Real-world experience with the cusp-overlap deployment technique in transcatheter aortic valve replacement: A propensity-matched analysis
- 1Department of Cardiology, Pulmonology and Vascular Medicine, Medical Faculty, Heinrich Heine University, Düsseldorf, Germany
- 2CardioVascular Center (CVC) Frankfurt, Frankfurt, Germany
- 3Cardiovascular Research Institute Düsseldorf (CARID), Medical Faculty, Heinrich Heine University, Düsseldorf, Germany
Background: The implantation depth (ID) is a critical condition for optimal hemodynamic and clinical outcomes in transcatheter aortic valve replacement (TAVR). The recently recommended cusp-overlap technique (COT) offers optimized fluoroscopic projections facilitating a precise ID. This single-center observational study aimed to investigate short-term clinical performance, safety, and efficacy outcomes in patients undergoing TAVR with self-expandable prostheses and application of COT in a real-world setting.
Materials and methods: From September 2020 to April 2021, a total of 170 patients underwent TAVR with self-expandable devices and the application of COT, while 589 patients were treated from January 2016 to August 2020 with a conventional three-cusp coplanar view approach. The final ID and 30-day outcomes were compared after 1:1 propensity score matching, resulting in 150 patients in both cohorts.
Results: The mean ID was significantly reduced in the COT cohort (−4.2 ± 2.7 vs. −4.9 ± 2.3 mm; p = 0.007) with an improvement of ID symmetry of less than 2 mm difference below the annular plane (47.3 vs. 57.3%; p = 0.083). The rate of new permanent pacemaker implantation (PPI) following TAVR was effectively reduced (8.0 vs. 16.8%; p = 0.028). While the fluoroscopy time decreased (18.4 ± 7.6 vs. 19.8 ± 7.6 min; p = 0.023), the dose area product increased in the COT group (4951 ± 3662 vs. 3875 ± 2775 Gy × cm2; p = 0.005). Patients implanted with COT had a shorter length of in-hospital stay (8.4 ± 4.0 vs. 10.3 ± 6.7 days; p = 0.007).
Conclusion: Transcatheter aortic valve replacement using the cusp-overlap deployment technique is associated with an optimized implantation depth, leading to fewer permanent conduction disturbances. However, our in-depth analysis showed for the first time an increase of radiation dose due to extreme angulations of the gantry to obtain the cusp-overlap view.
Introduction
Transcatheter aortic valve replacement (TAVR) is a fast-growing section in interventional cardiology. In the last decade, TAVR has become a safe and effective alternative to surgical valve replacement (SAVR) to treat symptomatic severe aortic valve stenosis across all surgical risk categories (1–3). Optimized implantation depth (ID) of transcatheter heart valves (THV) is an essential condition for valuable hemodynamic and clinical outcomes. Implantation located too high toward the aorta can result in complicated coronary reaccess, paravalvular leakage, or even valve embolization. In contrast, deep implantation in the left ventricular outflow tract (LVOT) is associated with aortic regurgitation and increased risk of conduction disturbances leading to higher rates of permanent pacemaker implantation (PPI). Despite advanced development of THV design, pre-procedural planning, and progressive implanters’ experience, current PPI rates following TAVR–especially with self-expandable valves–have remained high (4, 5).
In 2020, the manufacturer of the self-expandable THV CoreValve Evolut (Medtronic Inc., Minneapolis, MN, United States) introduced new best practice recommendations for valve deployment, including the cusp-overlap technique (COT). This is a series of procedural steps designed to provide optimized angiographic projections for TAVR with self-expandable devices (6). Application of COT during valve deployment has been shown to result in a reduced risk of interaction with the conduction system below the annular plane and significantly lower PPI rates (7–9). However, there is not sufficient evidence showing correlations to the achievement of an optimized ID as well as potential pitfalls of a more complex implantation process regarding prosthesis repositioning, radiation dose or amount of contrast medium used.
Therefore, this single-center observational study aimed to investigate short-term clinical performance, safety, and efficacy outcomes in patients undergoing transfemoral, self-expandable TAVR with newer-generation CoreValve Evolut THV regarding COT during valve deployment in a real-world setting.
Materials and methods
Study population
From 1530 consecutive patients who underwent transfemoral TAVR with newer-generation self-expandable CoreValve Evolut system (Medtronic Inc., Minneapolis, MN, United States) from January 2016 to April 2021 at the Heart Center Düsseldorf, 759 patients with completed datasets were included in the analysis (Figure 1). Most of the patients excluded from the final analysis due to missing data have lack of documentation in procedural characteristics (pre- and post-dilatation, resheathing, valve dislocation) or post-procedural evaluation of valve function by missing documentation of echocardiography.
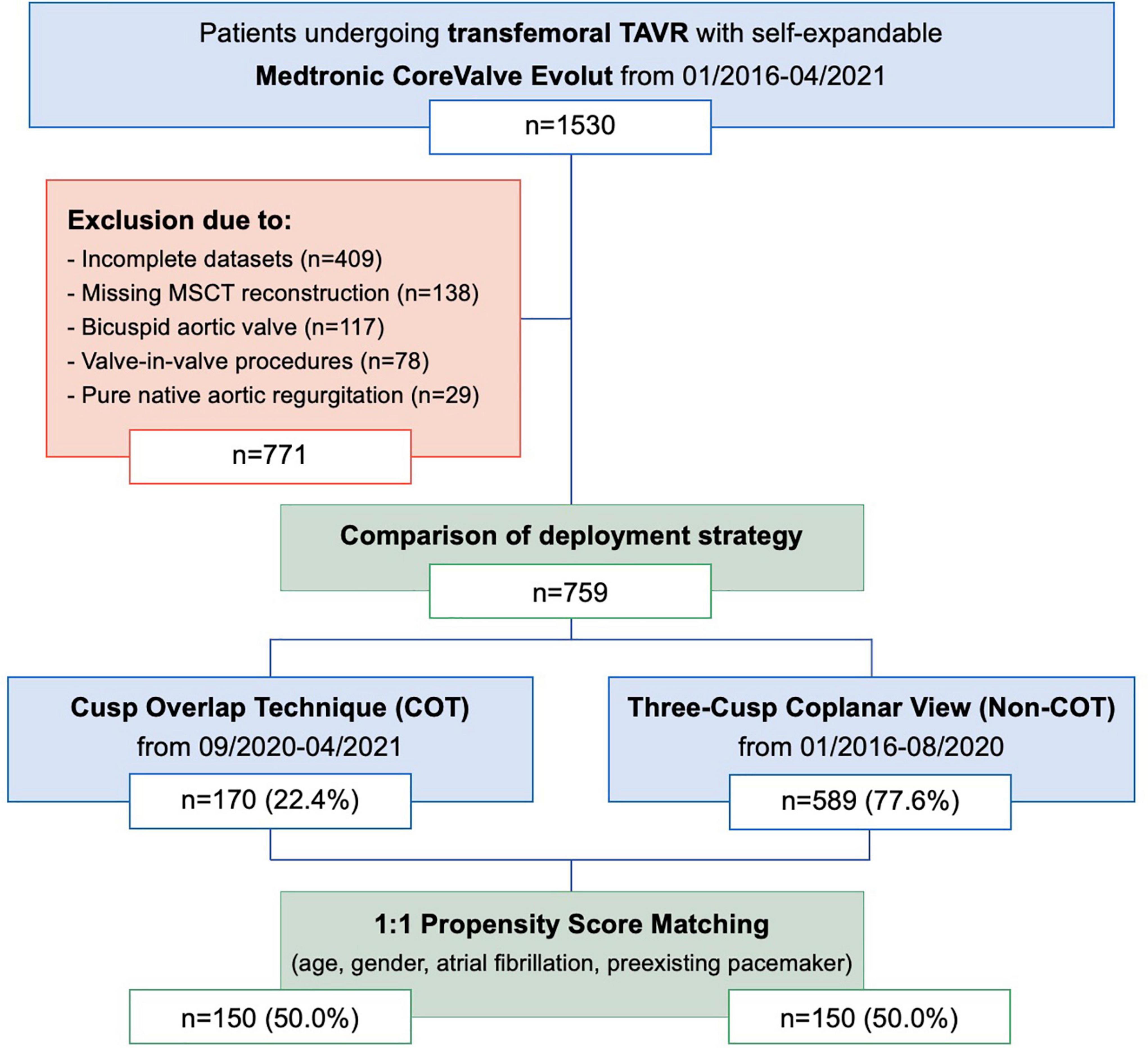
Figure 1. Modified CONSORT Diagram. From January 2016 to April 2021, a total of 1,530 patients underwent TAVR with the Medtronic CoreValve Evolut at the Heart Center Düsseldorf. A total of 759 patients were included in the comparison of the deployment strategy. A total of 1:1 propensity score matches analysis resulted in the final study cohort of 150 COT patients and 150 Non-COT patients. COT, cusp-overlap technique; MSCT, multislice computed tomography; TAVR, transcatheter aortic valve replacement.
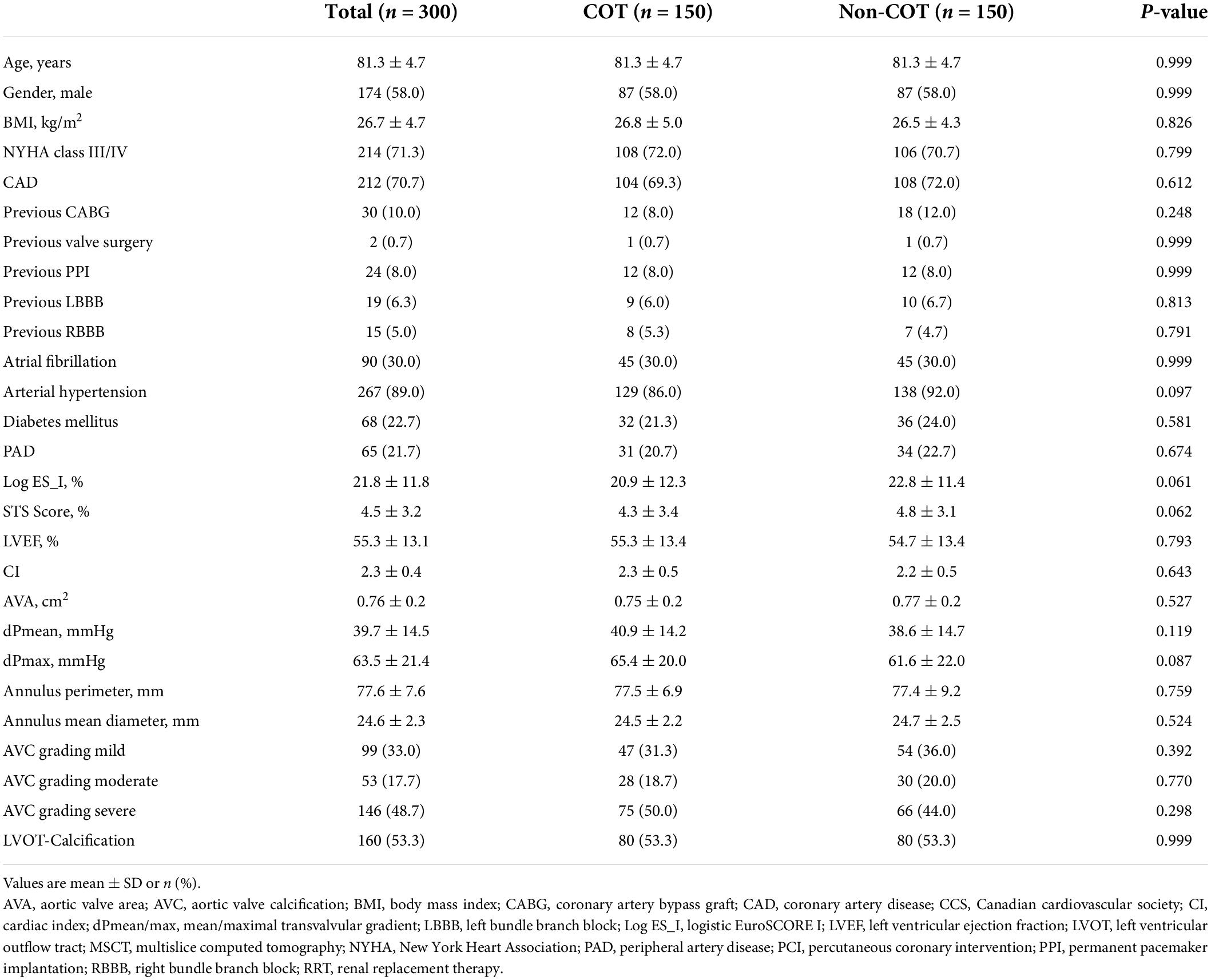
The study cohort was further separated into two groups. Patients undergoing TAVR according to Medtronic’s new best practice recommendations of 2020 regarding COT for prosthesis deployment and a target ID of 3 mm were analyzed prospectively from September 2020 to April 2021 (n = 170; 22.4%). The control group (Non-COT) treated with former manufacturer’s recommendations without COT but with a conventional three-cusp coplanar view and a target ID of 3–5 mm from January 2016 to August 2020 (n = 589; 77.6%) was analyzed retrospectively. To erase potential confounders of the treatment outcome relationship, we performed a 1:1 propensity-score matched analysis resulting in a final study cohort of 150 COT and 150 Non-COT patients (Figure 2). All included patients completed a 30-day follow-up examination to evaluate clinical outcome after TAVR based on Valve Academic Research Consortium-2 (VARC-2) definition (10).
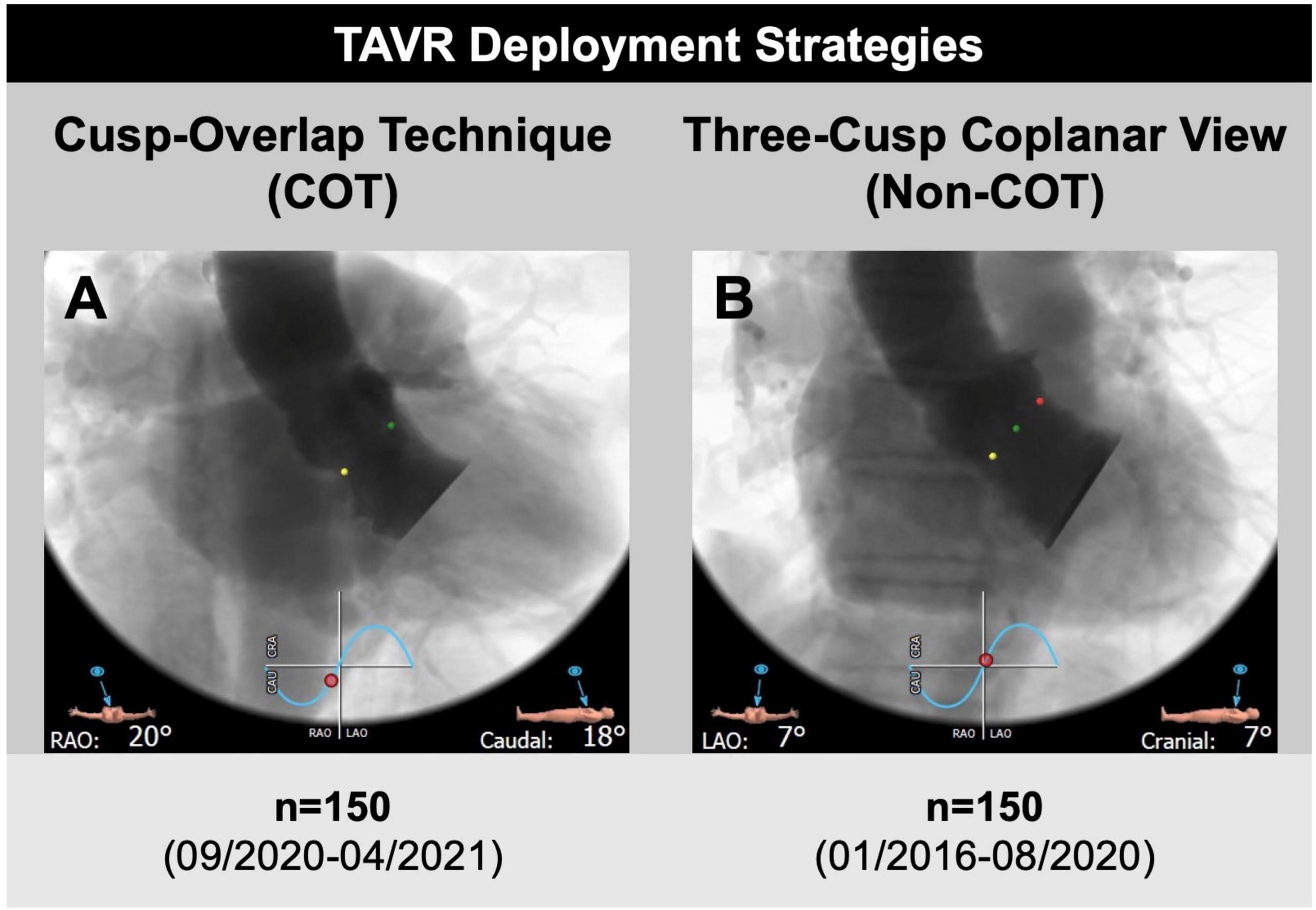
Figure 2. Transcatheter aortic valve replacement (TAVR) deployment strategies. (A) A total of 150 patients undergoing transfemoral TAVR with self-expandable Medtronic CoreValve Evolut prostheses and cusp-overlap deployment technique (COT) were compared to (B) 150 propensity-matched patients with application of a conventional three-cusp coplanar view only (Non-COT). The cusp-overlap view was generated by overlapping the right coronary cusp (RCC, green dot) and left coronary cusp (LCC, red dot) with isolation of non-coronary cusp (NCC, yellow dot), leading to reduction of parallax in the device and elongation of the left ventricular outflow tract.
The primary study endpoint was defined as the measurement of ID comparing valve deployment with and without COT during TAVR with self-expandable devices. Target ID is aspired to 3 mm for COT group, compared to target ID of 3–5 mm for Non-COT group, taking into account a measurement uncertainty of 1 mm each.
All participants in this study provided written informed consent. This study was approved by the institutional ethics committee of the Heinrich-Heine University of Düsseldorf and conducted in concordance with the Declaration of Helsinki. The study is registered at clinical trials (NCT01805739).
3D image analysis of multislice computed tomography
Multislice computed tomography was routinely performed as native and contrast-enhanced, electrocardiogram gated images. Pre-procedural MSCT data were transferred to a dedicated workstation for 3-dimensional volume-rendered reconstruction (3mensio Structural Heart; Pie Medical Imaging BV, Maastricht, Netherlands). Annular plane projection in the three-cusp view was routinely predicted from MSCT reconstruction and optimal angulation of the cusp-overlap view was generated by overlapping the right coronary cusp (RCC) and the left coronary cusp (LCC) on the MSCT annular plane toward right anterior oblique (RAO) and caudal angulation (6, 11).
Procedural details
Transcatheter aortic valve replacement procedures were conducted according to current guidelines and under local anesthesia. A total of four experienced operators were involved in this trial. In 2020, Medtronic issued new best practice advice for the CoreValve Evolut system, including cusp-overlap view with isolation of NCC for optimization of the ID during valve deployment. If extreme angulations of more than 30° RAO and/or 30°caudal were suggested for cusp-overlap view, a “near” cusp-overlap view with less extreme angulation was performed. Before final release, a three-cusp view was established to check for complete valve expansion and implantation depth in relation to the left coronary cusp.
The final ID of device implantation was determined angiographically in the three-cusp view after complete clearing of parallax in both COT and Non-COT groups for better visual differentiation of ID below NCC and LCC compared to the cusp overlap view (12). Distance measurements from the interventricular end of the prosthesis to the annular plane were performed afterward using the PACS system workstation (SECTRA IDS7, Linköping, Sweden). As described previously, the arithmetic means of the measured distances from the distal end of the prosthesis to the NCC and the LCC were assessed for final ID (13). Asymmetric valve deployment was defined as a more than 2 mm difference between the NCC and LCC distances. Two independent operators have performed ID measurement. The Pearson correlation coefficient was reported to assess intra- and interobserver reliability for the mean implantation depth measurements from the LCC and the NCC to the prosthesis in 50 randomly chosen cases. Results were interpreted as follows: >0.8, excellent agreement; 0.6 to 0.8, fair to good agreement; 0.4 to 0.6, moderate agreement; and <0.4, no agreement.
Statistical analysis
Continuous data are described as mean + standard deviation (SD) for normal distribution and comparisons were performed using unpaired Student’s t-test or Mann–Whitney U-test depending on the variable distribution. Categorical variables are presented as frequencies and percentages and comparisons were made using chi-square or Fisher exact test. All statistical tests were 2-tailed, and a value of p < 0.05 was considered statistically significant. To account for the differences in baseline characteristics of COT and Non-COT group, we performed 1:1 propensity score matched analysis using logistic regression and the nearest neighbor method with a caliper of 0.1 because of the observational nature of this study and the comparison of two non-contemporary cohorts with different group sizes. Covariates were chosen according to baseline differences between both cohorts listed in Table 1 (age, gender) as well as previous rhythm and conduction disturbances that could have a disturbing influence on the final analysis regarding new pacemaker dependency following TAVR (atrial fibrillation, preexisting pacemaker). Matching analysis resulted in a final 1:1 matched study cohort of 150 patients in both groups.
All statistical analyses were conducted using SPSS version 23.0 (IBM SPSS Inc., Chicago, IL, United States) and figures created with GraphPad Prism version 8.4 (GraphPad Software, San Diego, CA, United States).
Results
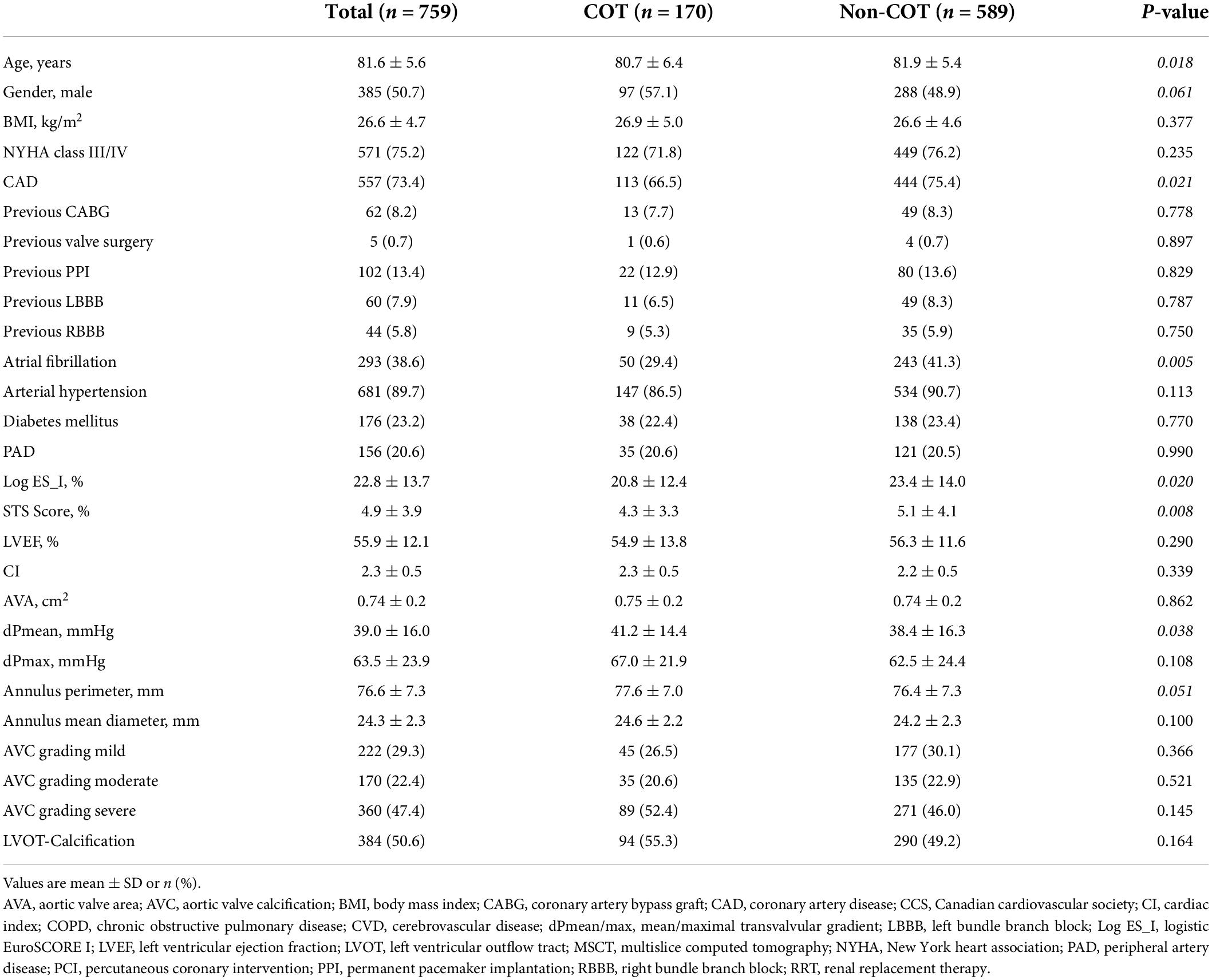
Baseline patient characteristics
In the unmatched cohorts (n = 759), patients undergoing TAVR with COT were younger (COT 80.7 ± 6.4 years vs. Non-COT 81.9 ± 5.4 years; p = 0.018), predominantly male (COT 57.1% vs. Non-COT 48.9%; p = 0.061) and had a lower incidence of coronary artery disease (COT 66.5% vs. Non-COT 75.4%; p = 0.021) as well as lower surgical risk (STS Score: COT 4.3 ± 3.3% vs. Non-COT 5.1 ± 4.1%; p = 0.008) (Table 1). COT patients showed less frequently atrial fibrillation (AF) (COT 29.4% vs. Non-COT 41.3%; p = 0.005). A total of 1:1 propensity score matching successfully eliminated any major differences between the two cohorts (Table 2).
Procedural characteristics
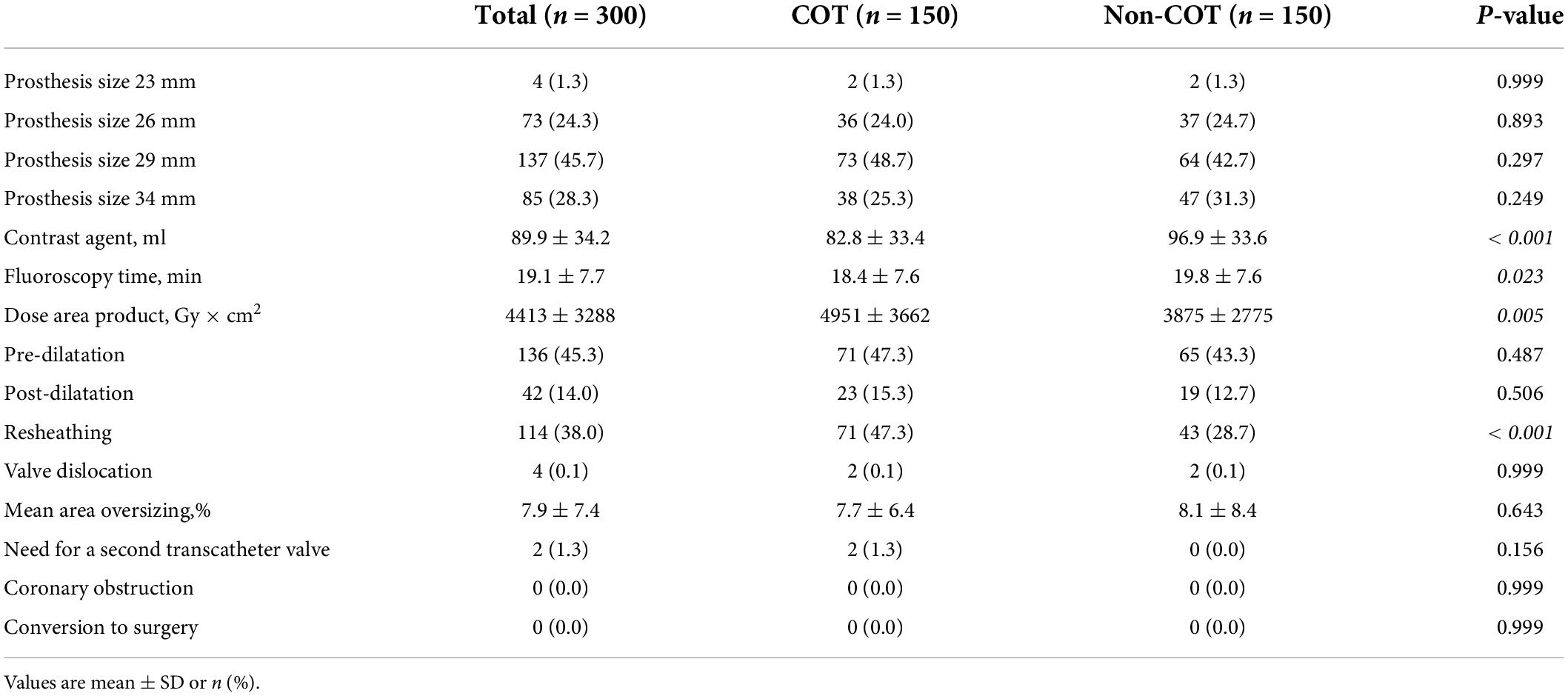
After pre-procedural MSCT planning to determine the optimal cusp-overlap projection angle, we achieved a projected cusp-overlap gantry view on CT reconstruction in 121 patients (80.7%) of patients and a near cusp-overlap projection in the remaining 29 cases (19.3%).
Valve size was equally distributed with most of the procedures performed with a 29-mm CoreValve Evolut prosthesis (45.7%). COT patients had less need of contrast agent volume (COT 82.8 ± 33.4 ml vs. Non-COT 96.9 ± 33.6 ml; p < 0.001) (Figure 3A) with a reduction in fluoroscopy time (COT 18.4 ± 7.6 min vs. Non-COT 19.8 ± 7.6 ml; p = 0.023) (Figure 3B). Patients undergoing TAVR with COT had a significantly higher radiation dose area product compared to the Non-COT group (COT 4951 ± 3662 Gy × cm2 vs. Non-COT 3875 ± 2775 Gy × cm2; p = 0.005) (Figure 3C). COT resulted in notably higher rates of device resheathing compared to the Non-COT group (COT 47.3% vs. Non-COT 28.7%; p < 0.001) (Figure 3D). Additional procedural data are shown in Table 3.
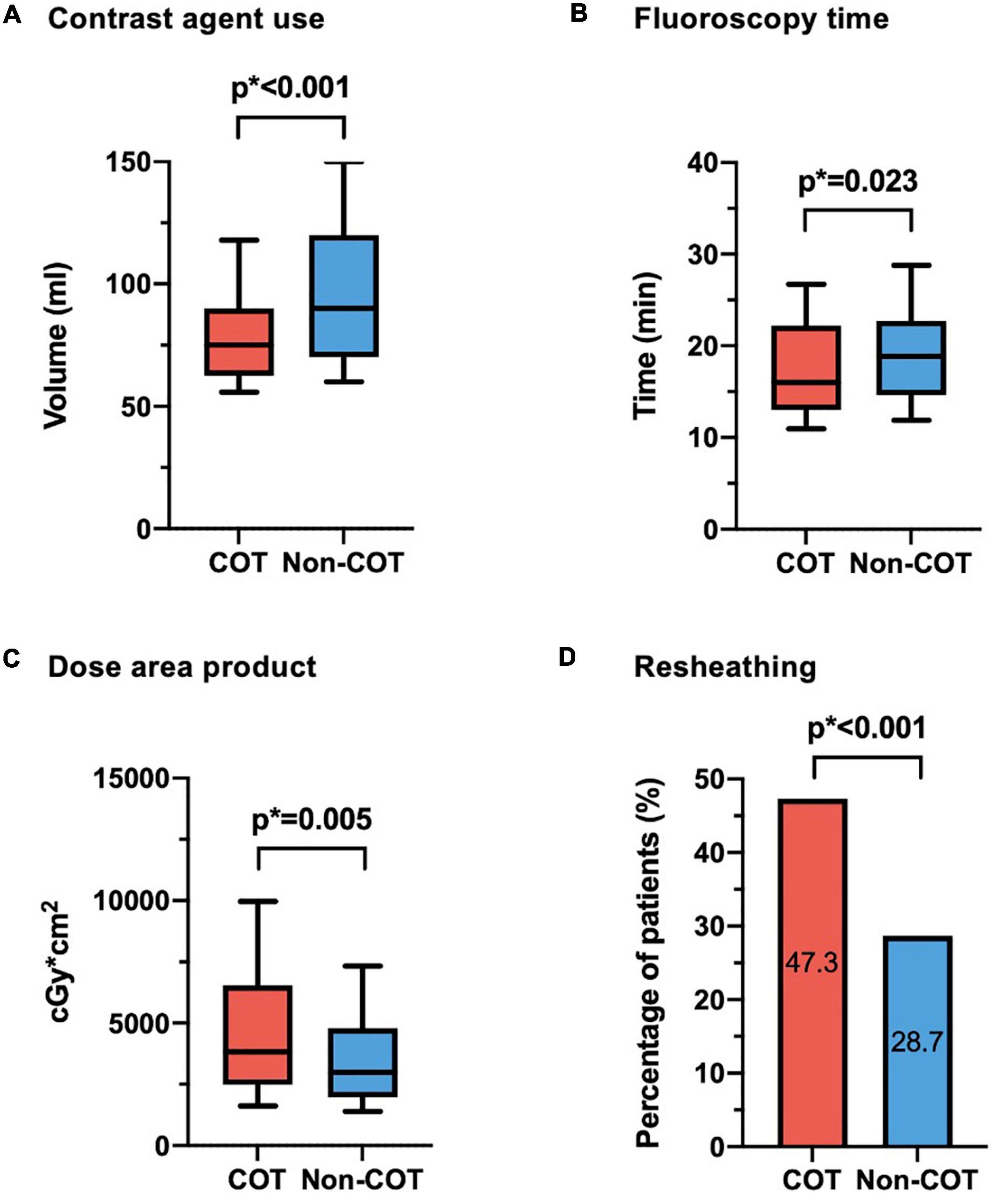
Figure 3. Procedural data. (A) Reduction of contrast agent use could be achieved in COT patients (COT 82.8 ± 33.4 ml vs. Non-COT 96.9 ± 33.6 ml; p < 0.001). (B) While fluoroscopy time was shorter (COT 18.4 ± 7.6 min vs. Non-COT 19.8 ± 7.6 ml; p = 0.023), (C) radiation dose area product was enhanced in the COT group (COT 4,951 ± 3,662 Gy × cm2 vs. Non-COT 3,875 ± 2,775 Gy × cm2; p = 0.005), probably due to more extreme angulations and (D) notably higher rates of device resheathing maneuvers (COT 47.3% vs. Non-COT 28.7%; p < 0.001) compared to the Non-COT group. COT, cusp-overlap technique.
Procedural and clinical outcome
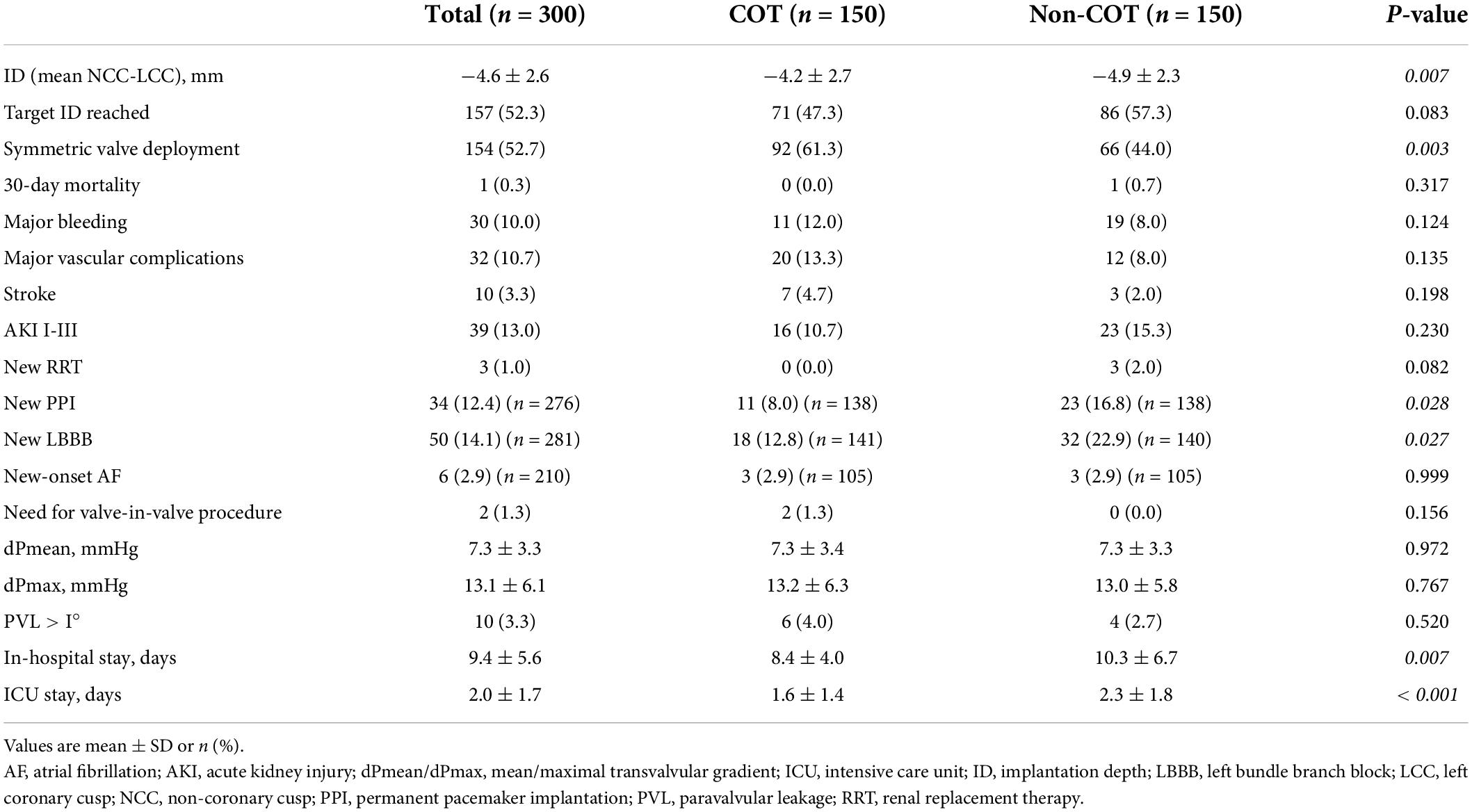
The final absolute mean ID was significantly reduced in the COT cohort (COT −4.2 ± 2.7 mm vs. Non-COT −4.9 ± 2.3 mm; p = 0.007) without a significant difference in the achievement of the target ID of 3 mm in the COT group and 3–5 mm in the Non-COT group (COT 47.3% vs. Non-COT 57.3%; p = 0.083) (Figures 4A,B). In COT patients valve deployment was conducted more symmetrically with a difference of less than 2 mm between NCC and LCC ID (COT 61.3% vs. Non-COT 44.0%; p = 0.003) (Figure 4C). Both the intra- and the interobserver reliability showed excellent agreement (r > 0.8) because we used a standardized measurement technique at our heart center for the analysis of ID after TAVR. Functional improvement was observed in both groups without significant differences concerning mean pressure gradients or paravalvular leakage assessed by TTE during 30-day follow-up (Figures 5A,B).
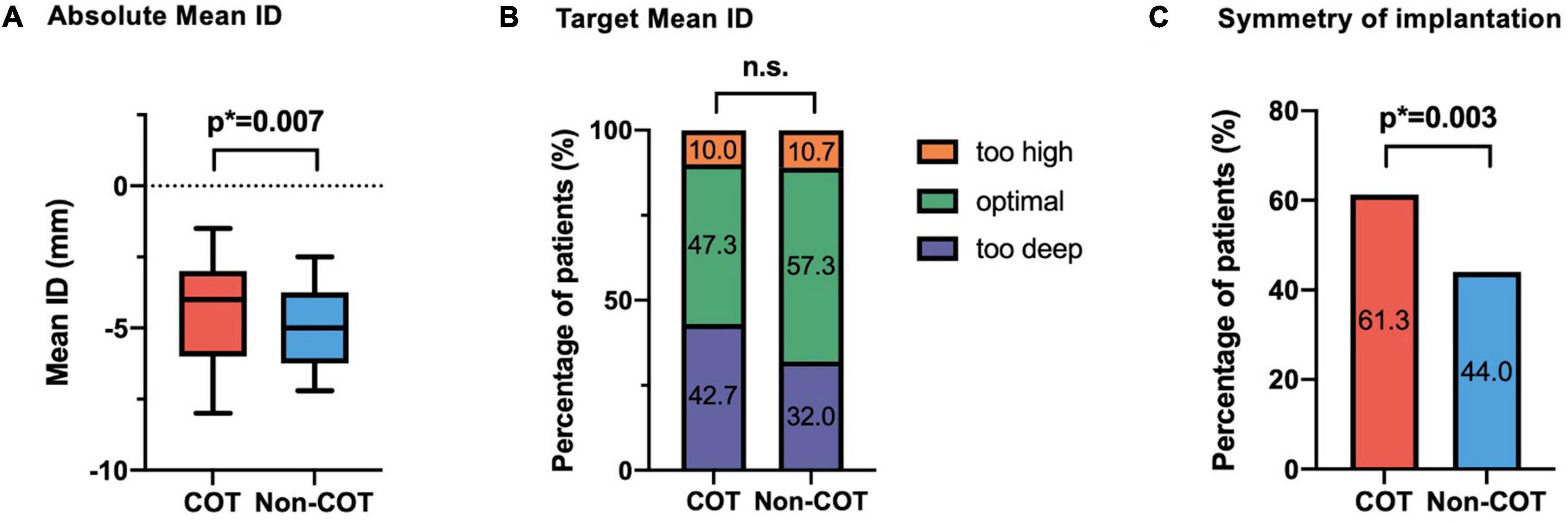
Figure 4. Impact of COT on implantation depth. (A) There was a significant reduction of final mean ID in the COT cohort (COT –4.2 ± 2.7 mm vs. Non-COT –4.9 ± 2.3 mm; p = 0.007), whereas (B) the target ID of 3 mm in the COT group and 3–5 mm in the Non-COT group was reached similarly often. (C) Reduction in asymmetric valve deployment with a difference of less than 2 mm between NCC and LCC ID could be achieved more often in the COT group (COT 61.3% vs. Non-COT 44.0%; p = 0.003). COT, cusp-overlap technique; ID, implantation depth.
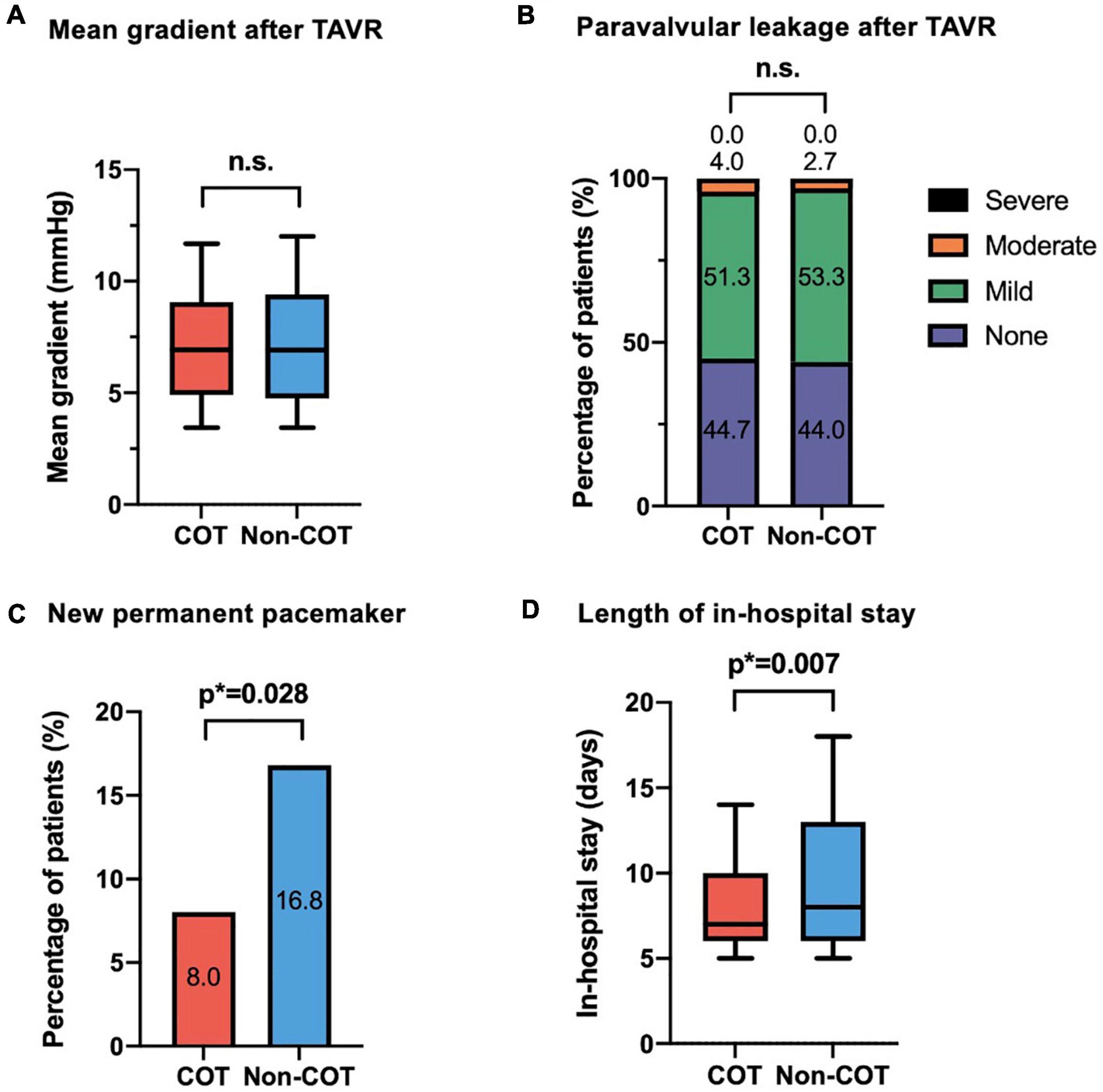
Figure 5. Efficacy outcome of COT. No difference could be observed in (A) mean aortic gradient and (B) paravalvular leakage following TAVR with different deployment techniques. In the COT group, fewer new permanent pacemaker implantations during 30-day follow-up were needed (COT 8.0% vs. Non-COT 16.8%; p = 0.028) (C), and a reduced length of in-hospital stay could be achieved (COT 8.4 ± 4.0 vs. Non-COT 10.3 ± 6.7 days; p = 0.007) (D). COT, cusp-overlap technique; TAVR, transcatheter aortic valve replacement.
The rate of new PPI following TAVR was markedly reduced in the COT group (COT 8.0% vs. Non-COT 16.8%; p = 0.028) (Figure 5C). The most frequent indication for pacemaker was high degree atrioventricular heart block by far (81 of 90 patients (90%) in the unmatched Non-COT group of 589 patients and 12 of 13 patients (92.3%) in the unmatched COT group of 170 patients). Other indications have been symptomatic bradycardia due to sick sinus syndrome or slowly conducted atrial fibrillation as well as bifascicular block. Therefore, indications for new pacemaker implantation after TAVR did not significantly change over time. Furthermore, the mean time from TAVR to pacemaker implantation has been 2.34 + 0.98 days in the Non-COT group and 2.12 + 0.84 days in the COT group (p = 0.289).
There was also an association between COT and lower incidence of new-onset left bundle branch block (LBBB) (COT 12.8% vs. Non-COT 22.9%; p = 0.027). Patients implanted with COT had a shorter length of ICU stay (COT 1.6 ± 1.4 days vs. Non-COT 2.3 ± 1.8 days; p < 0.001) and a shorter length of total in-hospital stay compared to Non-COT patients (COT 8.4 ± 4.0 vs. Non-COT 10.3 ± 6.7 days; p = 0.007) (Figure 5D). All procedures were performed successfully with only one case of 30-day mortality in the Non-COT group (one septic shock during in-hospital stay after TAVR). All 30-day post-procedural outcome parameters according to VARC-2 are shown in Table 4.
The outcome results between the unmatched cohorts are in large parts similar to those between the matched cohorts and do not differ in the main results, except for new left bundle branch block following TAVR. This parameter did not reach statistical significance in unmatched cohorts (COT 13.8% vs. Non-COT 16.9%; p = 0.364), but finally did after 1:1 propensity score matching (COT 12.8% vs. Non-COT 22.9%; p = 0.027). All procedural data and 30-day procedural outcome events of the unmatched study cohorts are shown in Supplementary Tables 1, 2.
Discussion
Until now, the impact of the recently recommended cusp-overlap technique on the implantation depth and efficacy outcome in a real-world setting using self-expandable devices is still being evaluated with many unknowns. This study demonstrates that application of COT during transfemoral TAVR with self-expandable THV causes.
(1) Optimization of implantation depth by both reducing the mean ID and improving the symmetry of ID between NCC and LCC,
(2) Significant reduction of permanent conduction disturbances with the need for new pacemaker implantation following TAVR,
(3) Consistent quality of hemodynamic outcome regarding valve pressure gradients and paravalvular leakage, and
(4) Increase of radiation dose due to extreme angulations.
After pre-procedural MSCT planning to determine the optimal cusp-overlap projection angle, we achieved a projected cusp-overlap gantry view on CT reconstruction in 81.9% of patients and a near cusp-overlap projection in the remaining cases. Finally, no increase in adverse events during 30-day follow-up could be observed compared to TAVR procedures without the usage of COT and a three-cusp coplanar view during valve deployment instead.
Improvement of implantation depth caused by cusp-overlap technique
To increase the accuracy of ID with Evolut CoreValve THV, we have performed COT according to the manufacturer’s recommendations since September 2020. In the three-cusp coplanar view, the device is often poorly aligned and there is no clear view of the native annulus relative to the conduction system due to the foreshortening of the LVOT (11). COT view results in an elongation of the LVOT, removement of the delivery catheter parallax, and accentuation of the NCC/RCC commissures in the center of the fluoroscopic view where the conduction system crosses the membranous septum below the atrioventricular node (6, 14). According to the new manufacturer’s advice, Evolut CoreValve’s target ID of 3 mm is recommended instead of a target ID of 3–5 mm as proposed before (15). Due to the wider range of former target ID of 3–5 mm in the Non-COT group (13), the present COT group with a narrow range of 3 mm target ID did not achieve higher proportions of successful target ID, but a significantly higher absolute mean ID was achieved by far. Not only the ID below a single coronary cusp but also the symmetry of valve implantation was improved. This is another benefit of COT during valve deployment because the NCC nadir is better visualized and both the device and the annulus are perpendicularly in-plane without parallax, allowing correct assessment of the true device depth below all three cusps (14).
Reliability of cusp-overlap technique in safety and clinical outcome
Although balloon-expandable valves exert higher radial forces, rates of new PPI are higher after implantation of self-expandable THV due to differences in the design of the prosthetic frame and technique of implantation. TAVR with supra-annular, newer-generation CoreValve Evolut prostheses have been associated with the need for new PPI in 14.7 to 26.7% of patients within 30 days after the intervention (4, 5).
The proximity of the aortic valve and the cardiac conduction system is one of the reasons for the occurrence of new conduction disturbances following TAVR (16, 17). The close relationship of the left bundle branch to the aortic root explains why LBBB is the most common conduction disturbance following TAVR and why ID greater than the membranous septum length is an independent predictor of PPI (18–20). Interaction of the THV with the surrounding tissue induces either direct injury by radial forces or indirect injury to the conduction tissue by localized edema or hematoma. Consequently, a complete AV block–the main indication for PPI by far in our analysis–may be either the result of total interruption of AV conduction or new-onset LBBB in patients with preexisting RBBB (21, 22).
Our single-center experience using COT for valve deployment showed that only 8% of patients without preexisting pacemaker required new PPI compared to 16.8% in Non-COT group, and the incidence of new-onset LBBB could also be reduced, what is in accordance with former studies (7–9). Because confounders like age and atrial fibrillation were eliminated by propensity score matching and other known procedural predictors like post-dilatation or oversizing did not differ between both groups, the difference in deployment technique seems to be the only reasonable explanation for this improvement in outcome.
A potential limitation of the COT is the implanter’s concern that a shallow THV implantation may lead to a higher rate of valve embolism with upward displacement (“pop-outs”), potentially resulting in increased procedural complexity and patient morbidity (23, 24). In our experience, we did not observe this event in COT group. Both cases with the need for a second valve in the COT group of our study were caused by moderate to severe valvular leakage after implantation of the first TAVR prosthesis due to infolding of the prosthesis and probably incorrect patient-prosthesis sizing.
The reasons for the reduction in length of in-hospital stay in the COT cohort are multifactorial, but one of them is very likely the reduced post-procedural conduction disturbances and therefore the decreased need for a temporary or permanent pacemaker. The clinical impact of new PPI following TAVR has been somehow controversial, but meta-analyses have suggested an increased risk of all-cause mortality at 1 year in patients receiving a new permanent pacemaker and a higher rate of heart failure rehospitalizations (25). Certainly, the requirement for PPI is associated with longer in-hospital stay and increased costs (26), leading to slower periprocedural recovery and increased resource utilization.
Impact of cusp-overlap technique on procedural performance during transcatheter aortic valve replacement
Similar to our results, there are already former studies examining the effect of COT on conduction disturbances following TAVR with observation of a significant reduction in both LBBB and PPI rate after TAVR (7–9). Mendiz et al. examined primary clinical outcomes without conclusions on procedural data like the achievement of target ID or the impact of COT on procedural aspects like the volume of contrast agent or the radiation dose used (7). Doldi et al. only reported the technical success according to the new 2021 VARC-3 criteria (27) without further procedural details (8). While Pascual et al. performed a propensity score analysis with different measurement techniques of target ID, no relevant information was published about possible procedural pitfalls of this complex implantation technique except for procedural success and fluoroscopic time without any significant difference between COT and Non-COT group (9).
At least in our hands, the price for optimized ID is a higher rate of resheathing due to a more accurate achievement of a target ID of exactly 3 mm than before with a range between 3 and 5 mm. Thus, higher rates of repositioning did not result in less device success or increased mortality of the COT group as described before during multiple resheathing (28). Thus, there is a trend toward increased stroke rates in the COT group compared to Non-COT. Although one could speculate that excessive manipulation in the aortic valve and its adjacent structures could affect stroke rates, no significant relationship has been found in former studies (28, 29). The non-randomized design of our study with different clinical and anatomical factors might have impacted this outcome, and confusion bias cannot be excluded.
Although even the length of fluoroscopy time could be reduced, the dose area product is higher in the COT group, what can be explained by more extreme angulations during cusp-overlap view with an automatic increment of radiation energy that is exposed to the patients’ body surface by the X-ray tube assembly (Figures 3B,C).
A significant reduction of contrast medium was observed in the COT cohort. This is the consequence of the need for less contrast agent usage to visualize the NCC only (2–4 ml) during most valve positioning up to the point of no return. A larger amount of contrast agent is only necessary for the final assessment of valve position before deployment, when, as a second angulation, a three-cusp view is obtained for position control and ID measurement. In contrast, during valve positioning in three-cusp only view, more contrast agent and longer fluoroscopy time were administered to delineate all three cusps.
Limitations
Several limitations should be considered when interpreting this study. This is a single-center observational study with a limited number of patients. Two non-contemporary groups were compared to assess differences between COT and 3-cusps coplanar view only technique with four different operators performing TAVR procedure with different levels of practical knowledge and experience. There may exist temporal, confounders and selection bias due to high rate of excluded patients with incomplete dataset that was not accounted for in our analysis. Therefore, larger multicenter randomized trials should be conducted to verify the usefulness of COT during THV deployment.
Conclusion
Transcatheter aortic valve replacement with self-expandable valves using the cusp-overlap deployment technique is associated with an optimized implantation depth, leading to fewer permanent conduction disturbances and a shortened length of in-hospital stay. Any prevention of conduction disturbances can potentially reduce rehospitalizations and late mortality rate, whereas the procedural and functional outcome is not remarkably influenced by COT. Thus, COT should be regularly performed for TAVR with a self-expandable prosthesis to achieve optimized ID, especially as TAVR is expanded to the low-risk population with higher life expectancy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of the Heinrich-Heine University of Düsseldorf (4080). The patients/participants provided their written informed consent to participate in this study.
Author contributions
OM: conception and design, investigation, analysis and interpretation of data, drafting of the manuscript, project administration, and final approval of the manuscript. KP, SB, NB, SA, AP, RW, PH, and CJ: analysis and interpretation of data, revision of the manuscript for important intellectual content, and final approval of the manuscript. KK: analysis and interpretation of data, data curation, revision of the manuscript for important intellectual content, and final approval of the manuscript. MK: conception and design, validation, drafting of the manuscript, and final approval of the manuscript. VV and TZ: conception and design, investigation, drafting of the manuscript, project administration, and final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was sponsored by Medtronic (Minneapolis, MN, United States) in the context of an external research program (ERP-2020-12514).
Acknowledgments
We thank Jenni Scharlau and Tatjana Wirachowski for their commitment to patient care and their substantial contribution to data acquisition and administration.
Conflict of Interest
VV, TZ, CJ, RW, AP, and OM had received consulting fees, travel expenses, or study honoraria from Medtronic (Minneapolis, MN, United States) and/or Edwards Lifesciences (Irvine, CA, United States).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.847568/full#supplementary-material
Abbreviations
BEV, balloon-expandable valve; COT, cusp-overlap technique; DLZ, device landing zone; ID, implantation depth; LCC, left coronary cusp; MSCT, multislice computed tomography; NCC, non-coronary cusp; PPI, permanent pacemaker implantation; RCC, right coronary cusp; SAVR, surgical aortic valve replacement; SEV, self-expandable valve; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve.
References
1. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91. doi: 10.1093/eurheartj/ehx391
2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
3. Popma JJ, Michael Deeb G, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380:1706–15. doi: 10.1056/NEJMoa1816885
4. Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi N, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. J Am Coll Cardiol Intv. (2021) 14:115–34. doi: 10.1016/j.jcin.2020.09.063
5. Van Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: A systematic review. Eur Heart J. (2018) 39:2003–13. doi: 10.1093/eurheartj/ehx785
6. Tang GHL, Zaid S, Michev I, Ahmad H, Kaple R, Undemir C, et al. “Cusp-Overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. J Am Coll Cardiol Intv. (2018) 11:1663–5. doi: 10.1016/j.jcin.2018.03.018
7. Mendiz OA, Noè M, Fava CM, Guti LA, Valdivieso R, Gada H, et al. Impact of cusp-overlap view for TAVR with self-expandable valves on 30-day conduction disturbances. J Interv Cardiol. (2021) 2021:9991528. doi: 10.1155/2021/9991528
8. Doldi PM, Stolz L, Escher F, Steffen J, Gmeiner J, Roden D, et al. Transcatheter aortic valve replacement with the self-expandable core valve evolut prosthesis using the cusp-overlap vs. tricusp-view. J Clin Med. (2022) 11:1561. doi: 10.3390/jcm11061561
9. Pascual I, Hernández-Vaquero D, Alperi A, Almendarez M, Avanzas P, Kalavrouziotis D, et al. Permanent pacemaker reduction using cusp-overlapping projection in TAVR. JACC Cardiovasc Interv. (2022) 15:150–61. doi: 10.1016/j.jcin.2021.10.002
10. Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The valve academic research consortium-2 consensus document (varc-2). Eur J Cardio-Thoracic Surg. (2012) 42:S45–60. doi: 10.1093/ejcts/ezs533
11. Piazza N, Mylotte D, Lauzier PT. Fluoroscopic “heart chamber” anatomy - The case for imaging modality-independent terminology. EuroIntervention. (2016) 12:Y9–15. doi: 10.4244/EIJV12SYA3
12. Hokken TW, Wolff QM, Schermers T, van Wiechen MP, Ooms JF, Adrichem R, et al. Cusp Overlap versus 3-cusps-aligned transcatheter aortic valve depth assessment with different angiography projections by multidetector computed tomography. JACC Cardiovasc Interv. (2022) 15:231–3. doi: 10.1016/j.jcin.2021.10.004
13. Piayda K, Hellhammer K, Veulemans V, Sievert H, Gafoor S, Afzal S, et al. Navigating the “Optimal Implantation Depth” with a self-expandable TAVR device in daily clinical practice. J Am Coll Cardiol Intv. (2020) 13:679–88. doi: 10.1016/j.jcin.2019.07.048
14. Ben-Shoshan J, Alosaimi H, Lauzier PT, Pighi M, Talmor-Barkan Y, Overtchouk P, et al. Double S-Curve Versus cusp-overlap technique: Defining the optimal fluoroscopic projection for TAVR with a self-expanding device. J Am Coll Cardiol Intv. (2021) 14:185–94. doi: 10.1016/j.jcin.2020.10.033
15. Petronio AS, Sinning JM, Van Mieghem N, Zucchelli G, Nickenig G, Bekeredjian R, et al. Optimal implantation depth and adherence to guidelines on permanent pacing to improve the results of transcatheter aortic valve replacement with the medtronic corevalve system: The CoreValve prospective, international, post-market ADVANCE-II study. J Am Coll Cardiol Intv. (2015) 8:837–46. doi: 10.1016/j.jcin.2015.02.005
16. Kawashima T, Sato F. Visualizing anatomical evidences on atrioventricular conduction system for TAVI. Int J Cardiol. (2014) 174:1–6. doi: 10.1016/j.ijcard.2014.04.003
17. Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. (2008) 1:74–81. doi: 10.1161/CIRCINTERVENTIONS.108.780858
18. Hamdan A, Guetta V, Klempfner R, Konen E, Raanani E, Glikson M, et al. Inverse relationship between membranous septal length and the risk of atrioventricular block in patients undergoing transcatheter aortic valve implantation. J Am Coll Cardiol Intv. (2015) 8:1218–28. doi: 10.1016/j.jcin.2015.05.010
19. Oestreich BA, Mbai M, Gurevich S, Nijjar PS, Adabag S, Bertog S, et al. Computed tomography (CT) assessment of the membranous septal anatomy prior to transcatheter aortic valve replacement (TAVR) with the balloon-expandable SAPIEN 3 valve. Cardiovasc Revascularization Med. (2018) 19:626–31.
20. Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol Intv. (2019) 12:1796–807. doi: 10.1016/j.jcin.2019.05.056
21. Siontis GCM, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J Am Coll Cardiol. (2014) 64:129–40. doi: 10.1016/j.jacc.2014.04.033
22. Siontis GCM, Praz F, Lanz J, Vollenbroich R, Roten L, Stortecky S, et al. New-onset arrhythmias following transcatheter aortic valve implantation: A systematic review and meta-analysis. Heart. (2018) 104:1208–15.
23. Hellhammer K, Piayda K, Afzal S, Veulemans V, Hennig I, Makosch M, et al. Micro-dislodgement during transcatheter aortic valve implantation with a contemporary self-expandable prosthesis. PLoS One. (2019) 14:e0224815. doi: 10.1371/journal.pone.0224815
24. Hachinohe D, Latib A, Laricchia A, Demir OM, Agricola E, Romano V, et al. Anatomic and procedural associations of transcatheter heart valve displacement following Evolut R implantation. Catheter Cardiovasc Interv. (2019) 93:522–9. doi: 10.1002/ccd.27827
25. Faroux L, Chen S, Muntané-Carol G, Regueiro A, Philippon F, Sondergaard L, et al. Clinical impact of conduction disturbances in transcatheter aortic valve replacement recipients: A systematic review and meta-analysis. Eur Heart J. (2020) 41:2771–81. doi: 10.1093/eurheartj/ehz924
26. Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: Analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT registry. J Am Coll Cardiol Intv. (2016) 9:2189–99. doi: 10.1016/j.jcin.2016.07.026
27. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur Heart J. (2021) 42:1825–57. doi: 10.1093/eurheartj/ehaa799
28. Bernardi FLM, Rodés-Cabau J, Tirado-Conte G, Amat Santos IJ, Plachtzik C, Cura F, et al. Incidence, predictor, and clinical outcomes of multiple resheathing with self-expanding valves during transcatheter aortic valve replacement. J Am Heart Assoc. (2021) 10:e020682. doi: 10.1161/JAHA.120.020682
Keywords: aortic stenosis, TAVR, implantation depth, cusp-overlap, permanent pacemaker
Citation: Maier O, Piayda K, Binnebößel S, Berisha N, Afzal S, Polzin A, Klein K, Westenfeld R, Horn P, Jung C, Kelm M, Veulemans V and Zeus T (2022) Real-world experience with the cusp-overlap deployment technique in transcatheter aortic valve replacement: A propensity-matched analysis. Front. Cardiovasc. Med. 9:847568. doi: 10.3389/fcvm.2022.847568
Received: 02 January 2022; Accepted: 15 August 2022;
Published: 31 August 2022.
Edited by:
Ronak Rajani, Guy’s and St Thomas’ NHS Foundation Trust, United KingdomReviewed by:
Tanja Katharina Rudolph, Heart and Diabetes Center North Rhine-Westphalia, GermanyGuillem Muntané Carol, Laval University, Canada
Martin Andreas, Medical University of Vienna, Austria
Copyright © 2022 Maier, Piayda, Binnebößel, Berisha, Afzal, Polzin, Klein, Westenfeld, Horn, Jung, Kelm, Veulemans and Zeus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Verena Veulemans, verenaveulemans@web.de
†These authors have contributed equally to this work
 Oliver Maier
Oliver Maier Kerstin Piayda2
Kerstin Piayda2  Amin Polzin
Amin Polzin Patrick Horn
Patrick Horn Christian Jung
Christian Jung Verena Veulemans
Verena Veulemans Tobias Zeus
Tobias Zeus