- 1Division of Endocrinology and Metabolism, Department of Medicine, Thyroid Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 2Division of Endocrinology, Department of Medicine, Sahmyook Medical Center, Seoul, South Korea
- 3Department of Medical Education, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
Background: Although the incidence of thyroid cancer had been increasing until a few years ago, a decrease has been observed in the last years, probably due to the reduction of the screening tests in Korea. Childhood thyroid cancer has been increasing in the past with the same trend as in adults, but there have been few reports on recent trends. We analyzed the trends of thyroid cancer in Korean children and related factors.
Methods: From national statistics and cancer register database, the data of age-specific incidence rate in Korean childhood thyroid cancer from 1999 to 2017 was obtained, and levels of seaweed intake, the number of computed tomography (CT) and neck ultrasonography (US), obesity prevalence rate, and smoking and alcohol consumption rates in children were analyzed.
Results: The age-specific incidence of thyroid cancer in Korean children has increased in both genders between 1999 and 2017 (2.0 in 1999 vs. 7.2 in 2017, per population of 100,000), especially in the age group of 14-18 years (1.5 in 1999 vs. 5.5 in 2017, per population of 100,000). During the same period, levels of seaweed intake, number of CT scans and neck US, and prevalence of obesity in children increased significantly, while childhood smoking and alcohol consumption rates decreased.
Conclusion: Unlike the adult thyroid cancer in Korea, childhood thyroid cancer continues to increase, and the cause might be accompanied by actual increases due to the environmental factors such as excessive iodine intake, exposure to medical radiation, and increased obesity prevalence as well as the screening effect.
Introduction
The worldwide incidence of thyroid cancer has been increasing (1, 2). This is mainly due to earlier detection of small sized thyroid carcinomas using high-resolution ultrasonography (US) (3). However, the incidence of larger thyroid cancers is also increasing (1, 4). Therefore, many experts have emphasized that early detection or over-diagnosis cannot completely explain the observed increase in thyroid cancer (1, 4, 5).
The understanding of predisposing genetic factors is increasing (6, 7). Czene et al. has suggested that genetic factors play a major role in the pathogenesis of thyroid cancer and that the population living in East Asia, including Korea, is genetically susceptible to thyroid cancer (7). Environmental factors, such as iodine intake, increased exposure to radiation, and rising rates of obesity may be the potential candidates to explain this phenomenon (8–12).
In the past, the incidence of childhood thyroid cancer had been increasing, although it remained very low. Siegel et al. also reported that annual percentage changes in the incidence of childhood thyroid cancer between 2001 and 2009 were increasing with a range from 4.3% to 6.6% in the United States (13). Our research team previously reported an increasing trend of childhood thyroid cancer in Korea until 2012 and since most children are not screened for thyroid cancer, 72% of childhood thyroid cancers were detected by palpation rather than screening (14). However, some experts stated that the increased incidence of childhood thyroid cancer is due to overdiagnosis by US-based screening (15).
The incidence of thyroid cancer has been decreasing from around 2012 based on the Korea Central Cancer Registry (KCCR), as US screening tests have decreased in Korea (16). The question arises whether the incidence of childhood thyroid cancer has also decreased since 2012 as in adults. Therefore, in the present study, we evaluated the incidence of childhood thyroid cancer in Korea between 1999 and 2017 to investigate its changes after 2012. We also analyzed the trends in iodine intake, medical radiation, and obesity in children and adolescents, which were known to be associated with the development of thyroid cancer by using data from national statistics and cancer register database.
Materials and Methods
Data from Statistics Korea (KOSTAT) and the KCCR were used to obtain incidences of thyroid cancer, brain tumors, and leukemia in total and childhood populations. The KCCR has been described in a previous study (14). Mid-year total and childhood populations were calculated as arithmetic averages of the resident registration populations on the first and last days of each year in supplementary data.
The total incidence of thyroid cancer by year was calculated as an age-adjusted incidence rate (population by age of resident registration in 2005). The annual incidence of childhood thyroid cancer was calculated as an age-specific incidence rate by dividing the number of newly diagnosed childhood thyroid cancer patients by the number of persons in the same age group (0-18 years). Incidences for brain tumors and leukemia were also calculated as age-specific incidence rates in supplementary data.
The Korea National Health and Nutrition Examination Survey (NHANES) has been conducted every three years since 1998. Since 2007, it has been operated as a regular annual survey conducted by the Korean Ministry of Health and Welfare (MOHW). This survey is administered to people above the age of 1 year in 11,520 households nationwide, and the consumption of each food group is assessed using a questionnaire in 192 districts and 4,416 households nationwide each year. Data on dietary iodine intake was obtained from the NHANES’s standardized daily seaweed intake trend for each food group. Dried kelp is a food ingredient commonly used to make broth in Korea, and if the ingredients were discarded, the food was treated as not comsumed before 2013. Since 2013, as broth foods were added to the survey, the actual nutrient intake was more accurately reflected, The annual trend data for seaweed intake is the result of standardization with the 2005 estimated population to compensate for the impact of age structure differences.
The number of CT scans in children (0-18 years) was identified through CT cases registered in the HIRA database. The age-specific CT scan rate was calculated as the number of childhood CT scans per year divided by the mid-year childhood population from 2007 to 2018. Moreover, since neck US was claimed for insurance from mid-2013, the number of US cases in children (4-18 years) from 2014 to 2017 was identified from the HIRA database.
Data on rates of childhood (13-18 years) obesity prevalence, smoking, and alcohol consumption were based on a Youth Health Behavior Survey, which is a survey of 800 middle and high schools and approximately 75,000 students, conducted by the MOHW form 2005. Childhood obesity prevalence rate is indicated as the percentage of survey respondents who are above the 95th percentile according to the body mass index (BMI) growth chart by age for children and adolescents in 2017. The childhood smoking rate was calculated as the percentage of survey respondents who have smoked more than one day in the last 30 days, and the childhood alcohol consumption rate was calculated as the percentage of survey respondents who have drunk more than one drink in the last 30 days.
Results
Trends in Incidence of Childhood Thyroid Cancer in Korea Between 1999 and 2017
From 1999 to 2017, the number of children with thyroid cancer per year ranges from 79 to 225. The age-adjusted incidence of thyroid cancer in Korea had gradually increased and peaked in 2012, but has been rapidly declining since 2013. The age-specific incidence of childhood (0-18 years) thyroid cancer in Korea also increased and peaked in 2013, followed by a decrease. However, it has been increasing again since 2015 (Figure 1). The age-specific incidence of childhood thyroid cancer saw a 3.6-fold increase from 2.0 per 100,000 children in 1999 to 7.2 per 100,000 children in 2017. As in adult, girls have higher incidence rates of thyroid cancer than boys, but while the rate of increase in incidence among girls was faster until 2012, the rate of increase in incidence in boys became faster from 2012 to 2017. Between 1999 and 2017, the 4.75-fold overall increase in the incidence rate in boys (0.8 vs. 3.8 per 100,000, respectively) was larger than the 3.1-fold increase seen in girls (3.5 vs. 10.8 per 100,000, respectively) (Figure 2A). According to the age-specific incidence rates, the age group of 14-18 years accounted for the majority, approximately 75%, of all childhood thyroid cancer cases, and this group’s rate continued to increase from 1.5 per 100,000 in 1999 to 5.5 per 100,000 in 2017 (Figure 2B).
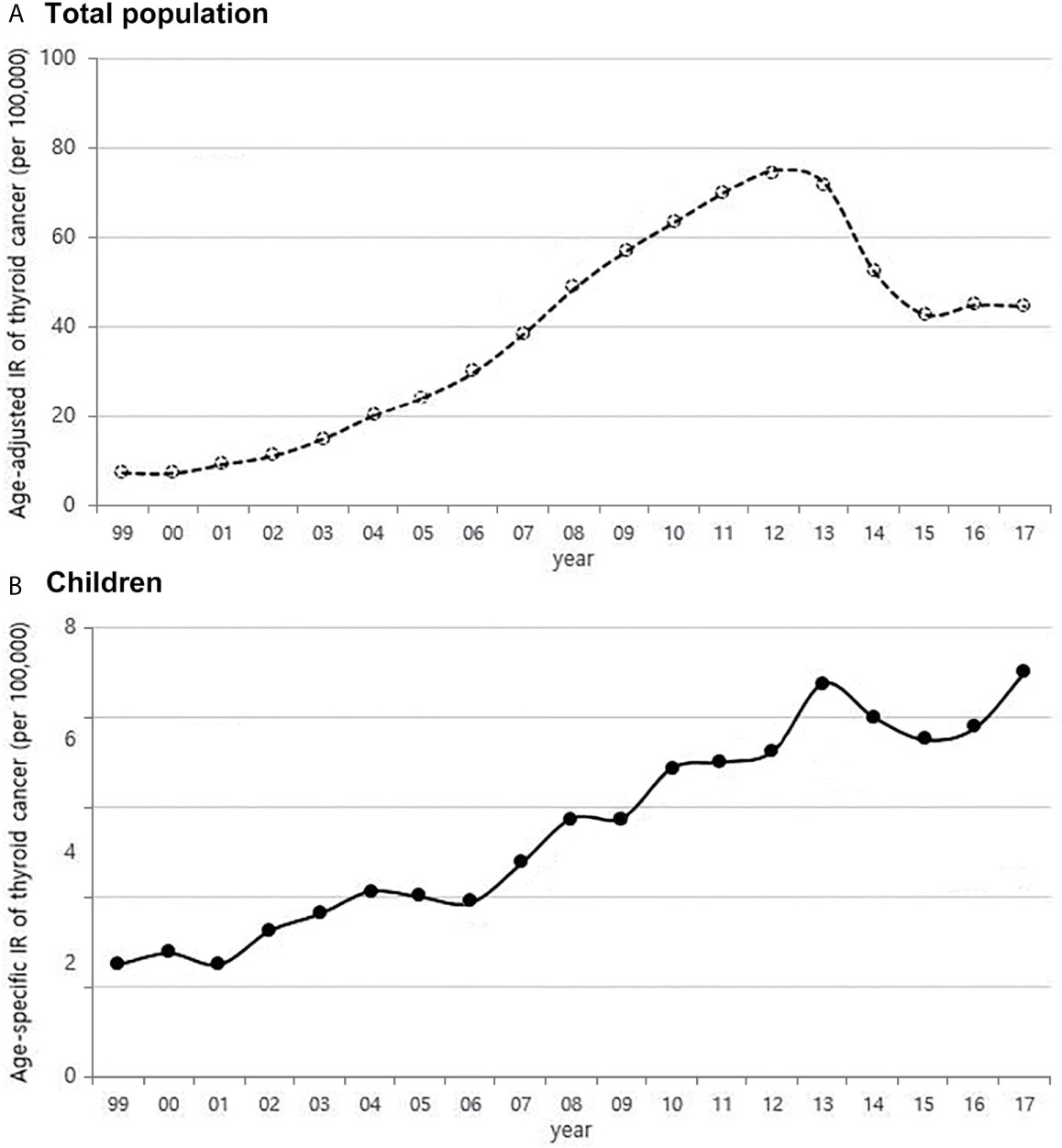
Figure 1 Age-adjusted incidence of thyroid cancer in Korea (A) and age-specific incidence rate of childhood thyroid cancer (0-18 years) (B) (1999-2017, per population of 100,000). IR, incidence rate.
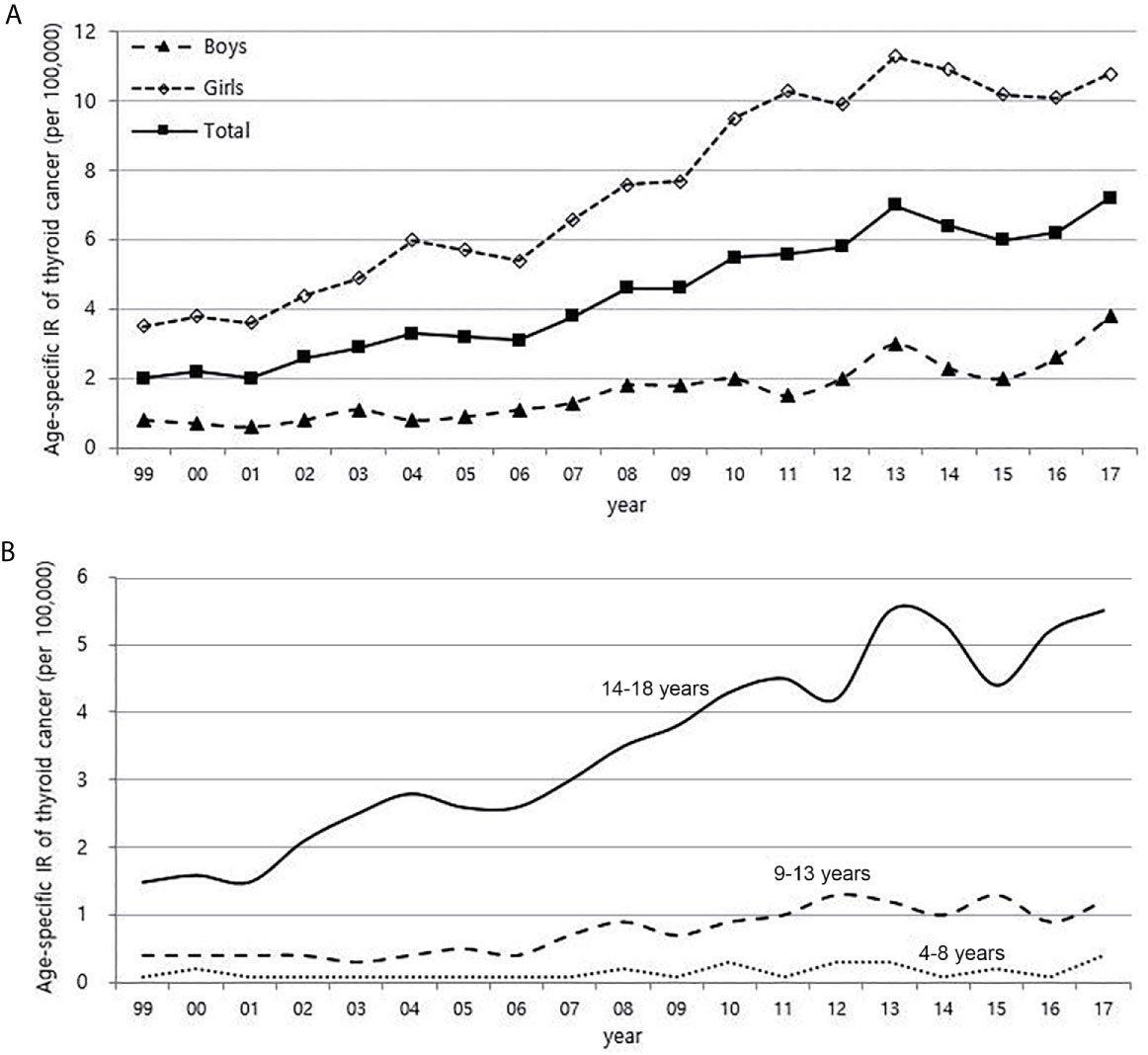
Figure 2 Age-specific incidence rate of childhood (0-18 years) thyroid cancer according to gender (A) and age (B) (1999-2017, per population of 100,000). IR, incidence rate.
Environmental Factors That Might Result in an Increase in Childhood Thyroid Cancer
Although it is not possible to compare before 2013 (broth food was not included), but the age-standardized rates of seaweed intake, which accounts for the bulk of dietary iodine consumption in Korea, have risen since 2013 when broth foods were added to the survey, based on the Korea NHANES (Figure 3). The number of CT scans in children and adolescents (0-18 years) has been increasing every year from approximately 260,000 cases in 2007 to 490,000 cases in 2018 (Figure 4). Adolescent (13-18 years) obesity rates from 2006 to 2018 also showed a steady increase in both genders (from 5.9% in 2006 to 10.8% in 2018), which corresponds to the increase in incidence of childhood thyroid cancer (Figures 5A, B). On the other hand, adolescent (13-18 years) cigarette smoking and alcohol consumption rates tended to decrease from 2005 to 2018 (11.8% to 6.7% and 27.0% to 16.1%, respectively), corresponding to a negative correlation with the incidence of childhood thyroid cancer (Figures 6A, B).
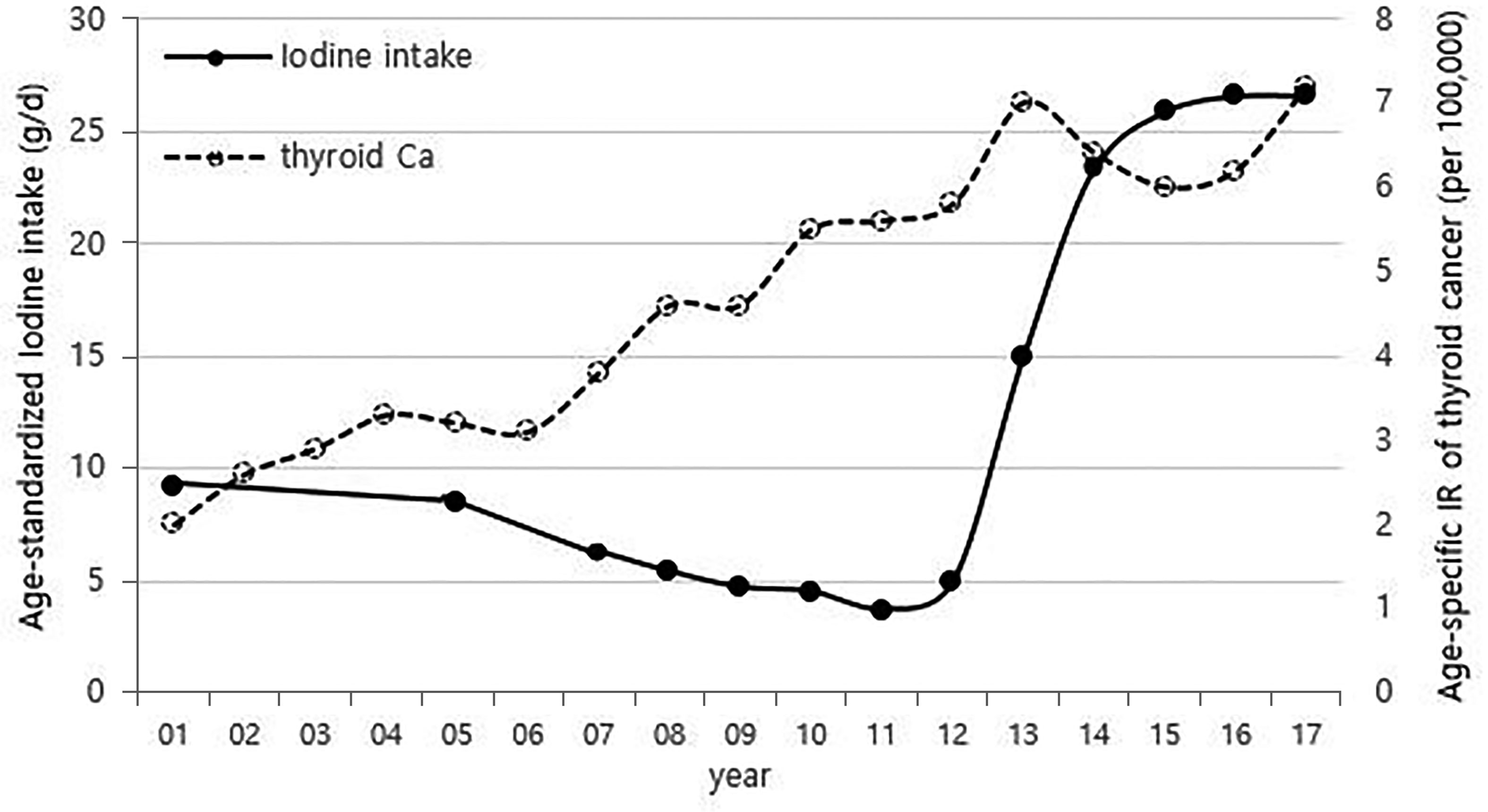
Figure 3 Correlation between age-standardized iodine intake (2007-2017) and age-specific incidence rate of childhood thyroid cancer (0-18 years) by year in Korea (1999-2017, per population of 100,000). Broth foods were added to the survey since 2013. Ca, cancer; IR, incidence rate.
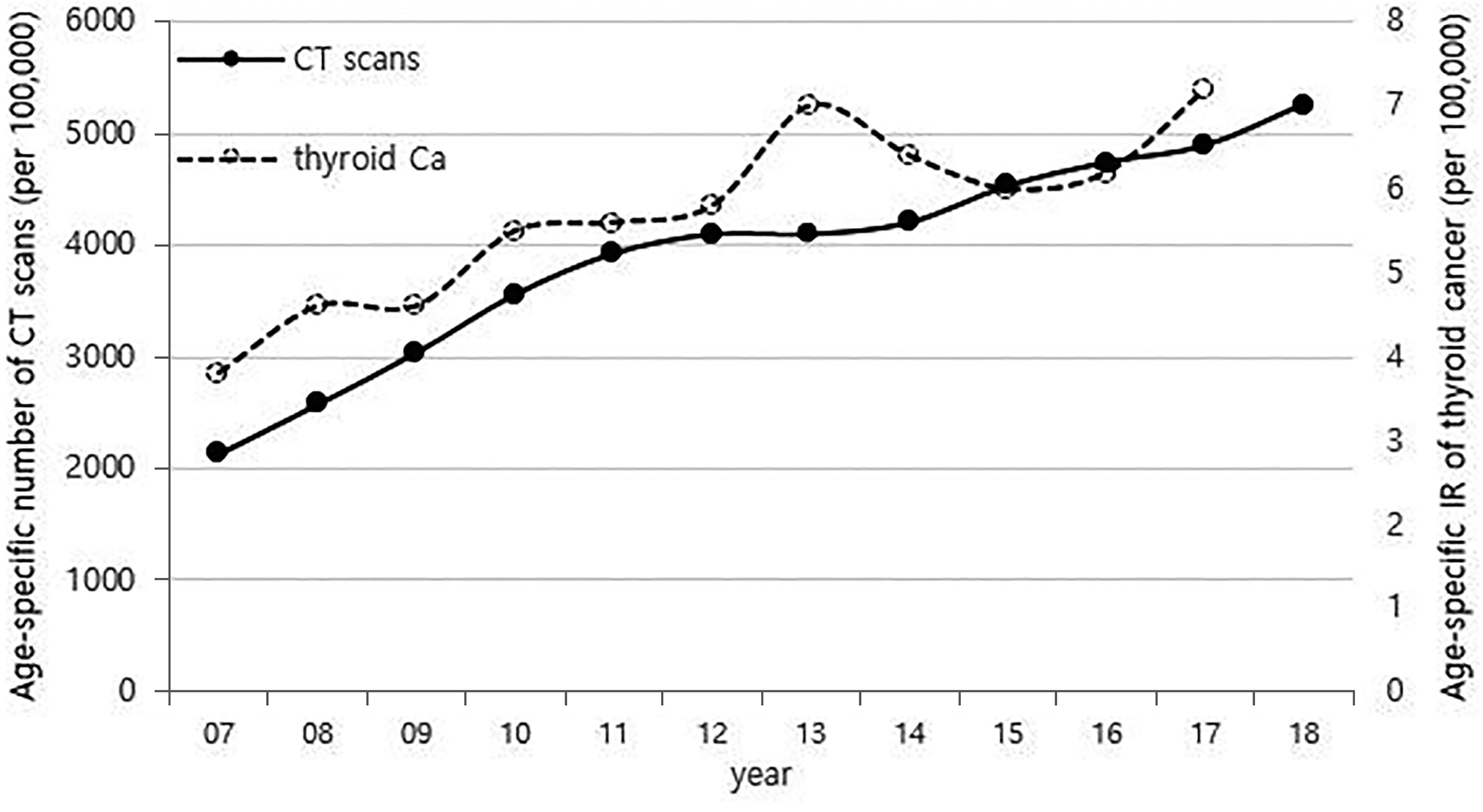
Figure 4 Correlation between the age-specific number of CT scans in childhood (0-18 years) (2007-2018, per population of 100,000) and age-specific incidence rate of childhood thyroid cancer (0-18 years) by year in Korea (2007-2017, per population of 100,000). CT, computed tomography; Ca, cancer; IR, incidence rate.
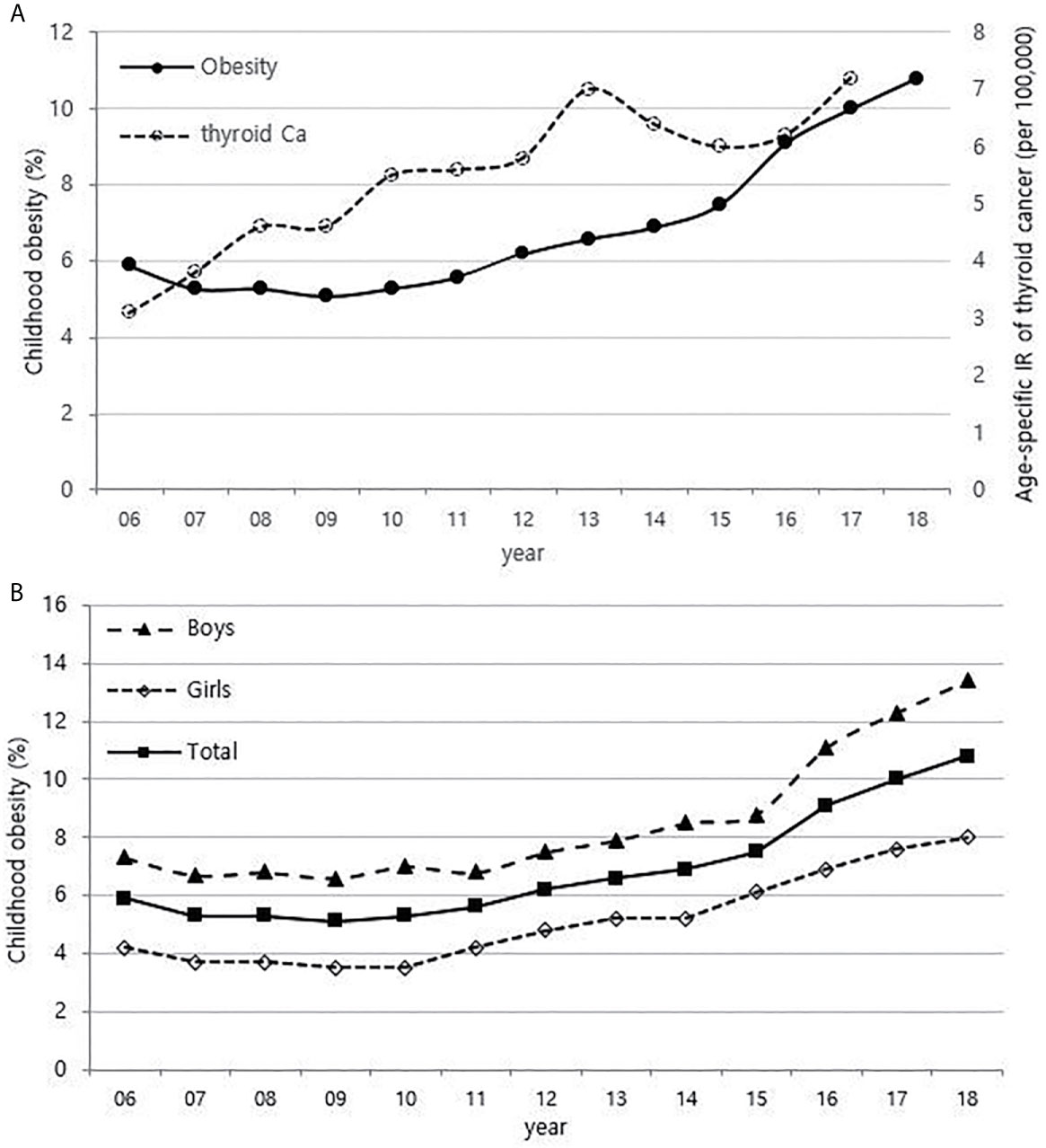
Figure 5 Correlation between childhood obesity prevalence (13-18 years) (2006-2018) and age-specific incidence rate of childhood thyroid cancer (0-18 years) by year in Korea (2006-2017, per population of 100,000) (A). Childhood obesity prevalence according to sex (B). Ca, cancer; IR, incidence rate.
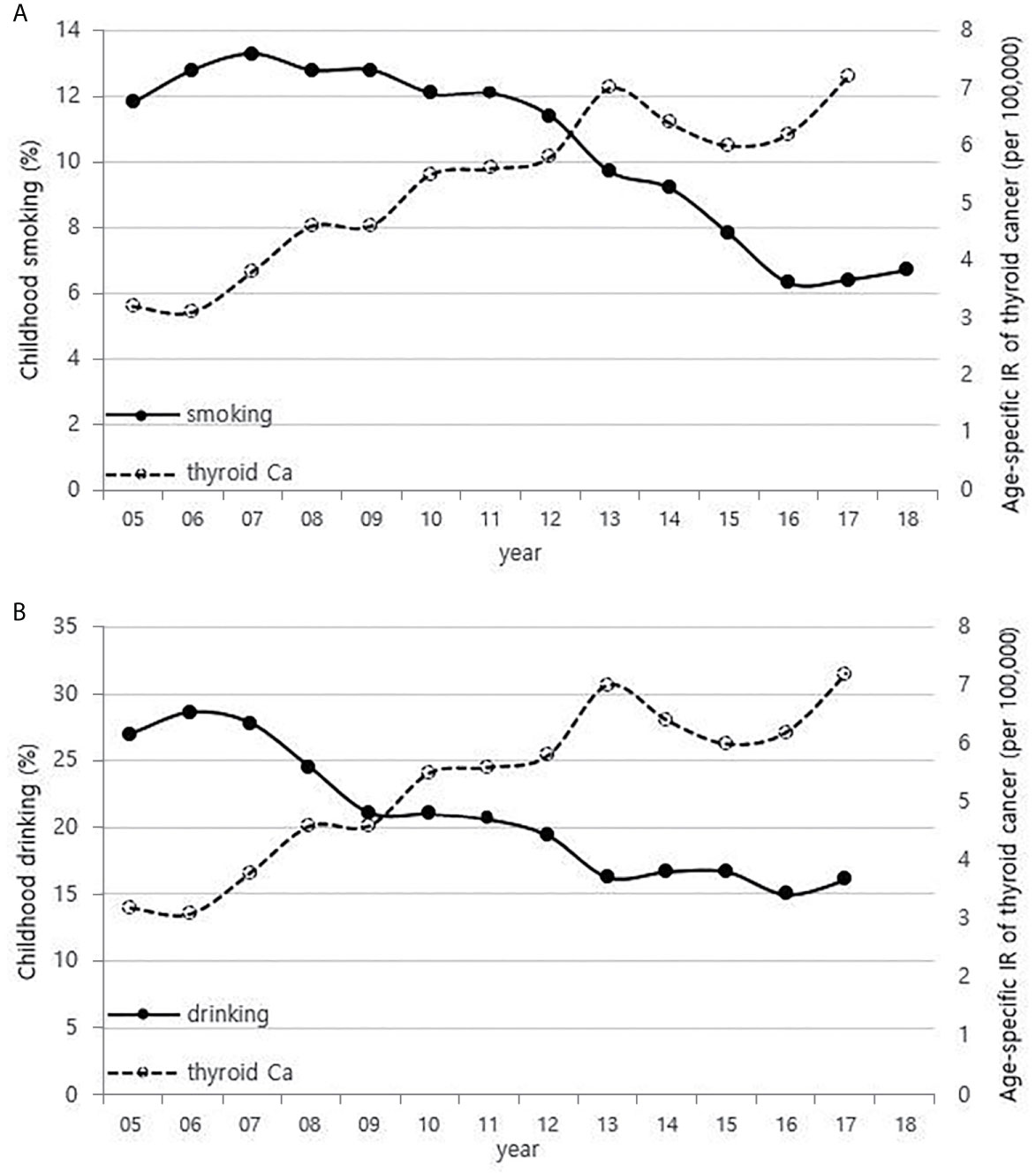
Figure 6 Correlation between childhood smoking (A) and drinking (B) rates (13-18 years) (2005-2018) and age-specific incidence rate of childhood thyroid cancer (0-18 years) in Korea (2005-2017, per population of 100,000). Ca, cancer; IR, incidence rate.
Trends in Neck US Exams in Korean Children Between 2014 and 2018
The national health insurance guarantee of neck US has begun from mid-2013, and the age-specific number of US exams for children charged with HIRA from 2014 to 2018 has increased sharply in all age groups (Figures 7A, B). This is different from the overall trend in incidence of childhood thyroid cancer, especially those aged 14-18 years (Figures 7B and 2B).
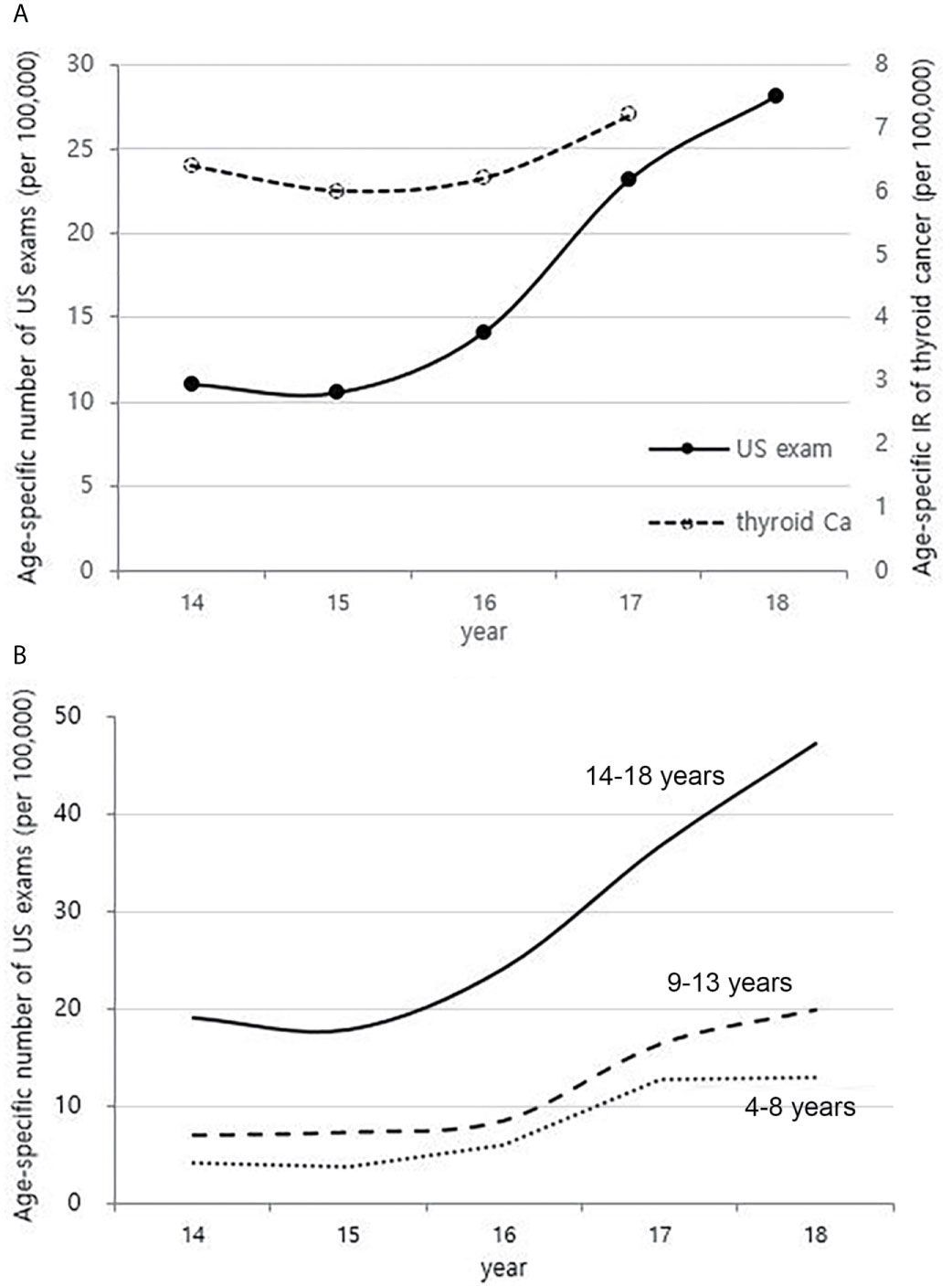
Figure 7 Correlation between the age-specific number of neck US exams in childhood (4-18 years) (2014-2018, per population of 100,000) and age-specific incidence rate of childhood thyroid cancer (0-18 years) by year in Korea (2014-2017, per population of 100,000) (A). Age-specific number of neck US exams in childhood (4-18 years) according to age groups (2014-2018, per population of 100,000) (B). US, ultrasonography; Ca, cancer; IR, incidence rate.
Discussion
We investigated trends in the incidence of childhood thyroid cancer in Korea over the past 18 years and also analyzed the trends in previously considered potential risk factors for thyroid cancer, such as the dietary iodine intake, the number of CT scans performed, and obesity prevalence in children or adolescents, by using data representing the Korean population.
Several studies have shown that iodine intake levels that are lower or higher than recommended result in an increase in thyroid cancer. Mousavi et al. showed that there were high rates of thyroid cancer among first-generation immigrants to Sweden from areas with inadequate or excessive levels of iodine intake (17). Michikawa et al. demonstrated that the incidence of thyroid cancer was significantly higher in Japanese women, who consumed seaweed more than 3-4 times per week, than in those who consumed seaweed fewer than 2 days per week, with hazard ratio 1.71 (95% CI: 1.01-2.90, p = 0.04) (18). In fact, there is a high level of iodine consumption in Korean due to the large amounts of seaweed intake (19). Iodine consumption in Korean children is higher than in adult groups (Figure S1) (20), and children have a relatively smaller volume of thyroid gland than adults, thus, it is expected that high iodine intake can have a greater impact on the development of thyroid cancer in children.
Radiation exposure of the thyroid, particularly in childhood, is a clearly established risk factor for the development of thyroid cancer, as demonstrated by the Chernobyl accident (21). There is a report that there was no significant increase in the incidence of pediatric thyroid cancer after the Fukushima Daiichi Nuclear Power Plant accident (22), but considering that the incidence of thyroid cancer increased rapidly after 4-5 years at the time of the Chernobyl accident, it is necessary to understand the latency phase. CT scans have been the largest contributor to radiation exposure in recent years. The childhood population in Korea steadily decreased (Figure S2), while the age-specific number of CT scans has steadily increased since 2007. The use of CT scans is rising more rapidly in the childhood population than in adults, and the thyroid glands of children are more sensitive to radiation than those of adults (23, 24). It is known that CT scans in children also increase the risk of developing brain tumors and leukemia as well as thyroid cancer, and the increase in risk is greatest for brain tumors (25, 26). However, while the incidence of childhood thyroid cancer is rapidly increasing, the incidence of brain tumors and leukemia in children has only been minimally increasing during the same period (Figure S3). Thus, the recent increase in childhood thyroid cancer in Korea may be associated with the increase in medical exposure, but it is not expected to be a major factor. In addition, as neck US exam of children has been also increasing recently (Figure 7), there must be some portion of the increase due to the incidental detection of small sized thyroid cancer. However, the increase in childhood thyroid cancer cannot be fully explained by the increase in incidental detection, and the true increase should be taken into account.
The relationship between obesity and thyroid cancer is still controversial, but there seems to be a positive association between BMI and the incidence of thyroid cancer, particularly in women (9, 27, 28). Thyroid cancer is almost three times more common in women than in men, and it has been suggested that the female sex hormones, especially estrogen, are related (29). Estrogen receptors are found in thyroid tissues. Therefore, the thyroid glands of women are more likely to be irritated by estrogen than those of men, causing inflammation and various thyroid diseases. There are also reports that estrogen promotes the proliferation of thyroid cancer cells (30, 31). In recent years, precocious puberty has been increasing with childhood obesity, and obesity also increases insulin resistance and inhibits sex hormone-binding proteins, which results in an increase in estrogen levels (32, 33). Since the prevalence of childhood obesity in Korea is rapidly increasing, exposure to estrogen at an early age might be contributing to the increase in childhood thyroid cancer. In particular, the prevalence of obesity in boys has been increasing more rapidly than in girls since 2015 in Figure 2A of this study, and this may be related to the recent steeper increase in the incidence of thyroid cancer in boys than in girls.
Cigarette smoking and alcohol consumption has an inverse association with thyroid cancer incidence. Many researchers have proposed that since higher thyroid-stimulating hormone (TSH) levels are associated with higher frequency and advanced stage of thyroid cancer (34), and low TSH levels are measured in smokers, suggesting that smokers have a lower risk of thyroid cancer (35, 36). Cho et al. found that men who are currently smoking had a lower risk of incident thyroid cancer even after adjusting for TSH and BMI in a cohort study of 96,855 Korean adults (37), and the reason is probably due to the anti-estrogenic effect of smoking (9, 35, 38). A meta-analysis showed that alcohol intake is responsible for reducing the risk of thyroid cancer (39), and Meinhold et al. demonstrated in a large prospective study that people who consume more than two alcoholic drinks a day have a reduced risk of thyroid cancer compared to those who do not drink alcohol (relative risk = 0.57, 95% CI 0.36–0.89, p-trend = 0.01) (40).
When considering the results from previous research and the positive correlations in overall trend between the incidence of thyroid cancer and dietary iodine consumption, the number of CT scans, and obesity prevalence that were observed among children in this study (Figures 1, 3–5), we might be tempted to suggest that when there is a genetic susceptibility, excessive iodine, medical radiation, and obesity are likely to cause thyroid cancer by acting as driving factors in children.
There are several limitations in this study. First, since this research is an ecological study of the entire population, it is not known whether the individual children who were diagnosed with thyroid cancer actually had a high dietary iodine intake, underwent several CT scans, or gained weight. Therefore, the results cannot be interpreted at an individual level, but hypotheses for the next step of research have been presented. Second, considering the potentially long latency periods between exposure and disease onset, it was recommended to refer to the amount of dietary iodine intake, the number of childhood CT scan use, and the childhood obesity prevalence data before 2005. However, since such earlier data were not available, it is meaningful to keep track of the trend of childhood thyroid cancer after 2017. Third, this analysis is the lack of data on tumor detection methods and characteristics such as histology, stage, and size at diagnosis. In addition, since the increase in childhood thyroid cancer is mainly seen in the population of 14-18 years, further analysis of this age group would be more informative, but there was a limit to further analysis as only the nationwide data already collected and published were used. Nevertheless, because there is not enough research on the development of childhood thyroid cancer, the comparison of childhood thyroid cancer incidence trends with total thyroid cancer incidence and the analysis of trends in prevalence of potential risk factors during the same period are a potentially valuable contribution.
In conclusion, the incidence of childhood thyroid cancer is constantly increasing. Although the number of thyroid US exams in children is also increasing, it cannot be concluded that the increase is only due to the screening effect. In addition, we cannot rule out the possibility that a true increase due to the potential risk factors may be implied. Since the environmental factors that can reduce or prevent childhood thyroid cancer can be overlooked, further studies are needed on the causes of the increase in childhood thyroid cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board in Samsung Medical Center (SMC-IRB number: 2020-04-041). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JP conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. HP designed the data collection instruments, collected data. TK, SK, and HJ reviewed and revised the manuscript. JC conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Samjung Scholarship Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.681148/full#supplementary-material
References
1. Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising Thyroid Cancer Incidence in the United States by Demographic and Tumor Characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev (2009) 18:784–91. doi: 10.1158/1055-9965.EPI-08-0960
2. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid Cancer Mortality and Incidence: A Global Overview. Int J Cancer (2015) 136:2187–95. doi: 10.1002/ijc.29251
3. Udelsman R, Zhang Y. The Epidemic of Thyroid Cancer in the United States: The Role of Endocrinologists and Ultrasounds. Thyroid (2014) 24:472–9. doi: 10.1089/thy.2013.0257
4. Chen AY, Jemal A, Ward EM. Increasing Incidence of Differentiated Thyroid Cancer in the United States, 1988-2005. Cancer (2009) 115:3801–7. doi: 10.1002/cncr.24416
5. Chung JH. Unfounded Reports on Thyroid Cancer. J Korean Med Sci (2014) 29:1033–4. doi: 10.3346/jkms.2014.29.8.1033
6. Adjadj E, Schlumberger M, de Vathaire F. Germ-Line DNA Polymorphisms and Susceptibility to Differentiated Thyroid Cancer. Lancet Oncol (2009) 10:181–90. doi: 10.1016/s1470-2045(09)70020-8
7. Czene K, Lichtenstein P, Hemminki K. Environmental and Heritable Causes of Cancer Among 9.6 Million Individuals in the Swedish Family-Cancer Database. Int J Cancer (2002) 99:260–6. doi: 10.1002/ijc.10332
8. Vigneri R, Malandrino P, Vigneri P. The Changing Epidemiology of Thyroid Cancer: Why is Incidence Increasing? Curr Opin Oncol (2015) 27:1–7. doi: 10.1097/cco.0000000000000148
9. Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, et al. Nonradiation Risk Factors for Thyroid Cancer in the US Radiologic Technologists Study. Am J Epidemiol (2010) 171:242–52. doi: 10.1093/aje/kwp354
10. Guan H, Ji M, Bao R, Yu H, Wang Y, Hou P, et al. Association of High Iodine Intake With the T1799A BRAF Mutation in Papillary Thyroid Cancer. J Clin Endocrinol Metab (2009) 94:1612–7. doi: 10.1210/jc.2008-2390
11. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-Mass Index and Incidence of Cancer: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Lancet (2008) 371:569–78. doi: 10.1016/s0140-6736(08)60269-x
12. Imaizumi M, Usa T, Tominaga T, Neriishi K, Akahoshi M, Nakashima E, et al. Radiation Dose-Response Relationships for Thyroid Nodules and Autoimmune Thyroid Diseases in Hiroshima and Nagasaki Atomic Bomb Survivors 55-58 Years After Radiation Exposure. JAMA (2006) 295:1011–22. doi: 10.1001/jama.295.9.1011
13. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer Incidence Rates and Trends Among Children and Adolescents in the United States, 2001-2009. Pediatrics (2014) 134:e945–55. doi: 10.1542/peds.2013-3926
14. Cho YY, Jang HW, Joung JY, Park SM, Jeong DJ, Kim SW, et al. Trends in Thyroid Cancer Incidence in Korean Children (1999-2012) Based on Palpation and Nonpalpation Detection Methods. Eur Thyroid J (2015) 4:252–9. doi: 10.1159/000442047
15. Takano T. Overdiagnosis of Juvenile Thyroid Cancer. Eur Thyroid J (2020) 9:124–31. doi: 10.1159/000503323
16. Jung K-W, Won Y-J, Kong H-J, Lee ES. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res Treat (2019) 51:417–30. doi: 10.4143/crt.2019.138
17. Mousavi SM, Brandt A, Sundquist J, Hemminki K. Risks of Papillary and Follicular Thyroid Cancer Among Immigrants to Sweden. Int J Cancer (2011) 129:2248–55. doi: 10.1002/ijc.25867
18. Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, et al. Seaweed Consumption and the Risk of Thyroid Cancer in Women: The Japan Public Health Center-Based Prospective Study. Eur J Cancer Prev (2012) 21:254–60. doi: 10.1097/CEJ.0b013e32834a8042
19. Han M-R, Ju DL, Park YJ, Paik H-Y, Song Y. An Iodine Database for Common Korean Foods and the Association Between Iodine Intake and Thyroid Disease in Korean Adults. Int J Thyroidol (2015) 8:170–82. doi: 10.11106/ijt.2015.8.2.170
20. Park SY, Kim HI, Oh HK, Kim TH, Jang HW, Chung JH, et al. Age- and Gender-Specific Reference Intervals of TSH and Free T4 in an Iodine-Replete Area: Data From Korean National Health and Nutrition Examination Survey IV (2013-2015). PLoS One (2018) 13:e0190738. doi: 10.1371/journal.pone.0190738
21. Pacini F, Vorontsova T, Demidchik EP, Molinaro E, Agate L, Romei C, et al. Post-Chernobyl Thyroid Carcinoma in Belarus Children and Adolescents: Comparison With Naturally Occurring Thyroid Carcinoma in Italy and France. J Clin Endocrinol Metab (1997) 82:3563–9. doi: 10.1210/jcem.82.11.4367
22. Iwaku K, Noh JY, Sasaki E, Suzuki N, Kameda T, Kobayashi S, et al. Changes in Pediatric Thyroid Sonograms in or Nearby the Kanto Region Before and After the Accident At the Fukushima Daiichi Nuclear Power Plant. Endocr J (2014) 61(9):875–81. doi: 10.1507/endocrj.ej14-0032
23. Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, et al. Primary Thyroid Cancer After a First Tumour in Childhood (the Childhood Cancer Survivor Study): A Nested Case-Control Study. Lancet (2005) 365:2014–23. doi: 10.1016/s0140-6736(05)66695-0
24. Brenner DJ, Hall EJ. Computed Tomography–An Increasing Source of Radiation Exposure. N Engl J Med (2007) 357:2277–84. doi: 10.1056/NEJMra072149
25. Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer Risk in 680,000 People Exposed to Computed Tomography Scans in Childhood or Adolescence: Data Linkage Study of 11 Million Australians. BMJ (2013) 346:f2360. doi: 10.1136/bmj.f2360
26. Shao YH, Tsai K, Kim S, Wu YJ, Demissie K. Exposure to Tomographic Scans and Cancer Risks. JNCI Cancer Spectr (2020) 4:pkz072. doi: 10.1093/jncics/pkz072
27. Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, et al. Obesity and the Risk of Papillary Thyroid Cancer: A Pooled Analysis of Three Case-Control Studies. Thyroid (2014) 24:966–74. doi: 10.1089/thy.2013.0566
28. Son H, Lee H, Kang K, Lee I. The Risk of Thyroid Cancer and Obesity: A Nationwide Population-Based Study Using the Korea National Health Insurance Corporation Cohort Database. Surg Oncol (2018) 27:166–71. doi: 10.1016/j.suronc.2018.03.001
29. Rahbari R, Zhang L, Kebebew E. Thyroid Cancer Gender Disparity. Future Oncol (2010) 6:1771–9. doi: 10.2217/fon.10.127
30. Santin AP, Furlanetto TW. Role of Estrogen in Thyroid Function and Growth Regulation. J Thyroid Res (2011) 2011:875125. doi: 10.4061/2011/875125
31. Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. Oestrogen Mediates the Growth of Human Thyroid Carcinoma Cells Via an Oestrogen receptor-ERK Pathway. Cell Prolif (2007) 40:921–35. doi: 10.1111/j.1365-2184.2007.00471.x
32. Ahmed ML, Ong KK, Dunger DB. Childhood Obesity and the Timing of Puberty. Trends Endocrinol Metab (2009) 20:237–42. doi: 10.1016/j.tem.2009.02.004
33. Holly JM, Smith CP, Dunger DB, Howell RJ, Chard T, Perry LA, et al. Relationship Between the Pubertal Fall in Sex Hormone Binding Globulin and Insulin-Like Growth Factor Binding Protein-I. A Synchronized Approach to Pubertal Development? Clin Endocrinol (Oxf) (1989) 31:277–84. doi: 10.1111/j.1365-2265.1989.tb01251.x
34. Boelaert K. The Association Between Serum TSH Concentration and Thyroid Cancer. Endocr Relat Cancer (2009) 16:1065–72. doi: 10.1677/erc-09-0150
35. Kreiger N, Parkes R. Cigarette Smoking and the Risk of Thyroid Cancer. Eur J Cancer (2000) 36:1969–73. doi: 10.1016/s0959-8049(00)00198-2
36. Kitahara CM, Linet MS, Beane Freeman LE, Check DP, Church TR, Park Y, et al. Cigarette Smoking, Alcohol Intake, and Thyroid Cancer Risk: A Pooled Analysis of Five Prospective Studies in the United States. Cancer Causes Control (2012) 23:1615–24. doi: 10.1007/s10552-012-0039-2
37. Cho A, Chang Y, Ahn J, Shin H, Ryu S. Cigarette Smoking and Thyroid Cancer Risk: A Cohort Study. Br J Cancer (2018) 119:638–45. doi: 10.1038/s41416-018-0224-5
38. Baron JA, La Vecchia C, Levi F. The Antiestrogenic Effect of Cigarette Smoking in Women. Am J Obstet Gynecol (1990) 162:502–14. doi: 10.1016/0002-9378(90)90420-c
39. Hong SH, Myung SK, Kim HS. Korean Meta-Analysis Study G. Alcohol Intake and Risk of Thyroid Cancer: A Meta-Analysis of Observational Studies. Cancer Res Treat (2017) 49:534–47. doi: 10.4143/crt.2016.161
Keywords: childhood, thyroid, cancer, incidence, increase, Korea
Citation: Park J, Park H, Kim TH, Kim SW, Jang HW and Chung JH (2021) Trends in Childhood Thyroid Cancer incidence in Korea and Its Potential Risk Factors. Front. Endocrinol. 12:681148. doi: 10.3389/fendo.2021.681148
Received: 16 March 2021; Accepted: 26 April 2021;
Published: 14 May 2021.
Edited by:
Akira Sugawara, Tohoku University, JapanReviewed by:
Toru Tateno, University of Alberta, CanadaDorina Ylli, MedStar Health Research Institute (MHRI), United States
Copyright © 2021 Park, Park, Kim, Kim, Jang and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae Hoon Chung, jaeh.chung@samsung.com
 Jun Park
Jun Park Hyunju Park1
Hyunju Park1