A Mini Review: The Potential Biomarkers for Non-invasive Diagnosis of Pulpal Inflammation
- 1Department of Oral and Maxillofacial Surgery, Rutgers School of Dental Medicine, Newark, NJ, United States
- 2Department of Oral Biology, Rutgers School of Dental Medicine, Newark, NJ, United States
- 3Department of Endodontics, Rutgers School of Dental Medicine, Newark, NJ, United States
For assessing the adequacy of vital pulp therapy for an inflamed pulp, the use of non-invasive diagnostic tools is necessary to avoid further damage to the teeth. Detection of biomarkers that are indicative of the inflammatory status in pulp can be a promising tool for this purpose. These biomarkers need to be reliably correlated with pulpal inflammation and to be easily detected without pulp exposure. This mini-review article aims to review biomarkers that are present in gingival crevicular fluid (GCF) in inflamed pulp conditions. Several studies have reported the availability of various biomarkers including cytokines, proteases, elastase, neuropeptides, and growth factors. Non-invasive pulpal diagnostic methods will be useful as well to determine reversibility, irreversibility, or necrosis of inflamed pulp. These types of molecular diagnoses via analyzing the proteome have revolutionized the medical field, and are one of the most promising empirical methodologies that a clinician can utilize for the proactive identification of pulpal disease.
Introduction
The decision to perform vital pulp therapy or to extirpate the pulp is based on the clinician's diagnosis regarding the inflammatory status of the pulp caused by a carious lesion or traumatic injury. The modalities for assessing the pulpal condition are dependent on the patient's chief complaint, the history of symptoms, clinical and radiographic assessment, pulpal sensibility, and periapical tests (1, 2). However, there is a dearth of high-quality studies claiming the insufficient accuracy of those modalities to determine the status of pulp inflammation (1). Additionally, there is no special test that measures the ability of the pulp to recover from inflammation. Thus, the diagnosis distinguishing reversible pulpitis from irreversible pulpitis lacks a strong foundation. Lastly, pulp diagnoses based on the current modalities have a poor correlation with the histopathological condition of the pulp (3–5).
As with any inflammatory process, molecular mediators are involved in the suppression and progression of the inflammatory response in the dental pulp. Gingival crevicular fluid (GCF) is easily collected, contains these mediators secreted from dental pulp via dentinal tubules (6). Therefore, the molecular mediators derived from the pulp in various stages of disease can be useful clinical tools to establish an accurate pulp diagnosis. The aim of this mini-review is to discuss the molecular mediators in inflamed dental pulp and evaluate the biomarkers present in GCF.
Pulpal Inflammation
Pulp inflammation is induced by a variety of causes: microbial intrusion via dental caries, cracks, or dentinal tubules; irritation by chemicals from etching and/or bonding materials for fillings; mechanical irritation during restorative procedures; or trauma caused by occlusion or orthodontic movement of the teeth (7). A series of inflammatory events are triggered to start the repair process (8). Pulp inflammation leads to changes in cellular composition and decrease cellular function, but in parallel, it facilitates an increase in mineral deposition from odontoblasts (9). The processes involved in the stages of inflammation occur both at cellular and molecular levels. The activation of the molecular response leads to the recruitment of immune cells (10). Dental pulp with reversible pulpitis shows coagulation and liquefaction in the tissue without bacteria, whereas dental pulp with irreversible pulpitis is characterized by the presence of acute inflammatory cells, mainly neutrophils, in the tissue underneath the lesion with bacterial infection (11–13). In irreversible pulpitis, lysosomal enzymes produced by neutrophils cause tissue damage and suppuration in dental pulp (14).
Methods For Non-invasive Pulp Diagnosis
Currently, five non-invasive pulp diagnosis methods are broadly recognized: cold pulp test, heat pulp test, electric pulp test (EPT), laser Doppler flowmetry (LDF), and pulse oximetry (PO). These methods, especially the latter two, are highly accurate (84, 72, 82, 97, and 97%, respectively) in testing pulp vitality (15). Importantly, the main purpose of these tests is to diagnose pulp vitality, not necessarily to distinguish reversible from irreversible pulpitis. The standard diagnostic procedure to distinguish between the two types of pulpitis is the cold pulp test. However, the response to thermal stimulus is affected by age of the patient. Individuals >53 years old have an increased incidence of painless pulpitis (non-lingering pain) than the ≤ 33 year old group (16), indicating that thermal test is not absolutely precise for pulp diagnosis.
Role of Biomarkers in Oral Diseases
The role of biomarkers for the diagnosis of oral cancers, temporomandibular joint disorders (TMD), periodontal disease, and caries have been actively investigated (17–19). Tumor necrosis factor (TNF) and TNF receptor (TNFR) 2 have been identified as biomarkers for diagnosis as well as treatment in terms of reduction of inflammation and pain of TMD (20). Salivary microRNAs have been studied as possible diagnostic and prognostic biomarkers for oral cancers and periodontal disease (18). Proteomic studies using mass spectrometry have been performed in order to correlate proteomes with periodontal disease conditions (21). Gingival crevicular fluids (GCF), saliva, and blood serum are mainly tested in those studies (22). Other promising biomarkers involved in periodontal inflammatory pathology are interleukin (IL)-1β, matrix metalloproteinase (MMP)-8, and serum carboxy-terminal telopeptide of type I collagen (ICTP) (23). In early childhood, a significant correlation has been established between the presence of cavities and the high concentrations of protective proteins in saliva, including IgA, IgG, immunoglobulins, β-defensin-2, and histatin-5 (24, 25), which have the potential to be utilized as caries biomarkers (19). Several genetic alterations and proteomic changes have been evaluated to identify the specific indicators of risk for development of oral cancers (26).
Biomarkers Associated With Pulpal Inflammation
This mini review provides an overview of molecules that are present and measurable during pulpal inflammation and therefore have the potential to serve as biomarkers for irreversible pulpitis by non-invasive detection methods such as GCF sampling. The pulp cavity communicates with the periodontal ligament via the apical foramina, accessory lateral and furcation canals, and dentinal tubules. Therefore, the molecular mediators of inflammation are present not only in pulp tissue and the blood, but also in GCF and dentinal fluid (14, 27, 28).
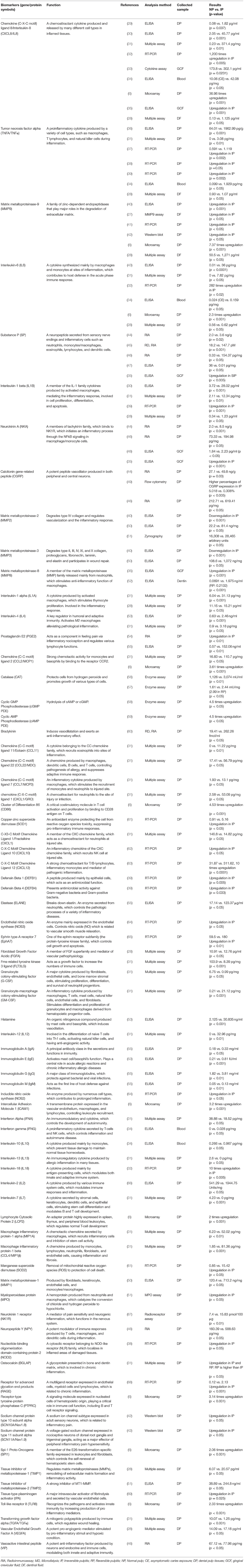
The potential biomarkers for the diagnosis of irreversible pulpitis are listed in Table 1. The selected biomarkers have shown statistical significance in previous literatures, when measuring cellular inflammation by the quantitative methods, such as enzyme-linked immunosorbent assay (ELISA), western blot analysis, quantitative reverse transcription PCR (RT-qPCR), radioimmunoassay, multiple assay, and enzyme assay.
The Selected Pulpitis Biomarkers Which Are Detected in GCF
The biomarkers described below are detected in GCF and are upregulated reflecting pulp inflammation. Since these biomarkers are upregulated also in the cases of periodontitis, measuring solely these markers may not distinguish pulp inflammation and periodontitis. There is no perfect solution to this problem, however, the detection of periodontitis markers might negatively select pulpitis patients. For example, cross-linked N-terminal telopeptides of type I collagen (NTx), a bone resorption marker, is reported to be detected only in the cases of periodontitis, but not in gingivitis cases (70). As bone resorption specifically occurs with periodontitis or orthodontic treatment, NTx should not be detected from pulpits patients without periodontitis. Likewise, the detection of the other bone resorption markers, such as Cathepsin-K, RANKL, or IL-34, can aid distinguishing periodontitis from pulpitis. This type of strategies to avoid “bias” by the biomarkers of periodontitis will be discussed later, as distinguishing pulpitis from other inflammatory conditions will be a central challenge to utilize biomarkers in GCF for non-invasive pulp diagnosis.
Matrix Metalloproteinase-8
MMP-8 is mainly produced by neutrophils, and it degrades type I, II and III collagens. It has a significant role in the regulation of the innate immune system, especially by activating IL-6 and IL-8 (71). MMP-8 is the most abundant collagenase in dentine (72). MMP-8 is detected in inflamed pulp and periapical root-canal exudates (73). Higher MMP-8 is included in dentin samples from reversible or irreversible pulpitis patients (52). In pulpitis patients, MMP-8 levels were significantly higher in the GCF samples compared with the healthy group (35). Patients' subjective pain levels were significantly related to both MMP-8 and substance P (SP) levels. MMP-8 and SP levels in GCF were decreased during root canal treatment, and they showed a positive correlation with each other (74). As described above, MMP-8 is one of the hopeful biomarkers for pulp inflammation diagnosis, however, MMP-8 is also detected in GCF from chronic periodontitis patients (75, 76).
Chemokine (C-X-C Motif) Ligand 8/Interleukin-8 (CXCL8/IL-8)
IL-8 is a chemoattractant cytokine produced and released by many different cell types in inflamed pulp tissues, such as monocytes/macrophages, lymphocytes, fibroblasts, endothelial cells, and odontoblasts (29). It recruits neutrophils and facilitate their accumulation at the injured site (77, 78). Levels of IL-8, as well as ratio of IL-6/IL-8 and IL-8/IL-10, have been shown to be higher in irreversible pulpitis and in caries-exposed pulp as compared to normal pulp (34). These levels can be assessed in the blood from caries-exposed teeth to evaluate if vital pulp therapy can be applied (34). In addition, Karapanou et al. and Akbal Dincer et al. have shown that IL-8 levels significantly increase in gingival crevicular fluid (GCF) of inflamed pulp teeth with a disease, such as irreversible pulpitis (33, 35). Since IL-8 is significantly increased in dentinal fluid of reversible and irreversible pulpitis compared to normal pulp, it can be useful for diagnosis of pulpitis (28). Therefore, IL-8 is a potential biomarker for pulp diagnosis using non-invasive method such as collection of GCF. However, IL-8 level is also enhanced in GCF of chronic periodontitis (79).
Substance P
SP and NKA (neurokinin A, described below) are the members of tachykinin family, which are involved in neuronal excitation, vasodilatation, plasma extravasation, nociception, and proinflammatory actions on immune and inflammatory cells (80). SP is a neuropeptide that is secreted from sensory nerve endings and inflammatory cells such as neutrophils, monocytes/macrophages, eosinophils, lymphocytes, and dendritic cells (81). SP stimulates macrophage's function by their NF-kB activation, which causes the increase of inflammatory chemokine secretion from macrophages (82). Several studies have shown by ELISA and radioimmunoassay that SP is significantly increased in inflamed pulp tissues (44–47). In addition, Akbal Dincer et al. have shown that SP is significantly higher in GCF from teeth with symptomatic irreversible pulpitis (SIP) in comparison with the healthy control group (35). However, SP and NKA are detected in GCF from periodontal disease patients with gingivitis or periodontitis (83).
Neurokinin A
NKA binds to NK1R, initiating an inflammatory process through the NF-kB signaling in macrophage/monocyte cells; whereas the neurological functions of NKA are primarily mediated by NK2R (84). Heidari et al. and Akbal Dincer et al. have shown that NKA is significantly enhanced in GCF of teeth with SIP in comparison with asymptomatic teeth (35, 48). Although SP and NKA are also upregulated in GCF of teeth with periodontitis, the levels are reduced after periodontal treatment (85).
The Selected Pulpitis Biomarkers Which Are Detected in Dentinal Fluid
The biomarkers listed below can be detected in dentinal fluid and they increase in the cases of pulp inflammation. The sampling of dentinal fluid requires the removal of enamel or soft dentine on caries; thus, it is not a non-invasive procedure presently. However, dentinal fluid can be collected without making access to patients' pulp and contains cues from the odontoblasts contiguous to inflammatory site (28). It would be supportive to list the biomarkers below, expecting the development of new methodology to collect them non-invasively in future.
Matrix Metalloproteinase-9
MMP-9 is a member of the MMPs, a family of zinc-dependent endopeptidases that play major roles in the degradation of extracellular matrix (ECM) during physiological and pathophysiological processes, including tissue remodeling (86). In inflammatory conditions, MMP-9 is locally produced and activated by infiltrating neutrophils (87, 88). MMP-9 is barely detectable in the healthy dental pulp, however, it is strongly increased in the dentinal fluid of inflamed pulp tissues with a disease, such as irreversible pulpitis (27, 28). However, since MMP9 is increased in dentinal fluid of both reversible and irreversible pulpitis (28), these two types of pulpitis cannot be distinguished by detecting MMP9 alone at this time.
Interleukin-1α
Interleukin 1 alpha is a cytokine produced mainly by activated macrophages, as well as neutrophils, epithelial cells, and endothelial cells. IL-1α is involved in a variety of immune reaction, including the stimulation of thymocyte proliferation by inducing IL-2 release, B-cell maturation and proliferation, and fibroblast growth factor activity. IL-1α is involved in the inflammatory response (32, 89). IL-1α is increased in the dentinal fluid of irreversible pulpitis compared to normal pulp, however, there is not significant difference compared to reversible pulpitis (28).
Interleukin-1β
IL-1β is a proinflammatory cytokine produced by activated macrophages. It induces neutrophil influx and activation, T-cell activation and cytokine production, B-cell activation and antibody production. It also plays a role in angiogenesis, working synergistically with TNF and IL-6 (90). IL-1β was found to increase in inflamed pulp tissue in analyses using ELISA (30), and RT-qPCR (39). IL-1β is increased in the dentinal fluid of irreversible pulpitis compared to normal pulp, however, it is not significantly different to reversible pulpitis (28).
Interleukin-6
IL-6 is a cytokine synthesized mainly by macrophages and monocytes at sites of inflammation, which contributes to host defense in the acute phase immune response. IL-6 induces acute phase proteins such as C-reactive protein and serum amyloid A (91). It also regulates acquired immune response such as T cell development (92). Although several articles have shown IL-6 is upregulated in the tissues of irreversible pulpitis (32, 43), upregulation of IL-6 level in GCF in irreversible pulpitis has not been demonstrated. IL-6 is upregulated in dentinal fluid of pulpitis, however, there is not significantly different between reversible and irreversible pulpitis (28).
Tissue Inhibitor Matrix Metalloproteinase 1
TIMP1 is a tissue inhibitor of metalloproteinase, playing a crucial role in ECM composition and wound healing (93). It also functions as a growth factor that regulates cell differentiation, migration, and apoptosis. After periapical lesion induction, the TIMP-1 mRNAs are expressed coordinately with mRNA of MMP-1, IL-6, and cyclooxygenase-2 (COX-2) (94). TIMP-1 is strongly detected in the dentinal tubuli of carious teeth, in contrast to sound teeth (95). TIMP1 is increased in the dentinal fluid of irreversible pulpitis compared to normal pulp, however, the intensity is not significantly different to reversible pulpitis (28).
Tumor Necrosis Factor Alpha
TNFα is a proinflammatory cytokine produced by a variety of cell types, such as macrophages, T lymphocytes, and natural killer cells during inflammation (96). It causes a disruption of the macrovascular and microvascular circulation, and also, stimulates apoptosis and acute inflammation (97, 98). Several studies have shown that TNFα is highly expressed in inflamed dental pulp tissue (36–38). In addition, TNFα is increased in the dentinal fluid of reversible and irreversible pulpitis (28).
Fibroblast Growth Factor 1
Fibroblast growth factor-1 plays a vital role in cell survival, cell division, angiogenesis, cell differentiation migration. It was reported to be involved in odontoblast differentiation together with TGFβ (99). FGF1 significantly increased in the dentinal fluid of irreversible pulpitis compared to reversible pulpitis or normal pulp (28). When FGF1, IL-1α, IL-6, and TIMP-1 biomarkers are combined, significant improvement of discrimination in the detection of IP versus RP diagnosis was shown (28).
Vascular Endothelial Growth Factor A
Vascular endothelial growth factor A induces angiogenesis, vasculogenesis, and endothelial cell growth, primarily through its interactions with the VEGFR1 and -R2 receptors on endothelial cells. It enhances the chemotaxis of cells to the inflamed site (89). It is an essential component also for dental pulp repair in response to damage (100). VEGF-A/VEGFR2 axis promoted the migration of hDPSCs via the FAK/PI3K/Akt and p38 MAPK signaling pathways (101). With a multiplex assay of dentinal fluid, Brizuela and colleague found that the concentration of VEGF-A is significantly higher in the fluid from teeth with SIP, compared to that from normal teeth or theeth with reversible pulpitis (28). This finding indicated the possibility of distinguishing irreversible pulpitis and reversible pulpitis.
Prospects For Non-invasive Pulp Diagnosis Using Biomarkers
Currently, GCF is the single source for biomarkers to non-invasively diagnose pulpitis. Since the contents of GCF reflect the condition of surrounding tissues including gingiva, periodontal ligament (PDL) and alveolar bone (102), periodontitis is a major cause of inflammatory biomarker contamination into the GCF (103). Several approaches to eliminate this ‘bias' have been proposed (14). In cases where the patients have periodontal inflammation, clinicians could: (i) average the values taken from several sites on one or multiple teeth, (ii) combine biomarker data with radiographic observations, or (iii) define a specific pattern of metabolites relevant to the pulp and not the periodontium. The third approach is theoretical at present because no such metabolites have been detected in the GCF, however, there is a set of reports predicting the feasibility of such a method. In teeth with caries, pulp cells were found to upregulate the expression of CARD18/ICEBERG, a caspase recruitment domain family member 18 gene, more than 10-fold that of normal pulp (104). In contrast, a group of genes regulating the inflammasome, including CARD18/ICEBERG, is downregulated in the gingival tissue cells from periodontal disease patients (105). These observations show an example of differential gene expression patterns between dental pulp and gingival tissue in inflammatory conditions. Although these gene products have not been detected in GCF, the development of protein-detection technology, and obviously, comprehensive gene profiling of pulp and gingival tissue in inflammatory condition, would make it possible to identify pulpitis-specific proteins in GCF.
Conclusion
In this mini review, we outlined the potential candidates for biomarkers in the context of pulpal diagnostics by non-invasive sampling methods. The most promising candidates are MMP-8, IL-8, Substance P, and Neurokinin A, owing to their presence in GCF and upregulation in SIP patients. The importance of identifying such biomarkers is obvious given the increasing need for vital pulp therapy, however, those candidates have also been reported to increase in GCF from periodontitis patient. In the future, comprehensive gene profiling of pulp and gingival tissue in inflammatory conditions and the development of protein detection technologies may aid in the identification of pulpitis-specific proteins in the GCF.
Author Contributions
BK and YK searched the literature, wrote the manuscript, and contributed to the tables. CC provided guidance, contributed to the table, and edited the manuscript. ES planned, provided guidance, wrote sections, contributed to the table, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
ES work contained within this mini review was supported by National Institutes of Dental and Craniofacial Research Grant R01-DE025885 (to ES).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mejàre IA, Axelsson S, Davidson T, Frisk F, Hakeberg M, Kvist T, et al. Diagnosis of the condition of the dental pulp: a systematic review. Int Endodontic J. (2012) 45:597–613. doi: 10.1111/j.1365-2591.2012.02016.x
2. Weisleder R, Yamauchi S, Caplan DJ, Trope M, Teixeira FB. The validity of pulp testing: a clinical study. J Am Dental Assoc. (2009) 140:1013–7. doi: 10.14219/jada.archive.2009.0312
3. Cuthrie TJ, McDonald RE, Mitchell DF. Dental pulp hemogram. J Dental Res. (1965) 44:678–82. doi: 10.1177/00220345650440041301
4. Dummer PM, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J. (1980) 13:27–35. doi: 10.1111/j.1365-2591.1980.tb00834.x
5. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. (1963) 16:969–77. doi: 10.1016/0030-4220(63)90201-9
6. Chen M, Zeng J, Yang Y, Wu B. Diagnostic biomarker candidates for pulpitis revealed by bioinformatics analysis of merged microarray gene expression datasets. BMC Oral Health. (2020) 20:279. doi: 10.1186/s12903-020-01266-5
7. Park SH, Ye L, Love RM, Farges JC, Yumoto H. Inflammation of the dental pulp. Mediators Inflamm. (2015) 2015:980196. doi: 10.1155/2015/980196
8. Goldberg M, Njeh A, Uzunoglu E. Is pulp inflammation a prerequisite for pulp healing and regeneration? Mediators Inflamm. (2015) 2015:347649. doi: 10.1155/2015/347649
10. Cooper PR, McLachlan JL, Simon S, Graham LW, Smith AJ. Mediators of inflammation and regeneration. Adv Dent Res. (2011) 23:290–5. doi: 10.1177/0022034511405389
11. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. (1963) 16:846–71. doi: 10.1016/0030-4220(63)90323-2
12. Ricucci D, Loghin S, Siqueira JF Jr. Correlation between clinical and histologic pulp diagnoses. J Endodontics. (2014) 40:1932–9. doi: 10.1016/j.joen.2014.08.010
13. Trowbridge HO. Pathogenesis of pulpitis resulting from dental caries. J Endodontics. (1981) 7:52–60. doi: 10.1016/S0099-2399(81)80242-7
14. Rechenberg DK, Galicia JC, Peters OA. Biological markers for pulpal inflammation: a systematic review. PLoS ONE. (2016) 11:e0167289. doi: 10.1371/journal.pone.0167289
15. Mainkar A, Kim SG. Diagnostic accuracy of 5 dental pulp tests: a systematic review and meta-analysis. J Endodontics. (2018) 44:694–702. doi: 10.1016/j.joen.2018.01.021
16. Michaelson PL, Holland GR. Is pulpitis painful? Int Endodontic J. (2002) 35:829–32. doi: 10.1046/j.1365-2591.2002.00579.x
17. Sultan M, Sadatullah S, Shaik M. Have biomarkers made their mark? A brief review of dental biomarkers. J Dental Res Rev. (2014) 1:37–41. doi: 10.4103/2348-3172.126167
18. Kim S-H, Lee S-Y, Lee Y-M, Lee Y-K. MicroRNAs as biomarkers for dental diseases. Singapore Dental J. (2015) 36:18–22. doi: 10.1016/j.sdj.2015.09.001
19. Hemadi AS, Huang R, Zhou Y, Zou J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci. (2017) 9:e1. doi: 10.1038/ijos.2017.35
20. Zwiri A, Al-Hatamleh MAI, W Ahmad WMA, Ahmed Asif J, Khoo SP, Husein A, et al. Biomarkers for temporomandibular disorders: current status and future directions. Diagnostics. (2020) 10:303. doi: 10.3390/diagnostics10050303
21. Tsuchida S. Proteome analysis of molecular events in oral pathogenesis and virus: a review with a particular focus on periodontitis. Int J Mol Sci. (2020) 21(15):5184. doi: 10.3390/ijms21155184
22. Rizal MI, Soeroso Y, Sulijaya B, Assiddiq BF, Bachtiar EW, Bachtiar BM. Proteomics approach for biomarkers and diagnosis of periodontitis: systematic review. Heliyon. (2020) 6:e04022. doi: 10.1016/j.heliyon.2020.e04022
23. González-Ramírez J, Serafín-Higuera N, Mancilla MC, Martínez-Coronilla G, Famanía-Bustamante J, López AL. Use of Biomarkers for the Diagnosis of Periodontitis, Periodontal Disease - Diagnostic and Adjunctive Non-surgical Considerations, Nermin Mohammed Ahmed Yussif. London: IntechOpen (2019). doi: 10.5772/intechopen.85394
24. Bagherian A, Jafarzadeh A, Rezaeian M, Ahmadi S, Rezaity MT. Comparison of the salivary immunoglobulin concentration levels between children with early childhood caries and caries-free children. Iranian J Immunol. (2008) 5:217–21.
25. Jurczak A, Kościelniak D, Papiez M, Vyhouskaya P, Krzyściak W. A study on β-defensin-2 and histatin-5 as a diagnostic marker of early childhood caries progression. Biol Res. (2015) 48:61. doi: 10.1186/s40659-015-0050-7
26. Cervino G, Fiorillo L, Herford AS, Romeo U, Bianchi A, Crimi S, et al. Molecular biomarkers related to oral carcinoma: clinical trial outcome evaluation in a literature review. Dis Markers. (2019) 2019:8040361. doi: 10.1155/2019/8040361
27. Zehnder M, Wegehaupt FJ, Attin T. A first study on the usefulness of matrix metalloproteinase 9 from dentinal fluid to indicate pulp inflammation. J Endodontics. (2011) 37:17–20. doi: 10.1016/j.joen.2010.10.003
28. Brizuela C, Meza G, Mercadé M, Inostroza C, Chaparro A, Bravo I, et al. Inflammatory biomarkers in dentinal fluid as an approach to molecular diagnostics in pulpitis. Int Endodontic J. (2020) 53:1181–91. doi: 10.1111/iej.13343
29. Huang GT, Potente AP, Kim JW, Chugal N, Zhang X. Increased interleukin-8 expression in inflamed human dental pulps. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics. (1999) 88:214–20. doi: 10.1016/S1079-2104(99)70118-6
30. Silva AC, Faria MR, Fontes A, Campos MS, Cavalcanti BN. Interleukin-1 beta and interleukin-8 in healthy and inflamed dental pulps. J Appl Oral Sci. (2009) 17:527–32. doi: 10.1590/S1678-77572009000500031
31. Abd-Elmeguid A, Abdeldayem M, Kline LW, Moqbel R, Vliagoftis H, Yu DC. Osteocalcin expression in pulp inflammation. J Endodontics. (2013) 39:865–72. doi: 10.1016/j.joen.2012.12.035
32. Zehnder M, Delaleu N, Du Y, Bickel M. Cytokine gene expression–part of host defence in pulpitis. Cytokine. (2003) 22:84–8. doi: 10.1016/S1043-4666(03)00116-9
33. Karapanou V, Kempuraj D, Theoharides TC. Interleukin-8 is increased in gingival crevicular fluid from patients with acute pulpitis. J Endodontics. (2008) 34:148–51. doi: 10.1016/j.joen.2007.10.022
34. Elsalhy M, Azizieh F, Raghupathy R. Cytokines as diagnostic markers of pulpal inflammation. Int Endodontic J. (2013) 46:573–80. doi: 10.1111/iej.12030
35. Akbal Dincer G, Erdemir A, Kisa U. Comparison of neurokinin a, substance p, interleukin 8, and matrix metalloproteinase-8 changes in pulp tissue and gingival crevicular fluid samples of healthy and symptomatic irreversible pulpitis teeth. J Endodontics. (2020) 46:1428–37. doi: 10.1016/j.joen.2020.07.013
36. Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res. (2002) 33:482–4. doi: 10.1016/S0188-4409(02)00396-X
37. Kokkas AB, Goulas A, Varsamidis K, Mirtsou V, Tziafas D. Irreversible but not reversible pulpitis is associated with up-regulation of tumour necrosis factor-alpha gene expression in human pulp. Int Endodontic J. (2007) 40:198–203. doi: 10.1111/j.1365-2591.2007.01215.x
38. Keller JF, Carrouel F, Staquet MJ, Kufer TA, Baudouin C, Msika P, et al. Expression of NOD2 is increased in inflamed human dental pulps and lipoteichoic acid-stimulated odontoblast-like cells. Innate Immun. (2011) 17:29–34. doi: 10.1177/1753425909348527
39. Paris S, Wolgin M, Kielbassa AM, Pries A, Zakrzewicz A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. J Endodontics. (2009) 35:520–3. doi: 10.1016/j.joen.2008.12.015
40. Gusman H, Santana RB, Zehnder M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. Euro J Oral Sci. (2002) 110:353–7. doi: 10.1034/j.1600-0722.2002.21347.x
41. Tsai CH, Chen YJ, Huang FM, Su YF, Chang YC. The upregulation of matrix metalloproteinase-9 in inflamed human dental pulps. J Endodontics. (2005) 31:860–2. doi: 10.1097/01.don.0000164851.55389.4e
42. Suwanchai A, Theerapiboon U, Chattipakorn N, Chattipakorn SC. NaV 1.8, but not NaV 1.9, is upregulated in the inflamed dental pulp tissue of human primary teeth. Int Endodontic J. (2012) 45:372–8. doi: 10.1111/j.1365-2591.2011.01986.x
43. Barkhordar RA, Hayashi C, Hussain MZ. Detection of interleukin-6 in human dental pulp and periapical lesions. Endodontics Dental Traumatol. (1999) 15:26–7. doi: 10.1111/j.1600-9657.1999.tb00744.x
44. Awawdeh L, Lundy FT, Shaw C, Lamey PJ, Linden GJ, Kennedy JG. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endodontic J. (2002) 35:30–6. doi: 10.1046/j.1365-2591.2002.00451.x
45. Bowles WR, Withrow JC, Lepinski AM, Hargreaves KM. Tissue levels of immunoreactive substance P are increased in patients with irreversible pulpitis. J Endodontics. (2003) 29:265–7. doi: 10.1097/00004770-200304000-00009
46. Caviedes-Bucheli J, Lombana N, Azuero-Holguín MM, Munoz HR. Quantification of neuropeptides (calcitonin gene-related peptide, substance P, neurokinin A, neuropeptide Y and vasoactive intestinal polypeptide) expressed in healthy and inflamed human dental pulp. Int Endodontic J. (2006) 39:394–400. doi: 10.1111/j.1365-2591.2006.01093.x
47. Kangarlou Haghighi A, Nafarzadeh S, Shantiaee Y, Naseri M, Ahangari Z. Relation between pulpal neuropeptides and dental caries. Iranian Endodontic J. (2010) 5:113–6.
48. Heidari A, Shahrabi M, Shahrabi MS, Ghandehari M, Rahbar P. Comparison of the level of substance P and neurokinin A in gingival crevicular fluid of sound and symptomatic carious primary teeth by ELISA. J Dentistry. (2017) 14:173–9. doi: 10.4103/1735-3327.201140
49. Caviedes-Bucheli J, Camargo-Beltrán C, Gómez-la-Rotta AM, Moreno SC, Abello GC, González-Escobar JM. Expression of calcitonin gene-related peptide (CGRP) in irreversible acute pulpitis. J Endodontics. (2004) 30:201–4. doi: 10.1097/00004770-200404000-00004
50. Shin SJ, Lee JI, Baek SH, Lim SS. Tissue levels of matrix metalloproteinases in pulps and periapical lesions. J Endodontics. (2002) 28:313–5. doi: 10.1097/00004770-200204000-00013
51. Accorsi-Mendonça T, Silva EJ, Marcaccini AM, Gerlach RF, Duarte KM, Pardo AP, et al. Evaluation of gelatinases, tissue inhibitor of matrix metalloproteinase-2, and myeloperoxidase protein in healthy and inflamed human dental pulp tissue. J Endodontics. (2013) 39:879–82. doi: 10.1016/j.joen.2012.11.011
52. Aguirre-López EC, Patiño-Marín N, Martínez-Castañón GA, Medina-Solís CE, Castillo-Silva BE, Cepeda-Argüelles O, et al. Levels of matrix metalloproteinase-8 and cold test in reversible and irreversible pulpitis. Medicine. (2020) 99:e23782. doi: 10.1097/MD.0000000000023782
53. Sattari M, Haghighi AK, Tamijani HD. The relationship of pulp polyp with the presence and concentration of immunoglobulin E, histamine, interleukin-4 and interleukin-12. Austral Endodontic J. (2009) 35:164–8. doi: 10.1111/j.1747-4477.2009.00160.x
54. Cohen JS, Reader A, Fertel R, Beck M, Meyers WJ. A radioimmunoassay determination of the concentrations of prostaglandins E2 and F2alpha in painful and asymptomatic human dental pulps. J Endodontics. (1985) 11:330–5. doi: 10.1016/S0099-2399(85)80039-X
55. Nakanishi T, Matsuo T, Ebisu S. Quantitative analysis of immunoglobulins and inflammatory factors in human pulpal blood from exposed pulps. J Endodontics. (1995) 21:131–6. doi: 10.1016/S0099-2399(06)80438-3
56. Esposito P, Varvara G, Caputi S, Perinetti G. Catalase activity in human healthy and inflamed dental pulps. Int Endodontic J. (2003) 36:599–603. doi: 10.1046/j.1365-2591.2003.00692.x
57. Esposito P, Varvara G, Murmura G, Terlizzi A, Caputi S. Ability of healthy and inflamed human dental pulp to reduce hydrogen peroxide. Euro J Oral Sci. (2003) 111:454–6. doi: 10.1034/j.1600-0722.2003.00062.x
58. Spoto G, Ferrante M, D'Intino M, Rega L, Dolci M, Trentini P, et al. Cyclic GMP phosphodiesterase activity role in normal and inflamed human dental pulp. Int J Immunopathol Pharmacol. (2004) 17(3 Suppl.):21–4.
59. Spoto G, Menna V, Serra E, Santoleri F, Perfetti G, Ciavarelli L, et al. Cyclic AMP phosphodiesterase activity in normal and inflamed human dental pulp. Int J Immunopathol Pharmacol. (2004) 17(3 Suppl.):11–5.
60. Lepinski AM, Hargreaves KM, Goodis HE, Bowles WR. Bradykinin levels in dental pulp by microdialysis. J Endodontics. (2000) 26:744–7. doi: 10.1097/00004770-200012000-00020
61. Bödör C, Matolcsy A, Bernáth M. Elevated expression of Cu, Zn-SOD and Mn-SOD mRNA in inflamed dental pulp tissue. Int Endodontic J. (2007) 40:128–32. doi: 10.1111/j.1365-2591.2006.01196.x
62. Adachi T, Nakanishi T, Yumoto H, Hirao K, Takahashi K, Mukai K, et al. Caries-related bacteria and cytokines induce CXCL10 in dental pulp. J Dental Res. (2007) 86:1217–22. doi: 10.1177/154405910708601215
63. Jiang HW, Ling JQ, Gong QM. The expression of stromal cell-derived factor 1 (SDF-1) in inflamed human dental pulp. J Endodontics. (2008) 34:1351–4. doi: 10.1016/j.joen.2008.07.023
64. Di Nardo Di Maio F, Lohinai Z, D'Arcangelo C, De Fazio PE, Speranza L, De Lutiis MA, et al. Nitric oxide synthase in healthy and inflamed human dental pulp. J Dental Res. (2004) 83:312–6. doi: 10.1177/154405910408300408
65. Dong Y, Lan W, Wu W, Huang Z, Zhao J, Peng L, et al. Increased expression of EphA7 in inflamed human dental pulp. J Endodontics. (2013) 39:223–7. doi: 10.1016/j.joen.2012.11.020
66. Rauschenberger CR, Bailey JC, Cootauco CJ. Detection of human IL-2 in normal and inflamed dental pulps. J Endodontics. (1997) 23:366–70. doi: 10.1016/S0099-2399(97)80184-7
67. Caviedes-Bucheli J, Gutierrez-Guerra JE, Salazar F, Pichardo D, Moreno GC, Munoz HR. Substance P receptor expression in healthy and inflamed human pulp tissue. Int Endodontic J. (2007) 40:106–11. doi: 10.1111/j.1365-2591.2006.01189.x
68. Tancharoen S, Tengrungsun T, Suddhasthira T, Kikuchi K, Vechvongvan N, Tokuda M, et al. Overexpression of receptor for advanced glycation end products and high-mobility group box 1 in human dental pulp inflammation. Mediators Inflamm. (2014) 2014:754069. doi: 10.1155/2014/754069
69. Huang FM, Tsai CH, Chen YJ, Liu CM, Chou MY, Chang YC. Upregulation of tissue-type plasminogen activator in inflamed human dental pulps. Int Endodontic J. (2005) 38:328–33. doi: 10.1111/j.1365-2591.2005.00951.x
70. Aruna G. Estimation of N-terminal telopeptides of type I collagen in periodontal health, disease and after nonsurgical periodontal therapy in gingival crevicular fluid: a clinico-biochemical study. Indian J Dental Res. (2015) 26:152–7. doi: 10.4103/0970-9290.159145
71. Thirkettle S, Decock J, Arnold H, Pennington CJ, Jaworski DM, Edwards DR. Matrix metalloproteinase 8 (collagenase 2) induces the expression of interleukins 6 and 8 in breast cancer cells. J Biol Chem. (2013) 288:16282–94. doi: 10.1074/jbc.M113.464230
72. Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. (2007) 52:121–7. doi: 10.1016/j.archoralbio.2006.08.009
73. Wahlgren J, Salo T, Teronen O, Luoto H, Sorsa T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) in pulpal and periapical inflammation and periapical root-canal exudates. Int Endodontic J. (2002) 35:897–904. doi: 10.1046/j.1365-2591.2002.00587.x
74. Shin SJ, Lee W, Lee JI, Baek SH, Kum KY, Shon WJ, et al. Matrix metalloproteinase-8 and substance P levels in gingival crevicular fluid during endodontic treatment of painful, nonvital teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics. (2011) 112:548–54. doi: 10.1016/j.tripleo.2011.04.026
75. Hernández M, Gamonal J, Tervahartiala T, Mäntylä P, Rivera O, Dezerega A, et al. Associations between matrix metalloproteinase-8 and−14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: a longitudinal study. J Periodontol. (2010) 81:1644–52. doi: 10.1902/jop.2010.100196
76. Leppilahti JM, Hernández-Ríos PA, Gamonal JA, Tervahartiala T, Brignardello-Petersen R, Mantyla P, et al. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J Clin Periodontol. (2014) 41:348–56. doi: 10.1111/jcpe.12223
77. Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. (1992) 307:97–101. doi: 10.1016/0014-5793(92)80909-Z
78. Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. (1993) 64(5 Suppl.):456–60.
79. Konopka L, Pietrzak A, Brzezińska-Błaszczyk E. Effect of scaling and root planing on interleukin-1β, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. J Periodontal Res. (2012) 47:681–8. doi: 10.1111/j.1600-0765.2012.01480.x
80. Burcher E, Shang F, Warner FJ, Du Q, Lubowski DZ, King DW, et al. Tachykinin NK2 receptor and functional mechanisms in human colon: changes with indomethacin and in diverticular disease and ulcerative colitis. J Pharmacol Exp Ther. (2008) 324:170–8. doi: 10.1124/jpet.107.130385
81. O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. (2004) 201:167–80. doi: 10.1002/jcp.20061
82. Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. (2008) 294:C1586–96. doi: 10.1152/ajpcell.00129.2008
83. Linden GJ, McKinnell J, Shaw C, Lundy FT. Substance P and neurokinin A in gingival crevicular fluid in periodontal health and disease. J Clin Periodontol. (1997) 24:799–803. doi: 10.1111/j.1600-051X.1997.tb01192.x
84. Sun J, Ramnath RD, Tamizhselvi R, Bhatia M. Neurokinin A engages neurokinin-1 receptor to induce NF-kappaB-dependent gene expression in murine macrophages: implications of ERK1/2 and PI 3-kinase/Akt pathways. Am J Physiol Cell Physiol. (2008) 295:C679–91. doi: 10.1152/ajpcell.00042.2008
85. Lundy FT, Mullally BH, Burden DJ, Lamey PJ, Shaw C, Linden GJ. Changes in substance P and neurokinin A in gingival crevicular fluid in response to periodontal treatment. J Clin Periodontol. (2000) 27:526–30. doi: 10.1034/j.1600-051x.2000.027007526.x
86. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology. (2013) 28:391–403. doi: 10.1152/physiol.00029.2013
87. Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Euro J Biochem. (1991) 198:391–8. doi: 10.1111/j.1432-1033.1991.tb16027.x
88. Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, et al. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. (2001) 103:2181–7. doi: 10.1161/01.CIR.103.17.2181
89. Hahn CL, Liewehr FR. Innate immune responses of the dental pulp to caries. J Endodontics. (2007) 33:643–51. doi: 10.1016/j.joen.2007.01.001
90. Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheumatism. (2003) 48:1521–9. doi: 10.1002/art.11143
91. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. (1990) 265:621–36. doi: 10.1042/bj2650621
92. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
93. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. (2000) 1477:267–83. doi: 10.1016/S0167-4838(99)00279-4
94. Lin SK, Kok SH, Kuo MY, Wang TJ, Wang JT, Yeh FT, et al. Sequential expressions of MMP-1, TIMP-1, IL-6, and COX-2 genes in induced periapical lesions in rats. Euro J Oral Sci. (2002) 110:246–53. doi: 10.1034/j.1600-0447.2002.11227.x
95. Okamoto M, Takahashi Y, Komichi S, Ali M, Watanabe M, Hayashi M. Effect of tissue inhibitor of metalloprotease 1 on human pulp cells in vitro and rat pulp tissue in vivo. Int Endodontic J. (2019) 52:1051–62. doi: 10.1111/iej.13099
96. Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryotic Gene Expression. (2010) 20:87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.10
97. Yang G, Shao GF. Elevated serum IL-11, TNF α, and VEGF expressions contribute to the pathophysiology of hypertensive intracerebral hemorrhage (HICH). Neurol Sci. (2016) 37:1253–9. doi: 10.1007/s10072-016-2576-z
98. Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. Role of TNF-alpha in vascular dysfunction. Clin Sci. (2009) 116:219–30. doi: 10.1042/CS20080196
99. Unda FJ, Martín A, Hilario E, Bègue-Kirn C, Ruch JV, Aréchaga J. Dissection of the odontoblast differentiation process in vitro by a combination of FGF1, FGF2, and TGFbeta1. Dev Dyn. (2000) 218:480–9. doi: 10.1002/1097-0177(200007)218:3<480::AID-DVDY1011>3.0.CO;2-O
100. Mullane EM, Dong Z, Sedgley CM, Hu JC, Botero TM, Holland GR, et al. Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. J Dental Res. (2008) 87:1144–8. doi: 10.1177/154405910808701204
101. Sun X, Meng L, Qiao W, Yang R, Gao Q, Peng Y, et al. Vascular endothelial growth factor A/Vascular endothelial growth factor receptor 2 axis promotes human dental pulp stem cell migration via the FAK/PI3K/Akt and p38 MAPK signalling pathways. Int Endodontic J. (2019) 52:1691–703. doi: 10.1111/iej.13179
102. Khurshid Z, Mali M, Naseem M, Najeeb S, Zafar MS. Human gingival crevicular fluids (GCF) proteomics: an overview. Dentistry journal. (2017) 5(1):12. doi: 10.3390/dj5010012
103. Fatima T, Khurshid Z, Rehman A, Imran E, Srivastava KC, Shrivastava D. Gingival crevicular fluid (GCF): a diagnostic tool for the detection of periodontal health and diseases. Molecules. (2021) 26(5):1208. doi: 10.3390/molecules26051208
104. Horst OV, Horst JA, Samudrala R, Dale BA. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. (2011) 12:9. doi: 10.1186/1471-2172-12-9
Keywords: vital pulp therapy, pulp diagnosis, pulp inflammation, non-invasive method, biomarkers, gingival crevicular fluid
Citation: Kaur B, Kobayashi Y, Cugini C and Shimizu E (2021) A Mini Review: The Potential Biomarkers for Non-invasive Diagnosis of Pulpal Inflammation. Front. Dent. Med. 2:718445. doi: 10.3389/fdmed.2021.718445
Received: 31 May 2021; Accepted: 01 December 2021;
Published: 20 December 2021.
Edited by:
Bruno Cavalcanti, University of Michigan, United StatesReviewed by:
Vivek Aggarwal, Jamia Millia Islamia, IndiaLama Adel Awawdeh, Jordan University of Science and Technology, Jordan
Copyright © 2021 Kaur, Kobayashi, Cugini and Shimizu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emi Shimizu, shimize1@sdm.rutgers.edu
†These authors have contributed equally to this work
 Brahmleen Kaur
Brahmleen Kaur Yoshifumi Kobayashi
Yoshifumi Kobayashi Carla Cugini
Carla Cugini Emi Shimizu
Emi Shimizu