- 1Department of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 2Department of Psychology, University of Campania “Luigi Vanvitelli”, Caserta, Italy
- 3Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy
- 4Department of Movement Sciences and Wellbeing, University of Naples “Parthenope”, Naples, Italy
Background: The present study examines the relationship between obesity, executive functions, and body image in a nonclinical population from southern Italy.
Methods: General executive functioning (Frontal Assessment Battery–15), and body image disturbances (Body Uneasiness Test) were assessed in a sample including 255 participants (138 females, M age = 43.51 years, SD = 17.94, range = 18–86 years; M body mass index (BMI) = 26.21, SD = 4.32, range = 18.03–38.79).
Findings: Multiple Linear Regression Analysis indicated that age, years of education, FAB15 score, body image concerns, and avoidance predicted the variance of BMI. A subsequent mediation analysis highlighted that the indirect effect of FAB15 on BMI through avoidance was statistically significant.
Interpretation: Our results suggest that more performing executive functioning predicts a decrease in BMI that is partially due to the mitigation of avoidance behaviors.
Introduction
Obesity is a chronic disease and a major public health challenge (1). In recent decades, it has been argued that excess body fat, in addition to being an important risk factor for many chronic diseases (2, 3), could represent a significant predictor of impaired cognitive performance, accelerated cognitive decline, and dementia (4–8). In this vein, excess body weight appears to be related to reduced brain volume in cognitively healthy older individuals, but in patients with cognitive impairment it may exert an additional detrimental effect (9). More specifically, increased body adiposity has been found to correlate with generalized structural alterations involving the orbital frontal cortex, temporal and parietal cortices, and hippocampus (10–12). The first generation of studies investigating the relationship between obesity and cognition found that obese subjects performed worse on tasks assessing global cognitive functioning, particularly impacting attention and memory (13, 14). However, more recently, attention has focused on more targeted cognitive abilities, such as executive functions (EFs) (15–20). Functionally related to the integrity of the prefrontal cortex, EFs are an umbrella term encompassing different higher order cognitive domains (e.g., abstraction, cognitive flexibility, inhibitory control, working memory, and planning) that are crucial for the guidance, direction and management of the general cognition, emotions, and goal-directed behaviors (21, 22). They enable individuals to respond adaptively to environmental demands, especially in conflict and unfamiliar contexts (23, 24). Therefore, it is expected that EFs play a critical role in modulating eating behavior.
It has been argued that EFs could predict body weight variability (18, 25). For instance, EFs were found to be related with increased intake of high-fat foods (26–31), poor energy expenditure (32), increased susceptibility to emotional eating (33), inability to learn from past experiences (34), and worse outcomes in treatments aimed at weight decrease (27, 35). In addition, neuroimaging evidence has highlighted a decreased neural activity in the prefrontal cortex of obese subjects (36–38). Nevertheless, conflicting results are also available. In fact, some studies have shown that executive performance of obese subjects were equal or better than those of normal-weight subjects (39–46). These observations support the “obesity paradox” (47–50), which lies in the hypothesis that obesity could play a protective role for health status, especially in the elderly population. Since several covariates could affect the relationship beween EFs and obesity (46, 51), this association deserves to be further investigated (52).
An interesting line of research has focused on the link between eating habits and body image disturbances in obesity (53, 54). Similar to patients with eating disorders, patients with obesity exhibit excessive body dissatisfaction (55), undue weight-related concerns (56–59), and body image distortion (60–62). Body image refers to a multidimensional construct underlying how individuals perceive, think, and feel about their bodies (63). It is usually assessed along a continuum ranging from unhealthy body perceptions (inaccurate perceptions and major negative qualities) to healthy body perceptions (accurate perceptions and predominantly positive attributes) (63, 64). It has been shown that body image-related dissatisfaction may predict the onset of psychopathological symptoms (including depression and low self-esteem) and unhealthy behaviors (e.g., smoking, unhealthy weight control behavior) (65, 66). According to cognitive theories, schemas related to appearance, body shape and body weight impact on body image (67, 68). Negative body image perception would be associated with biases that affect selective attention, information processing, memory, and reasoning/judgment skills. Such biases allegedly increase negative emotions resulting from body image dissatisfaction, which would promote unhealthy behaviors aimed at changing body shape and weight, such as eating disorders (68, 69). Analysis of cognitive biases has shown that attentional biases would underlie psychopathology; however, memory and judgment biases –the degree to which emotionally salient stimuli are encoded, recalled, perceived, and processed– could also play an important role in behavioral disorders (68, 69). Results from eye-tracking studies have suggested that individuals with high levels of body dissatisfaction tend to orient their attention more towards stimuli related to desired (70, 71) and feared (72, 73) appearance. Individuals with higher levels of body dissatisfaction seem to show greater attentional bias towards negative appearance-related (74, 75) as well as toward positive appearance-related stimuli (75–78). Some studies on patients with eating disorders have highlighted that subtle cognitive dysfunctions could alter body schema information processing. These might depend on the interaction between bottom-up and top-down mechanisms (79–81), in which frontal circuits that support EFs are involved. This interaction appears to be supported by neuroimaging evidence that detected the involvement of the limbic/paralimbic system, medial prefrontal cortex (mPFC) (82–84) and parietal lobe (85, 86) in patients with eating and weight disorders (EWDs) during self-image exposure tasks.
To date, a clear understanding of how EFs interact with body image perception in obese individuals is lacking. Thus, the aim of the present study was to offset this gap within the scientific literature, exploring the hypothesized relationship between EFs, body image and body weight in a nonclinical headings.
Methods
Participants
A convenience sampling method was used and data from 287 participants (155 females) were collected. Participants were Italian volunteers recruited across different districts of Southern Italy (i.e., Campania, Calabria, and Puglia regions). Inclusion criteria were as follows: age ≥ 18 years, formal schooling ≥ 5 years (i.e., primary school), and adjusted score greater than the normative cutoff (i.e., 23.80) on the Mini-Mental State Examination (MMSE) (87, 88). Exclusion criteria were as follows: neurocognitive, psychiatric or psychopathological diseases, past or present intellectual and/or linguistic deficits, and presence of serious health conditions (e.g., cancer or morbid obesity). None of the participants had history of alcohol or substance abuse/addiction and was treated with drugs interfering with cognitive functioning. Individuals with well-compensated chronic medical illnesses such as hypertension, type II diabetes, or cardiovascular diseases were not excluded to prevent the construction of a hyper-normal sample.
Materials and procedure
Participants were tested individually in a sound-proofed room. After acquisition of sociodemographic data (i.e., sex, age, and education), body mass index (BMI) was calculated according to Quetelet’s formula (kg/m2). Following the administration of MMSE, participants were administered the Frontal Assessment Battery-15 (FAB15) (89, 90) and the Body Uneasiness Test-Form A (BUT-A) (91). The FAB15 is a short neuropsychological screening battery employed to assess general executive functioning. It consists of five subtests exploring abstraction abilities, generativity, planning, sensitivity to interference, and inhibitory control. The scoring range is 0–15, with a higher score reflecting a better performance. In a recent normative study on a large sample of healthy individuals, the FAB15 demonstrated good internal consistency (Cronbach’s alpha = 0.72), a solid factorial structure (explained variance ranging from 53.80 to 73.79), and excellent interrater and test–retest reliabilities (90). The BUT-A is a 34-item self-report measures used to perform a multidimensional clinical assessment of body uneasiness. It simultaneously explores different areas of body-related psychopathology: dissatisfaction about the body and its weight; avoiding and compulsive control behavior; experience of separation and foreignness regarding the body; specific worries for certain body parts, characteristics or functions. It consists of five subscales: weight Phobia (WP, 8 items), body image concerns (BIC, 9 items), avoidance (AV, 6 items), compulsive self-monitoring (CSM, 5 items), and depersonalization (D, 6 items). The global severity index (GSI) is the average rating of all the 34 items. The BUT-A shows good internal consistency (Cronbach’s = 0.79–0.90), good test-retest reliability (correlation coefficients greater than 0.7), and satisfactory concurrent and discriminant validity (91). Participants were not aware of the specific aims of the study in order not to influence their response to the self-administered tests.
Ethics statement
All participants gave prior written informed consent to the study which was approved by the ethics committee of the University of Campania “Luigi Vanvitelli” and carried out according to the 1964 Declaration of Helsinki.
Statistical analyses
Statistical analyses were conducted in line with the generalized linear model. Descriptive statistics were expressed as frequency for nominal variables and mean and standard deviation for continuous variables. A multiple linear regression analysis (simultaneous entering method) was performed loading BMI as dependent variable and sex, age, education, FAB15 score, and BUT-A scores as predictors. The variance inflation factors (VIFs) and tolerance values were computed for determining the presence of a statistically significant multicollinearity (VIF = 1, no collinearity; VIF = 1 to 5, moderate collinearity; VIF > 5, high collinearity). Tolerance values ≥ 0.10 were deemed acceptable (92, 93). To establish whether, and how much, body image subdomains mediated the relationship between executive functioning (i.e., FAB15) and BMI, a mediation analysis was performed in line with results from multiple regression. To evaluate the significance of direct and indirect effects, bootstrapping procedure with 5,000 samples with replacement from the full sample to construct bias-corrected 95% confidence intervals (95% CI) was used (94). Statistical analyses were performed by means of IBM SPSS Statistics v. 26. Particularly, the SPSS Macro PROCESS was applied to execute mediation analysis. Finally, G*Power 3.1.9.4 was used to perform power analysis. A p-value ≤ 0.05 was considered statistically significant.
Results
Preliminary data analysis: normality assumptions and missing data
Univariate normality was assessed according to skewness and kurtosis indexes. More specifically, values ranging from -2 and +2 indicate the absence of appreciable deviations from normality. Square root transformation (√Xi) was applied to normalize variables in line with skewness parameter (|1|< γ< |2|). Univariate outliers, i.e., z-scores higher than 3 in absolute terms (95), were deleted from the dataset (n = 32). For diagnostics of multivariate outliers, Mahalanobis’ distance was calculated. No multivariate outliers were detected (mean = 11.91, df = 10, ps > 0.001). Multivariate normality was assumed by Mardia’s coefficient = 1.02< 120. Analysis of missing data showed a random missingness pattern (MCAR) that was handled via multiple imputation method.
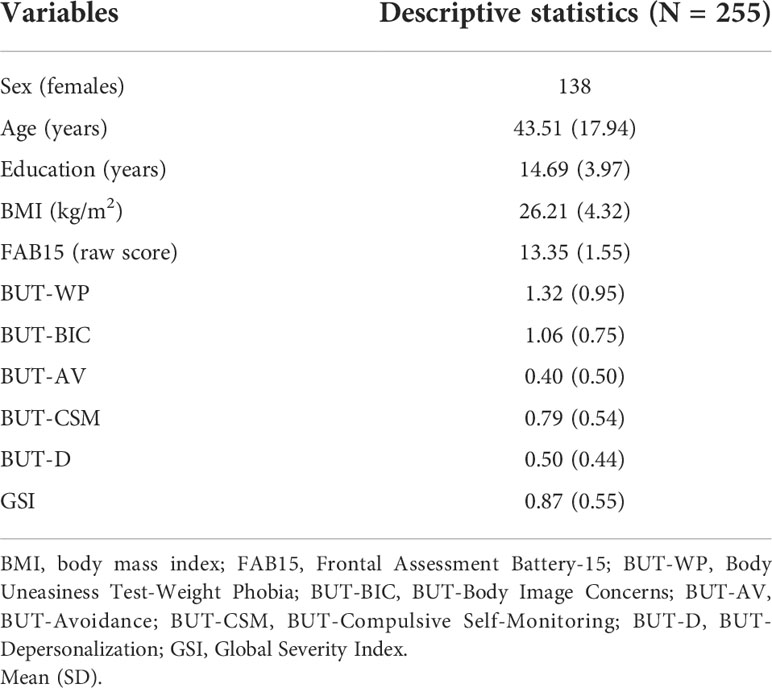
Descriptive statistics
Data from 255 volunteers (138 females) were analyzed (Table 1). Mean age of participants was 43.51 years (SD = 17.94, range = 18–86 years), whereas their mean education was 14.69 years (SD = 3.97, range = 5–20 years). Mean BMI value was 26.21 (SD = 4.32, range = 18.03–38.79; normal-weight, n = 101, mean BMI = 22.17, SD = 1.89; overweight, n = 98, mean BMI = 26.74, SD = 1.34; obese, n = 56, mean BMI = 32.56, SD = 2.33). On average, participants got a raw FAB15 score equal to 13.35 (SD = 1.55, range = 9–15). As for the BUT-A scores, mean values are the following: WP, mean = 1.32 (SD = 0.95, range = 0–3.88); BIC, mean = 1.06 (SD = 0.75, range = 0–3.33); AV, mean = 0.40 (SD = 0.50, range = 0–2.17); CSM, mean = 0.79 (SD = 0.54, range = 0–2.40); D, mean = 0.50 (SD = 0.44, range = 0–2.15). Mean GSI score was 0.87 (SD = 0.55, range = 0.03–2.59).
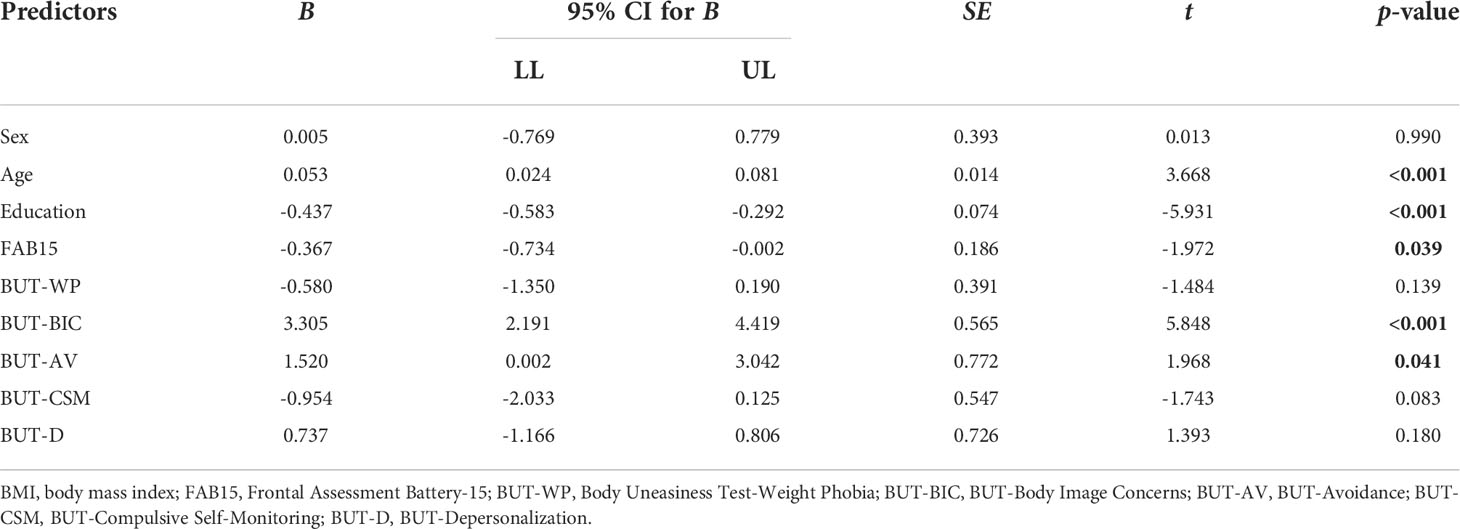
Multiple linear regression analysis
Assumptions of linear regression analysis were satisfied. Particularly, the relationship between predictors and dependent variable was linear, no violations of homoscedasticity was detected, and the standardized residuals followed the normal distribution. Furthermore, results from power analysis (nominal α = 0.05, power = 0.80, effect size f2 = 0.15, and number of predictors = 10) showed that a sample of 255 was more than acceptable (required sample size = 118). Due to abnormal VIF and tolerance values for the GSI score (VIF = 18.45, tolerance = 0.05), this variable was not included in the final model (see Table 2). The latter explained a significant amount of BMI variance (R2 = 0.46, F(9, 246) = 20.057, p< 0.001). The variables found to be significant predictors of BMI were age (B = 0.05, p< 0.001), education (B = -0.44, p< 0.001), FAB15 (B = -0.37, p = 0.04), and both BIC (B = 3.30, p< 0.001) and AV subscales (B = 1.52, p = 0.04) of the BUT-A.
Mediation analysis
To test the mediating role of BUT-AV and/or BUT-BIC in the relationship between FAB15 (general executive functioning) and BMI, a mediation analysis was performed. A graphical representation of the mediation model is displayed in Figure 1. The FAB15 score was a significant predictor of BUT-AV (a1 = -0.063, 95% CI [-0.105, -0.022], SE = 0.021, t = -3.034, p = 0.002) but not of BUT-BIC (a2 = -0.014, 95% CI [-0.080, 0.051], SE = 0.033, t = -0.432, p = 0.666). However, both BUT-AV (b1 = 1.977, 95% CI [0.466, 3.489], SE = 0.767, t = 2.578, p = 0.010) and BUT-BIC (b2 = 1.724, 95% CI [0.780, 2.669], SE = 0.479, t = 3.599, p< 0.001) showed a positive association with BMI. As a result, the indirect effect of FAB15 on BMI via BUT-AV (ab1) was statistically significant (ab1 = -0.125, 95% CI [-0.305, -0.009], SE = 0.076) while the indirect effect through BUT-BIC (ab2) was not (ab2 = -0.025, 95% CI [-0.166, 0.091], SE = 0.123). The direct effect of FAB15 on BMI (c′) was significant (c′ = -1.208, 95% CI [-1.550, -0.866], SE = 0.173, t = -6.965, p< 0.001); therefore, BUT-AV partially mediated the FAB15-BMI relationship. For the sake of clarity, we found that FAB15 exerted an indirect negative effect on BMI, which was partially explained by the negative association between FAB15 and BUT-AV. In other words, more performing executive functioning predicted a decrease in BMI that was partially due to the mitigation of avoidance behaviors.
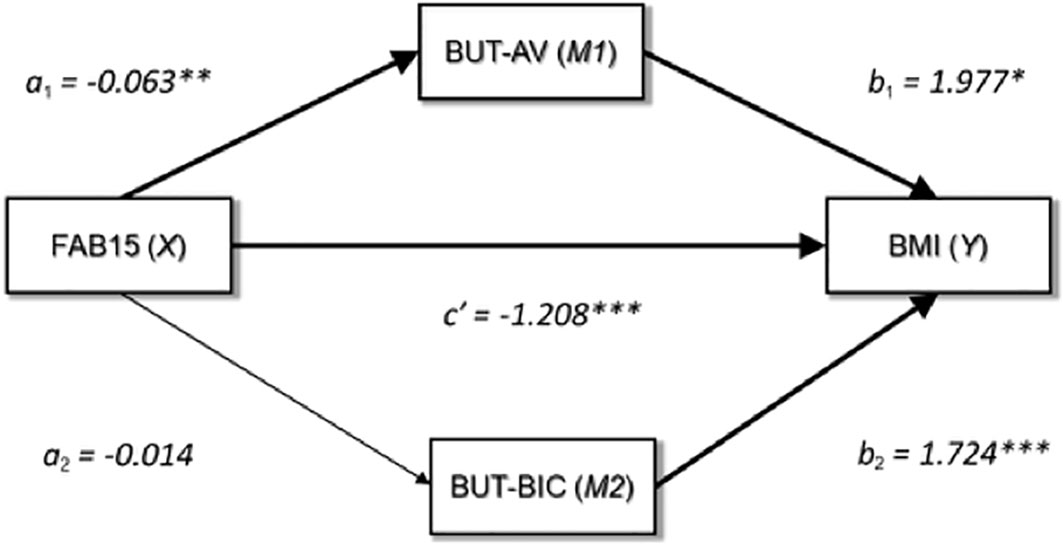
Figure 1 Graphical representation of the medication analysis output. FAB15, frontal assessment battery-15; BUT-AV, BUT-Avoidance; BUT-BIC, BUT-Body Image Concerns; BMI, body mass index; *p = <0.05; **p = <0.01; ***p = <0.001.
Discussion
With the aim to disentangle the unclear relationship between EFs and obesity (18–20, 30, 96, 97), the present study was designed to investigate the possible associations between EFs, body image and BMI in a nonclinical sample. Results from a preliminary multiple linear regression analysis showed that BMI was predicted by anthropometric variables (i.e., age and years of education), FAB15 score, in addition to both BUT-BIC and BUT-AV scores. Via a subsequent mediation analysis, we found that the negative association between EFs and BMI was partially explained by reduction of avoidance strategies.
Typically, avoidance is encountered in individuals with body image disorders. It leads people to avoid looking at themselves in the mirror and/or being looked at; in addition, thoughts concerned with alleged body defects significantly affect intimate, social, and work life. Avoidance was also associated with food choices (e.g., avoidance of sugary foods) and with interoceptive and emotional feelings inducing food ingestion (e.g., feeling of empty stomach or guilt after eating) (91).
To understand how EFs may interact with body weight and body image perception, examining the interaction between body weight and brain physiology is needed. Neuroimaging studies have shown hypoperfusion in the frontal territories and in the adjacent portions of the temporal and parietal cortices at increasing BMI levels (36–38). In addition, amygdala, ventral striatum, insula, and prefrontal regions (in particular, the orbitofrontal cortex and dorsomedial prefrontal cortex) functionally interact in order to promote approach conditions/behaviors, avoidance strategies/behaviors and decision-making processes (98, 99).
Decision making is a dynamic process, achieved after comparing and agreeing the current internal state with the expected one. Individuals constantly arbitrate between potential negative and/or rewarding outcomes when faced with a conflicting context. In this regard, excessive avoidance is presumably associated to overactivity of the amygdala and/or insular regions (100, 101). The increased signals related to the salience of anticipated emotional and interoceptive stimuli might decrease orbitofrontal activity that is, instead, aimed at integrating these signals. In this context, other relevant contingent information might be underestimated. Interestingly, a dysfunctional representation of approach conditions may be likely related to abnormal neural activity in the ventral striatum (102, 103). Attenuation of striatal activity may result in decreased motivation during effort-based decision-making tasks. Conversely, increased striatal activation could be related to overrepresentation of approach appraisal (104, 105).
The concomitant presence of conflicting motivational stimuli, such as approach- and avoidance-related stimuli, might overload the orbitofrontal cortex, leading to increased reaction time, as revealed during some decision-making paradigms, particularly those involving both reward and punishment, such as risk-based paradigms (106–108).
According to the “corticolimbic disconnection hypothesis” (109, 110), fronto-limbic suppressive mechanisms generate a state of emotional numbness. In particular, the prefrontal cortex would interact with the anterior cingulate cortex and amygdala, determining low emotionality, attentive difficulties, autonomic mitigation, and indifference to pain (100, 101, 111, 112). In addition, hypoactivity of posterior parietal regions has been associated with deficits in processing and integrating somatosensory information (111, 113–115) and low self-awareness (116).
The somatosensory pathways are involved in both conscious perception/recognition of one’s own body (i.e., the body image) (117) and in the body schema construction (118), i.e., a dynamic representation of one’s own body used to drive actions (119, 120). The terms body image and body schema are used to refer to two different dimensions of body representation, aware and unaware, respectively (121). Body image refers to “the body we perceive” and the conscious appraisal of one’s physical appearance (perceptual, cognitive, emotional) that is differentiated from the environment; conversely, body schema refers to “the body we act with” connected to the unconscious sensorimotor and postural control of one’s own body (sensory-motor capacities that function in communion with environment). However, the distinction is still unclear, and these terms have been and continue to be used in an arbitrary way (121).
In addition, the posterior parietal cortex, in concert with frontal cortex, the posterior insula and the angular gyrus, plays a pivotal role in integrating different input signals related to self-awareness in terms of enteroception, feelings of agency, and visceral sensations (122–129).
Overweight and obese subjects are more likely to misperceive their body features, i.e., they tend to underestimate (130, 131) or overestimate (60, 61, 127) their whole body or selective body parts (55, 62, 132, 133), particularly when these are perceived as unattractive (134–136).
Recently, the mirror exposure therapy (MET) has been proposed as an effective treatment for body image dissatisfaction (137, 138). MET may normalize interpretive biases by training individuals to assess their bodies in an objective, affectively neutral, or positive, manner (139, 140). Furthermore, MET may act by redirecting the focus of attention away from the negative body parts to the more balanced (141, 142), thereby gradually reducing self-focused attention. In other words, MET behaves as an exposure therapy by enhancing extinction through formation of a new safety memory that attenuates the negative response and/or through habituation (143, 144).
Our results showed that EFs exert a negative effect on BMI, which is partly justified by a detrimental action on avoidance, a subdimension of the body image construct. Although the relationship between body dissatisfaction and EFs is poorly investigated, our results suggest the involvement of executive abilities in modulating the cognitive processes underlying eating behavior and body weight control. In particular, EFs seem to indirectly influence the motivational systems involved in the processes of approaching/avoiding body image. Executive domains embrace the processes by which goal-directed actions are carried out, such as maintaining salient information in working memory and inhibiting non-goal-related responses (21–24). Successful self-regulation implies that individuals not only have sufficient motivation to reduce the discrepancy between the actual body image and the standard they are pursuing, but also the ability to achieve this reduction in the discrepancy. The ability to self-regulate is strongly related to EFs.
In a scientific context in which the link between EFs and obesity needs to be further explored, our findings may contribute to extend the debate on the matter.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by University of Campania “Luigi Vanvitelli”. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, MLM, AM, CRI and IV. methodology, MLM and CRI. software, CRI. validation, AM and IV. formal analysis, MLM and CRI. investigation, MLM, CRI, RP, MRS, MF, AC, and FS. resources, AM, GDM, IV and VM. data curation, CRI, RP, MRS, FS and GDM. writing—original draft preparation, MLM, AM, CRI and IV. writing—review and editing, MLM, CRI, IV and AM. visualization, MRS, MF, AC and FS. supervision, MLM, CRI, IV, AM, GMe and VM. project administration, MLM, GMe, IV and VM. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser (2000) 894:i–xii1, –253.
2. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Trans Med (2017) 5:161–1. doi: 10.21037/atm.2017.03.107
3. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
4. Miller AA, Spencer SJ. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behavior Immun (2014) 42:10–21. doi: 10.1016/j.bbi.2014.04.001
5. Bischof GN, Park DC. Obesity and aging: Consequences for cognition, brain structure, and brain function. Psychosomatic Med (2015) 77:697–709. doi: 10.1097/PSY.0000000000000212
6. Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc (2017) 76:443–54. doi: 10.1017/S0029665117002014
7. Leigh S-J, Morris MJ. Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. Biochim Biophys Acta (BBA) - Mol Basis Dis (2020) 1866:165767. doi: 10.1016/j.bbadis.2020.165767
8. Ma W, Zhang H, Wu N, Liu Y, Han P, Wang F, et al. Relationship between obesity-related anthropometric indicators and cognitive function in Chinese suburb-dwelling older adults. PloS One (2021) 16:e0258922. doi: 10.1371/journal.pone.0258922
9. Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Hum Brain Mapp (2009) 31:353–64. doi: 10.1002/hbm.20870
10. Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol (2005) 62:1545–8. doi: 10.1001/archneur.62.10.1545
11. Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol (2005) 5:23. doi: 10.1186/1471-2377-5-23
12. Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing (2006) 36:23–9. doi: 10.1093/ageing/afl123
13. Appelhans BM, French SA, Pagoto SL, Sherwood NE. Managing temptation in obesity treatment: A neurobehavioral model of intervention strategies. Appetite (2016) 96:268–79. doi: 10.1016/j.appet.2015.09.035
14. Higgs S, Williamson AC, Attwood AS. Recall of recent lunch and its effect on subsequent snack intake. Physiol Behav (2008) 94:454–62. doi: 10.1016/j.physbeh.2008.02.011
15. Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes Res Clin Pract (2015) 9:93–113. doi: 10.1016/j.orcp.2014.05.001
16. Sellaro R, Colzato LS. High body mass index is associated with impaired cognitive control. Appetite (2017) 113:301–9. doi: 10.1016/j.appet.2017.03.008
17. Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment: Obesity and cognitive function across lifespan. Obes Rev (2011) 12:740–55. doi: 10.1111/j.1467-789X.2011.00920.x
18. Yang Y, Shields GS, Guo C, Liu Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci Biobehav Rev (2018) 84:225–44. doi: 10.1016/j.neubiorev.2017.11.020
19. Fitzpatrick S, Gilbert S, Serpell L. Systematic review: Are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol Rev (2013) 23:138–56. doi: 10.1007/s11065-013-9224-7
20. Gettens KM, Gorin AA. Executive function in weight loss and weight loss maintenance: a conceptual review and novel neuropsychological model of weight control. J Behav Med (2017) 40:687–701. doi: 10.1007/s10865-017-9831-5
21. Strauss E, Sherman EMS, Spreen O, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. Oxford ; New York: Oxford University Press (2006). p. 1216.
22. Ardila A. On the evolutionary origins of executive functions. Brain Cogn (2008) 68:92–9. doi: 10.1016/j.bandc.2008.03.003
23. Burgess PW, Simons JS. Theories of frontal lobe executive function: clinical applications. In: Halligan PW, Wade DT, editors. The effectiveness of rehabilitation for cognitive deficits. (Oxford: Oxford University Press) (2005). p. 211–31. doi: 10.1093/acprof:oso/9780198526544.003.0018
24. Diamond A. Executive functions. Annu Rev Psychol (2013) 64:135–68. doi: 10.1146/annurev-psych-113011-143750
25. La Marra M, Ilardi CR, Villano I, Carosella M, Staiano M, Iavarone A, et al. Functional relationship between inhibitory control, cognitive flexibility, psychomotor speed and obesity. Brain Sci (2022) 12:1080. doi: 10.3390/brainsci12081080
26. Powell DJH, McMinn D, Allan JL. Does real time variability in inhibitory control drive snacking behavior? an intensive longitudinal study. Health Psychol (2017) 36:356–64. doi: 10.1037/hea0000471
27. Dassen FCM, Houben K, Allom V, Jansen A. Self-regulation and obesity: the role of executive function and delay discounting in the prediction of weight loss. J Behav Med (2018) 41:806–18. doi: 10.1007/s10865-018-9940-9
28. Lyke JA, Spinella M. Associations among aspects of impulsivity and eating factors in a nonclinical sample. Int J Eat Disord (2004) 36:229–33. doi: 10.1002/eat.20025
29. Steward T, Mestre-Bach G, Vintró-Alcaraz C, Lozano-Madrid M, Agüera Z, Fernández-Formoso JA, et al. Food addiction and impaired executive functions in women with obesity. Eur Eat Disord Rev (2018) 26:574–84. doi: 10.1002/erv.2636
30. Gluck ME, Viswanath P, Stinson EJ. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep (2017) 6:380–8. doi: 10.1007/s13679-017-0289-0
31. Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, et al. Cognitive function in morbidly obese individuals with and without binge eating disorder. Compr Psychiatry (2012) 53:490–5. doi: 10.1016/j.comppsych.2011.09.002
32. Riggs N, Chou C-P, Spruijt-Metz D, Pentz MA. Executive cognitive function as a correlate and predictor of child food intake and physical activity. Child Neuropsychol (2010) 16:279–92. doi: 10.1080/09297041003601488
33. Sims RC, Bennett NK, Mwendwa DT, Ali MK, Levy SA, Callender CO, et al. Executive function and negative eating behaviors in severely obese African americans. Ethn Dis (2014) 24:328–34.
34. Rodrigue C, Ouellette A-S, Lemieux S, Tchernof A, Biertho L, Bégin C. Executive functioning and psychological symptoms in food addiction: a study among individuals with severe obesity. Eat Weight Disord (2018) 23:469–78. doi: 10.1007/s40519-018-0530-1
35. Witbracht MG, Laugero KD, Van Loan MD, Adams SH, Keim NL. Performance on the Iowa gambling task is related to magnitude of weight loss and salivary cortisol in a diet-induced weight loss intervention in overweight women. Physiol Behav (2012) 106:291–7. doi: 10.1016/j.physbeh.2011.04.035
36. O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol (2017) 16:465–77. doi: 10.1016/S1474-4422(17)30084-4
37. Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging (2010) 31:1326–39. doi: 10.1016/j.neurobiolaging.2010.04.006
38. Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology (2004) 63:1876–81. doi: 10.1212/01.WNL.0000141850.47773.5F
39. Boeka A, Lokken K. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol (2008) 23:467–74. doi: 10.1016/j.acn.2008.03.003
40. Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, Zonderman AB. Longitudinal examination of obesity and cognitive function: Results from the Baltimore longitudinal study of aging. Neuroepidemiology (2010) 34:222–9. doi: 10.1159/000297742
41. Hartanto A, Yong J, Toh W. Bidirectional associations between obesity and cognitive function in midlife adults: A longitudinal study. Nutrients (2019) 11:2343. doi: 10.3390/nu11102343
42. Skinner JS, Abel WM, McCoy K, Wilkins CH. Exploring the “Obesity paradox” as a correlate of cognitive and physical function in community-dwelling black and white older adults. Ethn Dis (2017) 27:387. doi: 10.18865/ed.27.4.387
43. Volkow ND, Wang G-J, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phil Trans R Soc B (2008) 363:3191–200. doi: 10.1098/rstb.2008.0107
44. Monica D, Paulo M, Appolinário JC, De Freitas ,SR, Coutinho G, Santos C, et al. Assessment of executive functions in obese individuals with binge eating disorder. Rev Bras Psiquiatr (2010) 32:381–8. doi: 10.1590/S1516-44462010000400011
45. Favieri F, Forte G, Casagrande M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front Psychol (2019) 10:2126. doi: 10.3389/fpsyg.2019.02126
46. La Marra M, Villano I, Ilardi CR, Carosella M, Staiano M, Iavarone A, et al. Executive functions in overweight and obese treatment-seeking patients: Cross-sectional data and longitudinal perspectives. Brain Sci (2022) 12:777. doi: 10.3390/brainsci12060777
47. Artham SM, Lavie CJ, Milani RV, Ventura HO. The obesity paradox: Impact of obesity on the prevalence and prognosis of cardiovascular diseases. Postgraduate Med (2008) 120:34–41. doi: 10.3810/pgm.2008.07.1788
48. Childers DK, Allison DB. The ‘obesity paradox’: a parsimonious explanation for relations among obesity, mortality rate and aging? Int J Obes (2010) 34:1231–8. doi: 10.1038/ijo.2010.71
49. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA (2013) 309:71. doi: 10.1001/jama.2012.113905
50. Monda V, La Marra M, Perrella R, Caviglia G, Iavarone A, Chieffi S, et al. Obesity and brain illness: from cognitive and psychological evidences to obesity paradox. DMSO (2017) 10:473–9. doi: 10.2147/DMSO.S148392
51. La Marra M, Messina A, Ilardi CR, Verde G, Amato R, Esposito N, et al. The neglected factor in the relationship between executive functioning and obesity: The role of motor control. Healthcare (2022) 10:1775. doi: 10.3390/healthcare10091775
52. Villano I, Ilardi CR, Arena S, Scuotto C, Gleijeses MG, Messina G, et al. Obese subjects without eating disorders experience binge episodes also independently of emotional eating and personality traits among university students of southern Italy. Brain Sci (2021) 11:1145. doi: 10.3390/brainsci11091145
53. Sarwer DB, Thompson JK, Cash TF. Body image and obesity in adulthood. Psychiatr Clinics North America (2005) 28:69–87. doi: 10.1016/j.psc.2004.09.002
54. Thompson JK, Smolak L. Body image, eating disorders, and obesity in youth: Assessment, prevention, and treatment. Washington: American Psychological Association (2001). doi: 10.1037/10404-000
55. Mölbert SC, Sauer H, Dammann D, Zipfel S, Teufel M, Junne F, et al. Multimodal body representation of obese children and adolescents before and after weight-loss treatment in comparison to normal-weight children. PloS One (2016) 11:e0166826. doi: 10.1371/journal.pone.0166826
56. Bearman SK, Presnell K, Martinez E, Stice E. The skinny on body dissatisfaction: A longitudinal study of adolescent girls and boys. J Youth Adolescence (2006) 35:217–29. doi: 10.1007/s10964-005-9010-9
57. Caccavale LJ, Farhat T, Iannotti RJ. Social engagement in adolescence moderates the association between weight status and body image. Body Image (2012) 9:221–6. doi: 10.1016/j.bodyim.2012.01.001
58. Calzo JP, Sonneville KR, Haines J, Blood EA, Field AE, Austin SB. The development of associations among body mass index, body dissatisfaction, and weight and shape concern in adolescent boys and girls. J Adolesc Health (2012) 51:517–23. doi: 10.1016/j.jadohealth.2012.02.021
59. La Marra M, Messina A, Ilardi CR, Staiano M, Di Maio G, Messina G, et al. Factorial model of obese adolescents: The role of body image concerns and selective depersonalization–a pilot study. IJERPH (2022) 19:11501. doi: 10.3390/ijerph191811501
60. Docteur A, Urdapilleta I, Defrance C, Raison J. Body perception and satisfaction in obese, severely obese, and normal weight female patients. Obesity (2010) 18:1464–5. doi: 10.1038/oby.2009.418
61. Gardner RM, Gallegos V, Martinez R, Espinoza T. Mirror feedback and judgments of body size. J Psychosomatic Res (1989) 33:603–7. doi: 10.1016/0022-3999(89)90067-6
62. Ratcliff MB, Eshleman KE, Reiter-Purtill J, Zeller MH. Prospective changes in body image dissatisfaction among adolescent bariatric patients: the importance of body size estimation. Surg Obes Related Dis (2012) 8:470–5. doi: 10.1016/j.soard.2011.10.017
63. Cash TF, Phillips KA, Santos MT, Hrabosky JI. Measuring “negative body image”: validation of the body image disturbance questionnaire in a nonclinical population. Body Image (2004) 1:363–72. doi: 10.1016/j.bodyim.2004.10.001
64. Grogan S. Promoting positive body image in males and females: Contemporary issues and future directions. Sex Roles (2010) 63:757–65. doi: 10.1007/s11199-010-9894-z
65. Neumark-Sztainer D, Paxton SJ, Hannan PJ, Haines J, Story M. Does body satisfaction matter? five-year longitudinal associations between body satisfaction and health behaviors in adolescent females and males. J Adolesc Health (2006) 39:244–51. doi: 10.1016/j.jadohealth.2005.12.001
66. Neumark-Sztainer D, Wall M, Guo J, Story M, Haines J, Eisenberg M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: How do dieters fare 5 years later? J Am Dietetic Assoc (2006) 106:559–68. doi: 10.1016/j.jada.2006.01.003
67. Cash TF, Labarge AS. Development of the appearance schemas inventory: A new cognitive body-image assessment. Cognit Ther Res (1996) 20:37–50. doi: 10.1007/BF02229242
68. Williamson DA, White MA, York-Crowe E, Stewart TM. Cognitive-behavioral theories of eating disorders. Behav Modif (2004) 28:711–38. doi: 10.1177/0145445503259853
69. Williamson DA. Body image disturbance in eating disorders: A form of cognitive bias? Eating Disord (1996) 4:47–58. doi: 10.1080/10640269608250075
70. Cho A, Lee J-H. Body dissatisfaction levels and gender differences in attentional biases toward idealized bodies. Body Image (2013) 10:95–102. doi: 10.1016/j.bodyim.2012.09.005
71. Gao X, Deng X, Yang J, Liang S, Liu J, Chen H. Eyes on the bodies: An eye tracking study on deployment of visual attention among females with body dissatisfaction. Eating Behav (2014) 15:540–9. doi: 10.1016/j.eatbeh.2014.08.001
72. Gao X, Wang Q-C, Chen H, Wang B-Y, Zhao G. Time course of attentional bias components toward body-shape related pictures among women with fat negative physical self: An eye movement study: Time course of attentional bias components toward body-shape related pictures among women with fat negative physical self: An eye movement study. Acta Psychologica Sin (2013) 44:498–519. doi: 10.3724/SP.J.1041.2012.00498
73. Gao X, Li X, Yang X, Wang Y, Jackson T, Chen H. I Can’t stop looking at them: Interactive effects of body mass index and weight dissatisfaction on attention towards body shape photographs. Body Image (2013) 10:191–9. doi: 10.1016/j.bodyim.2012.12.005
74. Gao X, Deng X, Chen N, Luo W, Hu L, Jackson T, et al. Attentional biases among body-dissatisfied young women: An ERP study with rapid serial visual presentation. Int J Psychophysiol (2011) 82:133–42. doi: 10.1016/j.ijpsycho.2011.07.015
75. Rosser BA, Moss T, Rumsey N. Attentional and interpretative biases in appearance concern: An investigation of biases in appearance-related information processing. Body Image (2010) 7:251–4. doi: 10.1016/j.bodyim.2010.02.007
76. Glauert R, Rhodes G, Fink B, Grammer K. Body dissatisfaction and attentional bias to thin bodies. Int J Eat Disordu (2009) 43(1):42–9. doi: 10.1002/eat.20663
77. Joseph C. The impacts of attentional biases and implicit attitudes on body dissatisfaction. PhD Thesis. Rutgers University-Graduate School-Newark (2014). doi: 10.7282/T3125QWB
78. Onden-Lim M, Wu R, Grisham JR. Body image concern and selective attention to disgusting and non-self appearance-related stimuli. Body Image (2012) 9:535–8. doi: 10.1016/j.bodyim.2012.07.005
79. Epstein J, Wiseman CV, Sunday SR, Klapper F, Alkalay L, Halmi KA. Neurocognitive evidence favors “top down” over “bottom up” mechanisms in the pathogenesis of body size distortions in anorexia nervosa. Eat Weight Disord (2001) 6:140–7. doi: 10.1007/BF03339763
80. Fassino S, Pieró A, Daga GA, Leombruni P, Mortara P, Rovera GG. Attentional biases and frontal functioning in anorexia nervosa: Neuropsychological tests in AN. Int J Eat Disord (2002) 31:274–83. doi: 10.1002/eat.10028
81. Chieffi S, Iavarone A, La Marra M, Messina G, Villano I, Ranucci S, et al. Memory for proprioceptive targets in bulimia nervosa. J Psychiatry (2015) 18:297. doi: 10.4172/2378-5756.1000297
82. Ellison Z, Foong J, Howard R, Bullmore E, Williams S, Treasure J. Functional anatomy of calorie fear in anorexia nervosa. Lancet (1998) 352:1192. doi: 10.1016/S0140-6736(05)60529-6
83. Uher R, Brammer MJ, Murphy T, Campbell IC, Ng VW, Williams SCR, et al. Recovery and chronicity in anorexia nervosa. Biol Psychiatry (2003) 54:934–42. doi: 10.1016/S0006-3223(03)00172-0
84. Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. AJP (2004) 161:1238–46. doi: 10.1176/appi.ajp.161.7.1238
85. Seeger G, Braus DF, Ruf M, Goldberger U, Schmidt MH. Body image distortion reveals amygdala activation in patients with anorexia nervosa – a functional magnetic resonance imaging study. Neurosci Lett (2002) 326:25–8. doi: 10.1016/S0304-3940(02)00312-9
86. Wagner A, Ruf M, Braus DF, Schmidt MH. Neuronal activity changes and body image distortion in anorexia nervosa. NeuroReport (2003) 14:2193–7. doi: 10.1097/00001756-200312020-00012
87. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
88. Measso G, Cavarzeran F, Zappalà G, Lebowitz BD, Crook TH, Pirozzolo FJ, et al. The mini-mental state examination: Normative study of an Italian random sample. Dev Neuropsychol (1993) 9:77–85. doi: 10.1080/87565649109540545
89. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A frontal assessment battery at bedside. Neurology (2000) 55:1621–6. doi: 10.1212/WNL.55.11.1621
90. Ilardi CR, Chieffi S, Scuotto C, Gamboz N, Galeone F, Sannino M, et al. The frontal assessment battery 20 years later: normative data for a shortened version (FAB15). Neurol Sci (2022) 43:1709–19. doi: 10.1007/s10072-021-05544-0
91. Cuzzolaro M, Vetrone G, Marano G, Garfinkel PE. The body uneasiness test (BUT): Development and validation of a new body image assessment scale. Eat Weight Disord (2006) 11:1–13. doi: 10.1007/BF03327738
92. Daoud JI. Multicollinearity and regression analysis. J Phys: Conf Ser (2017) 949:12009. doi: 10.1088/1742-6596/949/1/012009
93. Ilardi A, Chieffi S, Ilardi CR. Predictive role of population density and use of public transport for major outcomes of SARS-CoV-2 infection in the Italian population: An ecological study. J Res Health Sci (2021) 21:e00518–8. doi: 10.34172/jrhs.2021.46
94. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods (2008) 40:879–91. doi: 10.3758/BRM.40.3.879
95. Ilardi CR, Garofalo E, Chieffi S, Gamboz N, La Marra M, Iavarone A. Daily exposure to digital displays may affect the clock-drawing test: from psychometrics to serendipity. Neurol Sci (2020) 41:3683–90. doi: 10.1007/s10072-020-04498-z
96. Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: A systematic review. Neurosci Biobehav Rev (2013) 37:279–99. doi: 10.1016/j.neubiorev.2012.11.008
97. Emery RL, Levine MD. Questionnaire and behavioral task measures of impulsivity are differentially associated with body mass index: A comprehensive meta-analysis. psychol Bull (2017) 143:868–902. doi: 10.1037/bul0000105
98. Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol (2010) 20:262–70. doi: 10.1016/j.conb.2010.03.001
99. Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog Neurobiol (2008) 86:216–44. doi: 10.1016/j.pneurobio.2008.09.001
100. Lemche E, Surguladze SA, Giampietro VP, Anilkumar A, Brammer MJ, Sierra M, et al. Limbic and prefrontal responses to facial emotion expressions in depersonalization. NeuroReport (2007) 18:473–7. doi: 10.1097/WNR.0b013e328057deb3
101. Medford N, Brierley B, Brammer M, Bullmore ET, David AS, Phillips ML. Emotional memory in depersonalization disorder: A functional MRI study. Psychiatry Research: Neuroimaging (2006) 148:93–102. doi: 10.1016/j.pscychresns.2006.05.007
102. Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron (2003) 40:1251–7. doi: 10.1016/S0896-6273(03)00724-4
103. Johansson ÅK, Hansen S. Increased alcohol intake and behavioral disinhibition in rats with ventral striatal neuron loss. Physiol Behav (2000) 70:453–63. doi: 10.1016/S0031-9384(00)00284-5
104. Floresco SB, JRSt O, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive Affective Behav Neurosci (2008) 8:375–89. doi: 10.3758/CABN.8.4.375
105. Rushworth MF, Mars RB, Summerfield C. General mechanisms for making decisions? Curr Opin Neurobiol (2009) 19:75–83. doi: 10.1016/j.conb.2009.02.005
106. Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci (2008) 28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008
107. O’Doherty JP. Lights, Camembert, action! the role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann New York Acad Sci (2007) 1121:254–72. doi: 10.1196/annals.1401.036
108. La Marra M, Caviglia G, Perrella R. Using smartphones when eating increases caloric intake in young people: An overview of the literature. Front Psychol (2020) 11:587886. doi: 10.3389/fpsyg.2020.587886
109. Reutens S, Nielsen O, Sachdev P. Depersonalization disorder. Curr Opin Psychiatry (2010) 23:278–83. doi: 10.1097/YCO.0b013e3283387ab4
110. Sierra M, Berrios GE. Depersonalization: neurobiological perspectives. Biol Psychiatry (1998) 44:898–908. doi: 10.1016/S0006-3223(98)00015-8
111. Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron (2006) 51:871–82. doi: 10.1016/j.neuron.2006.07.029
112. Chieffi S, Messina G, La Marra M, Iavarone A, Viggiano A, De Luca V, et al. Distractor interference in visual motor tasks. In: Horizon in neuroscience research. Hauppauge, NY, USA: Nova Science Publishers, Inc (2014).
113. Shomstein S. Cognitive functions of the posterior parietal cortex: top-down and bottom-up attentional control. Front Integr Neurosci (2012) 6:38. doi: 10.3389/fnint.2012.00038
114. Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. NeuroImage (2002) 15:596–603. doi: 10.1006/nimg.2001.1009
115. Chieffi S, Messina A, Villano I, Valenzano AA, Nigro E, La Marra M, et al. The use of velocity information in movement reproduction. Front Psychol (2017) 8:983. doi: 10.3389/fpsyg.2017.00983
116. Ilardi CR, Chieffi S, Iachini T, Iavarone A. Neuropsychology of posteromedial parietal cortex and conversion factors from mild cognitive impairment to alzheimer’s disease: systematic search and state-of-the-art review. Aging Clin Exp Res (2022) 34:289–307. doi: 10.1007/s40520-021-01930-y
117. de Vignemont F. Body schema and body image–pros and cons. Neuropsychologia (2010) 48:669–80. doi: 10.1016/j.neuropsychologia.2009.09.022
118. Dijkerman HC, de Haan EHF. Somatosensory processes subserving perception and action. Behav Brain Sci (2007) 30:189–201. doi: 10.1017/S0140525X07001392
119. Černelič-Bizjak M, Jenko-Pražnikar Z. Impact of negative cognitions about body image on inflammatory status in relation to health. Psychol Health (2014) 29:264–78. doi: 10.1080/08870446.2013.844807
120. Sedda A, Scarpina F. Dorsal and ventral streams across sensory modalities. Neurosci Bull (2012) 28:291–300. doi: 10.1007/s12264-012-1223-9
121. Cuzzolaro M, Cuzzolaro M, Fassino S. Body schema and body image: History and controversies. In: Body image, eating, and weight. Cham: Springer International Publishing (2018). p. 1–24. doi: 10.1007/978-3-319-90817-5_1
122. Boles DB, Givens SM. Laterality and sex differences in tactile detection and two-point thresholds modified by body surface area and body fat ratio. Somatosensory Motor Res (2011) 28:102–9. doi: 10.3109/08990220.2011.627068
123. Bud Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body: Insula and awareness. Ann New York Acad Sci (2011) 1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x
124. Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci (2004) 7:189–95. doi: 10.1038/nn1176
125. Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. NeuroImage (2003) 18:324–33. doi: 10.1016/S1053-8119(02)00041-1
126. Giesbrecht T, Merckelbach H, ter BL, Cima M, Simeon D. Acute dissociation predicts rapid habituation of skin conductance responses to aversive auditory probes. J Traum Stress (2008) 21:247–50. doi: 10.1002/jts.20323
127. Scarpina F, Castelnuovo G, Molinari E. Tactile mental body parts representation in obesity. Psychiatry Res (2014) 220:960–9. doi: 10.1016/j.psychres.2014.08.020
128. Sierra M, Senior C, Phillips ML, David AS. Autonomic response in the perception of disgust and happiness in depersonalization disorder. Psychiatry Res (2006) 145:225–31. doi: 10.1016/j.psychres.2005.05.022
129. Taylor-Clarke M, Jacobsen P, Haggard P. Keeping the world a constant size: object constancy in human touch. Nat Neurosci (2004) 7:219–20. doi: 10.1038/nn1199
130. Maximova K, McGrath JJ, Barnett T, O’Loughlin J, Paradis G, Lambert M. Do you see what I see? weight status misperception and exposure to obesity among children and adolescents. Int J Obes (2008) 32:1008–15. doi: 10.1038/ijo.2008.15
131. O’Connor JN, Golley RK, Perry RA, Magarey AM, Truby H. A longitudinal investigation of overweight children’s body perception and satisfaction during a weight management program. Appetite (2015) 85:48–51. doi: 10.1016/j.appet.2014.11.009
132. Braet C. Inpatient treatment for children with obesity: Weight loss, psychological well-being, and eating behavior. J Pediatr Psychol (2004) 29:519–29. doi: 10.1093/jpepsy/jsh054
133. Shaban LH, Vaccaro JA, Sukhram SD, Huffman FG. Perceived body image, eating behavior, and sedentary activities and body mass index categories in Kuwaiti female adolescents. Int J Pediatr (2016) 2016:1–7. doi: 10.1155/2016/1092819
134. Griffen TC, Naumann E, Hildebrandt T. Mirror exposure therapy for body image disturbances and eating disorders: A review. Clin Psychol Rev (2018) 65:163–74. doi: 10.1016/j.cpr.2018.08.006
135. Jansen A, Nederkoorn C, Mulkens S. Selective visual attention for ugly and beautiful body parts in eating disorders. Behav Res Ther (2005) 43:183–96. doi: 10.1016/j.brat.2004.01.003
136. Kollei I, Horndasch S, Erim Y, Martin A. Visual selective attention in body dysmorphic disorder, bulimia nervosa and healthy controls. J Psychosomatic Res (2017) 92:26–33. doi: 10.1016/j.jpsychores.2016.11.008
137. Hilbert A, Tuschen-Caffier B, Vögele C. Effects of prolonged and repeated body image exposure in binge-eating disorder. J Psychosomatic Res (2002) 52:137–44. doi: 10.1016/S0022-3999(01)00314-2
138. Rosen JC, Reiter J, Orosan P. Cognitive-behavioral body image therapy for body dysmorphic disorder. J Consulting Clin Psychol (1995) 63:263–9. doi: 10.1037/0022-006X.63.2.263
139. Delinsky SS, Wilson GT. Mirror exposure for the treatment of body image disturbance. Int J Eat Disord (2006) 39:108–16. doi: 10.1002/eat.20207
140. Luethcke CA, McDaniel L, Becker CB. A comparison of mindfulness, nonjudgmental, and cognitive dissonance-based approaches to mirror exposure. Body Image (2011) 8:251–8. doi: 10.1016/j.bodyim.2011.03.006
141. Glashouwer KA, Jonker NC, Thomassen K, de Jong PJ. Take a look at the bright side: Effects of positive body exposure on selective visual attention in women with high body dissatisfaction. Behav Res Ther (2016) 83:19–25. doi: 10.1016/j.brat.2016.05.006
142. Smeets R, Köke A, Lin C-W, Ferreira M, Demoulin C. Measures of function in low back pain/disorders: Low back pain rating scale (LBPRS), Oswestry disability index (ODI), progressive isoinertial lifting evaluation (PILE), Quebec back pain disability scale (QBPDS), and Roland-morris disability questionnaire. Arthritis Care Res (2011) 63:S158–73. doi: 10.1002/acr.20542
143. Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther (2008) 46:5–27. doi: 10.1016/j.brat.2007.10.003
Keywords: obesity, executive functions, BMI – body mass index, body image, avoidance
Citation: La Marra M, Ilardi CR, Villano I, Polito R, Sibillo MR, Franchetti M, Caggiano A, Strangio F, Messina G, Monda V, Di Maio G and Messina A (2022) Higher general executive functions predicts lower body mass index by mitigating avoidance behaviors. Front. Endocrinol. 13:1048363. doi: 10.3389/fendo.2022.1048363
Received: 20 September 2022; Accepted: 20 October 2022;
Published: 09 November 2022.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Sami Schiff, University of Padua, ItalyGiorgia Abete Fornara, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright © 2022 La Marra, Ilardi, Villano, Polito, Sibillo, Franchetti, Caggiano, Strangio, Messina, Monda, Di Maio and Messina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ines Villano, ines.villano@unicampania.it
 Marco La Marra
Marco La Marra Ciro Rosario Ilardi
Ciro Rosario Ilardi Ines Villano
Ines Villano Rita Polito
Rita Polito Maria Raffella Sibillo1
Maria Raffella Sibillo1 Giovanni Messina
Giovanni Messina Antonietta Messina
Antonietta Messina