Effect of estimated plasma volume status and left atrial diameter on prognosis of patients with acute heart failure
- Department of Cardiology, The Affiliated Hospital of Putian University, Putian University, Putian, China
Objective: Acute heart failure (AHF) is a frequent cardiovascular emergency presenting with high mortality as well as readmission rates. The aim was to investigate the predictive value of estimated plasma volume status (ePVs) and left atrial diameter (LAD) for the prognosis of patients with AHF.
Methods: Clinical profiles were collected from 259 cases of AHF patients at the Affiliated Hospital of Putian University between September 2019 and October 2021.
Results: Six patients lost follow-up, resulting in 253 patients enrolled. Cardiogenic death and heart failure readmission during follow-up were defined as major cardiovascular events (MACE) group, other patients were defined as Non-MACE group. Apart from age, no significant differences were found between the two groups in demographics and comorbidities. The comparison between the two groups was statistically significant in terms of ePVs, LAD, and N-terminal-pro B-type natriuretic peptide (Nt-pro-BNP). On binary logistic regression analysis, ePVs (OR = 2.061, 95% CI 1.322∼3.214, P = 0.001), LAD (OR = 1.054, 95% CI 1.012∼1.098, P = 0.011), and Nt-pro-bnp (OR = 1.006, 95% CI 1.003∼1.010, P = 0.036) as predicting factors for MACE. Kaplan-Meier analysis indicated that the risk for cardiogenic death increasing with ePVs (p < 0.05).
Conclusion: Estimated plasma volume status and LADs have some predictive value in assessing cardiogenic death and heart failure readmission in patients with AHF.
Introduction
Heart failure is a life-threatening and costly condition caused by structural and functional irregularities within the heart. It results in systolic and diastolic dysfunction, failure of cardiac output to meet the metabolic needs of the body, and inadequate perfusion of organs and tissues, leading to a series of clinical syndromes (1). Age-adjusted rates of heart failure may be declining in developed countries, possibly reflecting better management of cardiovascular disease, but overall rates are increasing due to aging. Currently, the prevalence of heart failure in Chinese residents aged 35 years or older is 1.3%, with approximately 13.7 million patients with heart failure (2). Acute heart failure is a symptom and sign that appears or worsens rapidly following abnormal cardiac function and is related to an increased in plasma natriuretic peptide levels.
Hematocrit is the proportion of erythrocytes to the whole blood volume and can reflect the severity of anemia and blood volume load. Anemia leads to myocardial ischemia and hypoxia, which results in compensatory increases in stroke volume and heart rate, secondary to myocardial remodeling, cardiac structural changes, and reduced cardiac systolic and diastolic functions (3). In aged patients who had preserved ejection fraction as well as mild reduced heart failure, decreased hematocrit was a risk factor for major adverse cardiovascular events in the vulnerable period. The lower the hematocrit, the worse the prognosis, the higher the readmission rate and mortality (4).
Plasma volume (PV) was a marker of volume overload based on the Duarte formula estimated on the basis of erythrocyte volume and hemoglobin (5). Estimated PV status (ePVs) was the use of hematocrit and hemoglobin from Strauss derivative Duarte calculated in the formula, specific as shown below: ePVs (mL/g) = 100 × (1–hematocrit)/hemoglobin (g/dL) (6). It was correlated with clinical outcomes in patients suffering from heart failure with reduced ejection fraction and in patients with acute heart failure. The higher the plasma volume status, the worse the prognosis (7).
The left atrium is an important factor in maintaining left ventricular filling, which has reserve function, pipeline function and pump function. Atrial function was closely related to the occurrence of heart failure. Left atrial dysfunction could be used as an independent predictor of the prognosis of heart failure (8). Left atrial enlargement indicated poor prognosis, and left atrial reduction after treatment could be used as a prognostic indicator (9).
Acute heart failure (AHF) is a common critical disease in the Department of cardiovascular medicine, with high mortality and readmission rate. Currently, there are few reports on the relationship between estimated plasma volume status and left atrial diameter on the prognosis of patients with acute heart failure. We have analyzed the factors influencing cardiovascular events in patients with acute heart failure in order to enhance the management of patients with heart failure and reduce readmission and mortality rates in patients with acute heart failure.
Materials and methods
Study populations
The study conformed to the declaration of Helsinki, and obtained the Affiliated Hospital of Putian University ethics review board for approval.
It was a retrospective study and collected data on the Hospital Digital Information System. Such data did not involve identifiable personal data; therefore, informed consent was not required to approve our study.
A total of 259 patients with acute heart failure presented at The Affiliated Hospital of Putian University from September 2019 to October 2021 were enrolled. Acute heart failure diagnosis in line with Universal definition and classification of heart failure published in the European Journal of Heart Failure in 2021 (10). Exclusion criteria: active bleeding and transfusion; new onset or previous history of atrial fibrillation/flutter; moderate to severe mitral valve disease; advanced renal disease requiring chronic renal replacement therapy; cerebral hemorrhage and other severe cerebrovascular disease; severe hepatic insufficiency; malignancy. We followed up all patients for 1 year. For the follow-up period, cardiogenic death and readmission due to heart failure were defined as the MACE group, and non-cardiogenic death and readmission not due to heart failure were defined as the non-MACE group.
Data collection
Demographic, clinical parameters and laboratory test results were assembled. All data collected through medical records.
Laboratory indicators
Blood samples were drawn on admission by the nurse in the vacuum vessel treated with ethylene diamine tetra-acetic acid. The automated hematology analyzer (CAL8000; Mindray, Shenzhen, China) measured parameters such as hematocrit and hemoglobin using the electrical impedance method for complete blood count. ePVs was derived by 100 × (1–hematocrit)/hemoglobin in g/dL. Automatic fluorescence immunoanalyzer (Getein1600; Geteinbiotech, Nanjing, China) measured N-terminal B-type natriuretic peptide (NT-pro-BNP) by dry immunofluorescence method.
Echocardiography
For all patients, measurements were taken using a GE Vivid 7 echocardiography device (General Electric, Boston, MA, USA). Measured the left atrial diameter (LAD) under the long axis of the parasternal left ventricle. Measurement of left ventricular ejection fraction (LVEF) by Simpson’s method under apical four-chamber and two-chamber views.
End-point
After discharge patients were followed up at 1, 2, 3, 6, and 12 months. Follow-up methods included outpatient follow-up, phone calls, WeChat, etc. The incidence of end-point events were recorded and follow-up was terminated after cardiogenic death. End-point events were defined as major cardiovascular events (MACE) during the follow-up period, including cardiogenic death and readmission for heart failure.
Statistical analysis
The statistical package for social sciences software (SPSS 22.0 for Windows, IBM, Armonk, NY, USA) was used for all statistical analyses. The continuous variables were shown as mean ± standard deviation and were tested by independent samples t-test for comparison. The categorical variables were shown as proportions, and the chi-square test was used for differences between categorical variables. All factors at entry that were statistically significant between groups with a p-value <0.05 and variables considered of relevant clinical interest were included in the logistic regression to identify the independent predictors of the endpoint event. Receiver operating characteristics (ROC) curve assess the predictive value of left atrial diameter and ePVs for MACE. Kaplan-Meier survival curves were used for survival analysis and log-rank tests were used for comparison of differences. The difference was considered statistically significant at a P-value < 0.05 (two-tailed test).
Results
Demographic characteristics
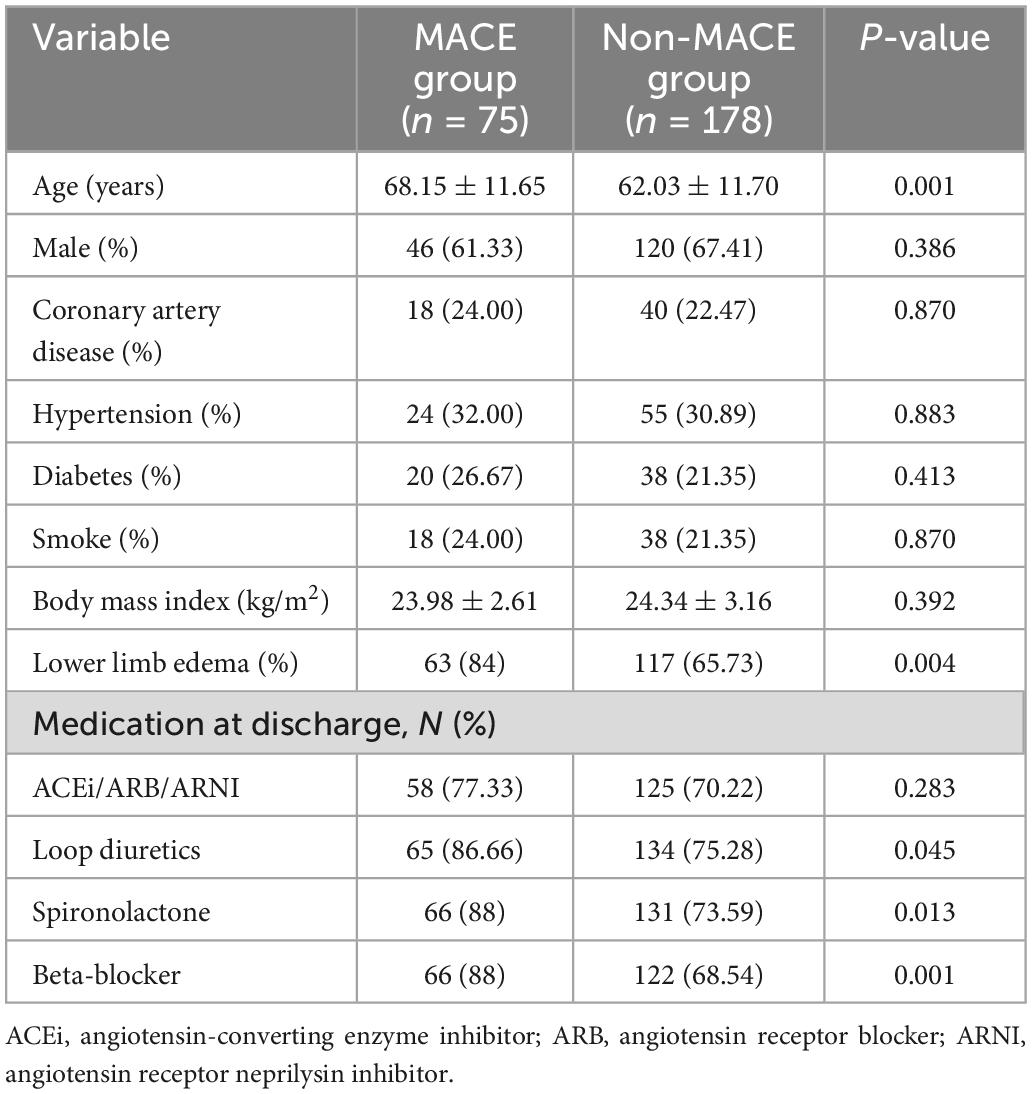
Six patients were lost to follow-up, and 253 patients were finally enrolled. There were 75 cases in the MACE group, of which 44 were cardiogenic deaths. There were 178 in the Non-MACE group. In the MACE group, 46 were males and 29 were females, average age was (68.15 ± 11.65), comprising 18 patients with coronary artery disease, 24 patients with hypertension and 20 patients with diabetes. In the non-MACE group, 120 were males and 58 were females, average age was (62.03 ± 11.70), comprising 40 patients with coronary artery disease, 55 patients with hypertension and 38 patients with diabetes mellitus. No statistical significance was found between the two groups in terms of gender, smoking, body mass index and comorbidities (p > 0.05). The age and rate of lower limb edema of MACE group was higher than that of the non-MACE group. The values were detailed in Table 1.
Baseline characteristics of patients
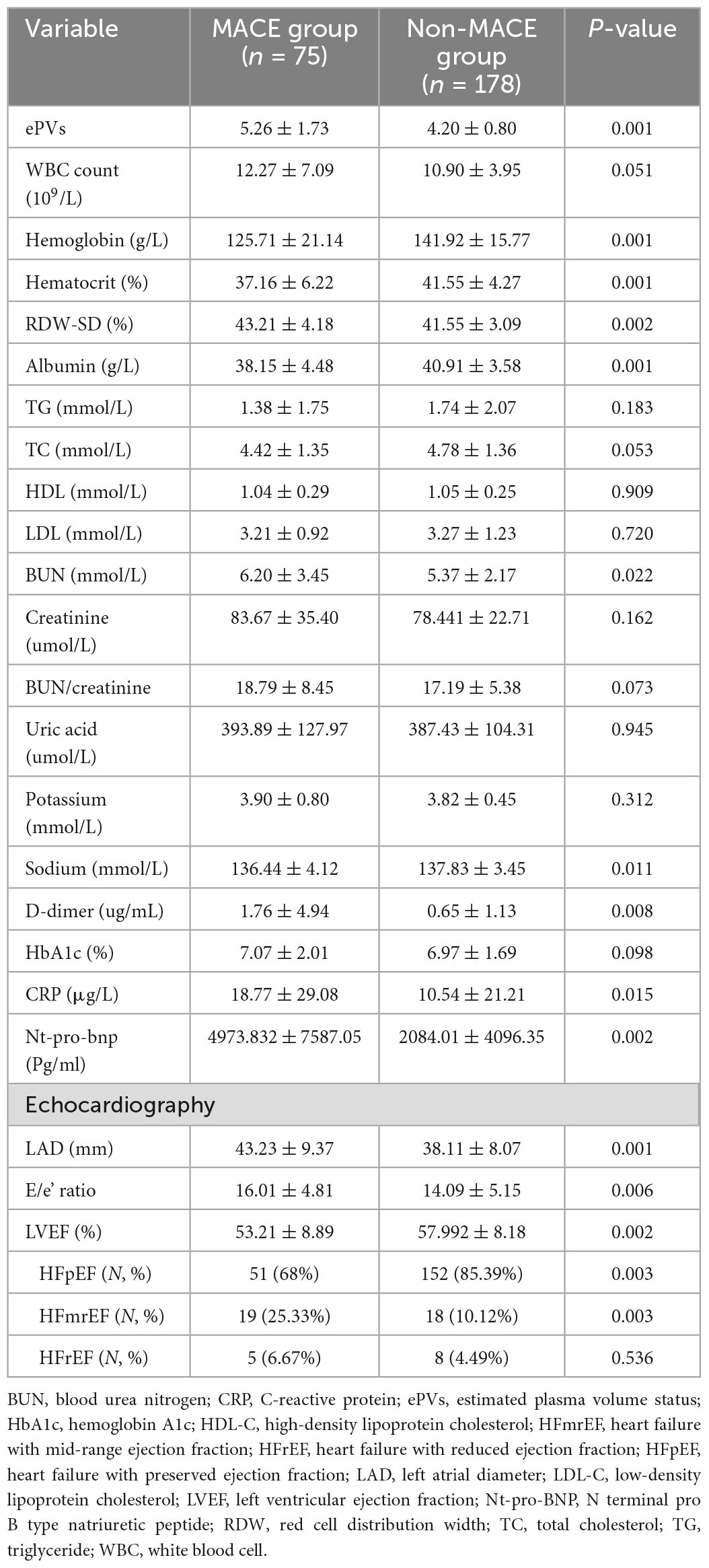
Hemoglobin, hematocrit, red cell distribution width (RDW), ePVs, blood urea nitrogen (BUN), Sodium, albumin, C-reactive protein (CRP), D-dimer, Nt-pro-bnp, LVEF, and LAD were all significantly different between the two groups. White-blood cells (WBC), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), creatinine, high-density lipoprotein cholesterol (HDL), BUN/creatinine, uric acid, triglycerides (TG), potassium, and hemoglobin A1c were not significantly different between the two groups. The values were detailed in Table 2.
Predictive indicators for MACE.
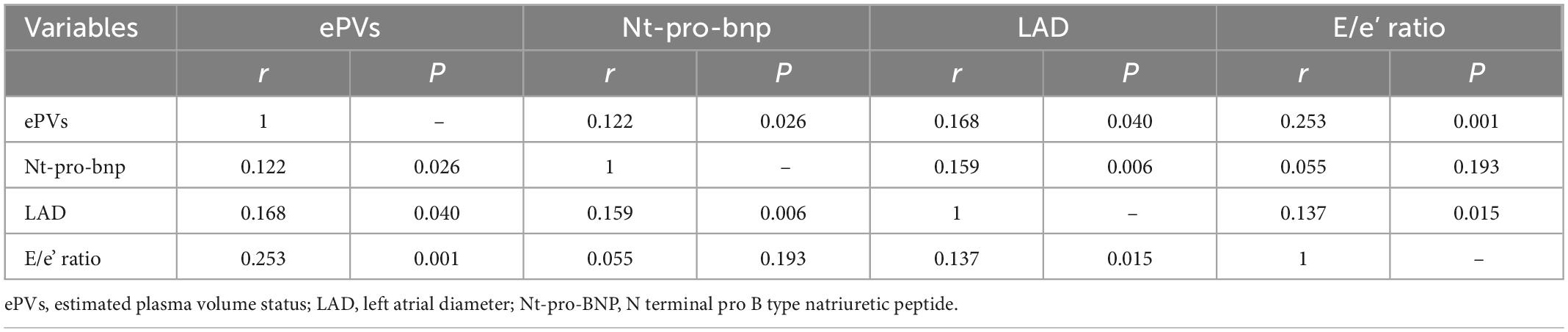
In correlation analysis, the ePVs was correlated with Nt-pro-bnp (r = 0.122, p < 0.05), LAD (r = 0.168, p < 0.05), E/e’ ratio (r = 0.253, p < 0.05). The Nt-pro-bnp was correlated with LAD (r = 0.159, p < 0.05). The LAD was correlated with E/e’ ratio (r = 0.137, p < 0.05). The values were detailed in Table 3.
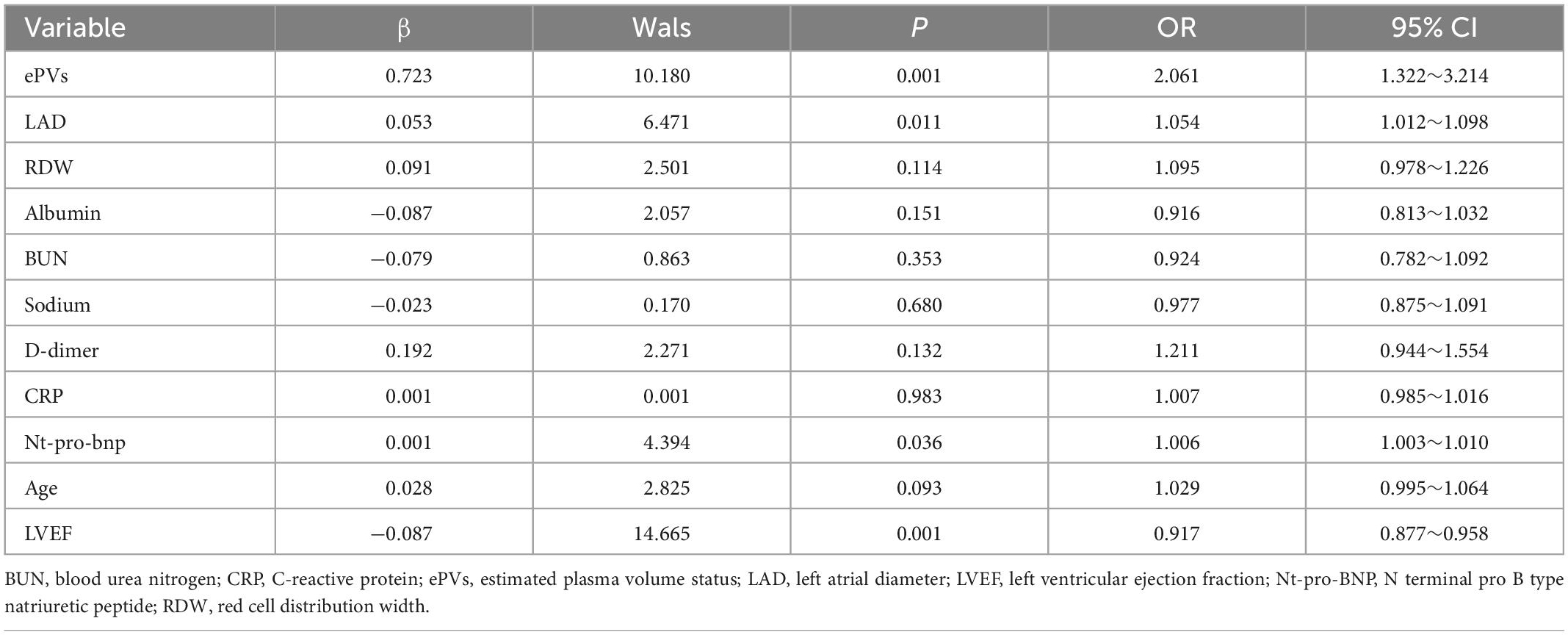
Analysis of binary logistic regression indicated that ePVs (OR = 2.061, 95% CI 1.322∼3.214, P = 0.001), LAD (OR = 1.054, 95% CI 1.012∼1.098, P = 0.011) and Nt-pro-bnp (OR = 1.006, 95% CI 1.003∼1.010, P = 0.036) were MACE predictors in acute heart failure patients. The values were detailed in Table 4.
Receiver operating characteristic curve
The ePVs area under the curve was 0.721 (95% CI: 0.648∼0.794, p < 0.001) with an optimal cut point value of 4.98 for predicting MACE, 64% sensitivity and 71.3% specificity; The LAD area under the curve was 0.668 (95% CI: 0.592∼0.745, p < 0.001) with an optimal cut point value of 38.5 mm for predicting MACE, 64% sensitivity and 70.8% specificity (Figure 1).
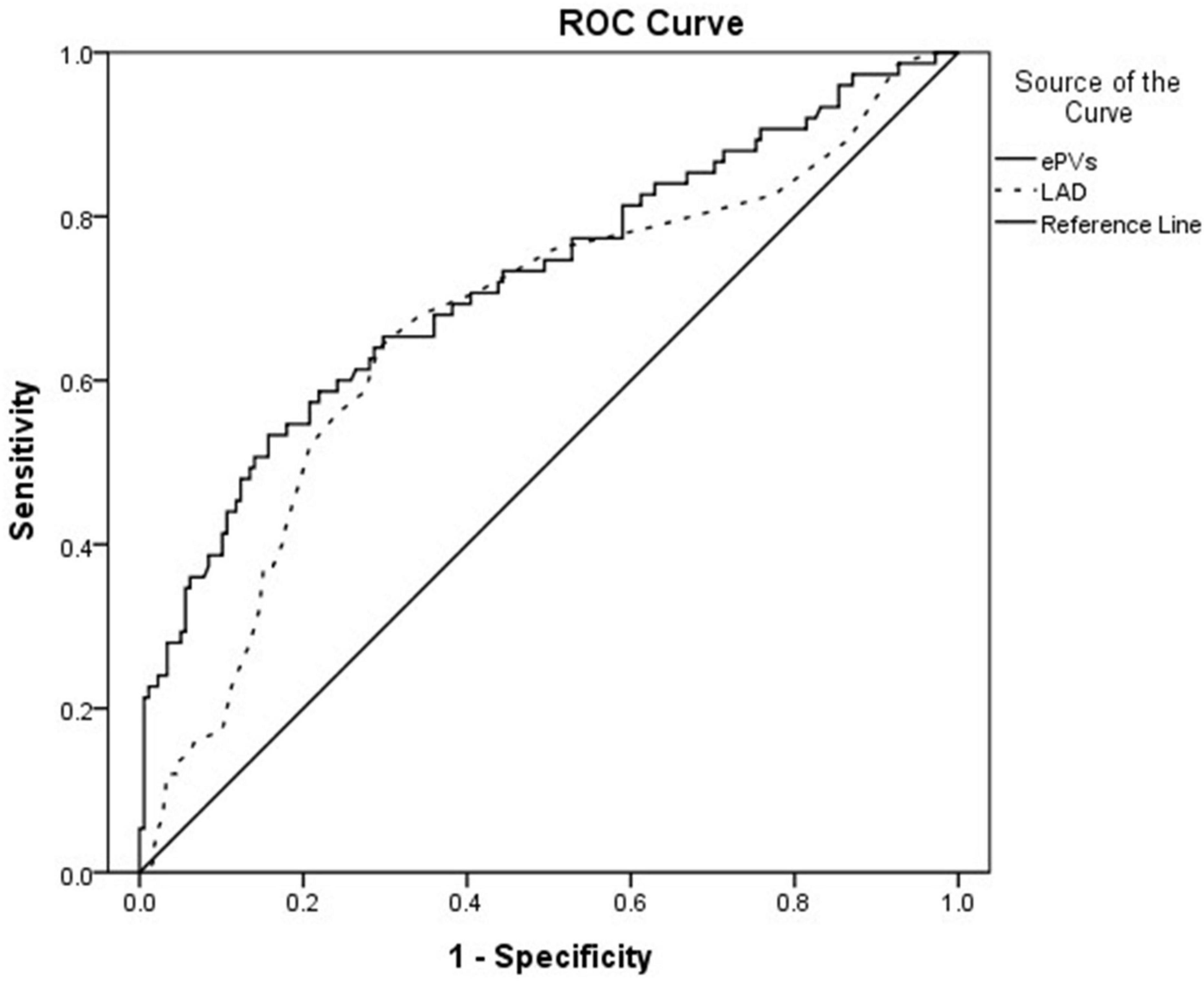
Figure 1. Receiver operating curve showing the area under the curve (AUC) for estimated plasma volume status (ePVs) and left atrial diameter (LAD).
The Kaplan–Meier survival analysis for cardiogenic death
Analysis of the ROC curve indicates an optimal ePVs threshold of 4.98 for MACE. Patients were divided into a high ePVs (ePVs ≥ 4.98) group (n = 70) and a low ePVs (ePVs < 4.98) group (n = 183). At 1 year follow-up, cardiac mortality was higher in the group with high ePVs than in the group with low ePVs (P = 0.027) (Table 5). Kaplan survival Meier analysis indicates a similar tendency (Figure 2).
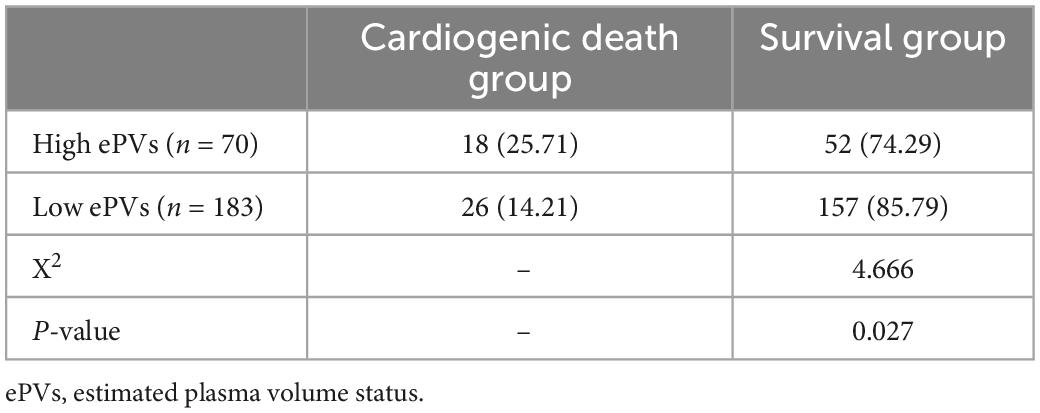
Table 5. One-year follow-up results of patients in the high and low estimated plasma volume status (ePVs) groups.
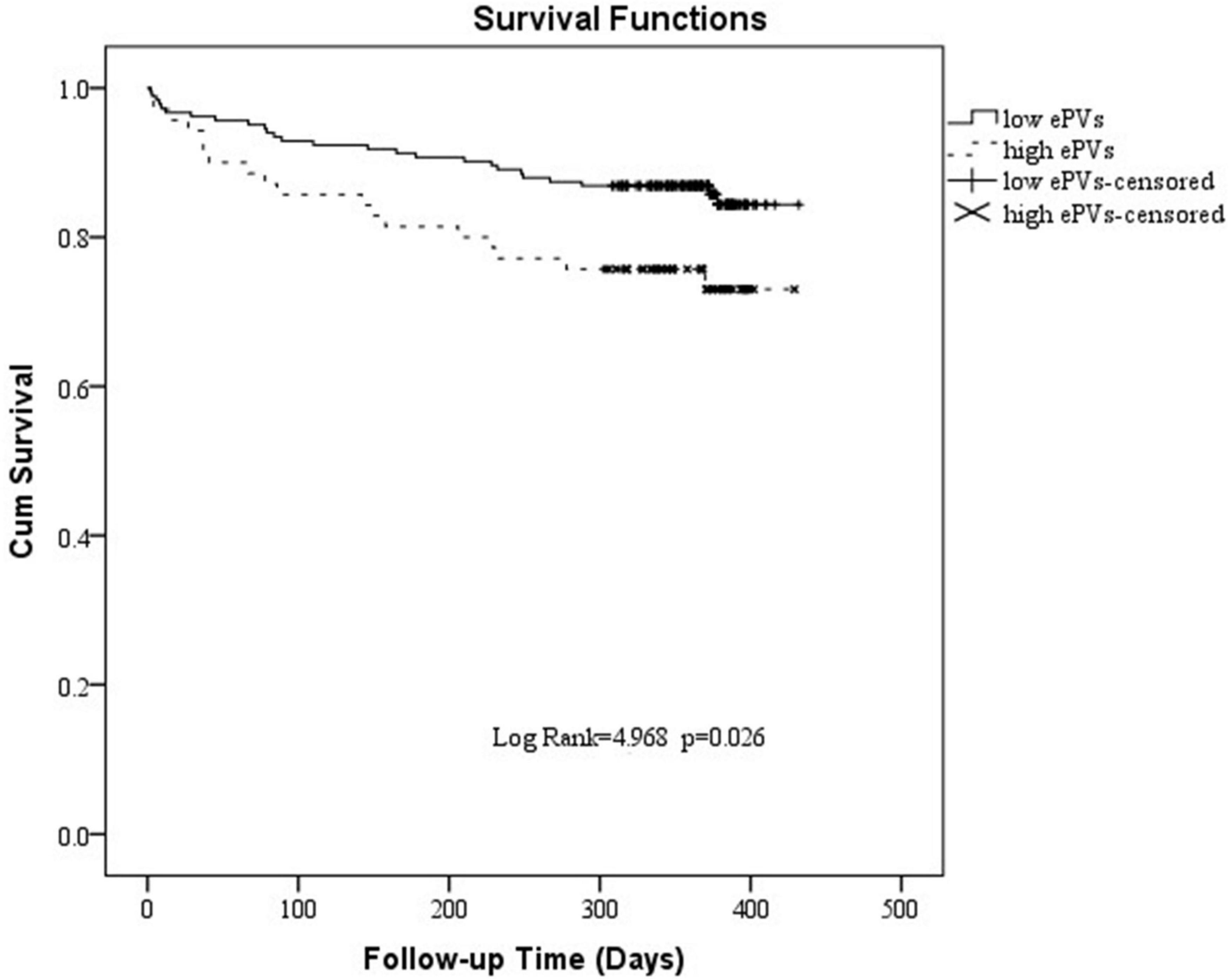
Figure 2. Kaplan-Meier survival curve of 1 year cardiogenic death in patients with high estimated plasma volume status (ePVs) and low ePVs groups.
Discussion
This single-center retrospective study evaluated the predictive value of ePVs and LAD in patients with AHF. The study showed that ePVs were higher in MACE group than in controls, and the LAD was larger than in controls. ePVs, LAD, and Nt-pro-bnp were MACE predictors in acute heart failure patients. ROC analysis showed that the optimal ePVs threshold for MACE was 4.98 with sensitivity of 64%, and specificity of 71.3%. At 1 year follow-up, cardiac mortality was higher in the group with high ePVs than in the group with low ePVs.
Acute heart failure is defined as signs and/or symptoms of rapid or progressive heart failure that are severe enough to warrant emergency medical attention. Patient with AHF required urgent assessment and initiation or intensification of treatment (11). AHF is the primary cause of hospitalization for patients aged >65 year and is related to high rates of mortality and readmission. Mortality rates in hospital range between 4 and 10% (12). One year after discharge, the mortality rate could be 25–30%, and the mortality or readmission rate could be more than 45% (13). In our study, the incidence of MACE events at 1 year follow-up was 29.64%. This may be related to race and region. We also found that the incidence of MACE events within 30 days was approximately 4% and the incidence of MACE events within 180 days was approximately 17%. The incidence of MACE events may be higher if the duration of follow-up is extended. And the study population was mainly composed of HFpEF or HFmrEF patients, only about 5% of patients with HFrEF. This may also be the reason for the low incidence of MACE.
Congestion was the main cause of hospitalization for acute decompensated heart failure and was associated with a poor prognosis (14). It was essential to assess the intensity of congestion in order to obtain the best possible treatment for heart failure. Plasma volume, which is the intravascular portion of the extracellular fluid volume, could be gauged with radio-labeled tracer molecules by standard dilution techniques (15). Non-invasive PV evaluation was essential for the treatment of heart failure patients, but was not possible due to the unreliability of clinical signs and symptoms (16). A correlation had now been demonstrated between PV estimated from hemoglobin/hematocrit and PV estimated from 125I-human serum albumin measurements (17). Several other studies had revealed an independent correlation between increased levels of PV estimates and increased clinical outcome risk (18–20). Hyponatremia and hypoproteinemia were also predictive factors for recent death in patients with heart failure (21). Our research also confirmed that high ePVs group had higher cardiac deaths and heart failure readmissions and were independent risk factors for them. Serum sodium and albumin concentrations were lower in the MACE group.
The left atrium could be compensated by pressor function in the early stage of heart failure. With the continuous increase of left ventricular end-diastolic pressure, the left atrium will continue to expand and eventually exceed its own regulatory range, and eventually decompensated, leading to the reduction of left atrial function in all aspects (22). Left atrial volume was an independent predictor of cardiovascular events, including atrial fibrillation, heart failure, stroke, and death (23). Left atrial diameter was proved to have a strong correlation with left atrial volume (24). The study also confirmed that the LAD was larger in the MACE group and was an independent risk factor for predicting MACE. Because fibrillation/flutter was closely associated with atrial remodeling, which affected the size of the left atrium. Therefore, atrial fibrillation/flutter was included as an exclusion criterion in our study. Considered the high prevalence of atrial fibrillation/flutter in a HF population aged >60 years-old, the exclusion of patients with atrial fibrillation/flutter may be an important limiting factor to generalize the conclusion of the study to all patients with acute failure.
There were some limitations to the study. Firstly, the major limitation of our study is the population size and the size of the MACE group, which are really small to confidently assess such a clinical endpoint. Secondly, due to the high clinical workload, the index of left atrial diameter was used instead of left atrial volume. Finally, there was no dynamic observation of the patient’s volume profile, which may have had some impact on the results.
Conclusion
Estimated plasma volume status and LAD have predictive value for assessing the incidence of cardiogenic death and heart failure readmission in acute heart failure patients. It is easy to detect, cheap and has promising utility for the prediction of cardiovascular death and heart failure readmission in patients with AHF.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical Review Board of the Affiliated Hospital of Putian University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
XC and GL designed and produced the manuscript. KX analyzed the data. CD conducted study and reviewed the manuscript. All authors contributed to the final manuscript, read and approved the submitted version.
Acknowledgments
The authors thank to all those involved in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Yuan X. Efficacy and renal tolerability of ultrafiltration in acute decompensated heart failure: a meta-analysis and systematic review of 19 randomized controlled trials. Cardiovasc Innov Appl. (2021) 6:1–16. doi: 10.15212/CVIA.2021.0020
2. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China hypertension survey, 2012-2015. Eur J Heart Fail. (2019) 21:1329–37. doi: 10.1002/ejhf.1629
3. Salgado M, Cao Z, Nagababu E, Mohanty J, Rifkind J. Red blood cell membrane-facilitated release of nitrite-derived nitric oxide bioactivity. Biochemistry. (2015) 54:6712–23. doi: 10.1021/acs.biochem.5b00643
4. Yiman L, Lu W, Xin Z. Influence of resting heart rate and erythrocyte volume fraction cardiovascular events in elderly patients with preserved or mid-range ejection fraction heart failure during the vulnerable phase. Chine J Geriatr. (2022) 41:798–803.
5. Kobayashi M, Douair A, Coiro S, Giacomin G, Bassand A, Jaeger D, et al. A combination of chest radiography and estimated plasma volume may predict in-hospital mortality in acute heart failure. Front Cardiovasc Med. (2022) 8:752915. doi: 10.3389/fcvm.2021.752915
6. Duarte K, Monnez J, Albuisson E, Pitt B, Zannad F, Rossignol P. Prognostic value of estimated plasma volume in heart failure. JACC Heart Fail. (2015) 3:886–93. doi: 10.1016/j.jchf.2015.06.014
7. Kobayashi M, Bercker M, Huttin O, Pierre S, Sadoul N, Bozec E, et al. Chest X-ray quantification of admission lung congestion as a prognostic factor in patients admitted for worsening heart failure from the ICALOR cohort study. Int J Cardiol. (2020) 299:192–8. doi: 10.1016/j.ijcard.2019.06.062
8. Nakanishi K, Jin Z, Russo C, Homma S, Elkind MS, Rundek T, et al. Association of chronic kidney disease with impaired left atrial reservoir function: a community-based cohort study. Eur J Prev Cardiol. (2017) 24:392–8. doi: 10.1177/2047487316679903
9. Vassiliou VS, Patel HC, Rosen SD, Auger D, Hayward C, Alpendurada F, et al. Left atrial dilation in patients with heart failure and preserved ejection fraction: insights from cardiovascular magnetic resonance. Int J Cardiol. (2016) 210:158–60. doi: 10.1016/j.ijcard.2016.02.101
10. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the Universal definition of heart failure: endorsed by the Canadian heart failure society, heart failure association of India, cardiac society of Australia and New Zealand, and Chinese heart failure association. Eur J Heart Fail. (2021) 23:352–80. doi: 10.1002/ejhf.2115
11. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed). (2022) 75:523. doi: 10.1002/ejhf.2333
12. Miró Ò, García Sarasola A, Fuenzalida C, Calderón S, Jacob J, Aguirre A, et al. Departments involved during the first episode of acute heart failure and subsequent emergency department revisits and rehospitalisations: an outlook through the NOVICA cohort. Eur J Heart Fail. (2019) 21:1231–44. doi: 10.1002/ejhf.1567
13. Tomasoni D, Lombardi C, Sbolli M, Cotter G, Metra M. Acute heart failure: more questions than answers. Prog Cardiovasc Dis. (2020) 63:599–606. doi: 10.1016/j.pcad.2020.04.007
14. Rubio-Gracia J, Demissei B, Ter Maaten J, Cleland J, O’Connor C, Metra M, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. (2018) 258:185–91.
15. Kalra P, Anagnostopoulos C, Bolger A, Coats A, Anker S. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. (2002) 39:1901–8.
16. Yancy CW, Jessup M, Bozkurt B, Butler J, Jr DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. (2013) 62:e147–239.
17. Dekkers C, Sjöström C, Greasley P, Cain V, Boulton D, Heerspink H. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab. (2019) 21:2667–73. doi: 10.1111/dom.13855
18. Kobayashi M, Girerd N, Duarte K, Preud’homme G, Pitt B, Rossignol P. Prognostic impact of plasma volume estimated from hemoglobin and hematocrit in heart failure with preserved ejection fraction. Clin Res Cardiol. (2020) 109:1392–401. doi: 10.1007/s00392-020-01639-4
19. Kobayashi M, Rossignol P, Ferreira J, Aragão I, Paku Y, Iwasaki Y, et al. Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin Res Cardiol. (2019) 108:549–61. doi: 10.1007/s00392-018-1385-1
20. Balderston J, Shah K, Paciulli S, Gertz Z. Usefulness of estimated plasma volume at postdischarge follow-up to predict recurrent events in patients with heart failure. Am J Cardiol. (2018) 122:1191–4. doi: 10.1016/j.amjcard.2018.06.057
21. Greene S, Fonarow G, Vaduganathan M, Khan S, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. (2015) 12:220–9.
22. Badano L, Nagueh S, Muraru D. Left atrial function: an overlooked metrics in clinical routine echocardiography. Eur J Heart Fail. (2019) 21:901–3. doi: 10.1002/ejhf.1475
23. Tsujiuchi M, Yamauchi T, Ebato M, Maezawa H, Nogi A, Ikeda N, et al. Prognostic value of left atrial size and functional indices measured by 3-dimensional speckle-tracking analysis. Circ J. (2019) 83:801–8. doi: 10.1253/circj.CJ-18-0554
Keywords: estimated plasma volume status, left atrial diameter, acute heart failure, hematocrit, hemoglobin
Citation: Chen X, Lin G, Dai C and Xu K (2023) Effect of estimated plasma volume status and left atrial diameter on prognosis of patients with acute heart failure. Front. Cardiovasc. Med. 10:1069864. doi: 10.3389/fcvm.2023.1069864
Received: 14 October 2022; Accepted: 06 January 2023;
Published: 25 January 2023.
Edited by:
Davide Stolfo, Azienda Sanitaria Università Integrata di Trieste, ItalyReviewed by:
Jiri Parenica, University Hospital Brno, CzechiaJulien Regamey, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2023 Chen, Lin, Dai and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoli Lin,  289055041@qq.com
289055041@qq.com
†These authors have contributed equally to this work and share first authorship
 Xiaomin Chen†
Xiaomin Chen†  Guoli Lin
Guoli Lin Kaizu Xu
Kaizu Xu