- 1Geriatric Diseases Institute of Chengdu, Department of Endocrine and Metabolism, Chengdu Fifth People’s Hospital (The Second Clinical Medical College, Affiliated Fifth People’s Hospital of Chengdu University of Traditional Chinese Medicine), Chengdu, China
- 2Department of Nephrology, Chengdu Third People’s Hospital, Chengdu, China
Background: With the increasing incidence of diabetes, diabetic foot ulcer(DFU) has become one of the most common and serious complications in people with diabetes. DFU is associated with significant morbidity and mortality, and can also result in significant economic, social and public health burdens. Due to peripheral neuropathy, peripheral vascular disease, hyperglycemic environment, inflammatory disorders and other factors, the healing of DFU is impaired or delayed, resulting in the formation of diabetic chronic refractory ulcer. Because of these pathological abnormalities in DFU, it may be difficult to promote wound healing with conventional therapies or antibiotics, whereas platelet-rich plasma(PRP) can promote wound healing by releasing various bioactive molecules stored in platelets, making it more promising than traditional antibiotics. Therefore, the purpose of this systematic review is to summarize and analyze the efficacy of PRP in the treatment of DFU.
Methods: A literature search was undertaken in PubMed, CNKI, EMB-ASE, the Cochrane Library, the WanFang Database and the WeiPu Database by computer. Included controlled studies evaluating the efficacy of PRP in the treatment of diabetic foot ulcers. The data extraction and assessment are on the basis of PRISMA.
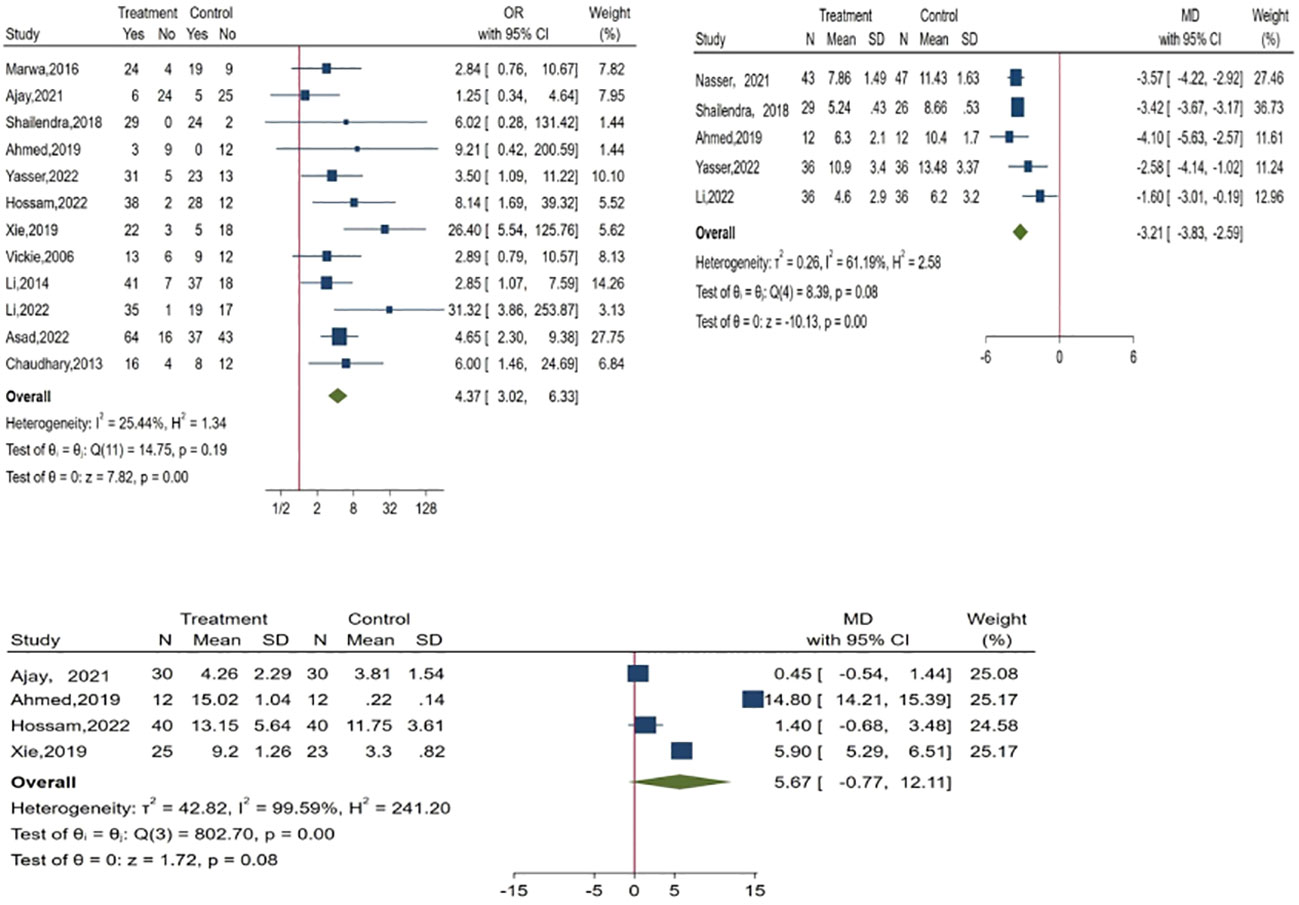
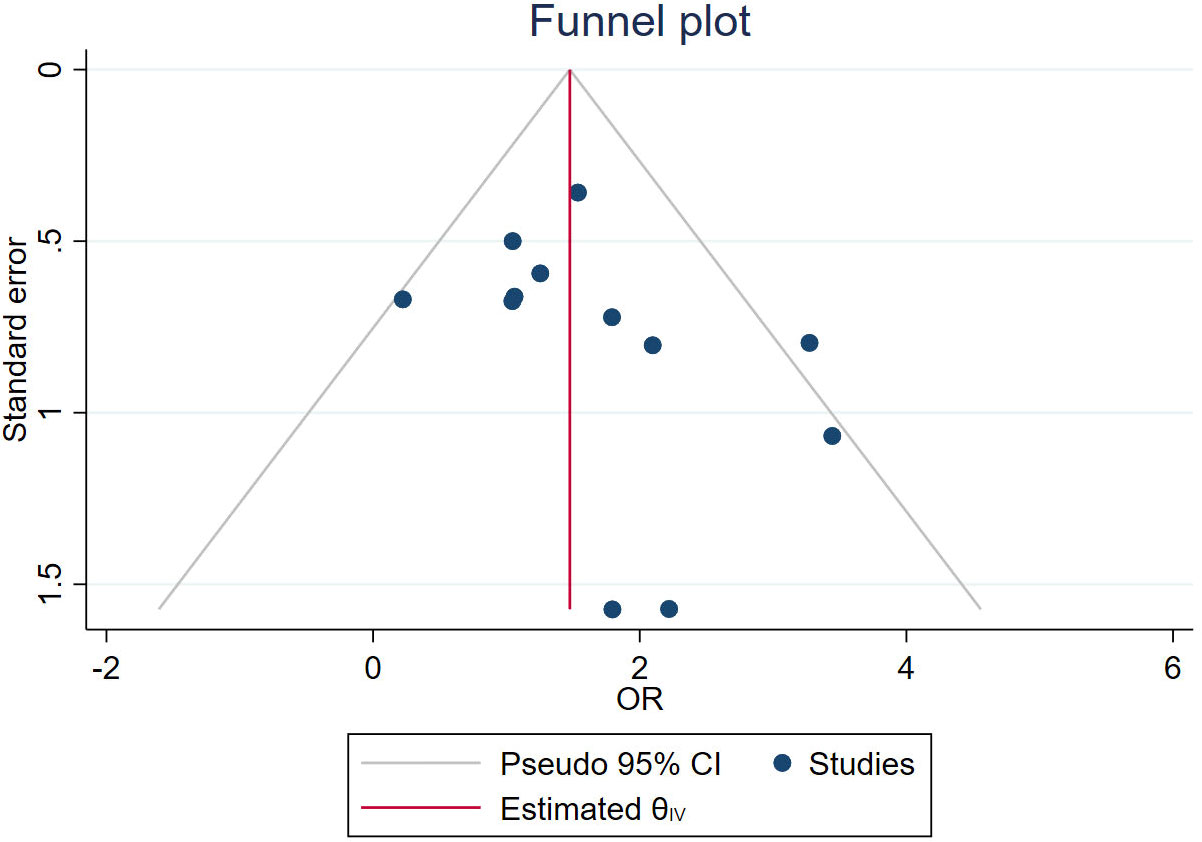
Results: Twenty studies were evaluated, and nineteen measures for the evaluation of the efficacy of PRP in DFU treatment were introduced by eliminating relevant duplicate measures. The efficacy measures that were repeated in various studies mainly included the rate of complete ulcer healing, the percentage of ulcer area reduction, the time required for ulcer healing, wound complications (including infection rate, amputation rate, and degree of amputation), the rate of ulcer recurrence, and the cost and duration of hospitalization for DFU, as well as subsequent survival and quality of life scores. One of the most important indicators were healing rate, ulcer area reduction and healing time. The meta-analysis found that PRP was significantly improve the healing rate(OR = 4.37, 95% CI 3.02-6.33, P < 0.001) and shorten the healing time(MD = -3.21, 95% CI -3.83 to -2.59,P < 0.001)of patients with DFU when compared to the conventional treatment, but there was no significant difference in reducing the of ulcer area(MD = 5.67, 95% CI -0.77 to 12.11,P =0.08>0.05 ).
Conclusion: The application of PRP to DFU can improve ulcer healing rate and shorten ulcer healing time, but more clinical data are needed to clarify some efficacy measures. At the same time, a standardized preparation process for PRP is essential.
Introduction
The global incidence of diabetes is increasing rapidly. The International Diabetes Federation(IDF) estimates that the prevalence of diabetes will increase from 10.5%(536.6 million people in the 20-79 age group) in 2021 to 12.2%(783.2 million people in the 20-79 age group) by 2045 (1). It is projected that nearly half of adults (44.7 percent; 239.7 million people in the 20-79 age group) do not know they have diabetes, and people may be more susceptible to microvascular and macrovascular complications in an asymptomatic diabetic state (2).
Diabetic foot ulcer (DFU) is one of the most common and serious complications in patients with diabetes (3–6) and is characterized by complex management, high morbidity, and high mortality (7). The annual incidence of diabetic foot around the world ranges from 9.1 million to 26.1 million (8, 9), with a global prevalence of about 6.3%, which mostly occurs in patients with type 2 diabetes, the elderly, and people with a longer duration of diabetes (10). In China, the prevalence of DFU is increasing with the increase of the incidence of diabetes year by year. According to statistics, the incidence of DFU in people over 50 years old in China is as high as 8.1% (3, 11). DFU continue to be an important cause of hospitalization in patients with diabetes and form the basis of 40-70% of diabetic non-traumatic lower limb amputations (12, 13).
Relevant reports have also shown that nearly 88% of lower leg amputations are associated with diabetic foot ulcers (14). In addition, the global annual cost of DFU treatment and amputation is approximately US $10.9 billion (15), and the cost of DFU treatment in China will rise from the current US $4.9 billion to US $7.4 billion by 2030 (16). Thus, DFU is associated with significant morbidity and mortality, as well as significant economic, social, and public health burdens.
Therefore, the treatment of DFU has become an urgent problem. Currently, the first-line routine treatment for DFU includes blood glucose control, conventional treatment (infection management, debridement, wound discharge, dressing), and angioplasty for ischemic peripheral artery disease (PAD) (17). However, the current treatment of DFU remains unsatisfactory. It has been reported that the median healing time of DFU without surgery is about 12 weeks (18), and about 20% of patients still have no healing after 1 year, with a recurrence rate of 40% in the same year (3). Therefore, the development of a fast, effective, and economical treatment for DFU is an important issue. In recent years, related studies have found that the use of stem cells or growth factors can form the basis of a new treatment, which can restore the body's normal healing process. Of these, PRP is of great interest because platelets possess a variety of growth factors, which are essential for tissue repair and regeneration, and have antibacterial properties in traumatic injuries (19, 20). PRP is a plasma preparation rich in platelets with a higher concentration than whole blood (21). The concentration of platelets in its plasma is above baseline, ranging from 150×103/dL to 400 x103/dL (22), which is 4-5 times higher than in whole blood (23, 24). The classic method of PRP preparation consists of two steps, the first step will be centrifugation to separate the blood components into three layers: a red blood cell layer, a light-colored coating layer (which contains most platelets and white blood cells) and poor quality platelet plasma, and the second step harvests concentrated platelets in a small volume of plasma, called PRP (25). The role of PRP in wound healing is mainly through the release of various bioactive molecules stored in platelets. In recent years, many studies have conducted relevant analysis on the efficacy of PRP in the treatment of DFU, but they are only limited to a few indicators, and there are still different conclusions, such as: Tasmania et al. concluded that the use of PRP in DFU promoted wound healing, reduced ulcer volume, reduced the time to complete wound healing, and reduced the incidence of adverse events, with no difference in the probability of wound complications (26). This is consistent with previous findings (27–31). However, Ajay et al. concluded that PRP had no significant effect on promoting ulcer healing (32). As one of the most important and common complications of diabetic patients, DFU has a profound impact on the prognosis, amputation and even death of patients. As a new method to treat DFU, the efficacy of PRP in DFU is worth further study. Therefore, the main purpose of this review is to review the results of various studies on the efficacy of PRP in the treatment of DFU.
Review method
Search strategy
This review was registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols(INPLASY).The registration number was INPLASY2023110003.A literature search was undertaken in PubMed, CNKI, EMBASE, the Cochrane Library, the WanFang Database and the WeiPu Database by computer. The retrieval time was from the establishment of the database to June 2023, using the combination of subject terms and free words. The search terms included "Diabetes", "Diabetic foot ulcer", "Platelet-rich plasma", "Diabetic complications" and "Efficacy”. The search strategies for each database were presented in Appendix 1.
Inclusion and exclusion criteria
The included studies were clinical trials (including randomized controlled trials、case-controlled trials、prospective observational) and retrospective studies (there were no language or location restrictions).We excluded case reports, letters, reviews. We included studies evaluating the efficacy of PRP in the treatment of DFU. Since the conventional first-line treatment of DFU includes blood glucose control, infection management, debridement, wound undressing, dressing, and vascular surgery for PAD (17), therefore, the relevant efficacy indicators included in our study mainly included wound healing rate, healing time, ulcer area reduction rate, ulcer recurrence rate, amputation rate or follow-up surgical treatment rate, infection rate, adverse event, length of stay, hospitalization cost, etc.
Data extraction and quality evaluation
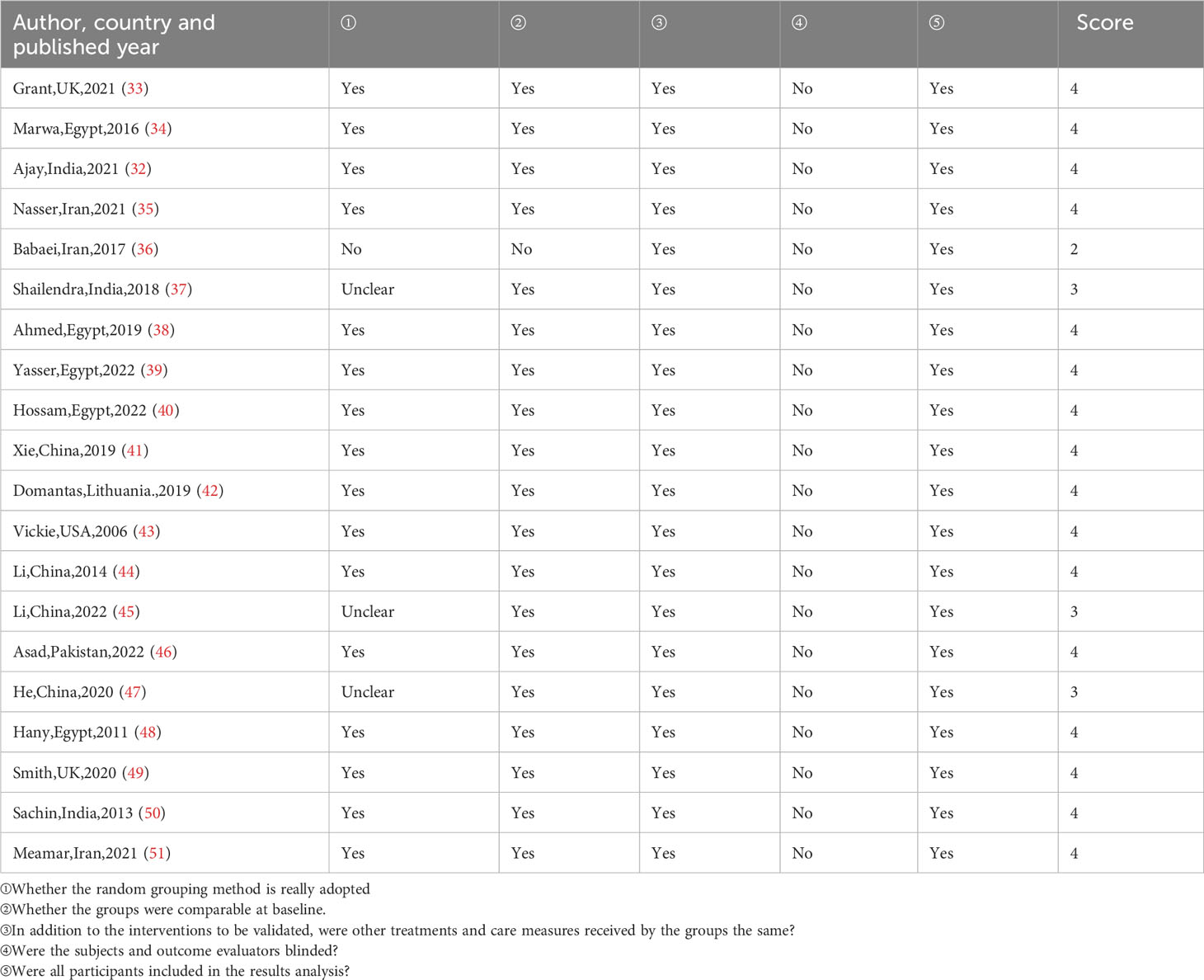
Two evaluators independently searched the database according to the inclusion and exclusion criteria, searched the full text of the initially included literatures, and extracted the literature data using a unified table, including the author's name, publication year, country, study type, research topic, number of studies, research time, main outcome indicators, and research conclusions. The included studies were evaluated from five aspects: randomization method, baseline comparability, intervention measures, blind method, and result analysis by using the Centre for Evidence-Based Medicine at Oxford University, UK. Evaluators made "yes", "no", "unclear" judgments for each evaluation item. We recorded the scores by using a scoring method ranging from 0 to 5, with 1 point for each project. The total score ≤2 points was considered as low-quality research, and ≥3 points was considered as high quality research.
Statistical analysis
We used the Stata or R software for statistical analysis, using relative hazard (RR) and 95% confidence interval (CI) as the evaluation index of the results, represented by mean difference and 95% CI. First, heterogeneity was assessed using the X2 test (a=0.05) and a quantitative analysis of I2 for heterogeneity (I2 ≥ 50%) conducted. In cases of no heterogeneity between the research results, the meta-analysis was conducted. In cases of statistical heterogeneity between the research results, the source of heterogeneity was further analyzed, and the random heterogeneity model was used after excluding the influence of obvious clinical heterogeneity. Funnel maps created using the Stata software were employed to detect publication bias.
Results
Study selection
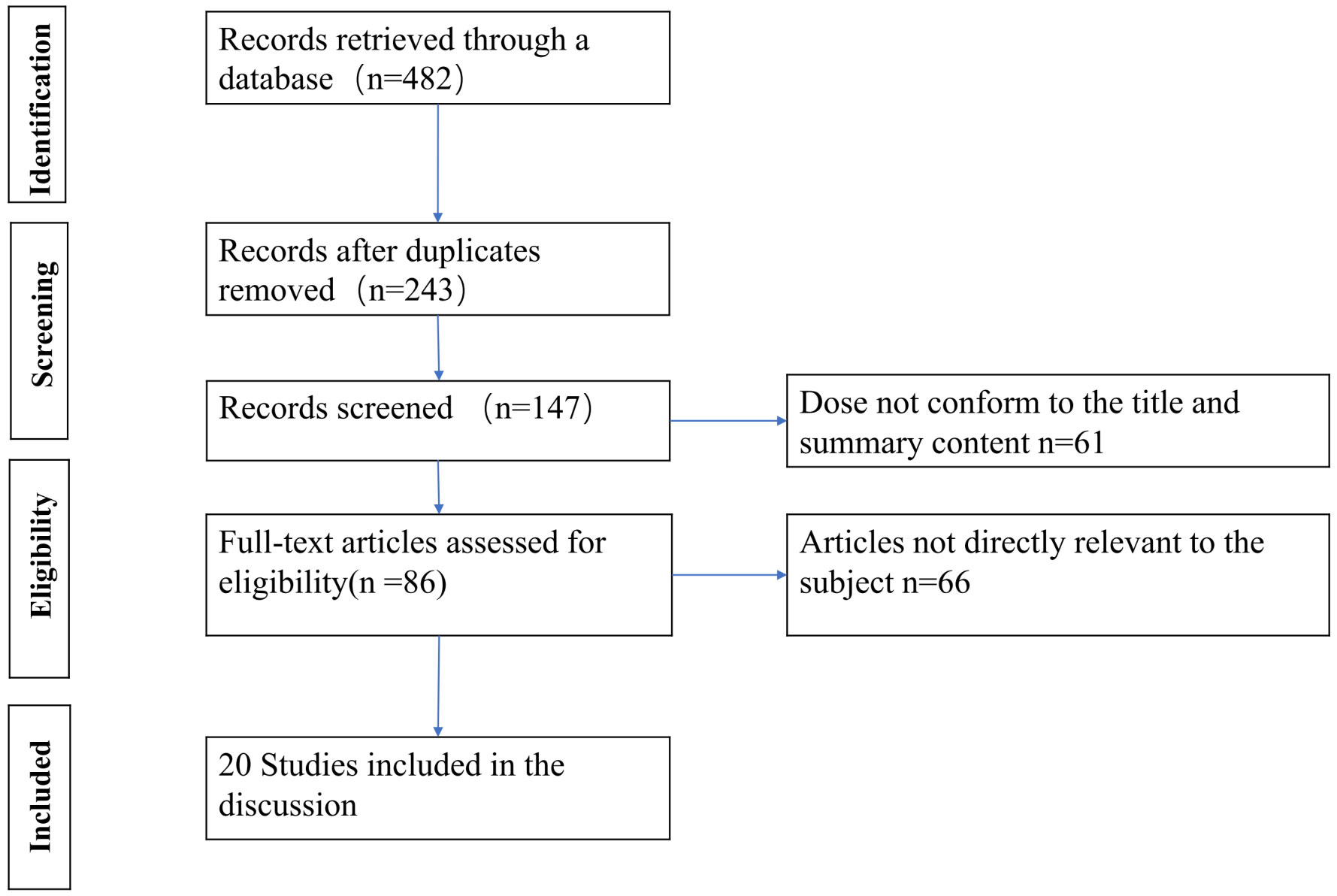
According to the pre-designed literature search strategy, a total of 482 articles were retrieved by June 2023, and after removing duplicate studies, we found 243. Then after reviewing the title and abstract, another 96 articles were excluded and 147 articles were reviewed in detail, of which 86 were deemed likely to qualify for this review. Ultimately, we included 20 studies in this review. The remaining 66 studies were excluded due to a lack of data on the efficacy of PRP in DFU in the full text. We adhered to reporting and conduct guidance based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (Figure 1).
Characteristics of studies
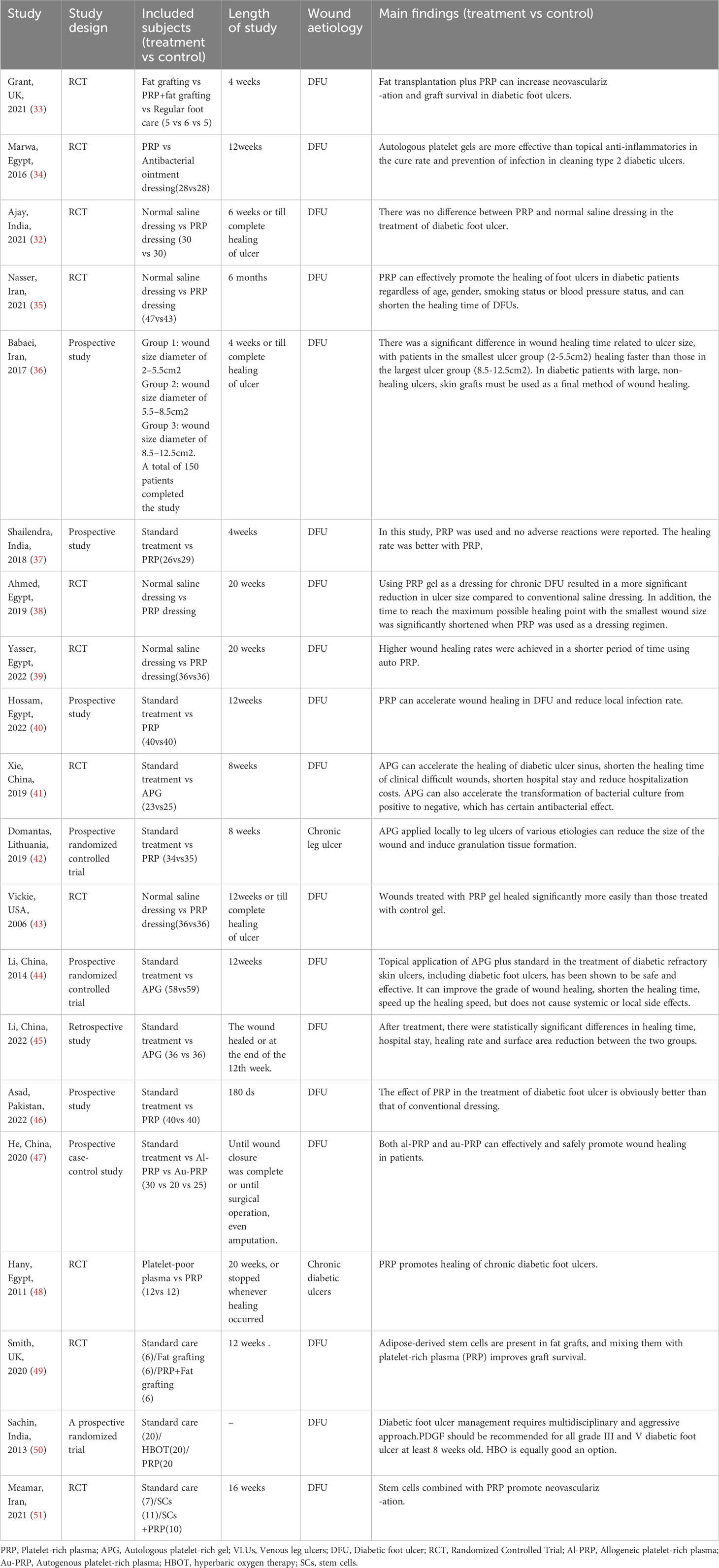
We summarized the basic characteristics of the included studies, as shown in Table 1. The included studies were published between 2005 and 2023, with five studies from Egypt (34, 38–40, 48), four from China (41, 44, 45, 52), three each from India (32, 37, 50) and Iran (35, 36, 51), two from the United Kingdom (33, 49), and one each from the United States (43), Pakistan (46), and Lithuania (42). The studies included seven different interventions: fat transplantation, fat transplantation +PRP, PRP, conventional therapy (standard care, saline dressing), hyperbaric oxygen therapy, stem cell transplantation, and stem cell transplantation +PRP. Among them, there were 14 studies comparing PRP with conventional treatment, 2 studies comparing fat transplantation, fat transplantation +PRP with conventional treatment, 1 study comparing different types of PRP, 1 study comparing hyperbaric oxygen and stem cell transplantation with PRP and conventional treatment respectively, and 1 study comparing the effect of PRP on diabetic feet with different wound sizes.
After quality evaluation, 19 studies scored ≥3 points, and only 1 study scored ≤2 points, which was good included in the study quality. The results were shown in Table 2. Many studies failed to score in the category of blinded or not, possibly because the study of DFU in the PRP group was an open-label study, both the patient and the investigator knew the nature of the study and the assignment of the study group, so a blind approach could not be implemented.
Overview of the efficacy of PRP in the treatment of DFU
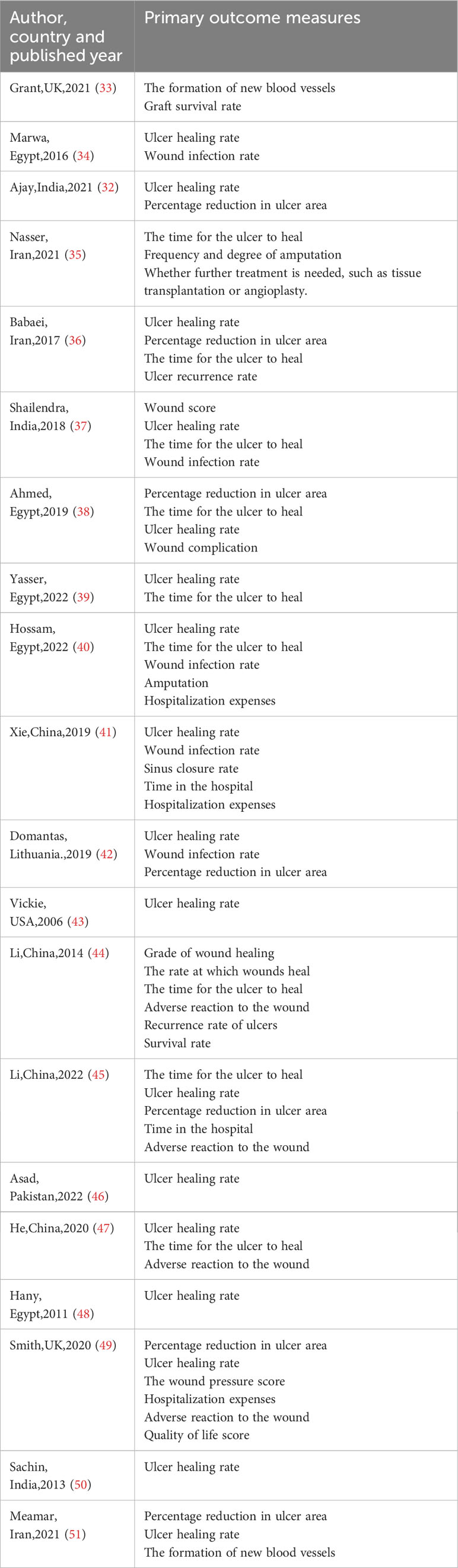
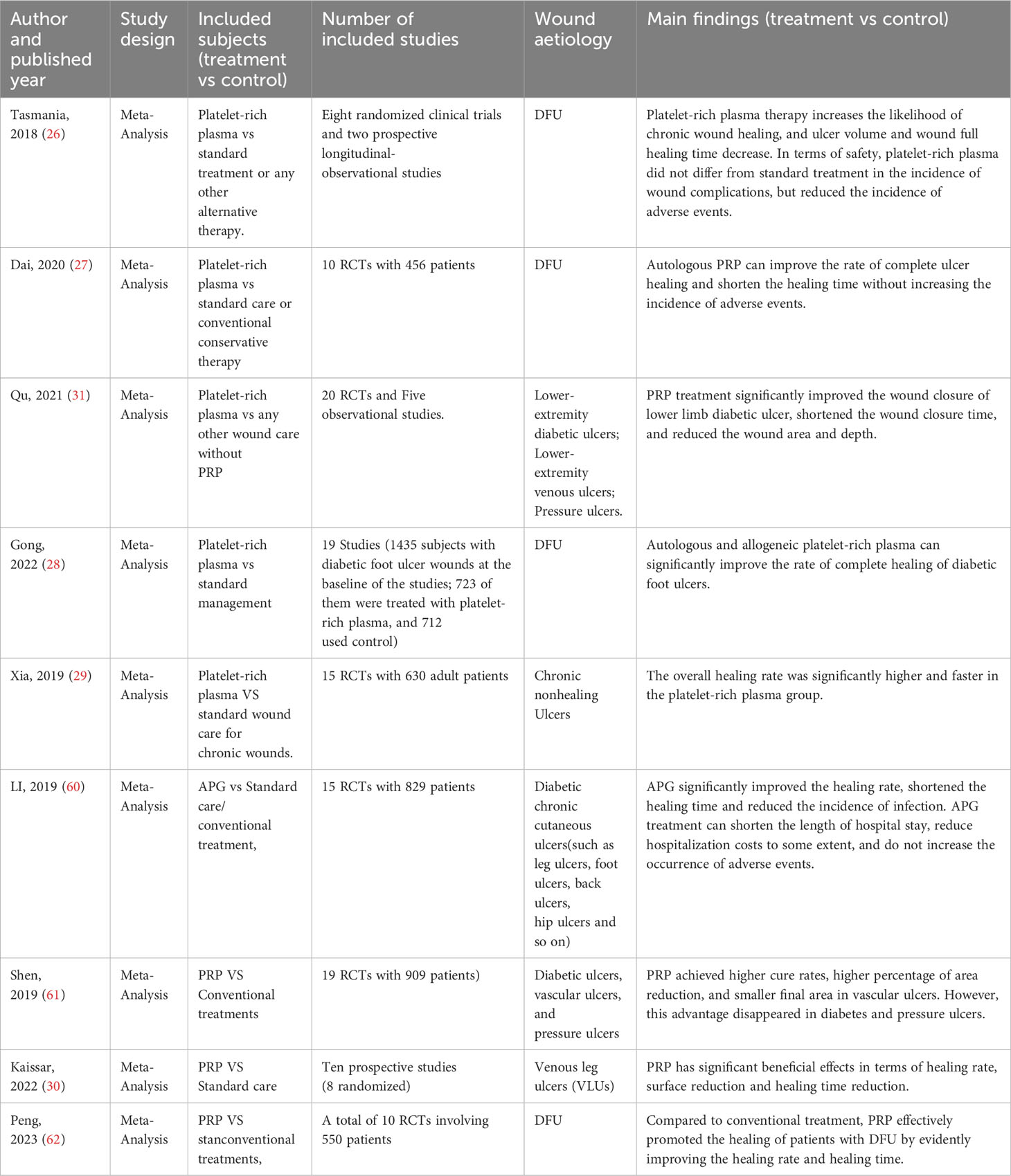
A total of 63 measures of efficacy of PRP for diabetic foot were reported (Table 3). By eliminating the duplicate measures reported in the study, we finally introduced 19 measures for the evaluation of the efficacy of PRP in DFU treatment. The efficacy measures that were repeated in various studies mainly included the rate of complete ulcer healing, the percentage of ulcer area reduction, the time required for ulcer healing, wound complications (including infection rate, amputation rate, and degree of amputation), the rate of ulcer recurrence, and the cost and duration of hospitalization for DFU, as well as subsequent survival and quality of life scores. Among these studies, twelve studies reported the complete healing rate of DFU patients after treatment, the results of meta-analysis showed that the use of PRP resulted in significantly higher complete-healed DFU compared to conventional treatment (OR = 4.37, 95% CI 3.02-6.33, P < 0.001) as shown in Figure 2.1. Five studies reported that the healing time of patients with DFU treated using PRP was significantly shorter compared to conventional treatment (MD=-3.21, 95% CI -3.83 to -2.59,P < 0.001)as shown in Figure 2.2. Four studies reported that the ulcer area of patients with DFU treated using PRP was no significantly reduced compared to conventional treatment(MD=5.67, 95% CI -0.77 to 12.11,P =0.08>0.05 )as shown in Figure 2.3. Figure 3 shows the funnel diagram after adjustments for comparison. Most studies on the funnel plot are symmetrically distributed on both sides of the vertical line at X = 0, indicating no significant publication bias.
Discussion
DFU is one of the most common, serious and costly complications of diabetes and the leading cause of hospitalization for people with diabetes worldwide (53). DFU is also the main cause of lower limb amputation in diabetic patients, which often leads to disability (54, 55), emotional disorders, socio-economic problems, and severely impaired quality of life, and even death in severe cases (56, 57). Studies have found that about 15%-25% of people with diabetes have experienced DFU during their lifetime (3, 57–59).
Through meta-analysis, we found that compared with conventional or standard care, the use of PRP in DFU can effectively improve the ulcer healing rate, and shorten the ulcer healing time, which is consistent with the results of previous studies (34, 37–40, 43, 46, 48). However, there was no significant difference in reducing the increase of ulcer area, which may be due to the small number of included study data and large heterogeneity.
In addition, a study has found that PRP can still effectively promote the healing of DFU and shorten the healing time after excluding factors such as age, gender, smoking status and blood pressure status of diabetic patients, but had no significant effect on reducing the need for amputation, the level of amputation, or the need for further treatment (such as graft or angioplasty) (35). He et al. divided PRP into autogenous PRP(au-PRP)and allogeneic PRP(al-PRP), and found that PRP in both groups could effectively and safely promote wound healing in DFU compared with conventional dressing treatment, suggesting that al-PRP could be used as a ready solution for DFU when au-PRP was limited (45). The efficacy of PRP is also different among ulcers of different sizes. Babaei et al. 's prospective study grouped diabetic foot wounds according to the size of ulcers and found a significant difference in wound healing time, which was related to the size of ulcers. Patients with the smallest ulceration group (2-5.5cm2) had faster wound healing time than those with the largest ulceration group (8.5-12.5cm2).According to this study, PRP is not recommended for large, non-healing ulcers in DFU, and skin graft must be used as the final method of wound healing (36). In the long-term follow-up of patients, Li et al. found that there was no significant difference in the long-term recurrence rate of ulcers between the PRP group and the control group (44). We collected meta-analyses related to PRP treatment of skin ulcers in the past 5 years and summarized them, as shown in Table 4. The results showed that PRP had a positive effect on promoting ulcer healing in the treatment of chronic skin ulcers, including DFU, venous ulcers of lower extremities and pressure ulcers. In two recent meta-analyses, Gong and Peng et al. found that PRP treatment of DFU increased the possibility of wound healing, reduced the ulcer volume, and reduced the time of complete wound healing (28, 63). This is consistent with the results of our meta-analysis. Secondly, the meta-analysis of Tasmania also showed that in terms of safety, platelet-rich plasma and standard treatment had no difference in the probability of wound complications or recurrence, but overall reduced the incidence of adverse events (26). These results are consistent with the findings of the meta-study by Dai,Qu (27–31, 62). Although there is no same conclusion about whether or not DFU treated by PRP has the same effect on patients' later amputation and the degree of amputation, it has been found in related studies that the overall amputation rate of DFU patients in PRP group is lower (62). PRP alone is used to treat DFU, which can effectively increase the healing rate of ulcers and shorten the healing time. In addition, relevant studies have also found that when PRP is combined with other treatment modalities, it can also work to a certain extent. Some studies reported that the addition of PRP to fat grafts resulted in increased angiogenesis in fat grafts and thus improved the viability of fat graft cells (33, 47, 49, 52, 60, 61, 64–67). In another study, the addition of PRP to mesenchymal stem cells also observed significant neovascularization and more significant wound reduction (51). Yin et al. found that VSD combined with PRP could also significantly shorten the healing time and improve the healing rate of ulcers (68).
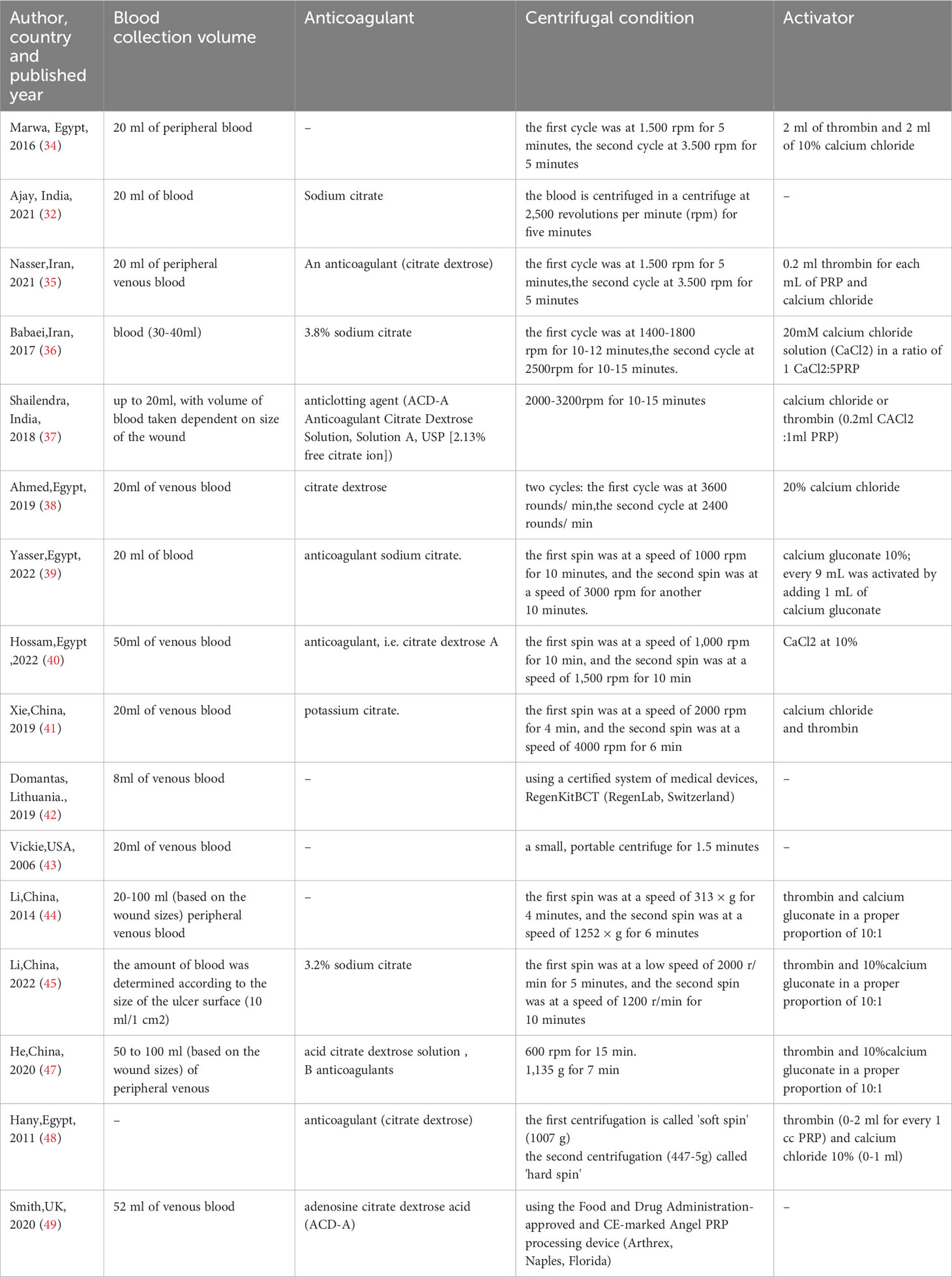
The activity of PRP is related to many factors in the preparation process, which to some extent affects the efficacy of PRP in the treatment of diabetic foot. We summarized the factors related to PRP preparation in the included studies, as shown in Table 5. We can find that an important factor affecting the preparation of PRP is the centrifuge conditions, such as force and duration, which are significantly different in different studies. Another important factor is the difference in activators, and different activators may affect the release of bioactive molecules and the cleavage of fibrinogen. In addition, we can see that PRP preparation is mostly extracted from patients' peripheral blood, and there are individual differences among different patients, which may also cause different PRP activities prepared under the same preparation conditions.
PRP for diabetic foot can effectively reduce the ulcer area, improve the ulcer healing rate, and to a certain extent reduce the infection rate of the wound and reduce the occurrence of complications. The reason why PRP can play such a curative effect is on the one hand due to the particularity of DFU healing, on the other hand, it depends on the mechanism of PRP. Changes in the micro-environment due to diabetes mellitus (DM) alter normal cell recruitment and activation and lead to impaired or delayed wound healing (69–71). In this way, the wound is disconnected from the normal process and does not heal for a long time, resulting in the formation of diabetic chronic refractory ulcers. At present, it is generally believed that peripheral neuropathy and peripheral vascular disease are the two main factors causing foot ulcers in diabetic patients (72). Secondly ,the hyperglycemic environment in diabetics also leads to increased production of advanced glycation end products (AGEs) and continued elevated levels of inflammatory cytokines (i.e., interleukin (IL-1ß) and tumor necrosis factor a (TNF-a), thus impedes the normal wound healing process (73, 74). At the same time, hyperglycemia also leads to an increased risk of infection, and the rapid spread of infection and high microbial burden also adversely affect the wound healing process. In addition, inflammation disorders are also a hallmark of diabetes and underlie many complications of diabetes, including diabetic ulcers (75). Given these pathological abnormalities present in diabetes, it is recommended that specific synthetic growth factors be used topically to manage diabetic foot ulcer wounds. PRP's effect on wound healing is mainly through the release of various bioactive molecules stored in platelets, including PDGF, transforming growth factor β(TGF-β), VEGF, epithelial growth factor (EGF), and adhesion molecules such as fibrin, fibronectin, and hyalenin (76, 77). These factors are known to regulate processes such as cell migration, attachment, proliferation, and differentiation, and to play an important role in wound healing and regeneration by binding to specific cell surface receptors to promote the accumulation of extracellular matrix(ECM) (76, 78–80). In addition to growth factors, PRP include many important proteins, such as fibrin, which not only provide scaffolds for tissue regeneration, but also promote wound contraction, blood clotting, and wound closure (81, 82). In addition, activated PRP contains a variety of antibacterial proteins. Previous studies have shown that activated PRP can inhibit staphylococcus aureus, staphylococcus epidermidis, escherichia coli, klebsiella pneumoniae, and methicillin-resistant staphylococcus aureus , without drug resistance, and has synergistic effects with antibacterial agents (83–85). The combination of these action characteristics makes PRP more promising than conventional antibiotic prescribing (85).
Limitations
In this study, employed a comprehensive search strategy for key review tasks that contains all of the studies that assessed the efficacy evaluation of PRP for DFU, however, it is possible that some unpublished data were missed. Second, there are too few data on some indicators of the efficacy of PRP in DFU treatment (such as amputation rate, degree of amputation, and need for further treatment (tissue transplantation or angioplasty), so more clinical data are needed for further study.
Conclusion
PRP can release various bioactive molecules and antibacterial proteins, which makes it effective in improving ulcer healing rate and shortening ulcer healing time when used in DFU. In the future, more studies are needed to further explore the efficacy of PRP. Secondly, the efficacy of PRP in the treatment of DFU is largely affected by various factors in the preparation process, so a standardized preparation process is essential.
Author contributions
HO: Investigation, Methodology, Writing – original draft, Writing – review & editing. YT: Writing – review & editing. FY: Writing – review & editing. XR: Data curation, Writing – review & editing. JY: Methodology, Writing – review & editing. HC: Formal Analysis, Writing – review & editing. YY: Validation, Writing – review & editing.
Funding
This project was supported by the Research and Development of intelligent system for sustainable Lifestyle Management of overweight or obese people of Chengdu Science and Technology Department(No. 2022-YF05-01340-SN) ,the Study on the efficacy and safety of different low carbohydrate diet patterns in the remission of Type 2 diabetes Mellitus of Chengdu Health Commission (No. : 2022357).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1256081/full#supplementary-material
Abbreviations
PRP, platelet-rich plasma; APG, autologous platelet-rich; VLUs:venous leg ulcers; DFU, diabetic foot ulcer; RCT, randomized controlled trial; Al-PRP, allogeneic platelet-rich plasma; Au-PRP, autogenous platelet-rich plasma; VSD, vacuum sealing drainage; ECM, extracellular matrix; IDF, international diabetes federation; DM, diabetes mellitus; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; AGEs, advanced glycation end products; IL-1ß, interleukin; TNF-a, tumor necrosis factor a; PAD, peripheral artery disease; TGF-β, transforming growth factor β; EGF, epithelial growth factor; PRISMA, the Preferred Reporting Items for Systematic Review and Meta-Analysis.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, et al. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract (2022) 183:109118. doi: 10.1016/j.diabres.2021.109118
3. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. New Engl J Med (2017) 376(24):2367–75. doi: 10.1056/NEJMra1615439
4. Lavery LA, Davis KE, Berriman SJ, Braun L, Nichols A, Kim PJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen (2016) 24:112–26. doi: 10.1111/wrr.12391
5. Zeng X, Tang Y, Hu K, Jiao W, Ying L, Zhu L, et al. Three-week topical treatment with placenta-derived mesenchymal stem cells hydrogel in a patient with diabetic foot ulcer: a case report. Med (2017) 96:e9212. doi: 10.1097/MD.0000000000009212
6. Bus SA. The role of pressure offloading on diabetic foot ulcer healing and prevention of recurrence. Plast Reconstr Surg (2016) 138(3 suppl):179S–87S. doi: 10.1097/PRS.0000000000002686
7. Dai J, Jiang C, Chen H, Chai Y. Assessment of the risk factors of multidrug-resistant organism infection in adults with type 1 or type 2 diabetes and diabetic foot ulcer. Can J Diabetes (2019). doi: 10.1016/j.jcjd.2019.10.009
9. Volmer-Thole M, Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci (2016) 17:917. doi: 10.3390/ijms17060917
10. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis†. Ann Med (2017) 49:106–16. doi: 10.1080/07853890.2016.1231932
11. Chinese Guidelines for prevention and treatment of diabetic foot (2019 edition). Chin J Diab (2019) 11(2):92–108.
12. Alves FLMT, Laporta GZ. Prevalence and factors associated with lower limb amputation in individuals with type II diabetes mellitus in a referral hospital in Fortaleza, Ceará, Brazil: A hospital-based cross-sectional study. Heliyon (2020) 6:e04469. doi: 10.1016/j.heliyon.2020.e04469
13. Sarfo-Kantanka O, Sarfo FS, Kyei I, Agyemang C, Mbanya JC. Incidence and determinants of diabetes-related lower limb amputations in Ghana, 2010-2015- a retrospective cohort study. BMCEndocr Disord (2019) 19:27. doi: 10.1186/s12902-019-0353-8
14. Alvarsson A, Sandgren B, Wendel C, Alvarsson M, Brismar K. A retrospective analysis of amputation rates in diabetic patients: can lower extremity amputations be further prevented? Cardiovasc Diabetol (2012) 11:1–11. doi: 10.1186/1475-2840-11-18
15. Jodheea-Jutton A, Hindocha S, Bhaw-Luximon A. Health economics of diabetic foot ulcer and recent trends to accelerate treatment. Foot (2022) 52:101909. doi: 10.1016/j.foot.2022.101909
16. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
17. Riedel U, Schüßler E, Härtel D, Keiler A, Nestoris S, Stege H. Wound treatment in diabetes patients and diabetic foot ulcers. Hautarzt (2020) 71:835–42. doi: 10.1007/s00105-020-04699-9
18. Morbach S, Furchert H, Gröblinghoff U, Hoffmeier H, Kersten K, Klauke G-T, et al. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care (2012) 35:2021–7. doi: 10.2337/dc12-0200
19. Drago L, Bortolin M, Vassena C, Taschieri S, Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol (2013) 13(1):47. doi: 10.1186/1471-2180-13-47
20. Cieslik-Bielecka A, Bielecki T, Gazdzik TS, Arendt J, Król W, Szczepanski T. Autologous platelets and leukocytes can improve healing of infected high-energy soft tissue injury. Transfus Apher Sci (2009) 41(1):9–12. doi: 10.1016/j.transci.2009.05.006
21. Malanga GA, Goldin M. PRP: review of the current evidence for musculoskeletal conditions. Curr Phys Med Rehab Rep (2014) 2:1–15. doi: 10.1007/s40141-013-0039-5
22. Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY, Chung HY, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. ArchPlast Surg (2013) 40(5):530–5. doi: 10.5999/aps.2013.40.5.530
23. Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg (2006) 118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf
24. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classifification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fifibrin (L-PRF). Trends Biotechnol (2009) 27:158–67. doi: 10.1016/j.tibtech.2008.11.009
25. Shao S, Pan R, Chen Y. Autologous platelet-rich plasma for diabetic foot ulcer. Trends Endocrinol Metab (2020). doi: 10.1016/j.tem.2020.10.003
26. Pino-Sedeño TD, Trujillo-Martín MM, Andia I. Platelet-rich plasma for the treatment of diabetic foot ulcers: A meta-analysis. Wound Repair Regener (2019) 27(2):170–82. doi: 10.1111/wrr.12690
27. Dai J, Jiang C, Sun Y. Autologous platelet-rich plasma treatment for patients with diabetic foot ulcers: a meta-analysis of randomized studies. J Diabetes Complications (2020) 34(8):107611. doi: 10.1016/j.jdiacomp.2020.107611
28. Gong F, Zhang Y, Gao. J. Effect of platelet-rich plasma vs standard management for the treatment of diabetic foot ulcer wounds: A meta-analysis. Int Wound J (2023) 20(1):155–63. doi: 10.1111/iwj.13858
29. Xia Y, Zhao J, Xie J. The efficacy of platelet-rich plasma dressing for chronic nonhealing ulcers: A meta-analysis of 15 randomized controlled trials. Plast Reconstr Surg (2019) 144(6):1463–74. doi: 10.1097/PRS.0000000000006281
30. Yammine K, Ghanimeh J, Agopian SJ. PRP versus standard of care for venous leg ulcers: A systematic review and meta-analysis of prospective comparative studies. Int J Low Extrem Wounds (2022) 14:15347346221094424. doi: 10.1177/15347346221094424
31. Qu W, Wang Z, Hunt C. The effectiveness and safety of platelet-rich plasma for chronic wounds: A systematic review and meta-analysis. Mayo Clin Proc (2021) 96(9):2407–17. doi: 10.1016/j.mayocp.2021.01.030
32. Gupta A, Channaveera C, Sethi S. Efficacy of intra-lesional platelet rich plasma in diabetic foot ulcer. J Am Podiatr Med Assoc (2021) 111(3):7. doi: 10.7547/19-149
33. Nolan GS, Smith OJ, Heavey S. Histological analysis of fat grafting with platelet-rich plasma for diabetic foot ulcers-A randomised controlled trial. Int Wound J (2022) 19(2):389–98. doi: 10.1111/iwj.13640
34. Ahmed M, Reffat S, Hassan A, Eskander F. Platelet rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg (2016) 38:206–11. doi: 10.1016/j.avsg.2016.04.023
35. Alamdari NM, Shafiee A, Mirmohseni A. Evaluation of the efficacy of platelet-rich plasma on healing of clean diabetic foot ulcers: A randomized clinical trial in Tehran, Iran. Diabetes Metab Syndr (2021) 15(2):621–6. doi: 10.1016/j.dsx.2021.03.005
36. Babaei V, Afradi H, Gohardani HZ. Management of chronic diabetic foot ulcers using platelet-rich plasma. J Wound Care (2017) 26(12):784–7. doi: 10.12968/jowc.2017.26.12.784
37. Singh SP, Kumar V, Pandey A. Role of platelet-rich plasma in healing diabetic foot ulcers: a prospective study. J Wound Care (2018) 27(9):550–6. doi: 10.12968/jowc.2018.27.9.550
38. Elsaid A, El-Said M, Emile S. Randomized controlled trial on autologous platelet-rich plasma versus saline dressing in treatment of non-healing diabetic foot ulcers. World J Surg (2020) 44(4):1294–301. doi: 10.1007/s00268-019-05316-0
39. Orban YA, Soliman MA, Hegab YH. Autologous platelet-rich plasma vs conventional dressing in the management of chronic diabetic foot ulcers. Wounds (2022) 33(2):36–42. doi: 10.25270/wnds/2022.3642
40. Hossam EM, Alserr AHK, Antonopoulos CN. Autologous platelet rich plasma promotes the healing of non-ischemic diabetic foot ulcers. A randomized controlled trial. Ann Vasc Surg (2022) 82:165–71. doi: 10.1016/j.avsg.2021.10.061
41. Xie J, Fang Y, Zhao Y. Autologous platelet-rich gel for the treatment of diabetic sinus tract wounds: A clinical study. J Surg Res (2020) 247:271–9. doi: 10.1016/j.jss.2019.09.069
42. Rainys D, Cepas A, Dambrauskaite K. Effectiveness of autologous platelet-rich plasma gel in the treatment of hard-to-heal leg ulcers: a randomised control trial. J Wound Care (2019) 28(10):658–67. doi: 10.12968/jowc.2019.28.10.658
43. Driver VR, Hanft J, Fylling CP. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage (2006) 52(6):68–70. 72, 74 passim.
44. Li L, Chen D, Wang C. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regener (2015) 23(4):495–505. doi: 10.1111/wrr.12294
45. He M, Guo X, Li T. Comparison of allogeneic platelet-rich plasma with autologous platelet-rich plasma for the treatment of diabetic lower extremity ulcers. Cell Transplant (2020) 29:963689720931428. doi: 10.1177/0963689720931428
46. Ullah A, Jawaid SI, Qureshi PNAA. Effectiveness of injected platelet-rich plasma in the treatment of diabetic foot ulcer diseasen. Cureus (2022) 14(8):e28292. doi: 10.7759/cureus.28292
47. Pires Fraga MF, Nishio RT, Ishikawa RS, Perin LF, Helene A, Malheiros CA. Increased survival of free fat grafts with platelet-rich plasma in rabbits. J Plast Reconstr Aesthet Surg (2010) 63(12):e818–22. doi: 10.1016/j.bjps.2010.07.003
48. Setta HS, Elshahat A, Elsherbiny K. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J (2011) 8(3):307–12. doi: 10.1111/j.1742-481X.2011.00797.x
49. Smith OJ, Leigh R, Kanapathy M, Macneal P, Jell G, Hachach-Haram N, et al. Fat grafting and platelet-rich plasma for the treatment of diabetic foot ulcers: A feasibility-randomised controlled trial. Int Wound J (2020) 17(6):1578–94. doi: 10.1111/iwj.13433
50. Khandelwal S, Chaudhary P, Poddar DD, Saxena N, Singh RAK, Biswal UC. Comparative study of different treatment options of grade III and IV diabetic foot ulcers to reduce the incidence of amputations. Clinics Pract (2013) 3:e9. doi: 10.4081/cp.2013.e9
51. Meamar R, Ghasemi-Mobarakeh L, Norouzi M-R, Siavash M, Hamblin MR, Fesharaki M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int Immunopharmacol (2021) 101:108282. doi: 10.1016/j.intimp.2021.108282
52. Nakamura S, Ishihara M, Takikawa M, Murakami K, Kishimoto S, Nakamura S, et al. Platelet rich plasma (PRP) promotes survival of fat-grafts in rats. Ann Plast Surg (2010) 65(1):101–6. doi: 10.1097/SAP.0b013e3181b0273c
53. Alavi A, Sibbald RG, Mayer D, Goodman L, Botros M, Armstrong DG, et al. Diabetic foot ulcers: part I. Pathophysiology and prevention. J Am Acad Dermatol (2014) 70(1):1.e1–1.e18. doi: 10.1016/j.jaad.2013.06.055
54. Chinese society of Diabetes, Chinese Society of Infectious Diseases, Chinese society of tissue repair and regeneration. Chinese guidelines for the prevention and treatment of diabetic foot (2019 edition)(I). Chin J Diab (2019) 11:92–108.
55. Bolton L. Diabetic foot ulcer: treatment challenges. Wounds (2022) 34:175–7. doi: 10.25270/wnds/2022.175177
56. Aragón-Sánchez J. Clinical–pathological characterization of diabetic foot infections: grading the severity of osteomyelitis. Int J Low Extrem Wounds (2012) 11(2):107–12. doi: 10.1177/1534734612447617
57. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diab (2015) 6(1):37. doi: 10.4239/wjd.v6.i1.37
58. Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower-extremity ulcers. N Engl J Med (2018) 378(3):302–3. doi: 10.1056/NEJMc1715237
59. Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: clinical review. J Tissue Viabil (2016) 25:229–36. doi: 10.1016/j.jtv.2016.06.005
60. DeRossi R, Coelho AC, Mello GS, Frazílio FO, Rejane C, Leal B, et al. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras (2009) 24(4):276–81. doi: 10.1590/S0102-86502009000400006
61. Findikcioglu F, Findikcioglu K, Yavuzer R, Lortlar N, Atabay K. Effect of intraoperative platelet-rich plasma and fibrin glue application on skin flap survival. J Craniofac Surg (2012) 23(5):1513–7. doi: 10.1097/SCS.0b013e3182597ce6
62. Li Y, Gao Y, Gao Y. Autologous platelet-rich gel treatment for diabetic chronic cutaneous ulcers: A meta-analysis of randomized controlled trials. J Diabetes (2019) 11(5):359–69. doi: 10.1111/1753-0407.12850
63. Peng Y, Wang J, Liu X, Zhou Y, Jia S, Xu J, et al. Efficacy of platelet-rich plasma in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. Ann Vasc Surg (2023) 22:S0890-5096(23)00332-1. doi: 10.1016/j.avsg.2023.05.045
64. Sönmez TT, Vinogradov A, Zor F, Kweider N, Lippross S, et al. The effect of platelet rich plasma on angiogenesis in ischemic flaps in VEGFR2-luc mice. Biomater (2013) 34(11):2674–82. doi: 10.1016/j.biomaterials.2013.01.016
65. Takikawa M, Sumi Y, Ishihara M, Kishimoto S, Nakamura S, Yanagibayashi S, et al. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J Surg Res (2011) 170(1):e189–96. doi: 10.1016/j.jss.2011.05.051
66. Li W, Enomoto M, Ukegawa M, Hirai T, Sotome S, Wakabayashi Y, et al. Subcutaneous injections of platelet-rich plasma into skin flaps modulate proangiogenic gene expression and improve sur vival rates. Plast Reconstr Surg (2012) 129(4):858–66. doi: 10.1097/PRS.0b013e3182450ac9
67. Atashi F, André-Lévigne D, Colin DJ, Germain S, Pittet-Cuénod B, Modarressi A. Does non-activated platelet-rich plasma (PRP) enhance fat graft outcome? An assessment with 3D CT-scan in mice. J Plast Reconstr Aesthet Surg (2019) 72(4):669–75. doi: 10.1016/j.bjps.2018.12.039
68. Yin X-L, Hu L, Li T, Zou Y, Li H-L. A meta-analysis on the efficacy of vacuum sealing drainage combined with autologous platelet-rich plasma in the treatment of Grade 2 and Grade 3 diabetic foot ulcers. Int Wound J (2022) 20(4):1033–41. doi: 10.1111/iwj.13956
69. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: A cellular perspective. Physiol Rev (2019) 99:665–706. doi: 10.1152/physrev.00067.2017
70. Shah A, Amini-Nik S. The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci (2017) 18(5):1068. doi: 10.3390/ijms18051068
71. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol (2020) 10(9):200223. doi: 10.1098/rsob.200223
72. Jørgensen TS, Hellsten Y, Gottlieb H, Brorson S. Assessment of diabetic foot ulcers based on pictorial material: an interobserver study. J Wound Care (2020) 29:658–63. doi: 10.12968/jowc.2020.29.11.658
73. Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. BioMed Res Int (2013) 2013:385641. doi: 10.1155/2013/385641
74. Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharm (Basel) (2020) 13. doi: 10.3390/ph13040060
75. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev (2013) 93:137–88. doi: 10.1152/physrev.00045.2011
76. Franchini M, Cruciani M, Mengoli C, Marano G, Pupella S, Veropalumbo E, et al. Effificacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus (2018) 16(6):502–13. doi: 10.2450/2018.0111-18
77. Borzini P, Mazzucco I. Platelet-rich plasma (PRP) and platelet derivatives for topical therapy. What is true from the biologic view point? ISBT Sci Ser (2007) 2(1):272–81.
78. Marti-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev (2015) 2015(10):CD008548. doi: 10.1002/14651858.CD008548.pub2
79. Martínez CE, Smith PC, Palma Alvarado VA. The inflfluence of platelet-derived products on angiogenesis and tissue repair: a concise update. Front Physiol (2015) 6:290(6). doi: 10.3389/fphys.2015.00290
80. Ueda M, Nishino Y. Cell-based cytokine therapy for skin rejuvenation. J Craniofac Surg (2010) 21:1861–6. doi: 10.1097/SCS.0b013e3181f43f0a
81. Romano F, Paolino FM, Rizzo BA, Russo A, Southworth S, Serra R, et al. The use of growth factors, CD34(+) cells and fibrin for the management of chronic venous ulcers. Int Wound J (2016) 13:1011–3. doi: 10.1111/iwj.12500
82. Houdek MT, Wyles CC, Stalboerger PG, Terzic A, Behfar A, Moran SL. Collagen and fractionated platelet-rich plasma scaffold for dermal regeneration. Plast Reconstr Surg (2016) 137:1498–506. doi: 10.1097/PRS.0000000000002094
83. Burnouf T, Chou ML, Wu YW, Su CY, Lee LW. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/ detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion (2013) 53(1):138–46. doi: 10.1111/j.1537-2995.2012.03668.x
84. Mariani E, Filardo G, Canella V, Berlingeri A, Bielli A, Cattini L, et al. Platelet-rich plasma affects bacterial growth in vitro. Cytotherapy (2014) 16:1294–304. doi: 10.1016/j.jcyt.2014.06.003
Keywords: diabetes, platelet-rich plasma, diabetic foot ulcer, therapeutic index, preparation condition
Citation: OuYang H, Tang Y, Yang F, Ren X, Yang J, Cao H and Yin Y (2023) Platelet-rich plasma for the treatment of diabetic foot ulcer: a systematic review. Front. Endocrinol. 14:1256081. doi: 10.3389/fendo.2023.1256081
Received: 10 July 2023; Accepted: 23 November 2023;
Published: 18 November 2023.
Edited by:
Dan Yan, Capital Medical University, ChinaReviewed by:
Rania ElBackly, Alexandria University, EgyptAjay Vikram Singh, Federal Institute for Risk Assessment (BfR), Germany
Copyright © 2023 OuYang, Tang, Yang, Ren, Yang, Cao and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Tang, paopaotangt@163.com; Fan Yang, yangfan102522@qq.com
†These authors have contributed equally to this work
 Hong OuYang
Hong OuYang Yi Tang1*†
Yi Tang1*†