- Department of Endocrinology, The Affiliated Hospital of Qingdao University, Qingdao, China
Circadian rhythm is an inherent endogenous biological rhythm in living organisms. However, with the improvement of modern living standards, many factors such as prolonged artificial lighting, sedentarism, short sleep duration, intestinal flora and high-calorie food intake have disturbed circadian rhythm regulation on various metabolic processes, including GLP-1 secretion, which plays an essential role in the development of various metabolic diseases. Herein, we focused on GLP-1 and its circadian rhythm to explore the factors affecting GLP-1 circadian rhythm and its potential mechanisms and propose some feasible suggestions to improve GLP-1 secretion.
Introduction
Glucagon-like peptide-1 (GLP-1) is an incretin mainly secreted by intestinal L cells (1), promoting insulin secretion in a glucose-dependent form. GLP-1 can also produce various non-glycemic effects through the systemic expression of a wide range of GLP-1 receptors (2) such as cardiovascular protection (3),lowering blood pressure (4), regulating lipid metabolism (4), and controlling gastrointestinal motility and delayed gastric emptying. A small amount of GLP-1 expression is found in the nucleus accumbens (5); because peripherally secreted GLP-1 does not cross the blood-brain barrier (6). Hence, only GLP-1 expressed in the nucleus accumbens acts on the central GLP-1R, which might be one of the reasons why GLP-1 can affect cognitive function and mood in addition to suppressing appetite (6). GLP-1 analogs are also approved as first-line drugs for type 2 diabetes and obesity (7).
Circadian rhythms are endogenous biological rhythms with a cycle of approximately 24 hours in organisms, mainly regulated in response to light and darkness changes, and are formed by various transcription factors and promoters that form an autoregulatory feedback loop (8). This feedback system is expressed not only in the supraoptic nucleus of the hypothalamus but also in peripheral tissues such as pancreatic islets, adipose tissue, gastrointestinal tract, liver and skeletal muscle (9, 10). Circadian rhythm stability is closely related to the stability of multiple metabolic pathways (8). However, the artificial lighting used to maintain a constant ambient temperature, sedentary lifestyle, and availability of cheap high-calorie food affects circadian program mechanisms (11). Disruption of circadian rhythms is a risk factor for metabolic disorders and can lead to various metabolic diseases, including impaired insulin secretion (12), abnormal glucose tolerance (12), obesity, and even diabetes (13).
This review focus on GLP-1 and its secretion rhythm as a clue to explore the factors influencing GLP-1 secretion rhythm and the role of exogenous GLP-1-like regulation in GLP-1 rhythm.
GLP-1 biological rhythm
GLP-1 is an incretin secreted by intestinal L cells. As a link between intestinal endocrine cells and pancreatic β-cells, GLP-1 can regulate insulin secretion in a glucose-dependent manner, and it is jointly responsible for approximately 50% of nutritionally induced insulin secretion with GIP (14). his phenomenon might be related to how L cells are stimulated by food to regulate GLP-1 secretion (15) and the fact that GLP-1 is rapidly hydrolyzed by the DPP-IV enzyme about 2 min after secretion (16). Thus, the temporal rhythm of GLP-1 secretion has not been found for a long time (17). Only in 2009, ola Lindgren et al. used N- and C-terminal directed antisera to measure GLP-1 concentrations after standardized food intake in healthy men and performed the first in vivo experiments revealing a temporal difference in GLP-1 secretion and demonstrating that early GLP-1 and GIP release was more pronounced in the morning than in the afternoon (18). A Further, a significant circadian rhythm in GLP-1 secretion was found in an in vivo GLP-1 test in response to OGTT in mice (19). Martchenko also identified an important role for the core biological clock gene Arnt1 in regulating time-dependent GLP-1 secretion in intestinal L cells in mice (20). Knockdown of the core biological clock gene Bmal1 in mice and transcriptional analysis of intestinal slices demonstrated that Bmal1 and its downstream target SNARE regulatory proteins are key regulatory proteins in maintaining GLP-1 circadian secretion (21–23). Additionally, Synaptotagmin-7 (24) is now considered a positive regulatory protein of GLP-1.
Furthermore, the intestinal flora regulation of GLP-1 secretion rhythm should not be neglected. The intestinal flora is not only necessary for maintaining the GLP-1 rhythm. For example, the rhythmic secretion of GLP-1 by L cells depends on the homeostasis of the intestinal flora environment (25). It also regulates central GLP-1 sensitivity and systemic metabolic processes through the microbial-gut-brain-liver axis (26, 27). This section will be discussed later.
In summary, GLP-1 has a physiological circadian secretory rhythm mediated by L cells and regulated by various core biological clock genes, as well as the intestinal environment. The homeostasis of this rhythm also plays a crucial role in connecting intestinal endocrine cells and pancreatic β-cells.
Disruption of GLP-1 secretion rhythm
Besides L-cells’ biological rhythms regulating GLP-1 release, dietary structure, obesity, prolonged light exposure, sleep disorders, and intestinal flora disorders can affect the rhythmic secretion of GLP-1.
Dietary structure
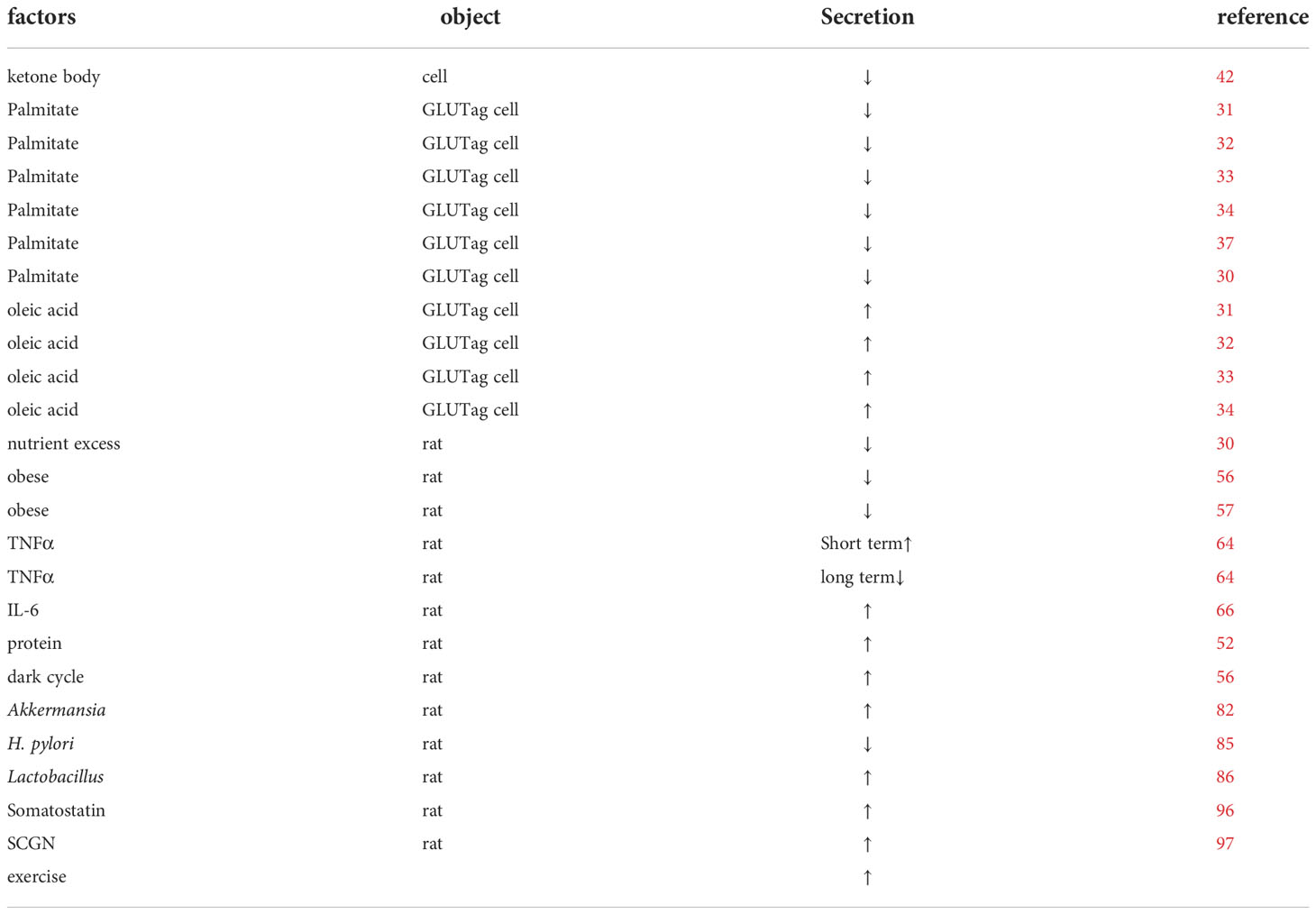
High-fat diets alter normal metabolic circadian rhythms in mice (28), and specific high-fat diets do not disrupt biological clock rhythms within the center, but can affect intestinal L-cell and islet β-cell rhythms (29). This might be related to L cells having an independent, autonomous rhythmic clock (30). The in vitro culture of the NCI-H716 human intestinal cell line revealed that nutrients such as palmitic acid, oleic acid and meat hydrolysates can stimulate GLP-1 secretion in a dose-dependent manner (31), however, long-term exposure to long-chain saturated fatty acids such as palmitic acid can lead to ceramide accumulation, caspase-3 activation, and increased DNA fragmentation leading to cell death in GLP-1- producing cells (32). It can also induce apoptosis through lipotoxicity in response to the endoplasmic reticulum (33). In contrast, long-chain unsaturated fatty acids such as oleic acid can have cytoprotective effects by reducing ceramide synthesis, attenuating reactive oxygen species (ROS) production, inhibiting caspase-3 activation, and reducing DNA fragmentation (32, 34, 35). Mice fed a high-fat diet, also disrupt L-cell circadian rhythms (36). So, in vitro cultures of mouse mGLUTag L cells (37, 38) and mouse assays (38) revealed that palmitate is a key factor affecting L cells as well as eliminating GLP-1 secretion rhythms, even at non-obesogenic doses, interfering with CLOCK : BMAL1 transcriptional activity, increasing Bmal1 transcriptional repression; and resulting in metabolic disorders (39). SIRT1 can regulate the transcription of CLOCK - and BMAL1 through the promoter E-box element (40), and regulate the expression of Dbp, Per1 and other circadian rhythm genes. SIRT1 can be affected by many factors. In hepatocytes, palmitic acid inhibits the splicing of BMAL1 and CLOCK through SIRT1 inhibition, which reduces the expression of hepatocyte genes, including Dbp and Per1 (41)。EX527, the inhibitor of SIRT1, was found to have the same inhibitory effect as palmitic acid. Resveratrol and CAY10591 were found to restore SIRT1 activity inhibited by palmitic acid. (Figure 1).

Figure 1 Palmitic acid affects the molecular clock in hepatocytes. SIRT1 can regulate the transcription of CLOCK - and BMAL1 through the promoter E-box element (40), and regulate the expression of Dbp, Per1 and other circadian rhythm genes(black lines). Palmitate can inhibit the expression of rhythm genes by suppressing BMAL1:CLOCK splicing in the form of SIRT1(orange line). EX527 is a SIRT1-specific antibody that produces the same effect as palmitate, have the same inhibitory effect as palmitic acid. CAY10591 can restore SIRT1 activity inhibited by palmitic acid.
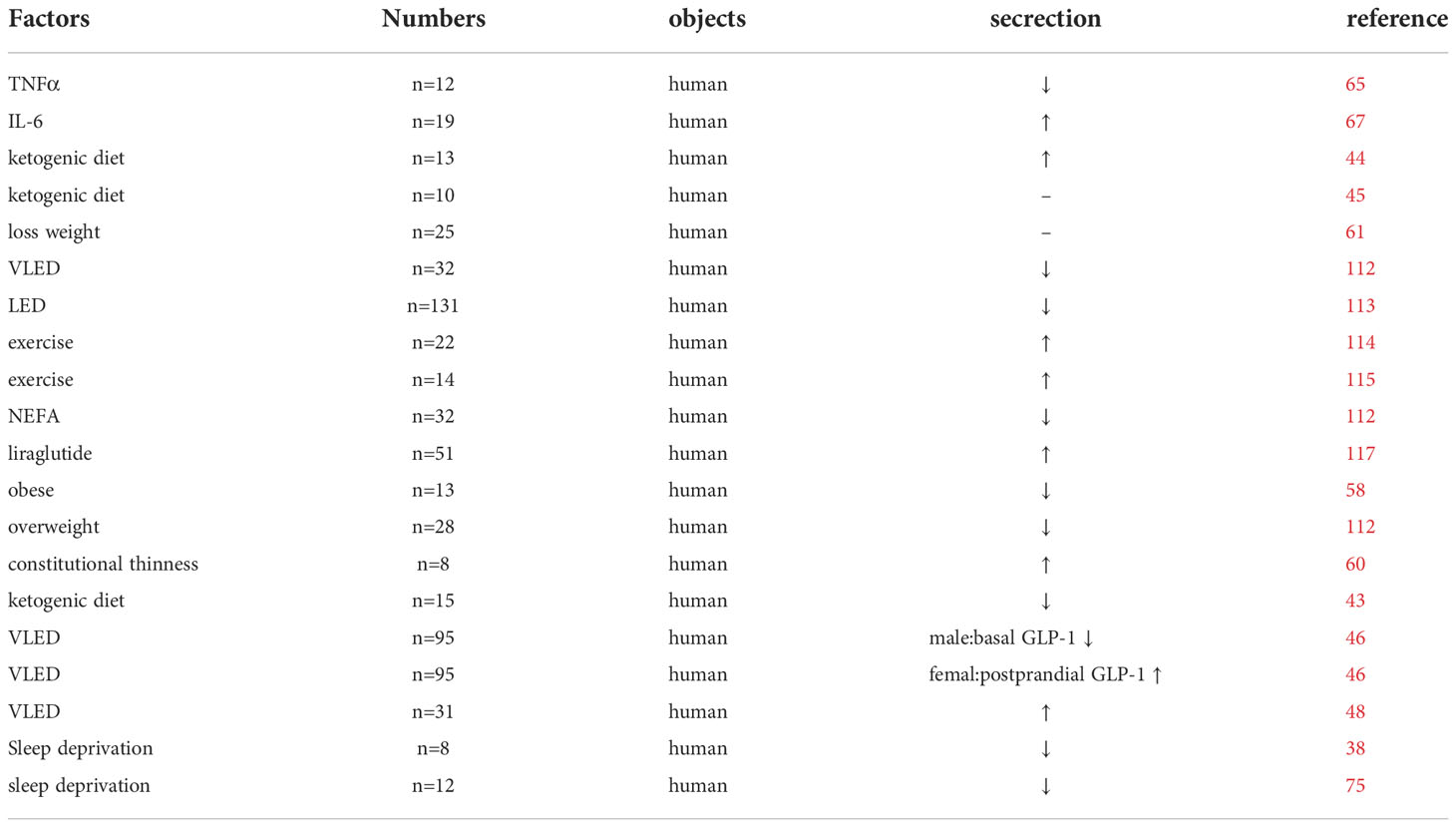
Ketogenesis might be another potential mechanism reducing GLP-1 secretion induced by a high-fat diet, as found by culturing primary intestinal endocrine cells in mice, where ketone bodies can inhibit approximately 40% of basal GLP-1 secretion (42). In clinical trials related to ketogenic diets, some short-term ketogenic diets or exercise resulted in lower fasting and postprandial levels of GLP-1 (43, 44). Nevertheless, another clinical trial in healthy men concluded that ketogenic diets do not affect GLP-1 secretion in humans (45). Moreover, some studies have found that the metabolic changes associated with a long-term ketogenic diet might have gender differences. One study has found that, after weight loss on a ketogenic diet, while basal GLP-1 levels significantly increased in both men and women, postprandial GLP-1 levels appeared significantly higher only in the female group and did not significantly differ in the male group (46). In contrast, other studies did not observe gender differences (47, 48). These differences might be related to the duration of the ketogenic diet, ketone body levels, and the metabolic differences between humans and experimental animals, but more studies are needed to prove this.
A ketogenic diet (KD) is formulated with a high fat proportion and low carbohydrate proportion and is designed to induce ketogenesis. Using unsaturated fatty acids is superior to consuming saturated fatty acids (49). A high-fat diet induces the expression of the ketogenic enzyme mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase (mhMGCs) in jejunal tissue and the production of functional ketones, which act on the fatty acid/ketone receptor FFAR3 expressed in the small intestinal epithelium to inhibit GLP-1 secretion (42). Additionally, ketone bodies, especially β-hydroxybutyric acid, inhibit inflammatory responses through multiple pathways, including the inhibition of inflammatory vesicles, especially NLRP3 production; ketone metabolism to increase adenosine levels, which are anti-inflammatory through the A1 and A1 receptor pathways; enhanced NADH oxidation; and inhibition of free radical formation (50); by increasing beneficial bacteriophages and reducing Firmicutes, improving the alpha diversity of the flora (51). This might also be why a ketogenic diet inhibits GLP-1 secretion in the short term and can improve GLP-1 in the long term.
Although carbohydrates and fats are the most important pro-secretors of GLP-1, proteins and peptides have recently been found to promote GLP-1. Shimizu’s study in rats showed that whey protein not only increased GLP-1 secretion but also prolonged GLP-1 action by inhibiting DPP-IV enzymatic activity (52). Besides, some plant proteins, such as those found in rice, maize, and peas, can also promote GLP-1 secretion (53). This might be related to various mechanisms such as increased intracellular calcium (54), extracellular signal-regulated kinase 1/2 (ERK1/2), mitogen-activated protein kinase (MAPK), and p38MAPK (55).
Obesity
Moghadam et al. have found that GLP-1 levels are lower in the obese rat group than in the lean rat group during the first 6 h of the dark cycle and in the middle of the light cycle (56). Meanwhile, postprandial GLP-1 secretion is similarly impaired in obese rats (57). Several clinical studies have found that obese patients have impaired basal and postprandial GLP-1 secretion compared to normal-weight patients (58, 59). Also, lighter-weight patients have higher levels of postprandial GLP-1 secretion compared to normal-weight or obese patients (60). In contrast, a clinical study has found that although obese and overweight patients have higher basal levels of GLP-1 than normal-weight, their secretion loss curves were flatter (61). Unlike the two previous trials, with normal-weight patients who reached standard weight through weight loss, the paradox might be because exercise weight loss alone did not restore normal GLP-1 secretion.
Lipid overload from obesity and validation might explain its effect on GLP-1 secretion. Inflammatory cell infiltration in adipose tissue, muscle, pancreas, and liver due to a saturated fatty acid diet, obesity, and elevated levels of inflammatory cytokines such as tumor necrosis factor, IL-1β, and IL-6 result in persistent chronic low-grade inflammation (62, 63). Notably, for a short time, TNFα promotes GLP-1 secretion to regulate insulin secretion after food intake to maintain glucose homeostasis (64). However, long-term exposure to TNFα impairs GLP-1 secretion (64, 65). Activation of the IL-6 transduction pathway can also increase GLP-1 secretion through the leptin pathway (66, 67). Overall, this mechanisms might be a protective compensatory measure of the organism.
Long light and short sleep
As shift and night work become more common in modern industrial societies, shorter night shift intervals do not provide sufficient recovery time to adjust circadian rhythms, resulting in poor sleep quality (68), prolonged artificial light exposure, and reduced sleep duration (69). The increase in the incidence of cancer, diabetes, cardiovascular disease, and psychiatric disorders (70–72), might also be related to disruption of sleep-wake rhythms, impaired secretion of melatonin from nighttime light, combined with obesity and a tendency to produce reactive oxygen species (73), which also affects the circadian rhythm of GLP-1 secretion.
Circadian regulation of L-cell activity in rats is highly sensitive to disturbances in circadian rhythms, as continuous light conditions eliminate normal changes in GLP-1 and insulin nutrient-induced responses and significantly impair glucose tolerance (19), Moghadam and his team similarly found that basal levels of GLP-1 were higher in rats under dark conditions (56), and sensitivity was highest (74). In a clinical trial on male volunteers, both sleep and prolonged light exposure interfered with GLP-1 secretion (30). The basal GLP-1 peak occurred at 6 am and was significantly lower after continuous light exposure compared to the normal light exposure group, although the node at which this peak occurred did not change. However, after experiencing continuous light, the postprandial GLP-1 peak increased by 24% compared to the previous one. A clinical trial by Benedict et al. in healthy men showed that patients after acute sleep deprivation had a delayed GLP-1 secretion peak after breakfast compared to normal sleep, despite no significant difference in the area under the total GLP-1 curve, for about 90 min (75).
Intestinal flora
In recent years, the role of intestinal flora in metabolism has received increasing attention. Dysbiosis is closely associated with various metabolic diseases such as obesity (76), gout (77), NAFLD (78), insulin resistance, diabetes mellitus and its complications (79, 80). Herein, we discuss the effects of the intestinal flora on the rhythmic secretion of GLP-1 and observe the mechanisms of related metabolic diseases from the perspective of GLP-1.
A homeostatic intestinal flora environment is necessary for the rhythmic secretion of GLP-1. Obesity, hyperglycemia and hyperlipidemia reduce the alpha and beta diversity of the intestinal flora (81). In germ-free mice without a 24-hour rhythm of insulin secretion, fecal transplantation returned the insulin rhythm, increased their fasting GLP-1 levels, and demonstrated that Akkermansia muciniphila and Lactobacillus are positively correlated to GLP-1 regulation (25, 82). This might be related to the glucagon 1-inducible protein P9, which induces GLP-1 secretion by activating GPCR-like downstream signals (83). Additionally, IL-6 deficiency blocks this pathway, demonstrating that Akkermansia induces GLP-1 secretion via the IL-6-P9 axis and that Lactobacillus can regulate bile acid secretion and increase GLP-1 secretion via the bile acid receptor FXR/TGR5 pathway (82). Although the roles of Firmicutes and Bacteroides in obesity need to be further clarified, they can still regulate GLP-1 secretion, and GLP-1 levels can be increased up to twofold in diet-induced obese patients treated with vancomycin compared to untreated patients (84). Helicobacter pylori eradication can also promote GLP-1 secretion and improve glucose metabolism, which may be associated with Lachnobacterium, Bifidobacterium adolescentis, Coriobacteriaceae, and other strain alterations (85). Besides, germ-free mice or antibiotic-induced mice can enhance central nervous sensitivity to leptin mediated by GLP-1RA (27).In contrast, mice supplemented with probiotic strains, such as Lactobacillus, can promote GLP-1 secretion (86–88). This increased secretion might be caused by reduced TNF-α and IL-6, inhibition of inflammation, antioxidant activity, increased short-chain fatty acid-related GLP-1 secretion, and regulation of bile acid secretion (89, 90).
Other factors
Current studies have demonstrated significant gender differences in both the structure of the supraoptic nucleus (91), electrophysiological activity (92) and the expression of androgen and estrogen receptors within the nucleus accumbens. Males express higher levels of androgens than females in the supraoptic nucleus, but lower levels of estrogen α receptors (93, 94). The expression levels of these receptors are influenced by circulating hormone levels, representing a direct interaction of gonadotropin levels with the central master clock, leading to sex differences in a wide range of physiological processes controlled by the circadian system, including the HPG axis, the HPA axis, and sleep-wake cycle (95).
Other factors also affect GLP-1 rhythm. For example, growth inhibitory hormone can act on growth inhibitory hormone receptor 5 on L cells to inhibit GLP-1 secretion (96). Knockdown of SCGN, an action-binding regulatory protein, in mice leads to a loss of GLP-1 circadian rhythm in response to glucose, demonstrating that SCGN is an important factor in maintaining GLP-1 circadian rhythm. This may be mediated by SCGN regulating secretory granules (97). The effect of diabetic models on GLP-1 rhythms is currently unclear, but a phase shift in circadian rhythm patterns can be found in high-fat diet/streptozotocin mouse models (98).
Obesity, diet, long light and short sleep, and dysbiosis of the gut flora can promote systemic chronic low-grade inflammation and oxidative stress leading to insulin resistance and increased risk of diabetes (56, 99–102). In recent years, gut flora has also been recognized as an important causative factor for diabetes (103). The GLP-1 and insulin secretion rhythm are consistent in both physiological and pathological states, and multiple factors might explain the pathological mechanisms of insulin resistance and diabetes from another perspective by altering the GLP-1 secretion rhythm by L cells.
GLP-1 circadian rhythm therapy
Disruption of GLP-1 rhythm leads to disruption of the corresponding insulin secretion rhythm. Therefore, by treating the above-related risk factors, the rhythmic secretion of re-GLP-1 can be restored and glucose metabolism can be improved.
As mentioned above, adequate sleep and a healthy diet such as a ketogenic diet can improve GLP-1 secretion through different mechanisms including inhibition of the inflammatory response and improved flora α diversity. Additionally, exercise is an important tool recommended by the ADA guidelines to prevent and treat obesity in diabetes mellitus patients (104) and can improve patients’ blood glucose levels and insulin resistance (105). Reduction of both insulin resistance after weight loss and chronic low-grade inflammation due to obesity contribute to the rhythmic recovery of GLP-1 levels. Exercise can affect the expression of various circadian rhythm-related genes (106) and influences the expression of the central hypothalamic clock, correlating with the expression of the clock genes per1 and per2 (107). Thomas et al. found that circadian rhythms could be phase-shifted by timed exercise interventions (108). They showed that early morning exercise advanced the melatonin phase, while late evening exercise delayed it. Exercise can also modulate the clock phase in skeletal muscle independent of the central clock (109). Exercise in obese mice under dark conditions increases the abundance of clock core proteins, such as BMAL1 and CLOCK proteins, in skeletal muscle (110). Adipose is an important endocrine tissue in the body, and white and brown adipose tissue are equally circadian (111). Exercise on adipose tissue can similarly regulate glucose and energy metabolism by modulating circadian gene expression in an adipose tissue-mediated manner (109).
However, weight loss through exercise and diet therapy alone does not fully restore rhythmic GLP-1 secretion, and the metabolic changes associated with diet control alone and exercise weight loss are inconsistent. Joaquín et al. showed that, despite a 5% reduction in body weight through diet control, unlike Ghrelin and YY peptide, GLP-1 levels did not change (61). Adam et al. found that after weight loss through a very-low-energy diet (VLED), GLP-1 levels were reduced compared to before weight loss (112). After 8 weeks of a low-energy diet (LED), Sloth similarly found a decrease in GLP-1 levels in subjects (113). In contrast, a decrease in GLP-1 levels was not found with exercise weight loss but rather a trend towards higher postprandial GLP-1 (114, 115). This might be related to epigenetic changes resulting from long-term obesity in patients who have lost weight after obesity. Changes in cellular stress, adipokine secretion, and lipolysis induced by weight loss (87), as well as biological drivers due to imbalances in energy supply and demand (88), contribute to rebound after weight loss. The vagus nerve might also play an important role in reducing GLP-1 secretion (116). The difference between diet and exercise might be because diet weight loss is a reduction in intake and inhibition of nonesterified fatty acids (NEFA), and elevated NEFA levels inhibit GLP-1 secretion (112). This might also be one of the reasons why dietary weight loss is more likely to rebound than exercise weight loss. From this perspective, exogenous supplementation of GLP-1 analogs can restore the autonomous GLP-1 secretion function of L cells (117) and effectively prevent weight loss failure. As the relationship between dysbiosis and metabolic diseases has been gradually studied, treatment by intestinal flora has received increasing attention. As mentioned earlier, antibiotic-induced strain changes can improve GLP-1 secretion rhythm. However, the abuse of antibiotics is not good. Therefore, supplementation with probiotics such as Lactobacillus is recommended to improve the alpha diversity of the intestinal flora (118). Additionally, dietary modification and weight loss treatment can help Firmicutes and Bacteroides abundance decrease, which might also help achieve improved intestinal flora. Nobiletin was found to improve the rhythm of GLP-1 secretion in high-fat-induced mice, and could increase fasting and postprandial GLP-1 levels. This may be related by improving lipid metabolism and modulating the structure of the intestinal flora (119, 120).
Furthermore, GLP-1 analogs, such as liraglutide, dulaglutide, and semaglutide, are now widely used in the clinic to treat patients with diabetes and obesity by various mechanisms, including anti-inflammation, emergency improvement, intestinal flora regulation, appetite suppression via the central nervous system, and weight reduction (121–123). Exogenous GLP-1 analog supplementation can restore the GLP-1 physiological secretion rhythm and the circadian rhythm of islet function (117, 124), which might be closely related to the aforementioned metabolic benefits when exogenously supplementing GLP-1 analogs.
We summarized the factors affecting the circadian rhythm GLP-1 and found that exercise can regulate the circadian rhythm (Tables 1, 2). Exercise and its associated weight loss can improve the GLP-1 secretion rhythm and might be more effective in preventing weight regain. However, the effects of diet, and dietary weight loss, are currently controversial. Short-term ketogenic diets are believed to reduce GLP-1 secretion, while long-term ketogenic diets might improve GLP-1 secretion levels, which needs further validation. Meanwhile, long-chain saturated fatty acids, represented by palmitic acid, have an inhibitory effect on circadian rhythms. Additionally, protein, peptides, and supplementation with intestinal probiotics contribute to GLP-1 secretion, while poor lifestyle habits such as long light and short sleep at night can impair GLP-1 secretion levels. Therefore, we recommend a good routine, appropriate exercise, healthy eating habits, and, if necessary, GLP-1 analogs or probiotic supplementation to improve the secretion rhythm.
In this review, we used GLP-1 and its circadian rhythm as a clue to explore the factors influencing the circadian rhythm of GLP-1 and its potential mechanisms and suggested some feasible recommendations to improve the secretory rhythm of GLP-1. This review might also provide some therapeutic recommendations for patients, help clarify the mechanisms of restoring GLP-1 secretion, and further develop relevant in treatments.
Author contributions
CL: Constructing ideas, reviewing literature, and writing papers. YL, YX: Reviewing literature and providing input. YW: Provide guidance. All authors contributed to the article and approved the submitted version.
Acknowledgements
We are grateful to all the authors who contributed to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
GLP-1, Glucagon-like peptide-1; ROS, reactive oxygen species; TNF, tumor necrosis factor α; mhMGCs, mitochondrial 3- hydroxy-3-methylglutaryl-coasynthase; MAPK, mitogen-activated protein kinase; ERK1/2, extracellular signal-regulated kinase ½; KD, ketogenic diet; SCGN, Recombinant Secretagogin; LED, low-energy diet; VLED, very-low-energy diet; NEFA, nonest-erified fatty acids; OGTT, oral glucose tolerance test.
References
1. Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab (2019) 30:72–130. doi: 10.1016/j.molmet.2019.09.010
2. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab (2013) 17(6):819–37. doi: 10.1016/j.cmet.2013.04.008
3. Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A, et al. GLP-1 receptor agonists (GLP-1RAs): Cardiovascular actions and therapeutic potential. Int J Biol Sci (2021) 17(8):2050–68. doi: 10.7150/ijbs.59965
4. Iorga RA, Bacalbasa N, Carsote M, Bratu OG, Stanescu AMA, Bungau S, et al. Metabolic and cardiovascular benefits of GLP-1 agonists, besides the hypoglycemic effect (Review). Exp Ther Med (2020) 20(3):2396–400. doi: 10.3892/etm.2020.8714
5. Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually Large intakes of food. Diabetes (2019) 68(1):21–33. doi: 10.2337/db18-0729
6. Katsurada K, Yada T. Neural effects of gut- and brain-derived glucagon-like peptide-1 and its receptor agonist. J Diabetes Investig (2016) 7 Suppl 1(Suppl 1):64–9. doi: 10.1111/jdi.12464
7. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 9. pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S125–s143. doi: 10.2337/dc22-S009
8. Gerhart-Hines Z, Lazar MA. Circadian metabolism in the light of evolution. Endocr Rev (2015) 36(3):289–304. doi: 10.1210/er.2015-1007
9. Brubaker PL, Gil-Lozano M. Glucagon-like peptide-1: The missing link in the metabolic clock? J Diabetes Investig (2016) 7 Suppl 1(Suppl 1):70–5. doi: 10.1111/jdi.12477
10. Harfmann BD, Schroder EA, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms (2015) 30(2):84–94. doi: 10.1177/0748730414561638
11. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest (2011) 121(6):2133–41. doi: 10.1172/JCI46043
12. Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: Endocrine and molecular mechanisms. Diabetol Metab Syndr (2015) 7:25. doi: 10.1186/s13098-015-0018-3
13. Gachon F, Loizides-Mangold U, Petrenko V, Dibner C. Glucose homeostasis: Regulation by peripheral circadian clocks in rodents and humans. Endocrinology (2017) 158(5):1074–84. doi: 10.1210/en.2017-00218
14. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab (2018) 27(4):740–56. doi: 10.1016/j.cmet.2018.03.001
15. Spreckley E, Murphy KG. The l-cell in nutritional sensing and the regulation of appetite. Front Nutr (2015) 2:23. doi: 10.3389/fnut.2015.00023
16. Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept (2005) 128(2):117–24. doi: 10.1016/j.regpep.2004.06.007
17. Schulz B, Ratzmann KP, Albrecht G, Bibergeil H. Diurnal rhythm of insulin sensitivity in subjects with normal and impaired glucose tolerance. Exp Clin Endocrinol (1983) 81(3):263–72. doi: 10.1055/s-0029-1210235
18. Lindgren O, Mari A, Deacon CF, Carr RD, Winzell MS, Vikman J, et al. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab (2009) 94(8):2887–92. doi: 10.1210/jc.2009-0366
19. Gil-Lozano M, Mingomataj EL, Wu WK, Ridout SA, Brubaker PL. Circadian secretion of the intestinal hormone GLP-1 by the rodent l cell. Diabetes (2014) 63(11):3674–85. doi: 10.2337/db13-1501
20. Martchenko SE, Martchenko A, Biancolin AD, Waller A, Brubaker PL. L-cell arntl is required for rhythmic glucagon-like peptide-1 secretion and maintenance of intestinal homeostasis. Mol Metab (2021) 54:101340. doi: 10.1016/j.molmet.2021.101340
21. Biancolin AD, Martchenko A, Mitova E, Gurges P, Michalchyshyn E, Chalmers JA, et al. The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol Metab (2020) 31:124–37. doi: 10.1016/j.molmet.2019.11.004
22. Wheeler SE, Stacey HM, Nahaei Y, Hale SJ, Hardy AB, Reimann F, et al. The SNARE protein syntaxin-1a plays an essential role in biphasic exocytosis of the incretin hormone glucagon-like peptide 1. Diabetes (2017) 66(9):2327–38. doi: 10.2337/db16-1403
23. Campbell JR, Martchenko A, Sweeney ME, Maalouf MF, Psichas A, Gribble FM, et al. Essential role of syntaxin-binding protein-1 in the regulation of glucagon-like peptide-1 secretion. Endocrinology (2020) 161(5). doi: 10.1210/endocr/bqaa039
24. Gustavsson N, Wang Y, Kang Y, Seah T, Chua S, Radda GK, et al. Synaptotagmin-7 as a positive regulator of glucose-induced glucagon-like peptide-1 secretion in mice. Diabetologia (2011) 54(7):1824–30. doi: 10.1007/s00125-011-2119-3
25. Martchenko SE, Martchenko A, Cox BJ, Naismith K, Waller A, Gurges P, et al. Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes (2020) 69(12):2589–602. doi: 10.2337/db20-0262
26. Wang SZ, Yu YJ, Adeli K. Role of gut microbiota in neuroendocrine regulation of carbohydrate and lipid metabolism via the microbiota-Gut-Brain-Liver axis. Microorganisms (2020) 8(4). doi: 10.3390/microorganisms8040527
27. Heiss CN, Mannerås-Holm L, Lee YS, Serrano-Lobo J, Håkansson Gladh A, Seeley RJ, et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep (2021) 35(8):109163. doi: 10.1016/j.celrep.2021.109163
28. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab (2007) 6(5):414–21. doi: 10.1016/j.cmet.2007.09.006
29. Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis Model Mech (2017) 10(6):679–89. doi: 10.1242/dmm.026609
30. Gil-Lozano M, Hunter PM, Behan LA, Gladanac B, Casper RF, Brubaker PL. Short-term sleep deprivation with nocturnal light exposure alters time-dependent glucagon-like peptide-1 and insulin secretion in male volunteers. Am J Physiol Endocrinol Metab (2016) 310(1):E41–50. doi: 10.1152/ajpendo.00298.2015
31. Reimer RA, Darimont C, Gremlich S, Nicolas-Métral V, Rüegg and K. Macé UT. A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology (2001) 142(10):4522–8. doi: 10.1210/endo.142.10.8415
32. Thombare K, Ntika S, Wang X, Krizhanovskii C. Long chain saturated and unsaturated fatty acids exert opposing effects on viability and function of GLP-1-producing cells: Mechanisms of lipotoxicity. PLoS One (2017) 12(5):e0177605. doi: 10.1371/journal.pone.0177605
33. Hayashi H, Yamada R, Das SS, Sato T, Takahashi A, Hiratsuka M, et al. Glucagon-like peptide-1 production in the GLUTag cell line is impaired by free fatty acids via endoplasmic reticulum stress. Metabolism (2014) 63(6):800–11. doi: 10.1016/j.metabol.2014.02.012
34. Ntika S, Thombare K, Aryapoor M, Kristinsson H, Bergsten P, Krizhanovskii C. Oleate increase neutral lipid accumulation, cellular respiration and rescues palmitate-exposed GLP-1 secreting cells by reducing ceramide-induced ROS. Biochimie (2019) 159:23–35. doi: 10.1016/j.biochi.2018.11.017
35. Morgan NG, Dhayal S. Unsaturated fatty acids as cytoprotective agents in the pancreatic beta-cell. Prostaglandins Leukot Essent Fatty Acids (2010) 82(4-6):231–6. doi: 10.1016/j.plefa.2010.02.018
36. Martchenko A, Brubaker PL. Effects of obesogenic feeding and free fatty acids on circadian secretion of metabolic hormones: Implications for the development of type 2 diabetes. Cells (2021) 10(9). doi: 10.3390/cells10092297
37. Martchenko A, Oh RH, Wheeler SE, Gurges P, Chalmers JA, Brubaker PL. Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Acta Physiol (Oxf) (2018) 222(4):e13007. doi: 10.1111/apha.13007
38. Gil-Lozano M, Wu WK, Martchenko A, Brubaker PL. High-fat diet and palmitate alter the rhythmic secretion of glucagon-like peptide-1 by the rodent l-cell. Endocrinology (2016) 157(2):586–99. doi: 10.1210/en.2015-1732
39. Tal Y, Chapnik N, Froy O. Non-obesogenic doses of fatty acids modulate the functionality of the circadian clock in the liver. Cell Mol Life Sci (2019) 76(9):1795–806. doi: 10.1007/s00018-019-03023-6
40. Liu J, Zhou B, Yan M, Huang R, Wang Y, He Z, et al. CLOCK and BMAL1 regulate muscle insulin sensitivity via SIRT1 in Male mice. Endocrinology (2016) 157(6):2259–69. doi: 10.1210/en.2015-2027
41. Tong X, Zhang D, Arthurs B, Li P, Durudogan L, Gupta N, et al. Palmitate inhibits SIRT1-dependent BMAL1/CLOCK interaction and disrupts circadian gene oscillations in hepatocytes. PLoS One (2015) 10(6):e0130047. doi: 10.1371/journal.pone.0130047
42. Wallenius V, Elias E, Elebring E, Haisma B, Casselbrant A, Larraufie P, et al. Suppression of enteroendocrine cell glucagon-like peptide (GLP)-1 release by fat-induced small intestinal ketogenesis: a mechanism targeted by roux-en-Y gastric bypass surgery but not by preoperative very-low-calorie diet. Gut (2020) 69(8):1423–31. doi: 10.1136/gutjnl-2019-319372
43. Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obes (Silver Spring) (2018) 26(2):269–73. doi: 10.1002/oby.22051
44. Okada TE, Quan T, Bomhof MR. Exogenous ketones lower post-exercise acyl-ghrelin and GLP-1 but do not impact ad libitum energy intake. Front Nutr (2020) 7:626480. doi: 10.3389/fnut.2020.626480
45. Vestergaard ET, Zubanovic NB, Rittig N, Møller N, Kuhre RE, Holst JJ, et al. Acute ketosis inhibits appetite and decreases plasma concentrations of acyl ghrelin in healthy young men. Diabetes Obes Metab (2021) 23(8):1834–42. doi: 10.1111/dom.14402
46. Lyngstad A, Nymo S, Coutinho SR, Rehfeld JF, Truby H, Kulseng B, et al. Investigating the effect of sex and ketosis on weight-loss-induced changes in appetite. Am J Clin Nutr (2019) 109(6):1511–8. doi: 10.1093/ajcn/nqz002
47. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur J Clin Nutr (2013) 67(7):759–64. doi: 10.1038/ejcn.2013.90
48. Nymo S, Coutinho SR, Jørgensen J, Rehfeld JF, Truby H, Kulseng B, et al. Timeline of changes in appetite during weight loss with a ketogenic diet. Int J Obes (Lond) (2018) 41(8):1224–31. doi: 10.1038/ijo.2017.96
49. Winters-van Eekelen E, Verkouter I, Peters HPF, Alssema M, de Roos BG, Schrauwen-Hinderling VB, et al. Effects of dietary macronutrients on liver fat content in adults: A systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr (2021) 75(4):588–601. doi: 10.1038/s41430-020-00778-1
50. Barrea L, Caprio M, Watanabe M, Cammarata G, Feraco A, Muscogiuri G, et al. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit Rev Food Sci Nutr (2022) p:1–17. doi: 10.1080/10408398.2022.2054935
51. Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell (2020) 181(6):1263–1275.e16. doi: 10.1016/j.cell.2020.04.027
52. Ishikawa Y, Hira T, Inoue D, Harada Y, Hashimoto H, Fujii M, et al. Rice protein hydrolysates stimulate GLP-1 secretion, reduce GLP-1 degradation, and lower the glycemic response in rats. Food Funct (2015) 6(8):2525–34. doi: 10.1039/C4FO01054J
53. Miguéns-Gómez A, Casanova-Martí À., Blay MT, Terra X, Beltrán-Debón R, Rodríguez-Gallego E, et al. Glucagon-like peptide-1 regulation by food proteins and protein hydrolysates. Nutr Res Rev (2021) 34(2):259–75. doi: 10.1017/S0954422421000019
54. Le Nevé B, Daniel H. Selected tetrapeptides lead to a GLP-1 release from the human enteroendocrine cell line NCI-H716. Regul Pept (2011) 167(1):14–20. doi: 10.1016/j.regpep.2010.10.010
55. Reimer RA. Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinases. J Endocrinol (2006) 191(1):159–70. doi: 10.1677/joe.1.06557
56. Moghadam AA, Moran TH, Dailey MJ. Alterations in circadian and meal-induced gut peptide levels in lean and obese rats. Exp Biol Med (Maywood) (2017) 242(18):1786–94. doi: 10.1177/1535370217732041
57. Dailey MJ, Moghadam AA, Moran TH. Nutrient-specific feeding and endocrine effects of jejunal infusions in obese animals. Am J Physiol Regul Integr Comp Physiol (2014) 306(6):R420–8. doi: 10.1152/ajpregu.00410.2013
58. Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab (2010) 95(2):872–8. doi: 10.1210/jc.2009-2054
59. Adam TC, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr (2005) 93(6):845–51. doi: 10.1079/BJN20041335
60. Germain N, Galusca B, Caron-Dorval D, Martin JF, Pujos-Guillot E, Boirie Y, et al. Specific appetite, energetic and metabolomics responses to fat overfeeding in resistant-to-bodyweight-gain constitutional thinness. Nutr Diabetes (2014) 4(7):e126. doi: 10.1038/nutd.2014.17
61. Galindo Muñoz JS, Jiménez Rodríguez D, Hernández Morante JJ. Diurnal rhythms of plasma GLP-1 levels in normal and overweight/obese subjects: lack of effect of weight loss. J Physiol Biochem (2015) 71(1):17–28. doi: 10.1007/s13105-014-0375-7
62. Esser N, Legrand-Poels S, Piette J, Scheen and N. Paquot AJ. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract (2014) 105(2):141–50. doi: 10.1016/j.diabres.2014.04.006
63. Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients (2019) 11(11). doi: 10.3390/nu11112579
64. Chen S, Wei W, Chen M, Qin X, Qiu L, Zhang L, et al. TNF signaling impacts glucagon-like peptide-1 expression and secretion. J Mol Endocrinol (2018) 61(4):153–61. doi: 10.1530/JME-18-0129
65. Lehrskov-Schmidt L, Lehrskov-Schmidt L, Nielsen ST, Holst JJ, Møller K, Solomon TP. The effects of TNF-α on GLP-1-stimulated plasma glucose kinetics. J Clin Endocrinol Metab (2015) 100(4):E616–22. doi: 10.1210/jc.2014-4244
66. Wueest S, Laesser CI, Böni-Schnetzler M, Item F, Lucchini FC, Borsigova M, et al. IL-6-Type cytokine signaling in adipocytes induces intestinal GLP-1 secretion. Diabetes (2018) 67(1):36–45. doi: 10.2337/db17-0637
67. Ellingsgaard H, Seelig E, Timper K, Coslovsky M, Soederlund L, Lyngbaek MP, et al. GLP-1 secretion is regulated by IL-6 signalling: a randomised, placebo-controlled study. Diabetologia (2020) 63(2):362–73. doi: 10.1007/s00125-019-05045-y
68. Kim JY, Chae CH, Kim YO, Son JS, Kim JH, Kim CW, et al. The relationship between quality of sleep and night shift rotation interval. Ann Occup Environ Med (2015) 27:31. doi: 10.1186/s40557-015-0084-x
69. Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris) (2014) 62(5):292–301. doi: 10.1016/j.patbio.2014.08.001
70. Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci (2017) 173:94–106. doi: 10.1016/j.lfs.2017.02.008
71. Smolensky MH, Sackett-Lundeen LL, Portaluppi F. Nocturnal light pollution and underexposure to daytime sunlight: Complementary mechanisms of circadian disruption and related diseases. Chronobiol Int (2015) 32(8):1029–48. doi: 10.3109/07420528.2015.1072002
72. Liu C, Tang X, Gong Z, Zeng W, Hou Q, Lu R. Circadian rhythm sleep disorders: Genetics, mechanisms, and adverse effects on health. Front Genet (2022) 13:875342. doi: 10.3389/fgene.2022.875342
73. Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev (2013) 17(4):273–84. doi: 10.1016/j.smrv.2012.08.003
74. Grasset E, Puel A, Charpentier J, Klopp P, Christensen JE, Lelouvier B, et al. Gut microbiota dysbiosis of type 2 diabetic mice impairs the intestinal daily rhythms of GLP-1 sensitivity. Acta Diabetol (2022) 59(2):243–58. doi: 10.1007/s00592-021-01790-y
75. Benedict C, Barclay JL, Ott V, Oster H, Hallschmid M. Acute sleep deprivation delays the glucagon-like peptide 1 peak response to breakfast in healthy men. Nutr Diabetes (2013) 3(6):e78. doi: 10.1038/nutd.2013.20
76. Wei M, Huang F, Zhao L, Zhang Y, Yang W, Wang S, et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine (2020) 55:102766. doi: 10.1016/j.ebiom.2020.102766
77. Chu Y, Sun S, Huang Y, Gao Q, Xie X, Wang P, et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes (2021) 7(1):66. doi: 10.1038/s41522-021-00235-2
78. Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord (2019) 20(4):461–72. doi: 10.1007/s11154-019-09512-0
79. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine (2020) 51:102590. doi: 10.1016/j.ebiom.2019.11.051
80. Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients (2020) 12(12). doi: 10.3390/nu12123719
81. Nizigiyimana P, Xu B, Liu L, Luo L, Liu T, Jiang M, et al. Gut microbiota is associated with differential metabolic characteristics: A study on a defined cohort of africans and Chinese. Front Endocrinol (Lausanne) (2022) 13:942383. doi: 10.3389/fendo.2022.942383
82. Chen H, Yao Y, Wang W, Wang D. Ge-Gen-Jiao-Tai-Wan affects type 2 diabetic rats by regulating gut microbiota and primary bile acids. Evid Based Complement Alternat Med 2021 (2021), 5585952. doi: 10.1155/2021/5585952
83. Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim JH, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol (2021) 6(5):563–73. doi: 10.1038/s41564-021-00880-5
84. Hwang I, Park YJ, Kim YR, Kim YN, Ka S, Lee HY, et al. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J (2015) 29(6):2397–411. doi: 10.1096/fj.14-265983
85. Cornejo-Pareja I, Martín-Núñez GM, Roca-Rodríguez MM, Cardona F, Coin-Aragüez L, Sánchez-Alcoholado L, et al. H. pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. J Clin Med (2019) 8(4). doi: 10.3390/jcm8040451
86. AlSuhaymi N, Darwish AM, Khattab AE. Protective and therapeutic effects of two novel strains of lactobacilli on diabetes-associated disorders induced by a high level of fructose. Mol Biol Rep (2021) 48(5):4333–40. doi: 10.1007/s11033-021-06448-0
87. Archer AC, Muthukumar SP, Halami PM. Lactobacillus fermentum MCC2759 and MCC2760 alleviate inflammation and intestinal function in high-fat diet-fed and streptozotocin-induced diabetic rats. Probiotics Antimicrob Proteins (2021) 13(4):1068–80. doi: 10.1007/s12602-021-09744-0
88. Singh S, Sharma RK, Malhotra S, Pothuraju R, Shandilya UK. Lactobacillus rhamnosus NCDC17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocintreated rats. Benef Microbes (2017) 8(2):243–55. doi: 10.3920/BM2016.0090
89. Wang G, Li X, Zhao J, Zhang H, Chen W. Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct (2017) 8(9):3155–64. doi: 10.1039/C7FO00593H
90. Yang X, Jiang W, Cheng J, Hao J, Han F, Zhang Y, et al. Reductions in intestinal taurine-conjugated bile acids and short-chain fatty acid-producing bacteria might be novel mechanisms of type 2 diabetes mellitus in otsuka long-Evans tokushima fatty rats. Exp Clin Endocrinol Diabetes (2022) 130(4):237–47. doi: 10.1055/a-1643-1689
91. Hofman MA, Fliers E, Goudsmit E, Swaab DF. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J Anat (1988) 160:127–43.
92. Kuljis DA, Loh DH, Truong D, Vosko AM, Ong ML, McClusky R, et al. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology (2013) 154(4):1501–12. doi: 10.1210/en.2012-1921
93. Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol (2000) 425(3):422–35. doi: 10.1002/1096-9861(20000925)425:3<422::AID-CNE7>3.0.CO;2-H
94. Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology (2002) 75(5):296–305. doi: 10.1159/000057339
95. Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol (2014) 35(1):111–39. doi: 10.1016/j.yfrne.2013.11.003
96. Jepsen SL, Albrechtsen NJW, Pedersen J, Engelstoft MS, Deacon CF, Holst JJ. GLP-1 secretion is increased upon blockade of the somatostatin receptor subtype 2 and 5 resulting in GLP-1 receptor-mediated lowering of blood glucose in mice. Diabetes (2018) 67(Supplement_1). doi: 10.2337/db18-1968-P
97. Biancolin AD, Srikrishnaraj A, Jeong H, Martchenko A, Brubaker PL. The cytoskeletal transport protein, secretagogin, is essential for diurnal glucagon-like peptide-1 secretion in mice. Endocrinology (2022) 163(11). doi: 10.1210/endocr/bqac142
98. Biancolin AD, Jeong H, Mak KWY, Yuan Z, Brubaker PL. Disrupted and elevated circadian secretion of glucagon-like peptide-1 in a murine model of type 2 diabetes. Endocrinology (2022) 163(9). doi: 10.1210/endocr/bqac118
99. Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med (2021) 42(3):199–214. doi: 10.1055/a-1263-0898
100. Aras M, Tchang BG, Pape J. Obesity and diabetes. Nurs Clin North Am (2021) 56(4):527–41. doi: 10.1016/j.cnur.2021.07.008
101. Kumar A, Sundaram K, Mu J, Dryden GW, Sriwastva MK, Lei C, et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun (2021) 12(1):213. doi: 10.1038/s41467-020-20500-w
102. Javeed N, Matveyenko AV. Circadian etiology of type 2 diabetes mellitus. Physiol (Bethesda) (2018) 33(2):138–50. doi: 10.1152/physiol.00003.2018
103. Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol (2020) 11:571731. doi: 10.3389/fimmu.2020.571731
104. Committee, A.D.A.P.P., 3. Prevention or delay of type 2 diabetes and associated comorbidities: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S39–s45. doi: 10.2337/dc22-S003
105. Ahn C, Ryan BJ, Schleh MW, Varshney P, Ludzki AC, Gillen JB, et al. Exercise training remodels subcutaneous adipose tissue in adults with obesity even without weight loss. J Physiol (2022) 600(9):2127–46. doi: 10.1113/JP282371
106. Schroder EA, Esser KA. Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc Sport Sci Rev (2013) 41(4):224–9. doi: 10.1097/JES.0b013e3182a58a70
107. Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci USA (1999) 96(26):15211–6. doi: 10.1073/pnas.96.26.15211
108. Thomas JM, Kern PA, Bush HM, McQuerry KJ, Black WS, Clasey JL, et al. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight (2020) 5(3). doi: 10.1172/jci.insight.134270
109. Dollet L, Zierath JR. Interplay between diet, exercise and the molecular circadian clock in orchestrating metabolic adaptations of adipose tissue. J Physiol (2019) 597(6):1439–50. doi: 10.1113/JP276488
110. Dalbram E, Basse AL, Zierath JR, Treebak JT. Voluntary wheel running in the late dark phase ameliorates diet-induced obesity in mice without altering insulin action. J Appl Physiol (1985) (2019) 126(4):993–1005. doi: 10.1152/japplphysiol.00737.2018
111. Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell (2014) 159(4):738–49. doi: 10.1016/j.cell.2014.10.029
112. Adam TC, Jocken J, Westerterp-Plantenga MS. Decreased glucagon-like peptide 1 release after weight loss in overweight/obese subjects. Obes Res (2005) 13(4):710–6. doi: 10.1038/oby.2005.80
113. Sloth B, Due A, Larsen TM, Holst JJ, Heding A, Astrup A. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br J Nutr (2009) 101(12):1846–58. doi: 10.1017/S0007114508137710
114. Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab (2010) 95(4):1609–16. doi: 10.1210/jc.2009-2082
115. Heiston EM, Eichner NZM, Gilbertson NM, Gaitán JM, Kranz S, Weltman A, et al. Two weeks of exercise training intensity on appetite regulation in obese adults with prediabetes. J Appl Physiol (1985) (2019) 126(3):746–54. doi: 10.1152/japplphysiol.00655.2018
116. Cigaina V, Hirschberg AL. Gastric pacing for morbid obesity: plasma levels of gastrointestinal peptides and leptin. Obes Res (2003) 11(12):1456–62. doi: 10.1038/oby.2003.195
117. Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. Chronic liraglutide therapy induces an enhanced endogenous glucagon-like peptide-1 secretory response in early type 2 diabetes. Diabetes Obes Metab (2017) 19(5):744–8. doi: 10.1111/dom.12858
118. Simon MC, Strassburger K, Nowotny B, Kolb H, Nowotny P, Burkart V, et al. Intake of lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes Care (2015) 38(10):1827–34. doi: 10.2337/dc14-2690
119. Martchenko A, Biancolin AD, Martchenko and P.L. Brubaker SE. Nobiletin ameliorates high fat-induced disruptions in rhythmic glucagon-like peptide-1 secretion. Sci Rep (2022) 12(1):7271. doi: 10.1038/s41598-022-11223-7
120. Morrow NM, Trzaskalski NA, Hanson AA, Fadzeyeva E, Telford DE, Chhoker SS, et al. Nobiletin prevents high-fat diet-induced dysregulation of intestinal lipid metabolism and attenuates postprandial lipemia. Arterioscler Thromb Vasc Biol (2022) 42(2):127–44. doi: 10.1161/ATVBAHA.121.316896
121. Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(-/-) and LDLr(-/-) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci (2018) 3(6):844–57. doi: 10.1016/j.jacbts.2018.09.004
122. Kim ER, Park JS, Kim JH, Oh JY, Oh IJ, Choi DH, et al. A GLP-1/GLP-2 receptor dual agonist to treat NASH: Targeting the gut-liver axis and microbiome. Hepatology (2022) 75(6):1523–38. doi: 10.1002/hep.32235
123. Chen X, Huang Q, Feng J, Xiao Z, Zhang and L. Zhao X. GLP-1 alleviates NLRP3 inflammasome-dependent inflammation in perivascular adipose tissue by inhibiting the NF-κB signalling pathway. J Int Med Res (2021) 49(2):300060521992981. doi: 10.1177/0300060521992981
Keywords: GLP-1, circadian rhythm, dietary structure, short sleep duration, intestinal flora
Citation: Liu C, Liu Y, Xin Y and Wang Y (2022) Circadian secretion rhythm of GLP-1 and its influencing factors. Front. Endocrinol. 13:991397. doi: 10.3389/fendo.2022.991397
Received: 11 July 2022; Accepted: 22 November 2022;
Published: 02 December 2022.
Edited by:
Ramasatyaveni Geesala, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Lisa Matz, University of Texas Medical Branch at Galveston, United StatesSowmya Mekala, University of Pittsburgh, United States
Copyright © 2022 Liu, Liu, Xin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yangang Wang, wangyg@qdu.edu
 Chuanfeng Liu
Chuanfeng Liu Yuzhao Liu
Yuzhao Liu Yu Xin
Yu Xin Yangang Wang
Yangang Wang