Research progress of aqueous Zn–CO2 battery: design principle and development strategy of a multifunctional catalyst
- 1College of Environmental Science and Engineering, Taiyuan University of Technology, Taiyuan, Shanxi, China
- 2Shanxi Key Laboratory of Compound Air Pollutions Identification and Control, Taiyuan University of Technology, Taiyuan, China
- 3School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan, Hubei, China
- 4Plasma Solar Fuels Devices, Dutch Institute for Fundamental Energy Research (DIFFER), Nieuwegein, Netherlands
- 5Shanxi Zhongke Huaneng Technology Co.,Ltd., Taiyuan, Shanxi, China
Aqueous Zn–CO2 battery possesses a large theoretical capacity of 820 mAh g-1 (5855 mAh cm-3) and high safety, showing a unique position in carbon neutrality and/or reduction and energy conversion and storage, which has developed rapidly in recent years. However, obstacles such as low value-added products, low current density, high overvoltage, and finite cycles impede its practical application. Cathode catalysts, as a key component, have a significant influence on gas cell performance. Despite many updated papers on cathode materials for aqueous Zn–CO2 batteries, a systematic summary has rarely been reported, and even less is mentioned about the design principle and development strategy for efficient catalysts. Relying on the structure and mechanism of the Zn–CO2 battery, this review discusses the research progress and existing challenges, and, more importantly, the design strategies and preparation methods of the efficient cathode are proposed, centering on material structure, charge distribution, and coordination environment. Finally, in this review, the opportunities for the development of a high-performance Zn–CO2 battery are highlighted, which enables enlightening the future exploration of next-generation energy storage systems.
1 Introduction
Excessive carbon dioxide (CO2) emissions caused by consumption of fossil fuels further aggravate the global energy crisis and the greenhouse effect (Chang et al., 2017; Asadi et al., 2018; Zhou et al., 2020). In recent years, the carbon capture, utilization, and storage (CCUS) (Chen et al., 2022c; de Oliveira Maciel et al., 2022; Jiang et al., 2022; Pfeiffer et al., 2022) technology has become a hot topic of research. Among them, metal–CO2 batteries adopt CO2 catalytic conversion and an energy storage solution where chemical energy is converted into green renewable electricity while reducing CO2 emissions (Chu et al., 2016; Ahmadiparidari et al., 2019; Eskezia Ayalew, 2021). Compared with traditional ion batteries, metal–CO2 batteries possess a higher specific capacity and energy density (Xiang Li et al., 2016; Qiao et al., 2017; Hu et al., 2019). For example, the energy density of a lithium–carbon dioxide (Li-CO2) battery is as high as 1876 Wh kg-1 (Zhang et al., 2017; Cai et al., 2018), approximately five times that of a lithium-ion (Li-ion) battery (387 Wh kg-1 (Khurram et al., 2018; Wu et al., 2021)). However, an alkali metal (Li, Na, and K)–CO2 battery using a toxic organic electrolyte is not environmentally friendly, and their solid products from carbon dioxide reduction (CO2RR) are chemically inert, resulting in over-accumulation and substances not subjectable to decomposition (Xie and Wang, 2019; Mu et al., 2020). Furthermore, the slow kinetics during the electrochemical process and thermodynamic instability hinder their application. For other metal–CO2 batteries with aqueous electrolytes, including Mg–CO2 and Al–CO2 batteries, their theoretical capacity densities are 6815 and 8076 Wh kg-1 (Ma et al., 2018), respectively, higher than that of Zn–CO2 units (984 Wh kg-1) (Aslam et al., 2023). However, the products of these metal–CO2 batteries are all metal carbonates and carbon, which cannot generate other value-added chemicals. Zinc metal reserves are abundant, and the price is low. In the derived Zn-gas batteries, Zn–CO2 batteries show no obvious advantages in energy density compared to Zn-air (1353 Wh kg-1) or Zn-O2 batteries but pose significant importance to CO2 utilization and conversion (Zhou et al., 2021). Relying on environment-friendly, higher safety and wider products, the Zn–CO2 battery is expected to be an ideal substitute for other metal batteries.
Based on its significant contribution to carbon neutrality (Chu et al., 2016; Ahmadiparidari et al., 2019; Eskezia Ayalew, 2021) and superiority in metal–gas batteries, the groundbreaking work of Zn–CO2 batteries is exhibited in Figure 1. Aqueous Zn–CO2 batteries originated from solar-powered CO2 splitting batteries and later developed into a rechargeable unit, subsequently to a dual-model and self-driven system, toward the extent of a reversible one. Nevertheless, the research on aqueous Zn–CO2 batteries is still in its infancy, and certain problems should be overcome: 1) the lack of effectively active catalysts with high selectivity leads to limited discharge products, mainly generating CO and formate, while rarely HCOOH or even less of other high value-added products (such as methanol, ethanol, and ethylene), causing the discharge–charge shuttle to be irreversible and potential application to be confined (Wang H. F. et al., 2020; Chen Y. et al., 2021). 2) The lack of stable catalytic behavior in the electrolyte environments. Most rechargeable Zn–CO2 batteries couple CO2RR and the oxygen evolution reaction (OER), in which CO2RR preferably proceeds in acidic/neutral solutions and OER favors basic environments, presenting a significant challenge to balance cathodic reduction and oxidation behavior (Guo Y. et al., 2022), while a completely reversible Zn–CO2 battery accompanied by CO2RR and formic acid oxidation reaction (FAOR) is built in neutral or slightly acidic electrolytes, triggering electrochemical corrosion or other undesired side reactions and significantly shortening the battery life. 3) The high overpotential and low current density (≤10 mA cm-2) severely compromise the power and energy density, where increasing energy consumption and poor long-term reversibility pose a significant obstacle toward practical application. These mentioned problems of a Zn–CO2 battery are mainly attributed to the high stability of the C=O bond (dissociation energy as high as 806 kJ mol-1) in CO2 (Zhou and Sun, 2017; Kim et al., 2020), finite product types, poor selectivity in CO2RR, and low electrochemical activity in the cathodic oxidation reaction. Indeed, it is tricky to obtain efficient long-term reversible cycles without effective catalysts (Hao et al., 2020; Hao et al., 2021). Moreover, the aqueous electrolytes present low operation potential and anode instability (like Zn self-corrosion or dendrite), resulting also in limited running cycles, but these topics are beyond our discussion in this review.
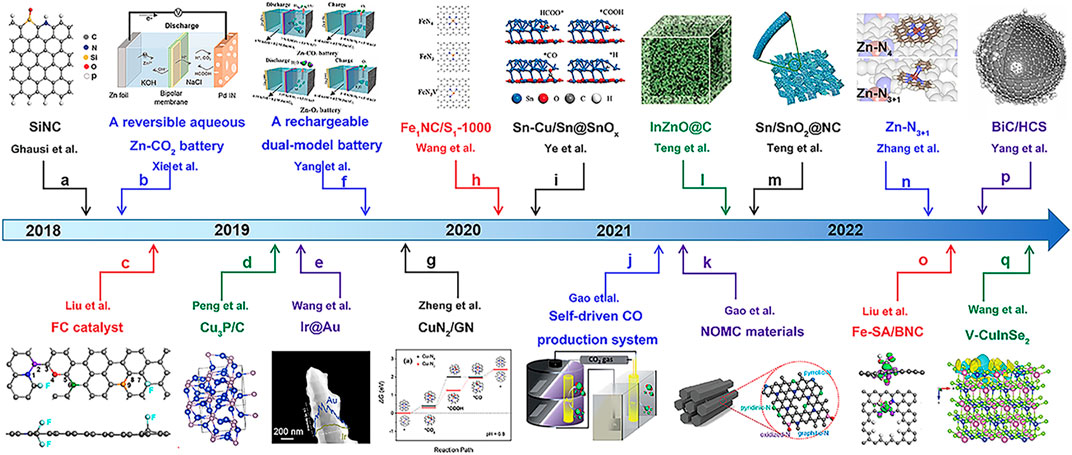
FIGURE 1. Timeline about the important developments of aqueous Zn–CO2 battery systems and cathode catalysts. (A) F-doped carbon (FC). Reproduced with permission (Xie et al., 2018b). Copyright 2018, Wiley. (B) Porous silicon–nitrogen-co-doped carbon (SiNC). Reproduced with permission (Ghausi et al., 2018). Copyright 2018, Wiley. (C) Reversible aqueous Zn–CO2 battery. Reproduced with permission (Xie et al., 2018a). Copyright 2018, Wiley. (D) Dendritic 3D Ir@Au. Reproduced with permission (Wang et al., 2019). Copyright 2019, Wiley. (E) Cu3P/C nanocomposites. Reproduced with permission (Peng et al., 2019). Copyright 2019, American Scientific Publishers. (F) Rechargeable dual-model battery. Reproduced with permission (Yang R. et al., 2019). Copyright 2019, Royal Society of Chemistry. (G) Free energy of Cu–N2 and Cu–N4 during CO2RR. Reproduced with permission (Zheng et al., 2019). Copyright 2019, Wiley. (H) Optimized adsorption configurations of Sn–Cu/Sn@SnOx for reaction intermediates. Reproduced with permission (Ye et al., 2020). Copyright 2020, Wiley. (I) Optimized atomic structures for FeN4, FeN3, and FeN3V embedded on the graphene layer. Reproduced with permission (Wang T. et al., 2020). Copyright 2020, Wiley. (J) InZnO@C. Reproduced with permission (Teng et al., 2021). Copyright 2021, Royal Society of Chemistry. (K) Self-driven CO production system. Reproduced with permission (Gao et al., 2021a). Copyright 2021, Royal Society of Chemistry. (L) N-doped ordered mesoporous carbon (NOMC). Reproduced with permission (Gao et al., 2021b). Copyright 2021, Wiley. (M) Sn/SnO2@NC. Reproduced with permission (Xue Teng et al., 2021). Copyright 2021, The Core Journal of China. (N) Optimized Zn-N4 and Zn-N3+1 structures. Reproduced with permission (Chen J. et al., 2022). Copyright 2022, Wiley. (O) Charge density differences maps of V-CuInSe2. Reproduced with permission (Wang Y.-X. et al., 2022). Copyright 2020, Wiley. (P) Fe-SA/BNC. Reproduced with permission (Liu et al., 2022c). Copyright 2022, Elsevier. (Q) Bi clusters (BiC) deposited on hollow carbon spheres (BiC/HCS). Reproduced with permission (Yang M. et al., 2022). Copyright 2022, Elsevier.
Therefore, it is crucial to design both effective and chemical/electrochemical stable catalysts to solve the aforementioned problems, tackling competitive adsorption–desorption between *COOH/*OCHO, *COH/*CO, *OH/*OOH, or *H in the catalytic process for the target products (Huang et al., 2019). Despite many updated papers on cathode materials (Table 1) for aqueous Zn–CO2 batteries, a systematic summary has rarely been reported (Wu et al., 2021), and even less is known about the principles of designing efficient catalysts. Herein, this review summarizes the structure and mechanism of Zn–CO2 battery and discusses the research progress and existing problems of cathode materials, and, more importantly design strategies and preparation methods for efficient cathode catalysts are proposed, centering on catalyst structure with adsorption performance of intermediate products, eventually highlighting the opportunities and challenges for high-performance Zn–CO2 batteries, which enables to enlighten the future development of the next-generation energy storage system.
The results were obtained from the data by providing directly from literature or estimating from the given information. NOTE: Dis-char means discharge–charge plateau. Ocv means open-circuit voltage. Otime means operation time. Cdensity means current density. For better performance evaluation, the data in red, blue, and green represent excellent, good, and average, respectively.
2 Structure and reaction mechanism of Zn–CO2 battery
2.1 Battery structure
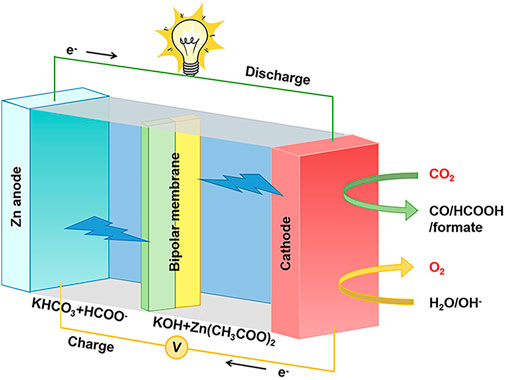
The Zn–CO2 battery typically consists of a catalyst cathode, Zn sheet anode, electrolyte, and diaphragm seen in Figure 2. Its uniqueness is the electrolyte, which is divided into two parts: the anode counterpart utilizes alkaline KOH and Zn(CH3COO)2 mixture solution, while the cathode counterpart uses near-neutral KHCO3 solution, and sometimes, buffer solution HCOO− is added (Asokan et al., 2020). Bipolar membranes are necessary to separate the electrodes, maintaining the pH and avoiding cross-contamination (Xie et al., 2018a).
2.2 Reaction mechanism
Based on the Frontier research progress of Zn–CO2 batteries, we discuss both anodic and cathodic reaction mechanisms and highlight the cathodic CO2 electrochemical reduction and opposite evolution mechanisms in alkaline and neutral electrolyte conditions.
2.2.1 Anodic reaction mechanism
The anodic reactions are based on Zn/Zn2+ redox couple, and the Zn2+ state is affected by the electrolyte’s pH shown as follows. In near-neutral electrolytes, Zn2+ exists as the charge carrier, while in alkaline solutions, Zn2+ initially appears as Zn(OH)42- and is further dehydrated to ZnO (Zhong et al., 2020).
In the neutral electrolyte: Eθ (Zn/Zn2+) = −0.76 V vs. SHE, where Eθ means the standard reduction potential
In the alkaline electrolyte: Eθ (Zn/ZnO) = −1.22 V vs. SHE
2.2.2 Cathodic reaction mechanism
CO2RR mechanism: When Zn–CO2 batteries discharge, CO2RR occurs on the gas–liquid–solid three-phase interface, and this complex process involves the adsorption and dissociation of CO2 together with the transfer of multiple protons and electrons (Li et al., 2017; Yang et al., 2018). CO2RR products are mainly present as HCOOH/formate and CO through different routes in the adsorption model on the catalytic site. The former tends to adsorb at the O end (*OCHO) to present HCOOH in near-neutral electrolytes and transit to formate in alkaline environments (Han et al., 2018), while the latter adsorbs at the C end (*COOH), as shown in Figure 3A.
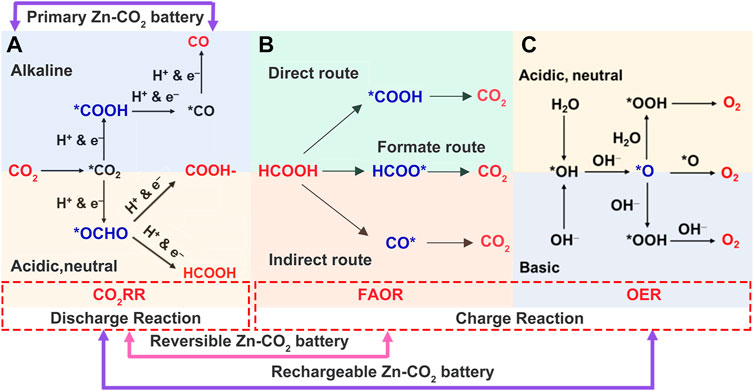
FIGURE 3. Cathodic reaction mechanism. (A) Possible pathways of aqueous CO2RR with diverse products. Possible pathways of (B) FAOR in direct, formate, and indirect routes and (C) OER in acidic (or neutral) and basic electrolytes. Reproduced with permission (Xie et al., 2019). Copyright 2019, Wiley. * represents the active site. The generation of intermediate (represented in blue) is the rate-determining step.
HCOOH in the neutral electrolyte:
Formate in the alkaline electrolyte:
CO in the alkaline electrolyte:
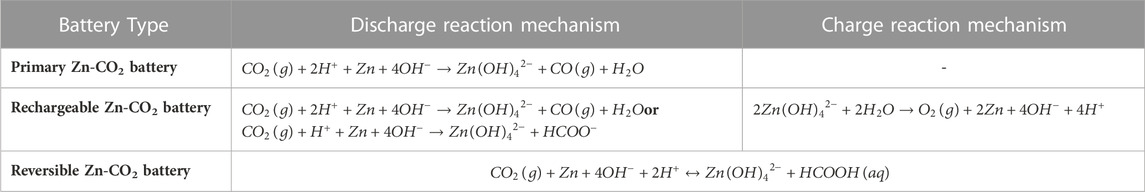
Relying on the reversibility of a discharge–recharge reaction, the Zn–CO2 secondary battery is classified into two types: the reversible battery covering the HCOOH product and FAOR charge process and the rechargeable one generating CO or HCOO− discharge products and coupling OER (simplified as Zn–CO2 battery) (Wang F. et al., 2021). We discuss the charge mechanism as follows.
FAOR mechanism: The reversible Zn–CO2 battery forms a closed loop between CO2 and HCOOH (El Sawy and Pickup, 2016; Xiong et al., 2020; Liang M. et al., 2021). FAOR generally occurs in the direct, indirect, or formate pathways (Wang et al., 2004; Zhu et al., 2021a), as seen in Figure 3B. The direct route follows the dehydrogenation of HCOOH with the C-H bond breaking and release of CO2, which occur quickly and effectively under low potential. As for the indirect route, the initial step is dehydration with C-O and C-H bonds breaking, and the adsorbed CO (CO*) is subsequently oxidized to CO2 at high potential. The residual CO* covers active sites and seriously represses the direct route, leading to catalyst poisoning or even inactivation (Calderón-Cárdenas et al., 2021). Different from the breaking sequence of the direct route, the formate route first breaks the O-H bond of HCOOH to HCOO* (i.e., *OCHO) and further dehydrogenates to CO2, requiring less binding energy than the aforementioned two paths, thus occurring at even lower potential (Xiong et al., 2020).
Direct route:
Indirect route:
Formate route:
To date, CO2RR products in the battery are mostly CO or formate and catalysts with high FAOR activity are rarely reported. The reversible Zn–CO2 batteries following the formate path are only achieved under bifunctional catalysts of porous Pd nanosheets (Xie et al., 2018a) and PdBi alloy (Gao S. et al., 2022).
OER mechanism: O2 is generated from H2O molecules in acidic electrolytes or from -OH groups in alkaline media during the OER, as shown in Figure 3C (Suen et al., 2017; Xie et al., 2019). Its symbiotic hydrogen evolution reaction (HER) seriously affects the battery’s Coulombic efficiency and should be inhibited.
To sum up, the reaction mechanisms of the Zn–CO2 battery are listed in Table 2. The continuous exploration of mechanisms enlightens the direction of efficient catalysts (Wang K. et al., 2020; Wang F. et al., 2021).
3 Cathode catalysts
The catalytic properties and electrolyte environment play a significant role in the electrochemical mechanism, influencing product state and reaction rate and ultimately determining its battery performance (Wang J. et al., 2021). Cathode catalysts (as the core component) are divided into three categories in this review: carbon-based metal-free catalysts, metal-based catalysts, and metal–carbon composites. When the battery discharges, CO2 is mostly reduced to CO and formate and rarely to HCOOH, depending on reaction intermediates. Specifically, accelerating the formation of *COOH tends to produce CO while reducing the reaction energy barrier of *OCHO, which promotes the generation of HCOOH in near-neutral electrolytes and formate in alkaline electrolytes (Xie et al., 2019). In the opposite charge process, it requires cathodes to show pertinent desorption catalysis of *COOH/HCOO*/CO* in FAOR or *OH/*OOH in the OER. The adsorption energy of intermediates reflects the catalytic activity and is influenced by the electronic structure and chemical environment of the catalytic sites (Selvakumaran et al., 2019; Peng et al., 2022b).
3.1 Carbon-based catalyst
This catalyst has the advantages of high conductivity, large specific surface area, superior stability, and low costs and involves carbon nanotubes, graphene, and other analogs (Lankone et al., 2017; Yu et al., 2020; Zhao et al., 2020; Chen B. et al., 2021). The catalytic performance is further regulated via doping N, F, Si, or P heteroatoms into the electronic structure and introduces defects (Xue et al., 2019; Wang C. et al., 2021; Gao Y. et al., 2022; Wang J. et al., 2022). Generally, the doped heteroatoms with strong electronegativity facilitate the combination with positively charged *COOH intermediates (Shi et al., 2021; Sawant et al., 2022; Thakur et al., 2022). N-doped carbon materials are widely used in this field. As reported in literature, N-doped ordered mesoporous carbon (NOMC) (Gao et al., 2021b) (Figures 4A, B) using the SBA-15 template is prepared via the pyrolytic-etching method, possessing ultra-high specific surface area, highly exposed N-C sites to promote the transport and storage of reactants/intermediates (Shakeri et al., 2021). The Faraday efficiency of the CO product (FECO) on NOMC is close to 100% at a low overpotential of 0.36 V, and a Zn–CO2 battery assembled with the NOMC cathode achieves long, stable cycles of 300 (100 h) even at 1.0 mA cm-2 (Figures 4C, D), indicating high catalytic performance and super stability. The presence of more electronegative F atoms induces positrons and arouses asymmetric spins, thereby rearranging the electron density of adjacent atoms (Wang G.-D. et al., 2021).
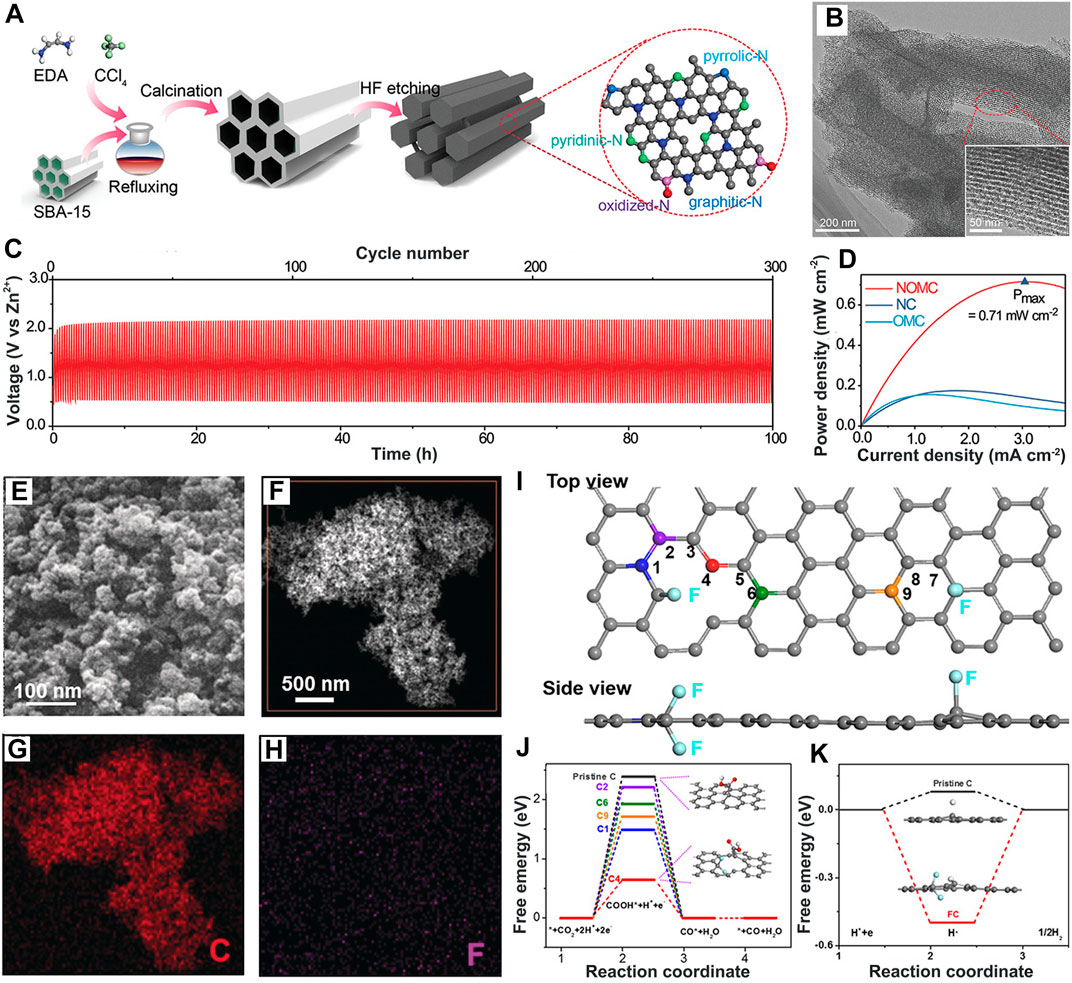
FIGURE 4. (A) Schematic illustration and (B) TEM image of NOMC. (C) Stability and (D) power density curves of Zn–CO2 battery with NOMC. Reproduced with permission (Gao et al., 2021b). Copyright 2021, Wiley. (E,F) SEM and HAADF images of FC and (G,H) related EDS mapping. (I) Top and side views of the DFT model for FC. Gray atom: C; other colorful atoms: C calculated as active sites. (J) Free energy diagram of CO2RR to CO on FC. (K) Schematic of the CO2RR pathway on FC. Reproduced with permission (Xie et al., 2018b). Copyright 2018, Wiley.
A few F atoms were adopted into carbon matrices forming F-doped carbon (FC) (Figures 4E–H) by the one-step pyrolysis method, and the FC catalyst was applied in Zn–CO2 for the first time (Xie et al., 2018b). Through density functional theory (DFT) calculation (Figures 4I–K), it was shown that *COOH is inclined to adsorb on the fourth C atom next to the CF2 bond with the lowest Gibbs free energy (ΔG) of 0.64 eV rather than the F-free doped C atom. Simultaneously, it endows the adjacent C atoms with higher positive charge density and asymmetric spin sites, effectively inhibiting the HER with strong binding ability to H*. As a result, its FECO is increased to 89.6%, and the assembled solar-driven CO2 battery achieves 13.6% photoelectric conversion efficiency, higher than that of SiNC materials (Ghausi et al., 2018) (12.5%). Such energy conversion–storage Zn–CO2 self-powered devices conform to the trends in the development of self-powered integrated devices.
In addition to heteroatom doping, the construction of intrinsic carbon defects causes charge delocalization, activating carbon atoms at defect sites for better catalytic properties. A defect-rich porous carbon (K-defect-C-1100) was synthesized by a K+-assisted strategy (Ling et al., 2022) and has 12-vacancy-type defects (V12) (Figures 5A–D). Its negative potential V12 defect attracts electrophilic CO2 molecules and renders the battery high FECO to 99% (Figure 5E) in a wide discharge potential range with excellent cyclic stability of about 200 cycles.
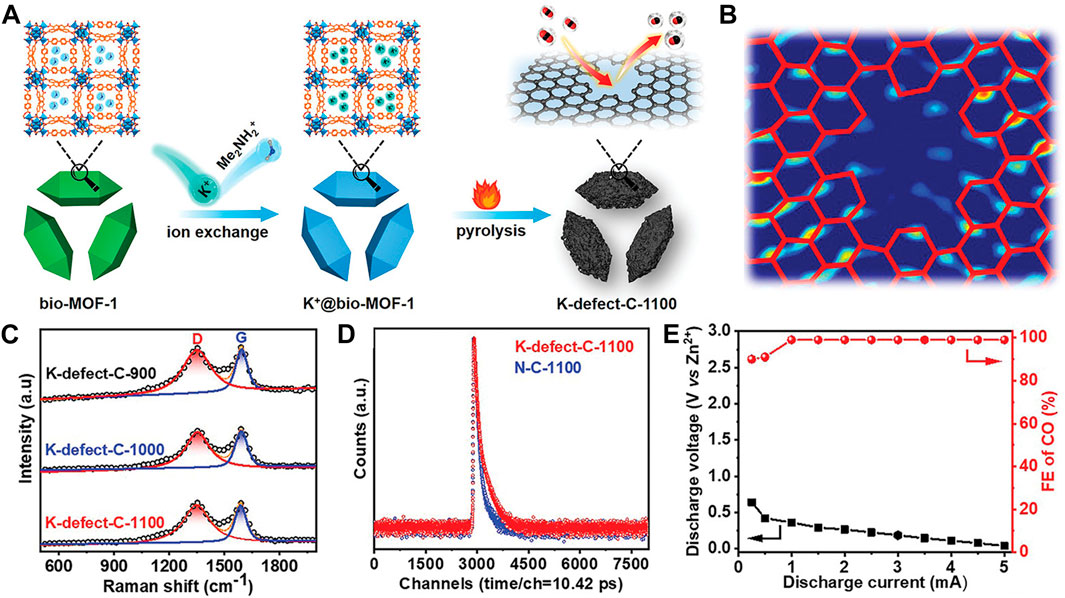
FIGURE 5. (A) Schematic illustration of K-defect-C-1100 preparation. (B) HAADF-STEM image of K-defect-C-1100 after fast Fourier transformation filtering. (C) Raman spectra and (D) PALS spectra of K-defect-C-1100. (E) Discharge voltages and the corresponding FECO profiles at different current densities. Reproduced with permission (Ling et al., 2022). Copyright 2022, Wiley.
Though electron delocalization is induced into carbon-based materials, the influence on the inertia of molecular activation is finite, which hardly meets application requirements.
3.2 Metal-based catalysts
Metal-based catalysts are generally composed of metal or alloy nanoparticles and metal compounds. Noble metal-based catalysts such as Au, Ru, Pd, and Ir have excellent catalytic properties, providing good acid and alkali resistance (Li D. et al., 2022; Zhao D. et al., 2022; Kang T. et al., 2022; Huang et al., 2022); the exposure states of active sites are easily made compatible via structure design and mass transfer capacity (Sui et al., 2021). Additionally, the catalyst’s lattice structure and charge distribution are evolved by introducing other metal or non-metallic atoms, which optimizes the interaction between the catalyst and intermediates, inhibits possible side reactions, and allows an elevation of the product selectivity (Feng et al., 2021).
As reported, Xie et al. (2018a) prepared porous three-dimensional interconnected Pd nanosheets (3D Pd NSs) with a rich pore structure, large specific surface area, sufficient active sites by electrodeposition (which adsorbs the *OCHO intermediate with high selectivity), and presents a good reversibility in conversion between CO2 and HCOOH under low potential. The assembled reversible Zn–CO2 battery steadily runs for more than 100 cycles (33 h), achieves a 788 Wh kg-1 discharge capacity, and shows an energy efficiency of 81.2%. Gao et al. (2021a) prepared coral-like Au catalyst with an irregular surface and initiated a self-driven CO production device that is co-assembled by a Zn–CO2 battery and H-type CO2 electrolyzer for the first time. *COOH is easily adsorbed on Au (111), with a lower reaction energy barrier (ΔG = 1.16 eV) than that in the common environment (ΔG = 1.27 eV). Unlike single metals, bimetal composites do achieve enhanced catalytic performance under the strong synergistic effect. Wang et al. (2019) built a dendritic 3D Ir@Au bifunctional catalyst and constructed a Zn–CO2 battery simulating the two-step plant photosynthesis to achieve CO2 fixation and H2O oxidation, which equably circulates for 30 h at 5 mA cm-2.
Although noble metals exhibit outstanding catalysis in Zn–CO2 batteries, their commercial application is restrained by high prices and low reserves. Alloys and transition metals with special catalytic properties are becoming their alternatives (Yang X. et al., 2022; Gao and Zhao, 2022; Lichchhavi and Shirage, 2022). Sn is rich in resources with low toxicity. In the electrochemical reaction, the Sn alloy and Sn2O3 are utilized as important promoters to reduce CO2 to C1 products (CO and HCOOH), but their poor conductivity is still an obstacle (Kang J. et al., 2022; Ansari et al., 2022). The hierarchical core-shell Sn–Cu/Sn@SnOx (Ye et al., 2020) (Figures 6A–C) was optimized with high conductivity by an Sn/SnOx interface. Its Sn–Cu/Sn core provides a sufficient Sn source to reconstruct the core-shell structure, thereby guaranteeing stability. Its in situ reconstructed Sn/SnOx shell increases the reaction energy barrier of *H and *COOH to suppress HER and CO formation; conversely, it reduces the *OCHO binding energy, which sets a decisive step toward obtaining a high selectivity of the HCOOH product and presents a high FEformate (Figures 6D, E). However, its partial current density of formate (jformate) is still lower than −0.3 A cm-2, which is far from application requirements. To solve this problem, Sn is incorporated with Li (s-SnLi) (Yan et al., 2021) on the surface, which stimulates local electron rearrangement and lattice strain that correspondingly occurred on the adjacent Sn atoms, showing more negative charge (Figure 6F).
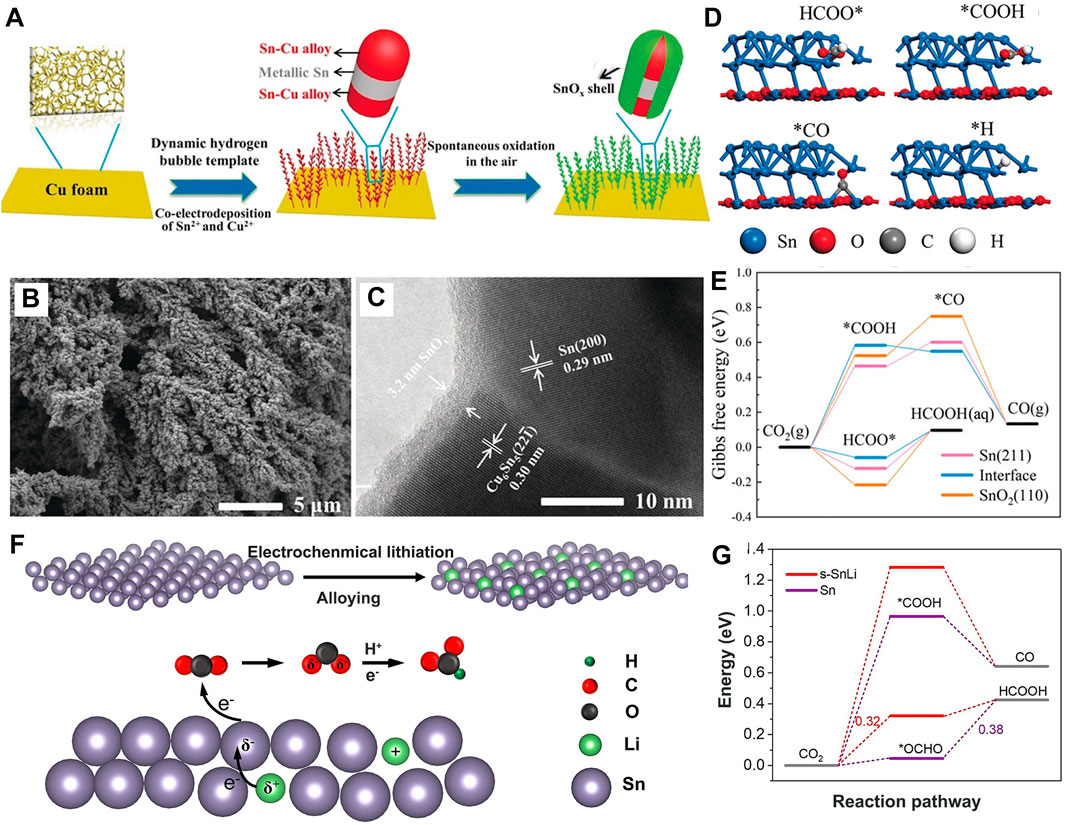
FIGURE 6. (A) Schematic illustration of Sn-Cu/Sn@SnOx. (B) SEM and (C) HRTEM images of Sn-Cu/Sn@SnOx. (D) Optimized adsorption configurations for reaction intermediates at the interface. (E) Gibbs free energy diagrams of (E) CO2RR on the Sn/SnOx interface. Reproduced with permission (Ye et al., 2020). Copyright 2020, Wiley. (F) Schematic illustration of s-SnLi and catalyst mechanisms for CO2RR to formate. (G) Mechanism investigations of CO2RR to HCOOH and CO on s-SnLi. Reproduced with permission (Yan et al., 2021). Copyright 2022, Royal Society of Chemistry.
Therefore, the s-SnLi with enhanced CO2 adsorption capacity and restricted ΔG*COOH and ΔG*OCHO (Figure 6G) showed both high catalysis and selectivity in reducing CO2 to formate. Thus, FEformate reaches 92%, the maximum power density of 1.24 mW cm-2, jformate is increased to −1.0 A cm-2, and the Zn–CO2 cell operates for over 800 cycles.
Metal oxides behave better in CO2RR performance than metal substances, but metal self-reduction may occur in the catalytic process. BiO2-x nanosheets (m-BiO2-x) with rich oxygen defects (Tan et al., 2022) and favorable localization of Bi p-orbital electrons were utilized to stabilize *OCHO and suppress *H adsorption for high selectivity in CO2-to-formate conversion. It offered a non-dopant pathway to boost electrochemical CO2RR. Inspired by the synergetic effect of multi-metals, Tian et al. (2021) embedded SnO2 nanoparticles on Bi2O3 sheets’ surfaces to assemble SnO2/Bi2O3 composites. Different work functions at the SnO2/Bi2O3 interface induce a built-in potential, thereby promoting the interface electron transfer and improving the conductivity of SnO2. The strong interface on SnO2/Bi2O3 effectively prevents SnO2 from electrolytic decomposition, which ensures catalyst stability and promotes CO2 adsorption and activation. In addition, it showed the enhanced adsorption of *OCHO on Bi2O3 to accelerate HCOOH generation and the very strong adsorption of *H to inhibit the HER (Figures 7A, B). The bimetallic oxides integrated stability and catalytic activity, achieving a multifunctional catalyst (Guo H. et al., 2022).
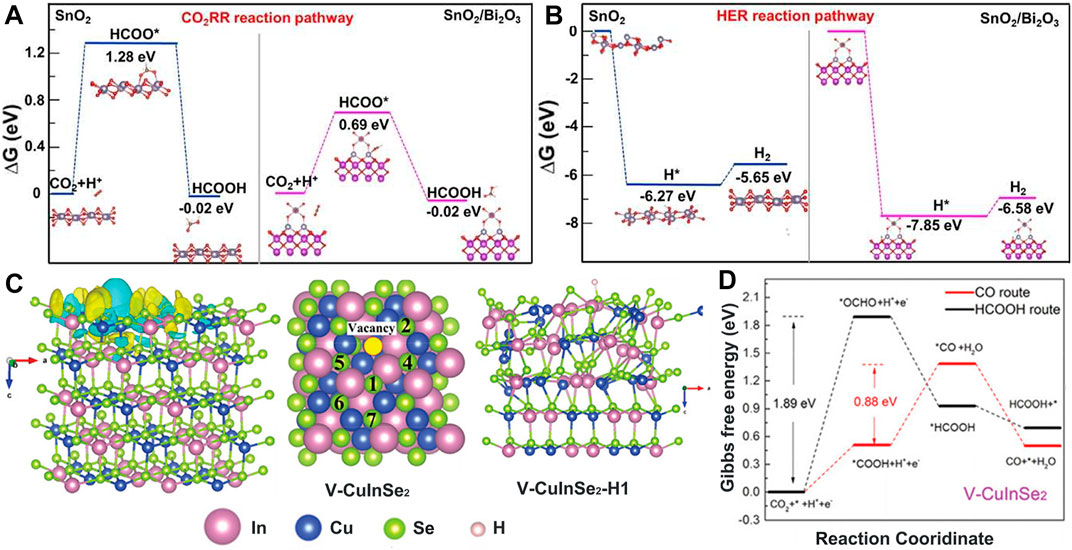
FIGURE 7. Reaction adsorption energy diagram of (A) CO2RR and (B) HER pathways on SnO2/Bi2O3. Reproduced with permission (Tian et al., 2021). Copyright 2021, Wiley. (C) Charge density differences maps of V-CuInSe2 (yellow and blue regions represent the accumulation and depletion of electrons, respectively). (D) Mechanism investigations of the CO2RR pathway on V-CuInSe2. Reproduced with permission (Wang Y.-X. et al., 2022). Copyright 2022, Wiley.
Moreover, metallic selenides are an option in CO2RR catalysts due to their low cost and unique physical and chemical properties, but their selectivity in products is poor (Hu H. et al., 2021). As reported, In2Se3 nanosheets reduce CO2 to CO with an FECO of 89% (Lü et al., 2019), and Cu1.63Se0.33 reduces CO2 to CH3OH with an FECH3OH of only 77.6% (Yang D. et al., 2019). Both examples show that catalyst activity and product selectivity are in great demand for improvement. Bimetallic selenides v-CuInSe2 (Wang Y.-X. et al., 2022) (Figure 7C) were prepared to possess the bimetallic orbital, which regulates the adsorption–desorption strength of *COOH being more conducive to CO generation and shows no stable *H adsorption sites, thereby significantly inhibiting the HER (Figure 7D). Thus, FECO was increased to 91% at −0.70 V (vs. RHE).
For metal-based catalysts, the structure and morphology of noble/transition metals are designed to avoid aggregation and self-reduction, where the hybridization and alloying strategy is adopted to modify the electron distribution for excellent catalytic behaviors.
3.3 Metal–carbon composite catalysts
The metal-based catalysts are prone to agglomerate, leading to a decrease in catalytic activity (Chen et al., 2022b). It is a strategy to isolate metal nanoparticles on carbon substrates (Lei et al., 2022) for catalytic performance and conductivity, thereby deriving metal-carbon catalysts. Such materials have excellent geometric structures and fully expose the specific active sites (Zhao S. N. et al., 2022). Their catalysis is not only related to the inherent properties of embedded metal and carbon frame but also regulated via the morphology and interface modification (creating defects and heterostructure) (Belotcerkovtceva et al., 2022; Cao et al., 2022; Fan et al., 2022; Su et al., 2022).
Metal Bi can enhance *OCHO adsorption and inhibit *H adsorption, but is easily oxidized due to its low melting point (Deng et al., 2020; Chhetri et al., 2022). To avoid oxidation, Bi is uniformly dispersed in a carbon layer, which creates an abundance of defects (Bi-D) (Wang J. et al., 2022), thereby showing hybrid crystalline–amorphous phases and heterojunctions, that render the FEformate as high as 90%; the maximum power density was 1.16 mW cm-2. Bi geometry was changed into nanotubes (Bi2O3 NTs) that accelerated the FEformate higher than 92.7%, and the maximum power density was up to 1.43 mW cm-2 (Gong et al., 2019). In addition, Bi clusters are deposited on hollow carbon spheres to form atomic dispersed BiC/HCS (Yang M. et al., 2022), improving CO2 adsorption capacity, increasing the FECO to 97% ± 2% (−0.6 V vs. RHE), achieving a record-breaking peak power density of 7.2 ± 0.5 mW cm-2, and a cycle number of more than 200 times.
The Sn/SnOx heterojunction presented higher catalytic activity than individual Sn, as previously mentioned. Multivalent Sn provides rich interfaces between Sn(0) and Sn(II) or Sn(IV), showing a special catalytic activity in stabilizing intermediates (Liu K. et al., 2022; Spada et al., 2022; Zhang et al., 2022). The Sn/SnO2 heterojunction was uniformly decorated on highly conductive N-doped carbon networks to construct Sn/SnO2@NC (Xue Teng et al., 2021). The FEformate is increased to 81%, the peak power density of the assembled battery reaches 0.9 mW cm-2, and the open-circuit voltage is 1.35 V. Furthermore, the carbon network was replaced by potential two-dimensional materials. Low-dimensional SnO2 quantum dots were spread among ultrathin Ti3C2Tx MXene nanosheets (SnO2/MXene) (Han L. et al., 2022) to reduce the reaction energy of CO2 hydrogenation to formate. This is carried out by destabilizing water and water dissociation in order to increase the surface coverage of *H and raise a new situation of electrochemical CO2-to-formate.
Similarly, bimetal or multimetal catalysts are rooted into carbon substrates to play a synergetic role via inducing geometric or electronic reconstructions (Zhang N. et al., 2021; Kong et al., 2022). For example, the In metal can reduce CO2 to formate, but is faced with many obstacles such as low jformate, poor stability, and limited coordination ability (Dong et al., 2018; Raknual et al., 2021).
To resolve these problems, In is fixed into Zn-MOF derivatives to prepare In/ZnO@C hollow nanocubes (Teng et al., 2021) (Figures 8A–D), thereby adjusting the adsorption of *OCHO on Zn catalytic sites and improving the product selectivity. Under the hollow nanocube framework, the synergetic effect of both Zn and In makes jformate as high as 23.5 mA cm-2, FEformate increases to 90% at a potential of −1.2 V (vs. RHE), long cycles of 153 (51 h) at the current density of 1 mA cm-2, and the peak power density of a Zn–CO2 battery is improved to 1.32 mW cm-2 (Figures 8E, F). Simultaneously, Fe and Ni nanoparticles are introduced into ZIF to synthesize Fe1–Ni1–N–C (Jiao et al., 2021) (Figure 6G), existing as Fe-N4 and Ni-N4 forms. Fe and Ni monoatomic pairs play a synergistic effect, where Ni atoms activate the adjacent Fe to increase the electron density between Fe and CO2 while reducing the free energy of *COOH (Figures 8H–L); thus, its FECO reaches 96.2% at −0.5 V. Furthermore, a bifunctional PdBi alloy anchored on the carbon substrate (BiPdC) (Gao S. et al., 2022) was fabricated via doping Pd into Bi-based MOF (CAU-17) (Figures 9A, B), in which Bi aims at conversion of CO2RR to HCOOH product and the Pd targets FAOR to the highly reversible conversion of CO2-to-HCOOH. This reversible Zn–CO2 battery exhibits 52.64% FEHCOOH (Figure 9C) and 45 h cycling durability. Despite the low FEHCOOH, it is of great significance to construct a truly reversible Zn–CO2 battery. In addition, the vital role of metal lattice revealed strains on CO2RR and strained PdNi alloy (s-PdNi) (Hao et al., 2022) was confined into carbon shells, presenting optimized *COOH adsorption and *CO desorption on s-PdNi-2.3% (111) surfaces through Bader charge analysis (Figures 9D, E).
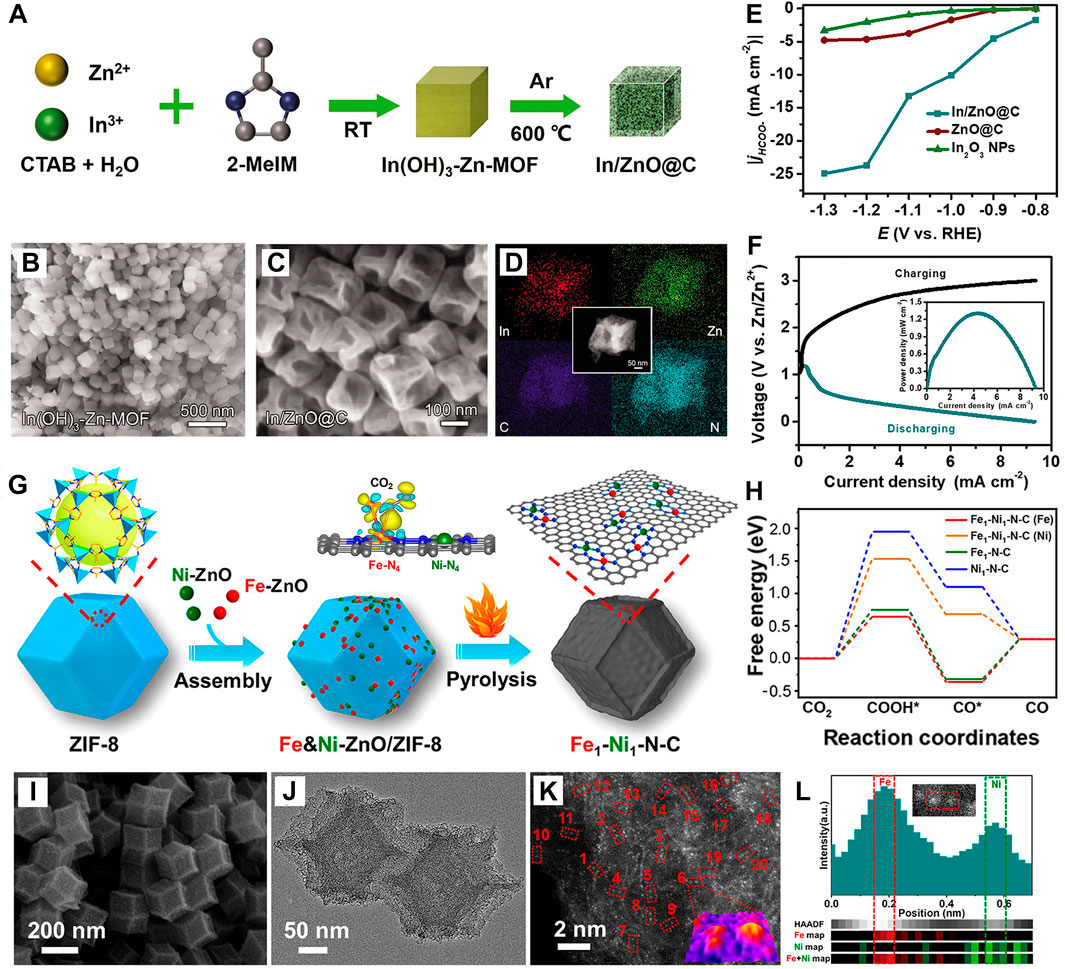
FIGURE 8. (A) Schematic illustrations of In/ZnO@C. SEM images of (B) In(OH)3-Zn-MOF precursors and (C) In/ZnO@C. (D) DEX mapping of In/ZnO@C. (E) jformate in CO2-saturated 0.5 M KHCO3 solutions. (F) Galvanostatic charge–discharge and power density curves of Zn–CO2 battery shown in the inset. Reproduced with permission (Teng et al., 2021). Copyright 2021, Royal Society of Chemistry. (G) Schematic illustrations of Fe1-Ni1-N-C. (H) Free energy diagrams of CO2RR on Fe1-Ni1-N-C. (I) SEM and (J) TEM images of Fe1-Ni1-N-C. (K) Aberration-corrected HAADF-STEM observation for Fe1-Ni1-N-C (inset: 3D atom-overlapping the Gaussian-function fitting map of region 6 in panel (K). (L) HAADF-STEM intensity profile and atomic resolution EELS mapping of the Fe–Ni pair. Reproduced with permission (Jiao et al., 2021). Copyright 2021, Wiley.
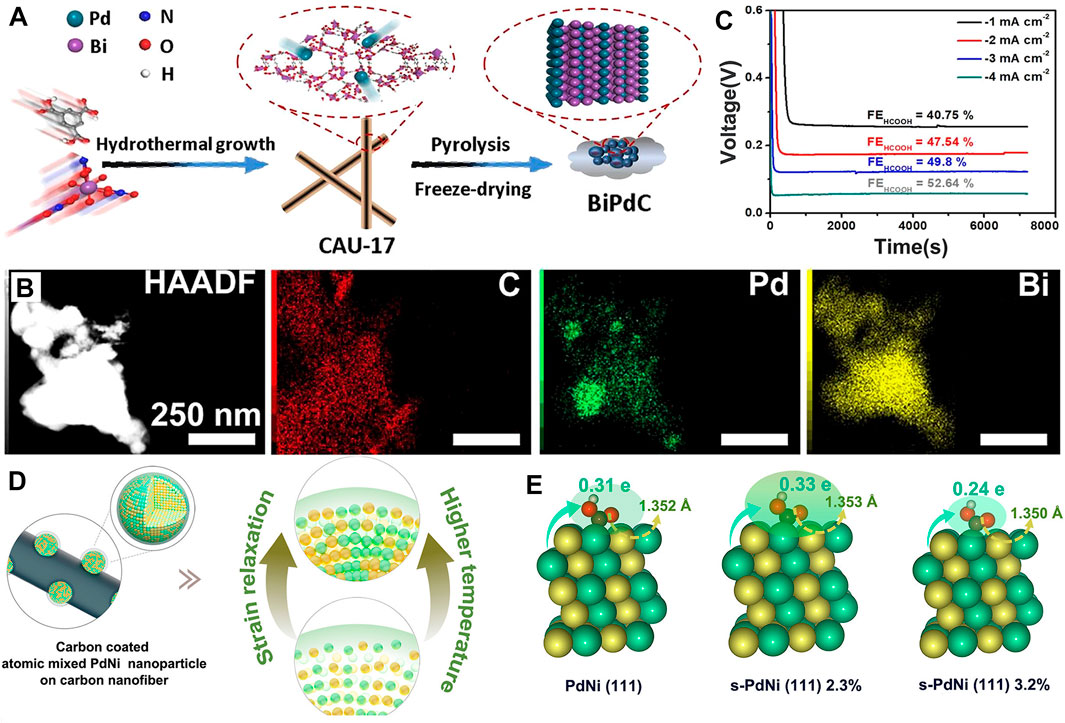
FIGURE 9. (A) Schematic illustration and (B) HAADF and DEX mapping of BiPdC. (C) Galvanostatic discharge curves with corresponding FEHCOOH. Reproduced with permission (Gao S. et al., 2022). Copyright 2022, American Chemical Society. (D) Schematic illustration of s-PdNi. Green, yellow, brown, and red balls refer to Ni, Pd, C, and O atoms, respectively. (E) Bader charge analyses of the optimized adsorption structure for intermediate *COOH on s-PdNi-2.3% (111) surfaces. Reproduced with permission (Hao et al., 2022). Copyright 2022, American Chemical Society.
For metal-carbon catalysts, introducing heteroatoms with large electronegativity (such as B, P, and S.) is mainly to regulate the local electron density of the metal, thereby providing appropriate adsorption energy for CO2RR (Maulana et al., 2021; Peng et al., 2022a; Ma et al., 2022). To achieve P doping, Fe–P nanocrystals were in situ implanted into a ZIF template and calcined at high temperatures to prepare Fe-P@NCPs (Liu et al., 2022d). During high-temperature pyrolysis, the ZIF-8 cage separates and encapsulates ferrocene, converts 2-methylimidazole into an N-C skeleton, and the evaporation of Zn ions simultaneously induces Fe reduction and phosphating, thus forming Fe–P nanocrystals with high interfacial charge transfer capability and catalytic performance. Its FECO can reach 95% at −0.55 V (vs. RHE). The Zn–CO2 battery with Fe-P@NCPs operates at ultra-high stability, exceeding 500 cycles (7 days) without obvious voltage attenuation. It was reported that P-doping promotes OER/ORR (Yang J. et al., 2019); Yang R. et al. (2019) co-doped Ni with N and P on graphene, forming Ni/PNG nanomaterials, and designed a dual-mode Zn–CO2/Zn–O2 battery realizing CO2RR/OER/ORR trifunctional catalysis. Ni-N on Ni/PNG mainly catalyzes CO2RR (He et al., 2020; Leverett et al., 2022) and assists the OER and ORR, while PG mainly works on the OER/ORR and inhibits the HER. The operational routine of dual-model batteries is controlled by supplying gas, which enlightens the integrated multifunctional energy conversion-storage devices.
Metal-carbon composite catalysts combine the superior catalytic performance of metal and the excellent electrical conductivity of carbon frames, presenting significant superiority in product selectivity and showing great potential as bifunctional catalysts.
3.4 Single-atom catalyst (SAC)
Derived from metal–carbon composite catalysts, the SAC is particularly noticeable, and the isolated single-metal atom behaves as an active center without interaction with adjacent metal atoms (Xu et al., 2018; Shah et al., 2021; Shah et al., 2022). Relying on the high atom utilization and unique coordination environment (Liu et al., 2020; Zhuang et al., 2020), the SAC shows great potential for excellent activity, selectivity, and stability (Sun et al., 2019) in CO2RR and oxidation reactions. However, in actual conditions, the reduction of a catalyst’s particle size leads to a sharp increase in surface energy, hardly maintaining the atomic level dispersion and driving metal atoms to gather, forming nanoparticles (Zhu et al., 2021b; Lin et al., 2021; Lin et al., 2022). Metal oxides, hydroxides, and carbon-based materials are accepted as substrates to separate metal atoms (Yang D. et al., 2020; Wang R. et al., 2021; Wan and Wang, 2021). Summarizing reports on SAC in Zn–CO2 batteries show that most substrates are carbon-based materials and the SAC exists in metal–nitrogen–carbon (M-N-C) mode (Chen Z. et al., 2022; Yujie Shi and Lou, 2022).
MOF with unique structures is becoming an alternative to preparing SAC and M-N-C catalysts mostly derived from ZIF-8 and ZIF-67 precursors (Ding et al., 2022; Song et al., 2022), which take dimethylimidazole as the organic ligand presenting the dodecahedral structure and fix the Zn2+ and Co2+ as ligand metals, respectively (Yang H. et al., 2020; Song et al., 2020; Mo et al., 2022). The in situ metal substitution and high-temperature calcination strategy is adopted to allow control over the coordination environment of metal center sites together with altering the porosity and conductivity of the carbon skeleton (Han W. et al., 2022; Fu et al., 2022). The Fe1NC/S1-1000 (Wang T. et al., 2020) catalyst with Fe-N3 sites balances the adsorption of *COOH and *CO, achieving a peak power density of 0.6 mW cm-2 and FECO as high as 96% at a potential of −0.5 V. Similarly, Zhang Y. et al. (2021) proposed the strategy of post-synthetic metal substitution. Zn-SAC was designed in advance to build a controllable coordination environment, and then Zn was replaced with Ni to generate Ni-SAC (Ni-Nx-C), increasing the FECO as high as 95.6%, and this Zn–CO2 battery circulates 100 times under 2 mA cm-2. Phthalocyanine (Pc) or porphyrin presents a similar plane symmetry structure as the M-N4-C catalyst and is also accepted as a precursor for the SAC. (Liang Z. et al., 2021; Cruz-Navarro et al., 2021; Ji et al., 2021; Wang H. et al., 2022). CoPc was uniformly distributed in three-dimensional N-doped hollow carbon spheres (NHC) and activated under CO2 atmosphere to prepare defected CoPc@DNHCS-T (Gong et al., 2022). Its high-density carbon defect with pyridine nitrogen establishes a double-electron absorption effect that regulates the electronic structure of the Co atom, accelerating CO2 activation and *COOH formation. As a result, the Zn–CO2 battery obtained a maximum power density of 1.02 mW cm-2 and circulated for 44 cycles (∼44 h) at 1 mA cm-2. Its FECO was up to 94%.
Importantly, the number and coordination mode of N atoms in the SAC induce a local electron density change around the central metal atom, regulating the adsorption of reaction intermediates and catalytic activity. (Yan et al., 2018; Lu et al., 2020) Liu et al. (2022c) anchored an Fe single atom on a B/N co-doped carbon matrix to form the Fe-SA/BNC catalyst that aroused significant electron transfer of *COOH and reduced the energy barrier of *CO on FeN3B sites in the desorption process (Figures 10A–C) compared with Fe-N4 sites based on DFT simulations. The Zn–CO2 battery assembled with Fe-SA/BNC obtained a high power density of 1.18 mW cm-2. Affected by the planar structure of M-N4, the coupling rate of proton–electron is slow in the CO2RR, and it requires higher reaction-free energy. Thus, the unique Fe-N5 sites with axial N coordination on defective porous carbon nanofibers (Fe-N5/DPCF) (Li Z. et al., 2022) (Figures 10D, E) were prepared via the facile dicyandiamide-assisted annealing method. Here, Fe is axially coordinated with N atoms on adjacent N-doped carbon layers, and the unstable pyrrole nitrogen and pyridine nitrogen sites on the CF substrate are removed, which leave intrinsic defects (Figures 10F, G). The theoretical calculations revealed that Fe-N5/DPCF plays a key role in boosting CO2RR (Figure 10H). Unlike plane Zn-N4 sites, highly active asymmetric Zn-N3+1 in Zn/NC NSs (Chen J. et al., 2022) (Figures 11A, B) was developed. The Zn atom center is coordinated with four N atoms at the edges of two adjacent graphite atoms to form a twisted Zn-N3+1 structure, which promotes H2O dissociation, accelerates proton–electron coupling, and reduces the energy barrier of *COOH (Figure 11C). Thus, its FECO is increased to 95% with a maximum battery power density of 1.8 mW cm-2. It performs stable runs for 100 cycles (∼26 h) under 1.5 mA cm-2.
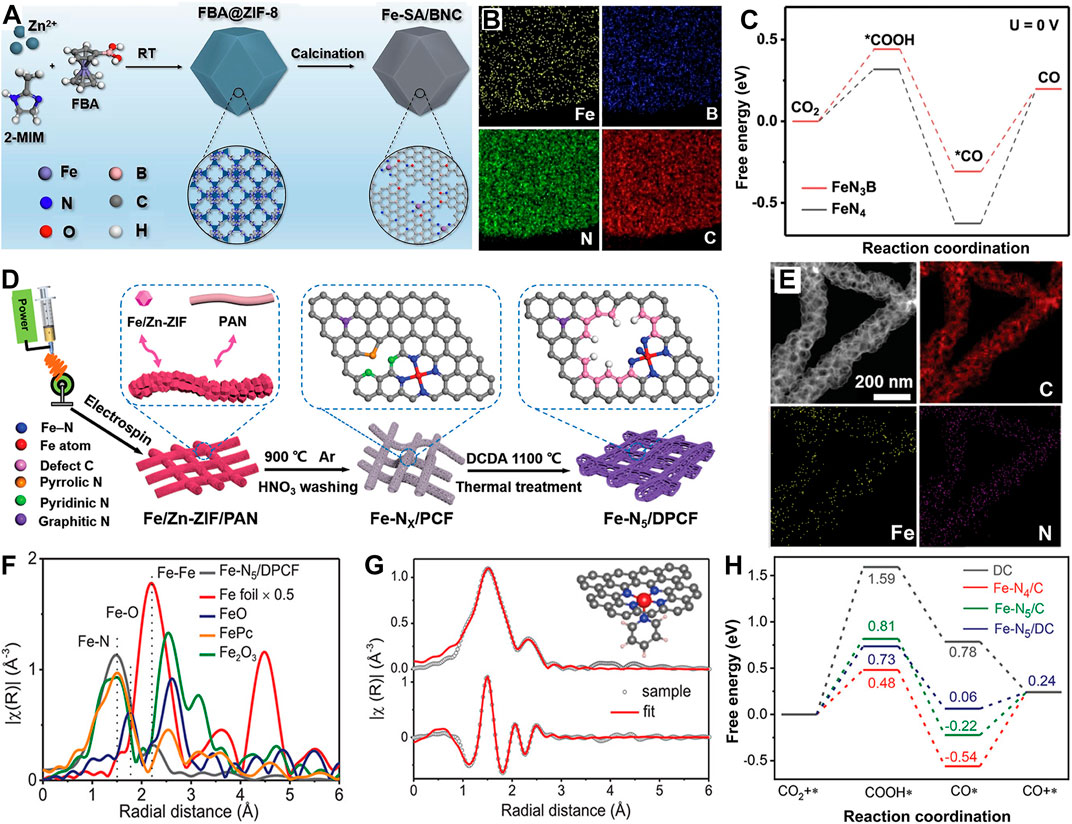
FIGURE 10. (A) Schematic illustrations and (B) EDX mapping of Fe-SA/BNC. (C) Mechanism research of CO2RR pathway on FeN4 and FeN3B. Reproduced with permission (Liu et al., 2022c). Copyright 2022, Elsevier. (D) Schematic illustrations of Fe-N5/DPCF. (E) HAADF-STEM image and STEM-EDS elemental maps of Fe-N5/DPCF. (F) Fourier transformed curves of Fe K-edge EXAFS spectra. (G) Fe K-edge EXAFS fitting result of Fe-N5/DPCF, inset shows the proposed 3D Fe-N5 structure. (H) Free-energy profiles of reaction intermediates in CO2RR. Reproduced with permission (Li Z. et al., 2022). Copyright 2022, Wiley.
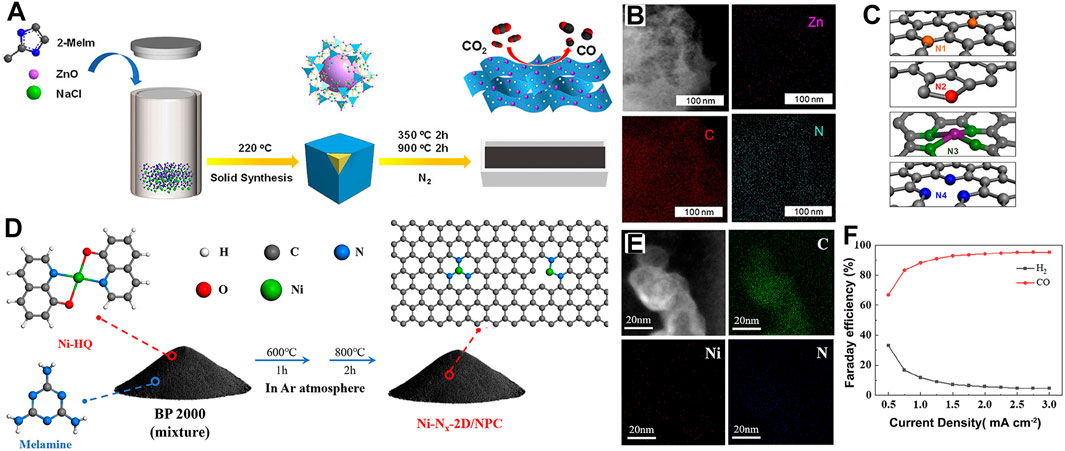
FIGURE 11. (A) Schematic illustrations and (B) TEM and EDS mapping of Zn/NC NSs. (C) Diagrams of different types of N atoms in Zn/NC NSs-T. Reproduced with permission (Chen J. et al., 2022). Copyright 2022, Wiley. (D) Schematic illustrations and (E) HAADF and corresponding EDX mappings of Ni-Nx-2D/NPC. (F) FE curves of Ni-Nx-2D/NPC cathode cells. Reproduced with permission (Zeng et al., 2021). Copyright 2021, Wiley.
As reported, the catalytic effect of N-C configurations in CO2RR is different and ranked as pyridine nitrogen > graphite nitrogen > pyrrole nitrogen, and these configurations can be altered via regulating the metal complex precursor or pyrolysis conditions (Hu et al., 2021b). For richer pyridine nitrogen, nickel 8-hydroxyquinoline complex (Ni-HQ) as a precursor mixed with melamine is pyrolyzed into Ni-doped graphite nanocarbon (Ni-Nx-2D/NPC) (Zeng et al., 2021) (Figures 11D–F). Pyridine nitrogen improves CO2 capture capacity, and Ni-N sites inhibit the HER, cooperatively promoting CO2RR selectivity under wide potential and high current density. The FECO is continuously raised above 90% and even nearly approaches 100% (Figure 7N).
SACs with fully exposed sites and a unique coordination environment improve the selective adsorption of specific functional groups, activation of reactants, and regulation of catalytic reactions (Chen et al., 2018; Liu L. et al., 2022). They show great potential in the field of catalysis and would play a vital role in future Zn–CO2 batteries.
4 Summary and outlook
For aqueous Zn-CO2 secondary batteries, the CO2RR process captures and transfers CO2 into value-added products such as COOH−/CH4 while also generating green electricity. In addition to the opposite charge process, it rarely produces CO2 again, consuming CO2 as a whole, which is in line with the CCUS strategy with broad prospects. However, problems such as unstable reaction kinetics, limited electrolyte environment, and poor product selectivity impede the application of Zn–CO2 batteries. Here, catalyst design plays a decisive role in obtaining high-performance Zn–CO2 batteries.
In this review, the structure and the involved reaction mechanism of CO2RR/FAOR/OER in the aqueous Zn–CO2 battery have been discussed; the preparation method and the strategy to develop cathode catalysts have also been summarized, especially for SAC materials. In summary, carbon-based catalysts contain excellent conductivity, large specific surface area, and abundant resources, showing obvious advantages in superior stability (Paul et al., 2019; Yuan and Lu, 2019; Hu et al., 2021a; Wang J. et al., 2021), but they are far from meeting application demands. Metal-based materials are considered effective catalysts with special geometry (Back et al., 2018), lattice defects, dispersion, and electron distribution (Jiang et al., 2018; Wang Y. et al., 2020). Alloys with low cost and toxicity are becoming their alternatives. Furthermore, metal–carbon composite catalysts are being developed to pursue the integration of excellent stability and catalytic activity (Ding et al., 2019; Wang H. F. et al., 2020; Wang and Astruc, 2020). To tap the potential of metal–carbon composite catalysts to the fullest extent possible, the following strategy is adopted: 1) isolating single-metal sites for fully exposed active sites; (Jiao et al., 2020); 2) steady and high-conductivity carbon support (Sun et al., 2021). Thus, single-atom catalysts (SACs) are initiated and applied in Zn–CO2 batteries.
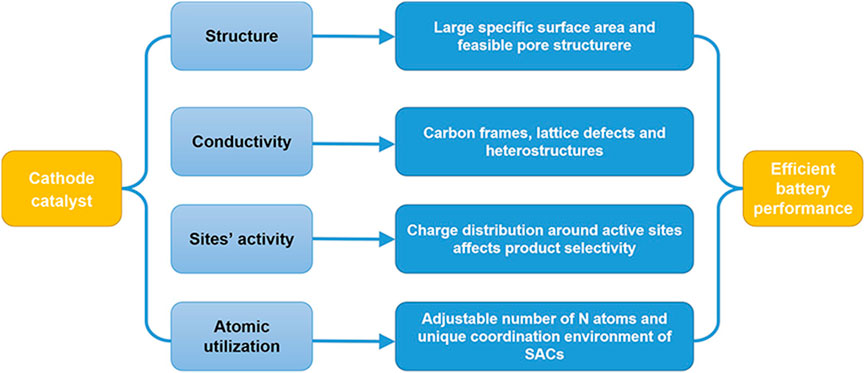
Cathode catalysts are expected to achieve high value-added and high yields of products. More attention should be paid to product selectivity for multi-carbon chemicals and their bi-functional properties in both reduction and oxidation reactions for Zn–CO2 systems. Catalyst design and structure regulation/optimization at the atomic level are recognized as feasible solutions. Herein, we propose the following strategies (Figure 12):
1) Reasonably design the catalyst structure.
The construction of catalysts with a large specific surface area and feasible pore structure is conducive to the accessibility of active sites where the rich mesoporous structure is profitable for the transport and storage of reactants and intermediates. Furthermore, this structure maintains the catalyst’s stability, thus preventing the collapse of the internal structure during battery operation.
2) Promote catalyst conductivity.
Conductivity is a vital factor for both electrochemical mass transfer and proton–electron coupling rates. It is possible to introduce carbon frames, lattice defects, and heterostructures.
3) Develop catalytic sites with intrinsic activity.
Charge distributions around active atoms influence the adsorption capacity of interaction intermediates/products, resulting in product selectivity and efficiency. Tackling competitive adsorption between *H, *COOH, *CO, and *OCHO in the catalytic process is crucial to the targeted products.
4) Improve atom utilization and create a unique coordination environment for SACs.
The adjustable number and coordination mode of N atoms in the SAC present an asymmetric or twisted structure that disrupts uniform charge distribution, resulting in highly desired catalytic properties.
In addition, aiming at the problems of self-corrosion, dendrite, and even hydrogen evolution in the anode of Zn–CO2 batteries, the reported SEI interface engineering strategy can effectively stabilize the charge–discharge reaction process of the anode, inhibit the formation of zinc dendrites, and reduce the HER process. The excessive reaction of zinc can also be slowed by adding additives to the zinc electrode or by using a polymer film to prevent direct contact between zinc and the water-based electrolyte. Furthermore, the presence of Zn can be altered. For example, Zn is deposited into the collectors as graphene or stainless steel mesh, alternatively, or combined with other metals such as Cu, forming alloys to enhance the anode’s stability.
At present, most Zn–CO2 battery devices are assembled in H-type electrolytic cells. Their open-circuit voltage is 1–1.5 V, the charge voltage is generally below 2.6 V, and the cycle number is limited, which is far from commercial requirements. This limited performance is caused by its special bipolar membrane in a two-sided electrolyte environment and huge mass/volume of the device. In order to further increase the current and voltage of the device, the overall volume of the device should be compressed as much as possible, and the asymmetric structure of the membrane electrode and gas diffusion electrode should be modified to reduce the contact resistance of each component. On one hand, the hydrophilic and hydrophobic properties on both sides of the membrane electrode can be used to extend the service life of the anode and accelerate the charge–discharge reaction at the three-phase boundary. In addition, the gas diffusion layer can be increased to improve the amount of gas dissolution and accelerate the process of proton–electron coupling.
On the aforementioned bases, advanced in situ characterization techniques such as Raman, XPS, XRD, DEMS, and EXAFS and related theoretical calculations are suggested to deeply explore further the electrochemistry mechanism/process in Zn–CO2 batteries, to guide the catalyst design, and to further increase the development toward high-performance batteries, multi-function devices, and/or multi-mode units with continuous expanding application potential.
Author contributions
WG and YW wrote the manuscript, and QY, ED, and LS revised the manuscript. PL, WS, and KQ designed and organized figures. XL conceived the idea, designed the structure of the review, revised the draft of the manuscript, supervised the work, and provided funding support. LG revised the draft of the manuscript and supervised the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supposed by the National Natural Science Foundation of China (No. 52103307), the Fundamental Research Program of Shanxi Province (Nos. 20210302124015 and 20210302124003), and the General Program of Shanxi Province (No. 202203021211150).
Conflict of interest
Authors XL was employed by Shanxi Zhongke Huaneng Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadiparidari, A., Warburton, R. E., Majidi, L., Asadi, M., Chamaani, A., Jokisaari, J. R., et al. (2019). A long-cycle-life lithium-CO2 battery with carbon neutrality. Adv. Mater 31 (40), e1902518. doi:10.1002/adma.201902518
Ansari, M. Z., Ansari, S. A., and Kim, S.-H. (2022). Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review. J. Energy Storage 53, 105187. doi:10.1016/j.est.2022.105187
Asadi, M., Sayahpour, B., Abbasi, P., Ngo, A. T., Karis, K., Jokisaari, J. R., et al. (2018). A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 555 (7697), 502–506. doi:10.1038/nature25984
Aslam, M. K., Wang, H., Chen, S., Li, Q., and Duan, J. (2023). Progress and perspectives of metal (Li, Na, Al, Zn and K)–CO2 batteries. Mater. Today Energy 31, 101196. doi:10.1016/j.mtener.2022.101196
Asokan, A., Lim, C., Kim, J., Kwon, O., Lee, H., Joo, S., et al. (2020). Carbon nanofibers encapsulated nickel-molybdenum nanoparticles as hydrogen evolution catalysts for aqueous Zn-CO2 system. ChemNanoMat 6 (6), 937–946. doi:10.1002/cnma.202000099
Back, S., Yeom, M. S., and Jung, Y. (2018). Understanding the effects of Au morphology on CO2 electrocatalysis. J. Phys. Chem. C 122 (8), 4274–4280. doi:10.1021/acs.jpcc.7b10439
Belotcerkovtceva, D., Maciel, R. P., Berggren, E., Maddu, R., Sarkar, T., Kvashnin, Y. O., et al. (2022). Insights and implications of intricate surface charge transfer and sp3-defects in graphene/metal oxide interfaces. ACS Appl. Mater Interfaces 14 (31), 36209–36216. doi:10.1021/acsami.2c06626
Cai, F., Hu, Z., and Chou, S. L. (2018). Progress and future perspectives on Li(Na)-CO2 batteries. Adv. Sustain. Syst. 2, 1800060–1800069. doi:10.1002/adsu.201800060
Calderón-Cárdenas, A., Hartl, F. W., Gallas, J. A. C., and Varela, H. (2021). Modeling the triple-path electro-oxidation of formic acid on platinum: Cyclic voltammetry and oscillations. Catal. Today 359, 90–98. doi:10.1016/j.cattod.2019.04.054
Cao, L., Fang, S., Xu, B., Zhang, B., Wang, C., Xiao, Z., et al. (2022). Enabling reversible reaction by uniform distribution of heterogeneous intermediates on defect-rich SnSSe/C layered heterostructure for ultralong-cycling sodium storage. Small 18 (26), e2202134. doi:10.1002/smll.202202134
Chang, Z., Xu, J., and Zhang, X. (2017). Recent progress in electrocatalyst for Li-O2 batteries. Adv. Energy Mater. 7 (23), 1700875. doi:10.1002/aenm.201700875
Chen, B., Wang, D., Zhang, B., Zhong, X., Liu, Y., Sheng, J., et al. (2021a). Engineering the active sites of graphene catalyst: From CO2 activation to activate Li-CO2 batteries. ACS Nano 15 (6), 9841–9850. doi:10.1021/acsnano.1c00756
Chen, J., Li, Z., Wang, X., Sang, X., Zheng, S., Liu, S., et al. (2022a). Promoting CO2 electroreduction kinetics on atomically dispersed monovalent ZnI sites by rationally engineering proton-feeding centers. Angew. Chem. Int. Ed. Engl. (2022a) 61 (7), e202111683. doi:10.1002/anie.202111683
Chen, S., Li, X., Kao, C. W., Luo, T., Chen, K., Fu, J., et al. (2022b). Unveiling the proton-feeding effect in sulfur-doped Fe-N-C single-atom catalyst for enhanced CO2 electroreduction. Angew. Chem. Int. Ed. Engl. (2022b) 61 (32), e202206233. doi:10.1002/anie.202206233
Chen, S., Liu, J., Zhang, Q., Teng, F., and McLellan, B. C. (2022c). A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 167, 112537. doi:10.1016/j.rser.2022.112537
Chen, Y., Ji, S., Chen, C., Peng, Q., Wang, D., and Li, Y. (2018). Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2 (7), 1242–1264. doi:10.1016/j.joule.2018.06.019
Chen, Y., Mei, Y., Li, M., Dang, C., Huang, L., Wu, W., et al. (2021b). Highly selective CO2 conversion to methane or syngas tuned by CNTs@non-noble-metal cathodes in Zn-CO2 flow batteries. Green Chem. 23 (20), 8138–8146. doi:10.1039/d1gc02496e
Chen, Z., Liu, J., Koh, M. J., and Loh, K. P. (2022d). Single-atom catalysis: From simple reactions to the synthesis of complex molecules. Adv. Mater 34 (25), e2103882. doi:10.1002/adma.202103882
Chhetri, K., Kim, T., Acharya, D., Muthurasu, A., Dahal, B., Bhattarai, R. M., et al. (2022). Hollow carbon nanofibers with inside-outside decoration of Bi-metallic MOF derived Ni-Fe phosphides as electrode materials for asymmetric supercapacitors. Chem. Eng. J. 450, 138363. doi:10.1016/j.cej.2022.138363
Chu, S., Cui, Y., and Liu, N. (2016). The path towards sustainable energy. Nat. Mater 16 (1), 16–22. doi:10.1038/nmat4834
Cruz-Navarro, J. A., Hernández-García, F., Mendoza-Huizar, L. H., Salazar-Pereda, V., Cobos-Murcia, J. . Á., Colorado-Peralta, R., et al. (2021). Recent advances in the use of transition-metal porphyrin and phthalocyanine complexes as electro-catalyst materials on modified electrodes for electroanalytical sensing applications. Solids 2 (2), 212–231. doi:10.3390/solids2020014
de Oliveira Maciel, A., Christakopoulos, P., Rova, U., and Antonopoulou, I. (2022). Carbonic anhydrase to boost CO2 sequestration: Improving carbon capture utilization and storage (CCUS). Chemosphere 299, 134419. doi:10.1016/j.chemosphere.2022.134419
Deng, P., Yang, F., Wang, Z., Chen, S., Zhou, Y., Zaman, S., et al. (2020). Metal-organic framework-derived carbon nanorods encapsulating bismuth oxides for rapid and selective CO2 electroreduction to formate. Angew. Chem. Int. Ed. Engl. 59 (27), 10807–10813. doi:10.1002/anie.202000657
Ding, J., Xue, H., Xiao, R., Xu, Y., Song, L., Gong, H., et al. (2022). Atomically dispersed Fe-nx species within a porous carbon framework: An efficient catalyst for Li-CO2 batteries. Nanoscale 14 (12), 4511–4518. doi:10.1039/d1nr08354f
Ding, M., Flaig, R. W., Jiang, H. L., and Yaghi, O. M. (2019). Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 48 (10), 2783–2828. doi:10.1039/c8cs00829a
Dong, Y., Ghuman, K. K., Popescu, R., Duchesne, P. N., Zhou, W., Loh, J. Y. Y., et al. (2018). Tailoring surface frustrated lewis pairs of In2O3-x (OH)y for gas-phase heterogeneous photocatalytic reduction of CO2 by isomorphous substitution of In3+ with Bi3+. Adv. Sci. (Weinh) 5 (6), 1700732. doi:10.1002/advs.201700732
El Sawy, E. N., and Pickup, P. G. (2016). Formic acid oxidation at Ru@Pt core-shell nanoparticles. Electrocatalysis 7 (6), 477–485. doi:10.1007/s12678-016-0328-8
Eskezia Ayalew, M. (2021). The role of nanotechnology for energy storage, conservation and post combustion CO2 capture in industry: A review. Int. J. Mater. Sci. Appl. 10 (3), 55. doi:10.11648/j.ijmsa.20211003.12
Fan, L., Xu, J., and Hong, Y. (2022). Defects in graphene-based heterostructures: Topological and geometrical effects. RSC Adv. 12 (11), 6772–6782. doi:10.1039/d1ra08884j
Feng, Z.-y., Peng, W.-j., Wang, Z.-x., Guo, H.-j., Li, X.-h., Yan, G.-c., et al. (2021). Review of silicon-based alloys for lithium-ion battery anodes. Int. J. Minerals, Metallurgy Mater. 28 (10), 1549–1564. doi:10.1007/s12613-021-2335-x
Fu, L., Chen, H., Wang, K., and Wang, X. (2022). Oxygen-vacancy generation in MgFe2O4 by high temperature calcination and its improved photocatalytic activity for CO2 reduction. J. Alloys Compd. 891, 161925. doi:10.1016/j.jallcom.2021.161925
Gao, S., Chen, S., Liu, Q., Zhang, S., Qi, G., Luo, J., et al. (2022a). Bifunctional BiPd alloy particles anchored on carbon matrix for reversible Zn-CO2 battery. ACS Appl. Nano Mater. 5 (9), 12387–12394. doi:10.1021/acsanm.2c02917
Gao, S., Jin, M., Sun, J., Liu, X., Zhang, S., Li, H., et al. (2021a). Coralloid Au enables high-performance Zn-CO2 battery and self-driven CO production. J. Mater. Chem. A 9 (37), 21024–21031. doi:10.1039/d1ta04360a
Gao, S., Liu, Y., Xie, Z., Qiu, Y., Zhuo, L., Qin, Y., et al. (2021b). Metal-free bifunctional ordered mesoporous carbon for reversible Zn-CO2 batteries. Small Methods 5 (4), 2001039. doi:10.1002/smtd.202001039
Gao, Y., and Zhao, L. (2022). Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 430, 132745. doi:10.1016/j.cej.2021.132745
Gao, Y., Zhao, Y., Liu, H., Shao, M., Chen, Z., Ma, T., et al. (2022b). N, P-doped carbon supported ruthenium doped Rhenium phosphide with porous nanostructure for hydrogen evolution reaction using sustainable energies. J. Colloid Interface Sci. 606, 1874–1881. doi:10.1016/j.jcis.2021.08.077
Ghausi, M. A., Xie, J., Li, Q., Wang, X., Yang, R., Wu, M., et al. (2018). CO2 overall splitting by a bifunctional metal-free electrocatalyst. Angew. Chem. Int. Ed. Engl. 57 (40), 13135–13139. doi:10.1002/anie.201807571
Gong, Q., Ding, P., Xu, M., Zhu, X., Wang, M., Deng, J., et al. (2019). Structural defects on converted bismuth oxide nanotubes enable highly active electrocatalysis of carbon dioxide reduction. Nat. Commun. 10 (1), 2807. doi:10.1038/s41467-019-10819-4
Gong, S., Wang, W., Zhang, C., Zhu, M., Lu, R., Ye, J., et al. (2022). Tuning the metal electronic structure of anchored cobalt phthalocyanine via dual-regulator for efficient CO2 electroreduction and Zn-CO2 batteries. Adv. Funct. Mater. 32, 2110649. doi:10.1002/adfm.202110649
Guo, H., Wang, X., Wang, H., Cui, W., Li, M., and Xie, W. (2022a). Double-exchange-induced effective increased CO2 capture of CaO by doping bimetallic oxides with variable valence state. Chem. Eng. J. 433, 134490. doi:10.1016/j.cej.2021.134490
Guo, Y., Zhang, R., Zhang, S., and Zhi, C. (2022b). Recent advances in Zn-CO2 batteries for the co-production of electricity and carbonaceous fuels. Nano Mater. Sci. doi:10.1016/j.nanoms.2022.09.004
Han, L., Peng, X., Wang, H. T., Ou, P., Mi, Y., Pao, C. W., et al. (2022a). Chemically coupling SnO2 quantum dots and MXene for efficient CO2 electroreduction to formate and Zn-CO2 battery. Proc. Natl. Acad. Sci. U. S. A. 119 (42), e2207326119. doi:10.1073/pnas.2207326119
Han, N., Wang, Y., Yang, H., Deng, J., Wu, J., Li, Y., et al. (2018). Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 9 (1), 1320. doi:10.1038/s41467-018-03712-z
Han, W., Xiong, L., Wang, M., Seo, W., Liu, Y., Taj Ud Din, S., et al. (2022b). Interface engineering via in-situ electrochemical induced ZnSe for a stabilized zinc metal anode. Chem. Eng. J. 442, 136247. doi:10.1016/j.cej.2022.136247
Hao, J., Li, X., Zeng, X., Li, D., Mao, J., and Guo, Z. (2020). Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy and Environ. Sci. 13 (11), 3917–3949. doi:10.1039/d0ee02162h
Hao, J., Yuan, L., Ye, C., Chao, D., Davey, K., Guo, Z., et al. (2021). Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew. Chem. Int. Ed. Engl. 60 (13), 7366–7375. doi:10.1002/anie.202016531
Hao, J., Zhuang, Z., Hao, J., Cao, K., Hu, Y., Wu, W., et al. (2022). Strain relaxation in metal alloy catalysts steers the product selectivity of electrocatalytic CO2 reduction. ACS Nano 16 (2), 3251–3263. doi:10.1021/acsnano.1c11145
He, Y., Li, Y., Zhang, J., Wang, S., Huang, D., Yang, G., et al. (2020). Low-temperature strategy toward Ni-NC@Ni core-shell nanostructure with Single-Ni sites for efficient CO2 electroreduction. Nano Energy 77, 105010. doi:10.1016/j.nanoen.2020.105010
Hu, A., Shu, C., Xu, C., Liang, R., Li, J., Zheng, R., et al. (2019). Design strategies toward catalytic materials and cathode structures for emerging Li-CO2 batteries. J. Mater. Chem. A 7 (38), 21605–21633. doi:10.1039/c9ta06506g
Hu, C., Dai, Q., and Dai, L. (2021a). Multifunctional carbon-based metal-free catalysts for advanced energy conversion and storage. Cell Rep. Phys. Sci. 2 (2), 100328. doi:10.1016/j.xcrp.2021.100328
Hu, C., Paul, R., Dai, Q., and Dai, L. (2021b). Carbon-based metal-free electrocatalysts: From oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 50 (21), 11785–11843. doi:10.1039/d1cs00219h
Hu, H., Wang, J., Cui, B., Zheng, X., Lin, J., Deng, Y., et al. (2021c). Atomically dispersed selenium sites on nitrogen-doped carbon for efficient electrocatalytic oxygen reduction. Angew. Chem. Int. Ed. Engl. 61, e202114441. doi:10.1002/anie.202114441
Huang, C., Ji, Q., Zhang, H., Wang, Y., Wang, S., Liu, X., et al. (2022). Ru-incorporated Co3O4 nanoparticles from self-sacrificial ZIF-67 template as efficient bifunctional electrocatalysts for rechargeable metal-air battery. J. Colloid Interface Sci. 606, 654–665. doi:10.1016/j.jcis.2021.08.046
Huang, Y., Wang, Y., Tang, C., Wang, J., Zhang, Q., Wang, Y., et al. (2019). Atomic modulation and structure design of carbons for bifunctional electrocatalysis in metal-air batteries. Adv. Mater 31 (13), e1803800. doi:10.1002/adma.201803800
Ji, W., Wang, T.-X., Ding, X., Lei, S., and Han, B.-H. (2021). Porphyrin- and phthalocyanine-based porous organic polymers: From synthesis to application. Coord. Chem. Rev. 439, 213875. doi:10.1016/j.ccr.2021.213875
Jiang, B., Zhang, X. G., Jiang, K., Wu, D. Y., and Cai, W. B. (2018). Boosting formate production in electrocatalytic CO2 reduction over wide potential window on Pd surfaces. J. Am. Chem. Soc. 140 (8), 2880–2889. doi:10.1021/jacs.7b12506
Jiang, K., Ashworth, P., Zhang, S., and Hu, G. (2022). Print media representations of carbon capture utilization and storage (CCUS) technology in China. Renew. Sustain. Energy Rev. 155, 111938. doi:10.1016/j.rser.2021.111938
Jiao, L., Zhang, R., Wan, G., Yang, W., Wan, X., Zhou, H., et al. (2020). Nanocasting SiO2 into metal-organic frameworks imparts dual protection to high-loading Fe single-atom electrocatalysts. Nat. Commun. 11 (1), 2831. doi:10.1038/s41467-020-16715-6
Jiao, L., Zhu, J., Zhang, Y., Yang, W., Zhou, S., Li, A., et al. (2021). Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction. J. Am. Chem. Soc. 143 (46), 19417–19424. doi:10.1021/jacs.1c08050
Kang, J., Rui, N., Rosales, R., Tian, Y., Senanayake, S. D., and Rodriguez, J. A. (2022a). Understanding the surface structure and catalytic activity of SnOx/Au(111) inverse catalysts for CO2 and H2 activation. J. Phys. Chem. C 126 (10), 4862–4870. doi:10.1021/acs.jpcc.2c00138
Kang, T., Nam, D., and Kim, J. (2022b). Activated palladium deposited on defect-rich carbon and cobalt oxide composite materials as bi-functional catalysts for stable rechargeable Zn-air battery. Appl. Surf. Sci. 582, 152442. doi:10.1016/j.apsusc.2022.152442
Khurram, A., He, M., and Gallant, B. M. (2018). Tailoring the discharge reaction in Li-CO2 batteries through incorporation of CO2 capture Chemistry. Joule 2 (12), 2649–2666. doi:10.1016/j.joule.2018.09.002
Kim, J., Yang, Y., Seong, A., Noh, H.-J., Kim, C., Joo, S., et al. (2020). Identifying the electrocatalytic active sites of a Ru-based catalyst with high Faraday efficiency in CO2-saturated media for an aqueous Zn-CO2 system. J. Mater. Chem. A 8 (30), 14927–14934. doi:10.1039/d0ta03050c
Kong, F., Liu, X., Song, Y., Qian, Z., Li, J., Zhang, L., et al. (2022). Selectively coupling Ru single atoms to PtNi concavities for high performance methanol oxidation via d-band center regulation. Angew. Chem. Int. Ed. Engl. 61, e202207524. doi:10.1002/anie.202207524
Lankone, R. S., Wang, J., Ranville, J. F., and Fairbrother, D. H. (2017). Photodegradation of polymer-CNT nanocomposites: Effect of CNT loading and CNT release characteristics. Environ. Sci. Nano 4 (4), 967–982. doi:10.1039/c6en00669h
Lei, Z., Sathish, C. I., Liu, Y., Karokoti, A., Wang, J., Qiao, L., et al. (2022). Single metal atoms catalysts-Promising candidates for next generation energy storage and conversion devices. EcoMat 4 (3). doi:10.1002/eom2.12186
Leverett, J., Yuwono, J. A., Kumar, P., Tran-Phu, T., Qu, J., Cairney, J., et al. (2022). Impurity tolerance of unsaturated Ni-N-C active sites for practical electrochemical CO2 reduction. ACS Energy Lett. 7 (3), 920–928. doi:10.1021/acsenergylett.1c02711
Li, D., Gao, Y., Xie, C., and Zheng, Z. (2022a). Au-coated carbon fabric as Janus current collector for dendrite-free flexible lithium metal anode and battery. Appl. Phys. Rev. 9 (1), 011424. doi:10.1063/5.0083830
Li, F., Chen, L., Knowles, G. P., MacFarlane, D. R., and Zhang, J. (2017). Hierarchical mesoporous SnO2 nanosheets on carbon cloth: A robust and flexible electrocatalyst for CO2 reduction with high efficiency and selectivity. Angew. Chem. Int. Ed. 129 (2), 505–509. doi:10.1002/anie.201608279
Li, Z., Jiang, J., Liu, X., Zhu, Z., Wang, J., He, Q., et al. (2022b). Coupling atomically dispersed Fe-N5 sites with defective N-doped carbon boosts CO2 electroreduction. Small 18 (38), e2203495. doi:10.1002/smll.202203495
Liang, M., Xia, T., Gao, H., Zhao, K., Cao, T., Deng, M., et al. (2021a). Modulating reaction pathways of formic acid oxidation for optimized electrocatalytic performance of PtAu/CoNC. Nano Res. 15 (2), 1221–1229. doi:10.1007/s12274-021-3629-z
Liang, Z., Wang, H. Y., Zheng, H., Zhang, W., and Cao, R. (2021b). Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem. Soc. Rev. 50 (4), 2540–2581. doi:10.1039/d0cs01482f
Lichchhavi, K. A., and Shirage, P. M. (2022). A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 22, 55. doi:10.1016/j.est.2022.105692
Lin, J., Ding, J., Wang, H., Yang, X., Zheng, X., Huang, Z., et al. (2022). Boosting energy efficiency and stability of Li-CO2 batteries via synergy between Ru atom clusters and single-atom Ru-N4 sites in the electrocatalyst cathode. Adv. Mater 34, e2200559. doi:10.1002/adma.202200559
Lin, L., Gerlak, C. A., Liu, C., Llorca, J., Yao, S., Rui, N., et al. (2021). Effect of Ni particle size on the production of renewable methane from CO2 over Ni/CeO2 catalyst. J. Energy Chem. 61, 602–611. doi:10.1016/j.jechem.2021.02.021
Ling, L. L., Jiao, L., Liu, X., Dong, Y., Yang, W., Zhang, H., et al. (2022). Potassium-assisted fabrication of intrinsic defects in porous carbons for electrocatalytic CO2 reduction. Adv. Mater 34 (42), e2205933. doi:10.1002/adma.202205933
Liu, D., He, Q., Ding, S., and Song, L. (2020). Structural regulation and support coupling effect of single-atom catalysts for heterogeneous catalysis. Adv. Energy Mater. 10 (32), 2001482. doi:10.1002/aenm.202001482
Liu, K., Meng, X., Yan, L., Fan, M., Wu, Y., Li, C., et al. (2022a). Sn/SnOx core-shell structure encapsulated in nitrogen-doped porous carbon frameworks for enhanced lithium storage. J. Alloys Compd. 896, 163009. doi:10.1016/j.jallcom.2021.163009
Liu, L., Li, M., Chen, F., and Huang, H. (2022b). Recent advances on single-atom catalysts for CO2 reduction. Small Struct. 4 (3), 2200188. doi:10.1002/sstr.202200188
Liu, S., Jin, M., Sun, J., Qin, Y., Gao, S., Chen, Y., et al. (2022c). Coordination environment engineering to boost electrocatalytic CO2 reduction performance by introducing boron into single-Fe-atomic catalyst. Chem. Eng. J. 437, 135294. doi:10.1016/j.cej.2022.135294
Liu, S., Wang, L., Yang, H., Gao, S., Liu, Y., Zhang, S., et al. (2022d). Nitrogen-doped carbon polyhedrons confined Fe-P nanocrystals as high-efficiency bifunctional catalysts for aqueous Zn-CO2 batteries. Small 18, e2104965. doi:10.1002/smll.202104965
Lu, B., Liu, Q., and Chen, S. (2020). Electrocatalysis of single-atom sites: Impacts of atomic coordination. ACS Catal. 10 (14), 7584–7618. doi:10.1021/acscatal.0c01950
Lü, F., Qi, G., Liu, X., Zhang, C., Guo, R., Peng, X., et al. (2019). Selective electrolysis of CO2 to CO on ultrathin In2Se3 nanosheets. Electrochem. Commun. 103, 127–132. doi:10.1016/j.elecom.2019.05.020
Ma, G., Ning, G., and Wei, Q. (2022). S-doped carbon materials: Synthesis, properties and applications. Carbon 195, 328–340. doi:10.1016/j.carbon.2022.03.043
Ma, W., Liu, X., Li, C., Yin, H., Xi, W., Liu, R., et al. (2018). Rechargeable Al-CO2 batteries for reversible utilization of CO2. Adv. Mater 30 (28), e1801152. doi:10.1002/adma.201801152
Maulana, A. L., Saputro, A. G., Prasetyo, Y., Mahyuddin, M. H., Iqbal, M., Yudistira, H. T., et al. (2021). Two-electron electrochemical reduction of CO2 on B-doped Ni-N-C catalysts: A first-principles study. J. Phys. Chem. C 125 (35), 19247–19258. doi:10.1021/acs.jpcc.1c04986
Mo, Z., Tai, D., Zhang, H., and Shahab, A. (2022). A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water. Chem. Eng. J. 443, 136320. doi:10.1016/j.cej.2022.136320
Mu, X., Pan, H., He, P., and Zhou, H. (2020). Li-CO2 and Na-CO2 batteries: Toward greener and sustainable electrical energy storage. Adv. Mater 32 (27), e1903790. doi:10.1002/adma.201903790
Paul, R., Dai, Q., Hu, C., and Dai, L. (2019). Ten years of carbon-based metal-free electrocatalysts. Carbon Energy 1 (1), 19–31. doi:10.1002/cey2.5
Peng, M., Ci, S., Shao, P., Cai, P., and Wen, Z. (2019). Cu3P/C nanocomposites for efficient electrocatalytic CO2 reduction and Zn-CO2 battery. J. Nanosci. Nanotechnol. 19 (6), 3232–3236. doi:10.1166/jnn.2019.16589
Peng, Y., Bai, Y., Liu, C., Cao, S., Kong, Q., and Pang, H. (2022a). Applications of metal–organic framework-derived N, P, S doped materials in electrochemical energy conversion and storage. Coord. Chem. Rev. 466, 214602. doi:10.1016/j.ccr.2022.214602
Peng, Y., Xu, J., Xu, J., Ma, J., Bai, Y., Cao, S., et al. (2022b). Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. 307, 102732. doi:10.1016/j.cis.2022.102732
Pfeiffer, O., Khurram, A., Olivetti, E. A., and Gallant, B. M. (2022). Life cycle assessment of CO2 conversion and storage in metal-CO2 electrochemical cells. J. Industrial Ecol. 26, 1306–1317. doi:10.1111/jiec.13266
Qiao, Y., Yi, J., Wu, S., Liu, Y., Yang, S., He, P., et al. (2017). Li-CO2 electrochemistry: A new strategy for CO2 fixation and energy storage. Joule 1 (2), 359–370. doi:10.1016/j.joule.2017.07.001
Raknual, D., Charoenphon, S., Reunchan, P., and Tubtimtae, A. (2021). Structural and electrochemical properties of undoped and In3+-doped multi-phase zinc-antimony oxide for a high-performance pseudocapacitor. Electrochimica Acta 389, 138773. doi:10.1016/j.electacta.2021.138773
Sawant, S. V., Patwardhan, A. W., Joshi, J. B., and Dasgupta, K. (2022). Boron doped carbon nanotubes: Synthesis, characterization and emerging applications - a review. Chem. Eng. J. 427, 131616. doi:10.1016/j.cej.2021.131616
Selvakumaran, D., Pan, A., Liang, S., and Cao, G. (2019). A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 7 (31), 18209–18236. doi:10.1039/c9ta05053a
Shah, S. S. A., Najam, T., Bashir, M. S., Peng, L., Nazir, M. A., and Javed, M. S. (2022). Single-atom catalysts for next-generation rechargeable batteries and fuel cells. Energy Storage Mater. 45, 301–322. doi:10.1016/j.ensm.2021.11.049
Shah, S. S. A., Najam, T., Javed, M. S., Bashir, M. S., Nazir, M. A., Khan, N. A., et al. (2021). Recent advances in synthesis and applications of single-atom catalysts for rechargeable batteries. Chem. Rec. 22, e202100280. doi:10.1002/tcr.202100280
Shakeri, M., Khatami Shal, Z., and Van Der Voort, P. (2021). An overview of the challenges and progress of synthesis, characterization and applications of plugged SBA-15 materials for heterogeneous catalysis. Mater. (Basel) 14 (17), 5082. doi:10.3390/ma14175082
Shi, Q., Liu, Q., Zheng, Y., Dong, Y., Wang, L., Liu, H., et al. (2021). Controllable construction of bifunctional CoxP@N,P-doped carbon electrocatalysts for rechargeable zinc-air batteries. Energy and Environ. Mater. 5 (2), 515–523. doi:10.1002/eem2.12208
Song, K., Feng, Y., Zhang, W., and Zheng, W. (2022). MOFs fertilized transition-metallic single-atom electrocatalysts for highly-efficient oxygen reduction: Spreading the synthesis strategies and advanced identification. J. Energy Chem. 67, 391–422. doi:10.1016/j.jechem.2021.10.011
Song, Z., Zhang, L., Doyle-Davis, K., Fu, X., Luo, J. L., and Sun, X. (2020). Recent advances in MOF-derived single atom catalysts for electrochemical applications. Adv. Energy Mater. 10 (38), 2001561. doi:10.1002/aenm.202001561
Spada, D., Bruni, P., Ferrari, S., Albini, B., Galinetto, P., Berbenni, V., et al. (2022). Self-Supported fibrous Sn/SnO2@C nanocomposite as superior anode material for lithium-ion batteries. Mater. (Basel) 15 (3), 919. doi:10.3390/ma15030919
Su, J., Yuan, M., Han, L., Deng, H., Chang, J., Zhuang, Y., et al. (2022). Ultrathin metal organic framework nanosheets with rich defects for enhanced fluoride removal. Chem. Eng. J. 451, 138989. doi:10.1016/j.cej.2022.138989
Suen, N. T., Hung, S. F., Quan, Q., Zhang, N., Xu, Y. J., and Chen, H. M. (2017). Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 46 (2), 337–365. doi:10.1039/c6cs00328a
Sui, X., Zhang, L., Li, J., Doyle-Davis, K., Li, R., Wang, Z., et al. (2021). Advanced support materials and interactions for atomically dispersed noble-metal catalysts: From support effects to design strategies. Adv. Energy Mater. 12 (1), 2102556. doi:10.1002/aenm.202102556
Sun, T., Mitchell, S., Li, J., Lyu, P., Wu, X., Perez-Ramirez, J., et al. (2021). Design of local atomic environments in single-atom electrocatalysts for renewable energy conversions. Adv. Mater 33 (5), e2003075. doi:10.1002/adma.202003075
Sun, T., Xu, L., Wang, D., and Li, Y. (2019). Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 12 (9), 2067–2080. doi:10.1007/s12274-019-2345-4
Tan, Z., Zhang, J., Yang, Y., Sha, Y., Duan, R., Zhong, J., et al. (2022). BiO2-x nanosheets with surface electron localizations for efficient electrocatalytic CO2 reduction to formate. CCS Chem. 5, 133–144. doi:10.31635/ccschem.022.202202068
Teng, X., Lu, J., Niu, Y., Gong, S., Xu, M., Meyer, T. J., et al. (2022). Selective CO2 reduction to formate on a Zn-based electrocatalyst promoted by tellurium. Chem. Mater. 34 (13), 6036–6047. doi:10.1021/acs.chemmater.2c01131
Teng, X., Niu, Y., Gong, S., Xu, M., Liu, X., Ji, L., et al. (2021)./ZnO@C hollow nanocubes for efficient electrochemical reduction of CO2 to formate and rechargeable Zn-CO2 batteries. Mater. Chem. Front. 5 (17), 6618–6627. doi:10.1039/d1qm00825k
Thakur, A. K., Kurtyka, K., Majumder, M., Yang, X., Ta, H. Q., Bachmatiuk, A., et al. (2022). Recent advances in boron- and nitrogen-doped carbon-based materials and their various applications. Adv. Mater. Interfaces 9 (11), 2101964. doi:10.1002/admi.202101964
Tian, J., Wang, R., Shen, M., Ma, X., Yao, H., Hua, Z., et al. (2021). Bi-Sn oxides for highly selective CO2 electroreduction to formate in a wide potential window. ChemSusChem 14 (10), 2247–2254. doi:10.1002/cssc.202100543
Wan, L., and Wang, P. (2021). Recent progress on self-supported two-dimensional transition metal hydroxides nanosheets for electrochemical energy storage and conversion. Int. J. Hydrogen Energy 46 (12), 8356–8376. doi:10.1016/j.ijhydene.2020.12.061
Wang, C., Li, J., Zhou, Z., Pan, Y., Yu, Z., Pei, Z., et al. (2021a). Rechargeable zinc-air batteries with neutral electrolytes: Recent advances, challenges, and prospects. EnergyChem 3 (4), 100055. doi:10.1016/j.enchem.2021.100055
Wang, F., Li, Y., Xia, X., Cai, W., Chen, Q., and Chen, M. (2021b). Metal-CO2 electrochemistry: From CO2 recycling to energy storage. Adv. Energy Mater. 11 (25), 2100667. doi:10.1002/aenm.202100667
Wang, G.-D., Wang, H.-H., Shi, W.-J., Hou, L., Wang, Y.-Y., and Zhu, Z. (2021c). A highly stable MOF with F and N accessible sites for efficient capture and separation of acetylene from ternary mixtures. J. Mater. Chem. A 9 (43), 24495–24502. doi:10.1039/d1ta05720k
Wang, H. F., Chen, L., Pang, H., Kaskel, S., and Xu, Q. (2020a). MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 49 (5), 1414–1448. doi:10.1039/c9cs00906j
Wang, H., Wu, Q., Cheng, L., Chen, L., Li, M., and Zhu, G. (2022a). Porphyrin- and phthalocyanine-based systems for rechargeable batteries. Energy Storage Mater. 52, 495–513. doi:10.1016/j.ensm.2022.08.022
Wang, J., Kong, H., Zhang, J., Hao, Y., Shao, Z., and Ciucci, F. (2021d). Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 116, 100717. doi:10.1016/j.pmatsci.2020.100717
Wang, J., Zheng, X., Wang, G., Cao, Y., Ding, W., Zhang, J., et al. (2022c). Defective bimetallic selenides for selective CO2 electroreduction to CO. Adv. Mater (2022b) 34 (3), e2106354. doi:10.1002/adma.202106354
Wang, K., Wu, Y., Cao, X., Gu, L., and Hu, J. (2020b). A Zn-CO2 flow battery generating electricity and methane. Adv. Funct. Mater. 30 (9), 1908965. doi:10.1002/adfm.201908965
Wang, Q., and Astruc, D. (2020). State of the art and prospects in metal-organic framework (MOF)-Based and MOF-derived nanocatalysis. Chem. Rev. 120 (2), 1438–1511. doi:10.1021/acs.chemrev.9b00223
Wang, R., Li, X., Nie, Z., Zhao, Y., and Wang, H. (2021e). Metal/metal oxide nanoparticles-composited porous carbon for high-performance supercapacitors. J. Energy Storage 38, 102479. doi:10.1016/j.est.2021.102479
Wang, T., Sang, X., Zheng, W., Yang, B., Yao, S., Lei, C., et al. (2020c). Gas diffusion strategy for inserting atomic iron sites into graphitized carbon supports for unusually high-efficient CO2 electroreduction and high-performance Zn-CO2 batteries. Adv. Mater 32 (29), e2002430. doi:10.1002/adma.202002430
Wang, X., Hu, J.-M., and Hsing, I. M. (2004). Electrochemical investigation of formic acid electro-oxidation and its crossover through a Nafion® membrane. J. Electroanal. Chem. 562 (1), 73–80. doi:10.1016/j.jelechem.2003.08.010
Wang, X., Xie, J., Ghausi, M. A., Lv, J., Huang, Y., Wu, M., et al. (2019). Rechargeable Zn-CO2 electrochemical cells mimicking two-step photosynthesis. Adv. Mater 31 (17), e1807807. doi:10.1002/adma.201807807
Wang, Y.-X., Rinawati, M., Huang, W.-H., Cheng, Y.-S., Lin, P.-H., Chen, K.-J., et al. (2022b). Surface-engineered N-doped carbon nanotubes with B-doped graphene quantum dots: Strategies to develop highly-efficient noble metal-free electrocatalyst for online-monitoring dissolved oxygen biosensor. Carbon 186, 406–415. doi:10.1016/j.carbon.2021.10.027
Wang, Y., Huang, Z., Lei, Y., Wu, J., Bai, Y., Zhao, X., et al. (2022d). Bismuth with abundant defects for electrocatalytic CO2 reduction and Zn-CO2 batteries. Chem. Commun. (Camb) 58 (22), 3621–3624. doi:10.1039/d2cc00114d
Wang, Y., Liu, Y., Liu, W., Wu, J., Li, Q., Feng, Q., et al. (2020d). Regulating the coordination structure of metal single atoms for efficient electrocatalytic CO2 reduction. Energy and Environ. Sci. 13 (12), 4609–4624. doi:10.1039/d0ee02833a
Wu, M., Zhang, G., Yang, H., Liu, X., Dubois, M., Gauthier, M. A., et al. (2021). Aqueous Zn-based rechargeable batteries: Recent progress and future perspectives. InfoMat. doi:10.1002/inf2.12265
Xiang Li, S. Y., Feng, N., He, P., and Zhou, H. (2016). Progress in research on Li-CO2 batteries: Mechanism, catalyst and performance. Chin. J. Catal. 37, 1016–1024. doi:10.1016/S1872-2067(15)61125-1
Xie, J., Huang, Y., Wu, M., and Wang, Y. (2019). Electrochemical carbon dioxide splitting. ChemElectroChem 6 (6), 1587–1604. doi:10.1002/celc.201801716
Xie, J., Wang, X., Lv, J., Huang, Y., Wu, M., Wang, Y., et al. (2018a). Reversible aqueous zinc-CO2 batteries based on CO2-HCOOH interconversion. Angew. Chem. Int. Ed. Engl. 57 (52), 16996–17001. doi:10.1002/anie.201811853
Xie, J., and Wang, Y. (2019). Recent development of CO2 electrochemistry from Li-CO2 batteries to Zn-CO2 batteries. Acc. Chem. Res. 52 (6), 1721–1729. doi:10.1021/acs.accounts.9b00179
Xie, J., Zhao, X., Wu, M., Li, Q., Wang, Y., and Yao, J. (2018b). Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution. Angew. Chem. Int. Ed. Engl. 57 (31), 9640–9644. doi:10.1002/anie.201802055
Xiong, Y., Dong, J., Huang, Z. Q., Xin, P., Chen, W., Wang, Y., et al. (2020). Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15 (5), 390–397. doi:10.1038/s41565-020-0665-x
Xu, H., Cheng, D., Cao, D., and Zeng, X. C. (2018). A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1 (5), 339–348. doi:10.1038/s41929-018-0063-z
Xue Teng, Y. N., Gong, S., Xuan, L., and Chen, Z. (2021). Selective CO2 reduction to formate on heterostructured Sn/SnO2 nanoparticles promoted by carbon layer networks. J. Electrochem. 2022, 27. doi:10.13208/j.electrochem.210844
Xue, X., Yang, H., Yang, T., Yuan, P., Li, Q., Mu, S., et al. (2019). P-coordinated fullerene-like carbon nanostructures with dual active centers toward highly-efficient multi-functional electrocatalysis for CO2RR, ORR and Zn-air battery. J. Mater. Chem. A 7 (25), 15271–15277. doi:10.1039/c9ta03828k
Yan, C., Li, H., Ye, Y., Wu, H., Cai, F., Si, R., et al. (2018). Coordinatively unsaturated nickel-nitrogen sites towards selective and high-rate CO2 electroreduction. Energy and Environ. Sci. 11 (5), 1204–1210. doi:10.1039/c8ee00133b
Yan, S., Peng, C., Yang, C., Chen, Y., Zhang, J., Guan, A., et al. (2021). Electron localization and lattice strain induced by surface lithium doping enable ampere-level electrosynthesis of formate from CO2. Angew. Chem. Int. Ed. Engl. 60 (49), 25741–25745. doi:10.1002/anie.202111351
Yang, D., Chen, D., Jiang, Y., Ang, E. H., Feng, Y., Rui, X., et al. (2020a). Carbon-based materials for all-solid-state zinc-air batteries. Carbon Energy 3 (1), 50–65. doi:10.1002/cey2.88
Yang, D., Zhu, Q., Chen, C., Liu, H., Liu, Z., Zhao, Z., et al. (2019a). Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 10 (1), 677. doi:10.1038/s41467-019-08653-9
Yang, H. B., Hung, S.-F., Liu, S., Yuan, K., Miao, S., Zhang, L., et al. (2018). Atomically dispersed Ni (I) as the active site for electrochemical CO2 reduction. Nat. Energy 3 (2), 140–147. doi:10.1038/s41560-017-0078-8
Yang, H., Chang, Z., Qiao, Y., Deng, H., Mu, X., He, P., et al. (2020b). Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew. Chem. Int. Ed. Engl. 59 (24), 9377–9381. doi:10.1002/anie.202001844
Yang, J., Guo, D., Zhao, S., Lin, Y., Yang, R., Xu, D., et al. (2019b). Cobalt phosphides nanocrystals encapsulated by P-doped carbon and married with P-doped graphene for overall water splitting. Small 15 (10), e1804546. doi:10.1002/smll.201804546
Yang, M., Liu, S., Sun, J., Jin, M., Fu, R., Zhang, S., et al. (2022a). Highly dispersed Bi clusters for efficient rechargeable Zn-CO2 batteries. Appl. Catal. B Environ. 307, 121145. doi:10.1016/j.apcatb.2022.121145
Yang, R., Xie, J., Liu, Q., Huang, Y., Lv, J., Ghausi, M. A., et al. (2019c). A trifunctional Ni-N/P-O-codoped graphene electrocatalyst enables dual-model rechargeable Zn-CO2/Zn-O2 batteries. J. Mater. Chem. A 7 (6), 2575–2580. doi:10.1039/c8ta10958c
Yang, X., Zhang, C., Chai, L., Zhang, W., and Li, Z. (2022b). Bimetallic rechargeable Al/Zn hybrid aqueous batteries based on Al-Zn alloys with composite electrolytes. Adv. Mater 34 (45), e2206099. doi:10.1002/adma.202206099
Ye, K., Zhou, Z., Shao, J., Lin, L., Gao, D., Ta, N., et al. (2020). In situ reconstruction of a hierarchical Sn-Cu/SnOx core/shell catalyst for high-performance CO2 electroreduction. Angew. Chem. Int. Ed. Engl. 59 (12), 4814–4821. doi:10.1002/anie.201916538
Yu, J., Feng, H., Tang, L., Pang, Y., Zeng, G., Lu, Y., et al. (2020). Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Prog. Mater. Sci. 111, 100654. doi:10.1016/j.pmatsci.2020.100654
Yuan, Y., and Lu, J. (2019). Demanding energy from carbon. Carbon Energy 1 (1), 8–12. doi:10.1002/cey2.12
Yujie Shi, Y. Z., and Lou, Y. (2022). * zupeng chen, haifeng Xiong, and yongfa Zhu*. Homogeneity of supported single-atom active sites boosting the selective catalytic transformations. Adv. Sci. (Weinh) 55, 40. doi:10.1002/advs.202201520
Zeng, Z., Mohamed, A. G. A., Zhang, X., and Wang, Y. (2021). Wide potential CO2-to-CO electroreduction relies on pyridinic-N/Ni-nx sites and its Zn-CO2 battery application. Energy Technol. 9 (8), 2100205. doi:10.1002/ente.202100205
Zhang, L., Han, L., Liu, H., Liu, X., and Luo, J. (2017). Back cover: Potential-cycling synthesis of single platinum atoms for efficient hydrogen evolution in neutral media (angew. Chem. Int. Ed. 44/2017) Angew. Chem. Int. Ed. 56 (44), 13900. doi:10.1002/anie.201709950
Zhang, N., Zhang, X., Tao, L., Jiang, P., Ye, C., Lin, R., et al. (2021a). Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction. Angew. Chem. Int. Ed. Engl. 60 (11), 6170–6176. doi:10.1002/anie.202014718
Zhang, S., Wang, J., Wang, J., Wang, K. Y., Zhao, M., Zhang, L., et al. (2022). A gradient Sn4+@Sn2+ core@shell structure induced by a strong metal oxide-support interaction for enhanced CO2 electroreduction. Dalton Trans. 51 (42), 16135–16144. doi:10.1039/d2dt02788g
Zhang, Y., Jiao, L., Yang, W., Xie, C., and Jiang, H. L. (2021b). Rational fabrication of low-coordinate single-atom Ni electrocatalysts by MOFs for highly selective CO2 reduction. Angew. Chem. Int. Ed. Engl. 60 (14), 7607–7611. doi:10.1002/anie.202016219
Zhao, D., Zhu, Y., Wu, Q., Zhou, W., Dan, J., Zhu, H., et al. (2022a). Low-loading Ir decorated cobalt encapsulated N-doped carbon nanotubes/porous carbon sheet implements efficient hydrogen/oxygen trifunctional electrocatalysis. Chem. Eng. J. 430, 132825. doi:10.1016/j.cej.2021.132825
Zhao, M., Feng, J., Yang, W., Song, S., and Zhang, H. (2020). Recent advances in graphitic carbon nitride supported single-atom catalysts for energy conversion. ChemCatChem 13 (5), 1250–1270. doi:10.1002/cctc.202001517
Zhao, S. N., Li, J. K., Wang, R., Cai, J., and Zang, S. Q. (2022b). Electronically and geometrically modified single-atom Fe sites by adjacent Fe nanoparticles for enhanced oxygen reduction. Adv. Mater 34 (5), e2107291. doi:10.1002/adma.202107291
Zheng, W., Yang, J., Chen, H., Hou, Y., Wang, Q., Gu, M., et al. (2019). Atomically defined undercoordinated active sites for highly efficient CO2 electroreduction. Adv. Funct. Mater. 30 (4), 1907658. doi:10.1002/adfm.201907658
Zhong, C., Liu, B., Ding, J., Liu, X., Zhong, Y., Li, Y., et al. (2020). Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc–manganese dioxide batteries. Nat. Energy 5 (6), 440–449. doi:10.1038/s41560-020-0584-y
Zhou, J., Cheng, J., Wang, B., Peng, H., and Lu, J. (2020). Flexible metal-gas batteries: A potential option for next-generation power accessories for wearable electronics. Energy and Environ. Sci. 13 (7), 1933–1970. doi:10.1039/d0ee00039f
Zhou, Y., Chen, G., Wang, Q., Wang, D., Tao, X., Zhang, T., et al. (2021). Fe-N-C electrocatalysts with densely accessible Fe-N4 sites for efficient oxygen reduction reaction. Adv. Funct. Mater. 31 (34), 2102420. doi:10.1002/adfm.202102420
Zhou, Z.-Y., and Sun, S.-G. (2017). A breakthrough in electrocatalysis of CO2 conversion. Natl. Sci. Rev. 4 (2), 155–156. doi:10.1093/nsr/nww083
Zhu, Y., Pan, Y., Zhu, Y., Jiang, H., Shen, J., and Li, C. (2021a). Efficient electrocatalytic formic acid oxidation over PdAu-manganese oxide/carbon. J. Colloid Interface Sci. 593, 244–250. doi:10.1016/j.jcis.2021.02.110
Zhu, Y., Yuk, S. F., Zheng, J., Nguyen, M. T., Lee, M. S., Szanyi, J., et al. (2021b). Environment of metal-O-Fe bonds enabling high activity in CO2 reduction on single metal atoms and on supported nanoparticles. J. Am. Chem. Soc. 143 (14), 5540–5549. doi:10.1021/jacs.1c02276
Keywords: Zn–CO2 batteries, multifunctional catalysts, reaction mechanism, catalyst design strategies, single-atom catalysts
Citation: Guo W, Wang Y, Yi Q, Devid E, Li X, Lei P, Shan W, Qi K, Shi L and Gao L (2023) Research progress of aqueous Zn–CO2 battery: design principle and development strategy of a multifunctional catalyst. Front. Energy Res. 11:1194674. doi: 10.3389/fenrg.2023.1194674
Received: 27 March 2023; Accepted: 16 May 2023;
Published: 31 May 2023.
Edited by:
Xiahui Zhang, Central South University, ChinaCopyright © 2023 Guo, Wang, Yi, Devid, Li, Lei, Shan, Qi, Shi and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuelian Li, lixuelian@tyut.edu.cn; Lili Gao, gaolili@tyut.edu.cn
†These authors have contributed equally to this work and share first authorship
 Wenqi Guo
Wenqi Guo Yukun Wang
Yukun Wang Qun Yi1,3
Qun Yi1,3  Xuelian Li
Xuelian Li Kai Qi
Kai Qi