- 1The First Clinical Medical College of Lanzhou University, Lanzhou, Gansu, China
- 2Department of Obstetrics and Gynecology, The First Hospital of Lanzhou University, Lanzhou, Gansu, China
Objective: To study the effect of type 2 diabetes mellitus(T2DM)on overall ovarian reserve and pregnancy outcomes during assisted reproductive technology (ART) among childbearing infertile women.
Design: Retrospective cohort study.
Setting: The Reproductive Medicine Special Hospital, The First Hospital of Lanzhou University, between January 2019 and December 2022.
Patient(s): A total of 265 infertile female patients aged 20–45 years who underwent in vitro fertilization-embryo transfer (IVF-ET), intracytoplasmic sperm injection-embryo transfer (ICSI-ET), or rescue intracytoplasmic sperm injection-embryo transfer (RICSI-ET) in the first fresh cycle.
Intervention(s): None.
Main Outcome Measure(s): Serum Anti-Müllerian Hormone (AMH) levels, clinical pregnancy rate (CPR), live birth rate (LBR), and abortion rate (AR) in the T2DM group and non-T2DM group.
Result(s): Patients with T2DM showed statistically decreased levels of AMH compared to the non-T2DM group. During ovarian stimulation, those with T2DM required significantly higher total and initial doses of gonadotropin (GN), although they had fewer retrieved oocytes and worse pregnancy outcomes than the non-T2DM group. Multivariate logistic regression analysis adjusting for confounding factors showed that T2DM alone was an independent risk factor for CPR and LBR (adjusted odds ratio [a OR], 0.458, adjusted 95% confidence interval [CI], 0.235-0.891, P = 0.022; a OR, 0.227, 95% CI, 0.101-0.513, P<0.001; respectively), and the abortion rate in the T2DM group was 3.316 times higher than the non-T2DM group(a OR, 3.316, 95%CI, 1.248-8.811, P = 0.016);
Conclusion: Infertile patients with T2DM have decreased ovarian reserve, and T2DM has a deleterious impact on clinical pregnancy outcomes during the ART process compared with non-T2DM infertile women.
Capsule: Infertile women with T2DM have decreased ovarian reserve and pregnancy outcomes during the assisted reproductive technology process compared with non-T2DM infertile women.
1 Introduction
Type 2 diabetes mellitus (T2DM) accounts for 90–95% of all diagnosed cases of diabetes, and, in parallel with the worldwide escalation in the prevalence of obesity, T2DM is becoming increasingly common on a global scale (1). As a common endocrine disease, T2DM is characterized by insulin resistance, relatively insufficient insulin secretion (2), and elevated fasting and postprandial blood glucose (3). The main complications of T2DM include cardiovascular disease (4), diabetic retinopathy (5), and neuropathy (6), as well as dysfunctional immunity (7) and a chronic inflammatory state (8). It is widely recognized that T2DM and its complications will bring profound psychological and physical distress to patients and put a huge burden on society (4).
In addition, studies have demonstrated that reproductive dysfunction is also a common but little studied complication of diabetes (9). Due to the state of hyperglycemia, women with diabetes may have substantially decreased fecundability (10) and higher proportions of spontaneous losses (11). Type 1 diabetes mellitus (T1DM), which is characterized by β-cell destruction and an absolute deficiency of insulin secretion (12), may lead to the accumulation of advanced glycation receptors and products (13) and appear more likely to have reproductive disorders (14), manifested by polycystic ovary syndrome (PCOS) (15), irregular menses (16, 17), and subfertility (18, 19). Studies also reported that the rates of spontaneous abortion among women with T1DM are increasing (20). T2DM, also known as non–insulin-dependent diabetes (21), although it has a shorter duration of diabetes than T1DM, both are equally at risk for reproductive disorders (9). There is growing evidence that T2DM impairs the function of the female reproductive system and negatively affects fertility (22). A recent review reported that T2DM was associated with lower rates of fertility (6). Another large prospective cohort study also found that women with T2DM prior to childbearing had a higher risk of miscarriage and infertility (23).
As the prevalence of T2DM rises sharply globally, especially among young people, more women of reproductive age will encounter severe challenges to ovarian reserve and pregnancy outcomes (6). This means that the increasing childbearing women with T2DM need to rely on assisted reproductive technology (ART) to complete fertility. However, comprehensive and holistic assessments of pregnancy outcomes in women with T2DM during the ART process have been rarely conducted (24), and few studies have illustrated associations between T2DM and reproductive outcomes in terms of pregnancy, live birth, or miscarriage, which attracted our attention.
Anti-Müllerian Hormone (AMH), a glycoprotein produced exclusively by granulosa cells of ovary (25, 26), is the most clinically useful marker of ovarian reserve (27) because of its strong correlation with primordial follicle pool and aging of ovary (28). An experimental study in a rat model revealed that AMH levels in the healthy control group were significantly higher than in the non-treated diabetic group (26). In addition, both a cross-sectional report in Chile (29) and a population-based study (14) reported that AMH concentrations were lower in women with T1DM. However, few studies have focused on the AMH levels in patients with T2DM, which requires further research.
Therefore, our purpose of this study was to evaluate the overall effects of T2DM on ovarian reserve and pregnancy outcomes during the ART process, and provide improved guidance that would increase clinical ART pregnancy success rates.
2 Materials and methods
2.1 Study population
It is a retrospective cohort study that was performed at the Reproductive Medicine Special Hospital, The First Hospital of Lanzhou University, between January 2019 and December 2022. This study was reviewed and approved by the Ethics Committee of the First Hospital of Lanzhou University (LDYYLL2019-44). Based on the inclusion and exclusion criteria, a total of 265 infertile female patients were enrolled. The clinical and follow-up information of the first fresh embryo transfer cycle of the 265 patients was eventually analyzed.
2.2 Inclusion criteria and exclusion criteria
Infertile female patients aged 20–45 years who underwent in vitro fertilization-embryo transfer (IVF-ET), intracytoplasmic sperm injection-embryo transfer (ICSI-ET), or rescue intracytoplasmic sperm injection-embryo transfer (RICSI-ET) in the first fresh cycle for female factor or (and) male factor infertility during January 2019 and December 2022 were enrolled.
Subjects with any of the following were excluded: (1) impaired fasting glucose, impaired glucose tolerance, or a diagnosis of type 1 diabetes, gestational diabetes, or other specific types of diabetes or glycemic abnormality; (2) incomplete follow-up information; (3) chromosome abnormality or (and) genetic disorder; (4) infectious or immunological diseases or suspicion of malignancy; (5) history of ovarian surgery, polycystic ovarian syndrome (PCOS), sexual hormone application within 3 months, and other endocrine disorders such as abnormal thyroid function and so on;
All the subjects were divided into two groups: the T2DM population and the non-T2DM reference population. The diagnostic criteria for T2DM, as defined by the International Diabetes Federation, are a fasting plasma glucose (FPG) level ≥7.0 mmol/L, a glycosylated hemoglobin (HbA1c) ≥6.5%, or a previous diagnosis of T2DM, and then the reference population was randomly selected after frequency matching with the T2DM group in terms of age (4 controls per T2DM patient). The final study comprised 53 infertile women with T2DM and a reference population of 212 who had never been diagnosed with any type of diabetes or glycemic abnormality.
2.3 Determination of sample size
We used PASS (version 21.0.3) to estimate whether the sample size met the requirements for conducting this study. To achieve 90% power at a 0.05 significance level, it was calculated that the sample size required to complete the analysis was the largest when calculated for clinical pregnancy rates. Given the results of the pre-analysis, the clinical pregnancy rates in the diabetic and control groups were 0.3 and 0.55, respectively. Consequently, the minimal total sample size that can satisfy the analysis for the purpose of this study was estimated to be 51 in the T2DM group and 204 in the non-T2DM group, which means that the sample size we included was able to meet the needs of the present study.
2.4 Data sources and measurement
In both study groups, the clinical data included anthropometric parameters and laboratory assessments like hormone levels and metabolic parameters.
2.4.1 Physical examination
Anthropometric parameters, including height, weight, and body mass index (BMI), were measured according to standardized protocols. BMI was defined as the weight in kilograms divided by the square of height in meters (kg/m2).
2.4.2 Laboratory assessment
Day 2 of menstruation was defined as the basal day, and blood samples were collected on day 2 of the menstrual cycle from 8:00 to 10:00 a.m. Fasting serum was collected on the basal day for further detection of sexual hormonal levels such as serum estradiol (E2), progesterone (P), follicle stimulating hormone (FSH), and luteinizing hormone (LH), which were measured by chemiluminescence (Roche Diagnostics, Germany). Fasting blood glucose was measured by the hexokinase method (Beckman Coulter, USA). Biomarkers that primarily represent ovarian reserve function, including Anti-Müllerian Hormone (AMH) and antral follicle count (AFC), were also measured among all female participants. Serum AMH concentrations were measured with a fully automated AMH electrochemiluminescence assay (ECLIA; Elecsys AMH assay, Roche Diagnostics, Germany) on the Cobas e 801 analyzer. Antral follicles were defined as 2-10 mm follicles visible in the ovaries by a vaginal ultrasound machine at 2-4 d of the menstrual cycle, and the total number of follicles in the ovaries was calculated bilaterally. Insulin was measured using the Immulite immunoassay. We used the homeostasis Model Assessment of Insulin resistance (HOMA-IR index): HOMA-IR index = fasting blood glucose (FBG) (mmol/L) ×fasting serum insulin (FINS) (μU/ml)/22.5 (30). The cut-off point of HOMA-IR value for defining insulin resistance was suggested to be 2.69, owing to previous Chinese studies showed that the cut-off point of HOMA-IR value was more consistent with the diagnostic criteria of insulin resistance in Chinese patients with T2DM (31–33).
2.5 Study procedures
Ovarian stimulation protocols, oocyte retrieval, and fertilization were performed according to standardized protocols, as recommended by the American Society for Reproductive Medicine and Chinese guidelines. Ovarian stimulation was performed under a long GnRH agonist, GnRH antagonist, or PPOS regimen.
2.5.1 Long GnRH agonist regimen
Human menopausal gonadotropin (HMG, Lizhu Group) and/or injectable urinary follicle stimulating hormone (Lishenbao, Lizhu Group) and/or recombinant human follicle stimulating hormone (Gonal-F, Merck Serono S.p.A.) were given intramuscularly for super-ovulation according to the basal condition and follicular development. Follicular development was monitored by dynamic ultrasound, and the levels of sex hormones FSH, LH, E2, and P were measured on the same day to assess the ovarian response to the ovulation stimulating drugs and to adjust the dosage of ovulation stimulating drugs. When at least 3 follicles reached 16mm in diameter or 2 follicles reached or exceeded 18mm in diameter, recombinant human chorionic gonadotropin (Azer, Merck Serono S.p.A.) 250 U with (or without) HCG (Zhuhai, Lizhu Group) 2000 U was given as a trigger.
2.5.2 GnRH antagonist regimen
Recombinant follicle-stimulating hormone (FSH; Gonal-F, Merck Serono) was started on day 2 or 3 of the menstrual cycle based on basal blood FSH, LH, E2, and AFC with an initiating start-up dose of 112.5-225 U and adjusted according to ovarian response. A gonadotropin-releasing hormone antagonist (Cetrorelix 250 mg; Merck Serono) was started when the lead follicle exceeded 12 mm. Follicular maturation was induced by GnRH-α (Daffelin, France) 0.2 mg combined with urinary human chorionic gonadotropin(HCG)2000 IU when 2 or more follicles measured 18 mm or more.
2.5.3 PPOS regimen
Oral dydrogesterone tablets (20 mg/d) were started on the 2nd to 5th menstrual day until the trigger day, and human menopausal gonadotrophin (HMG, Lizhu Group) and/or injectable urinary follicle stimulating hormone (Lishenbao, Lizhu Group) and/or recombinant human follicle stimulating hormone (Gonal-F, Merck Serono S.p.A.) were given intramuscularly for super-ovulation. The follicle size and the levels of sex hormones FSH, LH, E2, and P were monitored dynamically to assess ovarian responsiveness to ovulation stimulating drugs and to adjust the dosage of ovulation stimulating drugs. When at least 3 follicles reach 16mm in diameter or more than 2 follicles reach or exceed 18mm in diameter, human chorionic gonadotropin (HCG, Lizhu Group) 2000 U in combination with GnRH-a (Daffelin, France) 0.2mg was administered.
Oocyte retrieval was performed 34-36 hours later. Selection of different fertilization methods according to male semen parameters and infertility factors. Embryo transfer was performed 72 hours after oocyte retrieval.
2.6 Outcome measures and definition
The primary observation of ovarian reserve was AMH, and the main pregnancy outcome indicators included clinical pregnancy rate (CPR), live birth rate (LBR), and abortion rate (AR). MII oocyte rate, normal fertilization rate, and high-quality embryo rate were also compared to assess the effect of T2DM on oocyte maturation, fertilization, and embryo quality.
Clinical pregnancy rate (%) (CPR) = number of clinical pregnancy cycles/number of transplant cycles; A positive blood test for β-HCG 14 days after transplantation was considered a pregnancy; a clinical pregnancy was diagnosed on an ultrasound 30 days after transplantation if a gestational sac was observed. Live birth rate (%) (LBR) = number of live birth cycles/number of transplant cycles. Abortion rate (%) (AR) = number of abortion cycles/number of clinical pregnancy cycles; The fertilization rate for each individual patient was evaluated based on their insemination procedure. For patients who underwent conventional insemination IVF, the normal fertilization rate = 2PN/number of oocytes inseminated; For patients who underwent ICSI, the normal fertilization rate = 2PN/number of oocytes injected. High-quality embryo rate (%) = number of high-quality embryos per patient/number of available embryos per patient; MII oocyte rate (%) = number of MII oocytes per patient/number of oocytes retrieved per patient;
2.7 Statistical analysis
Statistical analysis was performed using SPSS 26.0; continuous variables were expressed as mean ± SD; quantitative variables were tested using the Mann–Whitney tests. Categorical variables were represented by frequency and percentage (%), and the differences between groups were assessed by the Pearson chi-square test (χ2-test) or Fisher exact probability method. Univariate and multivariate logistic regression were used to further investigate the effect of T2DM on clinical pregnancy outcomes. The binary outcome of this model was whether a clinical pregnancy, miscarriage, or live birth occurred. P < 0.05 was considered to indicate statistically significant differences.
3 Result
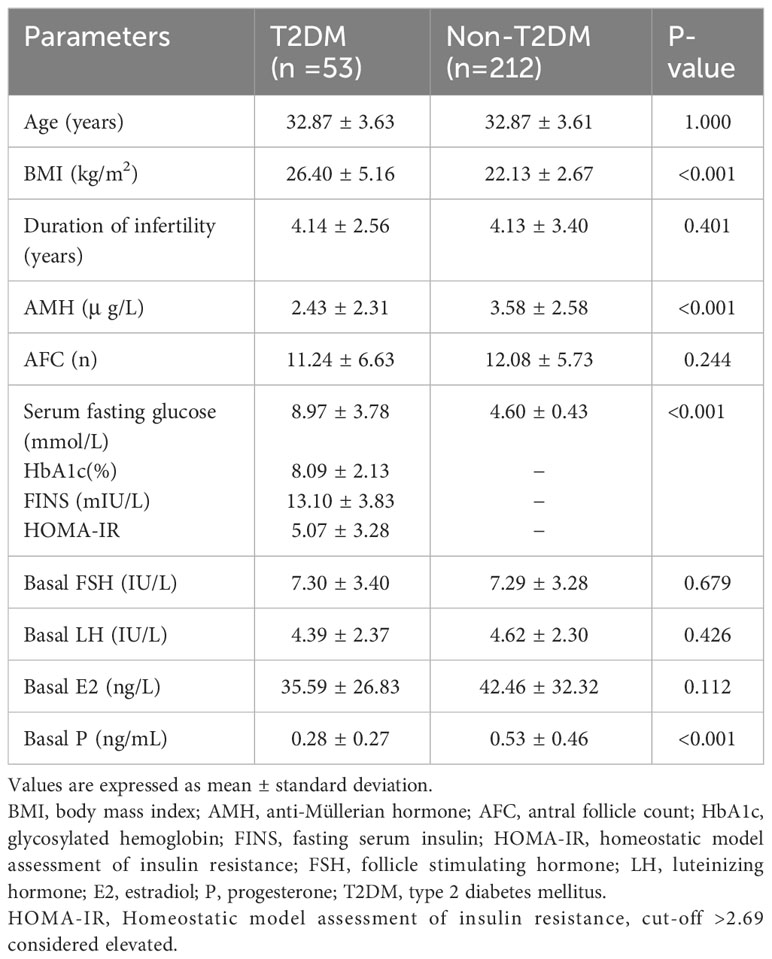
3.1 Baseline characteristics
We identified 53 infertile women with T2DM and selected 212 matched nondiabetic controls. The anthropometric and metabolic characteristics, sexual hormone, AMH levels and indicators related to diabetes and insulin resistance (IR) such as HbA1c, FINS, and HOMA-IR are shown in Table 1. The T2DM group had significantly higher BMIs and fasting blood glucose (26.4 ± 5.16 vs. 22.13 ± 2.67, P<0.001; 8.97 ± 3.78 vs. 4.60 ± 0.43, P<0.001, respectively) than the non-T2DM group. Furthermore, a significant decrease in AMH concentrations and basal progesterone levels was observed in patients with T2DM (2.43 ± 2.31 vs. 3.58 ± 2.58, P<0.001; 0.28 ± 0.27 vs. 0.53 ± 0.46, P<0.001, respectively; Table 1).
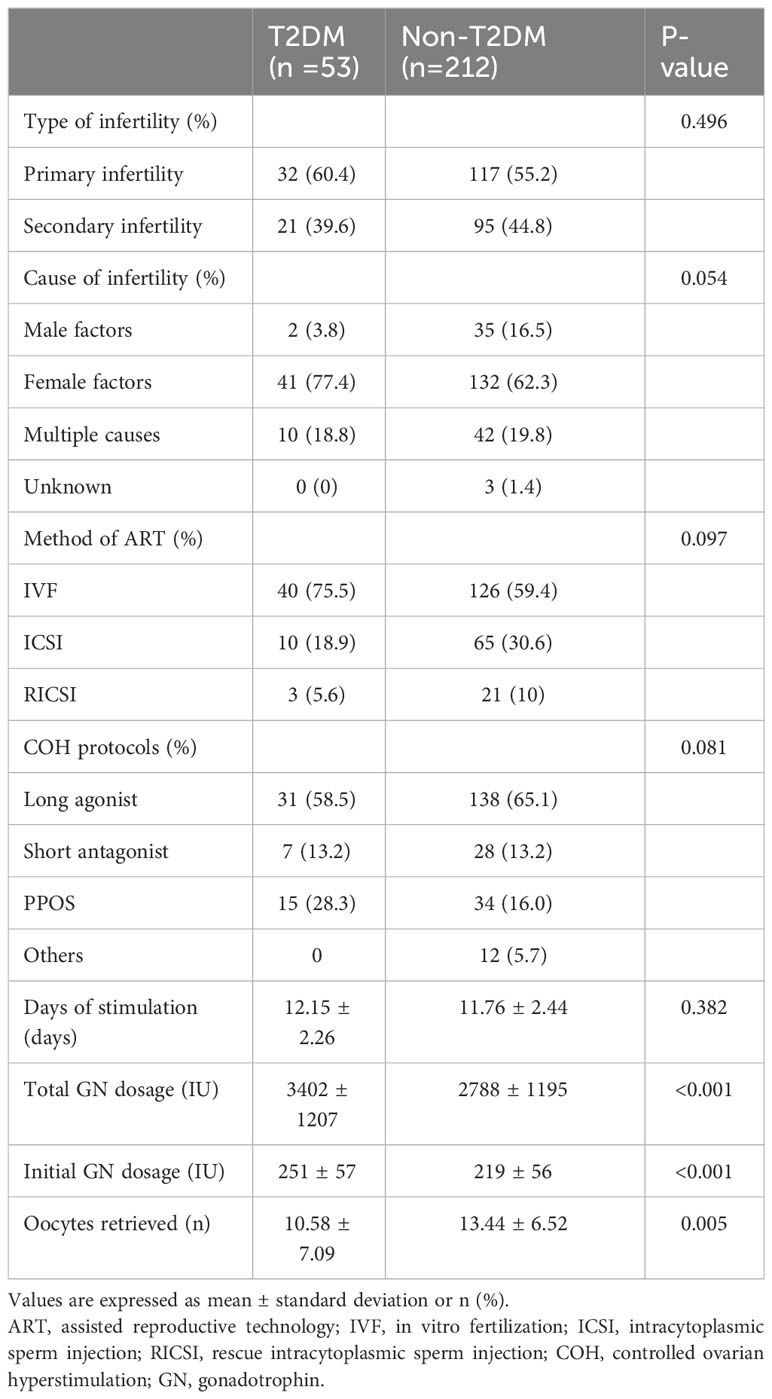
3.2 Comparison of infertility and cycle stimulation characteristics between two groups
As presented in Table 2, regarding ovarian stimulation, there was a significant difference among the two groups in terms of total and initial doses of gonadotropin (GN); those with T2DM required significantly higher initial and total doses of gonadotropin to support follicular development but had fewer retrieved oocytes compared to the non-T2DM group. The duration of ovarian stimulation was higher in the T2DM group but without a significant between-group difference (12.15 ± 2.26 vs. 11.76 ± 2.44, P = 0.382). The etiological classification and methods of assisting pregnancy, as well as ovulation stimulation protocols, did not differ between the two groups.
In order to further analyze the effect of blood glucose itself on the cycle stimulation characteristics such as stimulation time, GN doses and the number of oocytes retrieved during ovarian stimulation, we analyzed the correlation between blood glucose and the indicators of ovarian stimulation mentioned above. As shown in the Supplementary Table 1, blood glucose level was positively correlated with the total dose of gonadotrophin (GN), but no correlation was found between the blood glucose level and the number of oocytes retrieved or the time of ovarian stimulation.
3.3 The influence of T2DM on embryo development and pregnancy outcomes
The MII oocyte rate, fertilization rate, and high-quality embryo rate, as indicators of embryo development, were significantly lower in the T2DM group (86.8%, 56%, and 36.7%, respectively) compared with the non-T2DM group (89.9%, 63.4%, and 64.8%, respectively). The clinical pregnancy rate, abortion rate, and live birth rate, which represent pregnancy outcomes, are presented in Table 3. Apparently, by comparing the pregnancy outcomes of the two groups, patients in the T2DM group had significantly lower clinical pregnancy and live birth rates (30.2% vs. 52.8%, P = 0.003; 15.1% vs. 47.6%, P<0.001, respectively) but a significantly higher risk of miscarriage (50.0% vs. 9.8%, P<0.001) (Table 3).
Since diabetes-related indicators such as HbA1c, FINS, and HOMA-IR were accessible within the T2DM group, we conducted a univariate logistic regression analysis to delve deeper into the influence of these indicators on assisted reproductive outcomes among women with T2DM (refer to Supplementary Table 2). The results of the logistic regression analysis indicated that there was no significant impact of diabetes-related indicators on assisted reproductive outcomes in women with T2DM.
3.4 T2DM is an independent risk factor affecting assisted pregnancy outcomes
To further investigate the effect of T2DM on clinical pregnancy outcomes, a binary logistic regression model was performed. Univariate logistic regression was performed to identify potential confounders affecting CPR, LBR, and AR (Supplementary Tables 3, 5, 7), and variables with a value of P<0.1 were included in the multivariable logistic regression analysis (Supplementary Tables 4, 6, 8). Due to the involvement of blood glucose levels in the diagnosis of diabetes, the collinearity between the two is strong, and the blood glucose level is strongly correlated with diabetes in clinical significance and statistics. Therefore, only the diabetes group is included in the multivariate regression analysis. The odds ratios (ORs) with 95% confidence intervals (CIs) and P-values of assisted pregnancy outcomes between two groups were calculated, the results are shown in Table 4.
As shown in Table 4, T2DM is an independent risk factor for assisted reproductive outcomes, negatively affecting clinical pregnancy rate and live birth rate (adjusted odds ratio [a OR], 0.458, adjusted 95% confidence interval [CI], 0.235-0.891, P = 0.022; a OR, 0.227, 95% CI, 0.101-0.513, P<0.001);. The abortion rate in the T2DM group was 3.316 times higher than that in the non-T2DM group (a OR, 3.316, 95% CI, 1.248-8.811, P = 0.016; Table 4).
4 Discussion
In this four-year retrospective cohort study, we uniquely investigated clinical pregnancy rate (CPR), live birth rate (LBR), abortion rate (AR), and AMH concentrations among reproductive-aged women with T2DM who undergo ART treatments. The comparison of baseline parameters and assisted reproductive outcomes between the two groups showed that serum AMH levels decreased significantly and clinical pregnancy outcomes were poorer in the T2DM group, which demonstrated that T2DM adversely affects ovarian reserve and assisted reproductive outcomes.
The negative impact of diabetes on female fertility has been identified in several studies. A Chinese cohort study, comprising >2 million couples, reported that elevated pre-pregnancy maternal glucose levels were associated with the couple’s lower fecundability (34). Interestingly, another cohort study based on the Norwegian Mother and Child obtained the similar results (10). Besides, the incidence of spontaneous abortion (11) and the risk of pregnancy complications (35) are observed to be higher in diabetic women. The literature mentioned above strongly supports the results of this study, however, there are still some studies that remain controversial regarding the fertility in patients with T2DM, such as an observational, descriptive study in Iran that showed a higher rate of fertility in married women with T2DM (36). In addition to this, the study also found an unexpectedly low prevalence of polycystic ovary syndrome (PCOS) and hyperandrogenemia in the research group, which may be the reason why married women with T2DM have higher fertility rate. This is because PCOS was associated with increasing the probability of developing T2DM (9), and the onset of the two overlaps with each other. It is well known that PCOS is the most common endocrine disease in childbearing women, most often accompanied by metabolic and reproductive-related complications (37), such as anovulation and subfertility. Consequently, the reduced prevalence of PCOS in women with type 2 diabetes may lead to a pick-up in fertility.
Furthermore, regarding ovarian stimulation characteristics, we have found that the T2DM group required higher total and initial exogenous gonadotrophin (GN) dosage during the ART process. These findings may be the result of impaired ovarian sensitivity to exogenous GN stimulation under the influence of diabetes. A recent retrospective cohort study proposed that average GN dosage per follicle is a reliable index of ovarian response to exogenous GN, and lower exogenous GN requirements suggest the ovary response is more sensitive to GN stimulation (38). In addition, a longer duration of ovarian stimulation was also observed in the T2DM group despite lacks statistical significance. This may be due to the fact that the high total GN dosage, but not the duration of ovarian stimulation, is associated with decreasing the rate of live births in fresh cycles (39). Therefore, minimizing the total GN dose or limiting the duration of stimulation should be considered in fresh autologous cycles to improve pregnancy outcomes. Currently, there is a paucity of studies directly focused on the exogenous GN dosage and ovarian stimulation length in patients with T2DM, and thus further studies are necessary in the future.
Also, a significant decrease in the number of retrieved oocytes and poorer reproductive outcomes were observed in patients with T2DM; this might be influenced by the high glucose microenvironment during the progression of diabetes. Hyperglycemia induces the formation of reactive oxygen species (ROS) that promote oxidative stress and lead to mitochondrial dysfunction (7). Mitochondrion, as an energy source, provides energy for the development, maturation, and fertilization of oocytes. Meanwhile, the key step of steroid hormone biosynthesis also occurs in the mitochondria of granulosa cells, and accumulating evidence indicates that higher follicular estradiol levels correlate well with successful fertilization following ART (40). Moreover, the hyperglycemic condition may disturb the connexin expression and perturb the oocyte-granulosa gap junction communication (41) and through apoptosis, it can affect oocyte competence and pregnancy outcomes (42). Therefore, we speculate that hyperglycemia-induced mitochondrial dysfunction and granulosa cell apoptosis may contribute to the decline in quantity and quality of oocytes, a reduction in steroidogenesis, and a decreased fertilization rate, which ultimately jeopardize fertility.
AMH, produced by the granulosa cells of small ovarian follicles, represents the quantity and quality of follicles. Soto et al. (29) showed that AMH levels decreased earlier in diabetes mellitus (DM). Our data also showed a marked decline in women with T2DM. Several mechanisms may help explain the more rapid decline in ovarian reserve observed in diabetic women, including oxidative stress and NF-κB pathway activation, which have direct effects on granulosa cell injury (43). On the other hand, in the hyperglycemic state, a disturbed microenvironment may lead to the apoptosis of granulocytes. As one study has shown that elevated follicular glucose profoundly accelerated the pyroptosis of ovarian granulosa cells, affecting the synthesis of hormones (44) and AMH secretion. Thus, this may be another important reason for the decline in AMH levels in diabetic patients. Of course, due to the limitations of human knowledge, there may be many other unknown mechanisms that lead to diabetes-related reproductive dysfunction and decreased ovarian reserve, which need to be further explored in future studies.
As this was a retrospective study, a major limitation was the possibility of the potential bias due to missing data or incomplete medical records. Furthermore, another limitation of this study was a lack of detailed population characteristics, such as smoking and drinking status, as well as the duration of diabetes and the presence of diabetes-related complications, these indicators may affect fertility outcomes as unknown confounding factors. Finally, as in any cohort study, selection bias cannot be excluded. To minimize the limitations and get a more creditable conclusion, we consider conducting multi-center, prospective studies with larger sample sizes and more detailed medical data records in the future.
On the other hand, this study had certain strengths. All study populations were matched by age, as it is well known that age is the most critical factor affecting AMH levels. In addition to this, age was also adjusted in multivariate analysis, which achieved a doubly robust test or estimation. Lastly, our study uniquely investigated ovarian reserve and assisted reproductive outcomes in infertile women with T2DM requiring ART treatments during the reproductive years, the results may help to improve the success rate of assisted reproductive therapy.
In summary, our study concludes the adverse effects of T2DM on ovarian reserve and assisted reproductive outcomes during the ART process, emphasizes the importance of pre-pregnancy blood glucose screening and control for pre-pregnancy health care, and highlights that early intervention and preventive treatment for female partners with T2DM before ART treatment are necessary. Meanwhile, it is also a key measure to improve assisted reproductive outcomes and promote maternal and newborn health in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the Ethics Committee of the First Hospital of Lanzhou University (LDYYLL2019-44). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XQ and XL contributed to the central idea and design of the work. YL, QZ, and YXL guided the writing of the article. XQ, JD, and RH assisted in the analysis and interpretation of data. HL and XL provided critical feedback on the manuscript. The original draft was written by XQ. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82360303, No. 81960278), the Outstanding Youth Funds of Science and Technology Department of Gansu Province (No. 20JR5RA371), Longyuan Youth Innovation and Entrepreneurship Talent Project, and Youth Science Fund of the First Hospital of Lanzhou University (NO. ldyyyn2021-57).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1274327/full#supplementary-material
Supplementary Table 1 | Correlation between blood glucose levels with ovarian stimulation characteristics.
Supplementary Table 2 | Univariate logistic regression analysis of diabetes-related indicators and assisted reproductive outcomes in the T2DM group.
Supplementary Table 3 | Univariate logistic regression analysis on the clinical pregnancy rate (CPR).
Supplementary Table 4 | Multivariate analysis on the clinical pregnancy rate (CPR) with backward approach regression.
Supplementary Table 5 | Univariate logistic regression analysis on the live birth rate (LBR).
Supplementary Table 6 | Multivariate analysis on the live birth rate (LBR) with backward approach regression.
Supplementary Table 7 | Univariate logistic regression analysis on the abortion rate (AR).
Supplementary Table 8 | Multivariate analysis on the abortion rate (AR) with backward approach regression.
References
1. Nagore E, Martinez-Garcia MA, Gomez-Olivas JD, Manrique-Silva E, Martorell A, Bañuls J, et al. Relationship between type 2 diabetes mellitus and markers of cutaneous melanoma aggressiveness: an observational multicentric study in 443 patients with melanoma*. Br J Dermatol (2021) 185(4):756–63. doi: 10.1111/bjd.19813
2. Wang X, Xian T, Jia X, Zhang L, Liu L, Man F, et al. A cross-sectional study on the associations of insulin resistance with sex hormone, abnormal lipid metabolism in T2dm and igt patients. Medicine (2017) 96(26):e7378. doi: 10.1097/MD.0000000000007378
3. Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res (2018) 2018:1–9. doi: 10.1155/2018/7457269
4. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet (2017) 389(10085):2239–51. doi: 10.1016/S0140-6736(17)30058-2
5. Wang W-Y, Liu X, Gao X-Q, Li X, Fang Z-Z. Relationship between acylcarnitine and the risk of retinopathy in type 2 diabetes mellitus. Front Endocrinol (2022) 13:834205. doi: 10.3389/fendo.2022.834205
6. Creţu D, Cernea S, Onea CR, Pop R-M. Reproductive health in women with type 2 diabetes mellitus. Hormones (2020) 19(3):291–300. doi: 10.1007/s42000-020-00225-7
7. Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol (2020) 11:1582. doi: 10.3389/fimmu.2020.01582
8. Yu Y, Li J, Jiang Y, Zaid M, Zhao Q, Wang N, et al. Association between reproductive factors and type 2 diabetes: A cross-sectional study. Int J Environ Res Public Health (2022) 19(2):1019. doi: 10.3390/ijerph19021019
9. Thong EP, Codner E, Laven JSE, Teede H. Diabetes: A metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol (2020) 8(2):134–49. doi: 10.1016/S2213-8587(19)30345-6
10. Whitworth KW, Baird DD, Stene LC, Skjaerven R, Longnecker MP. Fecundability among women with type 1 and type 2 diabetes in the norwegian mother and child cohort study. Diabetologia (2011) 54(3):516–22. doi: 10.1007/s00125-010-2003-6
11. McGrogan A, Snowball J, de Vries CS. Pregnancy losses in women with type 1 or type 2 diabetes in the uk: an investigation using primary care records. Diabetes Med (2014) 31(3):357–65. doi: 10.1111/dme.12332
12. Diagnosis and classification of diabetes mellitus. Diabetes Care (2013) 36 Suppl 1(Suppl 1):S67–74. doi: 10.2337/dc13-S067
13. Codner E, Merino PM, Tena-Sempere M. Female reproduction and type 1 diabetes: from mechanisms to clinical findings. Hum Reprod Update (2012) 18(5):568–85. doi: 10.1093/humupd/dms024
14. Kim C, Karvonen-Gutierrez C, Kong S, Arends V, Steffes M, McConnell DS, et al. Antimüllerian Hormone among Women with and without Type 1 Diabetes: The Epidemiology of Diabetes Interventions and Complications Study and the Michigan Bone Health and Metabolism Study. Fertil Steril (2016) 106(6):1446–52. doi: 10.1016/j.fertnstert.2016.07.009
15. Codner E, Escobar-Morreale HF. Clinical review: hyperandrogenism and polycystic ovary syndrome in women with type 1 diabetes mellitus. J Clin Endocrinol Metab (2007) 92(4):1209–16. doi: 10.1210/jc.2006-2641
16. Strotmeyer ES, Steenkiste AR, Foley TP Jr., Berga SL, Dorman JS. Menstrual cycle differences between women with type 1 diabetes and women without diabetes. Diabetes Care (2003) 26(4):1016–21. doi: 10.2337/diacare.26.4.1016
17. Gaete X, Vivanco M, Eyzaguirre FC, López P, Rhumie HK, Unanue N, et al. Menstrual cycle irregularities and their relationship with hba1c and insulin dose in adolescents with type 1 diabetes mellitus. Fertil Steril (2010) 94(5):1822–6. doi: 10.1016/j.fertnstert.2009.08.039
18. Sjöberg L, Pitkäniemi J, Haapala L, Kaaja R, Tuomilehto J. Fertility in people with childhood-onset type 1 diabetes. Diabetologia (2013) 56(1):78–81. doi: 10.1007/s00125-012-2731-x
19. Jonasson JM, Brismar K, Sparén P, Lambe M, Nyrén O, Ostenson CG, et al. Fertility in women with type 1 diabetes: A population-based cohort study in Sweden. Diabetes Care (2007) 30(9):2271–6. doi: 10.2337/dc06-2574
20. Lorenzen T, Pociot F, Johannesen J, Kristiansen OP, Nerup J. A population-based survey of frequencies of self-reported spontaneous and induced abortions in danish women with type 1 diabetes mellitus. Danish iddm epidemiology and genetics group. Diabetes Med (1999) 16(6):472–6. doi: 10.1046/j.1464-5491.1999.00087.x
21. Artasensi A, Pedretti A, Vistoli G, Fumagalli L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules (2020) 25(8):1987. doi: 10.3390/molecules25081987
22. Lambrinoudaki I, Paschou SA, Armeni E, Goulis DG. The interplay between diabetes mellitus and menopause: clinical implications. Nat Rev Endocrinol (2022) 18(10):608–22. doi: 10.1038/s41574-022-00708-0
23. Mattsson K, Nilsson-Condori E, Elmerstig E, Vassard D, Schmidt L, Ziebe S, et al. Fertility outcomes in women with pre-existing type 2 diabetes—a prospective cohort study. Fertility Sterility (2021) 116(2):505–13. doi: 10.1016/j.fertnstert.2021.02.009
24. Qi L, Liu Y-p, Wang S-m, Shi H, Chen X-l, Wang N-n, et al. Abnormal bmi in male and/or female partners are deleterious for embryonic development and pregnancy outcome during art process: A retrospective study. Front Endocrinol (2022) 13:856667. doi: 10.3389/fendo.2022.856667
25. Brouwer J, Laven JSE, Hazes JMW, Schipper I, Dolhain RJEM. Levels of serum anti-müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis: serum amh and ovarian reserve in women with ra. Arthritis Care Res (2013) 65(9):1534–8. doi: 10.1002/acr.22013
26. Nayki U, Onk D, Balci G, Nayki C, Onk A, Gunay M. The effects of diabetes mellitus on ovarian injury and reserve: an experimental study. Gynecologic Obstetric Invest (2016) 81(5):424–9. doi: 10.1159/000442287
27. Cedars MI. Evaluation of female fertility—Amh and ovarian reserve testing. J Clin Endocrinol Metab (2022) 107(6):1510–9. doi: 10.1210/clinem/dgac039
28. Yang W, Lin C, Zhang M, Lv F, Zhu X, Han X, et al. Assessment of ovarian reserve in patients with type 1 diabetes: A systematic review and meta-analysis. Endocrine (2022) 77(2):205–12. doi: 10.1007/s12020-022-03091-y
29. Soto N, Iñiguez G, López P, Larenas G, Mujica V, Rey RA, et al. Anti-müllerian hormone and inhibin B levels as markers of premature ovarian aging and transition to menopause in type 1 diabetes mellitus. Hum Reprod (2009) 24(11):2838–44. doi: 10.1093/humrep/dep276
30. Yang X, Hu R, Wang Z, Hou Y, Song G. Associations between serum folate level and homa-ir in chinese patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes (2023) 16:1481–91. doi: 10.2147/dmso.S409291
31. Zhou M, Zhu L, Cui X, Feng L, Zhao X, He S, et al. The triglyceride to high-density lipoprotein cholesterol (Tg/hdl-C) ratio as a predictor of insulin resistance but not of β Cell function in a chinese population with different glucose tolerance status. Lipids Health Dis (2016) 15:104. doi: 10.1186/s12944-016-0270-z
32. Yang WB, Wang HL, Mao JT, Chen Z, Xu JW, Wang LH, et al. The correlation between ct features and insulin resistance levels in patients with T2dm complicated with primary pulmonary tuberculosis. J Cell Physiol (2020) 235(12):9370–7. doi: 10.1002/jcp.29741
33. Wang Z, Wang YJ, Liu ZY, Li Q, Kong YW, Chen YW, et al. Effect of insulin resistance on recurrence after radiofrequency catheter ablation in patients with atrial fibrillation. Cardiovasc Drugs Ther (2023) 37(4):705–13. doi: 10.1007/s10557-022-07317-z
34. Zhao J, Hong X, Zhang H, Dai Q, Huang K, Zhang X, et al. Pre-pregnancy maternal fasting plasma glucose levels in relation to time to pregnancy among the couples attempting first pregnancy. Hum Reprod (2019) 34(7):1325–33. doi: 10.1093/humrep/dez069
35. Ringholm L, Damm P, Mathiesen ER. Improving pregnancy outcomes in women with diabetes mellitus: modern management. Nat Rev Endocrinol (2019) 15(7):406–16. doi: 10.1038/s41574-019-0197-3
36. Tavakolian Arjmand A, Nouri M, Tavakolian Arjmand S. Surprisingly low infertility rate in married type 2 diabetic women: A rather curious paradox to the current opinion of insulin resistance as the joint pathogenesis of poly cystic ovary syndrome and type 2 diabetes mellitus. Diabetes Metab Syndr (2015) 9(4):201–4. doi: 10.1016/j.dsx.2015.08.007
37. Łebkowska A, Adamska A, Karczewska-Kupczewska M, Nikołajuk A, Otziomek E, Milewski R, et al. Serum anti-müllerian hormone concentration in women with polycystic ovary syndrome and type 1 diabetes mellitus. Metabolism (2016) 65(5):804–11. doi: 10.1016/j.metabol.2016.02.005
38. Liu S, Ma S, Li Y. The average gonadotrophin dosage per follicle is predictive of ovarian response and cumulative live birth chances after in vitro fertilization: A retrospective cohort study. BMC Womens Health (2023) 23(1):45. doi: 10.1186/s12905-023-02195-5
39. Gerber RS, Fazzari M, Kappy M, Cohen A, Galperin S, Lieman H, et al. Differential impact of controlled ovarian hyperstimulation on live birth rate in fresh versus frozen embryo transfer cycles: A society for assisted reproductive technology clinic outcome system study. Fertil Steril (2020) 114(6):1225–31. doi: 10.1016/j.fertnstert.2020.06.021
40. Sreerangaraja Urs DB, Wu WH, Komrskova K, Postlerova P, Lin YF, Tzeng CR, et al. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int J Mol Sci (2020) 21(10):3592. doi: 10.3390/ijms21103592
41. Ratchford AM, Esguerra CR, Moley KH. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Mol Endocrinol (2008) 22(12):2643–54. doi: 10.1210/me.2007-0495
42. Predheepan D, Daddangadi A, Uppangala S, Laxminarayana SLK, Raval K, Kalthur G, et al. Experimentally induced hyperglycemia in prepubertal phase impairs oocyte quality and functionality in adult mice. Endocrinology (2022) 163(9):bqac121. doi: 10.1210/endocr/bqac121
43. Pala HG, Pala EE, Artunc Ulkumen B, Aktug H, Yavasoglu A, Korkmaz HA, et al. The protective effect of granulocyte colony-stimulating factor on endometrium and ovary in a rat model of diabetes mellitus. Gynecol Obstet Invest (2014) 78(2):94–100. doi: 10.1159/000363239
Keywords: retrospective cohort study, type 2 diabetes mellitus, ovarian reserve, anti-mullerian hormone, embryonic development, assisted reproductive outcomes
Citation: Qin X, Du J, He R, Li Y, Zhu Q, Li Y, Li H and Liang X (2023) Adverse effects of type 2 diabetes mellitus on ovarian reserve and pregnancy outcomes during the assisted reproductive technology process. Front. Endocrinol. 14:1274327. doi: 10.3389/fendo.2023.1274327
Received: 08 August 2023; Accepted: 27 October 2023;
Published: 15 November 2023.
Edited by:
Vineet Kumar Maurya, Baylor College of Medicine, United StatesReviewed by:
Tsung-Hsien Lee, Chung Shan Medical University, TaiwanBei Shi, China Medical University, China
Copyright © 2023 Qin, Du, He, Li, Zhu, Li, Li and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolei Liang, liangxl07@lzu.edu.cn
 Xue Qin
Xue Qin Junhong Du
Junhong Du Yi Li1
Yi Li1 Xiaolei Liang
Xiaolei Liang