Frozen elephant trunk versus conventional proximal repair of acute aortic dissection type I
- Department of Cardiovascular Surgery, Robert-Bosch-Hospital, Stuttgart, Germany
Objective: The extent of surgery and the role of the frozen elephant trunk (FET) for surgical repair of acute aortic dissection type I are still subjects of debate. The aim of the study is to evaluate the short- and long-term results of acute surgical repair of aortic dissection type I using the FET compared to standard proximal aortic repair.
Methods: Between October 2009 and December 2016, 172 patients underwent emergent surgery for acute type I aortic dissection at our center. Of these, n = 72 received a FET procedure, while the other 100 patients received a conventional proximal aortic repair. Results were compared between the two surgery groups. The primary endpoints included 30-day rates of mortality and neurologic deficit and follow-up rates of mortality and aortic-related reintervention.
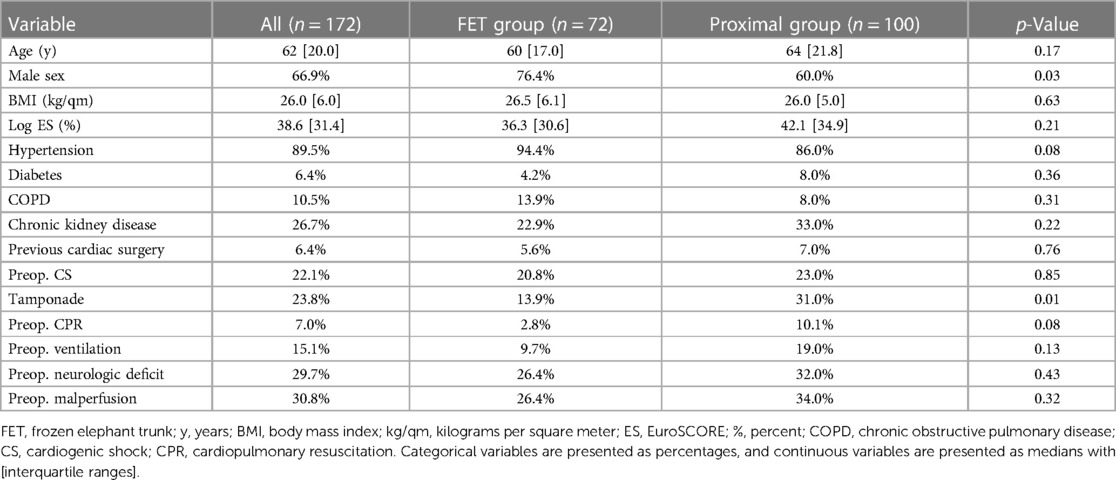
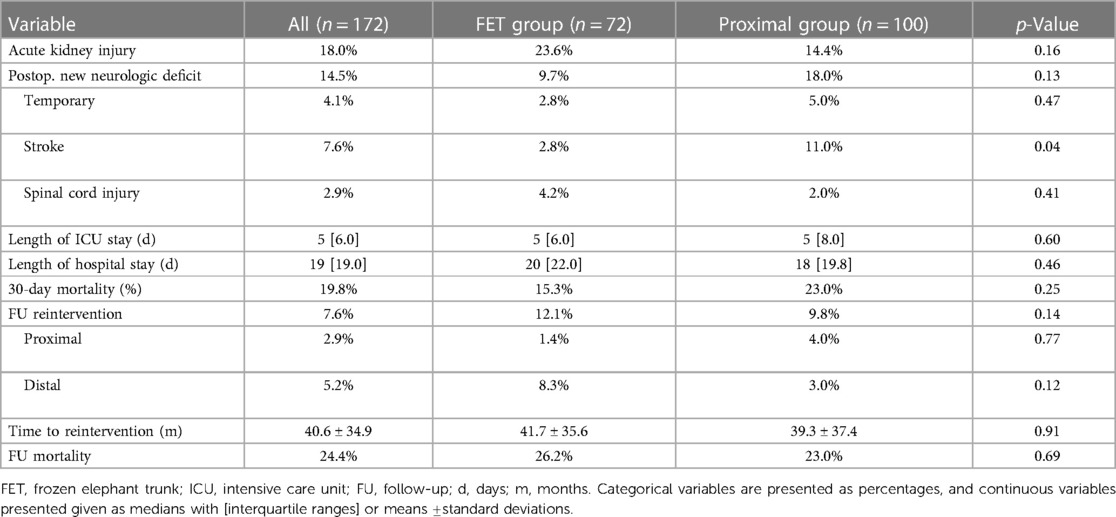
Results: Demographic data were comparable between the groups, except for a higher proportion of men in the FET group (76.4% vs. 60.0%, p = 0.03). The median age was 62 years [IQR (20), p = 0.17], and the median log EuroSCORE was 38.6% [IQR (31.4), p = 0.21]. The mean follow-up time was 68.3 ± 33.8 months. Neither early (FET group 15.3% vs. proximal group 23.0%, p = 0.25) nor late (FET group 26.2% vs. proximal group 23.0%, p = 0.69) mortality showed significant differences between the groups. There were fewer strokes in the FET patients (FET group 2.8% vs. proximal group 11.0%, p = 0.04), and the rates of spinal cord injury were similar between the groups (FET group 4.2% vs. proximal group 2.0%, p = 0.41). Aortic-related reintervention rates did not differ between the groups (FET group 12.1% vs. proximal group 9.8%, p = 0.77).
Conclusion: Emergent FET repair for acute aortic dissection type I is safe and feasible when performed by experienced surgeons. The benefits of the FET procedure in the long term remain unclear. Prolonged follow-up data are needed.
1 Introduction
The frozen elephant trunk (FET) technique has evolved into an effective and established therapy to treat the aortic arch and proximal descending aorta in a single procedure (1). In degenerative aortic aneurysm surgery, the second-stage downstream therapy, either open or endovascular, was substantially facilitated. However, the benefits of FET implantation in the setting of acute aortic dissection are still not fully elucidated. Therefore, the role of FET in acute dissection remains a topic of controversial discussion (2). In DeBakey type I dissection, conventional proximal aortic repair usually results in residual false lumen patency in the downstream aorta, which has been shown to be associated with elevated risk of dilation, rupture, and mortality (3). The FET enables stabilization of the distal arch and proximal descending aorta and therefore promotes aortic remodeling effectively (4). Especially in patients with distal re-entries and/or malperfusion, the FET enables false lumen decompression and restoration of true lumen perfusion (5). However, FET surgery is far more complex, potentially elevating perioperative risk in an already high-risk acute situation. Obviously, long-term benefits of the FET can only apply when the patient survives the acute operation. Moreover, not every attending surgeon is similarly skilled and familiar with this complex technique and inherited pitfalls. It has been proved that FET implantation in acute dissection is safe in experienced hands (6). We therefore compared our results of the FET vs. conventional proximal arch repair in DeBakey type I acute aortic dissections in a real-world setting.
2 Methods
2.1 Patients
Interrogation of our prospectively collected dissection database revealed 172 consecutive patients with DeBakey type I acute aortic dissection who underwent emergent surgery at our center between October 2009 and December 2016. Of these, 72 patients were treated using the FET technique (FET group), whereas 100 patients received conventional proximal surgical aortic repair (proximal group). Outcomes were retrospectively compared according to the type of surgery performed.
The study was approved by the authorized ethics review committee (University of Tuebingen, No. 069/2019BO2). Informed consent was obtained from all patients.
2.2 Endpoints
Primary endpoints included 30-day rates of mortality and neurologic deficit and follow-up rates of mortality and aortic-related reintervention including reoperation. Secondary endpoints included operative times, length of hospital and ICU stay, acute kidney injury, type of reintervention (aortic root vs. distal aorta), and mean time to reintervention.
The neurologic deficit was defined as stroke or spinal cord injury as assessed by imaging and/or specialist neurologic clinical examination. Acute kidney injury was defined according to KDOQI stage 3, i.e., requiring renal replacement therapy. Aortic-related reintervention was defined as any surgery on the aortic root and/or surgery or endovascular therapy on the distal aorta due to progressive aortic dilation or rupture.
2.3 Operative details
The institutional standard for the surgery of acute aortic dissection is an all-comers approach without delay in the timing of surgery, which is performed by the attending surgeon. In short, whenever possible, the right axillary artery is our primary arterial cannulation site, and we prefer direct vessel cannulation. Femoral or direct aortic arterial cannulation is rarely performed. Cardiocirculatory arrest is induced under moderate hypothermia of 28°C, combined with bilateral antegrade cerebral perfusion. The distal anastomosis is always accomplished in an open manner under circulatory arrest to control and correct the aortic arch for secondary (re-)entries, if applicable. Every attending surgeon performs acute dissection surgery, but only a few are trained and skilled with the FET technique. If surgical expertise was available, indication for the FET implantation included entry/re-entry in the aortic arch, proximal descending aorta, distal malperfusion, young patient age, and appropriate size of the proximal descending aorta. FET was implanted in arch zone 3, according to the classification of Ishimaru (7). The supra-aortic vessels were reimplanted as islands. The E-vita Open Plus prosthesis (Jotec GmbH/Artivion, Hechingen, Germany) was used in all cases for the FET procedure. The stentgraft length was 15 cm, but only with the most recent implantations, we introduced the shorter 13 cm version. Dacron vascular prostheses (Hemashield, Getinge, Gothenburg, Sweden) were used for all other aortic replacements. Anastomoses were sutured using 3-0 or 4-0 polypropylene; the use of reinforcing felt strips varied according to the surgeons' preferences.
2.4 Follow-up
All patients underwent regular clinical and imaging follow-up on an ambulatory basis, with the first visit scheduled 3–6 months after surgery, followed by annual visits thereafter. In stable conditions, the interval between visits was extended to every 2 years.
2.5 Statistical analysis
All statistical calculations were performed using SPSS Version 26.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, New York, United States). Categorical data are reported as numbers and percentages, and continuous data are reported as means ± standard deviations or medians with interquartile ranges, as appropriate. The assumption of normal distribution was tested with the Kolmogorov–Smirnov test. A comparison of categorical data was performed with Fisher's exact test or chi-square test. Normally distributed continuous data were compared using the t-test, and while the Mann–Whitney U-test was applied if the normal distribution was not met. Kaplan–Meier estimates were used to analyze the rates of survival and reintervention during follow-up, with groups compared using the log-rank test. Values of p < 0.05 were considered statistically significant.
3 Results
Demographic data were comparable between the groups, except for a higher proportion of men in the FET group (76.4% vs. 60.0%, p = 0.03). The median age was 62 years [IQR (20), p = 0.17], and the median log EuroSCORE was 38.6% [IQR (31.4), p = 0.21]. In addition, groups were well balanced regarding the mode of preoperative presentation: overall, 6.4% had previous cardiac surgery (p = 0.76), 22.1% presented in cardiogenic shock (p = 0.85), and 29.7% already had a preoperative neurologic deficit (p = 0.43). Complete demographics are summarized in Table 1.
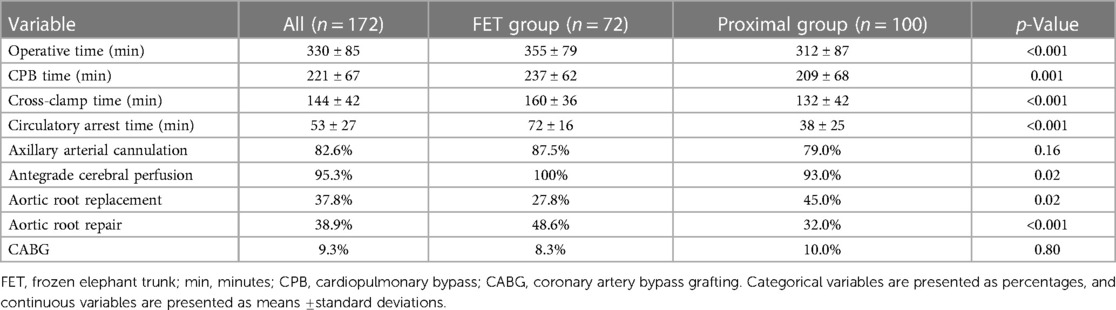
Intraoperative procedural times were all significantly longer in the FET group: operative time 355 ± 79 vs. 312 ± 87 min (p < 0.001), cardiopulmonary bypass time 237 ± 62 vs. 209 ± 68 min (p = 0.001), cross-clamp time 160 ± 36 vs. 132 ± 42 min (p < 0.001), and circulatory arrest time 72 ± 16 vs. 38 ± 25 min (p < 0.001). Application of antegrade cerebral perfusion was 100% in the FET group and 93% in the proximal group, which proved statistically significant (p = 0.02). There were significantly more aortic root repairs in the FET group (48.6% vs. 32.0%, p < 0.001). However, concomitant bypass surgeries (8.3% vs. 10.0%, p = 0.80) were distributed similarly between the groups; see Table 2 for intraoperative details.
The 30-day mortality rate was 15.3% in the FET group, which was lower than the 23.0% in the proximal group, but the difference did not reach statistical significance (p = 0.25). Overall postoperative neurologic deficit did not differ between the groups (14.5%, p = 0.13). There were significantly fewer strokes in the FET patients (2.8% vs. 11.0%, p = 0.04), and the rates of spinal cord injury were similar between the groups (FET group 4.2% vs. proximal group 2.0%, p = 0.41).
Follow-up was completed in 98.8% of cases. The mean follow-up time was 68.3 ± 33.8 months (range 1–131 months). Late mortality rates were well comparable between the groups (FET group 26.2% vs. proximal group 23.0%, p = 0.69), Figure 1. There were 13 reinterventions in follow-up, with 7 occurring in the FET group and 6 occurring in the proximal arch group; one patient required combined proximal and distal aortic repair. Overall aortic-related reintervention rates did not differ between the groups (FET group 12.1% vs. proximal group 9.8%, p = 0.14), nor did distal reintervention rates alone (FET group 8.3% vs. proximal group 3.0%, p = 0.12), Figure 2. The mean time to reintervention was 40.6 ± 34.9 (p = 0.91). All outcome data are summarized in Table 3.
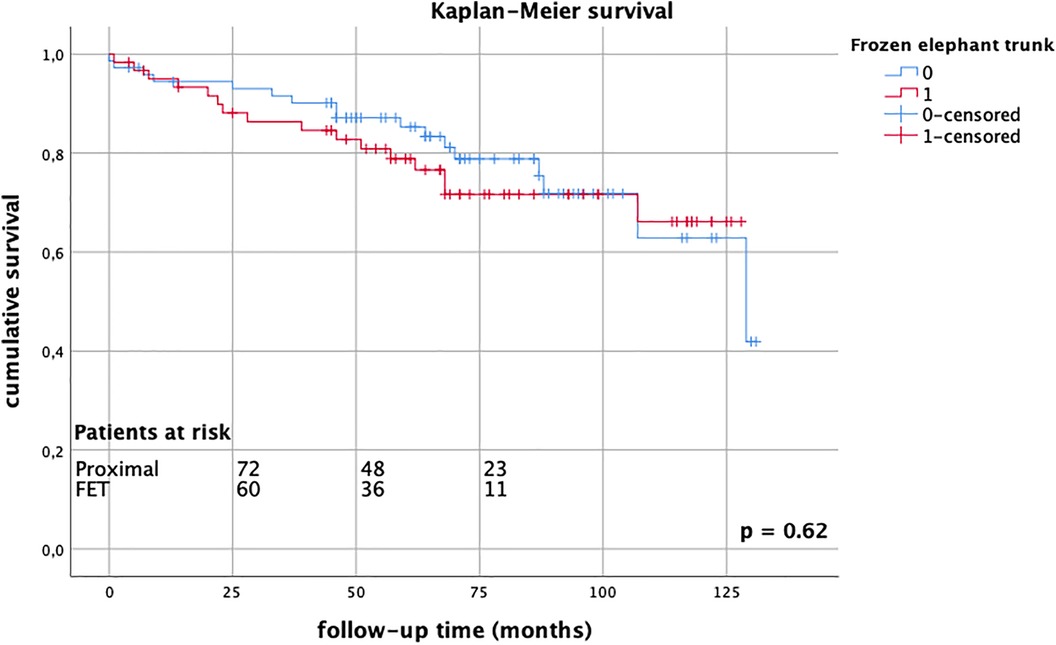
Figure 1. Kaplan–Meier survival estimates comparing FET group (red) vs. proximal group (blue) up to 10 years, without significant differences between groups, log-rank test p = 0.62.
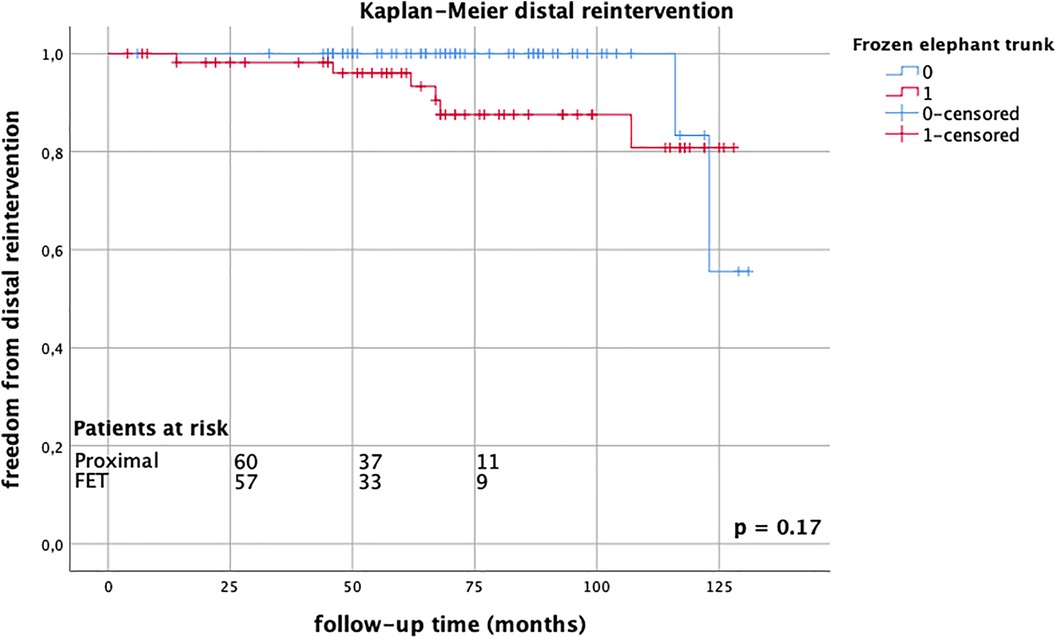
Figure 2. Kaplan–Meier estimates for distal reintervention comparing FET group (red) vs. proximal group (blue) up to 10 years, without significant differences between groups, log-rank test p = 0.17.
4 Discussion
The FET technique is increasingly used in acute aortic dissection surgery, but its role remains unclear. Our data confirm the safety and feasibility of FET implantation in acute aortic dissection but question the propagated beneficial long-term effects. At a mean follow-up time of 5.7 years, we did not observe any significant differences in survival and reintervention rates between the FET group and proximal repair-only patients.
Our 30-day mortality rates were comparable between the groups, regardless of the extent of surgery, as were the rates of postoperative new neurologic deficits. Moreover, these results are well comparable to contemporary reported outcomes: Overall 30-day mortality ranges between 16.9% in the GERAADA registry and 19.7% in the IRAD to 22% in the French registry vs. 19.2% at our center (8–12). Rates of postoperative permanent neurologic deficit range between 9.5% and 18.7% and were 10.5% in our patients (10, 11, 13–16).
Due to dismal outcomes, we discontinued FET implantation in patients after resuscitation during the study period; however, these patients represent only a small fraction of the cohort, but may explain the slightly elevated mortality rates in the proximal repair group due to selection bias, although it did not reach statistical significance. Uehara et al. proved the unfavorable prognosis of these patients and even suggested withdrawal of surgery on patients with prolonged cardiopulmonary resuscitation or without return of circulation after pericardiotomy due to the reduced chances of surviving without any neurologic deficit (17).
Of note, some proximal arch repair patients also experienced spinal cord injuries, without statistically significant differences between groups. Similar findings have been published by Poon et al. based on data from the large international ARCH registry: using propensity-score matching FET and conventional arch replacement, patients did not exhibit differences in stroke or spinal cord injury rates, emphasizing the multifactorial etiology of this devastating complication (16).
With both techniques parallelly in use, we provide a valid and appropriate comparison cohort, as all the perioperative settings and management are identical, and no unknown confounders need to be considered. Direct comparative studies reporting long-term outcomes are hardly found in the literature. Yoshitake et al. reported superior long-term survival rates with the FET technique, but reintervention rates were similar between FET and non-FET patients (18). These findings were confirmed by a recent meta-analysis comparing long-term Kaplan–Meier derived data of total arch and proximal arch repair patients: overall survival was better in the total arch group, but the risk of reoperation did not differ significantly. However, this held only true for the first 7 years after the index operation; thereafter, total arch patients had lower reoperation rates (19).
As early as 2015, Shrestha et al. already verbalized the concerns of overtaxing FET implantations in acute dissections (“are we pushing the limits too far”), emphasizing that indications have to be stated well-considered (20). A more restrictive approach seems to be reasonable in most hands and situations. A contemporary international multicenter study confirms the safety of a more limited surgical approach, showing even inferior survival rates with total arch repair and similar reoperation rates in follow-up compared to proximal arch surgery (9). Concentrating FET surgery in acute type A dissection repair to only experienced aortic centers might be a reasonable consequence (21, 22).
Neurologic complications are the most feared in dissection surgery as they seriously impact the quality of life and survival of these patients. With the FET technique, we face a dilemma: the longer the stentgraft portion with deep coverage of the descending aorta, the better the rate of aortic remodeling; however, at the same time, the risk of spinal cord injury is elevated (23). Shortening the stent-graft length and moving the proximal implantation level to arch zone 2 have nearly eliminated the devastating complication of spinal cord injury but carry the elevated risk of false lumen patency with the increased need for secondary interventions (24). Stroke rates were favorably low in our FET patients (2.8%), despite significantly longer circulatory arrest times than proximal arch repairs, which may be attributable to a strict cerebral perfusion protocol, whereas 7% of our conventional proximal group did not receive selective brain perfusion. Contemporary data report stroke rates of 2.7%–18% (21).
So far, secondary interventions have been associated with a substantial risk of mortality (14%–40%) (25, 26). The FET technique provides an excellent landing zone for further interventions, enabling endovascular therapy in most patients. In contrast, after proximal arch repair, a high percentage of patients required open surgery for distal reoperation, carrying a relevant risk of mortality (18). In the future, with further development of interventional and hybrid procedures, the second-stage intervention becomes potentially safer. Some contemporary data already demonstrate excellent outcomes of redo surgeries after limited dissection type I repair, as they can safely be performed in an elective setting at experienced centers (27, 28).
In our study, the follow-up rates of reintervention did not differ between the groups. Potentially, we are facing a paradox: while the FET easily enables distal endovascular extension, this procedure could be performed more liberally (and safer) than open surgery, which is more often needed after hemiarch repair (18); these patients may be deemed unsuitable for endovascular and inoperable for open reoperation. Moreover, FET patients adhere more strictly to follow-up visits. Therefore, more distal aortic dilations could be diagnosed in these patients compared to proximal-only patients without long-term surveillance. Accordingly, An et al. reported the paradox, wherein patients with guideline-adherent postoperative surveillance exhibit higher rates of reinterventions and mortality than those without (29). Therefore, each reintervention should be indicated wisely.
Surprisingly, the long-term risk of mortality was not affected by the extent of index surgery in this study. This is in contrast to the results of Yoshitake et al., who reported a survival benefit for FET patients (18). The explanation lies beyond follow-up time: the mean follow-up time was 68.3 months in our study but only 46.0 months in the study by Yoshitake et al. However, our patient cohort might have been too small to find significant differences. Moreover, geographical differences between Asia and Europe may play a role (30). Nevertheless, the enthusiasm about FET implantation in acute dissection has been tarnished. Further data are needed to clarify the role of the FET in acute dissection surgery.
In conclusion, the application of the FET in acute aortic dissection is as safe and feasible as conventional proximal arch repair in our study. After a mean follow-up of 5.7 years, the rates of survival and reintervention were similar between the groups, irrespective of the initial extent of surgery. A limited approach may be reasonable according to the estimated life expectancy of patients.
4.1 Limitations
Limitations of the study include its retrospective nature and the relatively small size of the single-center patient cohort.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of the medical faculty, University of Tuebingen, Tuebingen, Germany. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
NG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. SH: Data curation, Writing – review & editing. FH: Data curation, Writing – review & editing. YA: Investigation, Writing – review & editing. DB: Investigation, Resources, Supervision, Writing – review & editing. UF: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shrestha M, Bachet J, Bavaria J, Carrel TP, De Paulis R, Di Bartolomeo R, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the vascular domain of EACTS. Eur J Cardiothorac Surg. (2015) 47:759–69. doi: 10.1093/ejcts/ezv085
2. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. (2014) 35:2873–926. doi: 10.1093/eurheartj/ehu281
3. Fattouch K, Sampognaro R, Navarra E, Caruso M, Pisano C, Coppola G, et al. Long-term results after repair of type A acute aortic dissection according to false lumen patency. Ann Thorac Surg. (2009) 88:1244–50. doi: 10.1016/j.athoracsur.2009.06.055
4. Iafrancesco M, Goebel N, Mascaro J, Franke UFW, Pacini D, Di Bartolomeo R, et al. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg. (2017) 52:310–18. doi: 10.1093/ejcts/ezx131
5. Di Marco L, Pantaleo A, Leone A, Murana G, Di Bartolomeo R, Pacini D. The frozen elephant trunk technique: European Association for Cardio-Thoracic Surgery position and Bologna experience. Korean J Thorac Cardiovasc Surg. (2017) 50:1–7. doi: 10.5090/kjtcs.2017.50.1.1
6. Mousavizadeh M, Daliri M, Aljadayel HA, Mohammed I, Rezaei Y, Bashir M, et al. Hypothermic circulatory arrest time affects neurological outcomes of frozen elephant trunk for acute type A aortic dissection: a systematic review and meta-analysis. J Card Surg. (2021) 36:3337–51. doi: 10.1111/jocs.15700
7. Ishimaru S. Endografting of the aortic arch. J Endovasc Ther. (2004) 11(Suppl 2):Ii62–71. doi: 10.1177/15266028040110S609
8. Conzelmann LO, Weigang E, Mehlhorn U, Abugameh A, Hoffmann I, Blettner M, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg. (2016) 49:e44–52. doi: 10.1093/ejcts/ezv356
9. Biancari F, Juvonen T, Fiore A, Perrotti A, Hervé A, Touma J, et al. Current outcome after surgery for type A aortic dissection. Ann Surg. (2023) 278:e885–92. doi: 10.1097/SLA.0000000000005840
10. Chabry Y, Porterie J, Gautier CH, Nader J, Chaufour X, Alsac JM, et al. The frozen elephant trunk technique in an emergency: THORAFLEX French National Registry offers new insights.. Eur J Cardiothorac Surg. (2020) 59(2):458–66. doi: 10.1093/ejcts/ezaa325
11. Lee TC, Kon Z, Cheema FH, Grau-Sepulveda MV, Englum B, Kim S, et al. Contemporary management and outcomes of acute type A aortic dissection: an analysis of the STS adult cardiac surgery database. J Card Surg. (2018) 33:7–18. doi: 10.1111/jocs.13511
12. Hemli JM, Pupovac SS, Gleason TG, Sundt TM, Desai ND, Pacini D, et al. Management of acute type A aortic dissection in the elderly: an analysis from IRAD. Eur J Cardiothorac Surg. (2022) 61(4):838–46. doi: 10.1093/ejcts/ezab546
13. Chemtob RA, Fuglsang S, Geirsson A, Ahlsson A, Olsson C, Gunn J, et al. Stroke in acute type A aortic dissection: the Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD). Eur J Cardiothorac Surg. (2020) 58:1027–34. doi: 10.1093/ejcts/ezaa197
14. Conzelmann LO, Hoffmann I, Blettner M, Kallenbach K, Karck M, Dapunt O, et al. Analysis of risk factors for neurological dysfunction in patients with acute aortic dissection type A: data from the German Registry for Acute Aortic Dissection type A (GERAADA). Eur J Cardiothorac Surg. (2012) 42:557–65. doi: 10.1093/ejcts/ezs025
15. Dumfarth J, Kofler M, Stastny L, Gasser S, Plaikner M, Semsroth S, et al. Immediate surgery in acute type A dissection and neurologic dysfunction: fighting the inevitable? Ann Thorac Surg. (2020) 110:5–12. doi: 10.1016/j.athoracsur.2020.01.026
16. Poon SS, Tian DH, Yan T, Harrington D, Nawaytou O, Kuduvalli M, et al. Frozen elephant trunk does not increase incidence of paraplegia in patients with acute type A aortic dissection. J Thorac Cardiovasc Surg. (2020) 159:1189–96.e1. doi: 10.1016/j.jtcvs.2019.03.097
17. Uehara K, Matsuda H, Matsuo J, Inoue Y, Shijo T, Omura A, et al. Surgical outcomes of acute type A aortic dissection in patients undergoing cardiopulmonary resuscitation. J Thorac Cardiovasc Surg. (2021) 161:1173–80. doi: 10.1016/j.jtcvs.2019.11.135
18. Yoshitake A, Tochii M, Tokunaga C, Hayashi J, Takazawa A, Yamashita K, et al. Early and long-term results of total arch replacement with the frozen elephant trunk technique for acute type A aortic dissection. Eur J Cardiothorac Surg. (2020) 58:707–13. doi: 10.1093/ejcts/ezaa099
19. Sá MP, Jacquemyn X, Tasoudis PT, Van den Eynde J, Erten O, Sicouri S, et al. Long-term outcomes of total arch replacement versus proximal aortic replacement in acute type A aortic dissection: meta-analysis of Kaplan–Meier-derived individual patient data. J Card Surg. (2022) 37:4256–66. doi: 10.1111/jocs.16852
20. Shrestha M, Fleissner F, Ius F, Koigeldiyev N, Kaufeld T, Beckmann E, et al. Total aortic arch replacement with frozen elephant trunk in acute type A aortic dissections: are we pushing the limits too far?. Eur J Cardiothorac Surg. (2015) 47:361–6; discussion 66. doi: 10.1093/ejcts/ezu185
21. Murana G, Campanini F, Orioli V, Pagano V, Santamaria V, Di Marco L, et al. Frozen elephant trunk in acute aortic dissection: a literature review. Indian J Thorac Cardiovasc Surg. (2023) 39:315–24. doi: 10.1007/s12055-023-01624-2
22. Luthra S, Tsang GM. Concurrent stabilization of “downstream” aorta during acute type A aortic dissection repair. J Thorac Cardiovasc Surg. (2023) 165:586–88. doi: 10.1016/j.jtcvs.2021.06.042
23. Leontyev S, Borger MA, Etz CD, Moz M, Seeburger J, Bakhtiary F, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg. (2013) 44:1076–82; discussion 83. doi: 10.1093/ejcts/ezt252
24. Liebrich M, Charitos EI, Schlereth S, Meißner H, Trabold T, Geisbüsch P, et al. The zone 2 concept and distal stent graft positioning in TH 2-3 are associated with high rates of secondary aortic interventions in frozen elephant trunk surgery. Eur J Cardiothorac Surg. (2021) 60:343–51. doi: 10.1093/ejcts/ezab132
25. Kreibich M, Berger T, Rylski B, Chen Z, Beyersdorf F, Siepe M, et al. Aortic reinterventions after the frozen elephant trunk procedure. J Thorac Cardiovasc Surg. (2020) 159:392–99.e1. doi: 10.1016/j.jtcvs.2019.02.069
26. Arnold Z, Geisler D, Aschacher T, Winkler B, Lenz V, Crailsheim I, et al. Long-term results with 187 frozen elephant trunk procedures. J Clin Med. (2023) 12(12):4143. doi: 10.3390/jcm12124143
27. Ohira S, Malekan R, Kai M, Goldberg JB, Laskowski I, De La Pena C, et al. Aortic reoperation after prior acute type A aortic dissection repair: don't despair the repair. Ann Thorac Surg. (2023) 116:43–50. doi: 10.1016/j.athoracsur.2022.10.021
28. Wang H, Wagner M, Benrashid E, Keenan J, Wang A, Ranney D, et al. Outcomes of reoperation after acute type A aortic dissection: implications for index repair strategy. J Am Heart Assoc. (2017) 6(10):e006376. doi: 10.1161/JAHA.117.006376
29. An KR, de Mestral C, Tam DY, Qiu F, Ouzounian M, Lindsay TF, et al. Surveillance imaging following acute type A aortic dissection. J Am Coll Cardiol. (2021) 78:1863–71. doi: 10.1016/j.jacc.2021.08.058
Keywords: frozen elephant trunk, aortic dissection type I, aortic arch surgery, long-term outcomes, mortality, neurologic deficit, reintervention
Citation: Göbel N, Holder S, Hüther F, Anguelov Y, Bail D and Franke U (2024) Frozen elephant trunk versus conventional proximal repair of acute aortic dissection type I. Front. Cardiovasc. Med. 11:1326124. doi: 10.3389/fcvm.2024.1326124
Received: 22 October 2023; Accepted: 29 February 2024;
Published: 15 March 2024.
Edited by:
Antonio Miceli, Istituto Clinico Sant'Ambrogio, ItalyReviewed by:
Faizus Sazzad, National University of Singapore, SingaporeSuvitesh Luthra, University Hospital Southampton NHS Foundation Trust, United Kingdom
© 2024 Göbel, Holder, Hüther, Anguelov, Bail and Franke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nora Göbel nora.goebel@rbk.de
 Nora Göbel
Nora Göbel Simone Holder
Simone Holder  Ulrich Franke
Ulrich Franke