How to interpret serum creatinine increases during decongestion
- 1Nephrology Service, Hospital Civil de Guadalajara Fray Antonio Alcalde, Guadalajara, Mexico
- 2University of Guadalajara Health Sciences Center, Guadalajara, Mexico
- 3Heart Failure and Heart Transplant Clinic, Hospital de Cardiología, Instituto Mexicano del Seguro Social, Mexico City, Mexico
During decongestion in acute decompensated heart failure (ADHF), it is common to observe elevations in serum creatinine (sCr) values due to vascular congestion, a mechanism that involves increased central venous pressure that has a negative impact on the nephron, promoting greater absorption of water and sodium, increased interstitial pressure in an encapsulated organ developing “renal tamponade” which is one of main physiopathological mechanism associated with impaired kidney function. For the treatment of this syndrome, it is recommended to use diuretics that generate a high urinary output and natriuresis to decongest the venous system, during this process the sCr values can rise, a phenomenon that may bother some cardiologist and nephrologist, since raise the suspicion of kidney damage that could worsen the prognosis of these patients. It is recommended that increases of up to 0.5 mg/dL from baseline are acceptable, but some patients have higher increases, and we believe that an arbitrary number would be impractical for everyone. These increases in sCr may be related to changes in glomerular hemodynamics and true hypovolemia associated with decongestion, but it is unlikely that they are due to structural injury or truly hypoperfusion and may even have a positive connotation if accompanied by an effective decongestion and be associated with a better prognosis in the medium to long term with fewer major cardiovascular and renal events. In this review, we give a comprehensive point of view on the interpretation of creatinine elevation during decongestion in patients with ADHF.
Case presentation
A 66-year-old man was admitted to the emergency room with dyspnea and lower limb edema that worsened in the last 5 days. He has diabetes and hypertension in the past 14 years, chronic kidney disease G3aA2 (estimated glomerular filtration rate of 47 ml/min/1.73 m2 and albuminuria 122 mg) and heart failure with mildly reduced left ventricular systolic function. He last weight was 82 kg 1 month ago and now is 86 kg. He was taking losartan 50 mg every 12 h, dapagliflozin 10 mg, metformin 850 mg every 12 h, atorvastatin 20 mg and chlorthalidone 50 mg, the latter being stopped in the last 3 weeks because he forgot to take them on his short trip. His blood pressure was 176/96 mmHg, 92 beats per minute, 21 breaths per minute, oxygen saturation 93% at room air, jugular venous pressure at 20 cm H2O, lung ultrasound showed 16 B lines in 8 fields, a jugular vein radius < 2, VExUS score of 3, BNP 32,000 ng/dL, CA-125 88 mg/dL, and serum creatinine (sCr) 1.7 mg/dL (baseline 1.4 mg/dL 2 months ago). The patient received furosemide bolus followed by high-dose infusion, acetazolamide, continue the same previous doses of losartan, dapagliflozin and spironolactone for 3 days, seeking to achieve some of the results of the recent ADVOR and EMPULSE clinical trials, to promote greater natriuresis and urinary volume. At that time his urine output was 9.5 L, which resulted in improving dyspnea and lower limb edema, as well as a decrease in the VExUS score to 0. BNP decreased to 4,200 ng/dL, but sCr increased to 1.8, 2.5, and 2.9 mg/dL.
Introduction
Cardiorenal syndrome is the most studied organic interaction in medicine. The type 1, is the one that combines the acute worsening of kidney function (WKF) after the acute worsening of cardiac function (1), often observed in patients with acute decompensated heart failure (ADHF) who develop acute elevation of sCr. It carries high morbidity and mortality, increasing up to 5 times the risk of dying within 28 days (2), being the most lethal of the five types of cardiorenal syndromes (3, 4). This is not surprising when observing the intricate pathophysiological mechanism that it possesses, but today it can be established that congestion is one of the main determinants of kidney disfunction, secondary to neurohormonal, inflammatory, and hemodynamic activation, which worsens the kidney inability to excrete sodium and water (5, 6), promoting compression of the kidney parenchyma, an entity recently described as “congestive nephropathy” (7).
Impaired kidney function with ADHF
The worsening of the glomerular filtration rate (GFR) in congestive nephropathy could be explained by the following four mechanisms: (1) The kidney, being encapsulated, by increasing central venous pressure, promotes increased pressure in the interstitium, and inside the tubules, opposing the filtration force in Bowman’s capsule. (2) Enhanced activation of the renin angiotensin system (RAAS) and the sympathetic system increases interstitial oncotic pressure, which pulls water and Na+ from the tubule, leaving it without chloride and Na+. (3) The neurohormonal activation causes mesangial contraction, which decreases the surface area and capillary permeability, and (4) The hyperabsorption of Na+ increases the consumption of O2 in the renal tubule creating an hypoxic environment (8).
Decongestion of type 1 cardiorenal syndrome with diuretics
The relief of severe decongestion should be considered an emergency and all possible therapeutic efforts should be made to improve this condition as soon as possible. In these cases, it is recommended to use diuretics and in refractory cases even extracorporeal ultrafiltration (9). It has been reported that the faster the decongestion, the better the prognosis. The Heart Failure Association of the European Society of Cardiology proposes how to administer loop diuretics with the therapeutic objective of achieving a urinary volume of >3–4 L per day (10) until reaching decongestion.
Furosemide is the most studied loop diuretic, it is suggested to administer large doses to achieve the goals, and the doses of intravenous furosemide used in different clinical trials vary greatly from that reported in the technical data sheet of the drug, doses ranging from 125 to 1,500 mg per day (11). In refractory cases, it has been suggested to apply sequential blockade of the renal tubule with different drugs that prevent the absorption of sodium and water in different sites, obtaining a response in up to 62% of the cases as evidenced by greater urinary volume (12). Options include adding drugs such as thiazides (13), mineralocorticoid receptor blockers (14), carbonic anhydrase inhibitors (15), sodium glucose transporter type 2 inhibitors (16, 17), and even hypertonic saline solutions (18). In a clinical trial carried out in patients with cardiorenal syndrome type 1, a sequential blockade strategy (with stable furosemide dose) was compared with increasing doses of furosemide. The trial reported similar effect in achieving recovery of kidney function, decongestion, and adverse events (19).
Serum creatinine as a biomarker of kidney function in ADHF
Creatinine has great limitations when evaluating kidney function in patients with acute pathologies, where its values can change unpredictably and also take time to do so. Another great limitation is its generation because this is a complex process that depends on multiple metabolic steps that go from the interaction between arginine and glycine in the liver, pancreas and kidney, generating guanidinoacetate (glycocyamine), and then passing to the systemic circulation where it is metabolized to creatine and finally in the muscle is derived to phosphocreatine and creatinine (20). The latter is freely filtered and a small amount is secreted into the tubular lumen. During AKI the increase in sCr is due to a decrease in GFR and backleak through damaged proximal tubule cells (21), due to these complex steps, in different clinical scenarios, the increase in sCr does not always represent a true damage to the kidney parenchyma.
Another potential limitation of sCr is in states of fluid overload, commonly observed in AHF, where it might be possible Cr is affected by “dilution,” which interferes with its proper determination (22). That is one of the many reasons why the new biomarkers emerge with the intention to improve these limitations, improving the prediction of kidney injury, specifying the location of damage and ascertaining the severity and predicting the clinical evolution (23).
Serum creatinine elevation during decongestion
Given the hypothesis of congestive nephropathy, diuretics should improve kidney function by reducing venous pressure (24). But this does not happen in all patients. During decongestion in patients with AHF, it has been reported that approximately 50% of patients increase sCr levels (25) or experience a 20% change in sCr (26–28). It has been suggested based on an expert’s opinion that sCr elevation < 0.5 mg/dL during decongestion of patients with AHF could be permissive (9), but many patients who achieve a successful decongestion exceed this value of sCr. It is in these cases where clinicians begin to doubt whether, despite achieving the objectives, they’re going in the right way. The classical paradigm would be to expect negative connotations with these sCr increases, but this phenomenon does not always represent an adverse event. A clear example that Cr elevations are associated with a better clinical course, such as a decrease in the risk of dying or presenting cardiovascular events is observed in the first 2 weeks of starting of sodium glucose type 2 inhibitors in diabetes-associated nephropathy (29), the start of RAASi (30), or in intense blood pressure control (< 120/80 mmHg) (31). This elevation of sCr induced with some treatments should be interpreted in the correct clinical context, which would be completely the opposite to the elevation that occurs in sepsis (32, 33), obstructive nephropathy (34), or post-surgery, where it is known extensively its pathological significance. Another example of this controversy occurs in some cardiac intensive care units, where it has been considered the initiation of continuous renal replacement therapies in patients with elevated sCr who meet the AKI KDIGO 2–3 criteria (35) without consider the full context.
Despite these weaknesses sCr has continued to be used, and in fact it has performed better than other biomarkers at predicting negative events in ADHF. For example, in a cohort of 787 patients with ADHF who were decongested several biomarkers were measured including brain natriuretic peptide, high sensitivity cardiac troponin I, galectin 3, serum neutrophil gelatinase-associated lipocalin, and urine neutrophil gelatinase-associated lipocalin. When considering elevation of sCr as the criterion for WRF, it was observed that no biomarker predicted WRF better than creatinine. Importantly, in the multivariable Cox analysis, brain natriuretic peptide, and high sensitivity cardiac troponin I, but not WRF, were significantly associated with the 1-year composite of death or heart failure hospitalization (36).
The reason why some patients increase sCr is not clear. Different pathogenic mechanisms have been proposed (37), as shown in Figure 1. Thus, in the following sections we will discuss potential causes of sCr elevation during decongestion in patients with ADHF, including those with greater or lesser biological plausibility and scientific evidence. Among those that are considered to have low quality evidence would be the hypotheses of intense parenchymal injury that would have consequently acute tubular necrosis. True tubular parenchymal damage during this scenario is an unusual event. A post hoc analysis of the clinical trial CARRESS-HF evaluated other biomarkers in addition to sCr during decongestion. The authors described that effective decongestion was associated with increased urinary renal tubular injury biomarkers such as N-acetyl-glucosaminidase (NAG), kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL), and importantly, they reported that WKF increased up to 12 times the possibility that these tubular biomarkers increase. However, increases in urinary renal tubular biomarkers were paradoxically associated with better kidney recovery at 60 days (25) (Figure 1A).
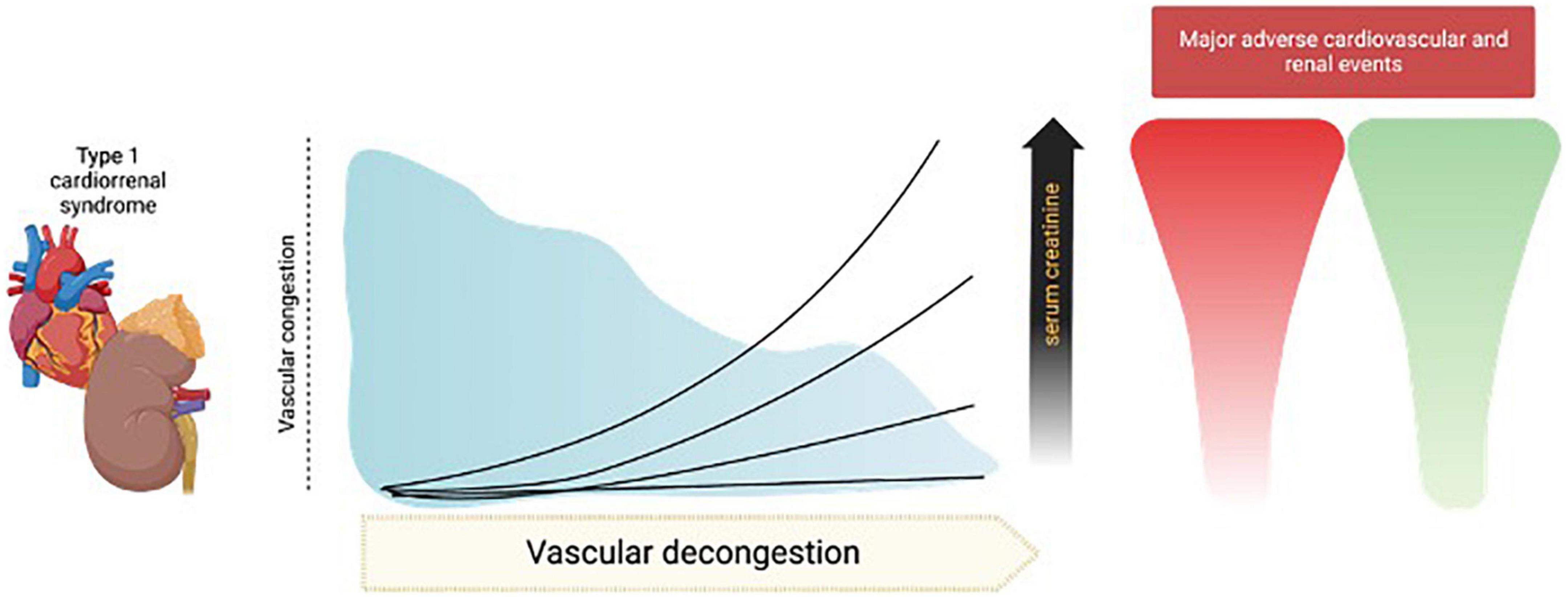
Figure 1. Causes of increase in serum creatinine (sCr) during decongestion. Hypotheses that are unlikely to play a critical role, such as ischemic damage that induces acute tubular necrosis and sCr hemoconcentration, are described in the panel (left). In the panel (right), the theories that are more likely and would have more prominence during the appearance of this event such as renal hypoperfusion, the induction of true intravascular hypovolemia and neurohormonal activation with hemodynamic alteration of the afferent and efferent glomerular arteries. The image was licensed from Biorender.
Another explanation for the elevation of sCr with scare clinical evidence is hemoconcentration that happens during diuretics in ADHF patients. Historically, it has been postulated that during intense decongestion, the increase in sCr may be due to an effect of hemoconcentration, but this was demystified in a post hoc study of the ROSE clinical trial. In that study 270 patients with ADHF and aggressive diuresis during 3 days were analyzed, and it was observed that the change in sCr may be due to hemoconcentration, only in those patients with urinary output > 7.5 L, an unusual event with the usual management, emphasizing that the hemoconcentration of sCr would not be the cause in most of these cases (38) (Figure 1A). On the other hand, the most plausible explanations would be alterations in renal perfusion, true intravascular hypovolemia, and neurohormonal activation with hemodynamic changes in the glomerular arteries.
This elevation of sCr could represent a state of relative hypovolemia, in which decreasing left ventricular end-diastolic pressure decreases renal perfusion to a pathological point. Loop diuretics can profoundly worsen tubuloglomerular feedback because they inhibit the sodium/potassium/2-chloride cotransporter, which promotes large amounts of these electrolytes to travel through the tubule, the macula densa sense chloride, then adenosine release falls, leading to afferent arteriolar vasodilation, and increased renin release from granular cells. Neurohormonal activation causes efferent and, to a lesser extent, afferent arteriolar vasoconstriction, causing decreased GFR due to hypoperfusion (39, 40) (Figure 1B).
Due to the changes and interactions of various markers and metrics during decongestion, it is relevant to consider the usefulness of guiding decongestion using a “multiparametric view” beyond sCr elevation objectively determining intravascular volume and assess bedside systolic volume with FoCUS as an indicator of intravascular hypovolemia.
Clinical significance of decongestion and creatinine increases
Bad creatinine elevation
Previous studies have shown that WKF during decongestion in ADHF has negative connotations, including very small increases in sCr (> 0.1 mg/dL) that would have a negative impact (41). In a study of 1,004 patients with ADHF, increased sCr > 0.3 mg/dL occurred in 1 out of 5 patients and was associated with prolonged hospitalization, and mortality (42). A meta-analysis of eight studies and 18,634 patients with HF, the authors defined WRF as increased sCr > 0.2 mg/dL or impaired GFR > 5 ml/min/1.73 m2, they reported that this event occurs in 25% of patients and was associated with increased 62% the risk of dead and had a stronger association with higher increases in sCr (43). An important limitation of this study was that they did not consider whether these patients reached decongestion during this process. Another meta-analysis of 28 studies and 49,890 patients reported that WRF occurs in 23% of cases and was associated with a 95% increased risk of death (44), but again, it does not analyze whether decongestion was achieved.
It is noteworthy that a study of 52 patients with type 1 cardiorenal syndrome refractory to diuretics where they were treated with kidney replacement therapy (KRT), the sCr values were not different between the groups of patients who recovered kidney function after follow-up, those who were dependent on KRT or those who died (45), a result that could be interpreted in favor of the fact that the severity of WKF is not assessable only with sCr levels.
But contemporary data have consistently indicated that WKF is not always associated with poor outcomes. And they have changed the notion that sCr elevations are always due to kidney pathological changes, it is alternatively proposed that hemodynamic and functional changes of the glomerulus may occur (46). It is important to always consider other etiologies of elevation of sCr, especially when this increase is out of the ordinary, such as in the presence of sepsis, bleeding, or nephrotoxic drugs.
Since the elevation of sCr in this context may raise doubts, attempts have been made to carry out these evaluations parametrically, together with other markers of decongestion, such as urinary sodium, NT-proBNP, BNP, CA-125 or the diuretic response to loop diuretics. The trajectory of some biomarkers that suggest decongestion in AHF has been considered, such as the decrease in BNP or NT-proBNP of at least 30%, body weight loss of 2.5 kg and decrease of CA-125. Likewise, hemoconcentration as assessed by increases in hemoglobin, hematocrit, serum albumin or total protein is accepted as a marker of decongestion (47).
Better prognosis with sCr elevations in decongestion
In this section, we discuss the evidence that has shown that the elevation of sCr in those who reach the goals of decongestion is associated with a better prognosis.
One of the first attempts to clarify this question was a study that evaluated the worsening of GFR according to decongestion parameters, such as increased hematocrit, total protein, or serum albumin. It was observed that decongested patients who increased these parameters were associated with a 69% less probability of dying, in comparison to those who did not (48).
In a post hoc of the ROSE study that evaluated changes in sCr and cystatin C in 238 patients with ADHF, decongestion was achieved with high doses of furosemide, reaching up to 8,726 ml in 3 consecutive days. It was observed that those patients who had an increase in both biomarkers were the group with the best survival after a follow-up of more than 150 days, reducing the probability of dead by 20% (49).
In another retrospective cohort of 4,182 patients with ADHF treated with diuretics, it was observed that 21% of them had elevated sCr and hematocrit at hospital discharge. Those patients had worse GFR, more days of hospitalization, higher dose of diuretics, but higher urine volume, greater fluid loss and weight loss. Notably, this translated to a 20% decrease in the probability of dying in a long follow-up of more than 3 years (50).
It has been reported in six cohorts and 1,232 hospitalized patients with AHF that a decrease in NTproBNP of > 30% during hospitalization is associated with a lower probability of dying, despite WKF (51).
The post hoc analysis of the AKINESIS study showed that the rapid decrease in BNP of >30% on the first day of decongestion in ADHF was associated with being free of congestion events in long follow-ups, this benefit was also accompanied by increases in sCr, and again, this WKF was irrelevant in the survival analysis (52).
In a post hoc of the DOSE study, they describe how the probability that the event composed of death, rehospitalization or visits to the emergency services 60 days after hospital discharge of patients with ADHF is increased in those patients who, during decongestion with diuretics, paradoxically have a decrease in sCr, an event that was not observed when it was increased (53).
The post hoc analysis of the EVEREST clinical trial, in 3,500 patients, showed that markers of aggressive decongestion that improved when treated with diuretics in ADHF, such as decreased values of BNP, NT-proBNP, congestion scores or increments of hematocrit, albumin and total proteins are associated with lower probabilities of being diagnosed with CKD grade 4 after a long follow-up of 10 months, reducing this risk by 70% (54).
In two cohorts (POROTEC and RELAX-AHF-2) the increases of sCr (> 0.3 mg/dL on day 4 of hospitalization) was analyzed according to the response to diuretics (defined as the reduction of 350 g of weight for every 40 mg of furosemide) and it was observed that the best survival at 180 days was in those who responded to diuretics, regardless of whether they presented WKF or not (55).
Therefore, the parameters that we currently use to describe decongestion in ADHF must be interpreted together and never alone. Therefore, the parameters we currently use to describe decongestion in ADHF should be interpreted together. A clear example of this suggestion is reported in the post hoc analysis of the EVEREST study, the authors described in 3,715 patients followed for 10 months, that the greatest changes in kidney function parameters lose negative prognostic value when analyzed together with the largest changes in BNP and hematocrit (54).
There is even a meta-analysis of 13 cohorts and 8,238 patients with type 1 cardiorenal syndrome in which the authors report that, if effective decongestion is not achieved, the appearance of AKI is associated with a > twofold increase in the risk of death, an effect that is nullified if decongestion was achieved (56).
Chirag R. Parikh and Steven G. Coca have proposed that these increases in sCr in decongestion should not be called AKI, at least not as the one described by KDIGO with its negative associations, instead terms such as “permissive hypercreatininemia” or “Permissive AKI” (24) and thereby change the landscape and traditional conceptualization.
How much should be acceptable in the magnitude of the sCr increase in AHF decongestion?
For decades, increases in sCr during decongestion have had many names, such as WKF or AKI, but authors have also been referred to solely as the numerical increase in sCr or as a percentage, increases in sCr > 0.3 mg/dL, > 20%, > 25%, or even > 50% have been proposed (56) (Table 1). We believe that a specific number has not yet been established with robust and convincing evidence and should not be systematically adopted for clinical decision making. There are patients who experience greater increases in sCr and still continue to have a favorable evolution, as during follow-up they recover their kidney function. However, we also consider that there are unacceptable values (for example, > 7.0 or 12 mg/dL). We believe that there could be an individual “sweet spot,” according to the clinical characteristics of each patient and the conditions established during management. Notwithstanding, for now, unfortunately, it continues to be an artisan response that involves clinical experience, profound physiological knowledge of intravascular volume, hemodynamics, diuretics, and decongestion (Figure 2).
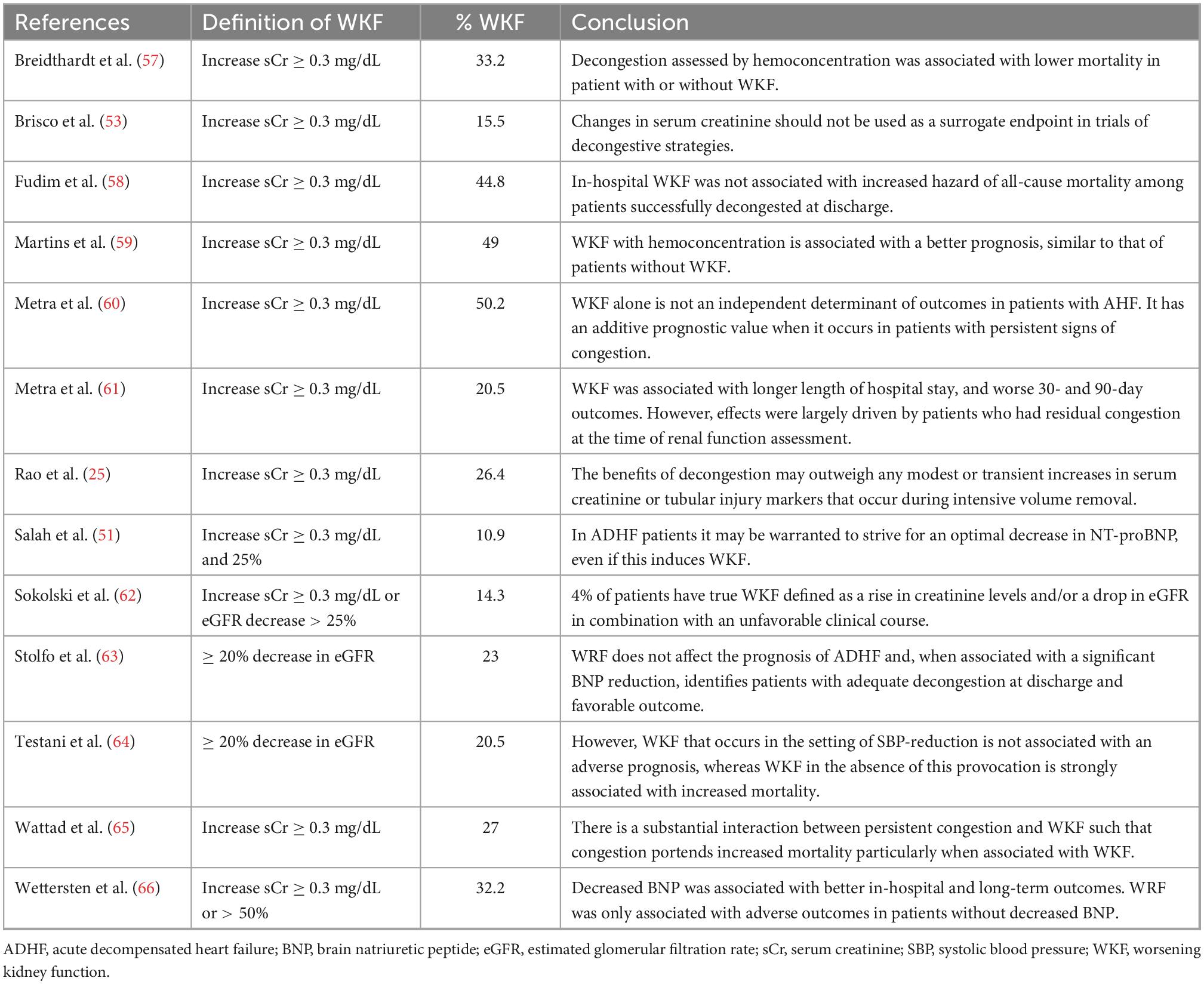
Table 1. Definitions of worsening kidney function, its frequency, and main outcomes during decongestion in acute decompensated heart failure.
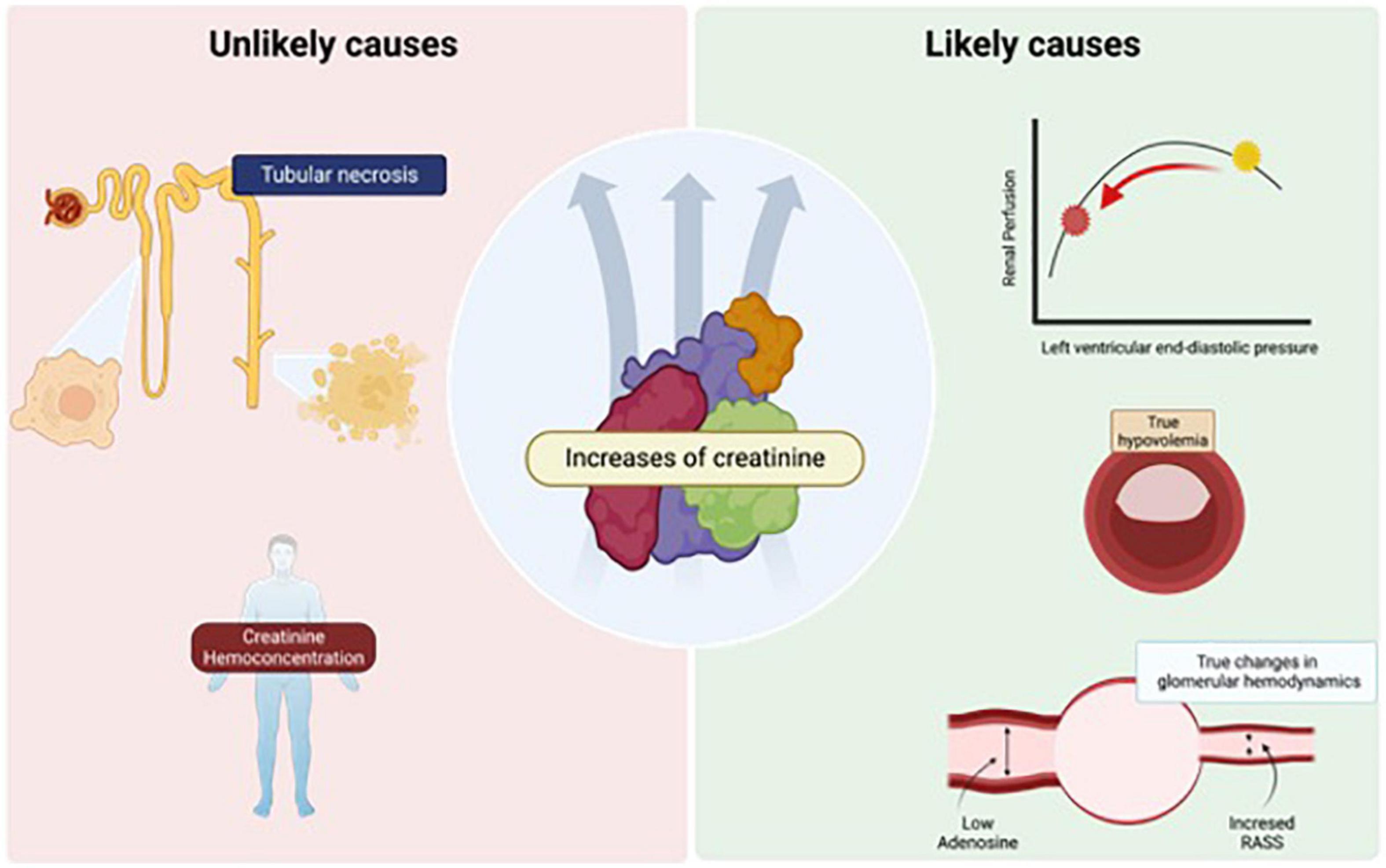
Figure 2. Different trajectories of serum creatinine (sCr) during decongestion in cardiorenal syndrome 1. During effective decongestion in acute decompensated heart failure (ADHF), the increase in sCr < 0.5 mg/dL has been considered acceptable, and is associated with fewer major cardiorenal events, this arbitrary number seems to be inconsistent with reality, where even greater increases in sCr could be associated with the same benefit, but there could be patients whose values have negative connotations and reflect a worsening of kidney function that exceeds the benefit of decongestion. The image was licensed from Biorender.
Back to the case
After 6 days of decongestion with diuretics, the dyspnea disappeared, BNP decreased to 2,100 ng/dL, the patient had a total urinary volume of 11 L, and the sCr value decreased to 1.6 mg/dL. Upon discharge, patient was prescribed with losartan 50 mg every 12 h, dapagliflozin 10 mg, metformin 850 mg, atorvastatin 20 mg, and chlorthalidone 50 mg, with strict instructions to not discontinue them. After 2 months of his hospitalization, he refers a normal life, with routine physical activity and without dyspnea or edema, blood pressure is 120/78 mmHg, sCr had returned to its baseline value 1.4 mg/dL and serum sodium is 136 mEq/L, potassium 4.1 mEq/L, glucose 122 mg/dL, and CA-125 is 11 mg/dL.
Author contributions
JC-Í, JI-M, FD, and JB-V contributed equally to the realization of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chávez-Iñiguez J, Sánchez-Villaseca S, García-Macías L. Cardiorenal syndrome: classification, pathophysiology, diagnosis and management. Literature review. Arch Cardiol Mex. (2022) 92:253–63. doi: 10.24875/ACM.20000183
2. Vandenberghe W, Gevaert S, Kellum J, Bagshaw S, Peperstraete H, Herck I, et al. Acute kidney injury in cardiorenal syndrome type 1 patients: a systematic review and meta-analysis. Cardiorenal Med. (2016) 6:116–28. doi: 10.1159/000442300
3. Gigante A, Liberatori M, Gasperini M, Sardo L, Di Mario F, Dorelli B, et al. Prevalence and clinical features of patients with the cardiorenal syndrome admitted to an internal medicine ward. Cardiorenal Med. (2014) 4:88–94. doi: 10.1159/000362566
4. Mavrakanas T, Khattak A, Singh K, Charytan D. Epidemiology and natural history of the cardiorenal syndromes in a cohort with echocardiography. Clin J Am Soc Nephrol. (2017) 12:1624–33. doi: 10.2215/CJN.04020417
5. Boorsma E, Ter Maaten J, Voors A, van Veldhuisen D. Renal compression in heart failure: the renal tamponade hypothesis. JACC Heart Fail. (2022) 10:175–83. doi: 10.1016/j.jchf.2021.12.005
6. Lo K, Rangaswami J. Mechanistic insights in cardiorenal syndrome. NEJM Evid. (2022) 1:1–13. doi: 10.1056/EVIDra2200053
7. Husain-Syed F, Gröne H, Assmus B, Bauer P, Gall H, Seeger W, et al. Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. (2021) 8:183–203. doi: 10.1002/ehf2.13118
8. Verbrugge F, Guazzi M, Testani J, Borlaug B. Altered hemodynamics and end-organ damage in heart failure: impact on the lung and kidney. Circulation. (2020) 142:998–1012. doi: 10.1161/CIRCULATIONAHA.119.045409
9. Felker G, Ellison D, Mullens W, Cox Z, Testani J. Diuretic therapy for patients with heart failure: JACC State-of-the-Art Review. J Am Coll Cardiol. (2020) 75:1178–95. doi: 10.1016/j.jacc.2019.12.059
10. Mullens W, Damman K, Harjola V, Mebazaa A, Brunner-La Rocca H, Martens P, et al. The use of diuretics in heart failure with congestion – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2019) 21:137–55. doi: 10.1002/ejhf.1369
11. de la Espriella R, Santas E, Zegri Reiriz I, Górriz J, Cobo Marcos M, Núñez J. Quantification and treatment of congestion in heart failure: a clinical and pathophysiological overview. Nefrologia. (2021) doi: 10.1016/j.nefro.2021.04.006 [Epub ahead of print].
12. Cox Z, Sarrell B, Cella M, Tucker B, Arroyo J, Umanath K, et al. Multinephron segment diuretic therapy to overcome diuretic resistance in acute heart failure: a single-center experience. J Card Fail. (2022) 28:21–31. doi: 10.1016/j.cardfail.2021.07.016
13. Piardi D, Butzke M, Mazzuca A, Gomes B, Alves S, Kotzian B, et al. Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: a randomized clinical trial. Sci Rep. (2021) 11:16474. doi: 10.1038/s41598-021-96002-6
14. Rossignol P, Cleland J, Bhandari S, Tala S, Gustafsson F, Fay R, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the eplerenone post-acute myocardial infarction heart failure efficacy and survival study. Circulation. (2012) 125:271–9. doi: 10.1161/CIRCULATIONAHA.111.028282
15. Mullens W, Dauw J, Martens P, Verbrugge F, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. (2022) 387:1185–95. doi: 10.1056/NEJMoa2203094
16. Damman K, Beusekamp J, Boorsma E, Swart H, Smilde T, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. (2020) 22:713–22. doi: 10.1002/ejhf.1713
17. Schulze P, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S, et al. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF). Circulation. (2022) 146:289–98. doi: 10.1161/CIRCULATIONAHA.122.059038
18. Liu C, Peng Z, Gao X, Gajic O, Dong Y, Prokop L, et al. Simultaneous use of hypertonic saline and iv furosemide for fluid overload: a systematic review and meta-analysis. Crit Care Med. (2021) 49:e1163–75. doi: 10.1097/CCM.0000000000005174
19. Chávez-Iñiguez J, Ibarra-Estrada M, Sánchez-Villaseca S, Romero-González G, Font-Yañez J, De la Torre-Quiroga A, et al. The effect in renal function and vascular decongestion in type 1 cardiorenal syndrome treated with two strategies of diuretics, a pilot randomized trial. BMC Nephrol. (2022) 23:3. doi: 10.1186/s12882-021-02637-y
20. Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. (2016) 20:299. doi: 10.1186/s13054-016-1478-z
21. Charlton J, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. (2014) 29:1301–11. doi: 10.1093/ndt/gft510
22. Seeley E. Updates in the management of acute lung injury: a focus on the overlap between AKI and ARDS. Adv Chronic Kidney Dis. (2013) 20:14–20. doi: 10.1053/j.ackd.2012.10.001
23. Claure-Del Granado R, Macedo E, Chávez-Íñiguez J. Biomarkers for Early Diagnosis of AKI: Could it backfire? Kidney360. (2022) 3:1780–4. doi: 10.34067/KID.0001012022
24. Parikh C, Coca S. “Permissive AKI” with treatment of heart failure. Kidney Int. (2019) 96:1066–8. doi: 10.1016/j.kint.2019.07.003
25. Rao V, Ahmad T, Brisco-Bacik M, Bonventre J, Wilson F, Siew E, et al. Renal effects of intensive volume removal in heart failure patients with preexisting worsening renal function. Circ Heart Fail. (2019) 12:e005552. doi: 10.1161/CIRCHEARTFAILURE.118.005552
26. Testani J, McCauley B, Chen J, Coca S, Cappola T, Kimmel S. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. (2011) 17:993–1000. doi: 10.1016/j.cardfail.2011.08.009
27. Testani J, McCauley B, Chen J, Shumski M, Shannon R. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. (2010) 116:206–12. doi: 10.1159/000316038
28. Testani J, McCauley B, Kimmel S, Shannon R. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. (2010) 106:1763–9. doi: 10.1016/j.amjcard.2010.07.050
29. Kraus B, Weir M, Bakris G, Mattheus M, Cherney D, Sattar N, et al. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. (2021) 99:750–62. doi: 10.1016/j.kint.2020.10.031
30. Chávez-Íñiguez J, Rifkin B. Dual RAAS Blockade in CKD: Does the Hype have Teeth? Kidney360. (2022) 3:1277–80. doi: 10.34067/KID.0000912022
31. Drawz P, Rai N, Lenoir K, Suarez M, Powell J, Raj D, et al. Effect of Intensive versus Standard BP Control on AKI and Subsequent Cardiovascular Outcomes and Mortality: findings from the SPRINT EHR Study. Kidney360. (2022) 3:1253–62. doi: 10.34067/KID.0001572022
32. Chávez-Iñiguez J, García-García G, Lombardi R. [Epidemiología y desenlaces de la lesión renal aguda en Latinoamérica]. Gac Med Mex. (2018) 154(Suppl. 1):S6–14. doi: 10.24875/GMM.M18000067
33. Chávez-Íñiguez J, Madero M. Global perspectives in acute kidney injury: Mexico. Kidney360. (2022) 3:737–9. doi: 10.34067/KID.0006592021
34. Chávez-Iñiguez J, Navarro-Gallardo G, Medina-González R, Alcantar-Vallin L, García-García G. Acute kidney injury caused by obstructive nephropathy. Int J Nephrol. (2020) 2020:8846622. doi: 10.1155/2020/8846622
35. Jentzer J, Bihorac A, Brusca S, Del Rio-Pertuz G, Kashani K, Kazory A, et al. Contemporary management of severe acute kidney injury and refractory cardiorenal syndrome: JACC Council Perspectives. J Am Coll Cardiol. (2020) 76:1084–101. doi: 10.1016/j.jacc.2020.06.070
36. Horiuchi Y, Wettersten N, Veldhuisen D, Mueller C, Filippatos G, Nowak R, et al. Potential utility of cardiorenal biomarkers for prediction and prognostication of worsening renal function in acute heart failure. J Card Fail. (2021) 27:533–41. doi: 10.1016/j.cardfail.2020.11.025
37. Bielecka-Dabrowa A, Godoy B, Schefold J, Koziolek M, Banach M, von Haehling S. Decompensated Heart Failure and Renal Failure: What Is the Current Evidence? Curr Heart Fail Rep. (2018) 15:224–38. doi: 10.1007/s11897-018-0397-5
38. Maulion C, Chen S, Rao V, Ivey-Miranda J, Cox Z, Mahoney D, et al. Hemoconcentration of creatinine minimally contributes to changes in creatinine during the treatment of decompensated heart failure. Kidney360. (2022) 3:1003–10. doi: 10.34067/KID.0007582021
39. Ellison D, Felker G. Diuretic treatment in heart failure. N Engl J Med. (2017) 377:1964–75. doi: 10.1056/NEJMra1703100
40. Felker G, Mentz R. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. (2012) 59:2145–53. doi: 10.1016/j.jacc.2011.10.910
41. Gottlieb S, Abraham W, Butler J, Forman D, Loh E, Massie B, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. (2002) 8:136–41. doi: 10.1054/jcaf.2002.125289
42. Forman D, Butler J, Wang Y, Abraham W, O’Connor C, Gottlieb S, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. (2004) 43:61–7. doi: 10.1016/j.jacc.2003.07.031
43. Damman K, Navis G, Voors A, Asselbergs F, Smilde T, Cleland J, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. (2007) 13:599–608. doi: 10.1016/j.cardfail.2007.04.008
44. Damman K, Valente M, Voors A, O’Connor C, van Veldhuisen D, Hillege H. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. (2014) 35:455–69. doi: 10.1093/eurheartj/eht386
45. Wu B, Yan W, Li X, Kong X, Yu X, Zhu Y, et al. Initiation and cessation timing of renal replacement therapy in patients with type 1 cardiorenal syndrome: an observational study. Cardiorenal Med. (2017) 7:118–27. doi: 10.1159/000454932
46. Damman K, Testani J. The kidney in heart failure: an update. Eur Heart J. (2015) 36:1437–44. doi: 10.1093/eurheartj/ehv010
47. McCallum W, Tighiouart H, Testani J, Griffin M, Konstam M, Udelson J, et al. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail. (2020) 8:537–47. doi: 10.1016/j.jchf.2020.03.009
48. Testani J, Chen J, McCauley B, Kimmel S, Shannon R. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. (2010) 122:265–72. doi: 10.1161/CIRCULATIONAHA.109.933275
49. Ahmad T, Jackson K, Rao V, Tang W, Brisco-Bacik M, Chen H, et al. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation. (2018) 137:2016–28. doi: 10.1161/CIRCULATIONAHA.117.030112
50. Griffin M, Rao V, Fleming J, Raghavendra P, Turner J, Mahoney D, et al. Effect on survival of concurrent hemoconcentration and increase in creatinine during treatment of acute decompensated heart failure. Am J Cardiol. (2019) 124:1707–11. doi: 10.1016/j.amjcard.2019.08.034
51. Salah K, Kok W, Eurlings L, Bettencourt P, Pimenta J, Metra M, et al. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail. (2015) 3:751–61. doi: 10.1016/j.jchf.2015.05.009
52. Horiuchi Y, Wettersten N, van Veldhuisen D, Mueller C, Filippatos G, Nowak R, et al. Relation of decongestion and time to diuretics to biomarker changes and outcomes in acute heart failure. Am J Cardiol. (2021) 147:70–9. doi: 10.1016/j.amjcard.2021.01.040
53. Brisco M, Zile M, Hanberg J, Wilson F, Parikh C, Coca S, et al. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. (2016) 22:753–60. doi: 10.1016/j.cardfail.2016.06.423
54. McCallum W, Tighiouart H, Testani J, Griffin M, Konstam M, Udelson J, et al. Rates of reversal of volume overload in hospitalized acute heart failure: association with long-term kidney function. Am J Kidney Dis. (2022) 80:65–78. doi: 10.1053/j.ajkd.2021.09.026
55. Emmens J, Ter Maaten J, Matsue Y, Figarska S, Sama I, Cotter G, et al. Worsening renal function in acute heart failure in the context of diuretic response. Eur J Heart Fail. (2022) 24:365–74. doi: 10.1002/ejhf.2384
56. Yamada T, Ueyama H, Chopra N, Yamaji T, Azushima K, Kobayashi R, et al. Systematic review of the association between worsening renal function and mortality in patients with acute decompensated heart failure. Kidney Int Rep. (2020) 5:1486–94. doi: 10.1016/j.ekir.2020.06.031
57. Breidthardt T, Weidmann Z, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, et al. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail. (2017) 19:226–36. doi: 10.1002/ejhf.667
58. Fudim M, Loungani R, Doerfler S, Coles A, Greene S, Cooper L, et al. Worsening renal function during decongestion among patients hospitalized for heart failure: findings from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Am Heart J. (2018) 204:163–73.
59. Martins J, Santos L, Faustino A, Viana J, Santos J. Worsening or ‘pseudoworsening’ renal function? The prognostic value of hemoconcentration in patients admitted with acute heart failure. Rev Port Cardiol. (2018) 37:595–602.
60. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. (2012) 5:54–62.
61. Metra M, Cotter G, Senger S, Edwards C, Cleland J, Ponikowski P, et al. Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: a post hoc analysis of the PROTECT data. Circ Heart Fail. (2018) 11:e004644. doi: 10.1161/CIRCHEARTFAILURE.117.004644
62. Sokolski M, Zymlinski R, Sokolska J, Biegus J, Banasiak W, Ponikowski P. True worsening renal function identifies patients with acute heart failure with an ominous outcome. Pol Arch Intern Med. (2019) 129:357–60. doi: 10.20452/pamw.4453
63. Stolfo D, Stenner E, Merlo M, Porto A, Moras C, Barbati G, et al. Prognostic impact of BNP variations in patients admitted for acute decompensated heart failure with in-hospital worsening renal function. Heart Lung Circ. (2017) 26:226–34. doi: 10.1016/j.hlc.2016.06.1205
64. Testani J, Coca S, McCauley B, Shannon R, Kimmel S. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. Eur J Heart Fail. (2011) 13:877–84.
65. Wattad M, Darawsha W, Solomonica A, Hijazi M, Kaplan M, Makhoul B, et al. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am J Cardiol. (2015) 115:932–7. doi: 10.1016/j.amjcard.2015.01.019
Keywords: AKI, cardiorenal syndrome 1, creatinine, decongestion, acute heart failure
Citation: Chávez-Íñiguez JS, Ivey-Miranda JB, De la Vega-Mendez FM and Borges-Vela JA (2023) How to interpret serum creatinine increases during decongestion. Front. Cardiovasc. Med. 9:1098553. doi: 10.3389/fcvm.2022.1098553
Received: 15 November 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Rafael De La Espriella, Hospital Clínico Universitario de Valencia, SpainReviewed by:
Gregorio Romero González, Hospital Germans Trias i Pujol, SpainLuis D’Marco, Universidad CEU Cardenal Herrera, Spain
Copyright © 2023 Chávez-Íñiguez, Ivey-Miranda, De la Vega-Mendez and Borges-Vela. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan S. Chávez-Íñiguez, ✉ jonarchi_10@hotmail.com,  @JonathanNefro; orcid.org/0000-0003-2786-6667
@JonathanNefro; orcid.org/0000-0003-2786-6667
 Jonathan S. Chávez-Íñiguez
Jonathan S. Chávez-Íñiguez Juan B. Ivey-Miranda3
Juan B. Ivey-Miranda3  Julian A. Borges-Vela
Julian A. Borges-Vela