- 1School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 2School of Nursing and Midwifery, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 3Department of Nursing, College of Health Sciences, Madda Walabu University, Shashamene, Ethiopia
- 4School of Public Health, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Introduction: Even though optimal blood glucose control reduces the risk of diabetes-related complications, many patients with type 2 diabetes (T2D) fail to achieve it for a variety of reasons. In the study area, there was a paucity of evidence regarding correlates of glycemic control. Therefore, this study aimed to find out the correlates of glycemic control among patients with T2D in Eastern Ethiopia.
Methods: A cross-sectional study was conducted among 879 adult patients with T2D on follow-up at two public hospitals in Harar. Data were collected through interviews, physical measurements, and record reviews. The level of glycemic control was determined from three consecutive fasting plasma glucose (FPG) measurements. A mean value of FPG measurements falling in the normal range (80–130 mg/dl) was considered as optimal glycemic control; otherwise, a mean FPG level that is below or above the normal range (<80 mg/dl or >130 mg/dl) was defined as suboptimal glycemic control. Descriptive statistics were used to summarize the data, while a linear regression model was used to find out the correlates of glycemic control. A beta coefficient and a 95% CI reported associations. The statistical significance was declared at a p-value ≤0.05.
Results: The mean age of the patients with T2D was 52.7 ( ± 13.3) years. The mean FPG level was 172 ± 56 mg/dl. Suboptimal glycemic control was found in 76% (95% CI: 73.41, 79.04) of patients with T2D. In a multivariable linear regression, khat chewing (β = 6.12; 95% CI: 1.55, 8.69), triglycerides (β = 0.56; 95% CI: 0.41.48, 0.65), comorbidity (β = 5.29; 95% CI: 1.39, 9.13), and poor level of self-care practices (β = 5.43; 95% CI: 1.41, 6.46) showed a significant correlation with glycemic control.
Conclusions: This study found that about three-fourths of patients with T2D had suboptimal glycemic control. Khat chewing, comorbidity, and poor level of self-care practices were independently correlated with glycemic control. Thus, suppressing glycemic levels through appropriate treatment and strict diabetes self-care practices including avoidance of Khat chewing is a useful approach to attaining glycemic target that subsequently reduces cardiovascular risks.
Introduction
Diabetes is one of the fastest-growing global health emergencies of the 21st century that has reached alarming levels (1). Type 2 diabetes (T2D) is a progressive illness associated with decreasing insulin secretion over time (2), and poorly controlled diabetes leads to multiple organ damage that can increase the overall risk of premature death (3).
Evidence shows that optimal glycemic control is a goal for diabetes management. The general target of glucose control is glycated hemoglobin (HbA1c) ≤7% for non-pregnant adults, and a less stringent HbA1C goal of 8% (64 mmol/mol) is optional for patients with a risk of severe hypoglycemia and advanced microvascular or macrovascular complications (4). However, HbA1c is expensive and unavailable in many places. Therefore, a fasting plasma glucose (FPG) level of 80–130 mg/dl without caloric intake for at least 8 h is one of the standard diagnostic criteria (5).
Growing evidence showed that optimal blood glucose control prevents acute and chronic complications, whereas a sustained hyperglycemic condition results in a severe diabetic condition by damaging the pancreatic β-cell and inducing insulin resistance (6, 7). Though a strong agreement, most diabetic patients do not attain their diabetic goals (8, 9). These problems are prominent in resource-limited countries including Ethiopia where access to diabetes health services and standard laboratory tests were inadequate (10).
The government of Ethiopia has made several efforts through policy formulation, designing strategies to identify risk factors, and developing management protocol for diabetes (7, 11); so far, two-thirds of patients fail to attain optimal glycemic control due to complex reasons (9). The most recurrently reported factors influencing glycemic control were younger age, male sex, marital status, rural residence, longer duration of diabetes, poor level of medication adherence, and self-monitoring blood glucose (9, 12–16). Specifically, studies indicated a higher prevalence of T2D and metabolic syndromes along with numerous barriers to self-care practices in the study area (17–19). Khat chewing habit has also been popular (20). However, evidence about correlates of glycemic control was scarce. Therefore, this study aimed to find out the correlates of glycemic control among patients with T2D in Eastern Ethiopia. This knowledge would provide valuable information for healthcare providers, local planners, and implementers to plan appropriate interventions that improve patients’ glycemia and lipid levels to reduce the extent of target organ damage.
Materials and Methods
Study Setting and Design
A cross-sectional study was conducted among patients with T2D at public health hospitals in Harar between December 1, 2020, to March 30, 2021. Harar city is found in the eastern part of the country, 526 km away from Addis Ababa. Hiwot Fana Specialized Comprehensive Hospital and Jugal General Hospital are two public hospitals that offer comprehensive health services for the entire community of eastern Ethiopia. The hospitals deliver services in medical, surgical, pediatric, obstetrics, and gynecology wards; intensive care units (ICUs); outpatient departments (OPDs); and radiology, pathology, laboratory, and pharmacy departments. Nursing services are also provided in all departments of the hospital with a team of allied disciplines in delivering patient care that aims at satisfying the nursing needs of the patients. Moreover, the hospitals have trained health and medical science students. In these hospitals, more than 1,985 patients with T2D were attending their follow-up care.
Population
Adult patients with a diagnosis of T2D who had diabetes follow-up at Hiwot Fana Specialized Comprehensive Hospital and Jugal Hospital were involved in the study. The patients with T2D who had at least three consecutive FPG measurements were randomly selected. However, two patients with T2D with severe illness and hearing impairment were excluded from the study, as they might not respond to our questions accurately and were unable to give valid consent.
Sample Size and Sampling Strategy
The estimated sample size was determined using a single and double population proportion formula using Epi-info version 7.1 software, where n is the sample size, Zα/2 = 1.96, d = 0.05 is the margin of error, P = 64.7% for the level of suboptimal glycemic control (12). Accordingly, a total of 891 patients with T2D were included in this study (598 = Jugal Hospital and 293 = Hiwot Fana Specialized Comprehensive Hospital). Then, the study participants were recruited randomly every two, while the first participant was selected by lottery method.
Data Collection and Measurements
Data were collected by interviewing eligible participants using a pretested semistructured questionnaire after reviewing various literature. The questionnaires consisted of demographic information and clinical characteristics diabetes duration, current regimen, comorbidity, complications, and biochemical data such as FPG, total cholesterol, and triglyceride levels. Summary diabetes self-care activity (SDSCA) has physical activities, dietary plan, medication adherence, blood glucose monitoring, foot care, smoking behaviors, and alcohol use (21). A wealth score was also computed using principal component analysis (PCA) from 23 variables that included household assets, farmland, and animals.
The blood glucose levels were determined by collecting the three most recent FPG measurements in the last three consecutive visits and calculating the mean values. The measurement of blood glucose was conventionally classified into two categories (controlled vs. uncontrolled or optimal vs. suboptimal). Based on the American Diabetic Association (ADA) standards of medical care, optimal glycemic control is an average FPG level of 80–130 mg/dl and suboptimal glycemic control refers to an average FPG level of <80 mg/dl and >130 mg/dl (4).
Blood pressure (BP) measurements were carried out using a digital automated BP monitor (Model UA-767F/UA-767FAC, manufactured by A&D Company, Limited, Japan) with patients sitting after resting for at least 15 min. BP measurement was made on the left arm with the elbow supported and the palm facing upward. Three consecutive measurements of BP were taken in a 3-min interval, and then the mean of the second and third readings was used for analysis (22). BP measurement ≥140/90 mmHg indicates raised BP or hypertension (23).
Anthropometric data [height, body weight, hip circumference (HC), and waist circumference (WC)] were collected by using standard procedures and calibrated instruments at the end of the interview. Height was measured using a stadiometer with removed footwear and hair gear and the patient’s face away from the wall, looking straight ahead with his or her heels together and the back as straight as possible. The head, shoulders, buttocks, and heels should be in contact with the vertical surface, and the height measurement was recorded to the nearest 0.1 cm. Body weight was measured barefooted and wearing light clothes using a Seca digital body weight scale made in Japan and measuring to the nearest 0.1 kg. Body mass index (BMI) was calculated as the patient’s weight in kilograms divided by height in square meters (kg/m2). BMI was categorized into four: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2) (22).
WC was measured in centimeters using a fixed tension tape at the midpoint of the line between the lower margin of the last palpable (12th) rib and the top of the iliac crest (hip bone) over light clothing without compressing the skin. The measurement was taken at the end of an expiration with the arms relaxed at the sides, and the measurement was recorded to the nearest 0.1 cm. The WC measurements were classified based on cutoffs recommended by the WHO into three health risk categories: low risk (men, WC = ≤93.9 cm; women, WC = ≤79.9 cm); increased risk (men, WC = 94.0–101.9 cm; women, WC = 80.0–87.9 cm); and high risk (men, WC = ≥102.0 cm; women, WC = ≥88.0 cm) (22).
HC was taken around the maximum circumference of the buttocks while the patients stand with their feet together with weight evenly distributed over both feet and holding their arms relaxed at the sides. The HC was measured using an inelastic measuring tape and recorded to the nearest 0.1 cm. Then, the waist-to-hip ratio (WHR) was calculated by dividing WC by HC in centimeters. The cutoff point used for WHR was ≤0.9 for the men and ≤0.85 for the women (22).
Data Quality Control
The data were collected using a standardized questionnaire written in English and translated into local languages. After that, it was retranslated into English by another expert to confirm that the instruments were consistent. Before the actual fieldwork, data collectors and supervisors were trained to ensure reliable and accurate data collection. The research instrument was also pretested in 50 patients with T2D who visited Dilchora Hospital for diabetic care, which is found about 52 km away from the study area. Moreover, the supervisors and principal investigator double-checked the data for completeness daily throughout the data collection period.
Statistical Analysis
The collected data were cleaned, coded, and entered to Epidata software version 3.1 and then exported to Stata version 14.0 for analysis. Descriptive statistics such as mean and standard deviation were used to report data of continuous variables, whereas frequencies and percentages were used to express categorical variables. A variable with p < 0.20 on a bivariate linear regression analysis was entered into a multivariate linear regression analysis model to identify the variable independently correlated with glycemic control. The data were summarized using beta coefficient with a corresponding 95% confidence interval. A variable with p ≤ 0.05 was statistically significant. The multicollinearity test was carried out to see the linear correlation among independent variables. The variance inflation factor and correlation coefficient did not prove the existence of multicollinearity. Model fitness was checked using the Hosmer–Lemeshow goodness-of-fit test.
Results
Demographic Characteristics
A total of 879 patients with T2D were included in the study, yielding a response rate of 99%. The female patients accounted for 56.1%. The mean age was 52.7 ± 13.3 years. Four hundred forty-seven (50.9%) were above the age of 55 years. Three hundred fifty-two patients (40.1%) had no formal education. Six hundred eighty-four (77.8%) of them were urban dwellers. Three hundred forty-five patients (39.4%) were in the medium wealth quantile category, while two-thirds (65.6%) of them had no health insurance coverage. The mean FPG level indicated persistently high glycemic levels across all demographic characteristics of the patients with T2D (Table 1).
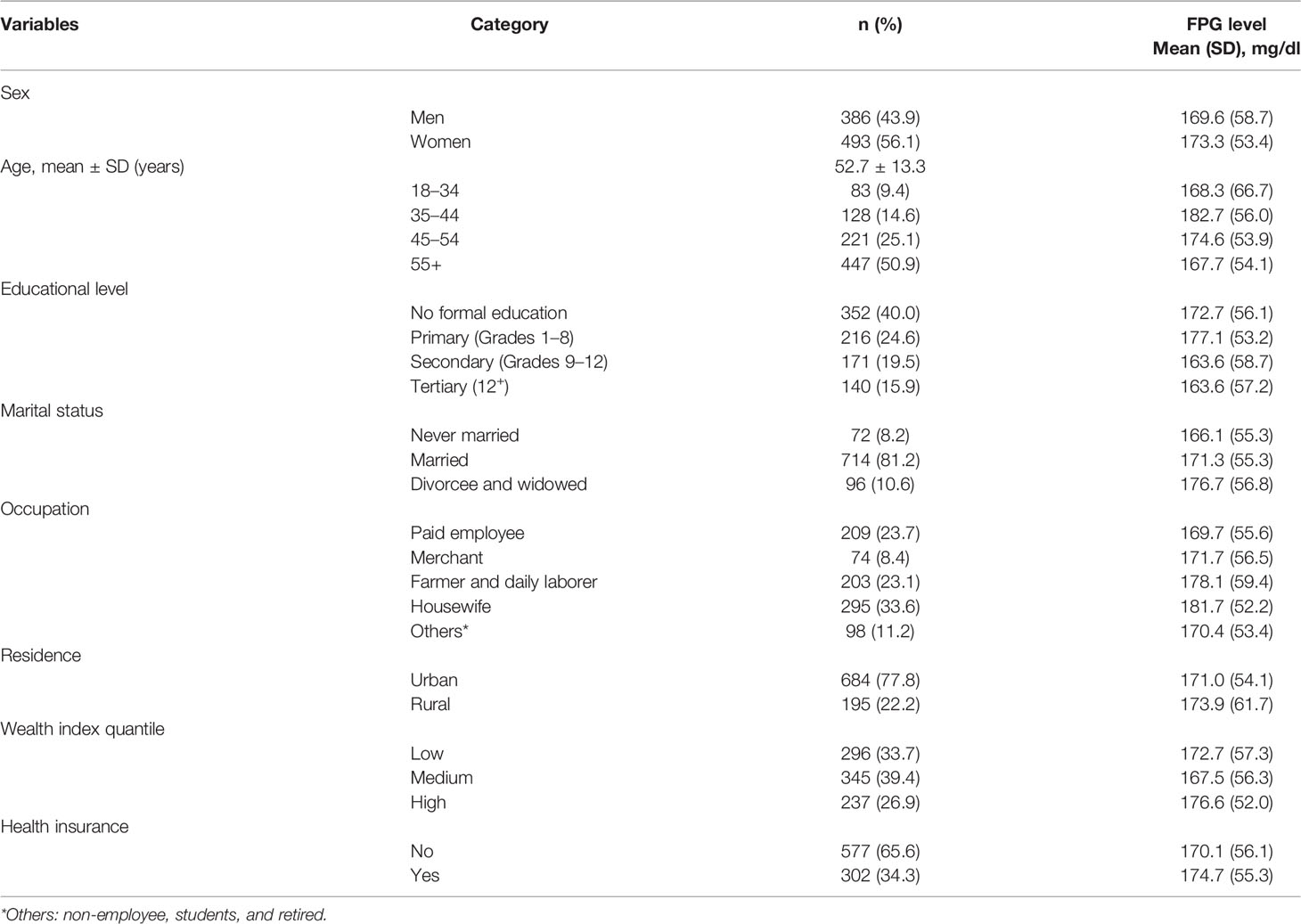
Table 1 Demographic characteristics by glycemic levels of patients with T2D in Eastern Ethiopia, 2020/2021 (n = 879).
In this study, 76% (95% CI: 73.4, 79.0) of T2D patients had suboptimal glycemic control (<80 mg/dl or >130 mg/dl). The average FPG level was 172 ± 56 mg/dl (95% CI: 167.9, 175.4). Female T2D patients showed slightly worse glycemic control than did male patients.
Clinical Characteristics
The mean glycemic control based on FPG level was 172.5 ± 56.2 mg/dl among patients with T2D who had less than 5 years of disease duration. The mean FPG level of patients with T2D who had no comorbidity was 173.9 ± 55.9 mg/dl. Although 300 (43.2%) patients with T2D were being treated with a combination of oral glycemic control agents (OGAs), their mean FPG level was 171.9 ± 55.7 mg/dl. The mean FPG level among patients with T2D who had no history of admission was 172.8 ± 56.5 mg/dl. More than half (58.8%) of patients with T2D with normal total cholesterol levels (<200 mg/dl) had a mean FPG level that was closer to normal (138.9 ± 33.9 mg/dl). Moreover, 315 (35.8%) patients with T2D had high to very high levels of triglycerides, and the corresponding mean FPG level was high (227.1 ± 40.9 mg/dl), and 65.7% of patients were found to have poor self-care practices with a mean FPG level of 173.5 ± 57.5 mg/dl (Table 2).
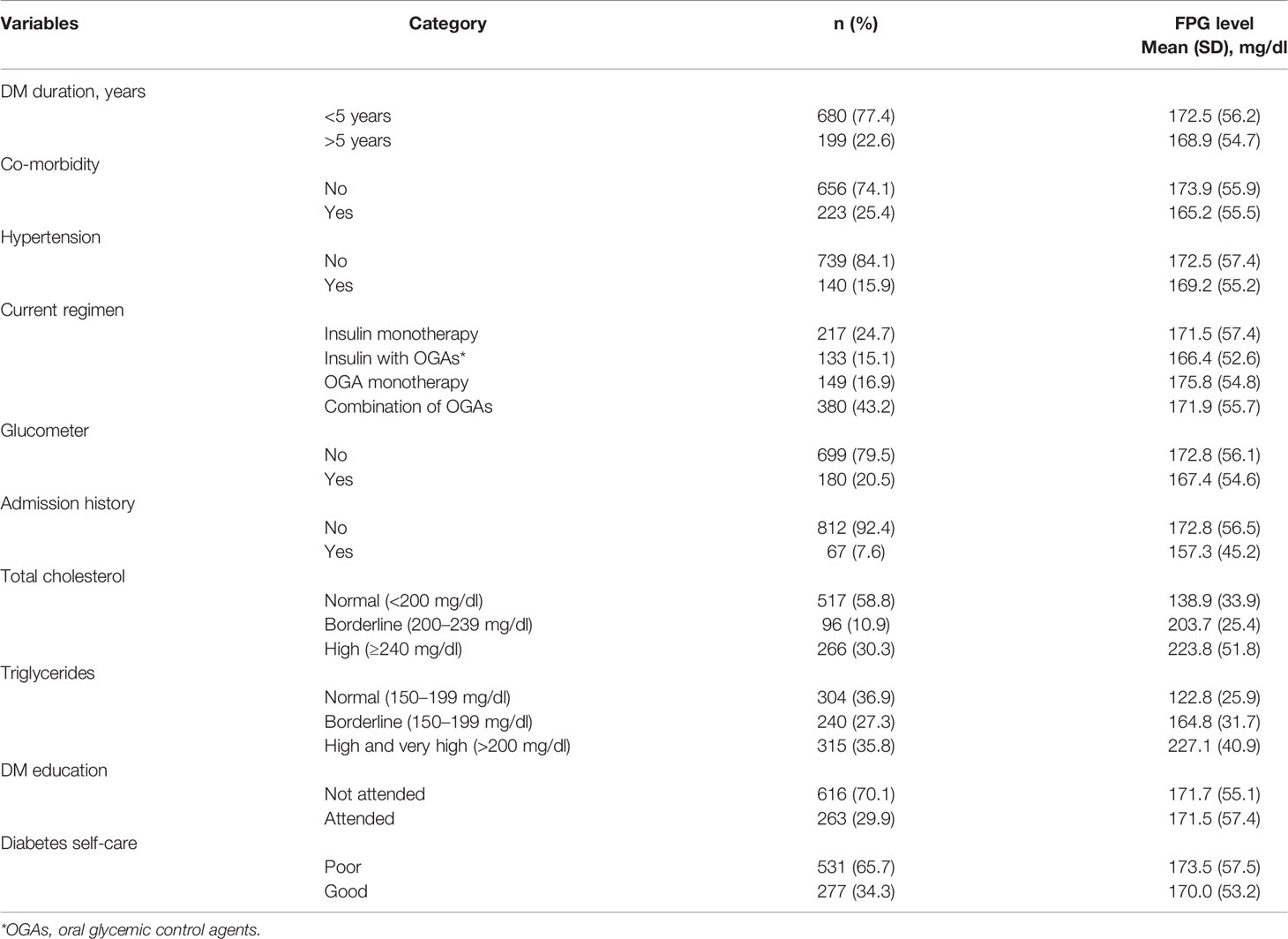
Table 2 Clinical characteristics by mean FPG level of the patients with T2D in Eastern Ethiopia, 2020/2021 (n = 879).
Anthropometric Measurements and Biochemical Tests
The mean ( ± SD) of BMIs of male and female participants were 25.0 ± 3.7 and 27.1 ± 5.5, indicating overweight, respectively. Likewise, the mean FPG, total cholesterol, and triglyceride levels had no significant differences in both men and women (Table 3).
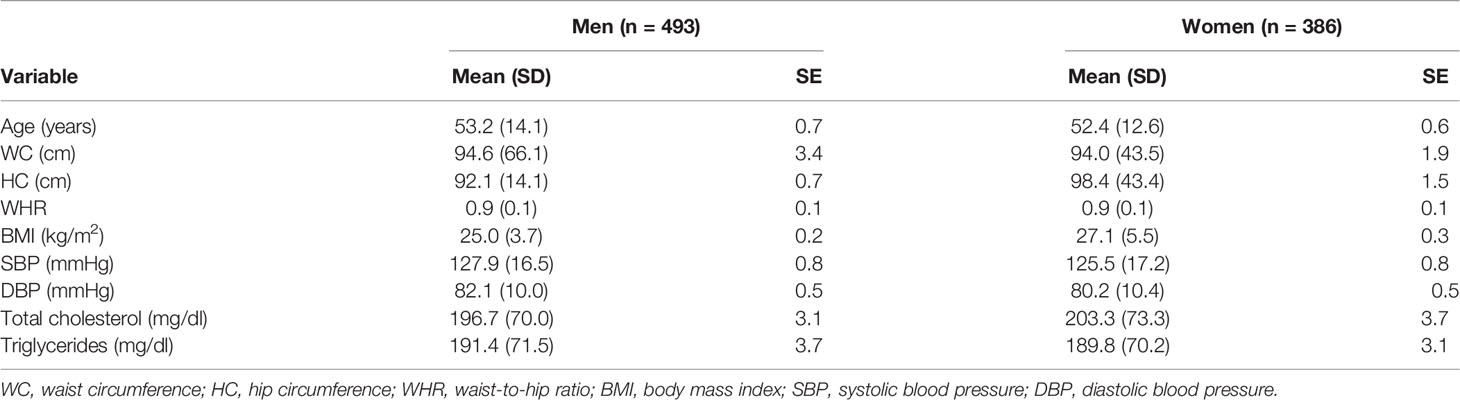
Table 3 Anthropometric indices and biochemical tests by sex of patients with T2D in Eastern Ethiopia, 2020/2021 (n = 879).
Correlates of Glycemic Control in Patients With Type 2 Diabetes
In bivariate analysis, participants’ age, educational level, health insurance, diabetes complication, admission history, attendance of religious fasting, total cholesterol, triglycerides, khat chewing, sugary drinks used during khat chewing, and self-care practices were statistically significant. After adjusting for potential confounding factors, total cholesterol, triglycerides, diabetes complications, chewing khat, and self-care practices showed a statistically significant association with glycemic control.
Keeping constant all variables in the model, an increase in the level of triglycerides by 1 unit increased the FPG level of patients with T2D by 0.51 mg/dl (β = 0.51; 95% CI: 0.48, 0.54). Khat chewing practices in patients with T2D indicated an increase in FPG level by 6.12 mg/dl (β = 6.12; 95% CI: 1.55, 8.69). Having comorbid conditions increased the FPG level of patients with T2D by 5.29 mg/dl (β = 5.29; 95% CI: 1.39, 9.13). In addition, poor levels of diabetes self-care practices increased the patients’ FPG level by 5.43 mg/dl (β = 5.43; 95% CI: 1.41, 6.46), while all variables in the model were kept constant (Table 4).
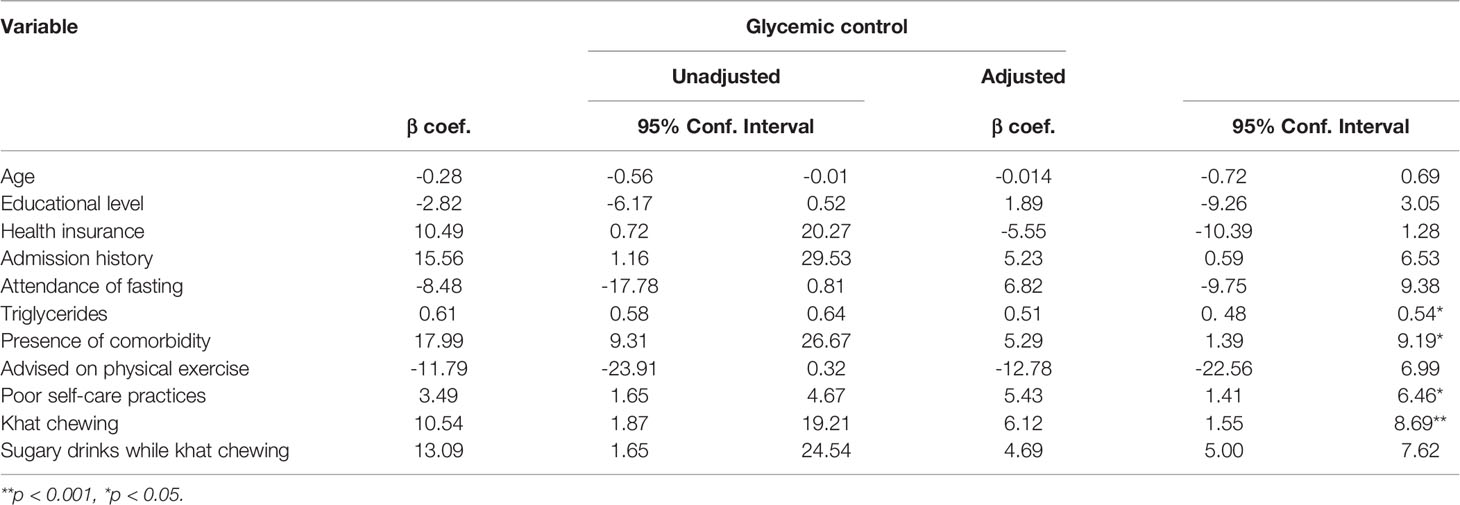
Table 4 Correlates of glycemic control among patients with T2D in Eastern Ethiopia, 2020/2021 (n = 879).
Discussion
This study explored correlates of glycemic control among patients with T2D in Eastern Ethiopia. Accordingly, 76% of patients with T2D (95% CI: 73.4, 79.0) had suboptimal glycemic control. The mean FPG level was 172 ± 56 mg/dl (95% CI: 167.9, 175.4), indicating uncontrolled blood glucose.
The findings of this study indicated that three-fourths of patients with T2D did not achieve the recommended glycemic target (FPG level of 80–130 mg/dl). This informed that most of the patients are at higher risk of diabetes complications (24, 25). The risk of premature death is usually higher in people with comorbidity (26). Its terrible economic loss affects the patients and their families and the national economy through medical costs and loss of work (3).
The finding was consistent with those of previous studies conducted in Saudi Arabia and India (15, 27, 28). The reasons for similarities might be that these studies shared common characteristics: there was a higher proportion of women who naturally have higher adipose tissue mass, free fatty acids, and intramyocellular lipid content that could promote insulin resistance and affect glycemic control (29). Most of the study participants were urban residents who had higher indices of overweight and obesity that potentially contribute to insulin resistance, reduce the uptake of glucose, and result in suboptimal glycemic control. Although regular physical exercise has a crucial role in increasing insulin sensitivity and improving glycemic management (30), physical inactivity (<150 min/week) was rampant. In addition, participants in Saudi Arabia were less educated, while more than three-fourths of patients with T2D had no formal education, which might limit their ability to understand the nature of the disease and access to adequate health care services.
However, the level of glycemic control in the current study was slightly lower than that of Bangladesh (31), Pakistan (32), and Addis Ababa, Ethiopia (33). Compared to Bangladesh and Pakistan studies, the proportion of comorbidities, overweight, and obesity that influence optimal glycemic control was lower. Moreover, the use of combination therapy was higher in the current study, which may influence glycemic control to some extent in contrast to single therapy. There was also a relative increment of the suboptimal level of glycemia in this study when compared with that of previous studies conducted in Mettu, Jimma, and Debre Tabor public hospitals of Ethiopia (34–36). The variation could be due to using a larger sample size, and the use of OGAs was the predominant regimen in the current study.
The current study also found that chewing khat, triglycerides, having comorbidity, and a poor level of self-care practices were independent correlates of glycemic control. Khat chewing showed a strong association with glycemic control. This result was supported by studies conducted in Sana’a and Hodeidah City, Yemen, that the patients who chewed khat demonstrated a higher mean HbA1c than non-khat chewer patients with T2D (37, 38). Another study in Malaysia disclosed that khat (cathinone) significantly increased the FPG level and decreased the insulin level of diabetic rats (39). This could be because most khat chewers used sugary drinks/beverages while chewing (40). Khat also has a cytotoxic effect on pancreatic cells and insulin synthesis, affecting regulation of blood glucose (41). However, there were contradictory findings about its effect on glycemic control (40, 42). Therefore, another study is suggested to determine the actual effects of khat on the blood glucose level.
The triglyceride level was also strongly correlated with glycemic control. This finding was consistent with studies conducted in Mainland and Zhejiang, China, and Nepal (43–45). Similarly, a study in Bosnia and Herzegovina showed a positive correlation between glycemic control and triglyceride level (46). In general, the lipid abnormality in patients with T2D might be explained based on insulin resistance that can occur due to genetic defects, ectopic lipid accumulation, physical inactivity, obesity, and inflammation (47–49). Insulin resistance reduces glycogen synthesis and protein catabolism in skeletal muscles while inhibiting lipoprotein lipase activity in adipocytes, resulting in a higher release of free fatty acids and inflammatory cytokines (50, 51). Moreover, insulin resistance impairs glucose output and fatty acid metabolism, increasing the triglyceride content from the liver (50, 52) and resulting in hypertriglyceridemia and increasing the synthesis of Very Low-Density Lipoprotein (VLDL), which increases cardiovascular risks by causing atherosclerosis (53).
In the current study, a poor level of self-care practices showed a correlation with glycemic control. This finding was comparable to that of a study done in Riyadh, Saudi Arabia, where a poor level of diabetes self-care behavior was associated with suboptimal glycemic control (54), which increases the risk of morbidity and mortality in diabetes patients (55). On the other hand, good self-care practices optimize glycemic control (32), reduce the risk of diabetes complications (56), and improve patients’ quality of life (57). The HbA1c level can be reduced up to 1% through self-care practices (58, 59).
Comorbidity appeared as a correlate of glycemic control in this study. This finding was congruent with those of previous studies conducted in Ethiopia (60) and Croatia that reported the effect of comorbidities on the level of HbA1c (61). This might be because patients with a comorbid condition have less energy to have physical exercise, which likely lowers the blood glucose level (62). Moreover, diabetic patients who had comorbidities received more drugs (63). The use of multiple medications might increase the chance of drug–drug interactions (64) and food–drug interactions (65), decreasing their medication adherence (66) and leading to suboptimal glycemic control (67).
Strengths and Limitations of the Study
The average of the three most recent FPG measurements was used to determine glycemic levels, which is more reliable than a single measurement. On top of that, an adequate sample size was used for meaningful results/conclusions. However, our study had some limitations. Self-report and social desirability biases might potentially influence the results of the study. In addition, the retrospective nature of chart review was a major limitation.
Conclusions
The findings suggested that considerable numbers of patients with T2D had suboptimal glycemic control usually linked to diabetes-associated complications. Khat chewing, triglycerides, comorbidity, and a poor level of diabetes self-care practices were independently correlated with glycemic control. Regular monitoring, suppressing triglyceride levels through strict self-care practices, and appropriate treatment can benefit the patients to attain optimal glycemic control that subsequently reduces diabetes-related complications. Diabetes self-management education that focuses on the avoidance of khat chewing needs to be instituted. Furthermore, a controlled trial study that generates robust evidence is recommended to elucidate the effect of khat chewing on the blood glucose level.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The study was reviewed and approved by the Institutional Health Research and Ethical Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University with the reference number (IHRERC/2017/2020). In addition, an official letter of cooperation was obtained from the college to get permission from the hospital administrators and diabetes units. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors had a significant contribution to the conception, study design, execution, analysis, and interpretation of the data; took part in drafting, critically reviewing the article; approved the final version for publication, and agree to be accountable for all aspects of the work.
Funding
This research was funded by Haramaya University, Ethiopia. The funder had no role in designing and data collection, analysis, writing, and submission of the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the study participants, data collectors, supervisors, Reginal Health Bureau, hospital administrators, and Haramaya University.
References
1. IDF. IDF Diabetes Atlas, 10th edition. (2021). https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf
2. WHO, IDF. Diagnosis and Management of Type 2 Diabetes(HEARTS-D). Geneva. (WHO/UCN/NCD/201) (2020), 1–35.
5. IDF. IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care. (2017), 1–36.
6. Giria B, Dey S, Dasb T, Sarkar M, Banerjeea J, Dasha SK, et al. Chronic Hyperglycemia Mediated Physiological Alteration and Metabolic Distortion Leads to Organ Dysfunction, Infection, Cancer Progression and Other Pathophysiological Consequences: An Update on Glucose Toxicity. Biomed Pharmacother (2018) 107:306–28. doi: 10.1016/j.biopha.2018.07.157
7. FMoH. Ethiopian National Guideline on Major NCDs 2016. Guidelines on Clinical and Programmatic Management of Major Non Communicable Diseases (2016), 33–50.
8. Aschner P, Gagliardino JJ, Mbanya JC, Ilkova H, Shestakova M. Persistent Poor Glycaemic Control in Individuals With Type 2 Diabetes in Developing Countries: 12 Years of Real-World Evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia (2020) 63:711–21. doi: 10.1007/s00125-019-05078-3
9. Gebreyohannes EA, Netere AK, Belachew SA. Glycemic Control Among Diabetic Patients in Ethiopia: A Systematic Review and Meta-Analysis. PloS One (2019) 14:e0221790. doi: 10.1371/journal.pone.0221790
10. Grant P. Management of Diabetes in Resource-Poor Settings. Clin Med (2013) 13:27–31. doi: 10.7861/clinmedicine.13-1-27
11. FMoH. National Strategic Action Plan (Nsap) For Prevention & Control Of Non-Communicable Diseases In Ethiopia. (2014), 1–60.
12. Abebe SM, Berhane Y, Worku A, Alemu S, Mesfin N. Level of Sustained Glycemic Control and Associated Factors Among Patients With Diabetes Mellitus in Ethiopia: A Hospital-Based Cross-Sectional Study. Diabetes Metab Syndr Obes (2015) 8:65–71. doi: 10.2147/DMSO.S75467
13. Benoit SR, Fleming R, Philis-Tsimikas A, Ji M. Predictors of Glycemic Control Among Patients With Type 2 Diabetes: A Longitudinal Study. BMC Public Health (2005) 5:36. doi: 10.1186/1471-2458-5-36
14. Yosef T, Nureye D, Tekalign E. Poor Glycemic Control and Its Contributing Factors Among Type 2 Diabetes Patients at Adama Hospital Medical College in East Ethiopia. Diabetes Metab Syndr Obes (2021) 14:3273–80. doi: 10.2147/DMSO.S321756
15. Alramadan MJ, Magliano DJ, Almigbal TH, Batais MA, Afroz A, Alramadhan HJ, et al. Glycaemic Control for People With Type 2 Diabetes in Saudi Arabia - an Urgent Need for a Review of Management Plan. BMC endocr Disord (2018) 18:62. doi: 10.1186/s12902-018-0292-9
16. Mamo Y, Bekele F, Nigussie T, Zewudie A. Determinants of Poor Glycemic Control Among Adult Patients With Type 2 Diabetes Mellitus in Jimma University Medical Center, Jimma Zone, South West Ethiopia: A Case Control Study. BMC endocr Disord (2019) 19:91. doi: 10.1186/s12902-019-0421-0
17. Motuma A, Gobena T, Roba KT, Berhane Y, Worku A. Metabolic Syndrome Among Working Adults in Eastern Ethiopia. Diabetes Metab Syndrome Obesity: Targets Ther (2020) 13:4941–51. doi: 10.2147/DMSO.S283270
18. Ayana DA, Bacha YD, Roba KT, Kebede DA. Type 2 Diabetes Mellitus Among Government Employees in Harar, Eastern Ethiopia: A Cross-Sectional Study. Res Rep Endocr Disord (2015) 5:71–7. doi: 10.2147/RRED.S82883
19. Ayele BH, Mengesha MM, Tesfa T. Predictors of Self-Care Activities of Outpatient Diabetic Residents in Harar and Dire Dawa: A Hospital-Based Cross-Sectional Study. SAGE Open Med Volume 7 (2019) 7:1–10. doi: 10.1177/2050312119865646
20. Reda AA, Moges A, Biadgilign S, Wondmagegn S. Prevalence and Determinants of Khat (Catha Edulis) Chewing Among High School Students in Eastern Ethiopia: A Cross-Sectional Study. PloS One (2012) 7:1–5. doi: 10.1371/journal.pone.0033946
21. Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities Measure:Results From 7 Studies and a Revised Scale. Diabetes Care (2000) 23:943–49.
22. WHO. The WHO STEPwise Approach to Noncommunicable Disease Risk Factor Surveillance Manual. (2020), 1-1-11–3-5-12.
23. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. International Society of Hypertension: Global Hypertension Practice Guidelines. Hypertension (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
24. UKPDS. Effect of Intensive Blood-Glucose Control With Metformin on Complications in Overweight Patients With Type 2 Diabetes (UKPDS 34). Lancet (1998) 352:854–55.
25. ADA. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41:S55–64. doi: 10.2337/dc18-S006
26. Eilat-Tsanani S, Margalit A, Golan LN. Occurrence of Comorbidities in Newly Diagnosed Type 2 Diabetes Patients and Their Impact After 11 Years' Follow-Up. Sci Rep (2021) 11:11071. doi: 10.1038/s41598-021-90379-0
27. Alzaheb RA, Altemani AH. The Prevalence and Determinants of Poor Glycemic Control Among Adults With Type 2 Diabetes Mellitus in Saudi Arabia. Diabetes Metab Syndr Obes (2018) 11:15–21. doi: 10.2147/dmso.s156214
28. Borgharkar SS, Das SS. Real-World Evidence of Glycemic Control Among Patients With Type 2 Diabetes Mellitus in India: The TIGHT Study. BMJ Open Diabetes Res Care (2019) 7:e000654. doi: 10.1136/bmjdrc-2019-000654
29. Mauvais-Jarvis F. Gender Differences in Glucose Homeostasis and Diabetes. Physiol Behav (2018) 187:20–3. doi: 10.1016/j.physbeh.2017.08.016
30. ADA. Lifestyle Management: Standards Ofmedical Care in Diabetesd. Diabetes Care (2019) 42:S46–60. doi: 10.2337/dc19-S005
31. Afroz A, Ali L, Karim MN, Alramadan MJ, Alam K, Billah DJMB, et al. Glycaemic Control for People With Type 2 Diabetes Mellitus in Bangladesh - An Urgent Need for Optimization of Management Plan. Sci Rep (2019) 9:10248. doi: 10.1038/s41598-019-46766-9
32. Bukhsh A, Khan TM, Nawaz MS, Ahmed HS, Chan KG, Goh B-H, et al. Association of Diabetes-Related Self-Care Activities With Glycemic Control of Patients With Type 2 Diabetes in Pakistan. Patient Prefer Adherence (2018) 12:2377–85. doi: 10.2147/ppa.s177314
33. Alemu T, Tadesse T, Amogne G. Glycemic Control and its Determinants Among Patients With Type 2 Diabetes Mellitus at Menelik II Referral Hospital, Ethiopia. SAGE Open Med (2021) 9:20503121211023000. doi: 10.1177/20503121211023000
34. Gudisa B, Gemechis B. The Incidence and Predictors of Poor Glycemic Control Among Adults With Type 2 Diabetes Mellitus in Ambulatory Clinic of Mettu Karl Referral Hospital, Southwestern Oromia, Ethiopia: A Prospective Cross Sectional Study. Diabetes Updates (2021) 7:1–6. doi: 10.15761/du.1000155
35. Kassahun T, Eshetie T, Gesesew H. Factors Associated With Glycemic Control Among Adult Patients With Type 2 Diabetes Mellitus: A Cross-Sectional Survey in Ethiopia. BMC Res Notes (2016) 9:78. doi: 10.1186/s13104-016-1896-7
36. Gebermariam AD, Tiruneh SA, Ayele AA, et al. Level of Glycemic Control and its Associated Factors Among Type II Diabetic Patients in Debre Tabor General Hospital, Northwest Ethiopia. Metabol Open (2020) 8:100056. doi: 10.1016/j.metop.2020.100056
37. Al-Sharafi BA, Gunaid AA. Effect of Habitual Khat Chewing on Glycemic Control, Body Mass Index, and Age at Diagnosis of Diabetes in Patients With Type 2 Diabetes Mellitus in Yemen. Clin Med Insights Endocrinol Diabetes (2015) 8:47–53. doi: 10.4137/cmed.s26045
38. Saghir SAM, Alhariri AEA, Alkubat SA, Almiamn AA, Aladaileh SH, Alyousefi NA, et al. Factors Associated With Poor Glycemic Control Among Type-2 Diabetes Mellitus Patients in Yemen. Trop J Pharm Res (2021) 18:1539–46. doi: 10.4314/tjpr.v18i7.26
39. Alsalahi A, Chik Z, Mohamed Z, Giribabu N, Alshawsh MA. Cathinone: An Alkaloid of Catha Edulis (Khat) Exacerbated Hyperglycemia in Diabetes-Induced Rats. Saudi J Biol Sci (2021) 28:4633–43. doi: 10.1016/j.sjbs.2021.04.072
40. Badedi M, Darraj H, Hummadi A, Najmi A, Solan Y, Zakry I, et al. Khat Chewing and Type 2 Diabetes Mellitus. Diabetes Metab Syndr Obes (2020) 13:307–12. doi: 10.2147/dmso.s240680
41. Alsalahi, AlshAwsh MA, Chik Z, Mohamed Z. Effect of Catha Edulis (Khat) on Pancreatic Functions in Streptozotocin-Induced Diabetes in Male Sprague-Dawley Rats Abdulsamad. Exp Anim (2018) 67:517–26. doi: 10.1538/expanim.18-0057
42. Mengistu Y, Dedefo G, Arkew M, Asefa G, Jebessa G, Atnafu A, et al. Effect of Regular Khat Chewing on Serum Fasting Sugar Level in Diabetic Patients Versus Healthy Individuals; A Comparative Study. Nutr Metab Insights (2021) 14:11786388211035220. doi: 10.1177/11786388211035220
43. Zheng D, Dou J, Liu G, Pan Y, Yan Y, Liu F, et al. Association Between Triglyceride Level and Glycemic Control Among Insulin-Treated Patients With Type 2 Diabetes. J Clin Endocrinol Metab (2019) 104:1211–20. doi: 10.1210/jc.2018-01656
44. Zhu H-T, Yu M, Hu H, He Q-F, Pan J, Hu R-Y, et al. Factors Associated With Glycemic Control in Community-Dwelling Elderly Individuals With Type 2 Diabetes Mellitus in Zhejiang, China: A Cross-Sectional Study. BMC Endocr Disord (2019) 19:1–11. doi: 10.1186/s12902-019-0384-1
45. Pandey T, Khanal J, Godar KC. Study of Association Between Glycated Hemoglobin and Lipid Profile in Type 2 Diabetes Mellitus in Tertiary Care Center. J Lumbini Med Coll (2020) 8:238–43. doi: 10.22502/jlmc.v8i2.387
46. Panjeta E, Jadrić R, Panjeta M, Ćorić J, Dervišević A. Correlation of Serum Lipid Profile and Glycemic Control Parameters in Patients With Type 2 Diabetes Mellitus. J Health Sci (2018) 8:110–6. doi: 10.17532/jhsci.2018.488
47. Westphal SA. Obesity, Abdominal Obesity, and Insulin Resistance. Clin Cornerstone (2008) 9:23–31. doi: 10.1016/S1098-3597(08)60025-3
48. Samuel VT, Shulman GI. Integrating Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell (2012) 148:852–71. doi: 10.1016/j.cell.2012.02.017
49. Samuel VT, Shulman GI. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell (2012) 148:852–71. doi: 10.1016/j.cell.2012.02.017
50. Ormazaba V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA, et al. Association Between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc Diabetol (2018) 17:1–14. doi: 10.1186/s12933-018-0762-4
51. Mooradian AD. Dyslipidemia in Type 2 Diabetes Mellitus. Nat Clin Pract Endocrinol Metab (2009) 5:150–59. doi: 10.1038/ncpendmet1066
52. Huang XL, Wang M, Hua Ning LA, Li Y, Sun C, et al. Lipoprotein Lipase Links Vitamin D, Insulin Resistance, and Type 2 Diabetes: A Cross-Sectional Epidemiological Study Yifan. Cardiovasc Diabetol (2013) 12:2–8. doi: 10.1186/1475-2840-12-17
53. Sherwani S, khan HA, Ekhzaimy H, Masood A, Sakharkar AMK. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. biomark Insights (2016) 11:95–104. doi: 10.4137/BMI.S38440
54. Al-Hayek AA, Robert AA, Alzaid AA, Nusair HM, Zbaidi NS, Al-Eithan MH, et al. Association Between Diabetes Self-Care, Medication Adherence, Anxiety, Depression, and Glycemic Control in Type 2 Diabetes. Saudi Med J (2012) 33:681–83.
55. Weledegebriel M, Mulugeta A, Hailu A. Evaluation of Self-Care Practice and Its Associated Factors in Adult Diabetic Patients, Ayder Diabetic Clinic, Mekelle, Ethiopia. Diabetes Metab Syndr Obes (2021) 14:2239–45. doi: 10.2147/dmso.s285181
56. Hai JA, Iftikhar S, Latif S, Herekar F, Patel MJ. Diabetes Self-Care Activities and Their Relation With Glycemic Control in Patients Presenting to The Indus Hospital, Karachi. Cureus (2019) 11:1–16. doi: 10.7759/cureus.6297
57. Shrivastava SR, Shrivastava PS, Ramasamy J. Role of Self-Care in Management of Diabetes Mellitus. J Diabetes Metab Disord (2013) 12:1–5. doi: 10.1186/2251-6581-12-14
58. Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A, et al. Intensive Glucose Control Versus Conventional Glucose Control for Type 1 Diabetes Mellitus. Cochrane Database Syst Rev (2014) 2014:CD009122. doi: 10.1002/14651858.CD009122.pub2
59. Nathan DM, Group DER. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care (2014) 37:9–16. doi: 10.2337/dc13-2112
60. Ejeta A, Abdosh T, Hawulte B, Lamessa A, Fite MB, Fekadu G. Diabetes Concordant Comorbidities and Associated Factors Among Adult Diabetic Out-Patients at Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia: A Cross-Sectional Study. Diabetes Metab Syndr Obes (2021) 14:2281–9. doi: 10.2147/dmso.s308553
61. Bralic Lang V, Bergman Markovic B. Prevalence of Comorbidity in Primary Care Patients With Type 2 Diabetes and its Association With Elevated HbA1c: A Cross-Sectional Study in Croatia. Scand J Prim Health Care (2016) 34:66–72. doi: 10.3109/02813432.2015.1132886
62. Abdulghani HM, AlRajeh AS, AlSalman BH, Alturki LS, Alnajashi NS, Irshad M, et al. Prevalence of Diabetic Comorbidities and Knowledge and Practices of Foot Care Among Diabetic Patients: A Cross-Sectional Study. Diabetes Metab Syndrome Obesity: Targets Ther (2018) 11:417–25. doi: 10.2147/DMSO.S171526
63. Dobrica EC, Gaman MA, Cozma MA, Bratu OG, Stoian AP, Diaconu CC, et al. Polypharmacy in Type 2 Diabetes Mellitus: Insights From an Internal Medicine Department. Med (Kaunas) (2019) 55:1–10. doi: 10.3390/medicina55080436
64. Teljeur C, Smith SM, Paul G, Kelly A, O’Dowd T. Multimorbidity in a Cohort of Patients With Type 2 Diabetes. Eur J Gen Pract (2013) 19:17–22. doi: 10.3109/13814788.2012.714768
65. Rodrigues MC, Oliveira C. Drug-Drug Interactions and Adverse Drug Reactions in Polypharmacy Among Older Adults: An Integrative Review. Rev Lat Am Enfermagem (2016) 24:e2800. doi: 10.1590/1518-8345.1316.2800
66. Bailey CJ, Kodack M. Patient Adherence to Medication Requirements for Therapy of Type 2 Diabetes. Int J Clin Pract (2011) 65:314–22. doi: 10.1111/j.1742-1241.2010.02544.x
Keywords: glycemic control, type 2 diabetes, total cholesterol, triglycerides, Harar, Ethiopia
Citation: Letta S, Aga F, Yadeta TA, Geda B and Dessie Y (2022) Correlates of Glycemic Control Among Patients With Type 2 Diabetes in Eastern Ethiopia: A Hospital-Based Cross-Sectional Study. Front. Endocrinol. 13:939804. doi: 10.3389/fendo.2022.939804
Received: 09 May 2022; Accepted: 14 June 2022;
Published: 22 July 2022.
Edited by:
Alok Raghav, Ganesh Shankar Vidyarthi Memorial Medical College, IndiaReviewed by:
Kirti Amresh Gautam, G D Goenka University, IndiaAlebachew Ashagre, University of Gondar, Ethiopia
Jamal Ahmad, Aligarh Muslim University, India
Copyright © 2022 Letta, Aga, Yadeta, Geda and Dessie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiferaw Letta, shife1973@gmail.com
 Shiferaw Letta
Shiferaw Letta Fekadu Aga2
Fekadu Aga2 Tesfaye Assebe Yadeta
Tesfaye Assebe Yadeta Yadeta Dessie
Yadeta Dessie