Bioconcentration and translocation of rare earth elements in plants collected from three legacy mine sites in Portugal
- 1Univ. Rennes, CNRS, Géosciences Rennes, UMR 6118, Rennes, France
- 2Centro de Ciências e Tecnologias Nucleares (C2TN), Instituto Superior Técnico, Universidade de Lisboa, Bobadela, Portugal
- 3Departamento de Engenharia e Ciências Nucleares (DECN), Instituto Superior Técnico, Universidade de Lisboa, Bobadela, Portugal
- 4EDM—Empresa de Desenvolvimento Mineiro, S.A., Lisbon, Portugal
Rare earth elements (REE), a group of emerging contaminants with commercial and technological applications, share many physical and chemical characteristics and have thus been used as accurate tracers of various environmental samples. They have been shown to increase in receiving waters following the dissolution of host-rock material during mining activities. In this study, spontaneous vegetation and related media were collected from three Portuguese legacy mine sites in November 2020 to evaluate the phytoavailability and fate of REE. Water, soil and plant data were analyzed in the context of the 1) prevailing geochemical context, 2) the mining context, and 3) plant effects. This study presents the REE signatures for different plant species and links the signatures to a potential source of bioavailable REE. The REE accumulated in plant tissue seems to reflect the REE signature of surface waters in the mining areas, showing enrichment in middle REE. Although the soils, sediments, and waters in this study had similar features, certain plants seemed better adapted to translocating Light REE and Eu over others. Given that REE are readily available within the field conditions of a mining site, this study shows how plant physiology and biologic preference towards particular REE contribute to the fractionation of REE and create a unique signature dependent on plant type.
1 Introduction
The rare earth elements (REE) are comprised of 17 elements: the lanthanoid series (La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu), plus Y and Sc (Pan et al., 2020). They can be characterized by similar chemical properties and closely related geochemical behaviors, resulting in similar distributions within the Earth’s crust (Taylor and McLennan, 1985). Though Y and Sc are officially defined as REE by the International Union of Pure and Applied Chemistry (IUPAC) (2005), the lanthanoid series (La to Lu) share electrical and thermodynamic properties (Joshi et al., 2018) and a unique electron configuration which results in lanthanide contraction. Therefore, this paper will only consider La-Lu when referencing the REE series.
Since the initial discovery of REE in 1794, they have become a critical part of the world economy (U.S. Geological Survey, 2022). Their versatile applications in industry, medical services, agriculture, and many other domains (Bau and Dulski, 1996; Tyler, 2004; Binnemans et al., 2013; Migaszewski and Gałuszka, 2015; Van Gosen et al., 2017) have inevitably led to the elevated release of REE in the environment (Li et al., 2013; Merschel and Bau, 2015) and an increased risk of occupational exposures (Rim et al., 2013). Yet the environmental risks related to REE have so far received little attention, overshadowed as they are by economic and geopolitical considerations. Evidence suggesting their potential toxicity (Pagano et al., 2015; Wakabayashi et al., 2016; Gong et al., 2021) has consequently led to REE being categorized as “emerging contaminants,” potentially making the anomalous supply of REE in the environment a major challenge for the years to come.
Historically the management of mining wastes has posed a risk to the environment, with many sites leaving a legacy of significant pollution to the surrounding environment (Perez-Lopez et al., 2010; Li et al., 2013; Soyol-Erdene et al., 2018). Economically valuable resources have often been mined, generating major anthropogenic waste streams and spurring geogenic pollution, exemplified by Acid Mine Drainage (AMD). AMD is characterized by the oxidation of iron-sulfide minerals leading to the perpetual formation of sulfuric acid, concomitant with an increase in soluble metal concentrations (Johnson and Hallberg, 2003; Herman and Maier, 2009). Several countries such as Australia, Bolivia, China, France, Germany, India, Portugal, Romania, Spain, Sweden, and the United States (Akcil and Koldas, 2006; Casiot et al., 2009; Grawunder et al., 2014; Strosnider et al., 2014; Marquez et al., 2018, and references therein) are impacted by AMD, making it a global concern. These mine waste sites, with large quantities of metals solubilized by AMD and mining practices, provide valuable insights into the behavior of REE in the environment. Because of their ubiquitous distribution in Earth’s crust, the dissolution of host-rock material during local mining activities has been shown to increase REE in receiving waters (Ferreira da Silva et al., 2009; Perez-Lopez et al., 2010; Prudêncio et al., 2015; Soyol-Erdene et al., 2018), even when the resources being mined are not REE.
While there are 199 cataloged abandoned mines in Portugal, this study focuses on three sites: São Domingos, Lousal, and Quinta do Bispo. These sites have been chosen as subjects of study due to their long history of mining activity in a variety of geologic contexts with different stages of remediation efforts in place. Though the context for each of these mines is vastly different, they share previously observed high REE concentration levels. Each site demonstrates anthropogenically driven changes to geochemistry as São Domingos and Lousal are subject to AMD evolution from waste rock piles, while low-grade uranium ores from Quinta do Bispo have been processed using in situ leaching (ISL). The Lousal site boasts a passive treatment system in an effort to remediate tailing leachate and AMD. Though no such treatment systems were in place at the other two sites as of November 2020, all three were monitored regularly with future remediation projects planned by Empresa de Desenvolvimento Mineiro (EDM). Since the geochemistry and background of each mine are unique, we are afforded the opportunity to study various control parameters, making them ideal targets for this investigation of the uptake and fractionation of REE by spontaneous vegetation as well as the concentration in corresponding soils and waters.
Plants potentially play a vital role in the fate and transport of REE (Tyler, 2004). Although some studies have suggested phytoremediation strategies to stabilize and remove REE from contaminated soils (Lima and Ottosen, 2021), the topic has been the subject of limited study. To date two plant types, Dichranopteris dichotoma (Chour et al., 2018; Liu et al., 2018) and Phytolacca Americana (Liu et al., 2018), have been identified as candidates for REE recovery when combined with enhanced extraction using electrokinetic methods (Lima and Ottosen, 2021).
The origin of REE fractionation in plant tissue is still subject to many questions as well. Very few authors have published on the entire REE series and their impact on the planted environment, instead opting to study a selection of REE considered representative of the LREE (light rare earth elements), MREE (middle rare earth elements), and HREE (heavy rare earth elements). However, the stability constants formed between individual REE and compatible ions vary (Millero, 1992), potentially creating differences in the bioavailability of REE in the natural environment, thus demonstrating the importance of studying the entire REE series. Moreover, while some studies have concluded that the geologic context and soil weathering are critical factors influencing plant REE uptake (Laul and Weimer, 1982; Pisciotta et al., 2017), others have suggested that a combination of plant type and geologic context is important to note in determining the phyto-availability of REE (Gałuszka et al., 2020).
Through the analysis of water, soil, and plant samples, this study presents REE signatures. The implication of each signature is discussed in the context of the environmental compartment, the geochemical background, and the mining influences. Using the bioconcentration and translocation factors, this study presents data on REE phyto-availability and fractionation.
2 Materials and methods
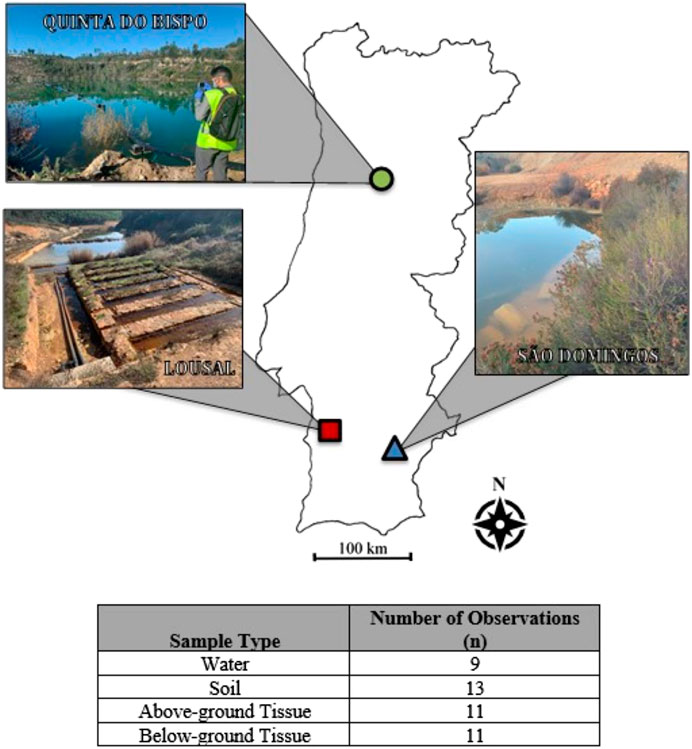
Nine water samples, 13 soil/aquatic sediment samples and 12 plant samples were recovered in November 2020 from three different legacy mine sites (Table 1) located in Portugal. Samples were collected from both inside and outside of mining sites to assess the legacy of mining activity. Site management and remediation is currently supervised by EDM, a State-owned enterprise, under a concession contract concluded with the Portuguese State for the environmental recovery of degraded mining areas (approved in Decree-Law n° 198-A/2001, 6/7/2001).
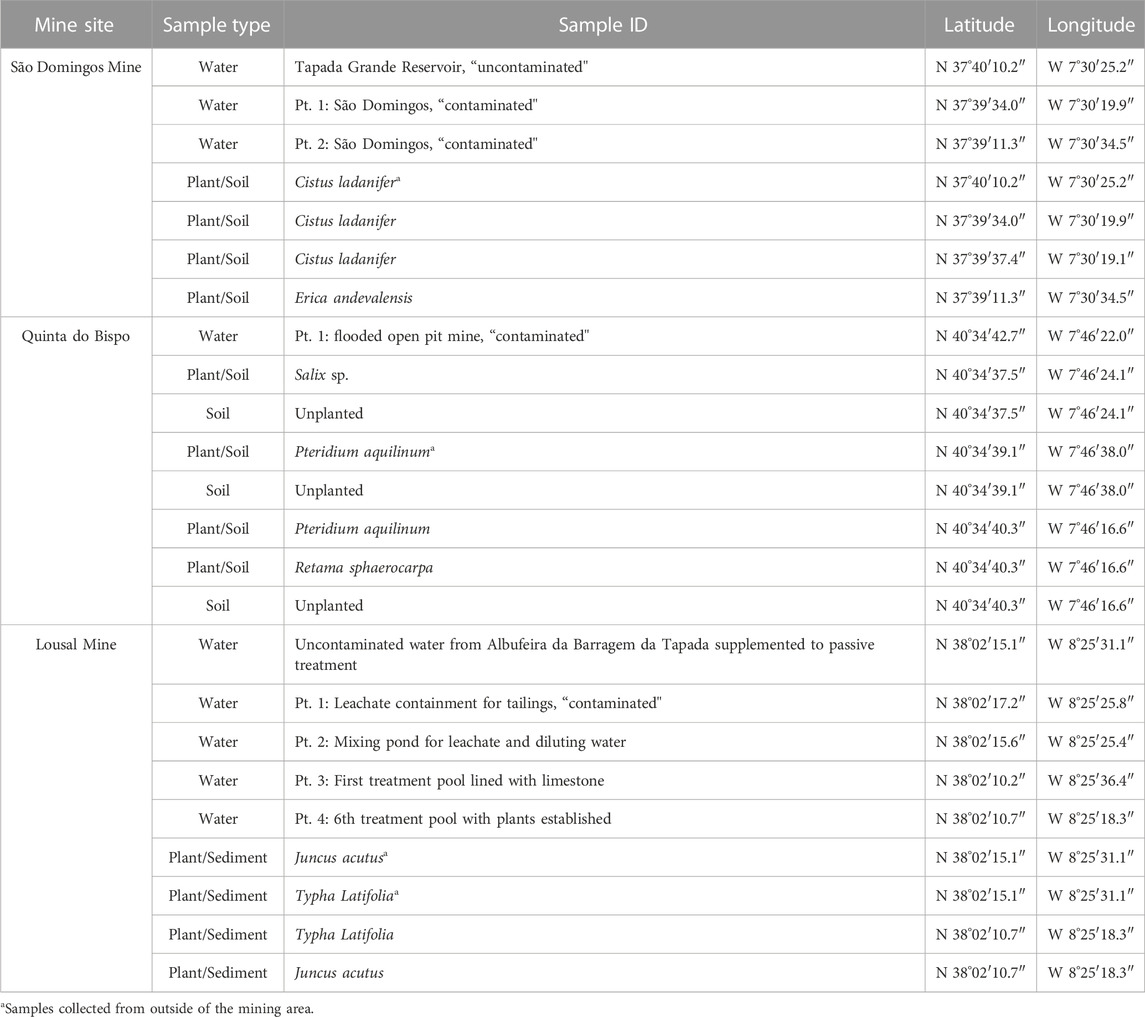
TABLE 1. Coordinates for all samples collected from mine areas in Portugal (São Domingos, Lousal and Quinta do Bispo).
2.1 Field site description and collected samples
The sample locations and types are reported in Table 1 and mapped in Figure 1. The geological characteristics of the sampling sites and the descriptions of the samples collected are detailed in the following section.
2.1.1 São Domingos
São Domingos, located in Southern Portugal, has been an established mining area since Chalcolithic times. Copper and sulfur ores were the dominant resources extracted from São Domingos mine till its closure in 1966 (Pérez-López et al., 2010). São Domingos covers 50 km2 of land in the Iberian Pyrite Belt (IPB) (Quental et al., 2002), an area infamously impacted by mining and the resulting AMD. The volcanogenic massive sulfides (VMS) in the IPB are rich in pyrite (FeS2) with inclusions of chalcopyrite (CuFeS2), sphalerite ((Zn,Fe)S), galena (PbS) and other polymetallic sulfide minerals. As the VMS deposits are exposed to the atmosphere, Fe(II) is oxidized to Fe(III) and S is oxidized to SO42−, forming H2SO4 in surface waters. The perpetual cycle of AMD has been a proven challenge for environmental recovery efforts. EDM’s strategy for rehabilitation prioritizes 1) the diversion of clean waters, 2) sealing and confinement of the mine waste deposits to prevent further AMD generation, 3) passive treatment of AMD, and 4) decontamination of the downstream Mosteirão River Valley. In order to mitigate the volume of contaminated waters, diversion of uncontaminated surface waters, as part of the first intervention, has already been completed. Following remediation, EDM intends to preserve the heritage of São Domingos and promote tourism.
The climate of São Domingos can be described as a temperate Mediterranean Kӧppen climate type with dry and hot summers (Csa) with an average annual air temperature of 17.5°C (Estatal de Meteorología (España) and Instituto de Meteorologia (Portugal), 2011). On average 500 mm of precipitation accumulates over the course of a year (Estatal de Meteorología (España) and Instituto de Meteorologia (Portugal), 2011). Species collected from São Domingos include: Cistus ladanifer and Erica andevalensis, both were prevalent species growing in the sampling area. Erica andevalensis is a metallophyte species endemic to the IPB, typically found growing on tailing piles or close to AMD-impacted surface waters (Cabezudo and Rivera, 1980). Since Erica andevalensis is identified as a vulnerable species (Law 8/2003 on Andalusian Wild Fauna and Flora) in Junta de Andalucia: Consejeria de Medio Ambiente’s list of threatened flora (Santa-Bárbara Carrascosa and Valdés, 2008/2008), this plant is subject to special protections.
2.1.2 Lousal
Lousal is an example of a legacy mine site under remediation and culturally revitalized as an educational center (Relvas, 2014). Located in the IPB of Southern Portugal, all mining activity ended at Lousal in 1988 (Relvas, 2014) when the extraction of S, Cu, and Zn from the polymetallic massive sulfide deposit became less profitable. Long established for the extraction of VMS ores, much like São Domingos, Lousal is impacted by AMD (Ferreira da Silva et al., 2009). In 2010, a passive treatment system consisting of 17 basins was built as part of EDM’s first phase of treatment works. The treatment system collects leachate from a sealed tailing pile. The leachate is supplemented with water from Albufeira da Barragem da Tapada reservoir during dry seasons to maintain the wetland system. Basins are structured in a cascade with a limestone barrier between each basin constituting a permeable dike and HDPE geomembrane lining underneath to contain treatment effluent until discharge into Corona Creek, a tributary of Sado River. In the first treatment stage, five basins are devoted to the neutralization, precipitation, and sedimentation of the leachate mixture. The consecutive basins are planted with macrophytes, which remediate the metal loading (Al, As, Cu, Fe, and Zn) from the tailing pile (Empresa de Desenvolvimento Mineiro [EDM] and Direcção Geral de Energia e Geologia [DGEG], 2011). According to the measurements made during the sampling campaign, by the time the effluent reaches Corona Creek for discharge the pH is increased from 2.3 to 3.2.
The climate of Lousal is similar to that of São Domingos, however Lousal accumulates 600 mm of rainfall annually on average (Estatal de Meteorología (España) and Instituto de Meteorologia (Portugal), 2011). The treatment system is dominated by aquatic species such as: Phragmites australis, Typha latifolia, and Juncus acutus. Due to sampling constraints and the large rooting system associated with Phragmites australis, only Typha latifolia and Juncus acutus were collected for analysis.
2.1.3 Quinta do Bispo
Quinta do Bispo is a legacy uranium mine site hosted in Hercynian granite and meta-sedimentary deposits (Ramalho et al., 2009) in Northern Portugal. Unlike São Domingos and Lousal, Quinta do Bispo is not subject to the geogenic phenomenon of AMD. Quinta do Bispo was Portugal’s largest open pit mine (European Commission Directorate-General for Energy, 2012). Between 1979 and 1987, 460,000 tons of ore were excavated, resulting in a 75-m deep pit (Ramalho et al., 2009). Later, in 1992 the open pit was used to extract low-grade uranium ores (300–500 ppm) using ISL, which employs strong acids—such as sulfuric acid—to leach precious metals from ore. The exploitation of hydrothermally deposited uranium seams has left the nearby surface waters with contamination from extraction processes (Pereira, 2014). Environmental remediation of ISL practices is ongoing at Quinta do Bispo. This site was selected for study because the lanthanide series has similar physiochemical characteristics to the actinide series.
The climate of Quinta do Bispo can be described by a temperate Mediterranean Kӧppen climate type with a dry and temperate summer (Csb) characterized by an average air annual temperature of 15°C and 1,400 mm annual rainfall (Estatal de Meteorología España and Instituto de Meteorologia Portugal, 2011). Due to site conditions being conducive to sample access the Quinta do Bispo site is the focus of the comparison between planted and unplanted soil environments. Species collection was limited to plants with large biomass and high prevalence on-site. Species collected included: Salix sp., Pteridium aquilinum and Retama sphaerocarpa.
2.2 Sample recovery and analyses
All materials used for the recovery of major cation and trace metal analysis were prepared in a clean room and decontaminated using a 24 h nitric acid (1.5 M HNO3) wash at 45°C, followed by a 24 h wash with deionized ultrapure water (18 MΩ-cm) at 45°C.
2.2.1 Water samples
In situ physiochemical water quality parameters were measured during sample collection using a Aquaread AP-2000 multimeter with an AquaPlus 2000 dynamic luminescence quenching optical dissolved oxygen (DO) combination electrical conductivity (EC) and temperature electrode, and a 3MPK1 silver chloride combination pH and oxidizing-reducing potential (ORP) probe. The ORP was converted to Eh by interpolating the half-cell potential of the reference electrode using manufacture provided potentials at variable temperatures.
For laboratory analyses, approximately 60 mL of sample were filtered through 0.2 μm polyethersulfone (PES) filters capable of resisting deterioration when exposed to low pH solutions. Filtered samples were collected and separated into two Nalgene bottles, one for dissolved organic carbon (DOC) and anion analysis, and one for major cation and trace metal analysis. Bottles for major cation and trace metal analysis were preserved with 2% HNO3 to maintain the integrity of the metals within the sample. The aliquots of sample reserved for dissolved organic carbon and anion analysis were kept refrigerated to preserve the integrity of analytes.
Major and trace cation concentrations were determined in water samples by ICP-MS (Agilent 7700x) using rhenium and rhodium as internal standards (Yéghicheyan et al., 2019). The international geostandard SLRS-6 was used to verify the validity and reproducibility of the results.
Dissolved Organic Carbon (DOC) and major anion concentrations of water samples were analyzed by Shimadzu TOC Analyzer, and by Dionex ICS-5000 ion chromatograph with an AS11 HC column (Dionex Method 123), respectively.
2.2.2 Plant samples
Plant samples were processed and analyzed in two mass fractions representative of the above-ground and below-ground tissues. Specimens from each species were collected within a 1-m radius and combined to represent one sample point. The above ground tissues were freeze dried and lyophilized, while below-ground tissue was washed with deionized water (18 MΩ-cm), and then dried in a 50°C oven for 4 days, and ground using a zircon ball-mill.
Above-ground plant samples were digested on a hot-plate using an adaption of the Krishnamurty (1976) method, whereas below-ground plant samples were digested more aggressively using a microwave (Anton Paar Multiwave 7000) with a modification of the preprogramed “organic” digestion method. Major and trace cation concentrations were determined using ICP-MS (Agilent 7700x) following an analogous protocol to the one performed on water samples, but with Tomato Leaves standard 1573a (NIST® SRM®) as a reference material for confirmation of the validity and reproducibility of results. Methodological error was determined using triplicate analysis of each sample.
2.2.3 Soil/aquatic sediment samples
Soils and aquatic sediments were collected from the same areas as the plant roots—representative of the rhizosphere - samples were dried at 40°C for 48 h and sieved to recover the <2 mm fraction. In addition to the rhizosphere soils collected for each plant, three bulk soils, identified by areas with no overlying vegetation, were collected from the Quinta do Bispo site.
Soil/aquatic sediment sample major and trace cation concentrations were analyzed by ICP-MS with alkaline fusion in the CNRS National SARM Analytical facility (https://sarm.cnrs.fr/index.html/) following the procedure described in Carignan et al. (2001), whereas soil/aquatic sediment texture, pHwater, pHKCl, cationic exchange capacity (CECMetson), and organic matter (OM) content were determined by LABOCEA. LABOCEA employed standard methods to determine extractable cations by ammonium acetate agitation at pH 7 (NF X 31-108) (Metson, 1956) and OM content by dry combustion of sample carbon (NF ISO 10694).
2.3 Data handling
In order to compare the enrichment or depletion of REE as a whole series, they are generally normalized to a standard set of values. For this study we normalized REE concentrations in soil and water samples to Taylor and McLennan (1989)’s Upper Continental Crust (UCC) values. Elements were further classified into three groups, the light REE (LREE) which range from elements La to Nd, middle REE (MREE) Sm to Tb, and heavy REE (HREE) Dy to Lu. For soil, sediment, and aqueous samples the Ce anomaly (Ce*) and Eu anomaly (Eu*) were calculated using a linear extrapolation of UCC-normalized REE, Eqs. 1, 2 respectively:
For comparisons of plant samples, the bioconcentration factor (BCF) and translocation factor (TF) were calculated using Eqs. 3, 4, accordingly, where [REEAG] is the concentration of REE in the above-ground tissue (mg kg−1) [REEBG] is the concentration of REE in the below-ground tissue (mg kg−1), and [REEsoil] is the concentration of REE in the soil or aquatic sediment collected from the plant rhizosphere (mg kg−1). The resulting BCF and TF values are dimensionless ratios. The BCF is reflective of the plant’s ability to accumulate an element from the soil/aquatic sediment and TF is reflective of the ability for the plant to translocate elements from the rooting system to aerial tissue. The Ce* and Eu* for plant samples were determined using BCF values calculated from Eqs 1, 2, instead of UCC-normalized values:
For statistical treatment, standard diagnostic tests were performed to determine whether data needed transformation. If a transformation was required, the box-cox method for transformation was used to determine the best fit. Data passing diagnostic tests were then tested with an Analysis of Variance (ANOVA), for statistical significance the p-value was limited to p < 0.05. The number, n, of all datasets analyzed is reported in Figure 1. Missing data are handled as random, allowing the analysis of imbalanced datasets, particularly soils and aquatic sediments. Post-hoc testing was completed using Tukey Honestly Significantly Difference (Tukey HSD).
3 Results
In the following section the REE concentrations and associated chemistry are presented for samples representing the water, soil/aquatic sediment, and plant environmental compartments.
3.1 Water sample REE signature
In situ physicochemical water quality parameters are displayed in Supplementary Table S1. Waters collected from outside of São Domingos and Lousal were circumneutral, pH 7.1 and pH 6.7, accordingly. Similar Eh values were observed for water collected from outside of São Domingos and Lousal, +397 mV and +360 mV, respectively. Of the water samples collected from inside of the mining sites, the open pit at Quinta do Bispo had the highest pH, 5.1. The sample collected at Quinta do Bispo still had an oxidizing Eh of +524 mV. Samples collected from the São Domingos River Valley display a pH ranging from 2.4 to 2.9 and were strongly oxidizing with an Eh ranging from +691 mV to +723 mV. Similarly, samples collected from the Lousal treatment system had a pH ranging from 2.3 to 3.0 with an Eh ranging from +615 mV to +808 mV.
The EC for the samples collected from the mining areas tended to be higher than the EC observed outside of the mining areas. The range of DOC for the samples collected ranges from 0.45 mg·L−1–9.71 mg·L−1. Supplementary Table S1 presents the concentrations of anions, measured in aqueous samples. The dominant inorganic anion observed in the mining sites was sulfate, in contrast to the dominant anion outside of the mining area which was chloride. The presence of sulfate is anticipated as sulfuric acid is generated through AMD and used during ISL.
Upper Continental Crust (UCC) normalized aqueous REE patterns are displayed in Figure 2 while REE concentrations are tabulated in Table 2. The aqueous samples collected from outside of the mining area contained less REE than the samples collected from inside of the mining areas. The samples collected outside of the mining area exhibited depletion of LREE relative to both the MREE and HREE. The depletion of LREE was punctuated by a negative Ce anomaly (Ce*) for samples collected outside the mining area. Samples collected from the Lousal passive treatment system had the highest REE concentrations with 628.84 μg·L−1 associated with the leachate treatment tank. The sample collected from the sixth treatment pool showed a marked decrease in dissolved REE with 238.68 μg·L−1. Aqueous samples collected from the AMD areas also contained higher concentrations of REE than in the open pit at Quinta do Bispo, perhaps indicating that AMD is a more potent mobilizer of REE than ISL. There were some similarities between the aqueous REE patterns, all mines showed a MREE enrichment as compared to the LREE and HREE. In addition to the MREE concavity, samples collected from São Domingos and Quinta do Bispo had a skewed pattern, with MREE > HREE > LREE. The open pit sample from Quinta do Bispo also exhibited a strong positive Eu anomaly (Eu*), not shared by the other sites.
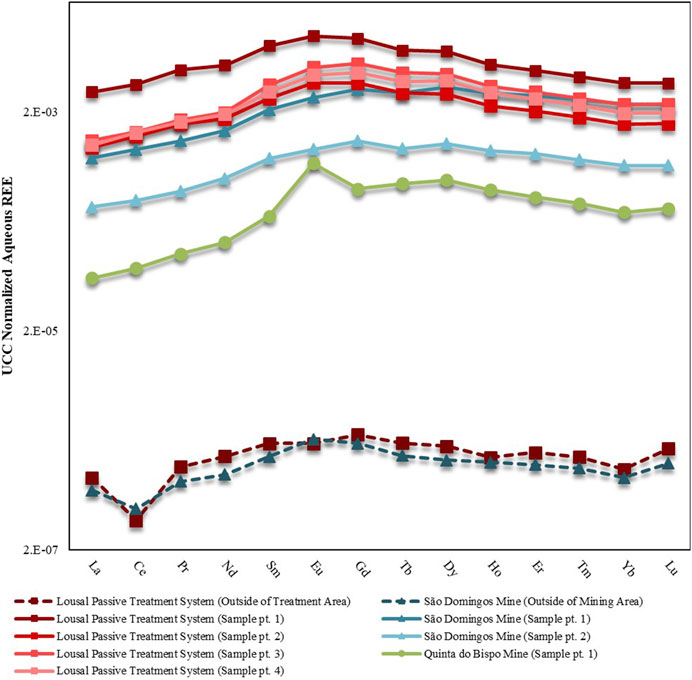
FIGURE 2. UCC-normalized REE patterns for aqueous samples collected from mine areas in Portugal (São Domingos, Lousal and Quinta do Bispo). Separate sampling points are distinguished using the abbreviation pt. Solid lines represent the samples collected inside of the mine, whereas dashed lines represent samples collected outside of the mining areas. Data for samples collected in São Domingos, Lousal and Quinta do Bispo are displayed by triangle, square and circle markers, respectively.
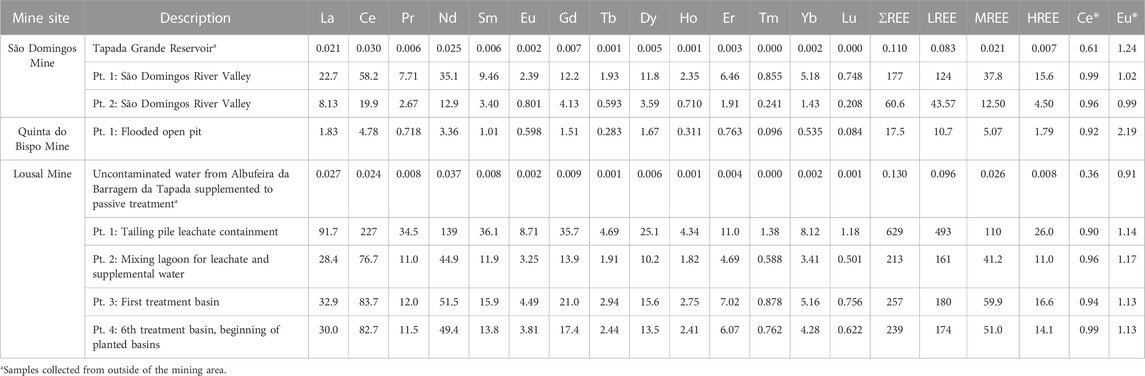
TABLE 2. Concentrations of REE in aqueous samples from mine areas in Portugal (São Domingos, Lousal and Quinta do Bispo). Separate sampling points are distinguished using the abbreviation pt. followed by a brief description of the sample location. Concentrations are reported in μg L−1; Ce and Eu anomalies (Ce* and Eu*, respectively) are also reported as dimensionless values.
3.2 Soil/aquatic sediment REE signature
A summary of major cation concentrations for water and soils/aquatic sediments reported in Supplementary Tables S2, S3, respectively. Table 3 displays the REE concentration of each individual soil. When evaluating soil REE concentrations there were three effects considered: 1) Prevailing geochemical context, 2) the mining context, and 3) rhizosphere effects.
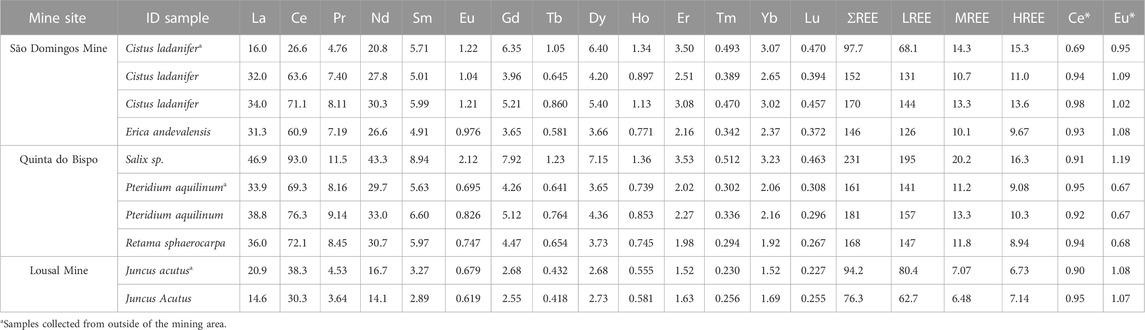
TABLE 3. The REE concentrations for all rhizosphere soils from mine areas in Portugal (São Domingos, Lousal and Quinta do Bispo) given in mg kg−1, as well as the Ce and Eu anomalies (Ce* and Eu*, respectively) given as dimensionless values.
3.2.1 Geochemical context
All of the water and soil/aquatic sediment sample data across all of the sites were used to make a correlation matrix and determine if any particular chemical couplings controlled REE behavior. As evidenced from Pearson’s correlations, HREE (Dy through Lu) in the soil have significant positive correlations (p < 0.05) with major cations such as Ca, Mg, and Mn. This correlation is also present in the water samples collected. Though no correlation between Fe concentration and REE was evident in soil samples, there was a correlation between Fe and REE in the water samples. These correlations are further contextualized in the results section which details how compatible cations may interact with REE.
Physiochemical characteristics of the soil/aquatic sediment were also taken into consideration for site geochemistry. Samples display contrasting pH, pHKCl, CECMetson, and OM concentrations (Supplementary Table S4). All of these characteristics can vary widely based on biotic and abiotic factors. The soils collected from São Domingos had high CECMetson and OM, while Lousal sediments had the lowest CECMetson and OM content.
3.2.2 Mining context
Based on the mining site there are distinct differences in the levels of REE enrichment observed at each site (Figure 3). Soil samples collected from Quinta do Bispo had the highest concentrations of REE (161–231 mg·kg−1), whereas aquatic sediment samples collected from Lousal exhibited the lowest concentrations (76–94 mg·kg−1) (Table 3). Statistically there was no difference between REE concentrations observed at Quinta do Bispo and São Domingos (p < 0.05). Similarly, there was no statistically significant difference between soils collected from São Domingos and aquatic sediments collected from Lousal (p < 0.05) with regards to the total REE concentrations. Quinta do Bispo and São Domingos share similarities in their environmental remediation status, since these legacy mine sites were both mitigated and maintained by EDM with environmental remediation planned in the near future. São Domingos and Lousal also share similarities, as both sites are in the IPB and subject to AMD.
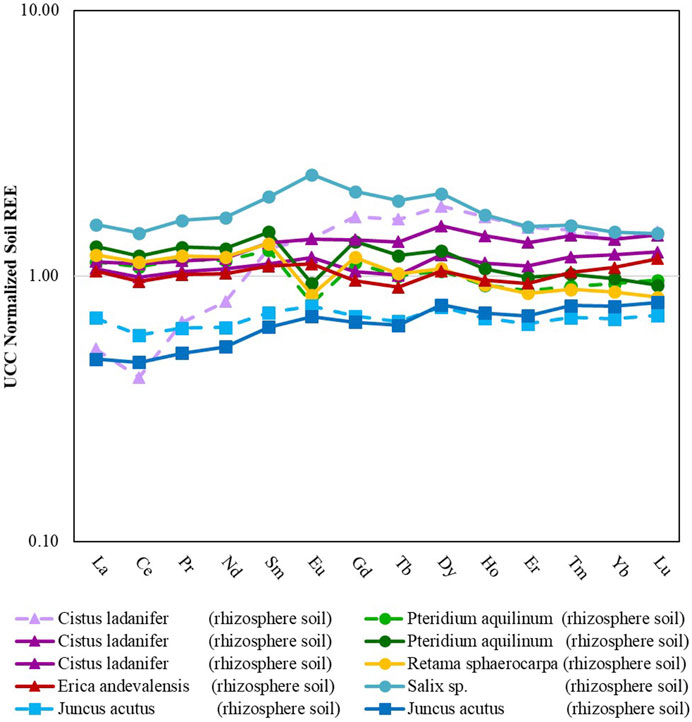
FIGURE 3. Rare earth element patterns for different rhizosphere soils from mine areas in Portugal (São Domingos, Lousal and Quinta do Bispo). Dashed lines represent soils collected outside of the mining areas. Data from soils collected in São Domingos, Lousal and Quinta do Bispo are displayed by triangle, square and circle markers, respectively.
Both Quinta do Bispo and São Domingos had statistically significantly higher LREE and MREE concentrations than Lousal. There was no statistical difference between the HREE for each of the mining sites. This can be attributed to the large spread of HREE concentrations. For Quinta do Bispo, a majority of the REE signatures show an enrichment of LREE and MREE with lower values of HREE when normalized to the UCC. One of the samples collected from Quinta do Bispo also displayed a positive Eu*, as opposed to the other Quinta do Bispo soil samples which display a negative Eu*. The REE signature for soils collected from São Domingos shows that the LREE and MREE had lower values than the HREE, with two of the samples showing significant enrichment. All of the aquatic sediment samples collected from Lousal were depleted in LREE, MREE, and HREE with respect to the UCC. The REE signatures for samples collected from Lousal display lower LREE values and higher MREE and HREE values.
There was no statistically significant difference for the soil LREE, MREE, HREE, or total REE concentrations among samples collected from inside or outside of the mining areas. As per the heterogeneous nature of soil, perhaps the spread of data between samples collected from inside and outside of the mining area is too large to make a distinction between the influences of the mining area without also taking into consideration the geochemical context. This is further highlighted by the fact that the REE signature for the soil sample collected from outside of Sao Domingos does not match samples collected from inside of the same site. The sample collected from outside of the mining area features depletion of the LREE and enrichment of the MREE and HREE with respect to the UCC, along with a negative Ce*. Due to sampling constraints encountered in this study there was not enough sample replication to determine the combined effects of mining and the surrounding geochemistry.
3.2.3 Rhizosphere effects
A comparison of the rhizosphere versus bulk soils was possible at the Quinta do Bispo site (Supplementary Table S5) and shows that the rhizosphere and bulk soils display similar patterns. This comparison should also show whether plants selectively depleted or enriched REE from the soil. There was ultimately no statistical difference for the LREE, MREE, HREE, or Total REE in soils collected from the plant rhizosphere when compared with bulk soils. This would indicate that the plant does not influence the soil REE observed in the area.
3.3 Plant sample REE signature
When considering the responses of plant REE concentration, BCF, and TF there were three main effects investigated: 1) Prevailing geochemical context, 2) the mining context, and 3) the plant type.
3.3.1 Geochemical context
There was no statistical difference between the BCF (Figure 4) or the TF of the Total REE (Figure 5) observed in samples collected from Quinta do Bispo, São Domingos, or Lousal. This would indicate that the geological background did not influence the uptake of REE. However, this conclusion is difficult to support, since among the plants collected from each site there were no shared species for comparison. Therefore, a potential area for expansion of this study could be to research REE in the same plant species found at different mining sites. Such a task may prove difficult since the spontaneous vegetation is specific to the growth conditions of the individual site. In the discussion section comparisons from literature are presented in order to account for some of the differences seen across plant types collected in different geological conditions.
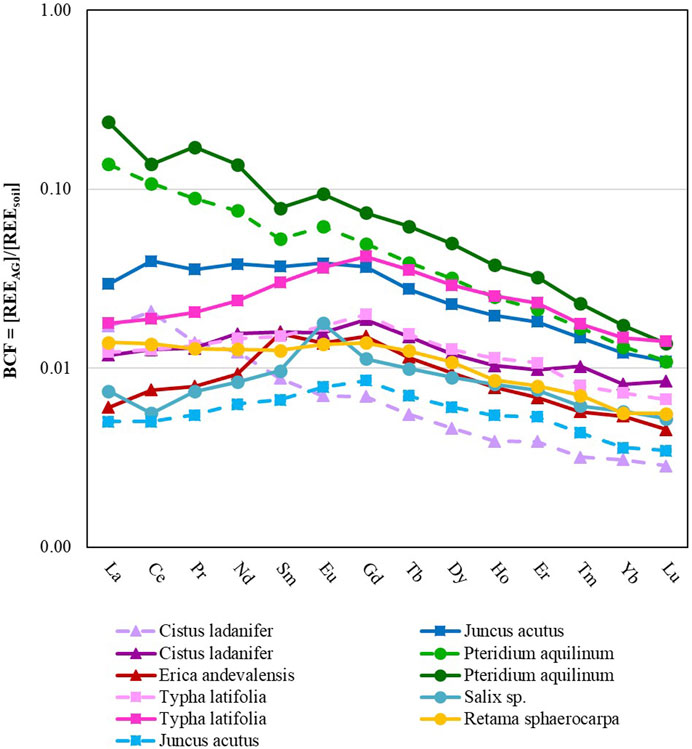
FIGURE 4. Plant BCF versus REE for above-ground tissue. Solid lines represent plant samples collected from inside of the mining sites while dashed lines represent samples collected from outside of the mining sites. Data from plants collected in São Domingos, Lousal and Quinta do Bispo (Portugal) are displayed by triangle, square and circle markers, respectively.

FIGURE 5. Plant TF versus REE. Solid lines represent plant samples collected from inside of the mining sites while dashed lines represent samples collected from outside of the mining sites. Data from plants collected in São Domingos, Lousal and Quinta do Bispo (Portugal) are displayed by triangle, square and circle markers, respectively.
3.3.2 Mining context
While it is difficult to make conclusions about the effects of geochemistry on the bioaccumulation of REE in plants, there were some differences in plants collected from inside of the mining site when compared with plants collected from outside of the mining site (Figure 4).
In general, the BCF and REE concentrations (Table 4) were elevated for plants collected from inside of the mining areas, when compared with plants collected from outside of the mining areas (approximately 76% more REE in the plants recovered from inside of the mining areas), indicating an enhancement in the uptake and bioconcentration of REE from the soil into the above-ground tissue. Specifically, the MREE and HREE were taken up at a greater rate when the plant was collected from inside of the mining area (94% more MREE and 106% more HREE, as compared with the LREE which was elevated by 75%). More specifically, plants had an elevated BCF (p < 0.05) for Sm, Eu, Gd, Tb, Dy, Ho, Er, Yb, and Lu, when collected from inside of the mining area. However, there was also no significant difference between the TF of samples collected inside vs. outside of the mining area. In summary, mining increased the bioavailability of all REE, in particular elevating the BCF for MREE and HREE over LREE. This increased availability of REE did not translate to an increased TF.

TABLE 4. Concentration of REE in above-ground and below-ground plant tissues (μg kg-1) collected on mine areas in Portugal (São Domingos, Lousal and Quinta do Bispo) as well as the Ce and Eu anomalies (Ce* and Eu*, respectively).
3.3.3 Plant type
Pteridium aquilinum showed the greatest affinity for REE, accumulating 27.3 mg·kg−1 of REE in the above-ground tissue. Similar to other studies, many of the plant types collected show higher concentrations of REE associated with the below ground-tissue as opposed to the above-ground tissue (Babula et al., 2009), with the exception of P. aquilinum which accumulated more La, Ce, and Pr in the above-ground plant tissue than in the below-ground plant tissue. Of the plants collected with replication (P. aquilinum, Cistus ladanifer, Typha latifolia, and Juncus acutus), plant type played a statistically significant role (p < 0.05) in the BCF of La, Ce, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Yb. P. aquilinum had a significantly higher BCF for all of the aforementioned elements except for Ho. For Ho, P. aquilinum had similar rates of accumulation to T. latifolia, but had higher accumulation rates when compared to J. acutus and C. ladanifer. T. latifolia also had a higher BCF when compared to C. ladanifer for Dy and Ho, and a higher BCF when compared to J. acutus for Tm.
Of the REE accumulated in P. aquilinum, the LREE tended to concentrate at a higher rate than MREE, and both LREE and MREE tended to concentrate more than the HREE. Lanthanum was three orders of magnitude more concentrated than Lu. Similarly, the C. ladanifer sample collected from outside of the mine exhibited the same downward trend of LREE>MREE>HREE. This trend was not conserved across all plant types. T. Latifolia and Erica andevalensis demonstrated a MREE concave pattern instead, MREE>LREE = HREE. The BCF of J. acutus and Retama sphaerocarpa displayed a pattern where LREE = MREE>HREE.
Three plant samples had a positive Eu*: the Salix sp. sample and both of the P. aquilinum samples. All of these samples were collected from Quinta do Bispo. This anomaly accounts for the initial soil Eu* as the plant tissue is normalized to the native soil when evaluating the BCF. Additionally, there was a positive Eu* for the TF, so though modest amounts of Eu* were incorporated into the below-ground tissue from the soil, there was a preferential transport and accumulation of Eu into the aerial plant tissue.
Moreover, the C. ladanifer sample collected from outside of the São Domingos mining area had a positive Ce* in the above-ground tissue and negative Ce* in the below-ground plant tissue, which is matched with a high TF for Ce. The total concentration of Ce in the plant tissue was not in fact inordinately high and when normalized to UCC values shows no such anomaly. This observation is matched with soil and water samples collected from the same area showing Ce depletion as well. In this particular sample, Ce was accumulated in the above-ground tissue of the plant at a higher rate and the root anomaly is an artifact of less plant-available Ce in the rhizosphere.
There are three distinct groups of TF rates (Figure 5). The highest TF is seen for both of the P. aquilinum (collected from both inside and outside of the Quinta do Bispo mining area), C. ladanifer (collected from inside of the São Domingos mining area), and R. sphaerocarpa (collected from inside of the Quinta do Bispo mining area). The middle group consisted of the C. ladanifer collected from outside of the mining area, T. latifolia, Salix sp., and E. andevalensis. The TF of the middle group was 3 times lower than the highest TF group. The lowest TF was observed in J. acutus, which had large quantities of REE associated with the below-ground tissue. Low TF resulted in low accumulation of REE in the above-ground tissue. The TF for J. acutus was 177 times less than the TF recorded for plants in the highest TF group. This observation indicates that a majority of the REE remained stabilized within the below-ground tissue of J. acutus. Plant type had a significant effect on the TF of total REE and LREE, but had no effect on the MREE and HREE. REE was preferentially translocated in the following order: LREE>MREE>HREE for select plants, particularly C. ladanifer and P. aquilinum samples. Many of the other plant samples have no relative slope regarding the TF for LREE, MREE, and HREE.
4 Discussion
4.1 Geochemical and mining context
Though none of the studied mines targeted the extraction of REE ores, REE can be distributed throughout silicate, carbonate, oxide, phosphate, and oxyhydroxide (Migaszewski and Gałuszka, 2015) minerals, which are susceptible to dissolution under low pH conditions or changes in reduction/oxidation conditions (Cao et al., 2001; Mihajlovic et al., 2017). The MREE enrichment in aqueous samples is a typical signature of AMD (Perez-Lopez et al., 2010). The pH range and Eh observed for both São Domingos and Lousal are also consistent with the geochemical context of AMD (Dold, 2014). The pH observed in Quinta do Bispo was relatively high when compared with the pH of São Domingos and Lousal. This is most likely due to the fact that sulfate mineral oxidation or AMD is negligible in the surrounding geology (Carvalho et al., 2005) and the ISL methods employed at Quinta do Bispo ended in 1999 (European Commission Directorate-General for Energy, 2012). The saturation of cations and anions in waste streams, represented by the EC measured in aqueous samples, favors the precipitation of metals (Nieto et al., 2007), and is therefore also a parameter used to evaluate the precipitation/dissolution dynamics at these sites. Low pH, high EC, and elevated sulfate concentrations were indicative of the geogenic and anthropogenic dissolution of primary minerals inside of the three legacy mine sites. These geochemical changes were induced by AMD processes or ISL practices.
In addition to dissolution, processed REE were susceptible to sorption and desorption processes, which were evaluated in this study based on the CECMetson and OM content of soils and aquatic sediments. A soil with higher CECMetson and OM content has a high metal loading capacity, whereas a soil with a low CECMetson and OM content has less potential metal-sorbing surfaces, thus the metal holding capacity of soils collected from São Domingos is higher and the metal holding capacity of aquatic sediments collected from Lousal is lower. The sediments collected from Lousal had lower concentrations of REE indicative of the lower metal holding capacity of these sediments relative to the other sampled sites. As a result, Lousal also displayed the highest aqueous REE concentrations.
All of the samples collected from inside of the mining sites are considered impaired simply on the basis of having low pH (pH < 6.5) (Baker et al., 1990). As Baker et al. (1990) reported, biological diversity is lost starting at pH < 6.5, and an interruption of nutrient cycling at pH < 5.0. While pH directly impacts the ecology of a system, the increased metal solubility and bioavailability caused by acidification (Baker et al., 1990; Cao et al., 2001; Batista et al., 2013) can also induce toxicity (Baker et al., 1990; Plante, 2007; Batista et al., 2013).
Inorganic anions typically associated with REE complexation include: SO42−, PO43−, Cl−, CO32−, and F− (Millero, 1992; Vaziri Hassas et al., 2021). As stated in the results, the mining areas were dominated by sulfate. A study by Zhimang et al. (2000) implicates sulfates in the bioavailability of REE to plants, mainly showing that REE accumulation in below-ground tissue is limited by sulfate. Secondary sulfate salts such as jarosite and alunogen have been shown to be temporary stabilizers of polyvalent cations (including REE) in AMD (Ferreira et al., 2021). Subsequent solubilization of metals associated with jarosite, alunogen, and other secondary sulfate minerals has been implicated as a cause for delayed revegetation (Ferreira et al., 2021). This demonstrates that plants growth in mining environments is dependent on the prevailing geochemistry, as primary and secondary minerals constitute a source or sink for potentially toxic metals.
Aside from sulfates being a sink for REE, sulfates have also been implicated in the selective partitioning of REE in soils and waters (Ferreira da Silva et al., 2009; Welch et al., 2009; Grawunder et al., 2014; Soyol-Erdene et al., 2018). REE have even been suggested as tracers of mining pollution due to their unique MREE-enriched signature in mining wastes (Merten et al., 2005; Perez-Lopez et al., 2010). This signature is presumably due to sulfate and sulfate intermediates showing higher stability in complexation with MREE (Perez-Lopez et al., 2010) and precipitation/sorption processes occluding LREE (Liu et al., 2019) and HREE (Byrne and Li, 1995) from solution, paired with dissolution/desorption of MREE-enriched Fe and S minerals (Welch et al., 2009; Perez-Lopez et al., 2010; Grawunder et al., 2014). The occurrence of a correlation between REE and Fe, as seen in the water samples collected in this study, may indicate that Fe-mineral dissolution contributes to the soluble REE in these systems (Cao et al., 2001; Merten, 2005; Prudêncio et al., 2015; Prudêncio et al., 2017; Riley and Dutrizac, 2017; Liu et al., 2022). Many geochemical factors may contribute to the observed high REE concentrations and unique MREE enrichment in AMD surface waters, a detailed study on REE speciation is still needed.
The DOC concentrations anticipated in AMD waters should be low as acidic pH promotes an increase of organic aggregation, inducing a decrease of their colloidal stability (Johnson and Hallberg, 2003; Pédrot et al., 2009). Though limited amounts of DOC and OM are associated with AMD systems, no broad generalizations can be made about the fractionation of REE by organics, which is specific to the type of organic matter. Organic matter forms a significant sink for REE, both as a sorbing surface and as a bridging ligand (Byrne and Li, 1995; Pourret et al., 2007). Organic ligands make a more stable complex with HREE, causing HREE to deplete from solution into complexes with sediment surface ligands (Byrne and Li, 1995). More complex organics, such as humic substances, have been shown to produce a fractionation of MREE enrichment over both LREE and HREE, specifically in natural systems characterized by a high metal loading like the sites studied (Pourret et al., 2007; Pédrot et al., 2008).
The oxidation state of REE is generally trivalent, with the exception of Eu2+ and Ce4+. In this study, a negative Ce* was associated with samples collected from outside the mining areas. Geochemically, the waters collected from outside of the mining area are less oxidizing and have a higher pH. Therefore, the speciation of metals may vary between waters collected from inside of the mining area versus those collected from outside of the mining area. The negative Ce* observed in surface waters collected from outside of the mining areas is indicative of preferential Ce4+ sorption to the solid phase, which is typical of circumneutral pH waters under oxidizing conditions (Bau, 1999).
The positive Eu* in liquors and ores associated with the open-pit can be attributed to the hydrothermal deposit where the Eu2+ oxidations state is preferentially incorporated into high-temperature hydrothermal seams over the other REE, leading to enriched Eu concentrations (Sverjensky, 1984; Danielson et al., 1992). The proceeding ISL processes increased mobilization of all REE contained in the Eu-enriched ore, accordingly producing an enrichment of Eu in the remaining liquor and analogous waste rock. This mechanism explains the positive Eu* seen in the open-pit water sample and the Salix sp. rhizosphere soil, which was collected from the waste rock pile. All other soil samples collected from Quinta do Bispo had a negative Eu*. Consequently, the conclusion can be drawn that the soils collected from the surrounding material were depleted in Eu as it was partitioned into the hydrothermal seam. Thus, in the soil samples collected from the surrounding areas, there is a negative Eu* corresponding to this partition.
4.2 Plant influences
In order for a plant type to be considered a REE hyperaccumulator, the REE concentration in the above ground-tissue must exceed 1,000 μg·g−1 (Liu et al., 2018). None of the plants collected in this study met the hyperaccumulator definition. Liu et al. (2018) acknowledges the importance of plant biomass, as high biomass can accrue significant amounts of REE. The concentrations of REE observed in the collected plants do not show any particular elevation or depletion when compared with other studies of plant REE (Wyttenbach et al., 1998; Liang et al., 2008; Migaszewski and Gałuszka, 2015). The P. aquilinum plant species did accumulate more REE than any other plant type: though this figure represents 6–53 times the amount accumulated by the other plant samples in the present study, the pteridophyte sample is not remarkably high. Pteridophytes are generally known to accumulate much higher concentrations of REE over other plant types (Tyler, 2004). P. aquilinum also tended to concentrate certain REE at a higher level in the above-ground tissue than in the below-ground tissue. Wang et al. (1997) made a similar observation in Dicranopteris dichotoma, another pteridophyte, which accumulated more REE in the above-ground tissue than in the below-ground tissue.
As mentioned in the materials and methods section, the BCF is reflective of biological occlusion or assimilation of particular REE from the surrounding media. The closer the BCF value is to 1, the closer the plant concentrations reflect the soil concentrations. In this study, any uptake of REE by the plant was insignificant in comparison with the soil pool. This was proven by a comparison of bulk and rhizosphere soils which showed no depletion or enrichment dependent on plant growth, and further supported by the BCF which for most plants was much less than 1.
The BCF can also be looked at in terms of slope and anomaly, indicating the effects of soil speciation and plant species selectivity towards the assimilation of particular REE into the above-ground tissue. Ultimately this study showed that for certain plant types (P. aquilinum), above-ground tissues were enriched in LREE relative to the surrounding soil. This finding is congruent with previous research, which demonstrated that LREE tend to be enriched in the above-ground tissue of plants compared to the MREE and HREE (Wang et al., 1997; Fu et al., 1998; Liang et al., 2008; Gonzalez et al., 2014). This may have to do with the plant’s affinity to uptake LREE (Brioschi et al., 2012) and porewater chemistry restricting available HREE (Millero, 1992; Byrne and Li, 1995; Fu et al., 1998; Tyler, 2004; Brioschi et al., 2012). However, this pattern was not maintained across all plant types. In fact, T. latifolia and Salix sp. showed an MREE-enrichment compared with LREE and HREE, supported by Wyttenbach et al. (1998).
Salix sp. and P. aquilinum had positive Eu* in their BCF signatures. Positive Eu* in plant tissue has been observed in other studies (Durães et al., 2014; Krzcuik and Gałuszka, 2019; Krzciuk and Gałuszka, 2020). There are a couple of to-date unconfirmed hypotheses on why plants may have Eu*. Potentially Eu3+ is transformed to Eu2+ and is taken up by plants under the redox conditions of the rhizosphere. This may be a result of diurnal patterns, which dictate oxygen supply to the roots (Krzcuik and Gałuszka, 2020), or it may be related to the acquisition of other essential nutrients; such as Fe, which is reduced at the root dermis (Marschner and Rӧ;mheld, 1994; Wyttenbach et al., 1998). In both scenarios, the supply of Eu and the redox conditions are a vital control on the bioavailability of Eu. Many authors (Wang et al., 2012; Brioschi et al., 2013; Durães et al., 2014; Thomas et al., 2014) have suggested that the origin of positive Eu* in plants is the result of Eu2+ substitution for an essential plant nutrient such as Ca2+ due to the similarity in ionic radius (Shannon, 1976). All of the above-ground tissue REE concentrations positively correlate with above-ground tissue Ca2+ concentrations for this study.
The investigation of the TF can be used to make conclusions about the transfer and relative accumulation of REE in and to different parts of the plant. In this study, the REE transfer from below-ground tissue to above-ground tissue did not relate to the geochemistry or mining context. It was only reflective of plant type differences. The subsequent enrichment in the LREE seen in the C. ladanifer and P. aquilinum plants is then an artifact of the preferential translocation of LREE into the above-ground tissue. Fu et al. (1998) posited that preferential uptake and translocation of REE in a pteridophyte was related to plant Si processes and that in situ recrystallization of Si would allow for substitution of MREE and HREE occluding the larger ionic radius of LREE, leaving the LREE more mobile and able to reach the distal and aerial tissues. Evidence supporting this may be seen in the strong positive correlation between all of the REE and Si (Supplementary Table S6) among all samples collected in this study.
Combining the information presented on the BCF enrichment of MREE with the lack of a TF MREE enrichment, it can be concluded that the MREE fractionation into the above-ground plant tissue, observed in T. latifolia and Salix sp., originates from the preferential uptake of MREE in the below-ground tissue. While the total bioavailable pool of metals can include the soil (water-soluble, ligand-exchangeable, and organic or carbonate bound metals), the dissolved metals in water are typically thought of as readily bioavailable (Adriano, 2001; Di Bonito et al., 2008). These dissolved concentrations could explain the elevated MREE in plants, as the enrichment of the MREE in plant tissue mirrors the enrichment of MREE in surface waters impacted by mining.
The information presented may show that the MREE enrichment seen in the roots is an artifact of the soluble REE pool in these mine sites, whereas the LREE enrichment and Eu* in the above ground plant tissue demonstrates plant-specific fractionation. Thus, this study provides evidence that the REE pattern displayed by each plant may result from an interaction between the plant type and growth medium. This conclusion is contrary to one posited by Laul and Weimer (1982). Laul and Weimer (1982) studied a selection of biological samples and concluded that the REE patterns closely resembled their growth medium when normalized to chrondrites, however the REE patterns for plants were never normalized to the soil media to confirm enrichment or depletion, which would have obviated any preferential uptake of specific REE. Wang et al. (1997) similarly found that the concentration of REE in most plants collected from sites with REE-rich ores had similar patterns to the soils they were collected from, but also notes an accumulation of LREE over HREE. Pisciotta et al. (2017) made a similar conclusion that the plant tissue reflected the geochemistry of the collection site, however this conclusion was based on one plant type in one geochemical context. Another study by Krzcuik and Gałuszka (2020) showed that plants generally had a similar pattern for uptake regardless of the land usage, proving quite the opposite of earlier studies by Laul and Weimer (1982) and Pisciotta (2017), since this would mean that regardless of geochemical background, plants had a certain physiological signature for REE-uptake. In the context of Krzcuik and Gałuszka (2020) it should be noted that researchers did find some differences based on collection of samples inside or outside of AMD areas. A study conducted by Barbera et al. (2021) actually showed that a planted system when spiked with REE did not necessarily lead to increased uptake of REE into the above-ground tissue. Barbera et al. (2021) instead shows increased REE concentration in the below ground tissue with limited transfer of REE into the above-ground tissue. Wyttenbach et al. (1998) similarly refuted the idea that the growth media was completely responsible for REE fractionation in above-ground tissues. Thus, there is evidence to support claims from each of these previous studies and create a more cohesive theory that both geochemical background and plant type contribute to REE patterns in plant tissues. The results from the present study seem to indicate that some plant types may be reflective of the area geochemistry, whereas others may show increased fractionation of soil REE, which demonstrates the importance of capturing many plant species in differing contexts.
5 Conclusion
Using samples from three Portuguese legacy mine sites, this study reaffirms previous findings that AMD waters tend to be enriched in MREE as compared with LREE and HREE. Through the coupled recovery of water, soil and plant samples, this research contributes to a growing body of work proving that 1) the substrate plays a role in the fractionation of REE into plant tissue and 2) the plant type can also contribute to REE fractionation.
Samples collected from inside of the mining sites had elevated dissolved REE concentrations in water samples, matched with a higher BCF in plant samples. This demonstrates the importance of the dissolved metal pool on the bioavailability of REE. Further sampling and identification of chemical speciation is recommended as the signatures for REE indicate some differences amongst soil types leading to differences in REE bioavailability. There were also no statistical differences in the BCF for plants based on the site in which they were collected from. This combined with the elevation of MREE in plant tissue may indicate that the dissolved REE pool plays a more significant role in plant REE uptake than the soil mineral REE. The concentration of REE in the soils collected from the rhizosphere and bulk soils were not statistically different, showing that the soil pool of REE in the plant rhizosphere was not significantly depleted when compared with unplanted soils.
Differences in uptake may be due to plant physiology as well. This hypothesis is supported by the fact that although the soils, aquatic sediments, and waters in this study had similar features, certain plants, such as P. aquilinum, seemed better adapted to extracting particular REE over others. Though it was anticipated that plant types commonly used in passive treatment systems, such as J. acutus, would perform best in extracting REE, very little REE was associated with the above-ground mass of these plants. Though the REE share physiochemical properties, there was observed preferential transfer of LREE and Eu into the aerial parts of certain plants. Thus, the unique geochemical context in which MREE is enriched in the dissolved phase, when combined with the preferential translocation of LREE and Eu based on plant type, creates each plant’s REE signature.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
AD and MD were responsible for funding acquisition. MP, RM, DR, CD, and KF organized sample retrieval. ID prepared plant samples for analysis. KF and ML performed laboratory analysis of the samples. KF compiled sample analysis and drafted the original manuscript. AD and MP significantly contributed to the revision of the manuscript. CD, EC, and RM read, edited, and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant Agreement No. 857989 (PANORAMA project). This publication is also supported by the European Union through the European Regional Development Fund (FEDER), the French ministry of Higher Education and Research, the French Region of Brittany and Rennes Métropole.
Acknowledgments
The authors are grateful for the support of the GeOHeLiS analytical platform of Rennes University with technical support from Maxime Pattier and Patrice Petitjean.
Conflict of interest
Access to legacy mine sites for sampling was provided by EDM, a State owned enterprise, under a legal regime of concession contract concluded with the Portuguese State for the environmental recovery of degraded mining areas (approved in Decree-Law no. 198-A/2001 and 6/7/2001).
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2023.1191909/full#supplementary-material
References
Adriano, D. C. (2001). “Bioavailablity of trace elements,” in Trace elements in terrestrial environments. Editor D. C. Adriano (New York: Springer), 61–89.
Akcil, A., and Koldas, S. (2006). Acid mine drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 14 (12-13), 1139–1145. doi:10.1016/j.jclepro.2004.09.006
Babula, P., Adam, V., Opatrilova, R., Zehnalek, J., Havel, L., and Kizek, R. (2009). “Uncommon heavy metals, metalloids and their plant toxicity: A review,” in Organic farming, pest control and remediation of soil pollutants sustainable agriculture reviews. Editor E. Lichtfouse (Dordrecht: Springer). doi:10.1007/978-1-4020-9654-9_14
Baker, J. P., Bernard, D. P., Christensen, S. W., Sale, M. J., Freda, J., Heltcher, K., et al. (1990). Biological effects of changes in surface water acid-base chemistry. Washington, D.C.: United States: Department of Energy. doi:10.2172/7255574
Barbera, M., Zuddas, P., Palazzolo, E., and Saiano, F. (2021). The distribution of rare earth elements discriminates the growth substrate of Vitis vinifera L. Chemosphere 266, 128993. doi:10.1016/j.chemosphere.2020.128993
Batista, M. J., de Oliveira, D. P. S., Abreu, M. M., Locutura, J., Shepherd, T., Matos, J., et al. (2013). Sources, background and enrichment of lead and other elements: Lower Guadiana River. Geoderma 193-194, 265–274. doi:10.1016/j.geoderma.2012.08.033
Bau, M., and Dulski, P. (1996). Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 143, 245–255. doi:10.1016/0012-821x(96)00127-6
Bau, M. (1999). Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta. 63, 67–77. doi:10.1016/s0016-7037(99)00014-9
Binnemans, K., Jones, P. T., Blanpain, B., Van Gerven, T., Yang, Y., Walton, A., et al. (2013). Recycling of rare earths: A critical review. J. Clean. Prod. 51, 1–22. doi:10.1016/j.jclepro.2012.12.037
Brioschi, L., Steinmann, M., Lucot, E., Pierret, M. C., Stille, P., Prunier, J., et al. (2013). Transfer of rare earth elements (REE) from natural soil to plant systems: Implications for the environmental availability of anthropogenic REE. Plant Soil 366, 143–163. doi:10.1007/s11104-012-1407-0
Byrne, R. H., and Li, B. (1995). Comparative complexation behavior of the rare earths. Geochim. Cosmochim. Acta. 59 (22), 4575–4589. doi:10.1016/0016-7037(95)00303-7
Cabezudo, B., and Rivera, J. (1980). Notas taxonómicas y corológicas sobre la Flora de Andalucía Occidental. Lagascalia 9 (2), 219–248.
Cao, X., Chen, Y., Wang, X., and Deng, X. (2001). Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 44, 655–661. doi:10.1016/s0045-6535(00)00492-6
Carignan, J., Hild, P., Mevelle, G., Morel, J., and Yeghicheyan, D. (2001). Routine analyses of trace elements in geological samples using flow injection and low pressure on-line liquid chromatography coupled to ICP-ms: A study of geochemical reference materials br, DR-N, UB-N, AN-G and gh. Newsl 25 (2-3), 187–198. doi:10.1111/j.1751-908x.2001.tb00595.x
Carvalho, F. P., Madruga, M. J., Reis, M. C., Alves, J. G., Oliveira, J. M., Gouveia, J., et al. (2005). “Radioactive survey in former uranium mining areas of Portugal,” in Environmental contamination from uranium production facilities and their remediation (Vienna, Austria: International Atomic Energy Agency), 29–40.
Casiot, C., Egal, M., Elbaz-Poulichet, F., Bruneel, O., Bancon-Montigny, C., Cordier, M., et al. (2009). Hydrological and geochemical control of metals and arsenic in a Mediterranean river contaminated by acid mine drainage (the Amous River, France); preliminary assessment of impacts on fish (Leuciscus cephalus). Appl. Geochem. 24 (5), 787–799. doi:10.1016/j.apgeochem.2009.01.006
Chour, Z., Laubie, B., Morel, J. L., Tang, Y., Qiu, R., Simonnot, M. O., et al. (2018). Recovery of rare earth elements from Dicranopteris dichotoma by an enhanced ion exchange leaching process. Chem. Eng. Process. – Process Intensif. 130, 208–213. doi:10.1016/j.cep.2018.06.007
International Union of Pure and Applied Chemistry (2005). Editors N. G. Connelly, T. Damhus, R. M. Hartshorn, and A. T. Hutton (Cambridge, U.K.: IUPAC).Nomenclature of inorganic chemistry
Danielson, A., Mӧller, P., and Dulski, P. (1992). The europium anomalies in banded iron formations and the thermal history of the oceanic crust. Geol 97, 89–100. doi:10.1016/0009-2541(92)90137-t
Di Bonito, M., Breward, N., Crout, N., Smith, B., and Young, S. (2008) “Chapter ten – overview of selected soil pore water extraction methods for the determination of potentially toxic elements in contaminated soils: Operational and technical aspects,” in environmental geochemistry , eds. B. de Vivo, H. E. Belkin, and A. Lima (Amsterdam, NL: Elsevier), 213–249.
Dold, B. (2014). Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals 4, 621–641. doi:10.3390/min4030621
Durães, N., Ferreira da Silva, E., Bobos, I., and Ávila, P. (2014). Rare earth elements fractionation in native vegetation from the moncorvo iron mines, NE Portugal. Procedia Earth Planet. Sci. 10, 376–382. doi:10.1016/j.proeps.2014.08.064
Empresa de Desenvolvimento Mineiro and Direcção Geral de Energia e Geologia (2011). The legacy of abandoned mines: The context and the action in Portugal. Lisboa, Portugal: EDM.
Estatal de Meteorología (España) and Instituto de Meteorologia (Portugal) (2011). Iberian climate atlas. Madrid, Spain: Estatal de Meteorologia.
European Commission Directorate-General for Energy (2012). Uranium sites environmental radioactivity and discharge monitoring and part of national monitoring system for environmental radioactivity: Portugal. Article 35 Technical Report – PT-11/01 (Brussels, Belgium: European Commision).
Ferreira da Silva, E., Bobos, I., Xavier Matos, J., Patinha, C., Reis, A. P., and Cardoso Fonseca, E. (2009). Mineralogy and geochemistry of trace metals and REE in volcanic massive sulfide host rocks, stream sediments, stream waters and acid mine drainage from the Lousal mine area (Iberian Pyrite Belt, Portugal). Appl. Geochem. 24 (3), 383–401. doi:10.1016/j.apgeochem.2008.12.001
Ferreira, R. A., Pereira, M. F., Magalhães, J. P., Maurício, A. M., Caçador, I., and Martin-Dias, S. (2021). Assessing local acid mine drainage impacts on natural regeneration-revegetation of São Domingos mine (Portugal) using a mineralogical, biochemical, and textural approach. Sci. Total Environ. 755 (1), 142825–142916. doi:10.1016/j.scitotenv.2020.142825
Fu, F., Akagi, T., and Shinotsuka, K. (1998). Distribution pattern of rare earth elements in Fern. Biol. Trace Elem. Res. 64, 13–26. doi:10.1007/bf02783321
Gałuszka, A., Migaszewski, Z. M., Pelc, A., Trembaczowski, A., Dołęgowska, S., and Michalik, A. (2020). Trace elements and stable sulfur isotopes in plants of acid mine drainage area: Implications for revegetation of degraded land. J. Environ. Sci. 94, 128–136. doi:10.1016/j.jes.2020.03.041
Gong, B., He, E., Romero-Freire, A., Ruan, J., Yang, W., Zhang, P., et al. (2021). Do essential elements (Pa and Fe) have mitigation roles in the toxicity of individual and binary mixture of yttrium and cerium in Triticum aestivum? J. Hazard. Mater. 416, 125761. doi:10.1016/j.jhazmat.2021.125761
Gonzalez, V., Vignati, D. A. L., Leyval, C., and Giamberini, L. (2014). Environmental fate and ecotoxicity of lanthanides: Are they a uniform group beyond chemistry? Environ. Int. 7, 148–157. doi:10.1016/j.envint.2014.06.019
Grawunder, A., Merten, D., and Büchel, G. (2014). Origin of middle rare earth element enrichment in acid mine drainage-impacted areas. Environ. Sci. Pollut. Res. 21, 6812–6823. doi:10.1007/s11356-013-2107-x
Herman, D. C., and Maier, R. M. (2009). “Consequences of biogeochemical cycles gone wrong,” in Environmental microbiology. Editors R. M. Maier, I. L. Pepper, and C. P. Gerba (Amsterdam, NL: Elsevier), 319–333.
Johnson, D. B., and Hallberg, K. B. (2003). The microbiology of acidic mine waters. Res. Microbiol. 154, 466–473. doi:10.1016/s0923-2508(03)00114-1
Joshi, M., Chandrasekar, A., and Ghanty, T. K. (2018). Theoretical investigation of M@Pb122-and M@Sn122- zintyl clusters (M=Lrn+, Lun+, La3+, Ac3+ and n= 0, 1, 2, 3. Phys. Chem. Chem. Phys. 20, 15253–15272. doi:10.1039/c8cp01056k
Krishnamurty, K. V., Shpirt, E., and Reddy, M. M. (1976). Trace metal extraction of soils and sediments by nitric acid-hydrogen peroxide. At. Absorpt. Newsl. 15 (3), 68–70.
Krzciuk, K., and Gałuszka, A. (2020). Presence and possible origin of positive Eu anomaly in shoot samples of Juncus effusus L. J. Trace Elem. Med. Biol. 58, 126432. doi:10.1016/j.jtemb.2019.126432
Krzciuk, K., and Gałuszka, A. (2019). Seasonal changes in concentrations of trace elements and rare earth elements in shoot samples of Juncus effusus L. collected from natural habitats in the Holy Cross Mountains, south-central Poland. Chemosphere 219, 954–960. doi:10.1016/j.chemosphere.2018.12.062
Laul, J. C., and Weimer, W. C. (1982). “Behavior of REE in geological and biological systems,” in The rare earths in modern science and technology. Editors G. J. McCarthy, J. J. Rhyne, and H. B. Silber (New York: Plenum Press), 531–535.
Li, X., Chen, Z., Chen, Z., and Zhang, Y. (2013). A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 93, 1240–1246. doi:10.1016/j.chemosphere.2013.06.085
Liang, T., Ding, S., Song, W., Chong, Z., Zhang, C., and Li, H. (2008). A review of fractionations of rare earth elements in plants. J. Rare Earths. 26, 7–15. doi:10.1016/s1002-0721(08)60027-7
Lima, A. T., and Ottosen, L. (2020). Recovering rare earth elements from contaminated soils: Critical overview of current remediation technologies. Chemosphere 265, 129163. doi:10.1016/j.chemosphere.2020.129163
Liu, C., Yuan, M., Liu, W. S., Guo, M. N., Huot, H., Tang, Y. T., et al. (2018). “Element case studies: Rare earth elements,” in Agromining: Farming for metals - extracting unconventional resources using plants. Editors A. van der Ent, G. Echevarria, A. J. M. Baker, and J. L. Morel Cham, Switzerland.
Liu, H., Guo, H., Pourret, O., Wang, Z., Liu, M., Zhang, W., et al. (2022). Geochemical signatures of rare earth elements and yttrium exploited by acid solution mining around an ion-adsorption type deposit: Role of source control and potential for recovery. Sci. Total Environ. 804, 150241. doi:10.1016/j.scitotenv.2021.150241
Liu, W. S., Guo, M. N., Liu, C., Yuan, M., Chen, X. T., Huot, H., et al. (2019). Water, sediment and agricultural soil contamination from an ion-adsorption rare earth mining area. Chemosphere 216, 75–83. doi:10.1016/j.chemosphere.2018.10.109
Marquez, J., Pourret, O., Faucon, M. P., Weber, S., Hoàng, T., and Martinez, R. (2018). Effect of cadmium, copper and lead on the growth of rice in the coal mining region of Quang Ninh, cam-pha (Vietnam). Sustainability 10, 1758. doi:10.3390/su10061758
Marschner, H., and Rӧmheld, V. (1994). Strategies of plants for acquisition of iron. Plant Soil 165 (2), 261–274. doi:10.1007/bf00008069
Merschel, G., and Bau, M. (1995). Rare earth elements in the aragonitic shell of freshwater mussel Corbicula fluminea and the bioavailability of anthropogenic lanthanum,samarium and gadolinium in river water. Sci. Total Environ. 533, 91–101. doi:10.1016/j.scitotenv.2015.06.042
Merten, D., Geletneky, J., Bergmann, H., Haferburg, G., Kothe, E., and Büchel, G. (2005). Rare earth element patterns: A tool for understanding processes in remediation of acid mine drainage. Chem. Erde 65 (S1), 97–114. doi:10.1016/j.chemer.2005.06.002
Metson, A. J. (1956). Methods of chemical analysis for soil survey samples. New Zealand: New Zealand Department of Scientific and Industrial Research.
Migaszewski, Z. H., and Gałuszka, A. (2015). The characteristics, occurrence, and geochemical behavior of rare earth elements in the environment: A review. Environ. Sci. Technol. 45, 429–471. doi:10.1080/10643389.2013.866622
Mihajlovic, J., Stärk, H., and Rinklebe, J. (2017). Rare earth elements and their release dynamics under pre-definite redox conditions in a floodplain soil. Chemosphere 181, 313–319. doi:10.1016/j.chemosphere.2017.04.036
Millero, F. J. (1992). Stability constants for the formation of rare earth inorganic complexes as a function of ionic strength. Geochim. Cosmochim. Acta. 56, 3123–3132. doi:10.1016/0016-7037(92)90293-r
Nieto, J. M., Sarmiento, A. M., Olías, M., Canovas, C. R., Riba, I., Kalman, J., et al. (2007). Acid mine drainage pollution in the tinto and odiel rivers (iberian pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva estuary. Int 33 (4), 445–455. doi:10.1016/j.envint.2006.11.010
Pagano, G., Aliberti, F., Guida, M., Oral, R., Siciliano, A., Trifuoggi, M., et al. (2015). Rare earth elements in human and animal health: State of art and research priorities. Environ. Res. 142, 215–220. doi:10.1016/j.envres.2015.06.039
Pan, J., Nie, T., Vaziri Hassas, B., Rezaee, M., Wen, Z., and Zhou, C. (2020). Recovery of rare earth elements from coal fly ash by integrated physical separation and acid leaching. Chemosphere 248, 126112. doi:10.1016/j.chemosphere.2020.126112
Pédrot, M., Dia, A., Davranche, M., Bouhnik-Le Coz, M., Henin, O., and Gruau, G. (2008). Insights into colloid-mediated trace element release at the soil/water interface. J. Colloid Interface Sci. 325, 187–197. doi:10.1016/j.jcis.2008.05.019
Pédrot, M., Dia, A., and Davranche, M. (2009). Double pH control on humic substance-borne trace elements distribution in soil waters as inferred from ultrafiltration. J. Colloid Interface Sci. 339, 390–403. doi:10.1016/j.jcis.2009.07.046
Pereira, R., Barbosa, S., and Carvalho, F. P. (2014). Uranium mining in Portugal: A review of the environmental legacies of the largest mines and environmental and human health impacts. Environ. Geochem. Health. 36, 285–301. doi:10.1007/s10653-013-9563-6
Pérez-López, R., Delgado, J., Nieto, J. M., and Márquez-García, B. (2010). Rare earth element geochemistry of sulphide weathering in the São Domingos mine area (iberian pyrite Belt): A proxy for fluid–rock interaction and ancient mining pollution. Chem. Geol. 276 (1-2), 29–40. doi:10.1016/j.chemgeo.2010.05.018
Pisciotta, A., Tutone, L., and Saiano, F. (2017). Distribution of YLOID in soil-grapevine system (Vitis vinifera L) as tool for geographical characterization of agro-food products. A two years case study on different grafting combinations. Food Chem. 221, 1214–1220. doi:10.1016/j.foodchem.2016.11.037
Plante, A. F. (2007). “Soil biogeochemical cycling of inorganic nutrients and metals,” in Soil microbiology, ecology, and biochemistry. Editor E. A. Paul (Amsterdam, NL: Elsevier), 389–432.
Pourret, O., Davranche, M., Gruau, G., and Dia, A. (2007). Rare earth elements complexation with humic acid. Chem. Geol. 243, 128–141. doi:10.1016/j.chemgeo.2007.05.018
Prudêncio, M. I., Valente, T., Marques, R., Braga, S. M. A., and Pamplona, J. (2015). Geochemistry of rare earth elements in a passive treatment system built for acid mine drainage remediation. Chemosphere 138, 691–700. doi:10.1016/j.chemosphere.2015.07.064
Prudêncio, M. I., Valente, T., Marques, R., Sequeira Braga, M. A., and Pamplona, J. (2017). Rare earth elements, iron and manganese in ochre-precipitates and wetland soils of a passive treatment system for acid mine drainage. Sci 17, 932–935. doi:10.1016/j.proeps.2017.01.024
Quental, L., Bourguignon, A., Sousa, A. J., Batista, M. J., Brito, M. G., Tavares, T., et al. (2002). MINEO Southern Europe environment test site: Contamination impact mapping and modelling — final Report. Report No. IST-1999-10337 (Alfragide, Portugal: Information Society Technologies). Available at: http://hdl.handle.net/10400.9/3268.
Ramalho, E., Carvalho, J., Barbosa, S., and Monteiro Santos, F. A. (2009). Using geophysical methods to characterize an abandoned uranium mining site, Portugal. J. Appl. Geophys. 67, 14–33. doi:10.1016/j.jappgeo.2008.08.010
Relvas, J. M. R. S., Pinto, A., Fernandes, C., Matos, J. X., Vieira, A., Mendonça, A., et al. (2014). Lousal: An old mine, a recent dream. A new reality. Comun. Geol. 101 (I), 1345–1347.
Riley, E., and Dutrizac, J. E. (2017). The behaviour of the rare earth elements during the precipitation of ferrihydrite from sulphate media. Hydrometallurgy 172, 69–78. doi:10.1016/j.hydromet.2017.05.004
Rim, K. T., Koo, K. H., and Park, J. S. (2013). Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health A. T. Work 4 (1), 12–26. doi:10.5491/shaw.2013.4.1.12
Santa-Bárbara Carrascosa, C., and Valdés, B. (2008). Guía de la Flora y Vegetación del Andévalo: Faja Pirítica España-Portugal (P, Silva, Trans). Junta de Andalucia Consejería de Medio Ambiente y Ordenación del Territorio.
Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767. doi:10.1107/s0567739476001551
Soyol-Erdene, T. O., Valente, T., Grande, J. A., and de la Torre, M. L. (2018). Mineralogical controls on mobility of rare earth elements in acid mine drainage environments. Chemosphere 205, 317–327. doi:10.1016/j.chemosphere.2018.04.095
Strosnider, W. H. J., Llanos López, F. S., LaBar, J. A., Palmer, K. J., and Nairn, R. W. (2014). Unabated acid mine drainage from cerro rico de Potosí, Bolivia: Uncommon constituents of concern impact the rio pilcomayo headwaters. Environ. Earth Sci. 71, 3223–3234. doi:10.1007/s12665-013-2734-z
Sverjensky, D. A. (1984). Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 67, 70–78. doi:10.1016/0012-821x(84)90039-6
Taylor, S. R., and McLennan, S. M. (1985). The continental crust: Its composition and evolution. Oxford, U.K.: Blackwell.
Thomas, P. J., Carpenter, D., Boutin, C., and Allison, J. E. (2014). Rare earth elements (REEs) effects on germination and growth of selected crop and native plant species. Chemosphere 96, 57–66. doi:10.1016/j.chemosphere.2013.07.020
Tyler, C. (2004). Rare earth elements in soil and plant systems - a review. Plant Soil 267, 191–206. doi:10.1007/s11104-005-4888-2
U.S. Geological Survey (2022). “Rare earths,” in Mineral commodity summaries 2022: U.S. Geological Survey. (Reston, VA: U.S. Geological Survey), 134–135. doi:10.3133/mcs2022
Van Gosen, B. S., Verplanck, P. L., Seal, R. R., Long, K. R., and Gambogi, J.II (2017). “Chapter O: Rare-earth elements,” in Critical mineral resources of the United States—economic and environmental geology and prospects for future supply. Editors K. J. Schulz, J. H. DeYoung, R. R. Seal, and D. C. Bradley (Reston, VA: U.S. Geological Survey), 1–31. doi:10.3133/pp1802O
Vaziri Hassas, B., Rezaee, M., and Pisupati, S. V. (2021). Effect of various ligands on the selective precipitation of critical and rare earth elements from acid mine drainage. Chemosphere 280, 130684. doi:10.1016/j.chemosphere.2021.130684
Wakabayashi, T., Ymamoto, A., Kazaana, A., Nakano, Y., Nojiri, Y., and Kashiwazaki, M. (2016). Antibacterial, antifungal and nematicidal activities of rare earth ions. Biol. Trace Elem. Res. 174 (2), 464–470. doi:10.1007/s12011-016-0727-y
Wang, C., Zhang, K., He, M., Jiang, C., Tian, L., Tian, Y., et al. (2012). Mineral nutrient imbalance, DNA lesion and DNA-protein crosslink involved in growth retardation of Vicia faba L. seedlings exposed to lanthanum ions. J. Environ. Sci. 24, 214–220. doi:10.1016/s1001-0742(11)60760-2
Wang, Y. Q., Sun, J. X., Chen, H. M., and Guo, F. Q. (1997). Determination of the contents and distribution characteristics of REE in natural plants by NAA. J. Radioanal. Nucl. Chem. 219 (1), 99–103. doi:10.1007/bf02040273
Welch, S. A., Christy, A. G., Isaacson, L., and Kirste, D. (2009). Mineralogical control of rare earth elements in acid sulfate soils. Geochim. Cosmochim. Acta. 73, 44–64.
Wyttenbach, A., Furrer, V., Schelppi, P., and Tobler, L. (1998). Rare earth elements in soil and soil-grown plants. Plant Soil 199, 267–273. doi:10.1023/a:1004331826160
Yéghicheyan, D., Aubert, D., Bouhnik-Le Coz, M., Chmeleff, J., Delpoux, S., Djouraev, I., et al. (2019). A new interlaboratory characterisation of silicon, rare earth elements and twenty-two other trace element concentrations in the natural river water certified reference material SLRS-6 (NRC-CNRC). Geostand. Geoanal. Res. 43 (3), 475–496. doi:10.1111/ggr.12268
Keywords: rare earth elements, acid mine drainage, abandoned mines, plant, soil, water, bioaccumulation, transfer factor
Citation: Forsyth K, Dia A, Marques R, Prudêncio MI, Diamantino C, Carvalho E, Russo D, Dionisio I, Davranche M, Bouhnik-Le-Coz M and Pédrot M (2023) Bioconcentration and translocation of rare earth elements in plants collected from three legacy mine sites in Portugal. Front. Environ. Sci. 11:1191909. doi: 10.3389/fenvs.2023.1191909
Received: 22 March 2023; Accepted: 25 July 2023;
Published: 09 August 2023.
Edited by:
Wei He, China University of Geosciences, ChinaReviewed by:
M. Dolores Basallote, University of Huelva, SpainŽeljka Fiket, Ruđer Bošković Institute, Croatia
Copyright © 2023 Forsyth, Dia, Marques, Prudêncio, Diamantino, Carvalho, Russo, Dionisio, Davranche, Bouhnik-Le-Coz and Pédrot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaisa Forsyth, kaisa.forsyth@gmail.com; Mathieu Pédrot, mathieu.pedrot@univ-rennes.fr
 Kaisa Forsyth
Kaisa Forsyth Aline Dia
Aline Dia Rosa Marques
Rosa Marques Maria Isabel Prudêncio2,3
Maria Isabel Prudêncio2,3  Melanie Davranche
Melanie Davranche Mathieu Pédrot
Mathieu Pédrot