Contemporary European practice in transcatheter aortic valve implantation: results from the 2022 European TAVI Pathway Registry
- 1Department of Cardiology, Algemeen Stedelijk Hospital, Aalst, Belgium
- 2Faculteit Geneeskunde, Vrije Universiteit Brussel (VUB), Brussels, Belgium
- 3Department of Cardiology, University Hospital Galway and National University of Ireland, Galway, Ireland
- 4Centrum Voor Hart- en Vaatziekten (CHVZ), Universitair Ziekenhuis Brussel (UZ Brussel), Brussels, Belgium
- 5Department of Cardiology, AZ Delta, Roeselare, Belgium
- 6Division of Cardiology, Department of Cardiology and Intensive Care, Clinic Ottakring, Medical University of Graz, Graz, Austria
- 7Centro Hospitalar de Lisboa Ocidental (CHLO), Hospital de Santa Cruz
- 8Nova Medical School, Centro de Estudo de Doenças Crónicas (CEDOC), Lisbon, Portugal
- 9Department of Cardiology, Sahlgrenska University Hospital, Gothenberg, Sweden
- 10Department of Molecular and Clinical Medicine, Institute of Medicine, Gothenburg University, Gothenburg, Sweden
- 11Institut Cardiovasculaire Paris Sud, Ramsay Santé, Massy, France
- 12Clinic of General and Interventional Cardiology, Heart and Diabetes Center Nordrhine Westfalia, Ruhr-University, Bad Oeynhausen, Germany
- 13Department of Cardiology, University Medical Center Groningen, Groningen, Netherlands
- 14Department of Cardiology, Guys and St Thomas’ NHS Foundation Trust London, London, United Kingdom
- 15Cardiac Catheterization Division, Cardiothoracic and Vascular Department, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
- 16Division of Interventional Cardiology, Reina Sofia Hospital, Maimonides Institute for Research in Biomedicine of Córdoba (IMIBIV), University of Córdoba, Córdoba, Spain
- 17Department of Cardiology, European Interbalkan Medical Centre, Thessaloniki, Greece
- 18University Clinic of Interventional Cardiology, Nicolae Testemitanu State University of Medicine and Pharmacy from Republic of Moldova, Chişinău, Moldova
- 19Medico-Surgical Department of Valvulopathies, CHU De Bordaux, Pessac, France
- 20CHU Lille, Institut Cœur Poumon, Pôle Cardiovasculaire et Pulmonaire, ACTION Group, Inserm U1011, Institut Pasteur de Lille, EGID, Université de Lille, Lille, France
- 21Department of Interventional Cardiology, Clinique Pasteur, Toulouse, France
- 22Department of Thoracic and Cardiovascular Surgery, Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany
- 23Department of Cardiology, St. Antonius Hospital, Nieuwegein, Netherlands
- 24Department of Cardiology and Structural Heart Diseases, 3 Division of Cardiology, Medical University of Silesia, Katowice, Poland
- 25Department of Cardiology, Leeds Teaching Hospitals, Leeds, United Kingdom
- 26Department of Interventional Cardiology, Erasmus University Medical Center, Rotterdam, Netherlands
- 27Chair and 1st Department of Cardiology, Poznan University of Medical Sciences, Poznan, Poland
- 28Department of Cardiology, Heart Center OLV Aalst, Aalst, Belgium
- 29Heart Center, Rigshospitalet, Copenhagen, Denmark
Background: A steep rise in the use of transcatheter aortic valve implantation (TAVI) for the management of symptomatic severe aortic stenosis occurred. Minimalist TAVI procedures and streamlined patient pathways within experienced Heart Valve Centres are designed to overcome the challenges of ever-increasing procedural volume.
Aims: The 2022 European TAVI Pathway Survey aims to describe contemporary TAVI practice across Europe.
Materials and methods: Between October and December 2022, TAVI operators from 32 European countries were invited to complete an online questionnaire regarding their current practice.
Results: Responses were available from 147 TAVI centres in 26 countries. In 2021, the participating centres performed a total number of 27,223 TAVI procedures, with a mean of 185 TAVI cases per centre (median 138; IQR 77–194). Treatment strategies are usually (87%) discussed at a dedicated Heart Team meeting. Transfemoral TAVI is performed with local anaesthesia only (33%), with associated conscious sedation (60%), or under general anaesthesia (7%). Primary vascular access is percutaneous transfemoral (99%) with secondary radial access (52%). After uncomplicated TAVI, patients are transferred to a high-, medium-, or low-care unit in 28%, 52%, and 20% of cases, respectively. Time to discharge is day 1 (12%), day 2 (31%), day 3 (29%), or day 4 or more (28%).
Conclusion: Reported adoption of minimalist TAVI techniques is common among European TAVI centres, but rates of next-day discharge remain low. This survey highlights the significant progress made in refining TAVI treatment and pathways in recent years and identifies possible areas for further improvement.
Introduction
Transcatheter aortic valve implantation (TAVI) now has a class IA indication for the treatment of symptomatic severe aortic stenosis in patients aged 75 years or more, regardless of surgical risk (1). Since the first-in-human TAVI was performed in 2002, procedural refinement achieved through successive design iterations, improved pre-procedural planning, and optimised implant technique have resulted in improved outcomes and rapid expansion of the technique. TAVI procedures now outnumber surgical aortic valve replacement (SAVR) in many countries, and the forecast is a further increase of its application due to population ageing and expanding procedural indications (2).
To preserve safety, efficiency and patient outcomes alongside accelerating TAVI demand, optimisation of TAVI patient pathways, and minimalist TAVI procedures are essential to balance the burden on healthcare systems and resources. There are no data available to define how TAVI is currently organised and performed across Europe and whether recommendations and guidelines are incorporated into a daily practice. The 2022 European TAVI Pathway Survey was set up to provide insights as to how contemporary TAVI pathways and procedures are organised and identify potential areas of improvement that may further improve healthcare impact.
Materials and methods
Study design
The 2022 European TAVI Pathway Survey is a non-funded, international, multi-centre, observational, transverse study. A list of TAVI centres in 32 European countries was generated based upon information provided by national cardiac societies, national registries, and additional PubMed searches. TAVI operators for each centre identified through the investigators’ network and PubMed searches were invited by email to participate. The questionnaire was set up on an electronic web-based platform (SurveyMonkey) and distributed via digital link. Participation was voluntary and anonymous. The questionnaire was requested to be completed by a TAVI operator. The survey was open between 1 October and 15 December 2022, and a second wave to further improve participation was open between 1 and 10 January 2023.
The survey consisted of 35 (mostly) multiple-choice questions and was compiled based on the specific requirements for a Heart Valve Centre defined in the 2021 European guidelines on valvular heart disease (Supplementary Table S1) (1). The questionnaire (Supplementary Table S2) was categorised into four main areas: (1) patient selection, (2) pre-procedural work-up, (3) TAVI procedure, and (4) patient flow/pathway. The participants were asked to submit their answers based on current TAVI practice in their centre for “regular” elective transfemoral TAVI cases. Only surveys with ≥85% completion and including a response to the question concerning country of origin and number of TAVI procedures performed in 2021 were included in analyses.
Definitions
Results were analysed overall and according to geographic region and centre procedural volume. Six geographic regions were defined: (1) DACH [Germany (D), Austria (A), Switzerland (CH)], (2) Nordic (Denmark, Finland, Iceland, Norway, Sweden), (3) BeNeFrance (Belgium, France, Luxembourg, the Netherlands), (4) UK/IRL (United Kingdom/ Republic of Ireland), (5) Southern Europe (Cyprus, Greece, Italy, Malta, Portugal, Spain), and (6) Eastern Europe (Albania, Bulgaria, Bosnia Herzegovina, Croatia, Czech Republic, Estonia, Hungary, Kosovo, Latvia, Lithuania, Montenegro, North Macedonia, Poland, Republic of Moldova, Romania, Serbia, Slovakia, Slovenia). Centre volume was defined by the number of TAVI cases performed in 2021 and divided into five categories: (1) <50 cases, (2) 50–99 cases, (3) 100–199 cases, (4) 200–499 cases, and (5) ≥500 cases. The number of TAVI cases per operator was calculated by dividing the number of TAVI cases by the number of TAVI operators in each centre and was not adjusted per local practice (e.g., one vs. two independent TAVI operators per case).
Alternative vascular access was reported as five separate approaches, each with two sub-categories: (1) transfemoral (balloon- or lithotripsy-assisted access), (2) transaxillary (direct percutaneous or surgical cutdown), (3) transthoracic (transapical or direct aortic approach), (4) transcarotid (direct percutaneous or surgical cutdown), and (5) transvenous (transcaval or transseptal approach).
Post-procedure transfer location was classified into high-care (intensive care unit), medium-care (recovery room, cardiac care unit, mid-care unit), and low-care (cardiology ward) unit.
Statistical analysis
Descriptive statistics were used to present the data. Continuous variables are reported as mean and standard deviation (±SD) in a normal number distribution and as median and interquartile range (IQR) for skewed number distribution. Categorical variables are presented as percentages.
Enrolment
In total, 688 European TAVI centres—with corresponding contact—were identified. One hundred and fifty-six centres (23%) responded to the survey: complete responses were available for 147 (94%) centres and 9 (6%) submitted partial results. Overall completion rate of the questionnaire was 94%, with an average time of 8 min and 24 s required. An overview of enrolment per country is provided in Supplementary Table S3.
Results
Centres
The 147 participating centres in 26 different countries performed a total of 27,223 TAVI cases in 2021 (Figure 1). Three quarters of respondents work in public hospitals (76%, n = 112), 14% (n = 21) in a private setting, and 10% (n = 14) in mixed public/private practice. The average number of TAVI cases per centre was 185 (median 138; IQR 77–194), the largest centre performing 1,010 cases and the smallest 20 cases (Figure 1). In 2021, the centres with the highest TAVI volume were situated in the DACH region (median 335; IQR 219–490), followed by the Nordic region (median 220; IQR 200–350), while the regions with the lowest number of TAVI cases per centre were in Southern (median 101; IQR 70–169) and Eastern Europe (median 100; IQR 53–122) (Supplementary Table S4). The average number of TAVI cases per operator was 52 (median 43; IQR 26–69) (Figure 1; Supplementary Table S5), and in 22% (n = 32) of participating centres, the mean number of TAVI cases per operator was <25 per annum.
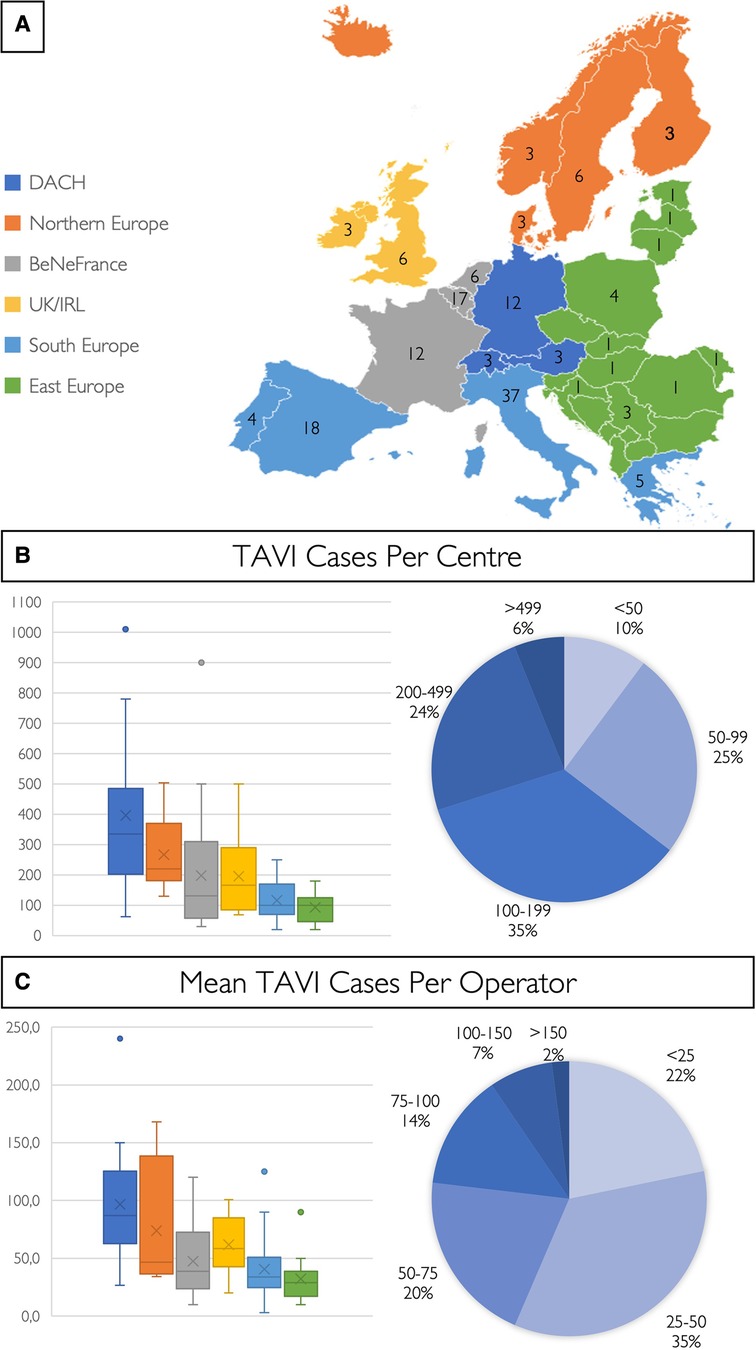
Figure 1. European TAVI pathway survey. (A) Colour coding for the six different European regions and number of centres of each country included in this study. The number of TAVI cases per centre (B) and the mean number of TAVI cases per operator (C) are shown in a box and whisker plot for the six different European regions, and cake diagrams showing categorised case and mean operator volumes [BeNeFrance, Belgium, France, Luxemburg, the Netherlands; DACH, Germany (D), Austria (A), Switzerland (CH); and UK/IRL, United Kingdom/Republic of Ireland].
Treatment strategy
Treatment strategy is guided by the Heart Team in 87% of centres (Figure 2) where a multi-slice computed tomography (MSCT) scan is available to assist decision-making in 90% of cases. Patient preference is considered in 72% of case discussions (Figure 2). Among patients with isolated severe aortic stenosis and favourable transfemoral access, 45% (n = 66) and 41% (n = 60) of operators use the ages of 75 and 80 years, respectively, as the threshold for TAVI as the default treatment strategy. In 1% (n = 1) and 5% (n = 8), the ages of 65 and 70 years are used as the threshold for TAVI as the preferred treatment strategy, while the remainder (6%, n = 9) indicated TAVI as the first choice in patients aged 85 years or older.
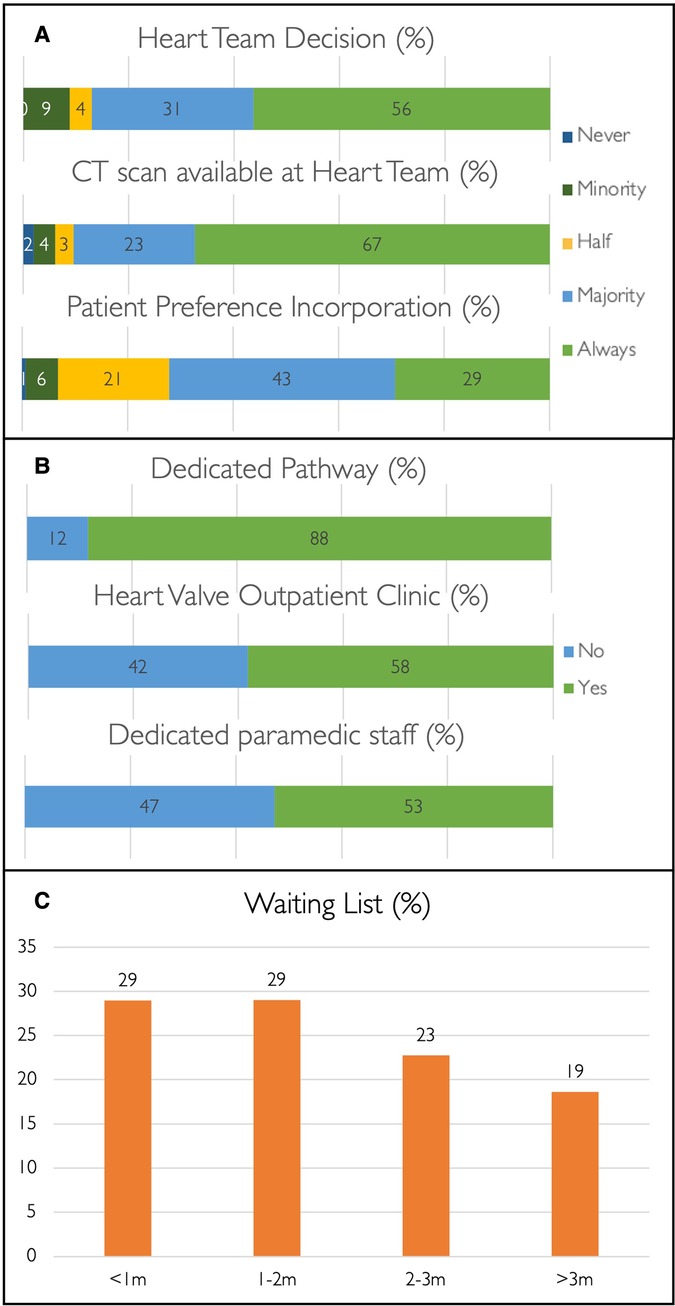
Figure 2. TAVI pathway. Bar charts and percentages demonstrating TAVI decision-making (A) and patient pathways (B), and TAVI waiting times (C).
Pathway
A structured TAVI pathway is present in 88% of centres (n = 129); 58% (n = 85) have a dedicated specialist-led outpatient heart valve clinic, and 53% (n = 77) have para-medical support staff (e.g., clinical nurse specialist or non-clinical coordinator) available (Figure 2). Most centres have waiting times of ≤3 months for TAVI (81%, n = 118), and this is even below 2 weeks in 10% (n = 14) but above 6 months in 5% (n = 7) (Figure 2). Waiting times of >3 months are observed across the spectrum of centre volume and appear irrespective of the availability of a structured pathway, a heart valve outpatient clinic, or presence of para-medical staff. DACH is the only region without a TAVI waiting time of >3 months (Supplementary Table S6).
Pre-procedural work-up
Transthoracic echocardiography (TTE), MSCT plus angiogram, and blood tests are the standard of care in the majority of centres (Figure 3). These pre-procedural investigations are performed either during hospital admission (25%, n = 36) or during one, two, or multiple separate outpatient visits in 20% (n = 29), 16% (n = 24), and 37% (n = 54) of institutions, respectively. The pre-TAVI MSCT is analysed by a TAVI operator in 60% (n = 88), a clinical specialist from a MedTech company in 20% (n = 30), a radiologist in 12% (n = 18), or a trained fellow/nurse in 3% (n = 5).
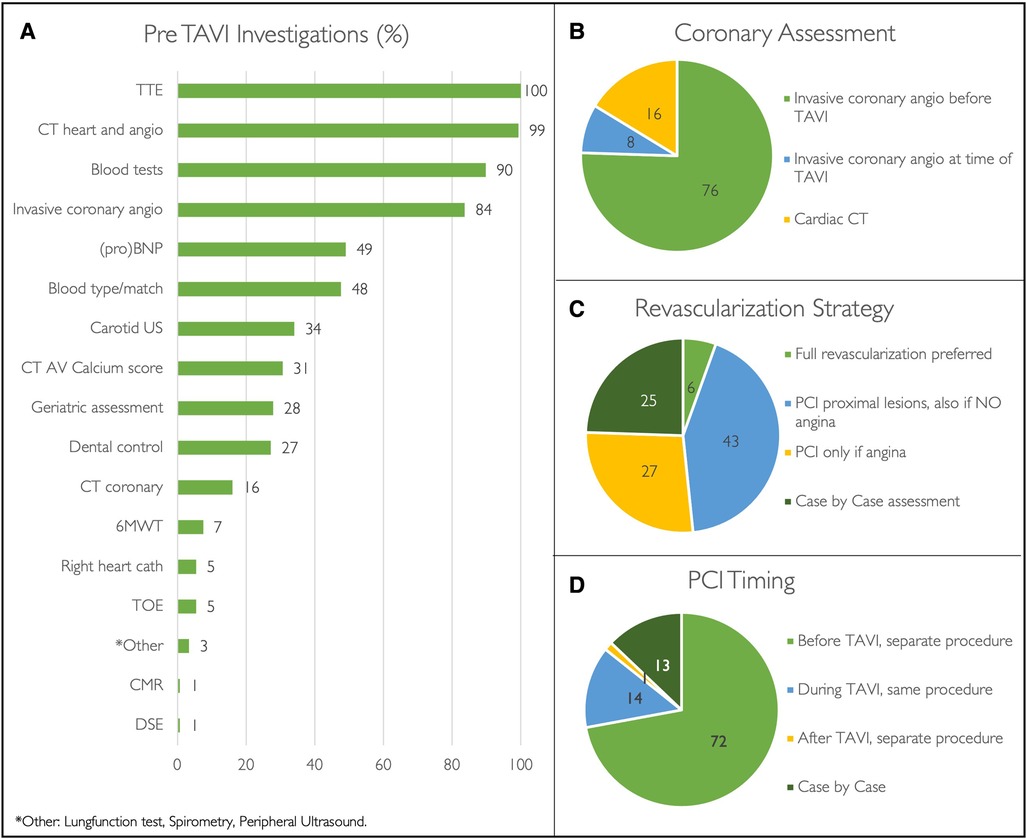
Figure 3. Pre-TAVI investigations. Pre-procedural investigations performed during routine TAVI work-up (A). Pie diagrams demonstrating coronary revascularization strategies (B-D) expressed as percentages. (Angio, angiogram; AV, aortic valve; CMR, cardiac magnetic resonance scan; CT, Computed tomography; DSE, dobutamine stress echocardiography; PCI, percutaneous coronary intervention; proBNP, N-terminal pro B-type natriuretic peptide; TOE, transoesophageal echocardiography; TTE transthoracic coronary echocardiography; US, ultrasound; 6MWT, six-minute walking test).
All surveyed centres perform coronary work-up prior to TAVI, either with invasive coronary angiography (84%, n = 123) or by means of the pre-TAVI MSCT (16%, n = 24) (Figure 2). Among patients with significant coronary artery disease that are accepted for TAVI, revascularisation strategy and timing of percutaneous coronary intervention (PCI) widely differ (Figure 2). When referring hospitals have a catheter laboratory onsite (87%, n = 128), PCI is performed in the referring centre in 23% (n = 30), in the TAVI centre in 17% (n = 22), and variably in either the TAVI centre or referring centre in the remaining 60% (N = 76) according to case complexity.
TAVI procedure
TAVI procedures are performed in most cases by interventional cardiologist teams only (60%), and the remainder are performed by mixed teams of interventionalists and cardiac surgeons (Figure 4).
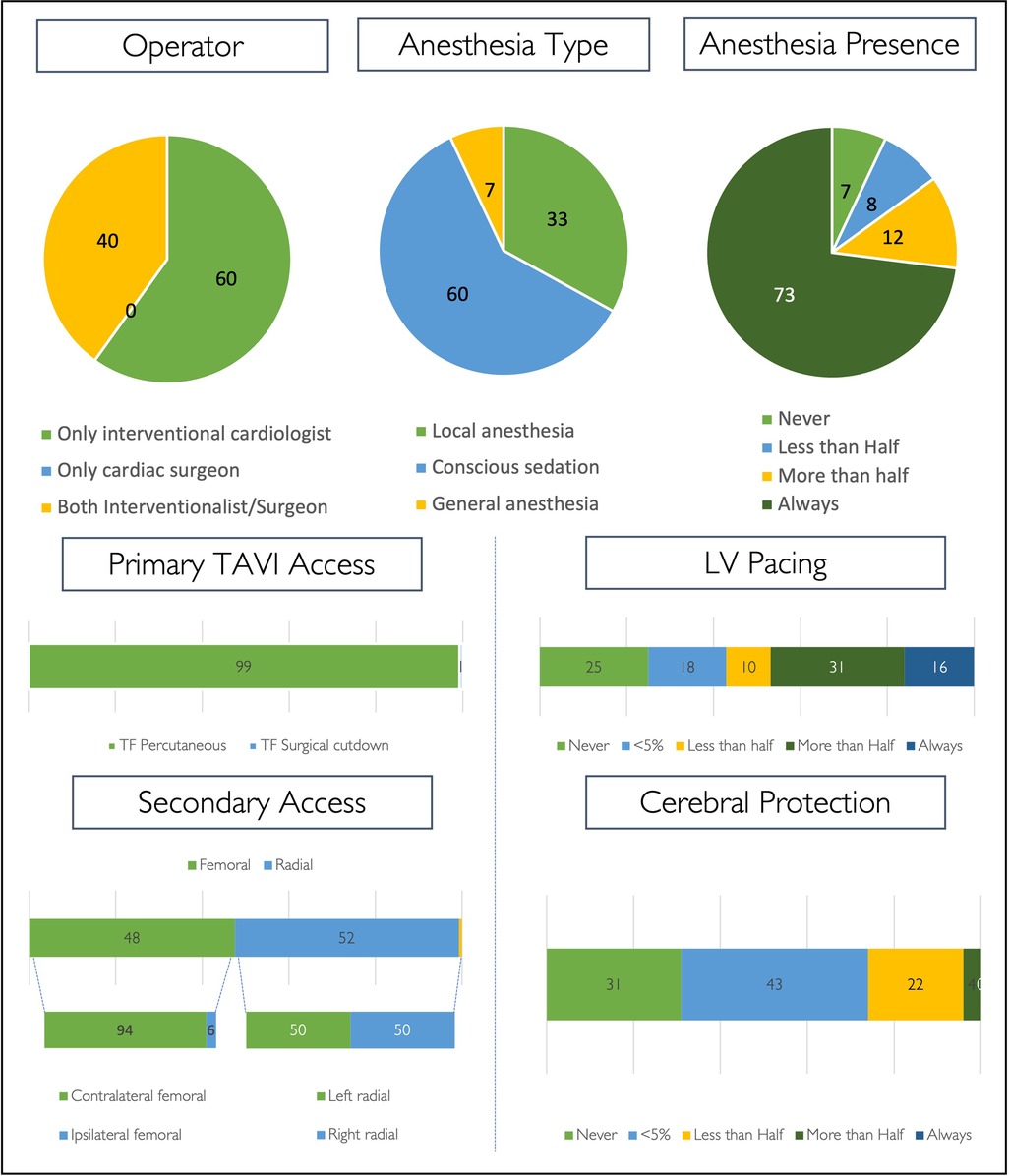
Figure 4. TAVI procedure. Procedural strategy for routine transfemoral TAVI cases, expressed as percentages. (LV, left ventricle).
Across all centres, local anaesthesia with conscious sedation is the most widely adopted anaesthetic strategy (60%) for routine TAVI. Local anaesthesia without conscious sedation is the default strategy in 33% (n = 49) of centres and most infrequently used in centres performing <50 cases per annum (7%). General anaesthesia is used in the minority of centres (7%, n = 10), is less implemented for regular TAVI cases in centres performing >100 cases per annum (4%, n = 4) as compared with centres performing <100 cases (12%, n = 6), and is only observed in the Southern European and BeNeFrance regions (Figure 4, Supplementary Table S7). Overall, an anaesthetic team is always present in the catheter laboratory during regular transfemoral TAVI in 73% of centres (n = 107). Further details on procedural strategy are shown in Figure 4.
Participants were asked to indicate alternative access sites employed in a sequence of preference/performance (Supplementary Table S8). Percutaneous transluminal angioplasty (PTA)-assisted transfemoral access—either with a plain or intravascular lithotripsy balloon—is the first choice for alternative access in 67% (78% plain balloon; 22% lithotripsy) and the second choice in 58% (28% plain balloon; 72% lithotripsy). The most preferred alternative access site as a third option is transaxillary (50%), more often using surgical cutdown (65%) as compared with a direct percutaneous approach (35%). Transapical access is chosen in 6%, 9%, and 12% as a first, second, and third choice for alternative access, respectively. When using the transcarotid approach, surgical cutdown is most often preferred (94%). Transvenous access is the least preferred approach for alternative access—the majority is transcaval (69%).
Post-procedural patient flow
After an uncomplicated transfemoral TAVI procedure, patients are transferred to a low-care facility (28%, n = 29) and discharged from the hospital on post-procedural day 1 (12%, n = 17) (Figure 5). Same-day discharge is never carried out, but 25% (N = 38) of participants would consider this strategy in selected cases. Results for in-hospital transfer and discharge timing according to region and centre size are shown in Figure 5.
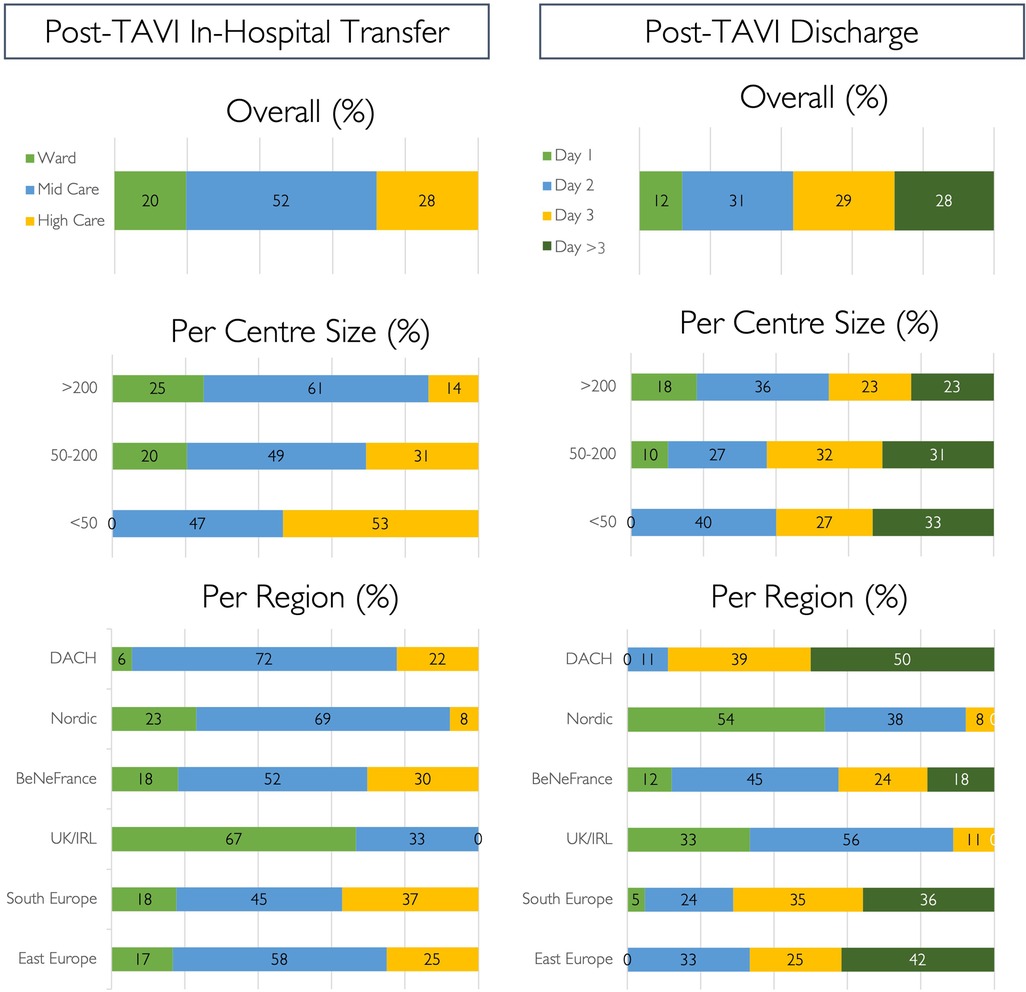
Figure 5. Post-TAVI strategy. Post-procedural strategy for in-hospital step down and discharge following routine uncomplicated transfemoral TAVI cases, expressed as percentages.
Registration/teaching
Most centres (92%, n = 132) record cases in a TAVI database at the institutional level, while only 74% (n = 99) and 12% (n = 11) register in national or international databases, respectively. Educational meetings (e.g., for medical staff, nurses, general practitioners, etc.) are organised on a regular basis in 67% (n = 98) of centres. In total, 84% (n = 122) of participants participate in research or clinical trials.
Discussion
The 2022 European TAVI Pathway Survey provides important insights as to how contemporary TAVI procedures and pathways are organised and executed across the continent. Data from 147 centres in 26 European countries were collected, and the salient observations were (1) guideline-directed Heart Team decision-making has been widely adopted, but patient preferences are not commonly incorporated in this discussion; (2) one-third of centres (35%) perform <100 TAVI cases per year; (3) most centres have a structured TAVI patient pathway, but only half have a dedicated heart valve clinic supported by para-medical staff; (4) although waiting times for TAVI are acceptable, one in five centres have a waiting list of >3 months; (5) the vast majority of cases are performed without general anaesthesia, but an anaesthetic team is still present in most cases (73%); and (6) post-procedure transfer to a low-care unit and next-day discharge after uncomplicated TAVI rarely occur.
A sharp increase in TAVI procedural volume is predicted within the next decades due to expanding indications and an ageing population (2, 3). TAVI consumes less healthcare resources than SAVR, mainly driven by shorter hospital stay, less post-procedural rehabilitation, and fewer short- and long-term complications (4). To pursue and sustain these advantages of TAVI in the face of ever-increasing demand, streamlined patient pathways and minimalistic procedures need to be incorporated into a daily practice. The current study reports how TAVI centres are organised across Europe for the first time and provides important insight concerning potential areas of improvement within current TAVI processes.
Multidisciplinary Heart Team-based decisions increase the application of guideline recommendations and subsequently improve patient outcomes (1). This survey demonstrates that Heart Team decisions are widely applied within the TAVI population (87% of centres) and that a MSCT scan is usually available to inform this discussion (90% of centres). Information gained from a pre-TAVI MSCT scan is essential for detailed risk stratification and procedural planning (SAVR vs. TAVI), reflecting the 2021 European Guidelines on valvular heart disease (1). Interestingly, 16% of centres consider the pre-procedural MSCT as an alternative to the invasive assessment of coronary artery disease. Expansion of this strategy has the potential to further simplify the TAVI pathway but requires further supportive evidence (5).
A patient-centred approach to treatment decisions is axiomatic in the setting of severe aortic stenosis and an important tenet of the 2021 European guidelines on valvular heart disease (1). Despite this guidance, patient preference does not appear to have a sufficient weight in Heart Team discussions. This finding suggests that the cardiovascular community should make greater efforts to more consistently implement this guideline recommendation, ideally within the setting of a dedicated heart valve clinic which provides the perfect opportunity to inform a patient (and caregiver) of potential treatment choices and subsequently discuss informed patient preferences.
Access to heart valve clinics improves adherence to guidelines and enhances detection of disease progression to ensure optimal timing of surgical or transcatheter intervention which is associated with improved patient outcomes (6–8). Heart valve clinics are essential to coordinate care, standardise patient flow, and limit TAVI waiting times. This study demonstrates that structured patient pathways are widely adopted (88%), but only half of the responding centres have a dedicated heart valve clinic supported by dedicated para-medical staff. Although the lack of specialised nurses or non-clinical coordinators is often financially driven, we hypothesise that their involvement provides a beneficial impact on efficiency and cost-effectiveness, as observed in heart failure programmes (9, 10). Potential areas of focus include the avoidance of futile or repeated pre-procedural tests, timely detection of disease progression, waiting list management, matching devices to Heart Team recommendation, and improving early discharge or access to stepdown care. While these assumptions have yet to be investigated, we believe that the role of dedicated heart valve clinics and para-medical staff will become even more apparent as global TAVI volumes increase.
Longer TAVI waiting times are associated with worse pre- and post-procedural outcomes (11, 12) and exceeded 3 months in nearly 20% of participating centres. This finding was observed in all categories of centre volume and organisation (but not in the DACH region) (Supplementary Table 6). These findings highlight the need for strategies to minimise delays in access to TAVI, especially in the face of increasing demand.
In 2019, 669 TAVI centres registered in the US TVT registry with an average procedure volume per site of 110 with a median of 84 (IQR 50–137) and 24% of centres performing <50 cases per annum were identified (13). In this contemporary European study, the average procedure volume per site was 185 with a median of 138 (IQR 77–194), with only 10% of centres performing <50 cases annually. Importantly, lower operator experience and volume have been associated with increased rates of adverse TAVI outcomes (14, 15). In the US consensus statements on advanced interventional cardiology training and the operator and institutional requirements for TAVI centres, a minimum of 50 cases per year is proposed for TAVI programmes, although recommendations concerning individual operator volumes are not specified (16, 17). The target of >50 cases per annum is reached in 90% of our study population. As with coronary interventions, European guidelines should inform policymakers of Heart Valve Centre requirements to avoid overgrowth of small, less-experienced centres.
A minimalist procedural approach should be applied to allow development of a streamlined TAVI programme. Local anaesthesia without the need for an anaesthetic team, secondary radial access, left ventricular pacing protocols, and early mobilisation have proven safety in multiple studies (18–24). Despite minimalist TAVI modifications being widely adopted, allowing faster post-procedural ambulation, this does not translate into greater post-procedural use of low-care facilities or faster discharge in this study. Indeed, uncomplicated TAVI patients are frequently transferred to medium- or high-care facilities (51% and 30%, respectively), and next-day discharge rates remain low (12%), with zero same-day discharge. Local protocols, politics, and/or legislation may influence these practices and unfortunately increase healthcare burden.
Study limitations
Although we identified 688 TAVI centres in 32 countries, an official database of European TAVI centres is unavailable, and some centres may therefore have been overlooked in the invitation to participate in this survey. Selection bias is possible, favouring centres with an underlying interest in scientific research—underestimation of the number of smaller volume centres within this study population is therefore possible. The questionnaire related only to the operator's practice at the time the survey was completed. Data on TAVI volume and practice in earlier periods were not included, nor were data on surgical valve replacement. The procedural volume of individual operators may be higher than represented since this depends on the distribution of procedures in each centre. Questions related only to regular transfemoral TAVI cases and baseline clinical profiles may differ between centres and countries.
Conclusion
The practice of TAVI across European centres varies widely. Heart Team decision-making, transfemoral access, and local anaesthesia are the norm. However, areas for improvement include greater account of individual patient preference into clinical decision-making, more frequent use of early discharge protocols, and continued attention to prolonged TAVI waiting times. These data identify areas for further refinement of TAVI pathways and procedures within the rapidly evolving European context.
Impact on a daily practice
A steep rise in TAVI has evolved in recent years, but no studies investigated how TAVI procedures and pathways are currently organised across Europe. In the 2022 European TAVI Pathway Registry, we collected data from 147 TAVI centres in 26 different European countries, including low- to high-volume centres. Adoption of minimalist TAVI techniques and guideline-directed Heart Team decision-making is common among European TAVI centres, but rates of next-day discharge and incorporation of patient’s preferences in the decision-making process remain low. The results highlight the significant progress made in refining TAVI treatment and pathways and their implementation but also identify possible areas for further improvement.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Commissie voor Medische Ethiek OG 052 ASZ Aalst, Belgium. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LR conducted the study and wrote the manuscript. DM and ODB extensively reviewed the manuscript. All other co-authors reviewed the manuscript with each significant contribution for the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
DM: consultant for Medtronic, Boston Scientific, and Microport. TR: proctor and advisor for JenaValve, speaker’s honoraria from Edwards Lifesciences, Boston Scientific, Medtronic, and JenaValve. JW: institutional grant from Medtronic and speaker’s honoraria (also to the institution) from Boston Scientific and Sinomed. SO: consulting fees from Medtronic and Edwards, speaker’s honoraria from Philips and World Medical, and research grant (PI21/00949) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). LL: proctoring and consulting honoraria for Abbott, Edwards, and Medtronic. DB: proctoring and consulting honoraria for Abbott, Edwards, and Medtronic. NM: institutional research grant support from Abbott Vascular, Boston Scientific, Biotronik, Medtronic, Daiichi Sankyo, Astra Zeneca, and PulseCath BV; and consultancy fees from Abbott Vascular, Boston Scientific, Biotronik, Medtronic, Daiichi Sankyo, Abiomed, Amgen, JenaValve, Anteris, and PulseCath BV. MG: Boston Scientific—research and travel grants, speaker’s honoraria, proctor, and advisory board member; Medtronic—research and travel grants, speaker’s honoraria, proctor, advisory board member; Abbott—speaker’s honoraria and travel grants; and Edwards Lifesciences—speakers honoraria and travel grants. BP: speaker’s fees from Edwards Lifesciences, Abbott, and Medtronic and consulting fees from Anteris and Microport. ODB: institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JS declared a past co-authorship with the author ODB to the handling editor.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1227217/full#supplementary-material
Abbreviations
MSCT, multi-slice computed tomography; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation; TTE, transthoracic echocardiography.
References
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the manamgement of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
2. Durko AP, Osnabrugge RL, Van Mieghem NM, Milojevic M, Mylotte D, Nkomo VT, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. (2018) 39:2635–42. doi: 10.1093/eurheartj/ehy107
3. Eurostat. Ageing Europe—statistics on population developments. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments#Older_people_.E2.80.94_population_overview (Accessed February 15, 2023).
4. Whou JY, Liew D, Duffy SJ, Walton A, Htun N, Stub D. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in low-risk patients with severe aortic stenosis. Heart Lung Circ. (2021) 30:547–54. doi: 10.1016/j.hlc.2020.09.934
5. Tarantini G, Tang G, Fovino LN, Blackman D, Van Mieghem NM, Kim W, Karam N, et al. Management of coronary artery disease in patients undergoing transcathteter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC working group on cardiovascular surgery. Eurointervention. (2023). doi: 10.4244/EIJ-D-22-00958
6. Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. (2018) 3:1060–8. doi: 10.1001/jamacardio.2018.3152
7. Chambers JB. Specialist valve clinic: why, who and how. Heart. (2019) 105:1913–20. doi: 10.1136/heartjnl-2019-315203
8. Paolisso P, Beles M, Belmonte M, Gallinoro E, De Colle C, Mileva N, et al. Outcomes in patients with moderate and asymptomatic severe aortic stenosis followed up in heart valve clinics. Heart. (2022) 0:1–9. doi: 10.1136/heartjnl-2022-321874
9. Kilpatrick K, Kaasalainen S, Donald F, Reid KJ, Carter N, Bryant-Lukosius D. The effectiveness and cost-effectiveness of clinical nurse specialist in outpatient roles: a systematic review. J Eval Clin Pract. (2014) 20:1106–23. doi: 10.1111/jep.12219
10. Driscoll A, Gao L, Watts JJ. Clinical effectiveness and cost-effectiveness of ambulatory heart failure nurse-led services: an integrated review. BMC Cardiovasc Disorders. (2022) 22:64. doi: 10.1186/s12872-022-02509-9
11. Albassam O, Henning KA, Qiu F, Cram P, Sheth TN, Ko DT, et al. Increasing wait-time mortality for severe aortic stenosis. A population-level study of the transition in practice from surgical aortic valve replacement to transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2020) 13:e009297. doi: 10.1161/CIRCINTERVENTIONS.120.009297
12. Roule V, Rebouh I, Lemaitre A, Sabatier R, Blanchart K, Briet C, et al. Impact of wait times on late postprocedural mortality after successful transcatheter aortic valve replacement. Sci Rep. (2022) 12:5967. doi: 10.1038/s41598-022-09995-z
13. The Society of Thoracic Surgeons. TAVR surges past surgery in US AVR treatment volume. Available at: https://www.sts.org/publications/sts-news/tavr-surges-past-surgery-us-avr-treatment-volume (Accessed February 15, 2023).
14. Carroll JD, Vemulapalli S, Dai D, Matsouaka R, Blackstone E, Edwards F, et al. Procedural experience for transcatheter aortic valve replacement and relation to outcomes: the STS/ACC TVT registry. J Am Coll Cardiol. (2017) 70:29–41. doi: 10.1016/j.jacc.2017.04.056
15. Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. (2019) 380:2542–50. doi: 10.1056/NEJMsa1901109
16. Bavaria JE, Tommaso CL, Brinids RG, Carroll JF, Deeb GM, Feldman RE, et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. (2019) 73:340–74. doi: 10.1016/j.jacc.2018.07.002
17. Bass TA, Abbott JD, Mahmud E, Parikh SA, Aboulhosn J, Ashwath ML, et al. 2023 ACC/AHA/SCAI advanced training statement on interventional cardioloy (coronary, peripheral vascular, and structural heart interventions): a report of the ACC competency management committee. Circ Cardiovasc Interv. (2023) 16:e000088. doi: 10.1161/HCV.0000000000000088
18. Thiele H, Kurz T, Feistritzer H, Stachel G, Hartung P, Lurz P, et al. General versus local anesthesia with conscious sedation in transcatheter aortic valve implantation. The randomized SOLVE-TAVI trial. Circulation. (2020) 142:1437–47. doi: 10.1161/CIRCULATIONAHA.120.046451
19. Saia F, Palmerini T, Marcelli C, Chiarabelli M, Taglieri N, Ghetti G, et al. Routine minimalistic transcatheter aortic valve implantation with local anesthesia only. J Cardiovasc Med. (2020) 21:805–11. doi: 10.2459/JCM.0000000000001030
20. Radhakrishnan SL, Ho KK. Transradial vs. transfemoral secondary access outcomes in transcatheter aortic valve implantation: a systematic review and meta-analysis. World J Cardiol. (2020) 12:571–83. doi: 10.4330/wjc.v12.i11.571
21. Jhand A, Apala DR, Dhawan R, Katta N, Aronow HD, Daniels MJ, et al. Meta-analysis comparing transradial versus transfemoral secondary access in transcatheter aortic valve implantation. Am J Cardiol. (2020) 131:74–81. doi: 10.1016/j.amjcard.2020.06.032
22. Faurie B, Souteyrand G, Staat P, Godin M, Caussin C, Van Belle E, et al. Left ventricular pacing via the vave delivery guidewire in transcatheter aortic valve replacement. J Am Coll Cardiol Cardiovasc Interv. (2019) 12:2449–59. doi: 10.1016/j.jcin.2019.09.029
23. Vendrik J, Vlastra W, van Mourik MS, Delewi R, Beijk MA, Lemkes J, et al. Early mobilisation after transfemoral transcatheter aortic valve implantation: result of the MobiTAVI trial. Neth Heart J. 2020;28:240–8. doi: 10.1007/s12471-020-01374-5
24. Wood DA, Lauck SB, Cairns JA, Humphries KH, Cook R, Welsh R, et al. The Vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilities safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. J Am Coll Cardiol Cardiovasc Interv. (2019) 12:459–69. doi: 10.1016/j.jcin.2018.12.020
Keywords: Transcatheter aortic valve implantation, aortic stenosis, multidisciplinary Heart Team, minimalist TAVI, early discharge
Citation: Rosseel L, Mylotte D, Cosyns B, Vanhaverbeke M, Zweiker D, Teles RC, Angerås O, Neylon A, Rudolph TK, Wykrzykowska JJ, Patterson T, Costa G, Ojeda S, Tzikas A, Abras M, Leroux L, Van Belle E, Tchétché D, Bleiziffer S, Swaans MJ, Parma R, Blackman DJ, Van Mieghem NM, Grygier M, Redwood S, Prendergast B, Van Camp G and De Backer O (2023) Contemporary European practice in transcatheter aortic valve implantation: results from the 2022 European TAVI Pathway Registry. Front. Cardiovasc. Med. 10:1227217. doi: 10.3389/fcvm.2023.1227217
Received: 22 May 2023; Accepted: 28 July 2023;
Published: 14 August 2023.
Edited by:
Luca Testa, IRCCS San Donato Polyclinic, ItalyReviewed by:
Janarthanan Sathananthan, St. Pauls Hospital, CanadaMayooran Namasivayam, St Vincent’s Hospital Sydney, Australia
© 2023 Rosseel, Mylotte, Cosyns, Vanhaverbeke, Zweiker, Teles, Angerås, Neylon, Rudolph, Wykrzykowska, Patterson, Costa, Ojeda, Tzikas, Abras, Leroux, Van Belle, Tchétché, Bleiziffer, Swaans, Parma, Blackman, Van Mieghem, Grygier, Redwood, Prendergast, Van Camp and De Backer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liesbeth Rosseel liesbeth.rosseel@asz.be
 Liesbeth Rosseel
Liesbeth Rosseel Darren Mylotte
Darren Mylotte Bernard Cosyns
Bernard Cosyns Maarten Vanhaverbeke5
Maarten Vanhaverbeke5  David Zweiker
David Zweiker Rui Campante Teles
Rui Campante Teles Oskar Angerås
Oskar Angerås Joanna J. Wykrzykowska
Joanna J. Wykrzykowska Giulia Costa
Giulia Costa Apostolos Tzikas
Apostolos Tzikas Marcel Abras
Marcel Abras Lionel Leroux
Lionel Leroux Sabine Bleiziffer
Sabine Bleiziffer Martin J. Swaans
Martin J. Swaans Radoslaw Parma
Radoslaw Parma Bernard Prendergast
Bernard Prendergast Guy Van Camp
Guy Van Camp Ole De Backer
Ole De Backer