Gender- and Age-Specific Associations of Visit-to-Visit Blood Pressure Variability With Anxiety
- 1School of Data Science, City University of Hong Kong, Hong Kong, China
- 2Cardiovascular Analytics Group, Laboratory of Cardiovascular Physiology, Hong Kong, China
- 3School of Life Sciences, The Chinese University of Hong Kong, Hong Kong, China
- 4Aston Medical School, Aston University, Birmingham, United Kingdom
- 5Faculty of Pharmaceutical Sciences, The University of British Columbia, Vancouver, BC, Canada
- 6Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular Disease, Department of Cardiology, Tianjin Institute of Cardiology, Second Hospital of Tianjin Medical University, Tianjin, China
- 7Division of Neurology, Department of Medicine, The University of Hong Kong, Hong Kong, China
- 8Division of Clinical Pharmacology and Therapeutics, Department of Medicine, The University of Hong Kong, Hong Kong, China
Background: There is a bidirectional relationship between blood pressure variability (BPV) and anxiety, but few studies have examined the gender- and age-specific effects of visit-to-visit BPV on incident anxiety. We examined the predictive value of BPV for the incidence of anxiety in a family clinic cohort.
Methods: Consecutive patients with a first attendance to family medicine clinics in Hong Kong between January 1, 2000, and December 31, 2002, with at least three blood pressure measurements available thereafter were included. The primary endpoint was incident anxiety as identified by ICD-9 coding.
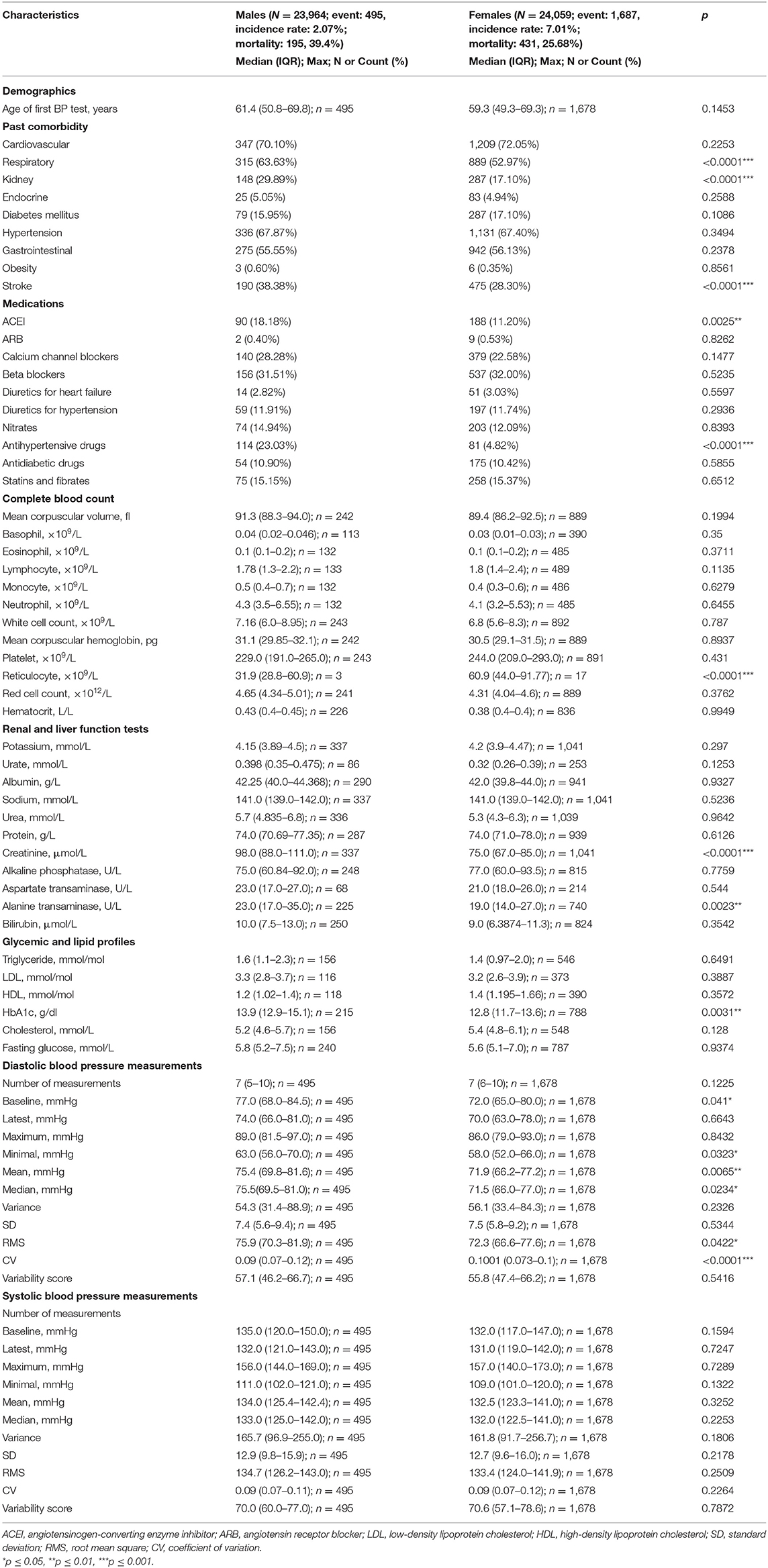
Results: This study included 48,023 (50% males) patients with a median follow-up of 224 [interquartile range (IQR): 217–229] months. Females were more likely to develop incident anxiety compared to males (incidence rate: 7 vs. 2%), as were patients of older age. Significant univariate predictors were female gender, older age, preexisting cardiovascular diseases, respiratory diseases, diabetes mellitus, hypertension, and gastrointestinal diseases, various laboratory examinations, and the number of blood pressure measurements. Higher baseline, maximum, minimum, standard deviation (SD), coefficient of variation (CV), and variability score of diastolic blood pressure significantly predicted incident anxiety, as did all systolic blood pressure measures [baseline, latest, maximum, minimum, mean, median, variance, SD, root mean square (RMS), CV, and variability score].
Conclusions: The relationships between longer-term visit-to-visit BPV and incident anxiety were identified. Female and older patients with higher blood pressure and higher BPV were at the highest risks of incident anxiety.
Introduction
Anxiety is a common symptom, and anxiety disorder includes a group of conditions characterized by excessive worry associated with fatigue, restlessness, muscle tension, irritability, sleeping difficulty, and concentration problems. It is a major public health problem in many countries, damaging not only psychological health but also physical health and quality of life. There is a bidirectional relationship between blood pressure variability (BPV) and incident anxiety. The presence of anxiety can exert effects on BPV. Patients with depressive symptoms presented a significantly lower nighttime systolic blood pressure (BP) fall compared with non-depressed patients after controlling for age, sex, and traditional cardiovascular risk factors (1). The control of negative emotions has been shown to influence BP control and BPV (2). Conversely, increased beat-to-beat BPV has been associated with incident anxiety (3). Longer-term visit-to-visit BPV has also been reported as an independent predictor of cognitive impairment in several cohort studies (4–6). With the widespread measurement of BP measurements at home, fluctuations in BP, as well as very high or low BP readings at home, can cause anxiety in patients. However, few previous studies have examined the longitudinal relationship between BPV and anxiety disorders in older cohorts. In this study, we investigated the gender- and age-specific associations of longer-period visit-to-visit BPV with the incidence of anxiety.
Methods
Research Design and Data Sources
The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee and Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. This was a retrospective cohort study of patients who attended family medicine clinics between January 1, 2000, and March 31, 2002, in the Hong Kong public sector. Patients with at least three BP measurements before being diagnosed with anxiety were included to calculate the variability measures. There were no exclusion criteria. The patients were identified from the Clinical Data Analysis and Reporting System (CDARS), a territory-wide database that centralizes patient information from individual local hospitals to establish comprehensive medical data, including clinical characteristics, disease diagnosis, laboratory results, and medication prescription details. The system has been previously used by both our team and other teams in Hong Kong to conduct studies on comparative drug action (7), specific diseases (8–10), model development (11), or visit-to-visit variability in metabolic parameters (12, 13). Data were obtained regarding consecutive patients diagnosed with anxiety, excluding those who died or discharged within 24 h after the first diastolic/systolic BP measurement and those with fewer than three diastolic/systolic BP measurements (study baseline). Mortality data were obtained from the Hong Kong Death Registry, a population-based official government registry with the registered death records of all Hong Kong citizens. Data on the clinical characteristics, disease diagnosis, laboratory results (including complete blood count, renal and liver function tests, glycemic and lipid profiles, and diastolic/systolic BP), and medication prescription details were extracted. Patients with anxiety were identified with the diagnosis codes 311, 296.3, 296.2, 308, 300.4, 292.84, 298, 300.02, 291.89, 293.84, 292.89, 294.9, 300.2, 309.24, 300.01, 309.21. The ICD-9 codes for past comorbidities and historical medication prescriptions are detailed in the Supplementary Material.
Primary Outcome and Statistical Analysis
The primary outcome was incident cases of anxiety from the study baseline in a time-to-event analysis. Follow-up was carried out until December 31, 2019. We extracted the baseline/latest/maximum/minimum values of diastolic and systolic BPs and calculated the temporal variability measures of diastolic and systolic BPs: (1) mean, (2) median, (3) standard deviation (SD), (4) root mean square (RMS) by first squaring all BP values and then calculating the square root of the mean of the squares, (5) coefficient of variation (CV) by dividing the BP SD by the mean BP and then multiplying by 100, and (6) a variability score [from 0 (low) to 100 (high)] defined as the number of changes in BP of 5 mmHg or more, i.e., 100 × (number of absolute BP change of each two successive measurements > 5)/number of measurements.
Clinical characteristics were summarized using statistical descriptive statistics. Continuous variables were presented as median [95% confidence interval (CI) or interquartile range (IQR)], and categorical variables were presented count (%). The Mann–Whitney U-test was used to compare continuous variables. The χ2-test with Yates' correction was used for 2 × 2 contingency data, and Pearson's χ2-test was used for contingency data for variables with more than two categories. Univariate Cox regression models were conducted based on cohorts of males and females, respectively, to identify the significant predictors of anxiety. Significant univariate predictors of demographics, prior comorbidities, clinical and biochemical tests, medication prescriptions, and BPV were used as input of a multivariate Cox analysis model, adjusted for demographics and comorbidities. Hazard ratios (HRs) with corresponding 95% CIs and p-values were reported. All significance tests were two-tailed and considered significant if p-values < 0.001. Data analysis was performed using the RStudio software (version: 1.1.456) and Python (version: 3.6).
Results
Baseline Clinical Characteristics and Anxiety Incidence
This study included a total of 48,023 (50% males) patients with a median follow-up of 224 (IQR: 217–229) months (Supplementary Figure 1). Among the 23,964 male patients, 495 (incidence rate: 2.1%, median age: 70 [IQR: 57–79] years old) developed anxiety. By contrast, females had a higher incidence rate, with 1,687 of 24,059 (incidence rate: 7.0%, median age: 68 [IQR: 56–78] years old) developing anxiety.
The clinical characteristics of the included patients are provided in Table 1. Compared with female patients, male patients were more likely to suffer from cardiovascular diseases (63.63 vs. 52.97%, p < 0.0001), kidney disease (29.89 vs. 17.10%, p < 0.0001), and stroke (38.38 vs. 28.30%, p < 0.0001). They were more likely to be prescribed angiotensinogen-converting enzyme inhibitors (ACEI) (18.18 vs. 11.20%) and other antihypertensive drugs (23.03 vs. 4.82%) than female patients.
Nevertheless, female patients had a higher reticulocyte level (median: 60.9, IQR: 44.0–91.77 vs. median: 31.9, IQR: 28.8–60.9, p < 0.0001), lower creatinine level (median: 75.0, IQR: 67.0–85.0 vs. median: 98.0, IQR: 88.0–111.0, p < 0.0001), lower alanine transaminase amount (median: 19.0, IQR: 14.0–27.0 vs. median: 23.0, IQR: 17.0–35.0, p = 0.0023), and lower HbA1c level (median: 12.8, IQR: 11.7–13.6 vs. median: 13.9, IQR: 12.9–15.1, p = 0.0031). Regarding diastolic BP measurements, female patients had a lower mean (median: 58.0, IQR: 52.0–66.0 vs. median: 63.0, IQR: 56.0–70.0, p = 0.0323), median (median: 71.9, IQR: 66.2–77.2 vs. median: 75.4, IQR: 69.8–81.6, p = 0.0065), RMS (median: 72.3, IQR: 66.6–77.6 vs. median: 75.9, IQR: 70.3–81.9, p = 0.0422), and CV (median: 0.1001, IQR: 0.073–0.1 vs. median: 0.09, IQR: 0.07–0.12, p < 0.0001).
Incidence of Anxiety on Follow-Up and Significant Predictors
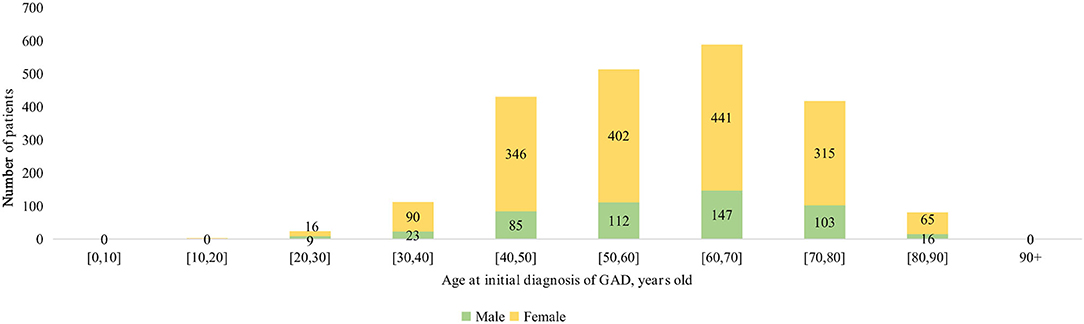
The age-specific incidences of anxiety among male and female subgroups are shown in Figure 1. The number of female patients developing anxiety was more than double that of male patients among those over 30 years of age. Kaplan–Meier survival curves in Figure 2 show that females had a higher risk of developing anxiety than males.
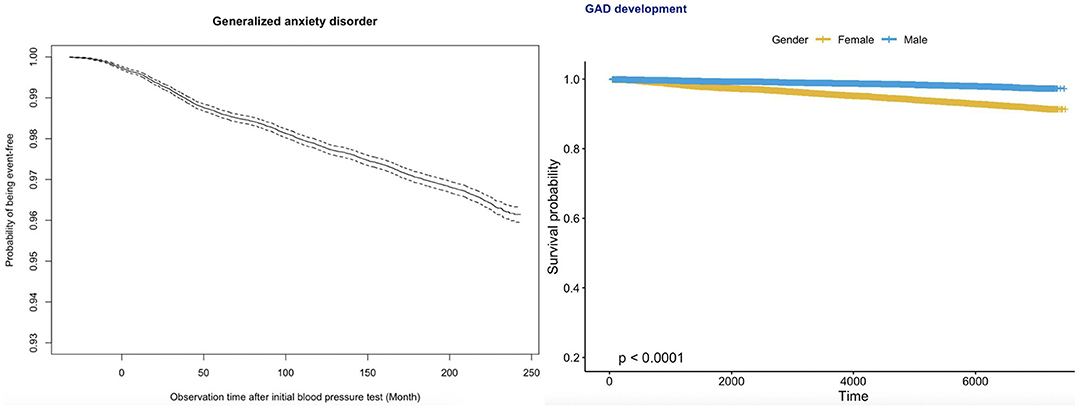
Figure 2. Kaplan–Meier survival curves of incident anxiety development for the whole cohort (top) and stratified by gender (bottom).
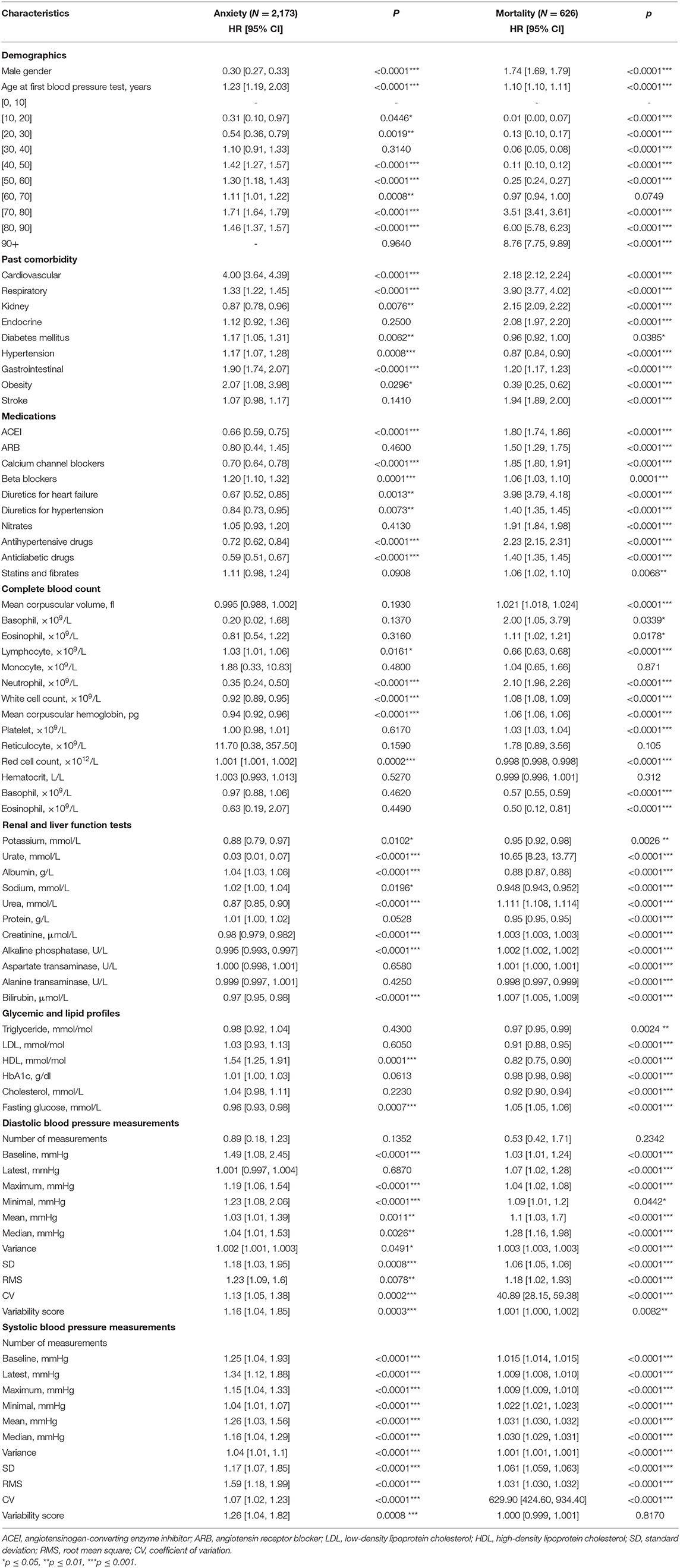
Univariate Cox regression demonstrated the following significant predictors for incident anxiety: demographics, namely, gender [female as comparison: HR for male: 0.30, 95% CI: [0.27, 0.33], p < 0.0001***] and older age [HR: 1.23, 95% CI: [1.19, 2.03], p < 0.0001]. The specific risks differed between age groups: [40, 50] years old [HR: 1.42, 95% CI: [1.27, 1.57], p < 0.0001], [50, 60] years old [HR: 1.30, 95% CI: [1.18, 1.43], p < 0.0001], [60, 70] years old [HR: 1.11, 95% CI: [1.01, 1.22], p = 0.0008], [70, 80] years old [HR: 1.71, 95% CI: [1.64, 1.79], p < 0.0001], [80, 90] years old [HR: 1.46, 95% CI: [1.37, 1.57], p < 0.0001]; past history of cardiovascular diseases [HR: 4.00, 95% CI: [3.64, 4.39], p < 0.0001], respiratory diseases [HR: 1.33, 95% CI: [1.22, 1.45], p < 0.0001], diabetes mellitus [HR: 1.17, 95% CI: [1.05, 1.31], p = 0.0062], hypertension [HR: 1.17, 95% CI: [1.07, 1.28], p = 0.0008], and gastrointestinal disorders [HR: 1.90, 95% CI: [1.74, 2.07], p < 0.0001]; laboratory parameters, namely, lower neutrophil [HR: 0.35, 95% CI: [0.24, 0.50], p < 0.0001], less white cell count [HR: 0.92, 95% CI: [0.89, 0.95], p < 0.0001], lower mean corpuscular hemoglobin level [HR: 0.94, 95% CI: [0.92, 0.96], p < 0.0001], higher red cell count [HR: 1.001, 95% CI: [1.001, 1.002], p = 0.0002], lower urate level [HR: 0.03, 95% CI: [0.01, 0.07], p < 0.0001], higher albumin level [HR: 1.04, 95% CI: [1.03, 1.06], p < 0.0001], lower urea level [HR: 0.87, 95% CI: [0.85, 0.90], p < 0.0001], lower creatinine level [HR: 0.98, 95% CI: [0.979, 0.982], p < 0.0001], lower alkaline phosphatase level [HR: 0.995, 95% CI: [0.993, 0.997], p < 0.0001], lower bilirubin level [HR: 0.97, 95% CI: [0.95, 0.98], p < 0.0001], higher high-density lipoprotein (HDL) level [HR: 1.54, 95% CI: [1.25, 1.91], p = 0.0001], and lower fasting glucose level [HR: 0.96, 95% CI: [0.93, 0.98], p = 0.0007]; diastolic BP measures, namely, higher baseline value [HR: 1.49, 95% CI: [1.08, 2.45], p < 0.0001], higher maximum value [HR: 1.19, 95% CI: [1.06, 1.54], p < 0.0001], higher minimum value [HR: 1.23, 95% CI: [1.08, 2.06], p < 0.0001], larger SD [HR: 1.18, 95% CI: [1.03, 1.95], p = 0.0008], larger CV [HR: 1.13, 95% CI: [1.05, 1.38], p = 0.0002], and larger variability score [HR: 1.16, 95% CI: [1.04, 1.85], p = 0.0003]; and larger values of all systolic BP measures (baseline, latest, maximum, minimum, mean, median, variance, SD, RMS, CV, and variability score) (HR: >1, p < 0.001).
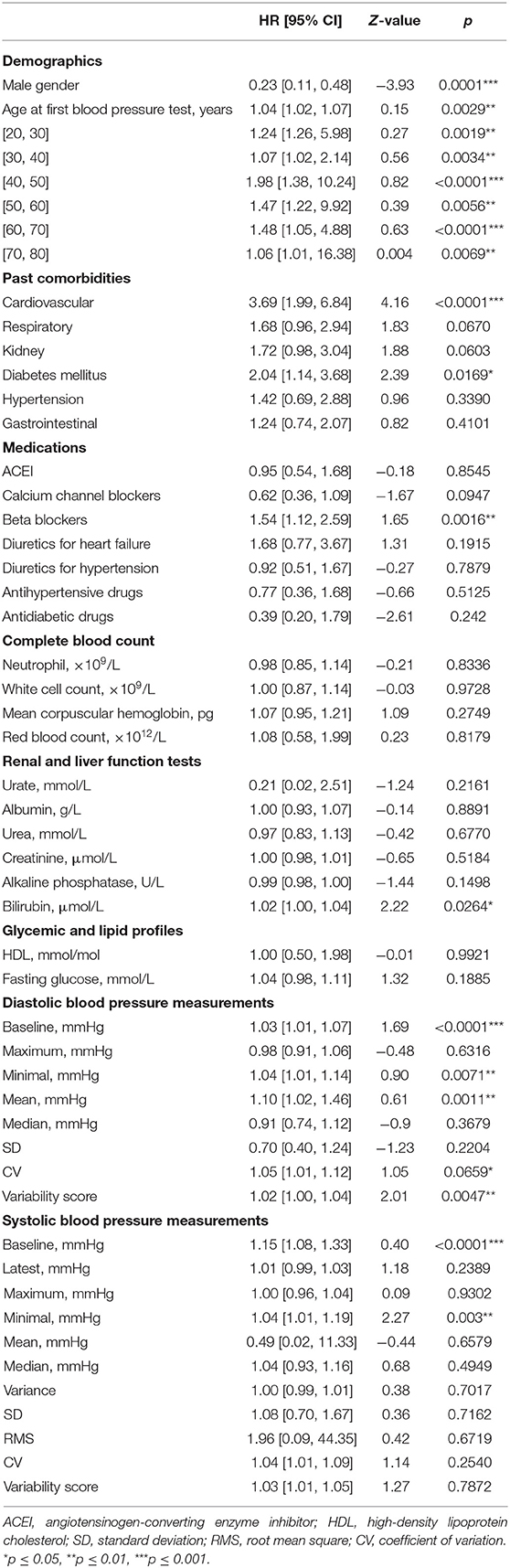
In addition, the significant univariate predictors of all-cause mortality after anxiety presentation were also identified (Table 2). Significant univariate predictors (p < 0.05) were entered into a multivariate Cox regression model, with most of the above univariate predictors remaining significant (Table 3). Next, we further analyzed different BP values in patients who developed anxiety with age stratification (Figures 3, 4 and Supplementary Table 2). There is an age-related increase in mean, median, and measures of variability for both diastolic and systolic BPs.

Figure 3. Box plots showing diastolic blood pressure (DBP) measurements stratified by gender and age at anxiety presentation.
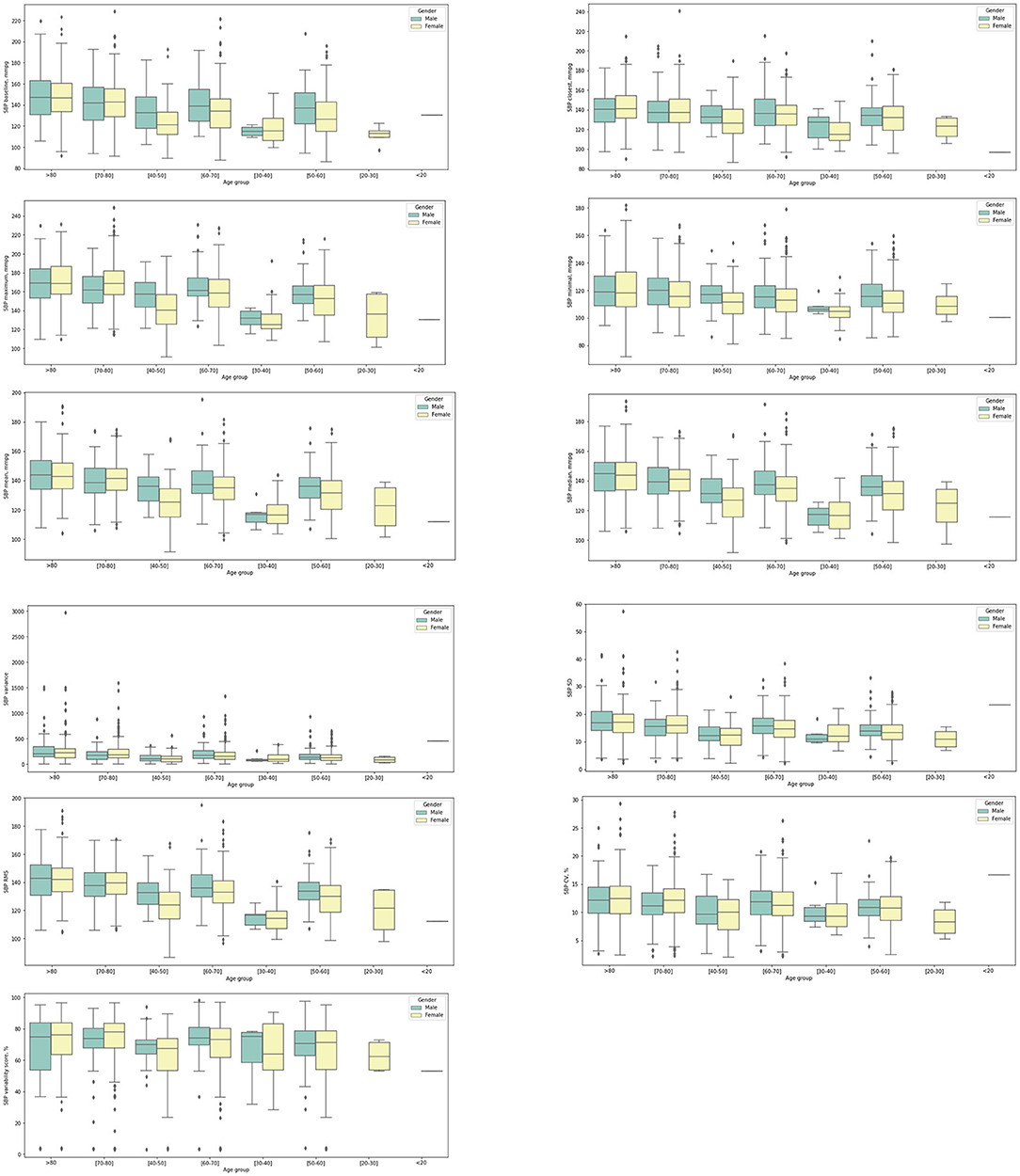
Figure 4. Box plots showing systolic blood pressure (SBP) measurements stratified by gender and age at anxiety presentation.
Comparisons of BP Measurements Before and After Anxiety Development
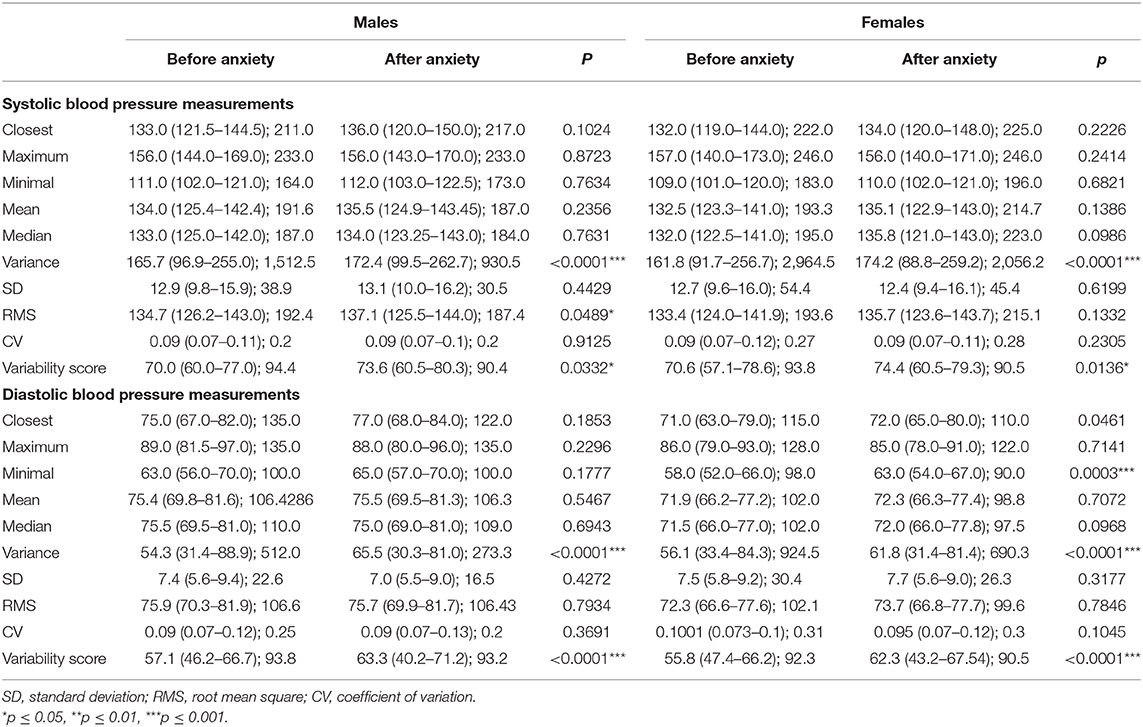
Gender-specific BP measure changes before and after initial anxiety presentation were identified as shown in Table 4. Regarding systolic BP, males had larger variance (median: 172.4, IQR: 99.5–262.7 vs. median: 165.7, IQR: 96.9–255.0, p < 0.0001), larger RMS (median: 137.1, IQR: 125.5–144.0 vs. median: 134.7, IQR: 126.2–143.0, p = 0.0489), and larger variability score (median: 73.6, IQR: 60.5–80.3 vs. median: 70.0, IQR: 60.0–77.0, p = 0.0332) after initial presentation of anxiety diseases; and females had larger variance (median: 174.2, IQR: 88.8–259.2 vs. median 161.8, IQR: 91.7–256.7, p < 0.0001) and larger variability score (median: 74.4, IQR: 60.5–79.3 vs. median: 70.6, IQR: 57.1–78.6, p = 0.0136) after initial presentation of anxiety diseases. In diastolic BP measurements, males had larger variance (median: 65.5, IQR: 30.3–81.0 vs. median: 54.3, IQR: 31.4–88.9, p < 0.0001) and larger variability score (median: 63.3, IQR: 40.2–71.2 vs. median: 57.1, IQR: 46.2–66.7, p < 0.0001) after initial presentation of anxiety; females had larger minimal test (median: 63.0, IQR: 54.0–67.0 vs. median: 58.0, IQR: 52.0–66.0, p = 0.0003), larger variance (median: 61.8, IQR: 31.4–81.4 vs. median: 56.1, IQR: 33.4–84.3, p < 0.0001), and larger variability score (median: 62.3, IQR: 43.2–67.54 vs. median: 55.8, IQR: 47.4–66.2, p < 0.0001).
Discussion
The main findings of this study are that (1) higher baseline, maximum, minimum, SD, CV, and variability score of diastolic BP significantly predicted anxiety, as did all systolic BP measures (baseline, latest, maximum, minimum, mean, median, variance, SD, RMS, CV, and variability score) and (2) female and older patients with higher BP and higher BPV were at the greatest risks of anxiety.
The effects of anxiety on BP and as a potential risk factor have been extensively examined in previous studies (14). However, whether BP influences the risk of incident anxiety has not been investigated in detail, and mixed results were seen in observational studies. Individuals with hypertension may be more likely to develop anxiety (14, 15), but this association is seen only when hypertension coexists with another chronic condition (16) or when the patients are aware of their hypertension diagnosis (17). Previously, higher beat-to-beat BPV has been associated with incident anxiety (3). Longer-term visit-to-visit BPV has also been reported as an independent predictor of neurological conditions such as cognitive impairment in cohort studies (4–6), but whether it can do so for incident anxiety has never been explored. In this population-based study of patients attending family medicine clinics in the public sector of Hong Kong, we established for the first time the predictive value of different metrics of BP and BPV on incident anxiety.
While the physiological mechanisms underlying the bidirectional relationship between hypertension and incident anxiety remain unclear, the phenomenon was reported in recent studies. Population-based studies demonstrated that patients with baseline anxiety had an increased risk of essential hypertension (18–20). By contrast, a territory-wide study of over two million patients in Sweden demonstrated that hypertensive patients were more likely to suffer from anxiety (21). The presence of anxiety increases the risk of poor drug compliance among hypertensive patients and thus worsens their BP control (22).
Various hypotheses have been proposed to explain the association between anxiety and hypertension. First of all, chronic stress, which induces a persistent maladaptive stress response that develops into anxiety, results in long-term cortisol elevation (23). Consequently, the hypothalamic–pituitary–adrenal axis becomes dysregulated and leads to hypertension (24). Furthermore, it is postulated that exaggerated neurobiological sensitivity toward threat results in prolonged activation of the hypothalamic–pituitary–adrenal axis, which results in both the autonomic dysregulation underlying hypertension and the biological change under anxiety (25). Other mechanisms including increased oxidative stress, physical inactivity, and hypercapnia were reported to be common in both the pathogenesis of hypertension and anxiety and thus may contribute to the association between the two diseases (26–28).
Similarly, hypotheses have been proposed to explain the predictive value of BPV for incident anxiety. Increased BPV has been shown to be due to reduced baroreflex sensitivity, which may reflect sympathovagal imbalance likely due to sympathetic hyperactivity, which is observed in anxiety patients (3, 29, 30). The BP instability may reflect compensatory hemodynamic changes under reduced arterial compliance and increased aortic stiffness under systemic inflammatory response, which is both a cause of hypertension and a consequence of anxiety (31, 32). Furthermore, the pathological worrying in anxiety may be associated with increased compliance toward antihypertensives, which are known to increase BPV (33). Moreover, the use of medications such as beta blockers also predicted incident anxiety. It may be that anxious patients are more likely to receive such medications to reduce the symptoms of anxiety (34).
Limitations
Several limitations should be noted for the present study. Given its observational nature, there is information bias with regard to issues of under-coding, missing data, and documentation errors. Moreover, data on lifestyle risk factors of hypertension, such as smoking and alcoholism, were unavailable; thus, their potential influence on the relationship between BP and anxiety cannot be assessed. Furthermore, the clinical circumstances of BP measurement during hospital visits were susceptible to the effects of circumstantial factors, which may introduce additional variables that affect patients' BP and BPV. Circadian changes in BP may be a good predictor of the adverse outcomes. However, our BP values were measured within the clinical setting. It was therefore not possible to obtain BP values at nighttime. Heart rate variability is also an important predictor, and this should be evaluated for its predictive value and incorporated into predictive risk models in subsequent studies. Finally, the diagnosis of anxiety was reliant on ICD-9 coding, and therefore, not all diagnoses were made by a specialist in psychiatry. However, results of psychological tools such as Generalized Anxiety Disorder 7 and Beck Anxiety Inventory are not routinely coded, and it was therefore not possible to precisely define the presence of anxiety disorder that fulfills the specialist definition of this disease.
Conclusions
The relationships between longer-term visit-to-visit BPV and incident anxiety were identified. Female and older patients with higher BP and higher BPV were at the highest risks of anxiety. Future studies should examine the interacting effects between BPV and medication use to influence incident anxiety and anxiety-related outcomes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee and Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JZ and SL: data analysis, data interpretation, statistical analysis, manuscript drafting, and critical revision of the manuscript. WW, KL, RN, PL, and TL: project planning, data acquisition, data interpretation, and critical revision of manuscript. BC: study supervision, data interpretation, statistical analysis, and critical revision of manuscript. QZ and GT: study conception, study supervision, project planning, data interpretation, statistical analysis, manuscript drafting, and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the following grants to QZ: National Natural Science Foundation of China (NSFC): 71972164, Health and Medical Research Fund of the Food and Health Bureau of Hong Kong: 16171991, Innovation and Technology Fund of Innovation and Technology Commission of Hong Kong: MHP/081/19, and National Key Research and Development Program of China, Ministry of Science and Technology of China: 2019YFE0198600.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.650852/full#supplementary-material
References
1. Scuteri A, Spalletta G, Cangelosi M, Gianni W, Assisi A, Brancati AM, et al. Decreased nocturnal systolic blood pressure fall in older subjects with depression. Aging Clin Exp Res. (2009) 21:292–7. doi: 10.1007/BF03324918
2. Symonides B, Holas P, Schram M, Sleszycka J, Bogaczewicz A, Gaciong Z. Does the control of negative emotions influence blood pressure control and its variability? Blood Press. (2014) 23:323–9. doi: 10.3109/08037051.2014.901006
3. Virtanen R, Jula A, Salminen JK, Voipio-Pulkki LM, Helenius H, Kuusela T, et al. Anxiety and hostility are associated with reduced baroreflex sensitivity and increased beat-to-beat blood pressure variability. Psychosom Med. (2003) 65:751–6. doi: 10.1097/01.PSY.0000088760.65046.CF
4. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. (2012) 30:1556–63. doi: 10.1097/HJH.0b013e3283552735
5. Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. (2013) 347:f4600. doi: 10.1136/bmj.f4600
6. Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, et al. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. (2014) 64:983–8. doi: 10.1161/HYPERTENSIONAHA.114.03978
7. Ju C, Lai RWC, Li KHC, Hung JKF, Lai JCL, Ho J, et al. Comparative cardiovascular risk in users vs. non-users of xanthine oxidase inhibitors and febuxostat vs. allopurinol users. Rheumatology. (2020) 59:2340–9. doi: 10.1093/rheumatology/kez576
8. Zhou J, Wang X, Lee S, Wu WKK, Cheung BMY, Zhang Q, et al. Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score-matched territory-wide study. Gut. (2020). doi: 10.1136/gutjnl-2020-323668. [Epub ahead of print].
9. Zhou J, Lee S, Guo CL, Chang C, Liu T, Leung KSK, et al. Anticoagulant or antiplatelet use and severe COVID-19 disease: a propensity score-matched territory-wide study. Pharmacol Res. (2021) 165:105473. doi: 10.1016/j.phrs.2021.105473
10. Zhou J, Tse G, Lee S, Liu T, Cao Z, Zeng DD, et al. Interaction effects between angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and steroid or anti-viral therapies in COVID-19: a population-based study. J Med Virol. (2021) 93:2635–41. doi: 10.1002/jmv.26904
11. Lee S, Zhou J, Guo CL, Wong WT, Liu T, Wong ICK, et al. Predictive scores for identifying patients with type 2 diabetes mellitus at risk of acute myocardial infarction and sudden cardiac death. Endocrinol Diabet Metabol. (2021) e00240. doi: 10.1002/edm2.240. [Epub ahead of print].
12. Lee S, Liu T, Zhou J, Zhang Q, Wong WT, Tse G. Predictions of diabetes complications and mortality using hba1c variability: a 10-year observational cohort study. Acta Diabetol. (2020) 58:171–80. doi: 10.1007/s00592-020-01605-6
13. Lee S, Zhou J, Wong WT, Liu T, Wu WKK, Wong ICK, et al. Glycemic and lipid variability for predicting complications and mortality in diabetes mellitus using machine learning. BMC Endocr Disord. (2021).
14. Pan Y, Cai W, Cheng Q, Dong W, An T, Yan J. Association between anxiety and hypertension: a systematic review and meta-analysis of epidemiological studies. Neuropsychiatr Dis Treat. (2015) 11:1121–30. doi: 10.2147/NDT.S77710
15. Player MS, Peterson LE. Anxiety disorders, hypertension, and cardiovascular risk: a review. Int J Psychiatry Med. (2011) 41:365–77. doi: 10.2190/PM.41.4.f
16. Grimsrud A, Stein DJ, Seedat S, Williams D, Myer L. The association between hypertension and depression and anxiety disorders: results from a nationally-representative sample of South African adults. PLoS ONE. (2009) 4:e5552. doi: 10.1371/journal.pone.0005552
17. Hamer M, Batty GD, Stamatakis E, Kivimaki M. Hypertension awareness and psychological distress. Hypertension. (2010) 56:547–50. doi: 10.1161/HYPERTENSIONAHA.110.153775
18. Bacon SL, Campbell TS, Arsenault A, Lavoie KL. The impact of mood and anxiety disorders on incident hypertension at 1 year. Int J Hypertens. (2014) 2014:953094. doi: 10.1155/2014/953094
19. Stein DJ, Aguilar-Gaxiola S, Alonso J, Bruffaerts R, De Jonge P, Liu Z, et al. Associations between mental disorders and subsequent onset of hypertension. Gen Hosp Psychiatry. (2014) 36:142–9. doi: 10.1016/j.genhosppsych.2013.11.002
20. Perez-Pinar M, Mathur R, Foguet Q, Ayis S, Robson J, Ayerbe L. Cardiovascular risk factors among patients with schizophrenia, bipolar, depressive, anxiety, and personality disorders. Eur Psychiatry. (2016) 35:8–15. doi: 10.1016/j.eurpsy.2016.02.004
21. Sandstrom YK, Ljunggren G, Wandell P, Wahlstrom L, Carlsson AC. Psychiatric comorbidities in patients with hypertension–a study of registered diagnoses 2009-2013 in the total population in Stockholm County, Sweden. J Hypertens. (2016) 34:414–20. doi: 10.1097/HJH.0000000000000824
22. Bautista LE, Vera-Cala LM, Colombo C, Smith P. Symptoms of depression and anxiety and adherence to antihypertensive medication. Am J Hypertens. (2012) 25:505–11. doi: 10.1038/ajh.2011.256
23. Patriquin MA, Mathew SJ. The neurobiological mechanisms of generalized anxiety disorder and chronic stress. Chronic Stress. (2017) 1:2470547017703993. doi: 10.1177/2470547017703993
24. Gold SM, Dziobek I, Rogers K, Bayoumy A, Mchugh PF, Convit A. Hypertension and hypothalamo-pituitary-adrenal axis hyperactivity affect frontal lobe integrity. J Clin Endocrinol Metab. (2005) 90:3262–7. doi: 10.1210/jc.2004-2181
25. O'donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev. (2013) 37:96–108. doi: 10.1016/j.neubiorev.2012.10.013
26. Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. (2010) 1359:178–85. doi: 10.1016/j.brainres.2010.08.093
27. Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. (2012) 37:1911–22. doi: 10.1038/npp.2012.38
28. Ushakov AV, Ivanchenko VS, Gagarina AA. Psychological stress in pathogenesis of essential hypertension. Curr Hypertens Rev. (2016) 12:203–14. doi: 10.2174/1573402112666161230121622
29. Januzzi JLJr, Stern TA, Pasternak RC, Desanctis RW. The influence of anxiety and depression on outcomes of patients with coronary artery disease. Arch Intern Med. (2000) 160:1913–21. doi: 10.1001/archinte.160.13.1913
30. Tully PJ, Tzourio C. Psychiatric correlates of blood pressure variability in the elderly: the Three City cohort study. Physiol Behav. (2017) 168:91–7. doi: 10.1016/j.physbeh.2016.10.024
31. Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res. (2009) 3:56–64. doi: 10.1016/j.artres.2009.02.002
32. Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. (2010) 375:938–48. doi: 10.1016/S0140-6736(10)60309-1
33. Parker G, Hyett M, Hadzi-Pavlovic D, Brotchie H, Walsh W. GAD is good? Generalized anxiety disorder predicts a superior five-year outcome following an acute coronary syndrome. Psychiatry Res. (2011) 188:383–9. doi: 10.1016/j.psychres.2011.05.018
Keywords: blood pressure variability, generalized anxiety disorder, risk prediction, visit-to-visit blood pressure variability, anxiety
Citation: Zhou J, Lee S, Wong WT, Leung KSK, Nam RHK, Leung PSH, Chau Y-LA, Liu T, Chang C, Cheung BMY, Tse G and Zhang Q (2021) Gender- and Age-Specific Associations of Visit-to-Visit Blood Pressure Variability With Anxiety. Front. Cardiovasc. Med. 8:650852. doi: 10.3389/fcvm.2021.650852
Received: 08 January 2021; Accepted: 22 March 2021;
Published: 07 May 2021.
Edited by:
Leonardo Roever, Federal University of Uberlandia, BrazilReviewed by:
Lilei Yu, Renmin Hospital of Wuhan University, ChinaJahaira Lopez-Pastrana, Temple University, United States
Copyright © 2021 Zhou, Lee, Wong, Leung, Nam, Leung, Chau, Liu, Chang, Cheung, Tse and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gary Tse, garytse86@gmail.com; Qingpeng Zhang, qingpeng.zhang@cityu.edu.hk
†These authors share first authorship
 Jiandong Zhou
Jiandong Zhou Sharen Lee
Sharen Lee Wing Tak Wong3
Wing Tak Wong3  Keith Sai Kit Leung
Keith Sai Kit Leung Yau-Lam Alex Chau
Yau-Lam Alex Chau Tong Liu
Tong Liu Bernard Man Yung Cheung
Bernard Man Yung Cheung Gary Tse
Gary Tse Qingpeng Zhang
Qingpeng Zhang