- 1Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Eight-Year Program of Clinical Medicine, Peking Union Medical College, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China
- 3Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Objective: Double pituitary adenomas (DPA) are a rare clinical condition, and our knowledge of them is limited. Missing the second lesion leading to incomplete biochemical remission after surgery is an important challenge in DPA management. This study aims to analyze independent prognostic factors in DPA patients and summarize clinical experiences to prevent surgical failure.
Methods: Two cases of DPA patients with Cushing’s disease diagnosed and surgically treated at Peking Union Medical College Hospital are reported. A literature review was performed on the online database Pubmed, and 57 DPA patients from 22 retrieved articles were included. Demographic characteristics, endocrine manifestations, diagnostic methods, tumor size, and immunohistochemical features of 59 patients were analyzed. Binary logistic regression models were used to identify independent prognostic factors affecting postoperative biochemical remission.
Results: Among 59 DPA patients, the mean ± SD age was 43.64 ± 14.42 years, with 61.02% being female (n = 36). The most common endocrine manifestations were Cushing’s syndrome (23/59, 38.98%) and acromegaly (20/59, 33.90%). The most prevalent immunohistochemical types were ACTH-immunopositive (31/118, 26.27%) and GH-immunopositive (31/118, 26.27%) tumors. Microadenomas (<1cm) were the most frequent in terms of tumor size (62/92, 67.39%). The detection rate for double lesions on 3.0T MRI was 50.00% (14/28), which significantly higher than 1.5T MRI (P = 0.034). Univariate analysis revealed that female, Cushing’s syndrome and only single lesion detected by surgical exploration were associated with significantly worse prognosis (P<0.05). Multivariate analysis identified double lesion detected by surgical exploration (OR = 0.08, P = 0.003) and contiguous type tumor (OR = 0.06, P = 0.017) as independent protective factors for DPA patients.
Conclusions: The double lesion detected by surgical exploration is independently associated with a better prognosis for DPA patients. Comprehensive intraoperative exploration are crucial measures to avoid missing causative lesions.
Introduction
Pituitary adenoma is the third most common intracranial tumor, following glioma and meningioma, accounting for about 15% of intracranial tumors (1, 2). Pituitary adenomas are the predominant pathological type of masses in the sellar and suprasellar region, accounting for up to 90% of cases (3). Clinically, pituitary adenomas present with endocrine symptoms associated with inappropriate pituitary hormone secretion as well as local mass effects. Typically benign, monoclonal, and slow-growing solid tumors, pituitary adenomas rarely exhibit invasive or malignant behavior (4). The estimated incidence of pituitary adenomas is approximately 25 cases per 100,000 individuals (5), with the vast majority of patients having a solitary tumor. The occurrence of two pituitary adenomas in one single patient is extremely low, with a prevalence of 0.2-2.6% among pituitary adenoma surgical cohorts (6).
Double pituitary adenoma (DPA) is defined as the synchronous presence of two distinct pituitary adenomas in the pituitary gland, exhibiting differences in morphology, immunohistochemistry, and ultrastructural features. They can be either two separate masses that exist independently of each other in the pituitary gland (separated type) or a single gross lesion with a histologically well-delineated border to distinguish two distinct cell populations (contiguous type). Notably, the concept of DPA should be distinguished from a plurihormonal adenoma, which is a single tumor capable of secreting two or more pituitary hormones simultaneously, composed of one homogeneous and histologically-uniform tumor cell population. The clinical management of patients with DPA presents significant challenges, including low imaging detection rates leading to diagnostic difficulties, challenges in achieving complete resection of pituitary adenoma tissue during surgery, and the potential risk for secondary surgery due to postoperative biochemical non-remission. Therefore, paying more attention to this rare clinical scenario is necessary.
Although there are review articles for DPA, to our knowledge, few authors have summarized this uncommon disease in a systematic way, especially the lack of prognostic analysis of clinical outcomes. In this article, we reported 2 patients with DPA presenting as Cushing’s syndrome and selected 57 cases of functional DPA from the previous literature. Finally, we conducted a systematic review of 59 functional DPA, summarizing their demographics, clinical features, and tumor characteristics, analyzing prognostic factors related to postoperative biochemical remission, and providing insights into diagnostic and therapeutic management practices for patients with DPA.
Cases presentation
Case 1
A 35-year-old female patient was admitted to the hospital due to a two-year history of facial roundness and redness. She exhibits the clinical features of classic hypercortisolism, including unexplained significant weight gain, acne on the face and back, and thinning of the skin with bruising. Symptoms exhibited seasonal fluctuations, with more severe manifestations in winter. Physical examination revealed signs of Cushing’s syndrome, including central obesity, moon face, buffalo hump, plethora, supraclavicular fat pad, increased vellus hair, and abdominal striae.
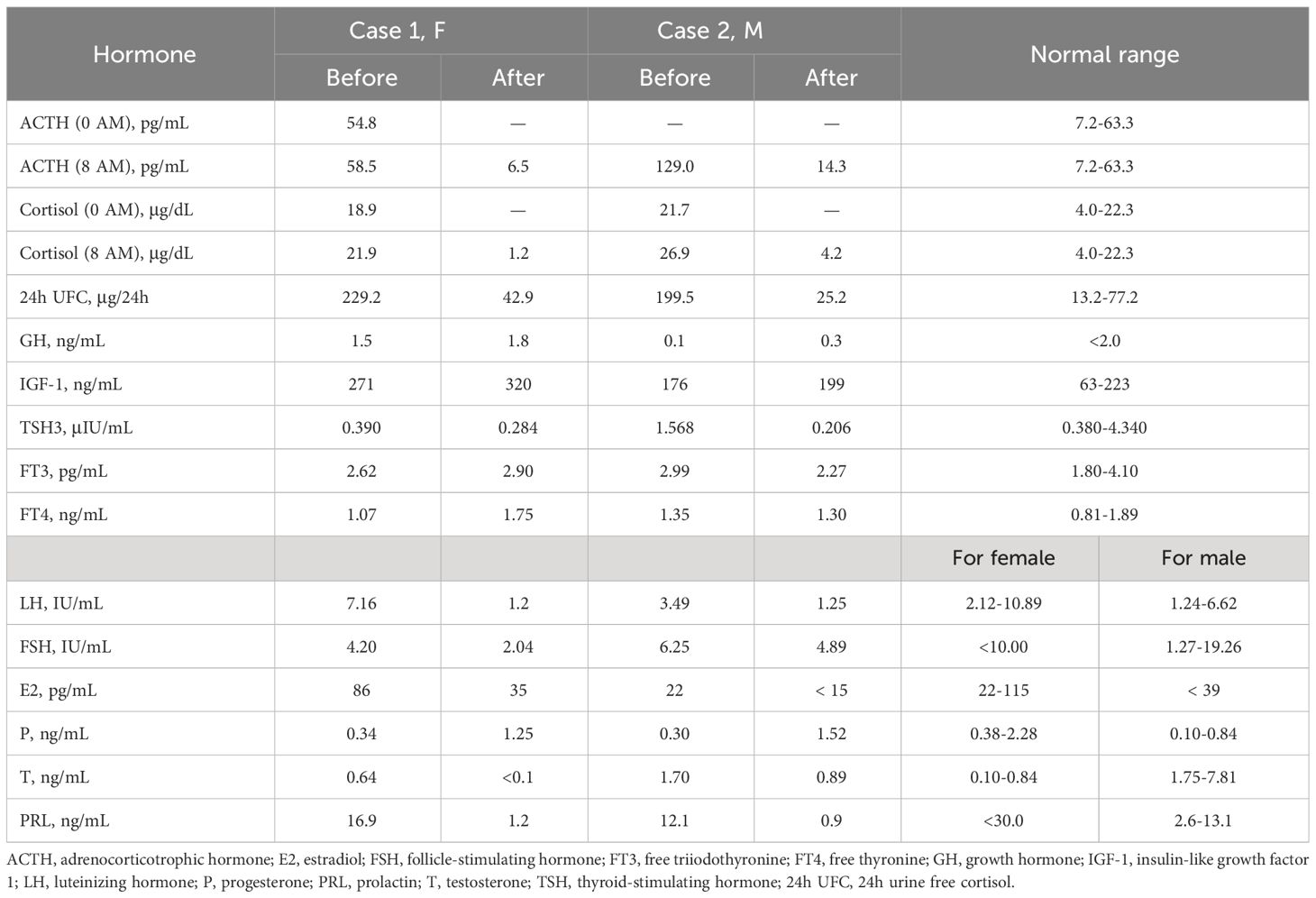
Biochemical results are shown in Table 1. Biochemical examination revealed elevated serum cortisol (21.9 μg/dL) and adrenocorticotrophic hormone (ACTH) levels (58.5 pg/mL) with the disappearance of circadian rhythm. The low-dose dexamethasone suppression test (LDDST) showed incomplete suppression of both blood cortisol and 24-hour urinary free cortisol (24h UFC). Continuous monitoring of 24h UFC revealed significant fluctuations (range: 347.7-992.3 μg/24h) and a “triple peak and double trough” pattern, confirming cyclic Cushing’s syndrome.
The high-dose dexamethasone suppression test (HDDST) completely suppressed serum cortisol and 24h UFC levels, supporting the diagnosis of Cushing’s disease. Dynamic contrast-enhanced MRI of the pituitary showed hypo-enhanced signal intensity on both sides of the pituitary gland, about 11.3 × 5.5 mm on the right side and 3 × 3.5 mm on the left side, which was considered as double pituitary adenomas (Figures 1A–C). Bilateral inferior petrosal sinus sampling (BIPSS) supported the left side as the dominant ACTH-secreting side.
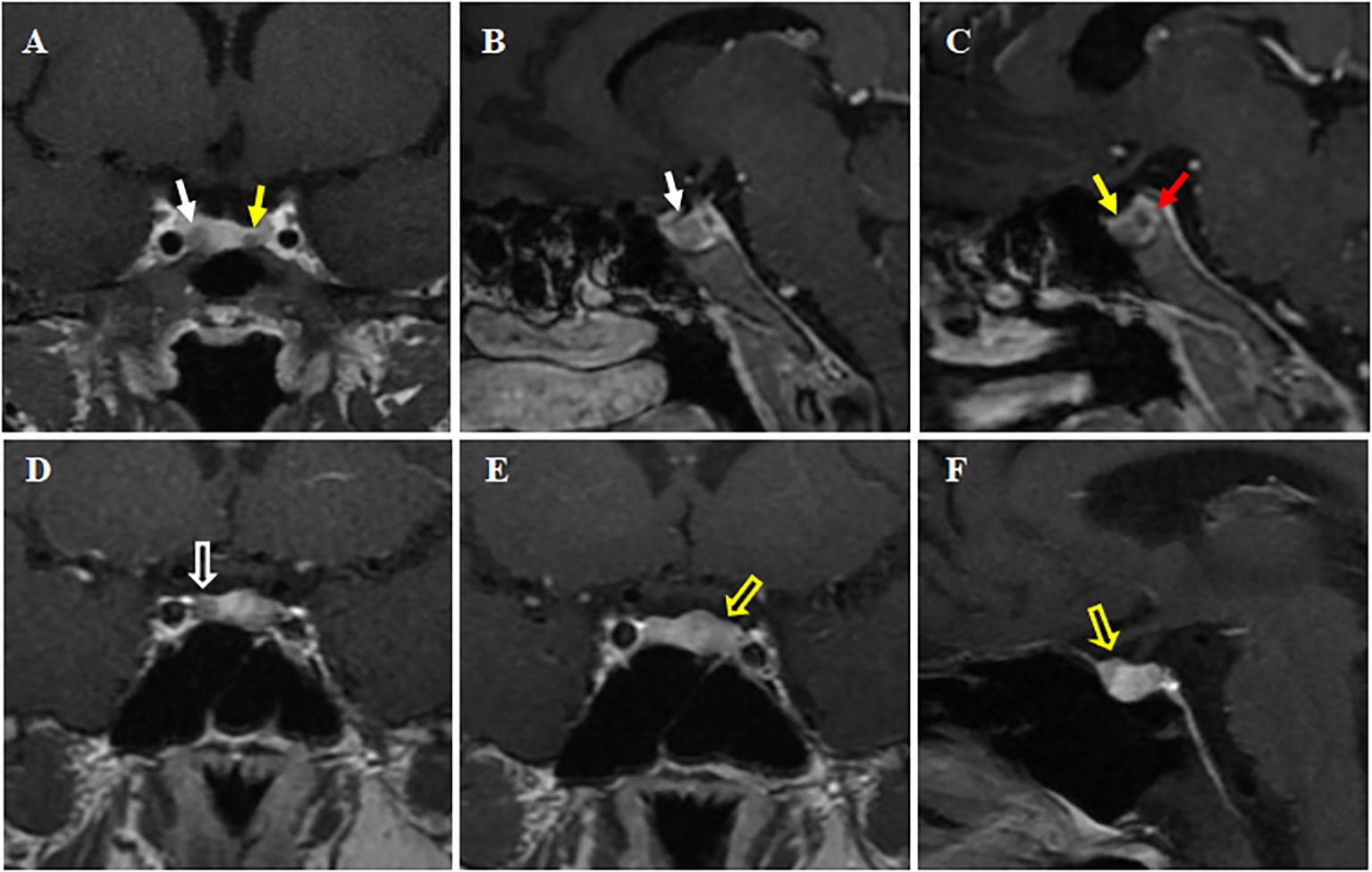
Figure 1 Preoperative dynamic contrast-enhanced MRI of the pituitary. (A-C) Case 1. The pituitary gland demonstrates heterogeneous enhancement, with bilateral areas of hypointense signal. Larger (about 11.3 × 5.5 mm) on right side (white solid arrows, (A, B); Smaller (about 3 × 3.5 mm) on left side (yellow solid arrow, A, C). A red solid arrow points to a non-enhancing area, representing a posterior pituitary cyst (C). (D-F) Case 2. A rounded hypo-enhanced signal about 5 mm in diameter is seen on the right side (white hollow arrow, in D), and a patchy area of reduced enhancement is observed on the left anterior side (yellow hollow arrows, in E, F). All A-F are post-contrast T1-weighted sequences. Coronal views in (A, D, E); Sagittal views in (B, C, F).
Preoperative evaluation showed normal anterior pituitary function except for the adrenal axis, and Cushing’s syndrome complications were managed symptomatically. In addition, we noticed the mild elevation of IGF-1 despite normal GH and performed glucose suppression test, which showed that GH could be completely suppressed and did not support a GH-secreting adenoma. The elevated IGH-1 was considered to be associated with long-term hypercortisolism. Ophthalmologic evaluation, including visual acuity and dynamic visual field test, revealed no abnormalities.
The patient underwent sellar lesions resection and sellar floor reconstruction through the neuroendoscopic endonasal transsphenoidal approach. Two soft gray-white tumors were found on the right and left sides of the intrasellar region and completely removed during the operation. A thorough endoscopic exploration of the sellar region was performed. Immunohistochemical examination confirmed strong positive ACTH staining and the expression of T-pit transcription factor in the left-sided adenoma, while the right-sided adenoma showed negative staining for any pituitary hormones and expressed the SF-1 transcription factor (Figure 2).
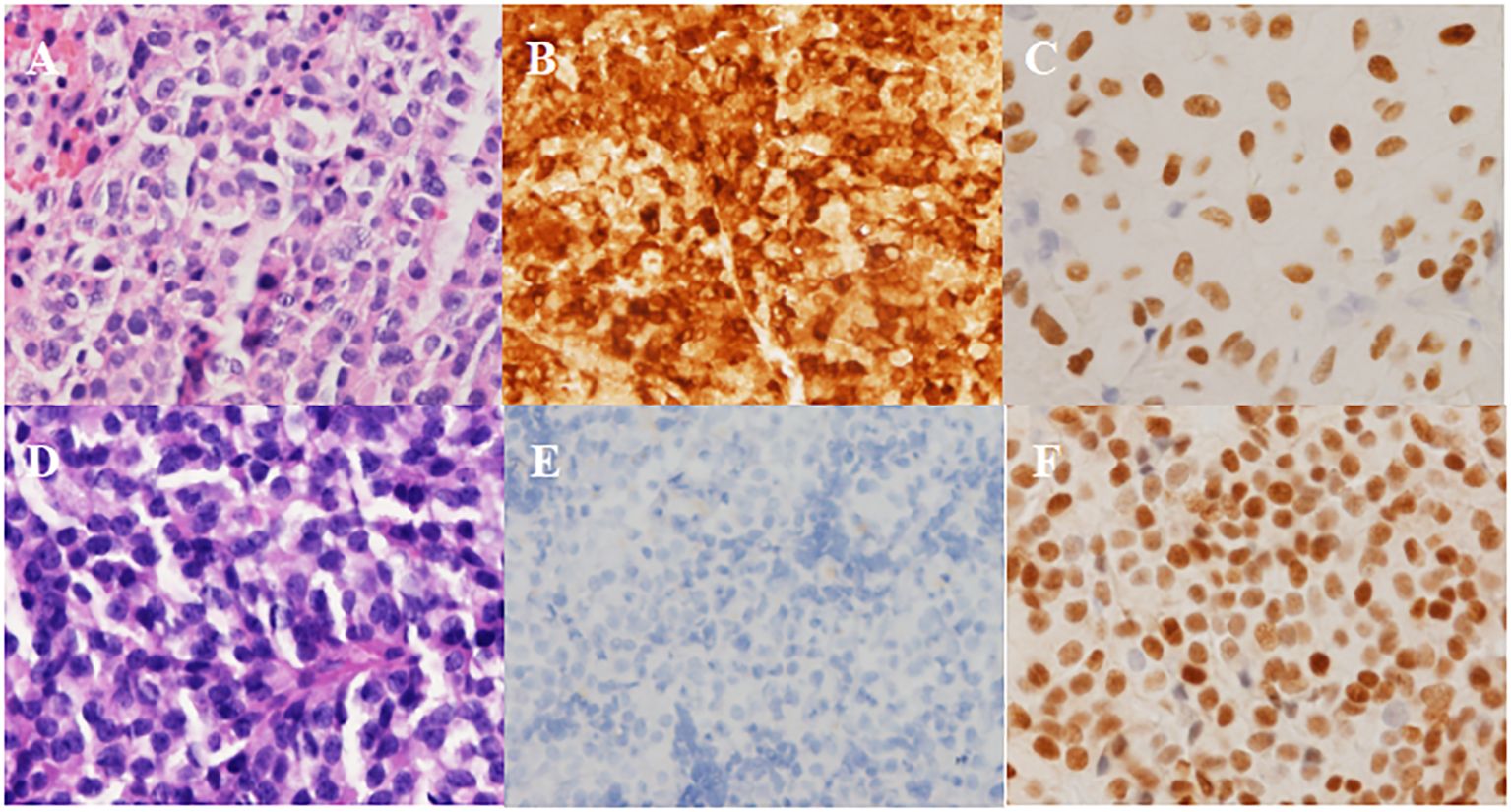
Figure 2 Pathological results in Case 1. (A-C) Left-side adenoma: strongly positive staining for ACTH and positive for T-pit. (A) H&E, (B) ICH for ACTH, (C) ICH for T-pit. (D-F) Right-side adenoma: negative staining for ACTH and positive for SF-1. (D) H&E, (E) ICH for ACTH, (F) ICH for SF-1. Magnification × 200.
On day 2 after the operation, a follow-up assessment of anterior pituitary function was conducted, and the patient achieved complete biochemical remission with significant decreases in serum ACTH, cortisol, and 24h UFC levels. Postoperative oral hydrocortisone replacement therapy was given because of hypopituitarism.
Case 2
A 35-year-old man was admitted to the hospital with complaints of unexplained weight gain for 4 years, with a weight increase of 25 kg in the last year. Three years ago, he noticed facial rounding and redness, abdominal striae, skin prone to bruising, and elevated blood pressure reaching a maximum of 180/120 mmHg. In the last 3 months, he experienced polydipsia, polyuria, lower limb and eyelid edema, and reduced sexual function, and was diagnosed as diabetes mellitus and hyperlipidemia. Physical examination demonstrated typical signs of Cushing’s syndrome, including central obesity, moon face, buffalo hump, thickened fat pad above the clavicles, abdominal and thigh striae, and limb bruising.
Biochemical tests revealed a loss of circadian rhythm in cortisol, which was as high as 26.9 μg/dL at 8 AM, with an elevated 24-h UFC of 199.5 μg/24 h and an elevated ACTH of 129.0 pg/mL. LDDST revealed non-suppressed cortisol levels (26.4 μg/dL). BIPSS supported a diagnosis of Cushing’s disease with left-side dominance. Dynamic contrast-enhanced pituitary MRI revealed a reduced enhancing area on the anterior left wing of the pituitary measuring approximately 5 × 3.5 mm, and another reduced enhancing area about 5mm in diameter on the right wing, suggesting the presence of double pituitary adenomas (Figures 1D–F).
Preoperative evaluation of other pituitary hormones showed no significant abnormality. Ophthalmological evaluation ruled out visual impairment and visual field defects.
The patient underwent sellar lesions resection and sellar floor reconstruction through neuroendoscopic endonasal transsphenoidal approach. Intraoperative pituitary adenomas on both sides were explored and found, completely excised and sent for pathologic examination separately. The immunohistochemical results confirmed that both pituitary adenomas stained positive for ACTH and expressed the T-pit transcription factor (Figure 3).
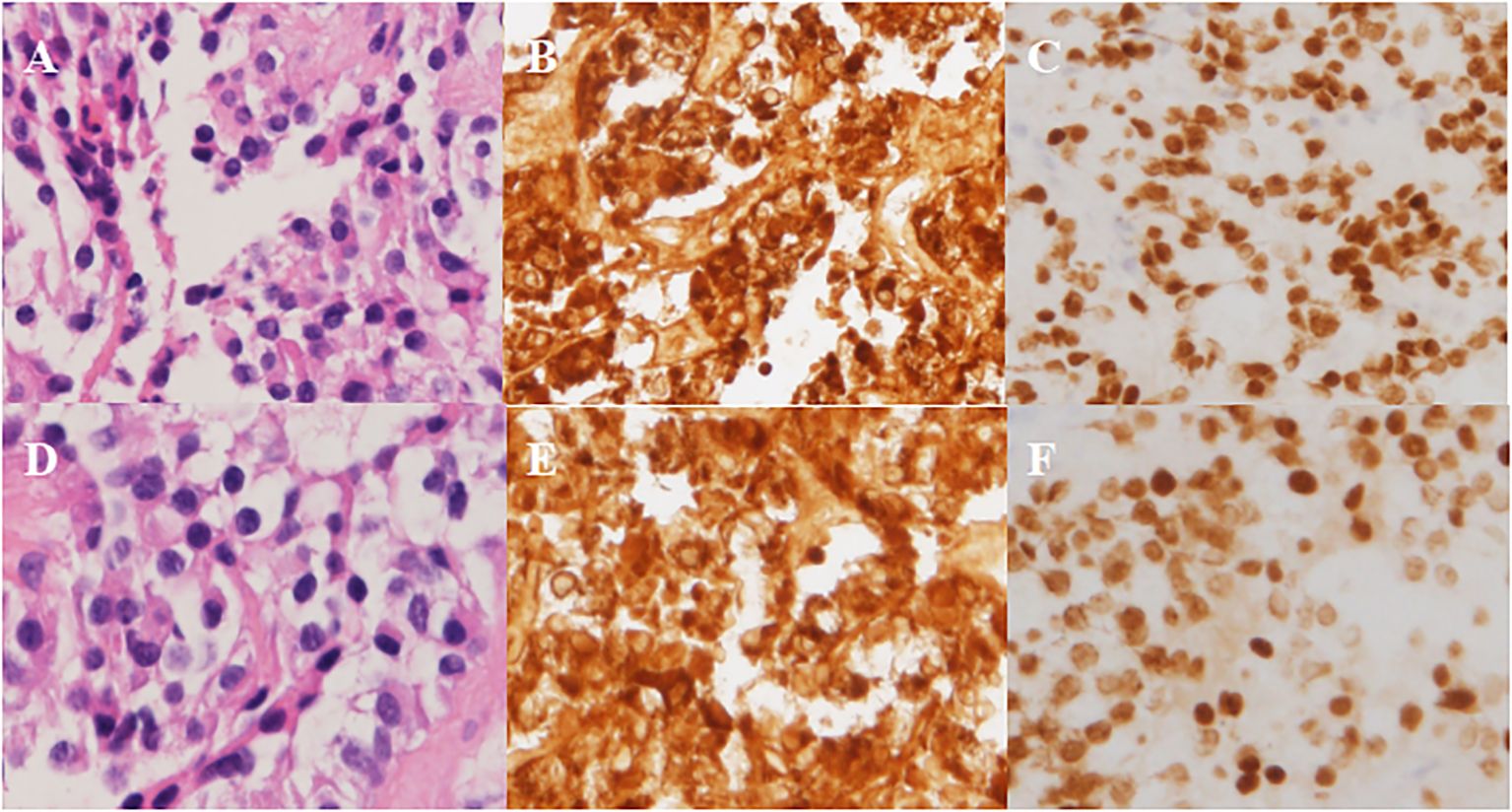
Figure 3 Pathological results in Case 2. (A-C) Left-side adenoma: positive staining for ACTH and T-pit. (A) H&E, (B) ICH for ACTH, (C) ICH for T-pit. (D-F) Right-side adenoma: positive staining for ACTH and T-pit. (D) H&E, (E) ICH for ACTH, (F) ICH for T-pit. Magnification × 200.
Day 3 after surgery, cortisol, ACTH, and 24h UFC levels had normalized, achieving complete biochemical remission. The patient’s pituitary function largely normalized, with a slight decrease in testosterone levels, and no subsequent replacement therapy was administered.
Methods
Search strategy and selection criteria
The online database Pubmed was searched using the keywords “double pituitary adenomas”, “bilateral pituitary adenomas” and “ concurrent pituitary adenomas”, with a publication date range from January 1990 to June 2023 and the article type was limited to case reports. After reviewing the titles and abstracts of the retrieved results, 28 articles were selected for further full-text assessment for inclusion. The following articles were excluded: (1) articles not published in English, (2) no surgical treatment was performed, (3) no hormonal abnormalities or related endocrine symptoms prior to surgery, (4) no mention of postoperative biochemical remission results in the text, (5) two tumors that were not concurrent, namely recurrent pituitary adenomas. Finally, 57 patients from 22 articles (7–28) met the inclusion criteria, adding 2 patients reported in the paper, a total of 59 patients with functional pituitary adenomas were included in the systematic review.
Statistical analysis
Continuous variables were described as mean ± standard deviation (SD). Categorical variables were described as numbers and percentages. The statistical significance in a 2 × 2 table was evaluated using Pearson’s chi-squared test or Fisher’s exact test. In the multivariate analysis, multivariate logistic regression was used to identify the independent prognostic factors. Two-tailed tests were used, and P-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 26 (IBM Corp., Armonk, New York) and GraphPad Prism 9 (GraphPad Software, California, USA).
Results
Demographics
Among the 59 cases of functional DPA, 61.02% (n = 36) were female patients, demonstrating a slight female predominance. The mean ± SD age at diagnosis was 43.64 ± 14.42 years. Age data showed that the patients with functional DPA tended to be diagnosed in the fourth decade of life, followed by in the sixth and third decades (Figure 4A). The age at diagnosis showed no statistically significant difference between genders (43.61 ± 13.99 years for male vs 43.67 ± 14.88 years for female). The majority of functional DPA patients were sporadic cases, but 8.5% of patients (n = 5) had a hereditary background, including 3 cases of familial multiple endocrine neoplasia type 1 (MEN 1) and 2 cases of familial pituitary adenoma unrelated to MEN 1.
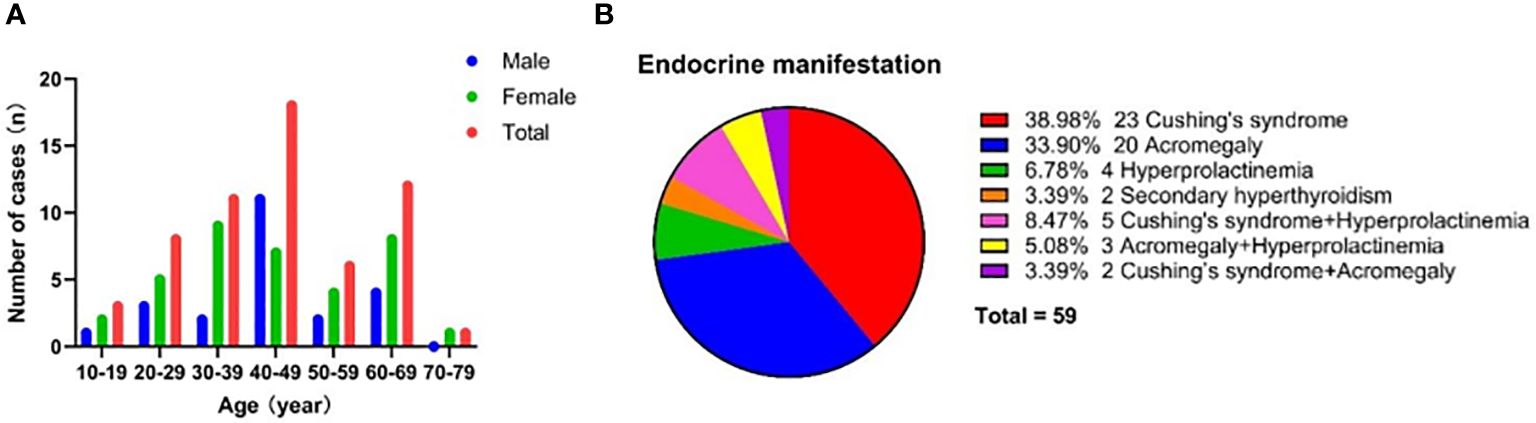
Figure 4 Demographic and clinical characteristics for patients with DPA. (A) Age and gander distribution for DPA patients. (B) Endocrine manifestations for DPA patients.
Clinical manifestations
The endocrine manifestations of functional DPA patients are summarized in Figure 4B. The most prevalent clinical manifestation was Cushing’s syndrome (n = 23, 38.98%), followed by acromegaly (n = 20, 33.90%). Furthermore, only a minority of patients presented with hyperprolactinemia (n = 4, 6.78%) or secondary hyperthyroidism (n = 2, 3.39%). It was worth noting that 16.95% of patients (n = 10) had two types of endocrine symptoms simultaneously, which may be due to the fact that both double pituitary adenomas had secreting function or one of the adenomas was a plurihormonal adenoma. Hyperprolactinemia tended to manifest as an incidental symptom coexisting with Cushing’s syndrome or acromegaly.
Diagnosis
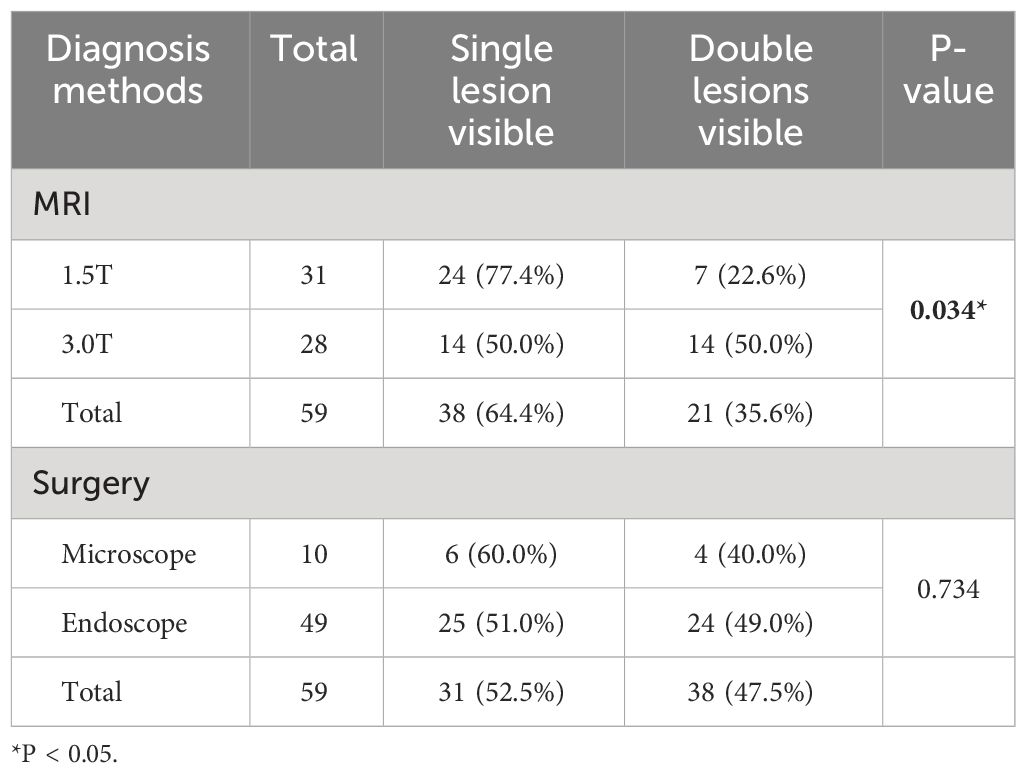
The diagnostic methods and detection rates of DPA are shown in Table 2. Twenty-one cases of DPA were diagnosed by preoperative MRI, with an total detection rate of 35.6%. The use of higher field strength MRI was beneficial in improving the DPA detection rate. 3.0T MRI had a detection rate of 50.0%, which was significantly higher than that of 1.5T MRI of 22.6%, and the difference reached a statistically significant level (P = 0.034). The DPA detection rate of surgical exploration was 47.5%, which was slightly higher than that of preoperative MRI, indicating that some MRI-negative adenomas were detected under direct surgical visualization. No statistically significant difference was seen when comparing the detection rates of the two surgical approaches, using endoscope and microscope (P = 0.734).
Of all 59 cases of DPA, the separated type accounted for 46 cases (78.0%) and the contiguous type accounted for 13 cases (22.0%). It is noteworthy that because the contiguous type DPA presented as a whole mass in appearance, neither MRI examination nor surgical exploration could detect the double lesions. All 13 cases of contiguous type DPA were definitively diagnosed on postoperative pathological examination.
Tumor characteristics
Figure 5 summarizes the tumor size and immunohistochemical characteristics of DPA. Among the 92 adenomas for which tumor size data were available in case reports, approximately two-thirds were microadenomas (n = 62, 67.39%), while about one-third were macroadenomas (n = 30, 32.61%). There are currently no reported cases of giant adenomas. The most common tumor size combination pattern for DPA is Micro+Micro (n = 23, 60.53%), followed by Micro + Macro (n =10, 26.32%), while Macro+Macro is the least common (n = 5, 13.16%).

Figure 5 Tumor characteristics for patients with DPA. (A) Tumor size for single adenoma and combination pattern for each DPA patient. Tumor size was assessed through MRI, surgery, or pathological examination. 38 cases reported sizes for both double pituitary adenomas, while another 16 cases reported size for only one of the double pituitary adenomas. (B) Pituitary hormone immunohistochemical staining of single adenoma and combination patterns for each DPA patients. ICH, immunohistochemistry. (C) Cell lineage for single adenoma and combination pattern for each DPA patient.
In terms of immunohistochemistry, among the 118 adenomas in 59 patients, the most common were ACTH-immunopositive (n = 31, 26.27%) and GH-immunopositive tumors (n = 31, 26.27%). The majority of these adenomas exhibited secretion activity (29/31 for ACTH and 28/31 for GH). PRL-immunopositive and FSH/LH-immunopositive adenomas accounted for 19.49% (n = 23) and 11.86% (n = 14), respectively, although they tended to be silent, with only eight PRL adenomas and one FSH/LH adenoma having secretory function. Notably, a proportion of DPA patients had plurihormonal adenomas (n = 11, 18.6%), the most common of which was GH-PRL mixed adenomas (n =4). The most frequent immunohistochemical combination patterns were ACTH+PRL (n = 15, 25.42%) and GH+LH/FSH (n = 9, 15.25%), typically associated with secretion in the former and silence in the latter.
According to the WHO 2022 classification of pituitary neuroendocrine tumors, they are categorized into three cell lineages: Pit-1 (GH-PRL-TSH adenomas), T-pit (ACTH adenomas), and SF1 (FSH-LH adenomas), accounting for 51.69% (n = 61), 26.27% (n = 31) and 11.86% (n = 14), respectively. The common combination patterns include Pit-1 + T-pit (n = 22, 37.29%), Pit-1 + SF1 (n = 12, 20.34%), and double Pit-1 (n =10, 16.95%).
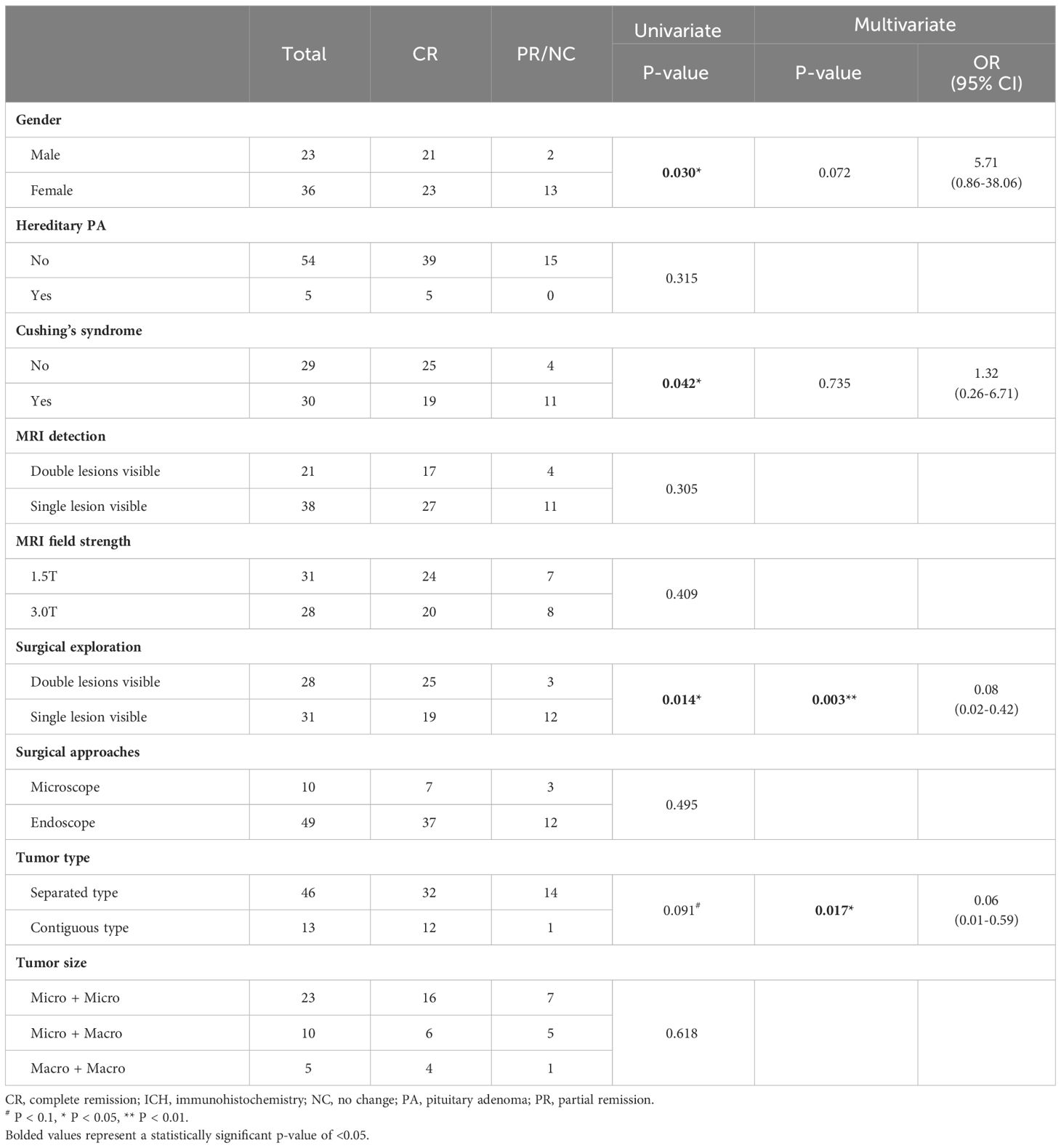
Clinical outcomes and prognostic factors
In the cohort of 59 DPA patients, the postoperative biochemical complete remission (CR) rate was 74.6% (n = 44), while 25.4 of patients (n = 15) achieved only biochemical partial remission (PR) or no change (NC), requiring ongoing medication or secondary surgery. Gender, hereditary PA, clinical features, MRI detection and field strength, surgical exploration and approach, tumor type, and tumor size were assessed as prognostic factors for their correlation with postoperative biochemical remission (Table 3). Immunohistochemistry and cell lineage were not included in the analysis due to the extensive variety of categories. In the univariate analysis, it was observed that female patients (P = 0.030) and patients presenting with Cushing’s syndrome (P = 0.042) had a worse prognosis, while double lesions were detected by surgical exploration exhibited a significantly better prognosis (P = 0.014). Contiguous type DPA also showed a tendency toward a more favorable prognosis, although statistical significance was not reached (P = 0.091). The four prognostic factors (gender, Cushing’s syndrome, surgical exploration, and tumor type) with a P-value of less than 0.1 in the univariate analysis were included in binary logistic regression. The results of the multivariate analysis demonstrated that the contiguous type (OR = 0.06, P = 0.017) and surgical exploration (OR = 0.08, P = 0.003) were independent protective factors for the prognosis of DPA patients.
Discussion
DPA is a rare clinical condition, and its incidence is one of the current research concerns. The incidence of DPA from randomly selected autopsy material ranges from 7.0-10.5% of adenomatous pituitary (29–31). The incidence of DPA estimated from surgical specimens varies over a wide range, from 0.16-2.6% has been reported (13, 16, 32, 33). The prevalence of DPA was higher in the surgical cohort of patients with Cushing’s disease, with 3.3% of 660 patients having double lesions (9). Comparing incidence from autopsy and surgical cohorts, we suggest that DPAs are not actually as rare in pituitary adenomas as they are seen clinically, but that the majority of DPAs are small-sized and non-functioning subclinical cases, leading to a significant underestimation of DPA incidence in surgical cohorts.
In this study, the mean age at diagnosis of DPA was 43.64 years, with a peak age of the fourth decade of life and a male-to-female ratio of approximately 2:3, suggesting that the age-sex profile of DPA is similar to that of the common solitary pituitary adenoma (34). Previous studies have concluded that Cushing’s disease and acromegaly are the most common clinical manifestations of DPA (6, 32), and this paper reconfirms this finding. The most frequent immunohistochemical types of DPA have reached a preliminary consensus (ACTH- and GH-immunopositive for single adenoma, ACTH+PRL and GH+FSH/LH for combination pattern), as reported in both this study and previous literature (35). Systematic summarization of clinical DPA cases revealed that PRL-immunopositive tumors tend to be silent in the setting of DPA, the mechanism of which is currently unknown. In contrast to surgical series, studies based on autopsy material have suggested that PRL-immunopositivity is the most common immunohistochemical type (29, 36). Therefore, it is hypothesized that PRL-immunopositive tumors have a high incidence in DPA but are typically distributed as silent forms in subclinical cases. Regarding tumor size, the traditional view based on autopsy series is that almost all DPAs are microadenomas (29, 37). However, this study found that macroadenomas accounted for a significant proportion (32.6%), which reflects the difference between subclinical and clinical cases.
In clinical practice, it is essential for clinicians to enhance their awareness of this rare condition — DPA, be vigilant for clinical scenarios suggestive of DPA, and identify patients at high risk for hidden dual lesions. The presence of two distinct endocrine manifestations or markedly elevated levels of two pituitary hormones is a crucial clue indicating DPA. Although hyperprolactinemia often manifests as an incidental symptom coexisting with Cushing’s syndrome or acromegaly, it must be distinguished from the pituitary stalk effect, with serum prolactin levels above 200 ng/mL supporting a primary prolactinoma diagnosis (38). Moreover, the elevation of two hormones may also result from a plurihormonal adenoma that simultaneously secretes both hormones (25), which requires confirmation upon pathological examination. Case reports have shown that bromocriptine treatment for prolactinomas may yield incomplete responses, where serum prolactin levels decreased but MRI did not show the expected degree of shrinkage of the tumor (39, 40). This phenomenon may be attributed to DPA, where the tumor visible on MRI consists partly or entirely of non-prolactinoma components, leading to poor response to medical treatment. In Cushing’s disease, if BIPSS suggests a dominant side inconsistent with the tumor’s MRI location, further evaluation is needed to avoid missing a hidden second lesion. In these specific clinical scenarios, clinicians should promptly consider the possibility of DPA and perform MRI reading and intraoperative exploration with extra care to achieve an early diagnosis.
The conventional perspective holds that the preoperative identification of DPA through imaging is challenging (8, 9, 18, 21), and this can be attributed to several factors: (1) Most DPAs typically include at least one small-sized adenoma (6), and (2) Cushing’s disease is the most common clinical manifestation of DPA, but MRI-negative tumors are more common among ACTH-secreting tumors (41, 42). Currently, the standard imaging method for diagnosing pituitary adenomas is dynamic contrast-enhanced pituitary MRI with thin-layer scanning and 3.0T field strength. In this study, we found 3.0T MRI significantly improved the detection rate of DPA compared with 1.5T MRI. This suggests that preoperative imaging detection of DPA depends on the development and clinical application of advanced imaging technologies. In addition to higher field strength, novel sequences and functional imaging fusion are also promising ways to improve image quality. Previous literature has demonstrated that using spoiled gradient recalled acquisition (SPGR) sequences can enhance the spatial resolution and sensitivity of MRI for the detection of ACTH-secreting adenomas, albeit with a mild loss of specificity (21, 43). What’s more (11).C-MET-PET/3.0T MRI showed higher sensitivity in the detection of ACTH-secreting pituitary microadenomas compared to traditional and dynamic MRI, which is an emerging imaging technology for future DPA diagnosis (44, 45).
Despite the use of 3.0T MRI, half of the DPA cases are not diagnosed on preoperative MRI. Therefore, preoperative imaging results should not be blindly trusted, and comprehensive intraoperative pituitary exploration is an important prerequisite for complete resection of double lesions. The importance for intraoperative exploration is further emphasized by the present study, which demonstrated double lesions detected by intraoperative exploration was an independent prognostic factor for postoperative biochemical remission. Preoperative MRI should be used as an imaging tool to guide intraoperative exploration by providing location information, but intraoperative exploration under direct visualization is an indispensable step regardless of how many lesions are detected by MRI. As mentioned above, elevated levels of two pituitary hormones, poor response of bromocriptine treatment, inconsistency between the dominant side of BIPSS and MRI, or pituitary abnormalities of undetermined significance on imaging should be considered as high-risk features for DPA. For high-risk patients, intraoperative exploration should be extra careful to exclude lesion missed. Pituitary surface exploration can largely reduce the omission of second lesions, as more than 50% of small tumors are distributed on the pituitary surface (46). Pituitary incisional exploration requires a patient-specific weighing of the importance of preserving pituitary function and avoiding missed lesions. The use of intraoperative pituitary imaging is beneficial for tumor identification and complete resection. Intraoperative ultrasound has been shown to detect some MRI-negative pituitary adenomas (47, 48), and the use of intraoperative MRI can help to identify residual tumors and increase the rate of complete resection (49, 50). For patients with Cushing’s disease, as MRI-negative tumors are more common among ACTH-secreting tumors, several cases of biochemical non-remission after surgery due to failing to remove the causative tumor have been reported (9, 12, 21). In this study, univariate analysis revealed significantly worse biochemical remission in patients with Cushing’s disease (P = 0.042), although no statistical significance was observed in multivariate analysis. Therefore, confirming the complete removal of tumors during surgery is particularly important for patients with Cushing’s disease. Determination of the attenuation of ACTH concentration after tumor resection by cavernous sinus blood sampling (51, 52) and quantitative analysis of ACTH in tumor tissues (53) are promising methods for confirming complete resection of ACTH-secreting tumors, but they are still not widely used in clinical practice.
Contiguous type DPA cannot be detected through preoperative MRI or intraoperative exploration, even if the images are retrospectively reviewed by experienced radiologists and the pituitary gland is carefully explored by experienced neurosurgeons (40). However, in this study, the rate of postoperative biochemical CR reached 92.3% in contiguous type DPA, which was an independent protective factor for postoperative biochemical remission. Contiguous type DPA is usually completely removed as a single mass during surgery, and the diagnosis can only be confirmed by the identification of two cell populations with distinct immunohistochemiscal features on postoperative pathological examination. Therefore, theoretically, there is no possibility of surgical failure due to missing one of the hormone-secreting tumors, which provides a reasonable explanation for its significantly better prognosis. Despite the favorable prognosis of contiguous type DPA compared to separated type DPA, it is not possible to accurately differentiate between these two tumor types until postoperative pathology is obtained. Therefore, there is no difference in clinical management between the two types of DPA, and careful MRI reading and comprehensive intraoperative exploration are not negligible for both tumor types.
This study is the first systematic review to analyze the prognostic factors affecting postoperative biochemical remission in patients with DPA and to provide meaningful insights into the clinical practice of this rare clinical condition of DPA. However, there are several limitations to this study. This is a retrospective study, whose data were derived from cases reported by our institution and collected from previous literature. Therefore, selection bias, missing data, and inaccurate information are unavoidable. Restricted by the very low incidence of DPA and the limited number of available case reports, the small sample size of this study was insufficient to produce more reliable and accurate results. The clinical information provided by the included case reports in this study exhibits significant heterogeneity. For example, tumor size was measured by different methods, including MRI, surgery, or pathology. Therefore, this article provides only preliminary experience in the prognosis and clinical management of DPA, and further research involving a larger number of patients is anticipated in the future.
Conclusion
The peak age of the occurrence of DPA is in the forties, with a slight female predominance. The most common clinical manifestations are Cushing’s disease and acromegaly. The most common immunohistochemical types are ACTH- and GH-immunopositive tumors, often occurring as ACTH+PRL and GH+FSH/LH combination patterns. PRL-immunopositive tumors tend to be silent in the setting of DPA. Double microadenomas are the most common tumor size combination, but macroadenomas+microadenomas are more frequent than previously recognized. 3.0T MRI significantly improved the detected rate of DPA compared with 1.5T MRI, and more novel imaging technology (eg. SPGR sequence, 11C-MET-PET/3.0T MRI) is promising. Double lesions detected by surgical exploration and contiguous type tumor are independent protective factors for postoperative biochemical remission in patients with DPA. Comprehensive intraoperative exploration is indispensable to avoid missing the second lesion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
YZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. XG: Formal Analysis, Funding acquisition, Writing – original draft, Writing – review & editing. JP: Methodology, Writing – review & editing. JL: Methodology, Writing – review & editing. ZY: Resources, Writing – review & editing. HZ: Resources, Writing – review & editing. LL: Resources, Writing – review & editing. HP: Methodology, Writing – review & editing. KD: Conceptualization, Supervision, Writing – review & editing. YY: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-B-114) from YY, and Youth Science Foundation of Peking Union Medical College Hospital (No. pumch201911867) from YZ.
Conflict of interest
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ogando-Rivas E, Alalade AF, Boatey J, Schwartz TH. Double pituitary adenomas are most commonly associated with GH- and ACTH-secreting tumors: systematic review of the literature. Pituitary. (2017) 20:702–8. doi: 10.1007/s11102-017-0826-6
2. Hagel C, Schüller U, Flitsch J, Knappe UJ, Kellner U, Bergmann M, et al. Double adenomas of the pituitary reveal distinct lineage markers, copy number alterations, and epigenetic profiles. Pituitary. (2021) 24:904–13. doi: 10.1007/s11102-021-01164-1
3. Gsponer J, De Tribolet N, Déruaz JP, Janzer R, Uské A, Mirimanoff RO, et al. Diagnosis, treatment, and outcome of pituitary tumors and other abnormal intrasellar masses. Retrospective analysis of 353 patients. Med (Baltimore). (1999) 78:236–69. doi: 10.1097/00005792-199907000-00004
4. Sav A, Rotondo F, Syro LV, Di Ieva A, Cusimano MD, Kovacs K. Invasive, atypical and aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am. (2015) 44:99–104. doi: 10.1016/j.ecl.2014.10.008
5. Davis FG, Kupelian V, Freels S, McCarthy B, Surawicz T. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol. (2001) 3:152–8. doi: 10.1093/neuonc/3.3.152
6. Budan RM, Georgescu CE. Multiple pituitary adenomas: A systematic review. Front Endocrinol (Lausanne). (2016) 7:1. doi: 10.3389/fendo.2016.00001
7. Kannuki S, Matsumoto K, Sano T, Shintani Y, Bando H, Saito S. Double pituitary adenoma–two case reports. Neurol Med Chir (Tokyo). (1996) 36:818–21. doi: 10.2176/nmc.36.818
8. Meij BP, Lopes MB, Vance ML, Thorner MO, Laws ER Jr. Double pituitary lesions in three patients with Cushing’s disease. Pituitary. (2000) 3:159–68. doi: 10.1023/a:1011499609096
9. Ratliff JK, Oldfield EH. Multiple pituitary adenomas in Cushing’s disease. J Neurosurg. (2000) 93:753–61. doi: 10.3171/jns.2000.93.5.0753
10. Syro LV, Horvath E, Kovacs K. Double adenoma of the pituitary: a somatotroph adenoma colliding with a gonadotroph adenoma. J Endocrinol Invest. (2000) 23:37–41. doi: 10.1007/bf03343674
11. Tosaka M, Kohga H, Kobayashi S, Zama A, Tamura M, Murakami M, et al. Double pituitary adenomas detected on preoperative magnetic resonance images. Case illustration. J Neurosurg. (2000) 92:361. doi: 10.3171/jns.2000.92.2.0361
12. McKelvie PA, McNeill P. Double pituitary adenomas: a series of three patients. Pathology. (2002) 34:57–60. doi: 10.1080/00313020120105651
13. Kim K, Yamada S, Usui M, Sano T. Preoperative identification of clearly separated double pituitary adenomas. Clin Endocrinol (Oxf). (2004) 61:26–30. doi: 10.1111/j.1365-2265.2004.02055.x
14. Shimizu C, Koike T, Sawamura Y. Double pituitary adenomas with distinct histological features and immunophenotypes. J Neurol Neurosurg Psychiatry. (2004) 75:140.
15. Andrioli M, Pecori Giraldi F, Losa M, Terreni M, Invitti C, Cavagnini F. Cushing’s disease due to double pituitary ACTH-secreting adenomas: the first case report. Endocr J. (2010) 57:833–7. doi: 10.1507/endocrj.k10e-140
16. Magri F, Villa C, Locatelli D, Scagnelli P, Lagonigro MS, Morbini P, et al. Prevalence of double pituitary adenomas in a surgical series: Clinical, histological and genetic features. J Endocrinol Invest. (2010) 33:325–31. doi: 10.1007/bf03346594
17. Rotondo F, Khatun N, Scheithauer BW, Horvath E, Marotta TR, Cusimano M, et al. Unusual double pituitary adenoma: a case report. Pathol Int. (2011) 61:42–6. doi: 10.1111/j.1440-1827.2010.02613.x
18. Zielinski G, Maksymowicz M, Podgorski J, Olszewski WT. Double, synchronous pituitary adenomas causing acromegaly and Cushing’s disease. A case report and review of literature. Endocr Pathol. (2013) 24:92–9. doi: 10.1007/s12022-013-9237-z
19. Mendola M, Dolci A, Piscopello L, Tomei G, Bauer D, Corbetta S, et al. Rare case of Cushing’s disease due to double ACTH-producing adenomas, one located in the pituitary gland and one into the stalk. Hormones (Athens). (2014) 13:574–8. doi: 10.14310/horm.2002.1503
20. Eytan S, Kim KY, Bleich D, Raghuwanshi M, Eloy JA, Liu JK. Isolated double pituitary adenomas: A silent corticotroph adenoma and a microprolactinoma. J Clin Neurosci. (2015) 22:1676–8. doi: 10.1016/j.jocn.2015.03.040
21. Mehta GU, Montgomery BK, Raghavan P, Sharma S, Nieman LK, Patronas N, et al. Different imaging characteristics of concurrent pituitary adenomas in a patient with Cushing’s disease. J Clin Neurosci. (2015) 22:891–4. doi: 10.1016/j.jocn.2015.01.001
22. Pu J, Wang Z, Zhou H, Zhong A, Jin K, Ruan L, et al. Isolated double adrenocorticotropic hormone-secreting pituitary adenomas: A case report and review of the literature. Oncol Lett. (2016) 12:585–90. doi: 10.3892/ol.2016.4673
23. Miyagi N, Doi R, Kuramoto T, Sakata K, Tahara S, Sugita Y, et al. Double pituitary adenomas associated with persistent trigeminal artery: a rare case report and the review of literature. Neurosurg Rev. (2018) 41:341–5. doi: 10.1007/s10143-017-0924-y
24. Collazo-Gutierrez N, de Jesus O, Villamil-Jarauta M, Alvarado M, Gonzalez L, Ramirez M, et al. Double pituitary adenomas with synchronous somatotroph and corticotroph clinical presentation of acromegaly and Cushing’s disease. World Neurosurg. (2019) 132:161–4. doi: 10.1016/j.wneu.2019.08.224
25. Gonzalez A, Saindane AM, Neill SG, Oyesiku NM, Ioachimescu AG. The intriguing case of a double pituitary adenoma. World Neurosurg. (2019) 126:331–5. doi: 10.1016/j.wneu.2019.02.242
26. Zielinski G, Sajjad EA, Maksymowicz M, Pekul M, Koziarski A. Double pituitary adenomas in a large surgical series. Pituitary. (2019) 22:620–32. doi: 10.1007/s11102-019-00996-2
27. Demirci H, Kahraman D, Kuzucu P, Senol O, Ugur KS, Ergun MA, et al. Growth hormone-releasing pituitary microadenoma overshaded by a macroadenoma: a case of double pituitary adenomas and review of the literature. Br J Neurosurg. (2022), 1–7. doi: 10.1080/02688697.2022.2076806
28. Ullah MT, Lopes MBS, Jane JA Jr., Hong GK, Love KM. Co-occurrence of functional gonadotroph adenoma and lactotroph adenoma: A case report and literature review. AACE Clin Case Rep. (2023) 9:5–9. doi: 10.1016/j.aace.2022.11.001
29. Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Multiple adenomas of the human pituitary. A retrospective autopsy study with clinical implications. J Neurosurg. (1991) 74:243–7. doi: 10.3171/jns.1991.74.2.0243
30. Parent AD, Bebin J, Smith RR. Incidental pituitary adenomas. J Neurosurg. (1981) 54:228–31. doi: 10.3171/jns.1981.54.2.0228
31. McComb DJ, Ryan N, Horvath E, Kovacs K. Subclinical adenomas of the human pituitary. New light on old problems. Arch Pathol Lab Med. (1983) 107:488–91.
32. Sano T, Horiguchi H, Xu B, Li C, Hino A, Sakaki M, et al. Double pituitary adenomas: six surgical cases. Pituitary. (1999) 1:243–50. doi: 10.1023/a:1009994123582
33. Powers SK, Wilson CB. Simultaneously occurring prolactinomas. Case report. J Neurosurg. (1981) 55:124–6. doi: 10.3171/jns.1981.55.1.0124
34. Daly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. (2020) 49:347–55. doi: 10.1016/j.ecl.2020.04.002
35. Iacovazzo D, Bianchi A, Lugli F, Milardi D, Giampietro A, Lucci-Cordisco E, et al. Double pituitary adenomas. Endocrine. (2013) 43:452–7. doi: 10.1007/s12020-013-9876-3
36. Tomita T, Gates E. Pituitary adenomas and granular cell tumors. Incidence, cell type, and location of tumor in 100 pituitary glands at autopsy. Am J Clin Pathol. (1999) 111:817–25. doi: 10.1093/ajcp/111.6.817
37. Burrow GN, Wortzman G, Rewcastle NB, Holgate RC, Kovacs K. Microadenomas of the pituitary and abnormal sellar tomograms in an unselected autopsy series. N Engl J Med. (1981) 304:156–8. doi: 10.1056/nejm198101153040306
38. Petersenn S, Fleseriu M, Casanueva FF, Giustina A, Biermasz N, Biller BMK, et al. Diagnosis and management of prolactin-secreting pituitary adenomas: a Pituitary Society international Consensus Statement. Nat Rev Endocrinol. (2023) 19(12):722–40. doi: 10.1038/s41574-023-00886-5
39. Coiré CI, Smyth HS, Rosso D, Horvath E, Kovacs K. A double pituitary adenoma presenting as a prolactin-secreting tumor with partial response to medical therapy. Case report. Endocr Pathol. (2010) 21:135–8. doi: 10.1007/s12022-009-9104-0
40. Roberts S, Borges MT, Lillehei KO, Kleinschmidt-DeMasters BK. Double separate versus contiguous pituitary adenomas: MRI features and endocrinological follow up. Pituitary. (2016) 19:472–81. doi: 10.1007/s11102-016-0727-0
41. Sabahi M, Shahbazi T, Maroufi SF, Vidal K, Recinos PF, Kshettry VR, et al. MRI-negative Cushing’s disease: A review on therapeutic management. World Neurosurg. (2022) 162:126–137.e1. doi: 10.1016/j.wneu.2022.03.076
42. Yang AB, Henderson F Jr., Schwartz TH. Surgical strategies in the treatment of MR-negative Cushing’s Disease: a systematic review and treatment algorithm. Pituitary. (2022) 25:551–62. doi: 10.1007/s11102-022-01239-7
43. Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, et al. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. (2003) 88:1565–9. doi: 10.1210/jc.2002-021438
44. Ikeda H. Demonstration of improved surgical outcome in patients with both ACTH and GH secreting adenomas diagnosed by a new sensitive method of MET-PET fusion 3T-MRI. Endocrinol Metab Syndr. (2013) 2.
45. Alzahrani AS, Farhat R, Al-Arifi A, Al-Kahtani N, Kanaan I, Abouzied M. The diagnostic value of fused positron emission tomography/computed tomography in the localization of adrenocorticotropin-secreting pituitary adenoma in Cushing’s disease. Pituitary. (2009) 12:309–14. doi: 10.1007/s11102-009-0180-4
46. Kontogeorgos G, Scheithauer BW, Horvath E, Kovacs K, Lloyd RV, Smyth HS, et al. Double adenomas of the pituitary: a clinicopathological study of 11 tumors. Neurosurgery. (1992) 31:840–9; discussion 849. doi: 10.1227/00006123-199211000-00003
47. Ram Z, Shawker TH, Bradford MH, Doppman JL, Oldfield EH. Intraoperative ultrasound-directed resection of pituitary tumors. J Neurosurg. (1995) 83:225–30. doi: 10.3171/jns.1995.83.2.0225
48. Marcus HJ, Vercauteren T, Ourselin S, Dorward NL. Intraoperative ultrasound in patients undergoing transsphenoidal surgery for pituitary adenoma: systematic review [corrected]. World Neurosurg. (2017) 106:680–5. doi: 10.1016/j.wneu.2017.07.054
49. Vitaz TW, Inkabi KE, Carrubba CJ. Intraoperative MRI for transphenoidal procedures: short-term outcome for 100 consecutive cases. Clin Neurol Neurosurg. (2011) 113:731–5. doi: 10.1016/j.clineuro.2011.07.025
50. Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M. Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-field magnetic resonance imaging. Acta Neurochir (Wien). (2014) 156:2233–43; discussion 2243. doi: 10.1007/s00701-014-2210-x
51. Czirják S, Bezzegh A, Gál A, Rácz K. Intraoperative bilateral cavernous sinus sampling for ACTH measurements during transsphenoidal pituitary surgery in patients with Cushing’s disease. Clin Neurol Neurosurg. (2002) 104:334–8. doi: 10.1016/s0303-8467(02)00028-8
52. Raff H, Shaker JL, Seifert PE, Werner PH, Hazelrigg SR, Findling JW. Intraoperative measurement of adrenocorticotropin (ACTH) during removal of ACTH-secreting bronchial carcinoid tumors. J Clin Endocrinol Metab. (1995) 80:1036–9. doi: 10.1210/jcem.80.3.7883819
Keywords: double pituitary adenomas (DPA), cushing disease, acromegaly, biochemical remission, prognostic analysis
Citation: Zhang Y, Gong X, Pu J, Liu J, Ye Z, Zhu H, Lu L, Pan H, Deng K and Yao Y (2024) Double pituitary adenomas: report of two cases and systematic review of the literature. Front. Endocrinol. 15:1373869. doi: 10.3389/fendo.2024.1373869
Received: 20 January 2024; Accepted: 18 March 2024;
Published: 02 April 2024.
Edited by:
Xiang’En Shi, Capital Medical University, ChinaReviewed by:
Aleksandra Gilis-Januszewska, Jagiellonian University Medical College, PolandBarry Pressman, Cedars Sinai Medical Center, United States
Copyright © 2024 Zhang, Gong, Pu, Liu, Ye, Zhu, Lu, Pan, Deng and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Yao, tigerfreeyy@126.com; Kan Deng, dcansums@126.com
†These authors have contributed equally to this work and share first authorship
 Yi Zhang
Yi Zhang Xinyue Gong2†
Xinyue Gong2† Hui Pan
Hui Pan