biol. 43, 131–140. doi: 10.1111/j.1600-048X.2012.05555.x
Mariette, M. M., and Griffith, S. C. (2015). The adaptive significance of provisioning and foraging coordination between breeding partners. Am. Nat. 185, 270–280. doi: 10.1086/679441
McNamara, J. M., Gasson, C. E., and Houston, A. I. (1999). Incorporating rules for responding into evolutionary games. Nature 401, 368–371.
McNamara, J. M., Houston, A. I., Barta, Z., and Osorno, J. L. (2003). Should young ever be better off with one parent than with two? Behav. Ecol. 14, 301–310. doi: 10.1093/beheco/14.3.301
Morvai, B., Nanuru, S., Mul, D., Kusche, N., Milne, G., Székely, T., et al. (2016). Diurnal and reproductive stage-dependent variation of parental behaviour in captive zebra finches. PLoS ONE 11:e0167368. doi: 10.1371/journal.pone.0167368
Nettle, D., Andrews, C., Reichert, S., Bedford, T., Kolenda, C., Parker, C., et al. (2017). Early-life adversity accelerates cellular ageing and affects adult inflammation: experimental evidence from the European starling. Sci. Rep. 740794. doi: 10.1038/srep40794
Oetting, S., Pröve, E., and Bischof, H.-J. (1995). Sexual imprinting as a two-stage process: mechanisms of information storage and stabilization. Anim. Behav. 50, 413–429. doi: 10.1006/anbe.1995.0254
Péter, A. (2015). Solomon Coder: A Simple Solution for Behavior Coding. Available online at: http://solomoncoder.com/ (accessed July 30, 2019).
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., and R Core Team (2019). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–140. Available online at: https://CRAN.R-project.org/package=nlme (accessed on 30 July, 2019).
Price, P. H. (1979). Developmental determinants of structure in zebra finch song. J. Comp. Physiol. Psychol. 93, 260–277.
R Core Team (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rehling, A., Spiller, I., Krause, E. T., Nager, R. G., Monaghan, P., and Trillmich, F. (2012). Flexibility in the duration of parental care: zebra finch parents respond to offspring needs. Anim. Behav. 83, 35–39. doi: 10.1016/j.anbehav.2011.10.003
Roper, A., and Zann, R. (2006). The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112, 458–470. doi: 10.1111/j.1439-0310.2005.01169.x
Royle, N. J., Hartley, I. R., and Parker, G. A. (2002a). Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 17, 434–440. doi: 10.1016/S0169-5347(02)02565-X
Royle, N. J., Hartley, I. R., and Parker, G. A. (2002b). Sexual conflict reduces offspring fitness in zebra finches. Nature 416, 733–736. doi: 10.1038/416733a
Royle, N. J., Smiseth, P. T., and Kölliker, M. (2012). The Evolution of Parental Care. Oxford, UK: Oxford University Press.
Savage, J. L., Browning, L. E., Manica, A., Russell, A. F., and Johnstone, R. A. (2017). Turn-taking in cooperative offspring care: by-product of individual provisioning behavior or active response rule? Behav. Ecol. Sociobiol. 71:162. doi: 10.1007/s00265-017-2391-4
Schielzeth, H., Burger, C., Bolund, E., and Forstmeier, W. (2008). Sexual imprinting on continuous variation: do female zebra finches prefer or avoid unfamiliar sons of their foster parents? J. Evol. Biol. 21, 1274–1280. doi: 10.1111/j.1420-9101.2008.01568.x
Schuett, W., Dall, S. R. X., and Royle, N. J. (2011). Pairs of zebra finches with similar ‘personalities’ make better parents. Anim. Behav. 81, 609–618. doi: 10.1016/j.anbehav.2010.12.006
Slagsvold, T., and Wiebe, K. L. (2007). Learning the ecological niche. Proc. R. Soc. B Biol. Sci. 274, 19–23. doi: 10.1098/rspb.2006.3663
Slagsvold, T., and Wiebe, K. L. (2011). Social learning in birds and its role in shaping a foraging niche. Philos. Trans. R. Soc. B Biol. Sci. 366, 969–977. doi: 10.1098/rstb.2010.0343
Swaddle, J. P., Cathey, M. G., Correll, M., and Hodkinson, B. P. (2005). Socially transmitted mate preferences in a monogamous bird: a non-genetic mechanism of sexual selection. Proc. R. Soc. B Biol. Sci. 272, 1053–1058. doi: 10.1098/rspb.2005.3054
Templeton, C. N., Philp, K., Guillette, L. M., Laland, K. N., and Benson-Amram, S. (2017). Sex and pairing status impact how zebra finches use social information in foraging. Behav. Processes 139, 38–42. doi: 10.1016/j.beproc.2016.12.010
Ten Cate, C., Los, L., and Schilperoord, L. (1984). The influence of differences in social experience on the development of species recognition in zebra finch males. Anim. Behav. 32, 852–860.
Therneau, T. (2015). A Package for Survival Analysis in S. version 2.38. Available online at: https://CRAN.R-project.org/package=survival (accessed July 30, 2019).
Vincze, O., Kosztolányi, A., Barta, Z., Küpper, C., Alrashidi, M., Amat, J. A., et al. (2017). Parental cooperation in a changing climate: fluctuating environments predict shifts in care division. Glob. Ecol. Biogeogr. 26, 347–358. doi: 10.1111/geb.12540
Vos, D. R. (1995). Sexual Imprinting in zebra-finch females - do females develop a preference for males that look like their father. Ethology 99, 252–262. doi: 10.1111/j.1439-0310.1995.tb00899.x
Yanagihara, S., and Yazaki-Sugiyama, Y. (2016). Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat. Commun. 7:11946. doi: 10.1038/ncomms11946
Keywords: parental coordination, negotiation, social learning, sex roles, parental care, zebra finch, Taeniopygia guttata
Citation: Pogány Á, Morvai B, Krause ET, Kitsios E, Böhm T, Ruploh T, von Engelhardt N, Székely T, Komdeur J, Miklósi Á and Krüger O (2019) Short- and Long-Term Social Effects of Parental Sex Roles in Zebra Finches. Front. Ecol. Evol. 7:294. doi: 10.3389/fevo.2019.00294
Received: 01 May 2019; Accepted: 24 July 2019;
Published: 07 August 2019.
Edited by:
James Luke Savage, University of Sheffield, United KingdomReviewed by:
Elizabeth Adkins-Regan, Cornell University, United StatesSimon Charles Griffith, Macquarie University, Australia
Copyright © 2019 Pogány, Morvai, Krause, Kitsios, Böhm, Ruploh, von Engelhardt, Székely, Komdeur, Miklósi and Krüger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ákos Pogány, akos.pogany@ttk.elte.hu
 Ákos Pogány
Ákos Pogány Boglárka Morvai1
Boglárka Morvai1  E. Tobias Krause
E. Tobias Krause Nikolaus von Engelhardt
Nikolaus von Engelhardt