- Department of Neurology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
Diabetic peripheral neuropathy is the most prevalent chronic complication of diabetes and is based on sensory and autonomic nerve symptoms. Generally, intensive glucose control and nerve nourishment are the main treatments. However, it is difficult to improve the symptoms for some patients; such cases are defined as refractory diabetic peripheral neuropathy (RDPN). In this paper, we present five patients treated with saline and mecobalamin by ultrasound-guided injection. The Visual Analog Scale and Toronto Clinical Scoring System were used to evaluate the symptoms, and the neuro-ultrasound scoring system and electrophysiological severity scale were evaluated by ultrasound and electrophysiological examination. In brief, ultrasound-guided hydrodissection may be a safe way to treat RDPN.
Introduction
Hydrodissection was first proposed in 1997. It is used in treating entrapment neuropathy and inflammatory peripheral neuropathy and plays an important role in separating adjacent tissue and protecting nerves and blood vessels via the injection of saline or other agents to build a non-existent surgical plane (1, 2). Previously, Smith has studied the treatment of carpal tunnel syndrome by hydrodissection (3).Without a unified standard, a visual needle is usually used to form a liquid clearance around nerves by ultrasound-guided injection with saline or another liquid (e.g., mecobalamin) to dissociate the nerves from their surroundings.
Currently, the treatment options for compressive neuropathy include: (1) medical treatment with oral drugs to provide nutrition to the nerves and blocking therapy (4) and (2) surgical treatment, after which symptoms may recur. Surgical procedures may damage the intraneural microcirculation, leading to pathological changes corresponding to chronic compressive neuropathy (5). However, hydrodissection minimizes the risk of nerve injury with its advantages of percutaneous punctures and blunt dissection. Ultrasound shows changes in the morphology, imaging, and echo of nerves, which could suddenly be attenuated at compressive sites, resulting in decreased echo intensity. In fact, honeycomb-like structures disappear on the axis, and the cross-sectional areas of proximal nerves increase due to edema. Therefore, ultrasound-guided neural hydrodissection represents a promising therapeutic strategy to treat various peripheral neuropathies.
Over the past decades, various investigations have been performed on neuropathies with mild to moderate, recurrent, and even severe nerve scars to explore the therapeutic effects of hydrodissection (6–9).
Hydrodissection has therapeutic effects on neural adhesion caused by inflammation, such as saphenous nerve ache. Fader has cured an athlete with compression of the right sural nerve on the right lateral foot and ankle using saline by hydrodissection to separate the sural nerve from the surrounding muscles; the athlete returned to participation in competitive sports without disability (10).
Based on 10 years of clinical experience, in this study, we demonstrate that ultrasound-guided neural hydrodissection has excellent therapeutic effects on compressive neuropathy and inflammatory adhesion and explore the therapeutic efficacy of ultrasound-guided injection in five patients with refractory peripheral neuropathy.
Patients and Methods
Five patients treated with intramuscular or oral mecobalamin were recruited. They were diagnosed with refractory diabetic peripheral neuropathy (RDPN) confirmed by electromyography (EMG). All patients, who had not experienced satisfactory effects after glycemic control and neurotrophic drug treatment, experienced symptoms of RDPN, such as pain, numbness, and sensory changes in the limb extremities. The exclusion criteria were cervical radiculopathy, history of trauma, immune dysfunction, severe cardiopulmonary insufficiency, and obvious myasthenia.
This study was approved by the institutional review board of the Affiliated Hospital of Guizhou Medical University (Guiyang, China), and written informed consent was obtained from all participants.
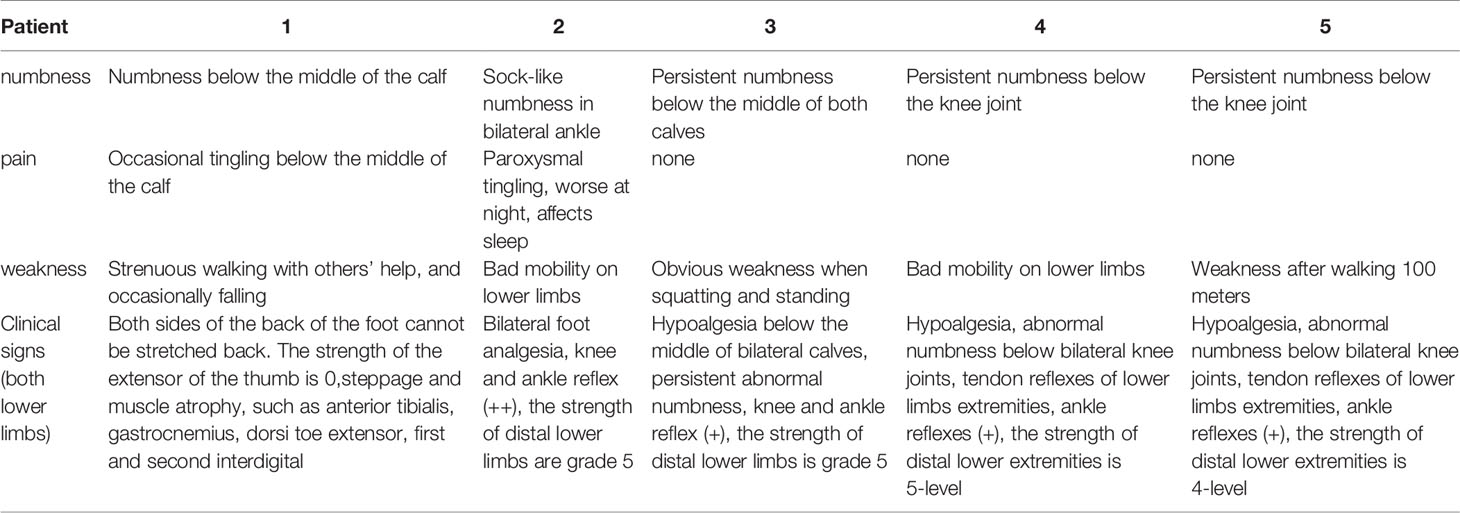
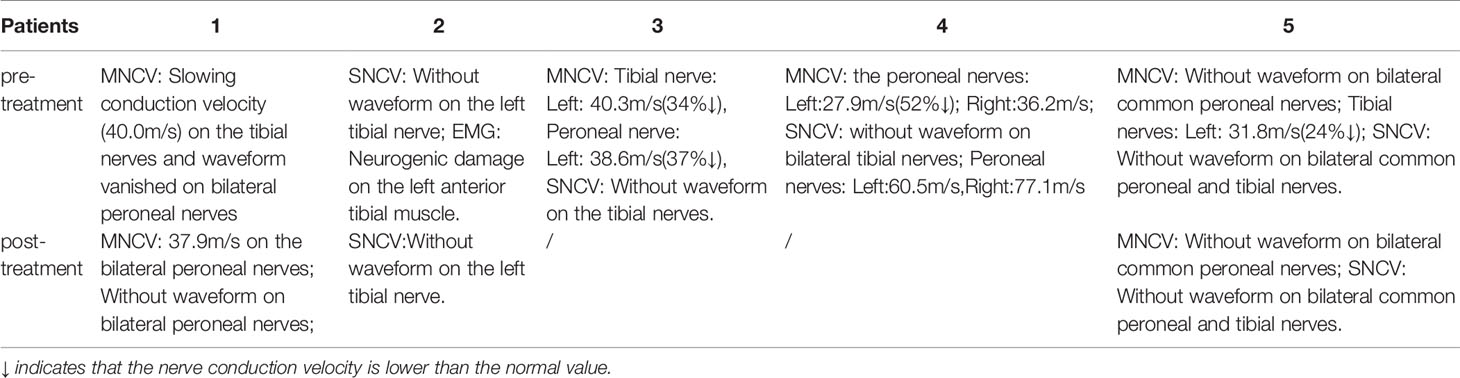
Case 1: A 60-year-old female with weak dorsiflexion of the feet and numbness for one year, occasionally with tingling, type 2 diabetes for five years, a maximum fasting blood glucose level of 15 mmol/L, and glycosylated hemoglobin of 6.9%. The diagnosis of DPN was based on numbness and tingling, slow conduction velocity (40.0 m/s) in the tibial nerves, and a waveform that vanished in the bilateral peroneal nerves as assessed by EMG examination. In the past year, the patient had experienced paresthesia and dyskinesia of the lower limbs. After treatment with insulin, her symptoms were somewhat relieved; subsequently, her glycemia was controlled with oral hypoglycemic drugs. The extensor digitorum brevis (EDB) muscle had significantly atrophied by 0.32 cm2 (Figure 1A), and the strength of the extensor of the thumb was grade 0/5 with steppage gait (Table 1).
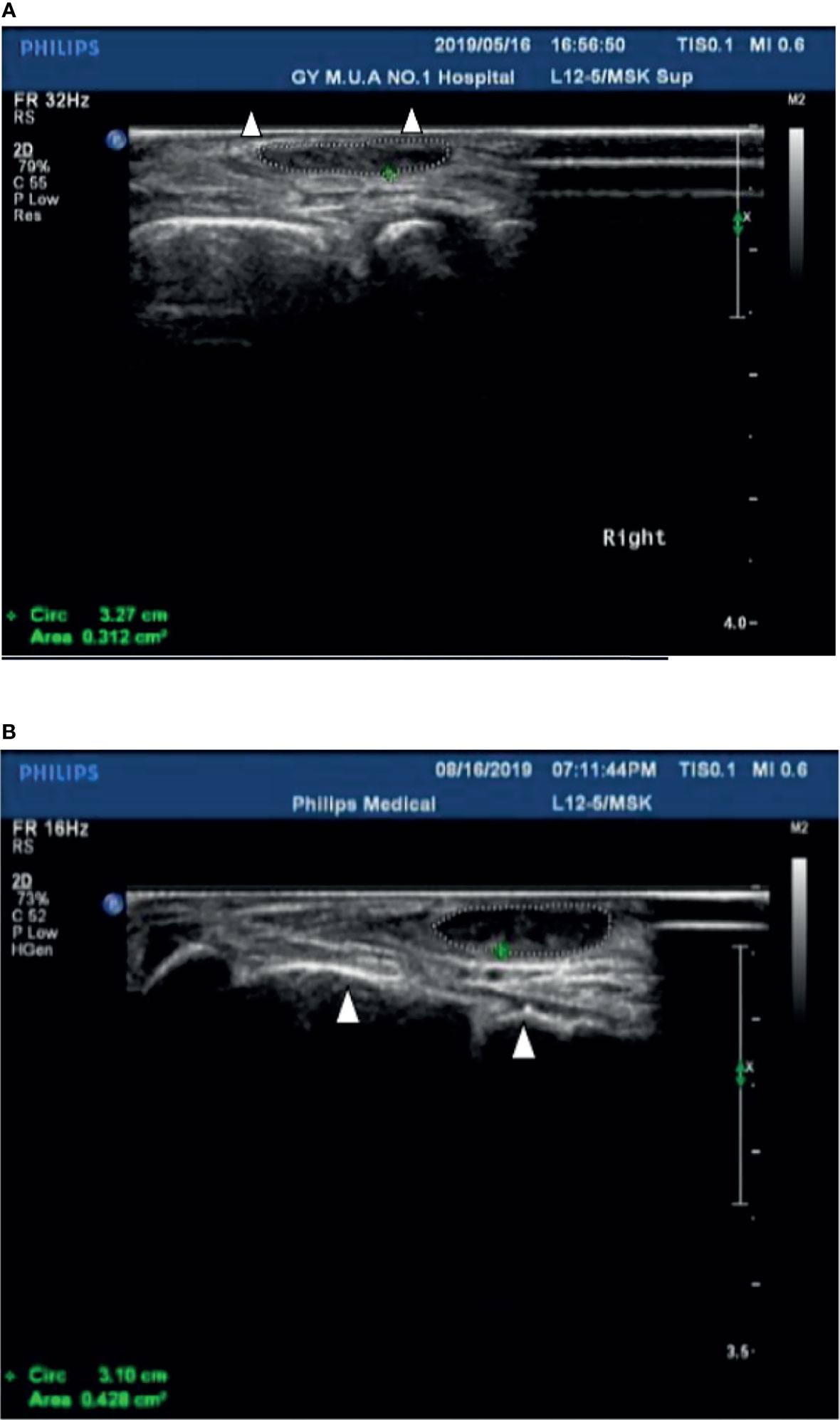
Figure 1 (A) The cross-sectional area of the extensor digitorum brevis of patient 1 before treatment was 0.312 cm2 (Circled by a dotted line); The Triangle refers to the fourth and fifth metatarsal bones. (B) The cross-sectional area of the extensor digitorum brevis increased to 0.428 cm2 (Circled by a dotted line) after two courses of treatment; The Triangle refers to the fourth and fifth metatarsal bones.
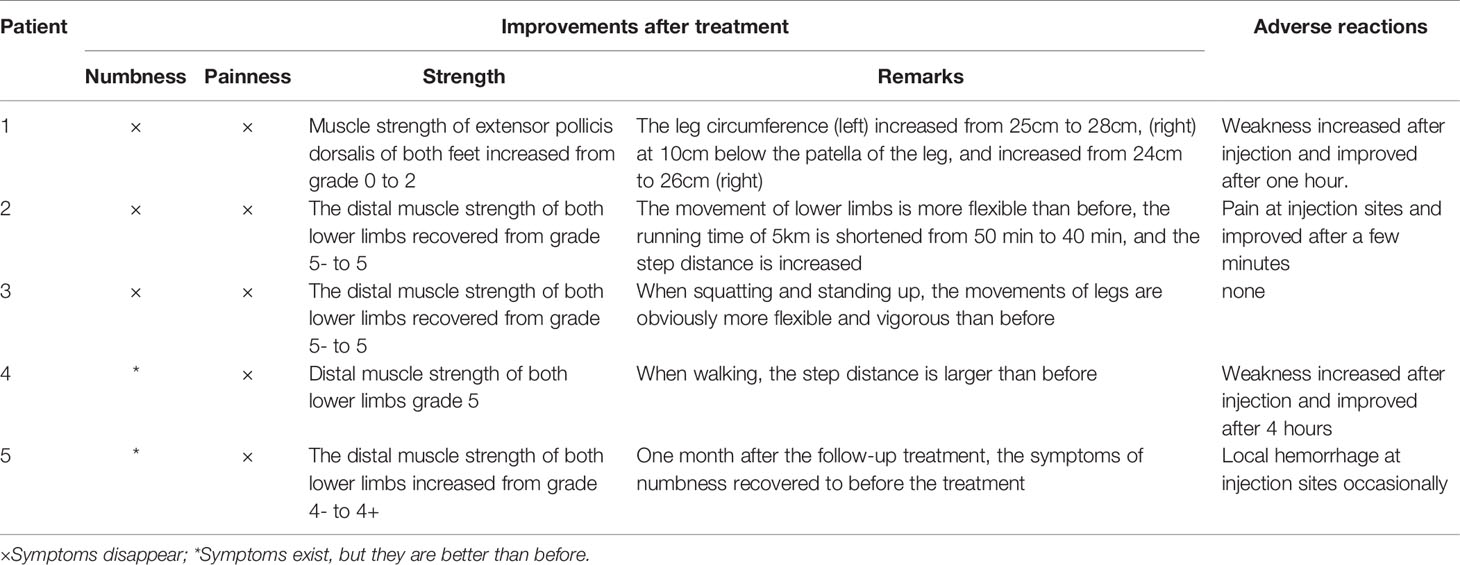
After hospitalization, mecobalamin was started via ultrasound-guided injection of an appropriate dose (1 mg in 10 ml 0.9% NaCl). After therapy, the patient improved gradually. After one course of treatment, the foot strength returned to grade 2/5, with the calf circumference increasing from 25 to 28 cm (left) and 26 to 24 cm (right). The EMG results showed an insignificant change of 37.9 m/s in the bilateral peroneal nerve conduction velocity (Table 2). The cross-sectional area of the EDB was 0.43 cm2 after two courses of treatment (Figure 1B). The other changes are shown in Tables 3 and 4.
Case 2: A 56-year-old male with type 2 diabetes for six years accompanied by renal function impairment. The diagnosis of DPN was based on characteristic sock-like numbness, inflexible movement of the lower limbs, no waveform in the tibial nerves, neurogenic damage to the left anterior tibial muscle upon electromyographic examination, and exclusion of other peripheral neuropathies. He had experienced paresthesia of the distal extremities within the previous two years. After treatment with mecobalamin and epalrestat based on strict control of blood glucose, these symptoms gradually worsened, with frequent nocturnal tingling affecting sleep. The foot strength was grade –5/5 with hypalgesia (Table 1).
After admission, mecobalamin was injected via ultrasound guidance at an appropriate dosage at the malleolus medialis of the posterior tibial nerves. Fortunately, the patient improved gradually after the therapy. After two courses, the foot strength returned to grade 5/5, the legs became more flexible, the walking distance increased, and there was no numbness or pain. An EMG examination showed no significant change in the bilateral peroneal nerves (Table 2). The other changes are shown in Tables 3 and 4.
Case 3: A 52-year-old male with type 2 diabetes mellitus for 11 years and retinopathy stage III diagnosed with DPN due to slow conduction velocity of the tibial and peroneal nerves and no tibial nerve waveform found with EMG. Mecobalamin and vitamin B1 were taken orally for nearly one year, but areflexia, distal numbness of the limbs, and weakness had occurred when squatting within the last six months (Table 1).
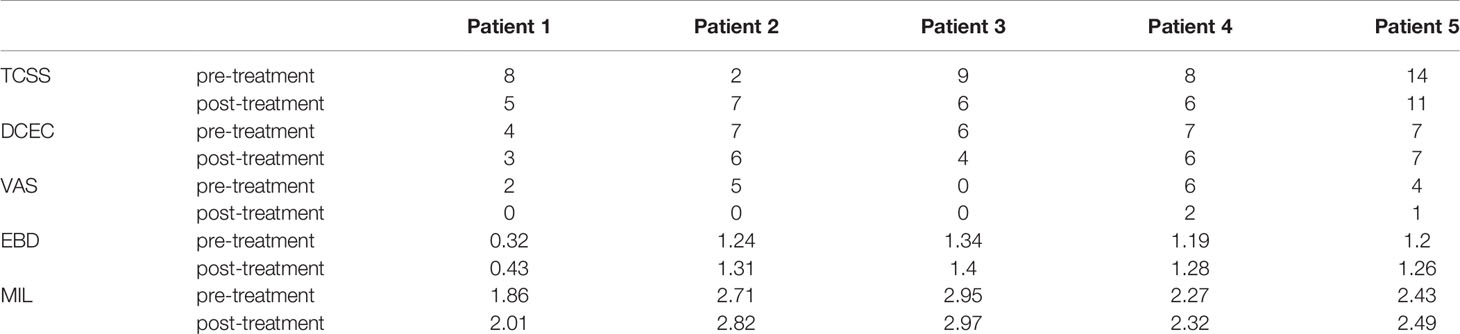
After two courses of treatment, the neurological examination showed that the muscle strength of the lower extremities was grade 5/5. Flexibility and numbness of the lower limbs improved, but no EMG results were obtained after treatment (Table 2). Moreover, after ultrasound-guided injection of saline and mecobalamin, the symptom and ultrasound image scores were improved (Table 3), and it was found that the EDB and muscles of the first interstitium (MIL) were larger than those before treatment (Table 3).
Case 4: A 76-year-old male with a history of type 2 diabetes for over 10 years diagnosed with DPN for 4 years. He had paresthesia and weakness in his lower limbs that was not relieved after treatment with mecobalamin, vitamin B1, and Maizhiling for more than two years. The EMG bilateral fibular motor nerve conduction velocity was 27.9 m/s (right, 52% ↓) and 36.2 m/s (left). The EMG showed that the conduction velocity of the bilateral tibial nerve waveforms had disappeared. The fibular sensory nerve values were 77.1 m/s (right) and 60.5 m/s (left) (Table 2). After one course of therapy, the patient’s walking distance increased, though with persistent numbness. The muscle strength of the lower limbs was not ameliorated at grade –5/5. The other relevant change scores, muscle cross-sectional areas, and thickness values are shown in Table 3.
Case 5: A 44-year-old male with type 2 diabetes for 13 years, renal failure and retinal complications, persistent numbness of the distal limbs, paresthesia, and weakness of the lower limbs after walking 100 m for nearly 5 years as a result of poor glycemic control, and 11.9% glycosylated hemoglobin. The patient had been treated with painkillers during this period. The waveform of the bilateral peroneal motor nerves had disappeared, and the conduction velocity of the tibial motor nerves was 31.8 m/s (24% ↓). Moreover, the waveforms of the bilateral tibial and peroneal sensory nerves were not revealed by EMG examination (Table 2).
After a course of treatment, the distal muscle strength of the lower limbs increased from grade –4/5 to +4/5, and the numbness was relieved gradually. Nevertheless, one month after follow-up, the symptoms appeared again. There was little change in the EMG results (Table 2). The other improvement indicators are shown in Tables 3 and 4.
Therapeutic Procedure
In our experience, the fibular head of the tibial nerves, the section at the start of the peroneal nerves, and the medial malleolus of the posterior tibial nerves should be chosen as treatment injection sites after ensuring there are no wounds on local skin sites. Herein, these injection sites were sterilized with povidone iodine, and the ultrasonic probe was covered with gloves after having been sterilized. Two syringes were prepared before injection (first syringe: 10 ml of 0.9% sodium chloride injection; second syringe: 0.5 mg of mecobalamin injection + 9 ml of 0.9% sodium chloride injection to prepare a 10-ml mixed solution with 1.5–2 ml for each injection site). The posterior tibial nerve was displayed with a clear neural boundary, an internal echo, and a honeycomb-like structure by high-resolution ultrasound (Figure 2A). The puncture needle was as perpendicular to the skin as possible, entering along the puncture point at a distance about 0.5 cm from the center of the probe. About 45°–60° was required between the needle and sonographic probe (Figure 2B). Real-time observation of the position of the puncture needle tip during injection was performed when the needle was connected to the first syringe, and 3–5 ml of saline was injected around the nerve to separate the nerve water from the surrounding tissue by ultrasound-guided injection. At the end of the hydrodissection, a hypoechoic ring could be observed on the transverse axis (Figure 2C). Then, the needle tip was kept still and substituted with the second syringe to inject 1.5–2 ml of solution into the target nerve at each injection point (Figure 2D). The surrounding liquid around the nerve was shown clearly on the longitudinal axis (Figure 2E), after which the needle was removed.
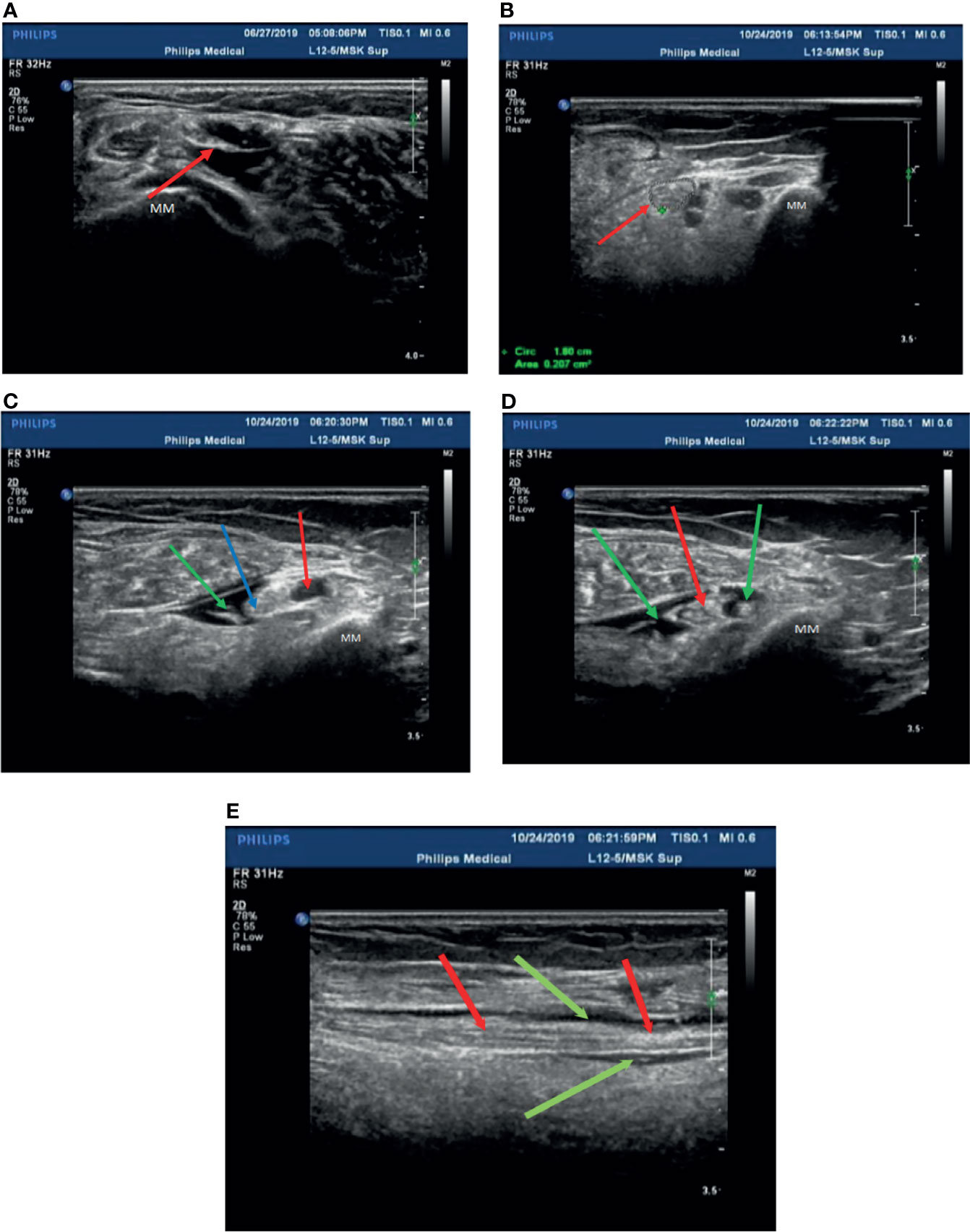
Figure 2 Patient 2 was treated with mecobalamin by ultrasound-guided hydrodissection injection at the malleolus medialis of the posterior tibial nerves (A–E). (A) A normal posterior tibial nerve is shown as a clear neural boundary (cribriform network), normal internal echo (the posterior tibial nerve is below the red arrowhead). (B) The posterior tibial nerve of patient 2 was thickened with a fuzzy boundary in the medial malleolus (the red arrowhead indicates the posterior tibial nerve). (C) Injection with 3 ml saline at the medial malleolus of the posterior tibial nerve (the posterior tibial nerve at the red arrowhead, the point of a needle at the blue arrowhead, and the green arrowhead points to saline after injection). (D) The posterior tibial nerve was injected with mecobalamin at the medial malleolus after injection in the transverse section, and it can be seen that the solution is evenly wrapped around the posterior tibial nerve (red arrowhead pointed to the posterior tibial nerve, saline + mecobalamin at the green arrowhead). (E) The medial malleolus of the posterior tibial nerve after injection with mecobalamin in the longitudinal section, it can be seen that the fluid evenly covers the posterior tibial nerve (the red arrowhead points to the posterior tibial nerve, and saline + mecobalamin at the green arrowhead). MM; Medial malleolus.
It was essential to observe whether the patients had side effects in their limbs, such as numbness, weakness, and chills, during the injection process. Injection took place once every other day into the bilateral limbs, i.e., three times per week for four weeks per treatment course. Evaluation of the effect occurred after the treatment course. The injection operation was carried out by an experienced clinician and a sonographer.
Assessment of Symptoms
The symptoms were evaluated with objectively measurable scores, including the VAS and Toronto Clinical Scoring System (TCSS).
The VAS is a scale to evaluate pain before and after therapy. The TCSS is a grading system for assessing the severity of DPN with a score from 1 to 19 (mild to severe) (11).
Electrophysiological Examination
Each patient was diagnosed with DPN following an electrophysiological examination, wherein nerves were assessed with an electrophysiological severity scale comprising motor and sensory nerve conduction velocity detection. These tests were performed by a single physician using a Nicolet EDX EMG system (Madison, Wisconsin, U.S.A.). The measured nerves included the median nerve, ulnar nerve, tibial nerve, and common peroneal nerve. The sural nerve was not measured as it is easily affected by many factors. The motor conduction velocity of the median nerve and sensory conduction velocity of the ulnar and sural nerves, action potential amplitudes of all three nerves, and distal latency period of the median nerve in the wrist segment were determined.
The results were measured against the normal values obtained from the EMG room of Beijing Peking Union Medical College Hospital in China (12). Judging criteria: the examined nerve did not elicit potential (the statistical data were 0); the conduction velocity was over 20% lower than the normal low limit; the latency of the distal motor nerve exceeded 130% of the normal high limit; the amplitude of motor or sensory potential was decreased; the latency of the F wave exceeded 130% of the normal high limit; and the occurrence rate of the evoked wave was less than 50%.
Sensory nerve conduction measurement: The amplitude of the action potential of the sensory nerve decreases (particularly at the extremities of the lower limbs) although the conduction velocity is relatively normal, consistent with the characteristics of length-dependent axonal peripheral neuropathy. When there is compressive peripheral neuropathy, the sensory nerve conduction velocity at the embedded sites may be slowed. Among patients with autonomic nervous symptoms, sensory conduction can be normal. Thus, sensory nerve conduction measurement is helpful for the detection of subclinical lesions.
Measurement of motor nerve conduction: The latency and nerve conduction velocities of distal movement are usually normal in the early stage, and there is no partial conduction block of motor nerves or abnormal wave form dispersion. In later stages, the amplitude of the compound muscle action potential decreases, and the conduction velocity slows down slightly, similar to mononeuropathy or lumbosacral plexus disease. In patients with embedded peripheral neuropathy, the conduction velocity at the embedded sites can be significantly slowed.
Sonographic Examinations
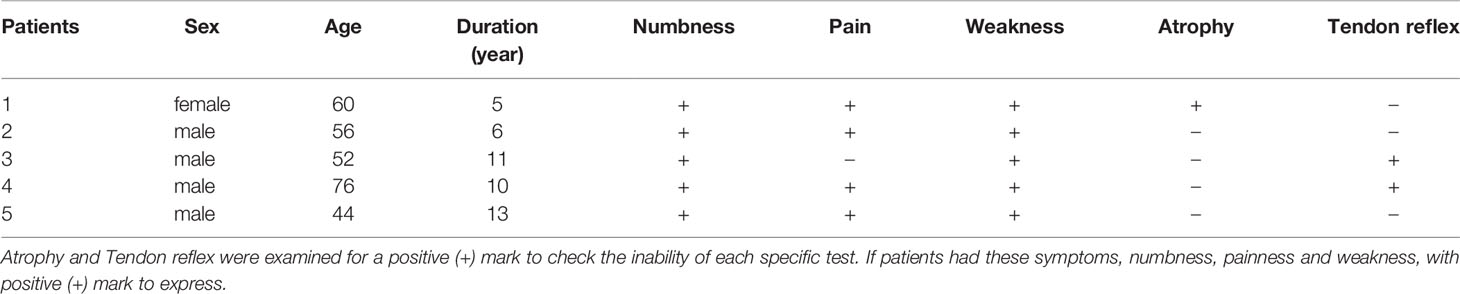
All nerve image examinations were performed using ultrasound guidance and evaluated by measuring the size of the morphologic changes of the nerves using an ultrasound system (Philips Ulrasound iU22, Holland) with a 5- to 12-MHz linear array transducer. The subjects lay on the examination bed and kept their lower limbs relaxed in the correct position according to different nerves. The cross-sectional areas of the tibial nerves were measured at the start of the nerves, the fibula head, and the medial malleolus of the posterior tibial nerves. Three averages of the cross-sectional area and three measurements for each site were used. The neuro-ultrasound scoring system included four aspects of the target nerves with ultrasound regarding the neuroimaging clarity of the cross-sectional area, internal echo, and the presence or absence of nerve entrapment (DCEC), of which the abbreviated form of the DCEC scoring system was used for each patient. The detailed scoring rules are shown in Table 5 (13). Meanwhile, the extensor digitorum brevis (EDB) muscle and MIL were measured by ultrasound (14). The participants kept the ankle joint relaxed, and the cross-sectional area at the EDB midpoint and thickness of the MIL were determined.
All patients were injected using ultrasound guidance. Before injection, the patients were examined for temperature, pinprick sensation (small fiber function), and vibration sensation using a 128-Hz tuning fork (for large fiber function). They were assessed with respect to neurophysiological changes, neural morphology, leg circumference, the EDB and MIL, and muscle strength in the bilateral limbs at the time of each injection.
Results
The patients were evaluated at the pre-injection timepoint as well as after one or two injection courses. The mean age in this study was 57.6 ± 11.87 years, and the mean duration of the participants with DPN was 9.0 ± 3.39 years at the pre-injection baseline (Table 6). All five patients showed numbness and weakness in the lower limbs. A positive tendon reflex was found in three patients, and two patients had paroxysmal tingling pain in the lower limbs. However, only one patient had distal muscle atrophy of the lower extremities (Table 1). There were some slight adverse reactions after the injection, such as short-term limb weakness, bleeding, and pain at the injection site. However, these did not have serious consequences, and the patients recovered within hours.
Among the symptom scores, the VAS was decreased after the ultrasound-guided injection, with a mean of 3.4 points pre-injection and 0.6 points after treatment. The TCSS and DCEC scores did not significantly decrease after injection (Table 3 and Figure 3).
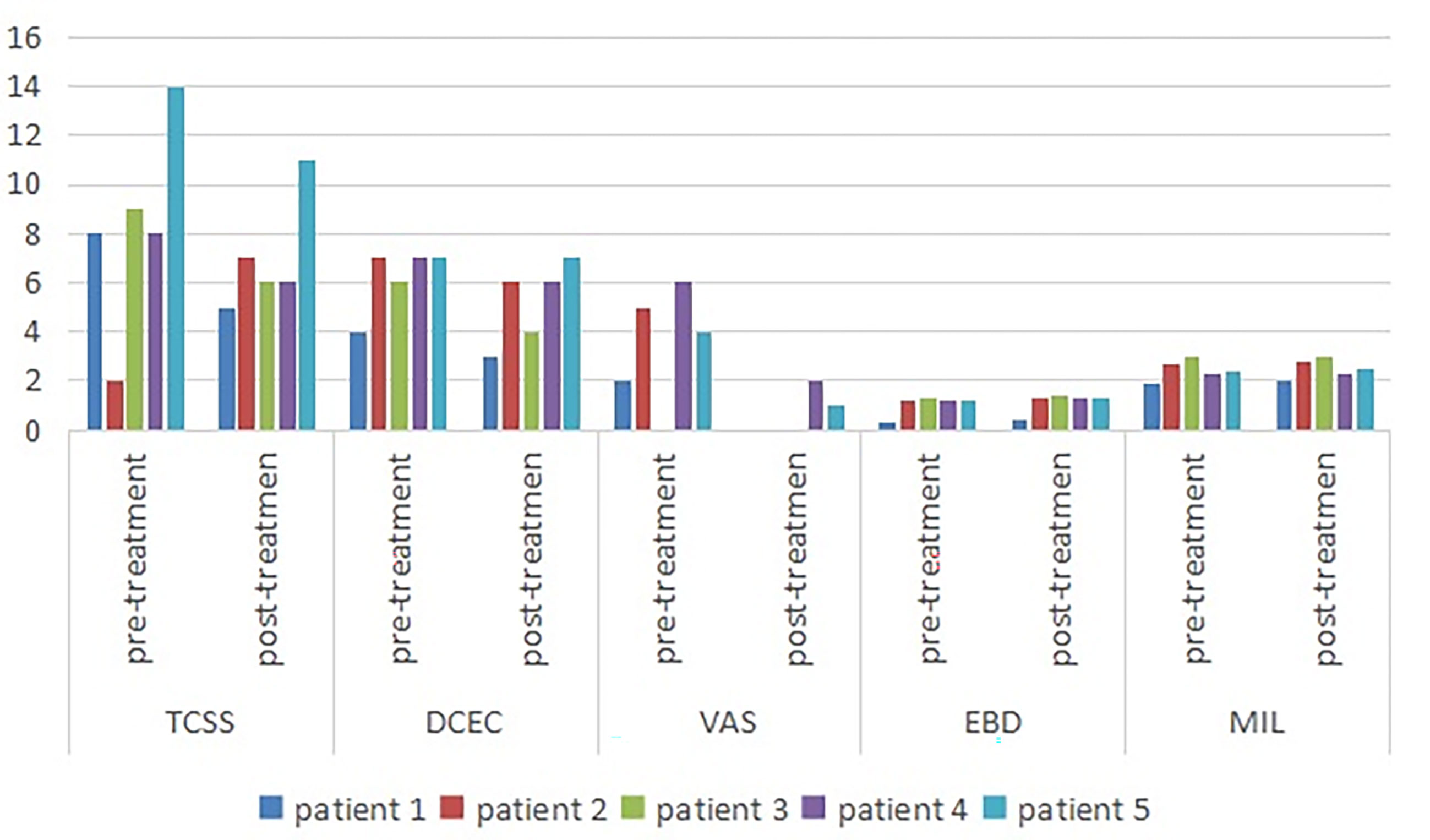
Figure 3 Symptom scores and morphologic changes were compared at baseline and post-injection. Among symptom scores, the VAS was significantly decreased, as compared to baseline. The DCEC score of nerves was measured by ultrasonography at limbs. Compared with baseline, the DCEC score and TCSS score showed a significant decrease post-injection. The EDB and MIL were increased after ultrasound-guided injection. DCEC, definition, cross sectional area, echogenicity and compression; TCSS, Toronto clinical scoring system; EDB, extensor digitorum brevis muscle; MIL, muscles of the first interstitium; VAS, visual analogy score.
The cross-sectional area of the EDB was measured by ultrasound at the midpoint of a straight line between the lateral malleolus and the fifth metatarsal tuberosity side of the dorsum pedis of the lower limbs, which determined the scanning plane. The cross-sectional areas of the EDB and MIL were decreased after injection (Figure 1B) compared with pre-injection, with a mean of 1.058 and 1.136 mm2, respectively (Table 3).
We observed that the electrophysiology outcomes did not significantly change compared with those pre-injection, though the conduction velocity from the left peroneal nerve increased slightly in one patient.
Discussion
The purpose of this investigation was to explore the clinical value of ultrasound-guided hydrodissection in the treatment of RDPN. The results showed that the TCSS and VAS of patients with RDPN treated with ultrasound-guided injection improved to some extent, with the latter improving the most. There was insignificant change in the DCEC scores and neuroelectrophysiological results. The improved neurotrophic effect was reflected in the increase of the leg circumference, EDB muscle, and MIL. It was obvious for Case 1 that the distal small muscle of the lower extremity atrophied but then grew after two courses of treatment. This was detected by ultrasonographic examination and measurement of the EDB and MIL from the innervation of the fibular nerve and tibial nerve, respectively. The degree of foot muscle atrophy was helpful in evaluating the neuropathology and the treatment effect on peripheral nerves.
The possible therapeutic mechanisms of ultrasound-guided hydrodissection are as follows: (1) Use the cutting force of fluid instead of needle to separate soft tissue. The primary objective of hydrodissection is to release the entrapment of the peripheral nerves by hydrodissecting the nerves (15). (2) By separating adhesion, reduce nerve compression, improve peripheral vein and lymph reflux, so as to promote nerve function (16). In addition, local injection of mecobalamin around nerves promotes axonal growth, which has significant effects on sensory symptoms and short courses (17). Mecobalamin injection after hydrodissection distributes evenly among the surrounding tissues, increases absorption, and plays a more efficient role.
DPN is a type of compressive disorder. In DPN treatment, especially in patients with pain, numbness, and other symptoms, in addition to controlling glycemia, we found that surgical decompression had an obvious therapeutic effect. As a result of nerve thickening, it could easily be compressed by the narrow pipe. The decompression operation relieved the nerve entrapment in the patients, improved the blood supply of axoplasmic transport in peripheral nerves, and repaired injured nerves, which significantly reduced the pain symptoms compared with the former (18, 19). This research demonstrates the advantages of reducing nerve compression, improving the blood supply of nerves, increasing compliance, and reducing secondary nerve injury. The surgical decompression point was selected as the injection point of the hydrodissection in five patients. Mecobalamin is a type of endogenous co-enzyme B12 and can easily be transferred to the organelles of nerve cells in the process of methionine synthesis from homocysteine. It can promote the synthesis of nucleic acids and proteins, the transport and regeneration of axons, and the synthesis of phosphatidylcholine in myelination, whereby it has obvious effects on damaged nerve cells (20). Li Yuxin et al. carried out a study of axillary brachial plexus block compared with the traditional perivascular injection method and identified that expansion of the peripheral nerve space by ultrasound-guided saline reduced anesthetic doses (21). Consistent with our findings, earlier studies by Ide et al. also described that intrathecal injection with mecobalamin improved symptoms in patients with RDPN; however, the observation time was short, and nerve repair was not detected by the electrophysiological examination, which may explain why the EMG outcomes, including autonomic neural function, were insignificantly improved (22).
Limitations
In this study, only five patients were observed for a short time, and a standard could not be formed. In this study, patients with refractory diabetic peripheral neuropathy have been treated with different hypoglycemic and vegetative nerve regimens. The severity of the lesions may be different before using ultrasound-guided hydrodissection. In addition, the objective score of patients with symptoms is relatively low, and the response to treatment may not be comprehensive. However, as a small case report, this study is reasonable from the comparison before and after treatment and the selection of research objects. Otherwise, without treatment with hydrodissection and local mecobalamin injection, we cannot combine the results with the efficacy of hydraulic exfoliation, local injection of mecobalamin combined with efficient nutrition, or both. Thus, a large number of participants and researchers will not be able to distinguish the results from the efficacy of hydrodissection, local injection of mecobalamin, or a combination of both. In the future, we will recruit more participants to further study the treatment and prognosis, and we need to design a large sample size, controlled and multicenter study to determine whether ultrasound-guided hydrodissection has satisfactory efficacy in patients with RDPN compared with traditional methods.
Conclusion
In our patients, the nerves were thickened and obviously damaged at compression sites, and the recovery time was measured by electrophysiology improvements. Owing to the advantages of nutrition and safety in relieving nerve compression, the pain of the patients with RDPN was relieved. Therefore, we believe that the most effective method for the treatment of DPN is to reduce nerve injury by controlling blood sugar.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Guizhou Medical University. The patients/participants provided their written informed consent to participate in this study. All participants provided written informed consent for the publication of the case reports (including all data and images).
Author Contributions
HQH and SW conceived the idea and conceptualized the study.HLH, CT, ML and JCL completed the ultrasound for patients. JH performed nerve conduction tests. HQH completed the process of hydrodissection injection.HLH and HQH collected and analyzed the datas. Finally, HLH drafted and reviesd the manuscript,then YL and SW reviewed the manuscript.All authors contributed to the article and approved the submitted version.
Funding
Application and industrialization project of scientific and technological achievements in Guizhou Province, “The regional pilot of precision medical poverty alleviation powered by technology— Sub Project III, diagnosis and treatment of epilepsy, Parkinson’s disease and headache”, [Achievements of science and technology synthesis in Guizhou (2018) 4615-3]; National Key R&D Program of China (NO.2018YFC1312901); Guiyang Science and Technology Program, “the establishment and clinical application of the neuroultrasound scoring system”, [architecture contracts (2019) 9-1-7].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
References
1. Guru KA, Perlmutter AE, Butt ZM, Peabody JO. Hydrodissection for Preservation of Neurovascular Bundle During Robot-Assisted Radical Prostatectomy. Can J Urol (2008) 15:4000. doi: 10.17709/2409-2231-2016-3-4-5
2. Bokey EL, Keating JP, Zelas P. Hydrodissection: An Easy Way to Dissect Anatomical Planes and Complex Adhesions. Aust New Z J Surg (1997) 67:643–4. doi: 10.1111/j.1445-2197.1997.tb04616.x
3. Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically Guided Carpal Tunnel Injection. J Ultrasound Med (2008) 27:1485–90. doi: 10.7863/jum.2008.27.10.1485
4. Jiang H, Yang W. Diagnosis and Treatment of Carpal Tunnel Syndrome. Chin J Integr Med Cardio Cerebrovasc Dis (2018) 16:65–7. doi: 10.12102/j.issn.1672-1349.2018.23.018
5. Rydevik B, Lundborg G, Nordborg C. Intraneural Tissue Reactions Induced by Internal Neurolysis. Scand J Plast Reconstr Surg Handb Surg (1976) 10:3–8. doi: 10.3109/02844317609169741
6. Delea SL, Chavez-Chiang NR, Poole JL, Norton HE, Sibbitt WL Jr, Bankhurst AD. Sonographically Guided Hydrodissection and Corticosteroid Injection for Scleroderma Hand. Clin Rheumatol (2011) 30:805–13. doi: 10.1007/s10067-010-1653-6
7. Wu YT, Chen SR, Li TY, Ho TY, Shen YP, Tsai CK, et al. Nerve Hydrodissection for Carpal Tunnel Syndrome: A Prospective, Randomized, Double-Blind, Controlled Trial. Muscle Nerve (2019) 59:174–80. doi: 10.1002/mus.26358
8. Fried SM, Nazarian LN. Ultrasound-Guided Hydroneurolysis of the Median Nerve for Recurrent Carpal Tunnel Syndrome. Hand (2019) 14:413–21. doi: 10.1177/1558944717731855
9. Lee JY, Park Y, Park KD, Lee JK, Lim OK. Effectiveness of Ultrasound-Guided Carpal Tunnel Injection Using in-Plane Ulnar Approach: A Prospective, Randomized, Single-Blinded Study. Med (Baltimore) (2014) 93:1–6. doi: 10.1097/MD.0000000000000350
10. Fader R, Mitchell J, Chadayammuri VP, Hill J, Wolcott ML. Percutaneous Ultrasound-Guided Hydrodissection of a Symptomatic Sural Neuroma. Orthopedics (2015) 38:e1046–50. doi: 10.3928/01477447-20151020-15
11. Chen MY, Cai HM, Chen JY, Zhang L, Wang R. Diagnostic Value of Michigan Diabetic Neuropathy Score and Toronto Clinical Scoring System in Diabetic Peripheral Neuropathy. Chin Gen Pract (2017) 20:427–31. doi: 10.3969/j.issn.1007-9572.2017.04.010
13. Ou YQ, Wu XH, Lin YC, Tang C, Wu S. Ultrasonic Diagnostic Score For Diabetic Peripheral Neuropathy. Chin J Nerv Ment Dis (2019) 45:197–201. doi: 10.3969/j.issn.1002-0152.2019.04.002
14. Wang X, Chen L, Liu W, Su B, Zhang Y. Early Detection of Atrophy of Foot Muscles in Chinese Patients of Type 2 Diabetes Mellitus by High-Frequency Ultrasonography. J Diabetes Res (2014) 2014:1–6. doi: 10.1155/2014/927069
15. Cass SP. Ultrasound-Guided Nerve Hydrodissection: What is it? A Review of the Literature. Curr Sports Med Rep (2016) 15(1):20–2. doi: 10.1249/JSR.0000000000000226
16. Lam KHS, Hung CY, Chiang YP, Onishi K, Su DCJ, Clark TB, et al. Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J Pain Res (2020) 13:1957–68. doi: 10.2147/JPR.S247208
17. Suzuki K, Tanaka H, Ebara M, Uto K, Matsuoka H, Nishimoto S, et al. Electrospun Nanofiber Sheets Incorporating Methylcobalamin Promote Nerve Regeneration and Functional Recovery in a Rat Sciatic Nerve Crush Injury Model. Acta Biomaterialia (2017) 53:250–9. doi: 10.1016/j.actbio.2017.02.004
18. Liao CL, Yang M, Zhong WX, Zhang WC. Role of Pain Distribution in Surgical Decompression for Diabetic Peripheral Neuropathy. Chin J Minim Invasive Neurosurg (2015) 20:545–8. doi: 10.11850/j.issn.1009-122X.2015.12.006
19. Hu JD, Chen ZG. Surgical Treatment of Diabetic Peripheral Neuropathy. Fudan Univ J Med Sci (2016) 43:615–9. doi: 10.3969/j.issn.1672-8467.2016.05.018
20. Zhang YJ, Ding JQ, Li JH. Application of Mecobalamin in Nervous System Diseases. China Med Pharmac (2012) 2:19–21. doi: CNKI:SUN:GYKX.0.2012-14-011
21. Li YX, Xu Y, Hu Q, Cui D-R. Application of Perineural Space Expansion by Normal Saline in Axillary Brachial Plexus Block. J Shanghai Jiaotong Univ (Med Edition) (2018) 38:510–3. doi: CNKI:SUN:SHEY.0.2018-05-005
Keywords: hydrodissection, peripheral nerve compression, the scoring system of ultrasound, electrophysiological, refractory diabetic peripheral neuropathy
Citation: Hu HQ, Huang H, Huang J, Leng JC, Li M, Tang C, Li Y and Wu S (2021) Case Report: Successful Outcome for Refractory Diabetic Peripheral Neuropathy in Patients With Ultrasound-Guided Injection Treatment. Front. Endocrinol. 12:735132. doi: 10.3389/fendo.2021.735132
Received: 19 July 2021; Accepted: 21 September 2021;
Published: 28 October 2021.
Edited by:
Bogdan Timar, Victor Babes University of Medicine and Pharmacy, RomaniaReviewed by:
Gabriela Radulian, Carol Davila University of Medicine and Pharmacy, RomaniaNikolaos Papanas, Democritus University of Thrace, Greece
Copyright © 2021 Hu, Huang, Huang, Leng, Li, Tang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Wu, wushan9685@163.com
†These authors have contributed equally to this work
 Hua Qiong Hu†
Hua Qiong Hu† Shan Wu
Shan Wu