- 1Tianjin Medical University General Hospital, Tianjin, China
- 2Shanghai Tenth People’s Hospital, Tongji University, Shanghai, China
Purpose: To assess predictive value of clinical and pathological characteristics for metastatic radioactive iodine-refractory differentiated thyroid carcinoma (RAIR-DTC) in early stage retrospectively.
Methods: We studied 199 metastatic DTC patients who were divided into two groups (TgAb negative and TgAb positive). The stimulated Tg (Sti-Tg) at the first and second radioiodine therapy (RIT) were defined as Sti-Tg1 and Sti-Tg2, the suppressed Tg (Sup-Tg) were designated as Sup-Tg1 and Sup-Tg2, while the TgAb were defined as TgAb1 and TgAb2, respectively. Univariate analysis and Logistic regression were used to investigate the effects of 13 observed factors to predict RAIR-DTC.
Results: In TgAb negative group, ROC curve analysis showed that cut-off values of age, Sti-Tg2/Sti-Tg1 and Sup-Tg2/Sup-Tg1 to predict RAIR-DTC were 40 years old, 57.0% and 81.0%, respectively. Age, extrathyroid invasion, Sti-Tg2/Sti-Tg1, Sup-Tg2/Sup-Tg1 and BRAF gene mutation were proved to be independent factors predicting RAIR-DTC. In TgAb-positive group, ROC curve analysis showed that cut-off values of age, TgAb1 and TgAb2/TgAb1 to predict RAIR-DTC were 55 years old, 297 IU/ml (14.8 times higher than the upper limit) and 53.6%, respectively.
Conclusions: For TgAb-negative DTC, age over 40, extraglandular invasion, mutated BRAF gene, Sti-Tg decreased less than 43%, and Sup-Tg decreased less than 19% after the first two courses of RIT were independent predictors for RAIR-DTC. For TgAb-positive DTC, age over 55, extraglandular invasion, mutated BRAF gene, distant metastasis before RIT, TgAb level 14.8 times higher than the upper limit, TgAb dropped less than 46.4% after two courses of RIT were influencing factors.
Background
Thyroid carcinoma is the most common endocrine malignancy, most of which are differentiated thyroid carcinoma (DTC). For decades, “thyroidectomy + radioiodine therapy (RIT) + L-T4 suppression therapy” has been considered as standard treatment, the clinical efficacy is well documented (1, 2). However, about 30% of patients with distant metastases become radioactive iodine-refractory DTC (RAIR-DTC) (3, 4), and the 10-year survival rate is less than 10%. Patients would benefit little from RIT if they do not respond or become refractory to 131I. Therefore, timely diagnosis of RAIR-DTC can avoid unnecessary RIT and allow patients to move towards other relatively effective treatments. Till now, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18FDG-PET/CT) is recommended in clinical guidelines for the identification of RAIR-DTC, combining with 131I-whole body scan (131I-WBS), computed tomography (CT) and serum thyroglobulin (Tg) level after multiple RIT (3). How to early evaluate, diagnose, and timely adopt effective therapy for metastatic DTC (m-DTC) is a great clinical challenge. This study analyzed the detailed clinical and pathological characteristics of such patients, and proposed a prediction methodology for RAIR-DTC at an early stage.
Materials and Methods
Study Conduct
Each patient was provided with written information about the principles of the therapy and regulations of radiological protection. Further, written informed consent was obtained from all patients for participating the study. The selection and drop outs of study participants were shown in Flowchart Figure (Figure 1).
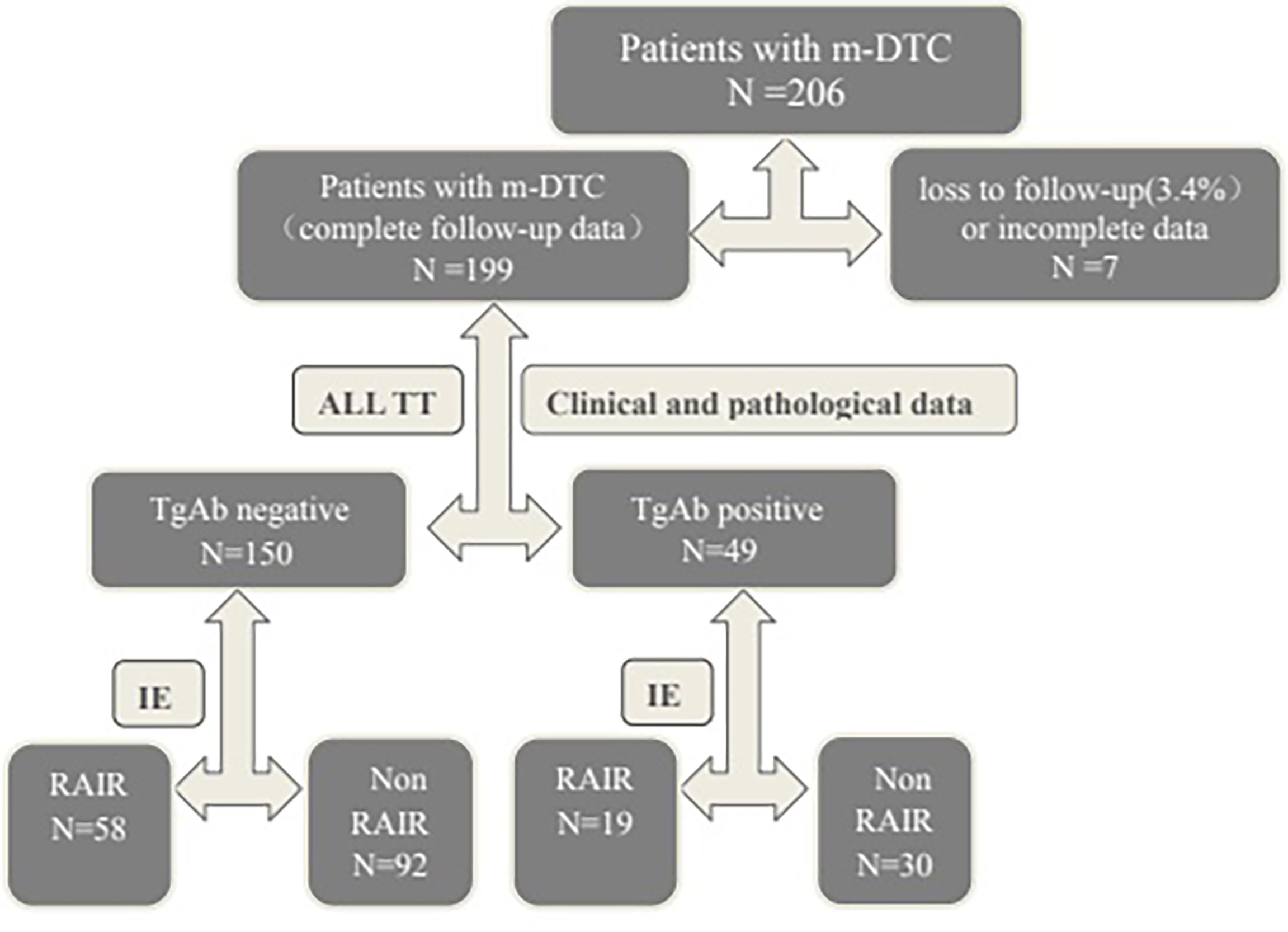
Figure 1 Flowchart of the participants. m-DTC, metastatic differentiated thyroid carcinoma; TT, total thyroidectomy; TgAb, thyroglobulin antibody; IE, imaging examination(WBS, CT, MRI, bone imaging or 18F-FDG PET-CT); RAIR, Radioactive iodine-refractory.
Patients
This retrospective study was conducted between January 2003 and October 2019. There were 199 of 206 cases with complete follow-up data. Loss to follow-up rate was 3.4%. Collectively, 199 m-DTC patients (71 males and 128 females; age range: 11-73 years) were enrolled. The average follow-up time was (50.2 ± 20.3) months. The inclusion criteria were as follows: (1) received total or near total thyroidectomy, (2) metastatic lesions existed, (3) received at least two courses of RIT, (4) both Tg and thyroglobulin antibody (TgAb) measured at the same time and scored according to the results. Patients were divided into TgAb negative group and TgAb positive group.
Patients were defined as lung, lymph node, bone or other site of metastasis with the following facts (3): (1) metastasis site confirmed by histopathology, (2) 131I-WBS showed focal or diffuse lung, lymph node, bone or other metastatic uptake, with or without other positive findings (by chest CT, X-rays, MRI, bone imaging or 18F-FDG PET/CT) and with or without Tg (TgAb) level elevation, (3) m-DTC patients diagnosed with different metastases site by post-therapeutic or diagnostic 131I-WBS after thyroid remnant ablation.
Clinical and Pathological Data
The clinical and histopathological data evaluated in this study included gender, age, primary tumor size, extrathyroid invasion, single or multiple primary tumors, AJCC TNM staging (4), BRAF gene mutation, lung metastasis size, and lymph nodes metastases size. Among them, lung metastasis, and lymph node metastasis were all divided into <1cm and ≥1cm; AJCC T staging was divided into T4 and T3 or below; AJCC N staging was divided into N0 and N1; AJCC M staging was divided into M0 and M1; BRAF gene mutations were detected by PCR.
Postoperative RIT and Follow-Up Strategy
A serum thyroid stimulating hormone (TSH) level of higher than 30 μIU/mL was achieved by thyroid hormone withdrawal (THW) before RIT. All patients were instructed with a low iodine diet from the beginning of THW till 3 weeks after RIT. Patients were administrated with a radioactive iodine dose of 3.7–7.4GBq (100-200mCi) according to the Chinese guideline (5). The second RIT was performed about 6 months after first therapy (5). All patients were under TSH suppressive therapy by using sodium levothyroxine with TSH <0.1 μIU/mL.
The functional detection sensitivities of Tg and TgAb by electrochemiluminescence immunoassay (Abbott Laboratories, Chicago, IL, USA) were 0.2 ng/mL and 20 IU/mL. TSH was determined by chemiluminescence immunoassay (Abbott Laboratories, Chicago, IL, USA), with a measuring range from 0.35 to 4.94 μIU/mL. A serum stimulated or suppressed Tg, TSH, TgAb were usually followed 1 day before RIT and 2–3 months after RIT.
131I-WBS was obtained by using Discovery NM/CT 670 (General Electric Medical Systems, Milwaukee, Wisconsin, USA) equipped with high-energy parallel-hole collimators, and a 20% energy window was centered at 364 keV, at a table speed of 14 cm/min for a total time of 20 min (256×1024 matrix). Post-therapeutic whole-body scan (Rx-WBS) was performed 3-6 days after RIT (5).
Tg and TgAb Assessment Profiles
The patients were divided into TgAb negative group and TgAb positive group. The stimulated Tg (defined as Sti-Tg) at the first and second RIT were defined as Sti-Tg1 and Sti-Tg2, the suppressed Tg (defined as Sup-Tg) were designated as Sup-Tg1 and Sup-Tg2, while TgAb were defined as TgAb1 and TgAb2, respectively.
Assessment of Radioactive Iodine-Refractory Thyroid Carcinoma
Till the last time of follow-up, RAIR-DTC was defined if the patient met any of the followings criteria (3): (1) the malignant/metastatic tissue did not ever concentrate RAI, (2) tumor tissue lost the ability to concentrate RAI after previous evidence of RAI-avid disease (absence of 131I contamination), (3) RAI was avidly concentrated in some lesions but not in others, and (4) metastatic disease progressed despite avidity of RAI.
Statistical Analysis
Univariate analysis, t test and Mann-Whitney test were used for qualitative variables, and chi-square test was used to compare categorical data. Logistic regression was used for multivariate analysis. ROC (Receiver operating characteristic) curve analysis was used to determine the critical value for predicting RAIR-DTC. Statistical analysis was carried out using SPSS (version 22.0; SPSS Inc, Chicago, Illinois, USA). A p value of less than 0.05 was considered significant.
Results
Patient Clinical and Pathological Characteristics
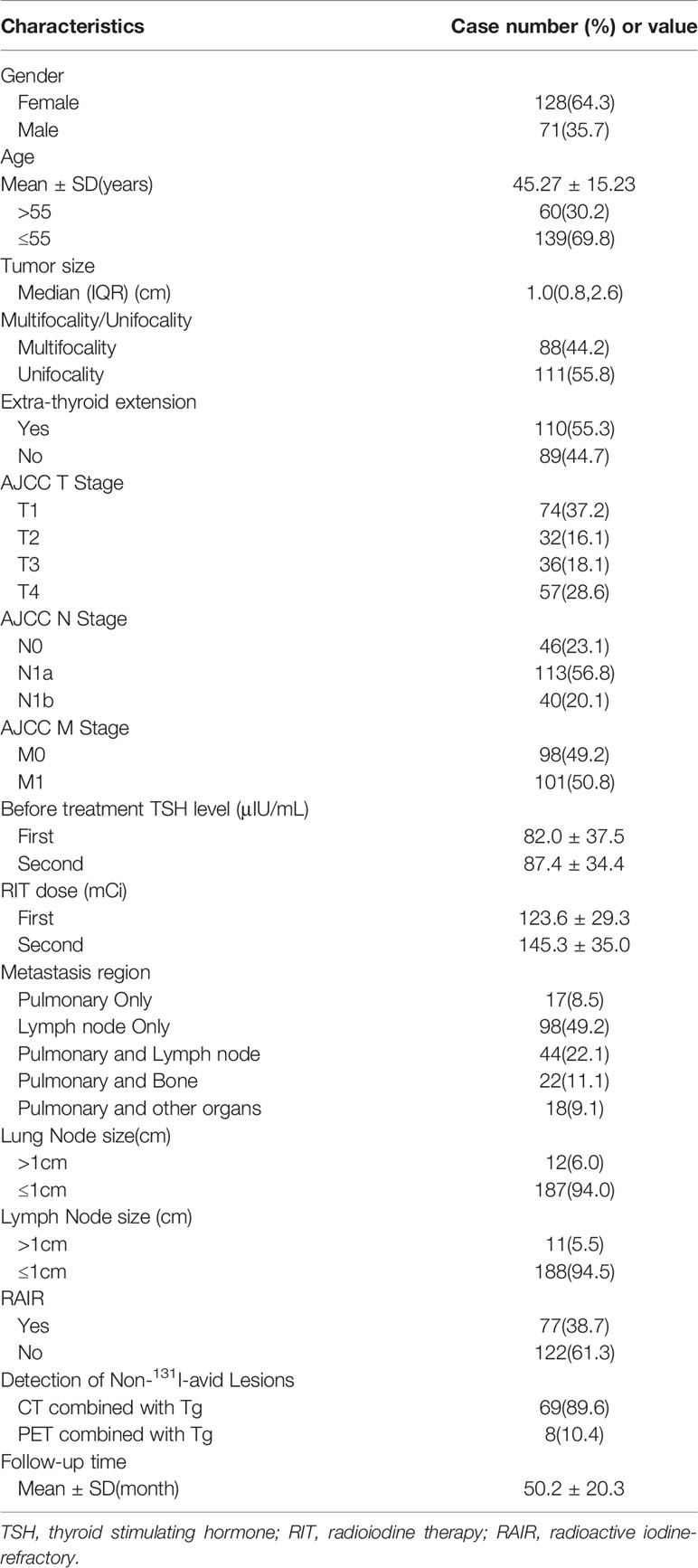
A total of 199 m-DTC patients (71 males and 128 females; age range: 11-73 years) were involved in this study, 150 were in the TgAb negative group and 49 in the TgAb positive group. Among them, 4 cases were follicular thyroid carcinoma (FTC) and 195 cases were papillary thyroid carcinoma (PTC). A total of 77 patients were considered as RAIR-DTC. The average follow-up time was (50.2 ± 20.3) months. The clinical and pathological characteristics of the included patients were shown in Table 1.
Univariate Analysis
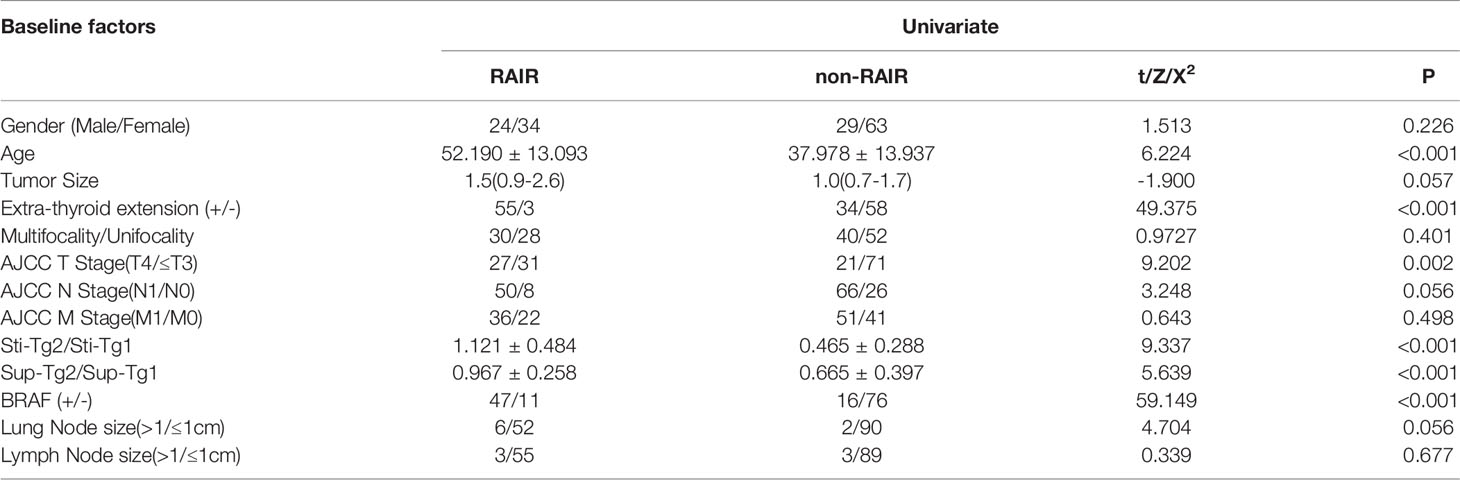
Univariate analyses on the factors to predict RAIR-DTC were given in Tables 2, 3. The results of TgAb negative group showed that cases with older age, extrathyroidal invasion, higher AJCC T stage, BRAF V600E mutation positivity, lower Sti-Tg2/Sti-Tg1 and lower Sup-Tg2/Sup-Tg1 after the first two courses of RIT would lead to poorer prognosis (P=0.000, 0.000, 0.002, 0.000, 0.000 and 0.000, respectively). However, we found no statistically significant differences in gender, primary tumor size, multifocality, AJCC N stage, AJCC M stage, lung metastasis size and lymph node metastasis size.
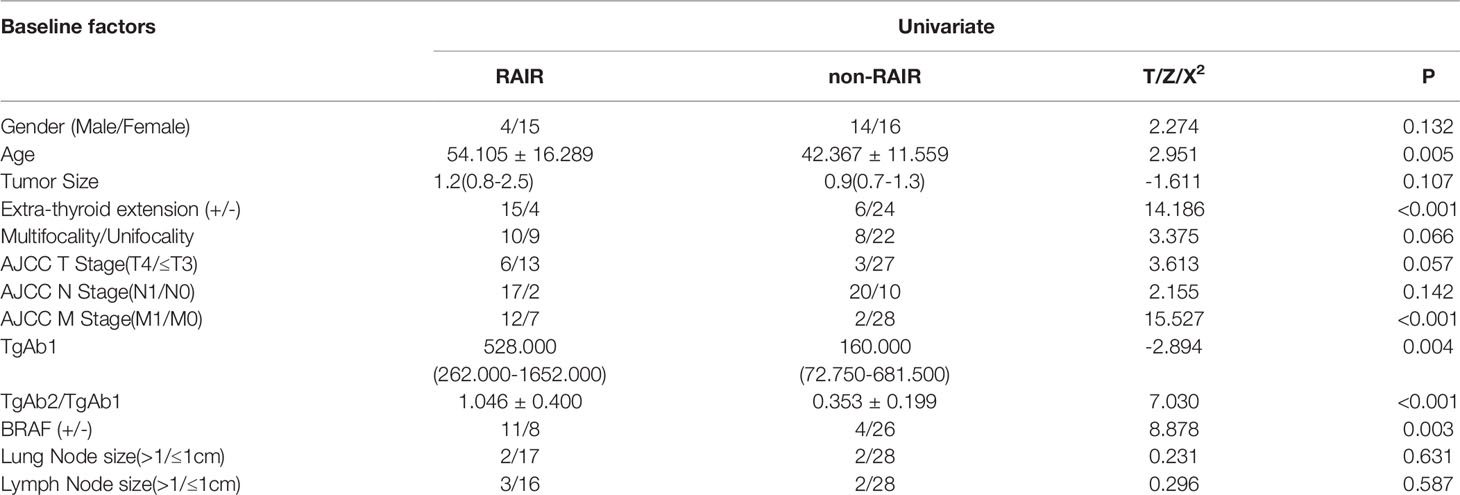
The results of TgAb positive group showed that cases with older age, extrathyroidal invasion, higher AJCC M stage, higher TgAb1 level, BRAF V600E mutation positivity, and lower TgAb2/TgAb1 would more likely progress to RARI-DTC (P=0.005, 0.000, 0.000, 0.004, 0.003 and 0.000, respectively). However, we found no statistically significant differences in gender, primary tumor size, primary tumor multifocality, AJCC T stage, AJCC N stage, lung metastasis size, and lymph node metastasis size. In specific, cases over 55 years old, with extraglandular invasion, positive BRAF gene mutation, distant metastasis before the first RIT, TgAb level 14.8 times higher than the upper limit, TgAb dropping less than 46.4% after the first two courses of RIT were influencing factors for becoming RAIR DTC.
ROC Curve Analysis and Diagnostic Performance
Data with statistical significance in the above univariate analyses were further assessed by ROC curve.
In TgAb negative group, ROC curve showed that age, Sti-Tg2/Sti-Tg1 and Sup-Tg2/Sup-Tg1 had good performances in predicting RARI-DTC (Figure 2). A cut-off value of age was 40 years old, with a sensitivity of 81.0% and specificity of 60.9% and an area under the curve (AUC) of 0.772. Cut-off value of Sti-Tg2/Sti-Tg1 was 57.0%, with a sensitivity of 98.3% and specificity of 80.4% and AUC of 0.921. Cut-off value of Sup-Tg2/Sup-Tg1 was 81.0%, with a sensitivity of 82.8% and specificity of 65.2% and AUC of 0.740.
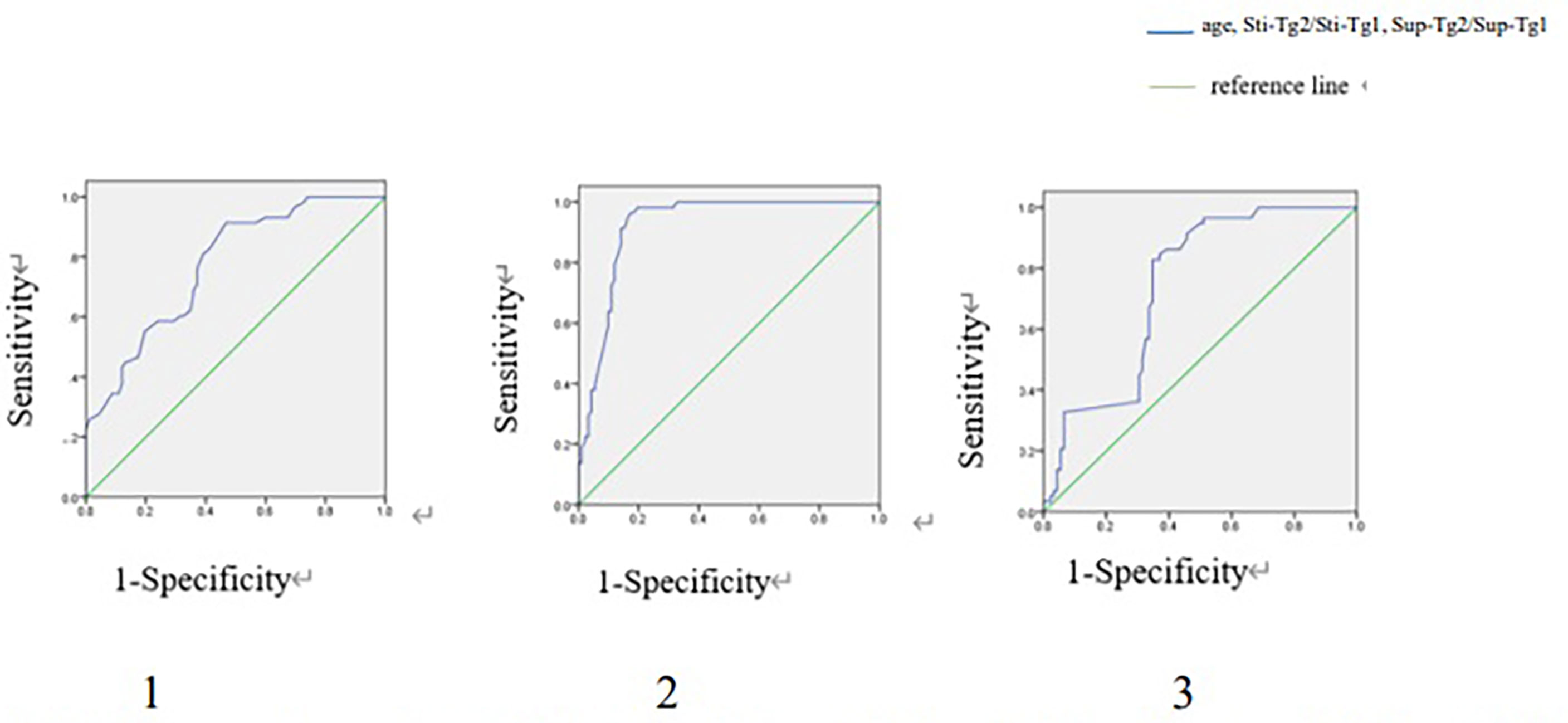
Figure 2 ROC curves of age, Sti-Tg2/Sti-Tg1 and Sup-Tg2/Sup-Tg1 for detecting RAIR in TgAb negative group. ROC, Receiver operating characteristic; RAIR, radioactive iodine refractory. 1. ROC curve of age for detecting RAIR in TgAb negative group. Area under the ROC (AUC) curve 0.772 (95% CI:0.699-0.846), cut-off value 40, sensitivity 0.810, specificity 0.609. 2. ROC curve of Sti-Tg2/Sti-Tg1 for detecting RAIR in TgAb negative group. AUC 0.921 (95% CI:0.878-0.965), cut-off value 0.570, sensitivity 0.983, specificity 0.804. 3. ROC curve of Sup-Tg2/Sup-Tg1 for detecting RAIR in TgAb negative group. AUC 0.740 (95% CI:0.662-0.819), cut-off value 0.810, sensitivity 0.828, specificity 0.652.
In TgAb positive group, ROC curves were drawn regarding age, TgAb1 level and TgAb2/TgAb1 in predicting RARI-DTC (Figure 3). The cut-off values of age, TgAb1 level and TgAb2/TgAb1 were 55 years old, 297 IU/ml (14.8 times higher than the upper limit) and 53.6%, with corresponding sensitivities of 68.4%, 73.7% and 94.7%, specificities of 86.7%,70.0% and 86.7%, and AUC values of 0.712, 0.747 and 0.974, respectively.
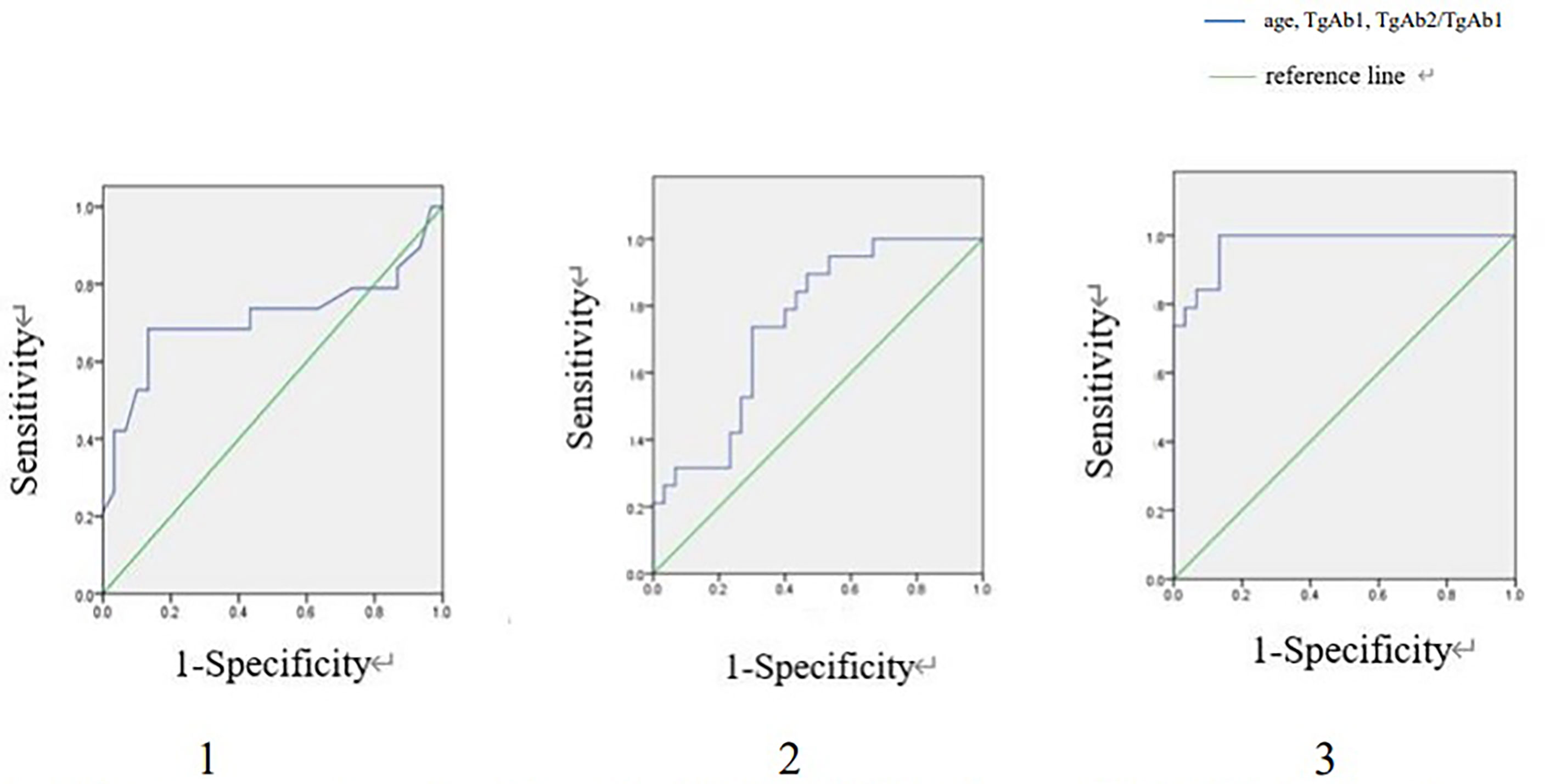
Figure 3 ROC curves of age, TgAb1 and TgAb2/TgAb1 for detecting RAIR in TgAb positive group. ROC, Receiver operating characteristic; RAIR, radioactiveiodine refractory. 1. ROC curve of age for detecting RAIR in TgAb positive group. Area under the ROC curve (AUC) 0.712 (95% CI:0.539-0.885), cut-off value 55, sensitivity 0.684, specificity 0.867. 2. ROC curve of TgAb1 for detecting RAIR in TgAb positive group. AUC 0.747 (95% CI:0.612-0.883), cut-off value:297, sensitivity: 0.737, specificity 0.700. 3. ROC curve of TgAb2/TgAb1 for detecting RAIR in TgAb positive group. AUC 0.974 (95% CI:0.000-1.000), cut-off value 0.536, sensitivity 0.947, specificity 0.867.
Multivariate Logistic Regression Analysis
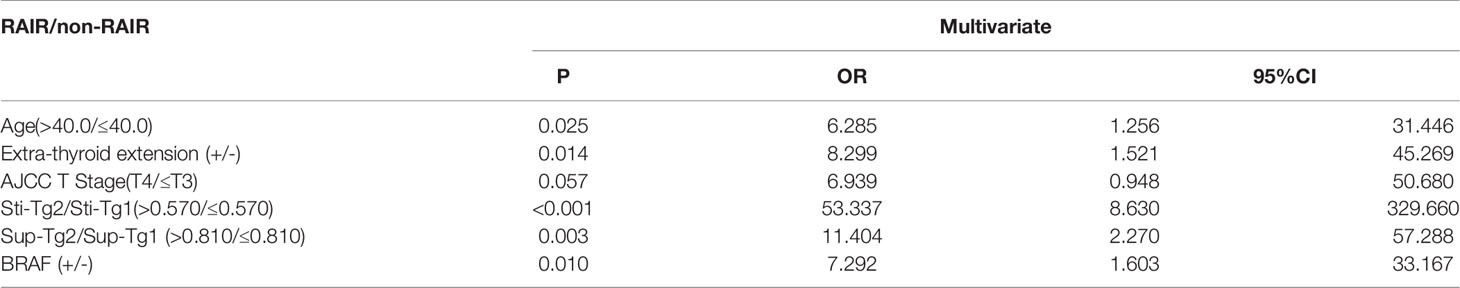
In TgAb negative group, multivariate logistic regression (Table 4) revealed that age (OR: 6.285, 95% CI: 1.256–31.446, P=0.025), extrathyroid invasion (OR: 8.299, 95% CI: 1.521–45.269, P=0.014), Sti-Tg2/Sti-Tg1 (OR: 53.337, 95% CI: 8.630–329.660, P=0.000), Sup-Tg2/Sup-Tg1 (OR: 11.404, 95% CI: 2.270–57.288, P=0.003) and BRAF gene mutation (OR: 7.292, 95% CI: 1.603–33.167, P=0.010) were independent factors in predicting RAIR-DTC. Specifically, age over 40 years old, with extraglandular invasion, BRAF gene mutation positivity, Sti-Tg decreased less than 43% and Sup-Tg decreased less than 19% were independent predictors for RAIR-DTC.
Due to small number of cases in TgAb positive group, the logistic regression analysis was not conducted.
Discussion
Currently, RIT is the mainstay treatment for m-DTC, especially for patients with lung, lymph node, bone or other metastases, while its efficacy depends on the ability of radioiodine uptake in the metastatic lesions. Non-avidity of the lesions usually indicates a tendency towards RAIR and less favorable outcome. The prognosis of patients with m-DTC is affected by many factors, and the loss of iodine uptake capacity in metastases will eventually transform into RAIR-DTC leading to a poorer prognosis (6–8). And the increasing risk of adverse effect and the progression during THW each time from repeated high-dose RIT limited its benefits. The current advancements in RAIR-DTC diagnosis are made according to post-131I therapy evaluation, under which circumstance the patients may have been exposed to unnecessary RAI and missed the opportunity to receive more effective interventions (such as targeted drug therapy). There is a need to be able to predict RAIR-DTC before 131I therapy and direct to a more individualized treatment (9). How to quickly evaluate and diagnose RAIR-DTC in an early stage, and timely adopt more effective methods for m-DTC is an important issue that needs to be solved urgently (10–12). In the current study, we envision whether metastatic RAIR-DTC can be early predicted by analyzing the clinical and pathological characteristics of these patients.
Multiple studies have confirmed Tg as a specific biomarker of DTC metastasis (13, 14). Song et al. (8) reported that the decline of Tg levels after RIT could link to the efficacy of 131I. Serum Tg level is the most sensitive and easily available bio-marker, and an indicator of recurrence or metastasis during the follow-up monitoring after thyroidectomy and 131I ablation in DTC patients. The quantitative changes of Tg could provide the basis for tumor response to RIT, and could be used as an index to predict RAIR-DTC (15). Miyauchi et al. (16) found that Tg-doubling time (Tg-DT) was a prominent independent predictor of RAIR-DTC prognosis. Patients with Tg-DT shorter than 1 year showed a 10-year-survival rate of 50%, which was significantly less than 95% of those with Tg-DT for 1-3 years. However, some patients with positive TgAb would hamper the accuracy of Tg determination and makes serum Tg level unreliable. In these patients, Tg cannot be used as DTC marker (17, 18).
In the current management guideline (3), the concept of using TgAb as DTC marker has not been taken into account with a full consensus. There are still conflicts regarding the association between TgAb and DTC outcomes (19–23). Some previous studies suggested that a rising trend of TgAb itself could indicate cancer recurrence or persistence (19–22). However, in another study (23), TgAb was not found to predict disease status in DTC patients. In order to avoid the above inconsistency, this study divided patients into TgAb-negative group and TgAb-positive group for further separate analysis.
Regarding the influencing factors, it is known that age is an independent prognostic factor for the efficacy of RIT (24–26). It could be due to the fact that elderly patients have longer disease duration, lower radiation sensitivity, poor uptake of 131I and weaker immune system. Therefore, close follow-up monitoring and/or other management strategies should be carried out for these elderly patients to avoid unnecessary or excessive RIT.
Regarding BRAFV600E mutation in DTC, literature indicates conflicting results. Some viewpoints suggest that BRAFV600E mutation is associated with DTC recurrence and poor prognosis (27–30). The BRAFV600E mutation negatively regulates iodine metabolism genes (NIS, TSHR, Tg, TPO, etc) via the abnormal activation of the mitogen-activated protein kinase (MAPK) pathway (31, 32). The opposite viewpoint exists showing BRAFV600E has limited predictive value for DTC prognosis, but, TERT and BRAF double mutations as a biomarker for predicting a worse outcome (33, 34). Our study demonstrated that the positive BRAF gene mutation was a predictor of RAIR-DTC.
In our research, we also focused on whether extrathyroid invasion could lead to RAIR-DTC. Ha’s study suggested that aggressive histopathologic subtypes were significantly associated with the recurrent or metastatic lesions in RAI-negative DTC (35). Robertson et al. (36) conducted a multivariate analysis of 231 patients with thyroid cancer and found that lymphadenopathy was the only important risk factor for recurrence (p<0.001), and the presence of extrathyroid invasion (p=0.013) leaded to high mortality. Aboelnaga (37) found that the survival rate of PTC was significantly affected by age, extrathyroid invasion and extensive distant metastasis. Our study also showed that the presence of extrathyroid invasion could be used as a predictive factor for the diagnosis of RAIR-DTC.
Li et al. (38) evaluated the clinical features of patients with RAIR-DTC, which indicated that tumor size, BMI, gender and TNM staging were statistically nonsignificant trend, however, smoking, tumor type (follicular thyroid cancer), extrathyroid extension, lymph node metastasis number (≥4), lymph node metastasis rate (≥53%), and pN stage (N1) were positively correlated with the prevalence of RAIR-DTC. Our study made some amendments based on the above literature and increased the predictive value of changes in TG and TGAb for RAIR-DTC, and showed negative predictive value for lymph node metastasis size. In the TgAb negative group, those who were over 40 years old, with obvious extraglandular invasion, BRAF gene mutation, Sti-Tg decreased less than 43% and Sup-Tg decreased less than 19% would develop into RAIR-DTC. Even if these patients continue RIT, they would not benefit from it, treatment and follow-up strategy should be adjusted. However, we found no statistically significant differences in gender, primary tumor size, multifocality, AJCC N stage, AJCC M stage, lung metastasis size and lymph node metastasis size. In the TgAb-positive group, the results found that both TgAb levels and trends between RAIR and non-RAIR-DTC were quite different. The persistently high TgAb level may indicate high aggressiveness of the tumor, which was a manifestation of poor prognosis. If the TgAb level did not decrease significantly after the second RIT, the patient would not benefit from the therapy.
This study had limitations. Firstly, the design of retrospective studies has shortcomings and should be verified by future prospective studies. Secondly, the number of TgAb positive cases was small, and it was difficult to conduct multivariate analysis. Thirdly, there may be interactions between positive results obtained by univariate analysis.
Conclusion
In summary, this study showed for TgAb-negative DTC patients, age over 40, extraglandular invasion, mutated BRAF gene, Sti-Tg decreased less than 43%, and Sup-Tg decreased less than 19% after the first two courses of RIT were independent predictors for RAIR-DTC. For TgAb-positive DTC patients, age over 55, extraglandular invasion, mutated BRAF gene, distant metastasis before the first RIT, level 14.8 times higher than the upper limit, TgAb dropped less than 46.4% after the first two courses of RIT were influencing factors.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of Tianjin Medical University General Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
JC and RW contributed to the conception and design of the study. JC, RZ assisted with data acquisition. JC, RZ, and RW conducted the statistical analyses and drafted the manuscript. JT, ZM, QJ and GZ critically revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Donohoe KJ, Aloff J, Avram AM, Bennet KG, Giovanella L, Greenspan B, et al. Appropriate Use Criteria for Nuclear Medicine in the Evaluation and Treatment of Differentiated Thyroid Cancer. J Nucl Med Off publication Soc Nucl Med (2020) 61):375–96. doi: 10.2967/jnumed.119.240945
2. Simsek FS, Balci TA, Donder Y, Ugur K, Kilinc F. How Important is the Timing of Radioiodine Ablation in Differentiated Thyroidal Carcinomas: A Referral Centre Experience. Rev espanola medicina Nucl e imagen Mol (2020) 39):157–62. doi: 10.1016/j.remn.2019.08.004
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2016) 26):1–133. doi: 10.1089/thy.2015.0020
4. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More "Personalized" Approach to Cancer Staging. CA: Cancer J Clin (2017) 67):93–9. doi: 10.3322/caac.21388
5. Tan J, Jiang NY, Li L, Lin YS, Lu HK, Gao ZR, et al. Guidelines for Radioiodine Therapy of Differentiated Thyroid Cancer (2014 Edition). Chin J Nucl Med Mol Imaging (2014) 34):264–78. doi: 10.3760/cma.j.issn.2095-2848.2014.04.002
6. Yu L, Hong H, Han J, Leng SX, Zhang H, Yan X. Comparison of Survival and Risk Factors of Differentiated Thyroid Cancer in the Geriatric Population. Front Oncol (2020) 10):42. doi: 10.3389/fonc.2020.00042
7. Cho SW, Choi HS, Yeom GJ, Lim JA, Moon JH, Park DJ, et al. Long-Term Prognosis of Differentiated Thyroid Cancer With Lung Metastasis in Korea and its Prognostic Factors. Thyroid Off J Am Thyroid Assoc (2014) 24):277–86. doi: 10.1089/thy.2012.0654
8. Song HJ, Qiu ZL, Shen CT, Wei WJ, Luo QY. Pulmonary Metastases in Differentiated Thyroid Cancer: Efficacy of Radioiodine Therapy and Prognostic Factors. Eur J Endocrinol (2015) 173):399–408. doi: 10.1530/eje-15-0296
9. Mu ZZ, Zhang X, Lin YS. Identification of Radioactive Iodine Refractory Differentiated Thyroid Cancer. Chonnam Med J (2019) 55):127–35. doi: 10.4068/cmj.2019.55.3.127
10. Hińcza K, Kowalik A, Pałyga I, Walczyk A, Gąsior-Perczak D, Mikina E, et al. Does the TT Variant of the rs966423 Polymorphism in DIRC3 Affect the Stage and Clinical Course of Papillary Thyroid Cancer? Cancers (2020) 12):423. doi: 10.3390/cancers12020423
11. Li S, Dong S, Xu W, Jiang Y, Li Z. Polymer Nanoformulation of Sorafenib and All-Trans Retinoic Acid for Synergistic Inhibition of Thyroid Cancer. Front Pharmacol (2019) 10):1676. doi: 10.3389/fphar.2019.01676
12. Araque KA, Gubbi S, Klubo-Gwiezdzinska J. Updates on the Management of Thyroid Cancer. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones metabolisme (2020) 52):562–77. doi: 10.1055/a-1089-7870
13. Liu L, Zhang X, Tian T, Huang R, Liu B. Prognostic Value of Pre-Ablation Stimulated Thyroglobulin in Children and Adolescents With Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2020) 30):1017–24. doi: 10.1089/thy.2019.0585
14. Yang X, Liang J, Li T, Zhao T, Lin Y. Preablative Stimulated Thyroglobulin Correlates to New Therapy Response System in Differentiated Thyroid Cancer. J Clin Endocrinol Metab (2016) 101):1307–13. doi: 10.1210/jc.2015-4016
15. Wang C, Zhang X, Li H, Li X, Lin Y. Quantitative Thyroglobulin Response to Radioactive Iodine Treatment in Predicting Radioactive Iodine-Refractory Thyroid Cancer With Pulmonary Metastasis. PloS One (2017) 12):e0179664. doi: 10.1371/journal.pone.0179664
16. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic Impact of Serum Thyroglobulin Doubling-Time Under Thyrotropin Suppression in Patients With Papillary Thyroid Carcinoma Who Underwent Total Thyroidectomy. Thyroid Off J Am Thyroid Assoc (2011) 21):707–16. doi: 10.1089/thy.2010.0355
17. Feldt-Rasmussen U, Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, et al. Thyroglobulin Autoantibodies as Surrogate Biomarkers in the Management of Patients With Differentiated Thyroid Carcinoma. Curr medicinal Chem (2014) 21):3687–92. doi: 10.2174/0929867321666140826120844
18. Spencer CA. Clinical Review: Clinical Utility of Thyroglobulin Antibody (TgAb) Measurements for Patients With Differentiated Thyroid Cancers (DTC). J Clin Endocrinol Metab (2011) 96):3615–27. doi: 10.1210/jc.2011-1740
19. Hsieh CJ, Wang PW. Sequential Changes of Serum Antithyroglobulin Antibody Levels are a Good Predictor of Disease Activity in Thyroglobulin-Negative Patients With Papillary Thyroid Carcinoma. Thyroid Off J Am Thyroid Assoc (2014) 24):488–93. doi: 10.1089/thy.2012.0611
20. Viola D, Agate L, Molinaro E, Bottici V, Lorusso L, Latrofa F, et al. Lung Recurrence of Papillary Thyroid Cancer Diagnosed With Antithyroglobulin Antibodies After 10 Years From Initial Treatment. Front Endocrinol (2018) 9):590. doi: 10.3389/fendo.2018.00590
21. Qiu ZL, Shen CT, Sun ZK, Song HJ, Zhang GQ, Luo QY. Lung Metastases From Papillary Thyroid Cancer With Persistently Negative Thyroglobulin and Elevated Thyroglobulin Antibody Levels During Radioactive Iodine Treatment and Follow-Up: Long-Term Outcomes and Prognostic Indicators. Front Endocrinol (2019) 10):903. doi: 10.3389/fendo.2019.00903
22. Sun D, Zheng X, He X, Huang C, Jia Q, Tan J, et al. Prognostic Value and Dynamics of Antithyroglobulin Antibodies for Differentiated Thyroid Carcinoma. Biomarkers Med (2020) 14):1683–92. doi: 10.2217/bmm-2019-0432
23. Smooke-Praw S, Ro K, Levin O, Ituarte PH, Harari A, Yeh MW. Thyroglobulin Antibody Levels Do Not Predict Disease Status in Papillary Thyroid Cancer. Clin Endocrinol (2014) 81):271–5. doi: 10.1111/cen.12421
24. Wierts R, Brans B, Havekes B, Kemerink GJ, Halders SG, Schaper NN, et al. Dose-Response Relationship in Differentiated Thyroid Cancer Patients Undergoing Radioiodine Treatment Assessed by Means of 124I PET/Ct. J Nucl Med Off publication Soc Nucl Med (2016) 57):1027–32. doi: 10.2967/jnumed.115.168799
25. Chen P, Feng HJ, Ouyang W, Wu JQ, Wang J, Sun YG, et al. Risk Factors for Nonremission and Progression-Free Survival After I-131 Therapy in Patients With Lung Metastasis From Differentiated Thyroid Cancer: A Single-Institute, Retrospective Analysis in Southern China. Endocrine Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists (2016) 22):1048–56. doi: 10.4158/ep151139.Or
26. Schneider DF, Chen H. New Developments in the Diagnosis and Treatment of Thyroid Cancer. CA: Cancer J Clin (2013) 63):374–94. doi: 10.3322/caac.21195
27. Cho JW, Kim WW, Lee YM, Jeon MJ, Kim WG, Song DE, et al. Impact of Tumor-Associated Macrophages and BRAF(V600E) Mutation on Clinical Outcomes in Patients With Various Thyroid Cancers. Head Neck (2019) 41):686–91. doi: 10.1002/hed.25469
28. Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers. J Clin Endocrinol Metab (2019) 104):1417–28. doi: 10.1210/jc.2018-01478
29. Wang P, Lun Y, Fu Y, Wang F, Zhao S, Wang Y, et al. Generation of a Potential Prognostic Matrix for Papillary Thyroid Cancer That Assesses Age, Tumor Size, Transforming Growth Factor-β, and BRAFV600E Mutation. Oncol Res Treat (2017) 40):586–92. doi: 10.1159/000477909
30. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association Between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2015) 33):42–50. doi: 10.1200/jco.2014.56.8253
31. Xing M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat Rev Cancer (2013) 13):184–99. doi: 10.1038/nrc3431
32. Yan C, Huang M, Li X, Wang T, Ling R. Relationship Between BRAF V600E and Clinical Features in Papillary Thyroid Carcinoma. Endocrine connections (2019) 8):988–96. doi: 10.1530/ec-19-0246
33. Luo Y, Jiang H, Xu W, Wang X, Ma B, Liao T, et al. Clinical, Pathological, and Molecular Characteristics Correlating to the Occurrence of Radioiodine Refractory Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10):549882. doi: 10.3389/fonc.2020.549882
34. Meng Z, Matsuse M, Saenko V, Yamashita S, Ren P, Zheng X, et al. TERT Promoter Mutation in Primary Papillary Thyroid Carcinoma Lesions Predicts Absent or Lower (131) I Uptake in Metastases. IUBMB Life (2019) 71):1030–40. doi: 10.1002/iub.2056
35. Ha LN, Iravani A, Nhung NT, Hanh NTM, Hutomo F, Son MH. Relationship Between Clinicopathologic Factors and FDG Avidity in Radioiodine-Negative Recurrent or Metastatic Differentiated Thyroid Carcinoma. Cancer Imaging Off Publ Int Cancer Imaging Soc (2021) 21):8. doi: 10.1186/s40644-020-00378-z
36. Robertson B, Parker M, Shepherd L, Panieri E, Cairncross L, Malherbe F, et al. Nodal Disease Predicts Recurrence Whereas Other Traditional Factors Affect Survival in a Cohort of South African Patients With Differentiated Thyroid Carcinoma. Cancers Head Neck (2018) 3):10. doi: 10.1186/s41199-018-0037-5
37. Aboelnaga EM, Ahmed RA. Difference Between Papillary and Follicular Thyroid Carcinoma Outcomes: An Experience From Egyptian Institution. Cancer Biol Med (2015) 12):53–9. doi: 10.7497/j.issn.2095-3941.2015.0005
Keywords: radioiodine therapy (RIT), radioactive iodine-refractory differentiated thyroid carcinoma, receiver-operating characteristic curve (ROC), thyroglobulin, thyroglobulin antibody (TgAb)
Citation: Chai J, Zhang R, Zheng W, Zhang G, Jia Q, Tan J, Meng Z and Wang R (2022) Predictive Value of Clinical and Pathological Characteristics for Metastatic Radioactive Iodine-Refractory Differentiated Thyroid Carcinoma: A 16-year Retrospective Study. Front. Endocrinol. 13:930180. doi: 10.3389/fendo.2022.930180
Received: 27 April 2022; Accepted: 30 May 2022;
Published: 28 June 2022.
Edited by:
Laura Sterian Ward, State University of Campinas, BrazilReviewed by:
Jose Miguel Dora, Federal University of Rio Grande do Sul and Hospital de Clínicas de Porto Alegre, BrasilFabian Pitoia, Hospital de Clínicas José de San Martín, Argentina
Copyright © 2022 Chai, Zhang, Zheng, Zhang, Jia, Tan, Meng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaowei Meng, zmeng@tmu.edu.cn; Renfei Wang, roslyn_en@163.com
 Jinyan Chai1
Jinyan Chai1 Ruiguo Zhang
Ruiguo Zhang Zhaowei Meng
Zhaowei Meng Renfei Wang
Renfei Wang