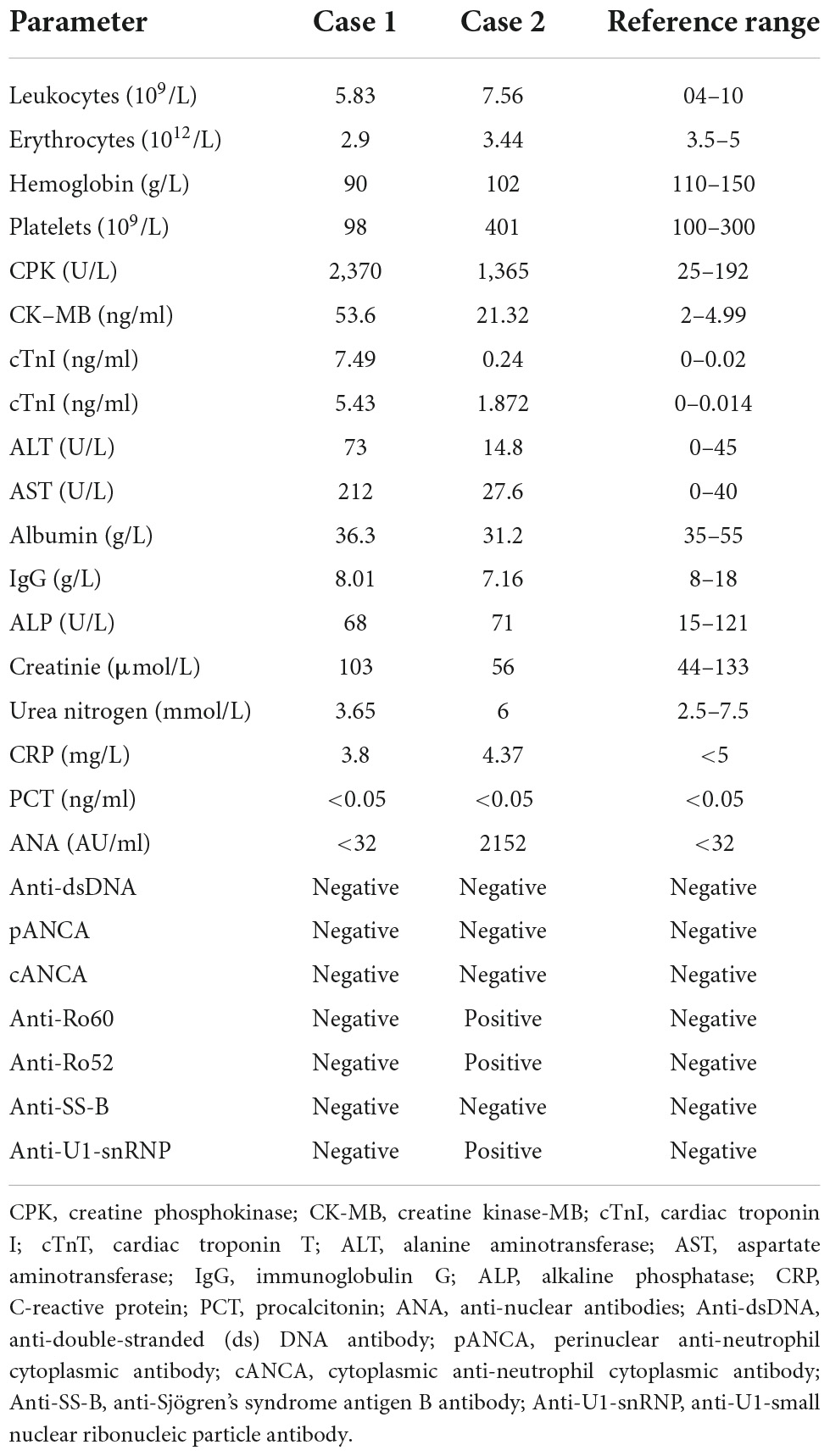
Multisystem immune-related adverse events due to toripalimab: Two cases-based review
- 1The Second Clinical Medical College, Jinan University, Shenzhen, China
- 2Department of Rheumatology and Immunology, Shenzhen People’s Hospital, The Second Clinical Medical College, Jinan University, Shenzhen, China
- 3The First Affiliated Hospital, Southern University of Science and Technology, Shenzhen, China
Immune checkpoint inhibitors (ICIs) have significantly improved the survival of patients with advanced tumors. However, immune-related adverse events (irAEs) caused by ICIs, especially high-grade irAEs, are of growing concern. High-grade multisystem irAEs due to toripalimab, a programmed cell death-1 (PD-1) inhibitor, have been rarely reported. Two patients with malignant metastatic tumors were treated with anti-PD-1 immunotherapy. However, both patients developed high-grade multisystem irAEs based on myocarditis, with chest discomfort and malaise as the main clinical manifestation. Both patients had an elevation of cardiac enzymes, abnormal electrocardiography and left ventricular wall motion. Patient 2 was also diagnosed with organizing pneumonia. Immunotherapy was suspended. High-dose intravenous methylprednisolone was immediately initiated. The patients’ symptoms were significantly relieved in a short period of time. Immunosuppressants were discontinued at the 6th month follow-up in patient 1 without relapse. However, patient 2 was lost to follow up due to financial reasons. To the best of our knowledge, this is the first report regarding ICI-associated myocarditis-pneumonia due to toripalimab, indicating the significance of early recognition and management of high-grade multisystem irAEs in clinical practice.
Introduction
Immune checkpoint inhibitors (ICIs) have dramatically extended the survival of patients with advanced tumors. However, immune-related adverse events (irAEs) caused by ICIs, especially high-grade irAEs, pose a significant threat to patients’ lives, with an incidence ranging from 54 to 76% (1). Excessive immune activation by ICIs can occasionally induce multiple irAEs in different organs (2). The incidence of multisystem high-level irAEs may be underestimated due to the high rate of misdiagnosis that may result from its individualized clinical presentation.
Toripalimab, a human monoclonal antibody against programmed cell death-1 (PD-1), has been developed and received conditional approval as salvage treatment for unresectable or metastatic melanoma in China since 2018 (3). Yet high-grade multisystem irAEs caused by toripalimab have been rarely reported. Here we report two Chinese patients with advanced tumors who experienced a severe storm of multisystem irAEs after receiving toripalimab treatment, indicating the significance of early diagnosis and timely management of multisystem irAEs due to toripalimab.
Case presentation
Case 1
A 43 years-old Chinese female patient had a 6-year history of mixed liposarcoma of the right upper extremity with bilateral lower extremity metastases. The patient received 240 mg of toripalimab every 2 weeks from May 2019. After four cycles of toripalimab treatment, she presented to the emergency department at our institution with complaints of a 5-day generalized rash and malaise, as well as a sudden onset of severe chest pain with palpitation and dyspnea lasting for 2 h. She denied a history of diabetes mellitus, cardiovascular diseases, or thyroid diseases. Blood pressure on admission was 101/58 mmHg and the heart rate was irregular with a frequency of 131 beats/min. The respiratory rate was 36 breaths/min with normal oxygen saturation. Physical examination revealed generalized multiforme-like rash as well as muffled heart sounds and a grade II/VI holosystolic murmur typical of mitral regurgitation. Neurological examination revealed a grade IV muscle strength in her both lower limbs.
Laboratory tests showed that serum creatine phosphokinase (CPK) was 2,370 U/L (normal 25∼192 U/L), with increased levels of creatine kinase isoenzyme MB (CK-MB 53.6 ng/ml, normal 2∼4.99 ng/ml), cardiac troponin I (cTnI 7.49 ng/ml, normal 0∼0.02 ng/ml), cardiac troponin T (cTnT 5.43 ng/ml, normal 0∼0.014 ng/ml), myoglobin (214 ng/ml, normal 0∼46.6 ng/ml), lactate dehydrogenase (LDH 616 U/L, normal 110∼240 U/L), alanine aminotransferase (ALT 73 U/L, normal 0–40 U/L), aspartate aminotransferase (AST 212 U/L, normal 0–45 U/L), and N-terminal pro brain natriuretic peptide (NT-proBNP 4999 pg/ml, normal 0∼450 pg/ml). Further tests revealed decreased levels of free triiodothyronine (2.44 pmol/L, normal 3.28∼6.47 pmol/L) and free thyroxine (3.72 pmol/L, normal 7.64∼11.3 pmol/L), with elevated levels of thyrotropin (68.29 mIU/L, normal 0.38∼5.91 mIU/L), anti-thyroid peroxidase antibodies (94.99 mIU/ml, normal 0∼34 mIU/ml), and anti-thyroglobulin antibodies (572 IU/ml, normal 0∼115 IU/ml) (Table 1). There was no evidence of infection, and her autoimmune antibodies, including myositis-associated and myositis-specific antibodies, were also negative. Electrocardiogram showed paroxysmal ventricular tachycardia (104 beats/min). Emergency coronary angiography and left heart catheterization were unremarkable. Echocardiography revealed the myocardial motion of the anterior wall and the apical segment of the anterior septum of the left ventricle was diminished. The left atrium and left ventricle were enlarged (anterior-posterior left atrial diameter was 38 mm). In contrast, the right atrium and right ventricle were normal, with moderate mitral and tricuspid regurgitation and a small amount of pericardial effusion, with an ejection fraction (EF) of 55%. Electromyography demonstrated varying degrees of myogenic damage in the proximal muscles of the extremities, without abnormal changes in the distal muscles. Thyroid ultrasound and chest computed tomography (CT) scan showed no abnormalities. Cardiac magnetic resonance (MR) and muscle biopsy was not allowed due to the rapid deterioration of chest pain. She was transferred to the intensive care unit.
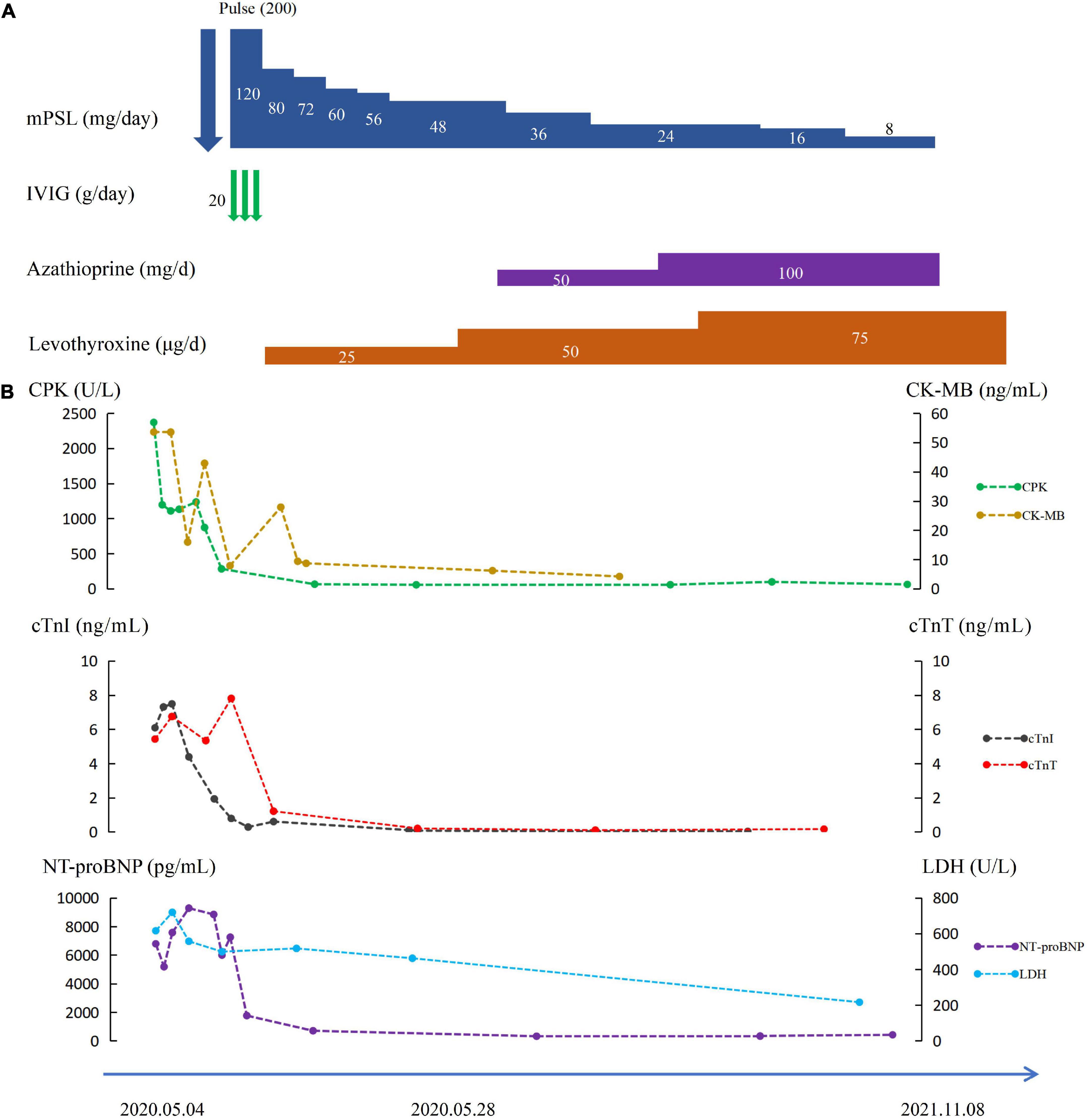
The patient was finally diagnosed with multisystem irAEs resulting from anti-PD-1 therapy according to the results of the multidisciplinary discussion. The diagnoses of ICI-associated myocarditis (grade 4), myositis (grade 3), Hashimoto’s thyroiditis (grade 2), and skin toxicity (grade 2) were made based on the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE). On day 3, intravenous methylprednisolone (200 mg/day) was initiated for 3 days, with oral levothyroxine replacement therapy (50 μg/day). Her chest pain and dyspnea were significantly relieved and the rash disappeared within 10 days. CPK, CK-MB, and troponin gradually decreased (Figure 1). On day 7, laboratory tests showed serum CPK to be 284 U/L, cTnI 0.279 ng/ml, cTnT 1.367 ng/ml, myoglobin 34 ng/ml, CK-MB 7.91 ng/ml, and NT-proBNP 1792 pg/ml. Echocardiography suggested an increase in EF to 60%.
Her methylprednisolone was gradually tapered. After 3 weeks, methylprednisolone was reduced to 48 mg/day, and she again developed chest pain and malaise. Laboratory tests showed a normal CPK level but an elevated CK-MB level to 28.7 ng/ml. Therefore, azathioprine (50 mg/day) was added, and her symptoms were gradually relieved within 2 weeks. Methylprednisolone was gradually tapered accordingly and was discontinued after 12 weeks, with azathioprine (100 mg/day) as maintenance therapy. At 6-month follow-up, laboratory tests revealed CPK to be 212 U/L, CK-MB 3.84 ng/ml, CTnI 0.08 ng/ml, and CTnT 0.016 ng/ml (Figure 1). At the time of writing this report, she was only taking oral levothyroxine (75 μg/day) replacement therapy and refused to receive any other anti-PD-1 therapies due to the potential risk for recurrence of severe multisystem irAEs, without evidence of tumor progression.
Case 2
A 64 years-old Chinese woman was diagnosed with advanced pancreatic adenocarcinoma with multiple metastases (liver, adrenal glands, and multiple lymph nodes) in January 2021. Due to her intolerance to chemotherapy, toripalimab (240 mg every 2 weeks) was initiated and clinical improvement was gradually achieved. Before the sixth treatment, she developed chest tightness and malaise that lasted for 2 days, without fever or cough. She took amlodipine for 7 years due to hypertension, and she denied prior history of lung diseases or autoimmune diseases. Physical examination on admission showed that the patient had an oxygen saturation of 87% (room air, at rest) and wet rales in the right lower lungs, with normal cardiac auscultation.
Laboratory tests showed that serum CPK was 1322 U/L, CK-MB 101.2 ng/ml, CTnI 0.72 ng/ml, CTnT 1.872 ng/ml, myoglobin 146.3 ng/ml, and NT-proBNP 3964.8 pg/ml. The white blood cell count was 9.18 × 109/L (normal 4∼10 × 109/L), and calcitonin (<0.05 ng/ml, normal <0.05 ng/ml) and C-reactive protein (2.73 mg/L, normal <5 mg/L) levels were normal. Arterial blood gas analysis showed an oxygenation index of 281. Further examination revealed positive anti-nuclear antibodies (ANA, 2152 AU/ml, normal 0∼32 AU/ml) by chemiluminescent immunoassay and positive anti-U1-small nuclear ribonucleic particles (snRNP), anti-Ro60, and anti-Ro52 antibodies by line immunoassay (Table 1). Her electrocardiogram showed a complete right bundle branch conduction block. The echocardiogram showed reduced anterior interventricular wall segmental motion in the left ventricle with an EF of 49%. Chest CT of the chest showed consolidative opacities predominantly in the right basal parenchyma and a small amount of right pleural fluid. Lymphocyte subpopulation testing of the patient’s bronchoalveolar lavage fluid showed that CD3+ T cells accounted for 95.8% of lymphocytes, without evidence of infection. Lung biopsy showed alveolar wall edema suggestive of alveolitis, with a large amount of fibrinous exudate and lymphocytic infiltrate in the alveolar space, with no hyaline membrane formation (Figure 2). No malignant cells were seen in the patient’s bronchoalveolar lavage fluid or lung tissue biopsy.
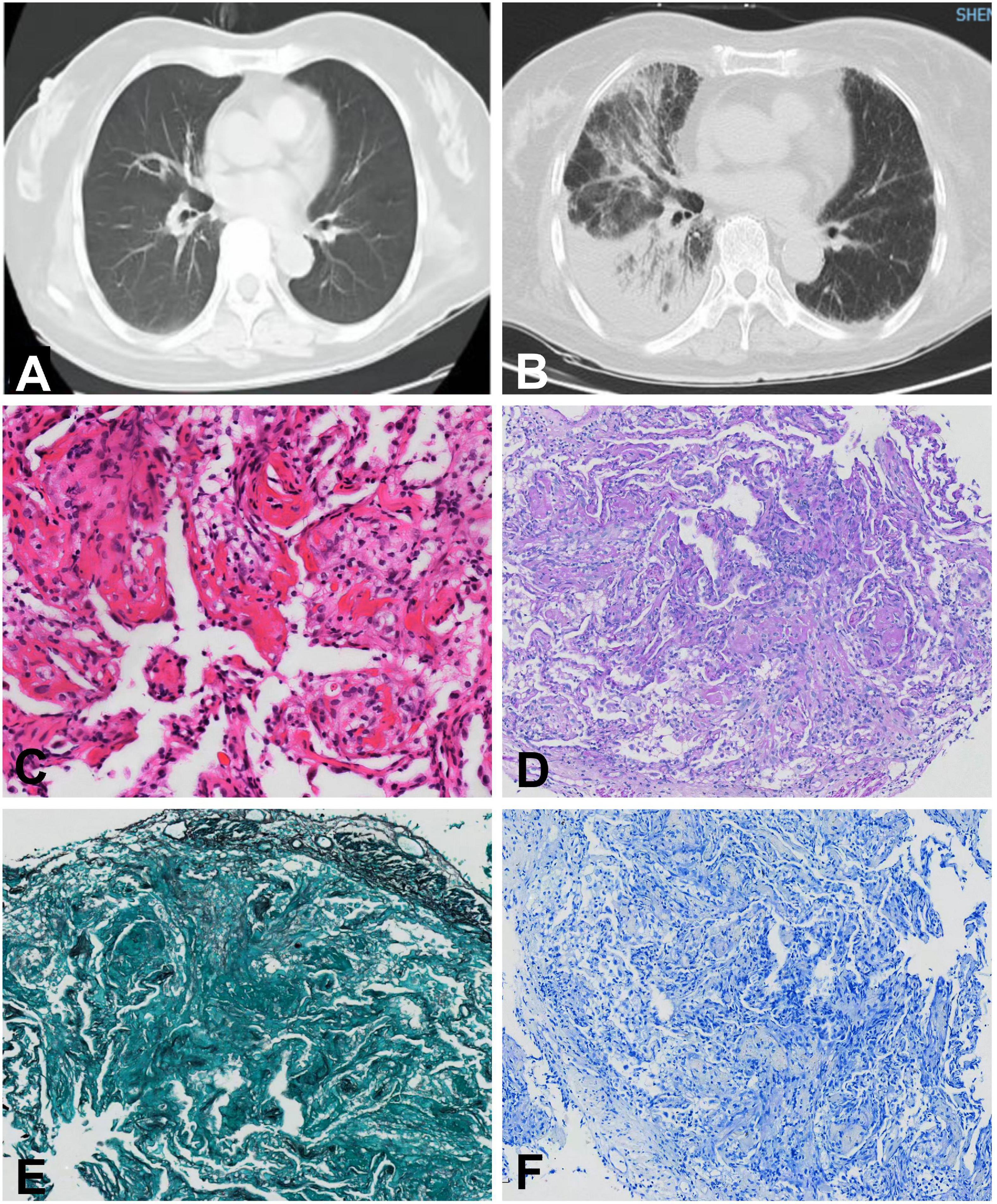
Figure 2. Chest computed tomography and histopathological changes of the lung biopsy in case 2. Chest computed tomography before (A) and after (B) onset of pneumonia. (C) Hematoxylin-eosin staining (×20). (D) Periodic acid-Schiff staining (×10). (E) Hexosamine silver staining (×10). (F) Antacid staining (×10).
The patient was diagnosed with ICI-associated myocarditis (grade 3) and ICI-associated pneumonia (grade 3) based on NCI CTCAE. Intravenous methylprednisolone (200 mg/day) was started on the second day of admission, along with intravenous immunoglobulin (20 g/day) for 3 days. The patient’s chest tightness improved and the oxygenation index increased to 323. On day 4 of admission, the patient was discharged without a repeated CT scan according to her wish.
Discussion
Immune checkpoint inhibitors such as those targeting PD-1, PD-1 ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) have been the most significant breakthroughs in cancer immunotherapy in recent years. These ICIs enhance immune surveillance and reduce the immune escape of cancer cells by “releasing the brakes” on the T-cell activation pathway, which may affect multiple organs. The NCI-CTCAE classifies irAEs into five levels, from mild, moderate, severe, life-threatening, to fatal (4). The most common fatal irAEs include myocarditis, pneumonia, encephalitis, and fulminant hepatitis, with a mortality ranging from 0.3 to 1.3% (2). Toripalimab is a relatively new ICI developed in China and was generally well-tolerated in clinical trials in Chinese patients with advanced malignancies. The high-grade irAEs (grade 3 or higher) associated with toripalimab was most common in the hematologic and hepatic system (3). Multisystem irAEs and fatal irAEs, such as myocarditis and pneumonia, caused by toripalimab have been rarely reported. Here we report two Chinese patients with advanced tumors who suffered from fatal multisystem irAEs after receiving toripalimab treatment. To the best of our knowledge, this is the first study describing a patient who concurrently experienced ICI-associated myocarditis and pneumonia, which indicates the significance of early recognition and management of multisystem irAEs due to toripalimab.
Cardiac immune-related events include the development of myocarditis, pericarditis, pericardial effusion, arrhythmias, myocardial infarction, and heart failure. The mechanisms by which ICIs cause myocarditis are unclear. Most of the existing studies have been attributed to the presence of shared antigens between the tumor and myocardium (5). Several studies have confirmed that PD-1 is found in the myocardium of patients with ICI-associated myocarditis with expansion of T cell clones. T cell receptors bind to homologous muscle antigens and tumor antigens, causing damage similar to viral myocarditis (5, 6). Even though ICI-associated myocarditis only seems to account for about 2.4% of all irAEs (5, 7), its mortality rate may reach 50% (8). The first 3 months of receiving ICIs are considered as a high-risk period for developing immune myocarditis (7–9). Mahmood et al. (7) showed that the median time from the first exposure to ICIs to the onset of myocarditis was 34 days. Remarkably, cases of immune myocarditis that received combination immunotherapy occurred even after the first receipt (10). PD-1/PD-L1 inhibitors are more likely to cause myocarditis and pericardial disease than CTLA-4 inhibitors (11). Accordingly, both patients in this study developed myocarditis within the first 3 months of receiving toripalimab, suggesting an extra attention should be paid during the high-risk period after toripalimab treatment. Therefore, informing patients receiving ICIs for the first time is recommended to pay special attention to symptoms such as chest pain and palpitations and to seek prompt medical attention. For patients with a history of ICIs and symptoms such as chest tightness, chest pain, and weakness, clinicians should consider ICIs-cardiomyopathy and perform electrocardiography, cardiac enzymology, and echocardiography.
The initial symptoms of ICI-associated myocarditis are heterogeneous and may include vague manifestations such as discomfort, fatigue, and weakness associated with the primary diseases, which are difficult to distinguish and can be easily overlooked. Almost all patients with myocarditis had elevated troponin and CPK levels, and 89% of cases showed electrocardiographic arrhythmias, including atrial fibrillation, premature ventricular beats, conduction block, and ventricular tachycardia. Cardiac ultrasound was suggested as a baseline assessment tool because about three-fourth of the patients showed abnormal left ventricular EF values after the onset of the disease (12). Enhancement of cardiac MR is an important modality in the available non-invasive diagnosis of myocarditis and 48% of patients with myocarditis may demonstrate late gadolinium enhancement (13). Endomyocardial biopsy is the gold standard for the diagnosis of myocarditis, which is characterized by myocardial infiltrates, including CD4 and CD8-positive T lymphocytes as well as macrophages (14). However, myocardial biopsy has its technical limitations, especially in cases of patchy or focal myocardial infarction-associated myocarditis (15). Furthermore, coronary angiography can help make a differential diagnosis in some cases with myocarditis that mimic coronary artery diseases, as demonstrated in patient 1 in this study. Most patients with clinically advanced tumors refuse to undergo this invasive procedure after a difficult tumor identification and treatment. Enhanced MR is considered an important non-invasive tool for diagnosing myocarditis, but patients prefer to spend their limited treatment costs on anti-tumor drugs due to their high price. In addition, although cardiac MR and endomyocardial biopsy was not performed in these two patients, myocardial abnormalities, such as significantly elevated levels of myocardial enzymes, ventricular dysfunction and arrhythmias, strongly supported the diagnosis of myocarditis.
Shao et al. reported an overall incidence of 4.5% for ICI-associated pneumonia (16). The incidence of severe pneumonia (grade 3 or higher) was reported to be 0.8–1.5% (17). Studies have shown that lung cancer patients treated with PD-1 or PD-L1 inhibitors were more likely to develop pneumonia compared to CTLA-4 inhibitors (2.7 vs. 1%) (17–19). ICI-associated pneumonia mainly includes organizing pneumonia (OP), non-specific interstitial pneumonia, hypersensitivity pneumonia, diffuse alveolar injury, acute interstitial pneumonia, and acute respiratory distress syndrome (20–22). OP was the predominant type of ICI-associated pneumonia, accounting for 77.8% of cases (22). Bronchoalveolar lavage and transbronchial lung biopsy were recommended in patients with ICI-associated pneumonia (14, 23). Based on Naidoo et al. criteria for ICI-associated pneumonia (24), the diagnosis of OP (grade 3) can be made in case 2. In addition, the incidence of thyroid dysfunction due to anti-PD-1 antibody treatment (5–10%) is higher than that after CTLA-4 inhibitors (0–5%) (25–27). Thyroiditis caused by ICIs usually causes transient hyperthyroidism, which often progresses to hypothyroidism. Notably, in this study, the diagnoses of myocarditis, myositis, Hashimoto’s thyroiditis, and skin toxicity were concurrently made in patient 1, and ICI-associated myocarditis and pneumonia was made in patient 2, which indicates multisystem irAEs in both of these two patients.
In a clinical study including 623 patients, 5.8% of patients experienced multisystem irAEs due to ICIs, with the combination of hepatitis-thyroiditis (10%) and dermatitis-pneumonia (10%) being the most common (28). ICI-associated myocarditis was most commonly accompanied by myositis (29%) and hepatitis (21%), followed by thyroiditis (12%) (9). It has been shown that myositis was the second frequent rheumatic and musculoskeletal irAEs (accounting for 36.1%), in which the mortality was 24% for myositis and 56.7% for concurrent myocarditis (29). The mechanism of myocarditis-myositis is unclear. Still, the theory of shared antigens between myocardium, skeletal muscle, and tumors has also been highlighted in the previous studies (6, 29). Importantly, myocarditis with myositis and/or myasthenia gravis overlap syndrome was reported to have a 60% mortality rate and is a hot issue in ICI-associated cardiomyopathy involving multisystem irAEs (30, 31). Therefore, it is reasonable to speculate that patients with multisystem irAEs based on myocarditis could have a higher mortality. Early recognition and diagnosis of multisystem irAEs are pivotal in the management of patients undergoing ICI treatment. Multisystem irAEs based on myocarditis reported previously are summarized in Table 2. Furthermore, to our knowledge, case 2 is the first report of myocarditis-pneumonia caused by toripalimab.
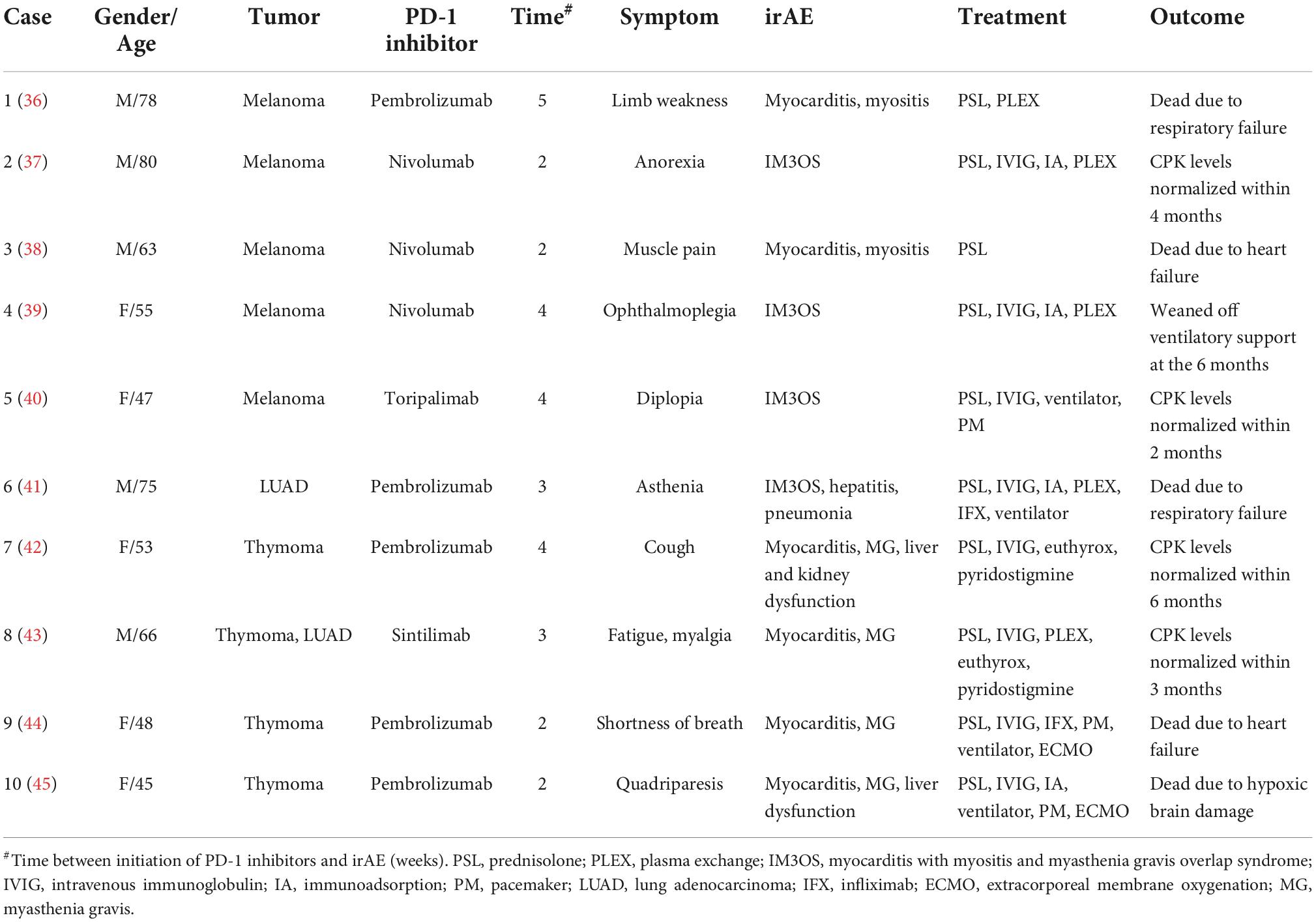
Table 2. Case reports regarding multisystem immune-related adverse events (irAEs) based on myocarditis induced by programmed cell death-1 (PD-1) inhibitors.
Low-grade irAEs respond rapidly to steroid therapy and generally do not result in hospitalization or termination of treatment with ICIs. Permanent discontinuation of ICIs is recommended for patients with severe myositis or myocarditis and myositis-carditis overlap (32). Patients with ICI-associated myocarditis required immediate initiation of intravenous corticosteroid therapy and consideration of escalation to immunoglobulin, cyclophosphamide, rituximab, azathioprine, or methotrexate if patients do not respond well to corticosteroid alone (4, 14). Infliximab is the recommended second-line agent for ICI-associated myocarditis. Both abciximab and tacrolimus have been reported (1, 33), and tofacitinib has recently been reported to have significant efficacy in immune-associated myocarditis (34). Notwithstanding, even based on the above treatment, the mortality rate of myocarditis still reaches 50% (35). Hence, an early recognition and intervention of myocarditis is beneficial to improve the prognosis. Notably, both patients in this study had a positive response to glucocorticoids. Patient 1 experienced a recurrence of symptoms during glucocorticoid tapering, and a positive effect was obtained with the addition of azathioprine. In case 2, the patient also showed improvement after methylprednisolone and immunoglobulin therapy.
Conclusion
In conclusion, we described two Chinese patients with advanced tumors suffering from severe multisystem irAEs based on after receiving toripalimab treatment. Improved awareness and an early identification of multisystem irAEs are of great importance in management of patients undergoing treatment of ICIs.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XH initiated and reviewed the manuscript. YRC and YLC were involved in the data collection and writing of the manuscript. JX provided the clinical details. DL was responsible for steering the direction of the manuscript. All authors read and approved the final manuscript for submission.
Funding
This work was supported by Shenzhen Key Medical Discipline Construction Fund (No. SZXK011).
Acknowledgments
We greatly appreciate the patients and their family for their kind cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martins F, Sykiotis GP, Maillard M, Fraga M, Ribi C, Kuntzer T, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. (2019) 20:e54–64. doi: 10.1016/S1470-2045(18)30828-3
2. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
3. Keam SJ. Toripalimab: first global approval. Drugs. (2019) 79:573–8. doi: 10.1007/s40265-019-01076-2
4. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw. (2020) 18:230–41. doi: 10.6004/jnccn.2020.0012
5. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
6. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
7. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
8. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. (2018) 391:933. doi: 10.1016/S0140-6736(18)30533-6
9. Atallah-Yunes SA, Kadado AJ, Kaufman GP, Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. (2019) 145:1527–57. doi: 10.1007/s00432-019-02927-x
10. Guo CW, Alexander M, Dib Y, Lau PKH, Weppler AM, Au-Yeung G, et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur J Cancer. (2020) 124:15–24. doi: 10.1016/j.ejca.2019.09.009
11. Esposito R, Fedele T, Orefice S, Cuomo V, Prastaro M, Canonico ME, et al. An emergent form of cardiotoxicity: acute myocarditis induced by immune checkpoint inhibitors. Biomolecules. (2021) 11:785. doi: 10.3390/biom11060785
12. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. (2017) 136:2085–7. doi: 10.1161/CIRCULATIONAHA.117.030571
13. Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. (2020) 41:1733–43. doi: 10.1093/eurheartj/ehaa051
14. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
15. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. (2016) 2:1346–53. doi: 10.1001/jamaoncol.2016.1051
16. Shao J, Wang C, Ren P, Jiang Y, Tian P, Li W. Treatment- and immune-related adverse events of immune checkpoint inhibitors in advanced lung cancer. Biosci Rep. (2020) 40:BSR20192347. doi: 10.1042/BSR20192347
17. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.2453
18. Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. (2010) 11:155–64. doi: 10.1016/S1470-2045(09)70334-1
19. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. (2018) 13:1930–9. doi: 10.1016/j.jtho.2018.08.2035
20. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. (2016) 22:6051–60. doi: 10.1158/1078-0432.CCR-16-1320
21. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
22. Larsen BT, Chae JM, Dixit AS, Hartman TE, Peikert T, Roden AC. Clinical and histopathologic features of immune checkpoint inhibitor-related pneumonitis. Am J Surg Pathol. (2019) 43:1331–40. doi: 10.1097/PAS.0000000000001298
23. Puzanov I, Diab A, Abdallah K, Bingham CO III, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
24. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. (2015) 26:2375–91. doi: 10.1093/annonc/mdv383
25. Gonzalez-Rodriguez E, Rodriguez-Abreu D. Spanish group for cancer I-B. immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist. (2016) 21:804–16. doi: 10.1634/theoncologist.2015-0509
26. Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. (2018) 4:173–82. doi: 10.1001/jamaoncol.2017.3064
27. Hasegawa S, Ikesue H, Nakao S, Shimada K, Mukai R, Tanaka M, et al. Analysis of immune-related adverse events caused by immune checkpoint inhibitors using the Japanese adverse drug event report database. Pharmacoepidemiol Drug Saf. (2020) 29:1279–94. doi: 10.1002/pds.5108
28. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. (2020) 6:1952–6. doi: 10.1001/jamaoncol.2020.5012
29. Allenbach Y, Anquetil C, Manouchehri A, Benveniste O, Lambotte O, Lebrun-Vignes B, et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev. (2020) 19:102586. doi: 10.1016/j.autrev.2020.102586
30. Steven NM, Fisher BA. Management of rheumatic complications of immune checkpoint inhibitor therapy - an oncological perspective. Rheumatology. (2019) 58:vii29–39. doi: 10.1093/rheumatology/kez536
31. Pathak R, Katel A, Massarelli E, Villaflor VM, Sun V, Salgia R. Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist. (2021) 26:1052–61. doi: 10.1002/onco.13931
32. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis. (2021) 80:36–48. doi: 10.1136/annrheumdis-2020-217139
33. Ma R, Wang Q, Meng D, Li K, Zhang Y. Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems. BMC Cancer. (2021) 21:38. doi: 10.1186/s12885-020-07741-0
34. Liu Y, Jiang L. Tofacitinib for treatment in immune-mediated myocarditis: the first reported cases. J Oncol Pharm Pract. (2020). [Epub ahead of print]. doi: 10.1177/1078155220947141
35. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. (2016) 4:50. doi: 10.1186/s40425-016-0152-y
36. Haddox CL, Shenoy N, Shah KK, Kao JC, Jain S, Halfdanarson TR, et al. Pembrolizumab induced bulbar myopathy and respiratory failure with necrotizing myositis of the diaphragm. Ann Oncol. (2017) 28:673–5. doi: 10.1093/annonc/mdw655
37. Kimura T, Fukushima S, Miyashita A, Aoi J, Jinnin M, Kosaka T, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. (2016) 107:1055–8. doi: 10.1111/cas.12961
38. Behling J, Kaes J, Münzel T, Grabbe S, Loquai C. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res. (2017) 27:155–8. doi: 10.1097/CMR.0000000000000314
39. So H, Ikeguchi R, Kobayashi M, Suzuki M, Shimizu Y, Kitagawa K. PD-1 inhibitor-associated severe myasthenia gravis with necrotizing myopathy and myocarditis. J Neurol Sci. (2019) 399:97–100. doi: 10.1016/j.jns.2019.02.023
40. Luo YB, Tang W, Zeng Q, Duan W, Li S, Yang X, et al. Case report: the neuromusclar triad of immune checkpoint inhibitors: a case report of myositis, myocarditis, and myasthenia gravis overlap following toripalimab treatment. Front Cardiovasc Med. (2021) 8:714460. doi: 10.3389/fcvm.2021.714460
41. Fuentes-Antrás J, Peinado P, Guevara-Hoyer K, Díaz Del Arco C, Sánchez-Ramón S, Aguado C. Fatal autoimmune storm after a single cycle of anti-PD-1 therapy: a case of lethal toxicity but pathological complete response in metastatic lung adenocarcinoma. Hematol Oncol Stem Cell Ther. (2020). doi: 10.1016/j.hemonc.2020.04.006
42. Shen L, Chen H, Wei Q. Immune-therapy-related toxicity events and dramatic remission after a single dose of pembrolizumab treatment in metastatic thymoma: a case report. Front Immunol. (2021) 12:621858. doi: 10.3389/fimmu.2021.621858
43. Xing Q, Zhang ZW, Lin QH, Shen LH, Wang PM, Zhang S, et al. Myositis-myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti-programmed cell death-1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med. (2020) 8:250. doi: 10.21037/atm.2020.01.79
44. Portolés Hernández A, Blanco Clemente M, Escribano García D, Velasco Calvo R, Núñez García B, Oteo Domínguez JF, et al. Checkpoint inhibitor-induced fulminant myocarditis, complete atrioventricular block and myasthenia gravis-a case report. Cardiovasc Diagn Ther. (2021) 11:1013–9. doi: 10.21037/cdt-21-147
Keywords: immune checkpoint inhibitors, PD-1, immune-related adverse events, myocarditis, pneumonia
Citation: Chen Y, Chen Y, Xie J, Liu D and Hong X (2022) Multisystem immune-related adverse events due to toripalimab: Two cases-based review. Front. Cardiovasc. Med. 9:1036603. doi: 10.3389/fcvm.2022.1036603
Received: 05 September 2022; Accepted: 24 October 2022;
Published: 25 November 2022.
Edited by:
Tianshu Liu, Fudan University, ChinaReviewed by:
Teruhiko Imamura, University of Toyama, JapanMarco Giuseppe Del Buono, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2022 Chen, Chen, Xie, Liu and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Hong, hong_xiaoping@hotmail.com, orcid.org/0000-0002-2062-8394
†These authors have contributed equally to this work
 Yanran Chen1†
Yanran Chen1†  Dongzhou Liu
Dongzhou Liu Xiaoping Hong
Xiaoping Hong