Metal-Free Aminohalogenation of Quinones With Alkylamines and NXS at Room Temperature
- 1Department of Neurology, First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Orthopaedics Surgery, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 3School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou, China
A simple and practical strategy for intermolecular aminohalogenation of quinone with alkyl amines and NXS was developed, in which haloamines generated in situ were employed as bifunctional reagents. The reaction system is reliable, efficient and wide in substrate range, which is suitable for the two-fold aminochlorination of 1, 4-benzoquinones, large-scale reaction and late-stage modification of pharmaceuticals.
Introduction
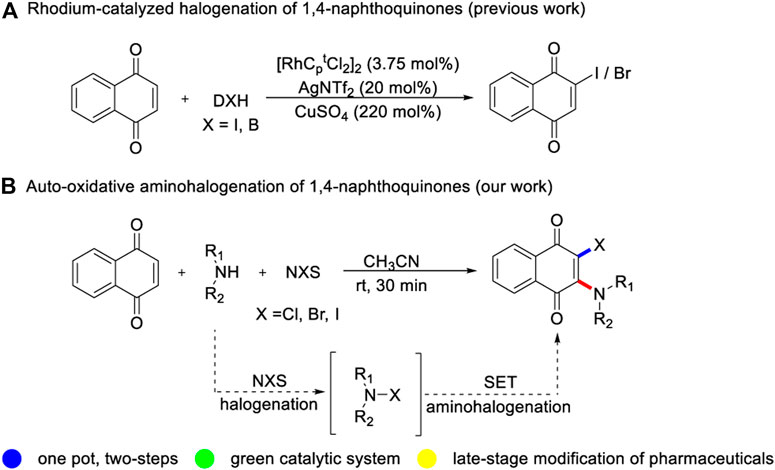
2-amino-3-halogenated naphthoquinones are of great academic and industrial value with widespread applications in medicinal chemistry areas (Calil et al., 2019). Some of these compounds possess low cytotoxicity and tumor growth ability in multiple myeloma (Prachayasittikul et al., 2014). They are used as multipotent agents for treating Alzheimer’s disease and potential new drug for treating leber’s hereditary optic neuropathy (Varricchio et al., 2020). Historically, the developed synthetic approaches relied on dihalogenation reaction/nucleophilic substitution step-wise strategy (Shvartsberg et al., 2009; Sieveking et al., 2014) and halogenation of 2-amino naphthoquinones (Vaidya et al., 2020). Despite the success of these reliable methods, the access of 2-amino-3-iodinated naphthoquinones is still an impalpable assignment. The associated issues related to substrate pre-functionalization, poor chemical selectivity (Shi et al., 1996) and step economy would limit their potential applications in the design and development of new naphthoquinone drugs. Therefore, the development of novel synthetic approach toward highly functionalized 2-amino-3-halogenated naphthoquinones derivatives based on direct aminohalogenation, would be a more promising and attractive protocol.
In the past decade, transition-metal catalyzed C-H functionalization of naphthoquinone with different cross-coupling partners has made incredible progress mainly exploiting its’ high electrophilic properties (Fujiwara et al., 2011; Lisboa et al., 2011; Ilangovan et al., 2013; Galloway et al., 2017; Yu et al., 2018; Liu et al., 2019; Dong et al., 2020; Zhu et al., 2020). In contrast, the cross-coupling of naphthoquinone with electrophiles is still challenging but significant, and only scattered cases have been reported (Zhang et al., 2015). Wang and Bi group described a copper-catalyzed radical trifluoromethylation reaction between naphthoquinone and Togni reagent (Wang et al., 2013; Fang et al., 2014). Bower and coworkers developed a rhodium-catalyzed bromination and iodination of naphthoquinone, using electrophilic halogenation reagent (Jardim et al., 2016a; Jardim et al., 2016b) (Scheme 1A). These studies indicate that cheap and readily available naphthoquinone has broad application prospects, and monofunctional naphthoquinone derivatives can be facilely synthesized by two-component reaction. Nevertheless, there is still a great demand for developing novel multi-component cascade reaction to strealmine the variety of 2,3-difunctionalized quinones without transition metal catalysis (Zeng et al., 2019). Herein, we disclose a metal-free auto-oxidative aminohalogenation of quinones with a series of alkyl amines and NXS (X = Cl, Br, I) at room temperature (Scheme 1B). This synthetic strategy provides a concise and efficient strategy for the preparation of structurally diverse 2-amino-3-halogenated quinones derivatives with the formation of C-N and C-X bonds. In addition, the practical utility of current transformation was applied in large-scale reaction, late-stage functionalization of atomoxetine and two-fold aminochlorination of benzoquinone.
Results and Discussion
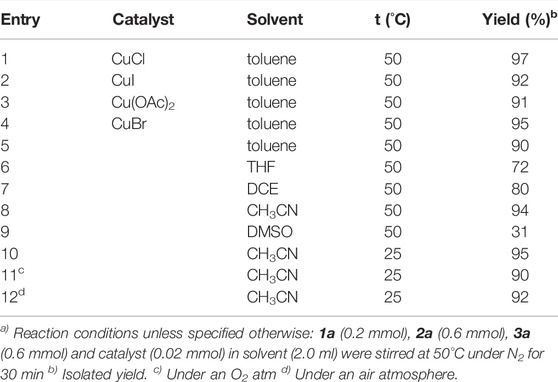
We began our studies using 1,4-naphthoquinone 1a, morpholine 2a and NCS 3a as model substrates to test the feasibility of multi-component aminohalogenation reactions (Table 1). To our delight, when the model reaction was catalyzed by CuCl in toluene at 50 °C under N2 atmosphere for 30 min, the desired 2-chloro-3-morpholino naphthoquinone was isolated in 97% yield (entry 1). It is noteworthy that the transformation efficiency was not profound affected by the choice of different copper catalysts (entries 1–4). To our surprise, the target product 4a was still obtained in excellent yield, even if the reaction was performed in the absence of copper salt (entry 5). This result clearly indicates that the copper salts only worked as a promoter rather than a catalyst. Further solvent screening showed that the high efficiency and high yields relied on polarity of solvent, and the reaction was not effective in polar solvent (entries 6–9). Remarkably, the yield of 4a is almost unaffected by lowering the reaction temperature to room temperature (entry 10). In addition, it is worth mentioning that this transformation is insensitive to reaction atmosphere, whether it is air or oxygen (entries 11–12).
With the optimized reaction condition in hand, an array of alkylamines was examined for current three-component oxidative aminochlorination (Scheme 2). It was found that various cyclic secondary amines (2a-2h, 2l) reacted smoothly, and the corresponding product (4a-4h, 4l) were obtained with moderate to excellent yields. Many important synthetic functional groups, such as methyl, acyl, sulfonyl, ester, hydroxyl, cyano, and halogen, are well compatible under the standard reaction condition. It is worth noting that base-sensitive substrates 2c and 2e-2g are also competent coupling partners, and they are commonly fragile in strongly basic medium. Apart from cyclic organic amines, chain organic amines (2i-2k) are also suitable for vinylchlorination, showing diversity of substrate range. In addition, N-Methyl-4-bromobenzylamine (2k) is feasible and could be utilized for further transformation by classical cross-coupling reactions. Importantly, aromatic heterocycles such as pyrimidine was tolerated and afforded the desired product in 80% yield.
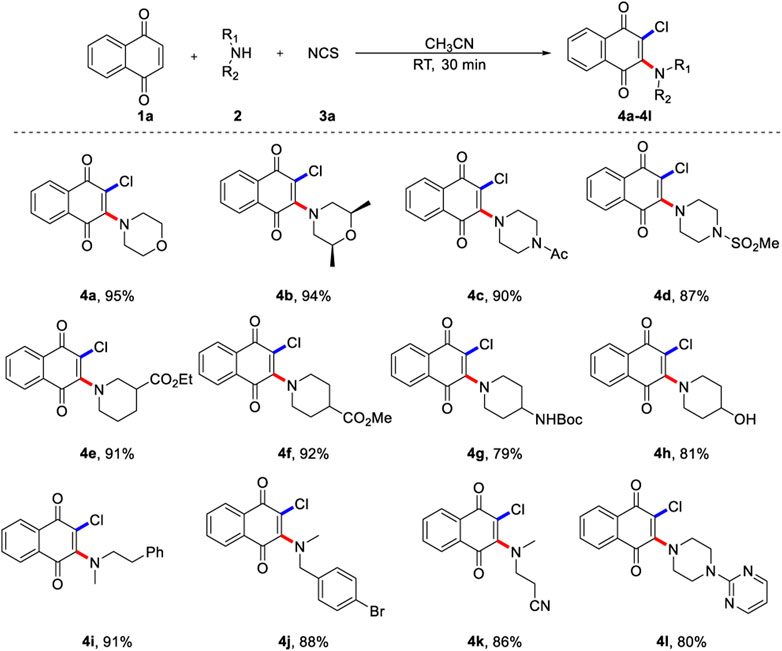
SCHEME 2. Scope of 1,4-naphthoquinone aminochlorinationa. a) Reaction conditions unless specified otherwise: 1a (0.2 mmol), 2 (0.6 mmol) and NCS (0.6 mmol) in CH3CN (2.0 ml) were stirred at room temperature under N2 for 30 min, isolated yields are given.
Encouraged by the versatility of the aminochlorination of 1,4-naphthoquinone, we turned our attention to evaluating the feasibility of aminobromination and aminoiodination (Scheme 3), because these compounds could not be accessed by conventional methods. In these cases, morpholine (6a, 6b), piperidine (5b, 5d-5f, 6c) and piperazine (5a, 5c, 6d-6f) all showed good tolerance, and the corresponding products could be obtained in excellent yields. Interestingly, the aminobromination and aminoiodination of 1,4-naphthoquinone displayed a similar reaction trends to that of aminochlorination reaction. Surprisingly, the aminoiodination of 1,4-naphthoquinone can be completed quickly in a short reaction time, which only takes 2 min, and the TLC silica gel plate was very clean. It is possible that the reactivity of N-X is enhanced by increasing the radius of halogen atom.
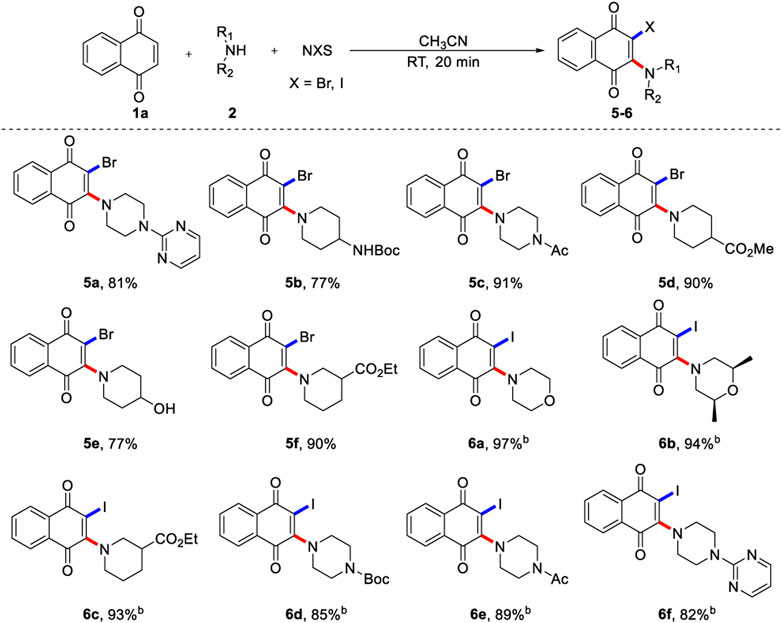
SCHEME 3. Scope of 1,4-naphthoquinone aminobromination and aminoiodinationa. a) Reaction conditions unless specified otherwise: 1a (0.2 mmol), 2 (0.6 mmol) and NBS (0.6 mmol) in CH3CN (2.0 ml) were stirred at room temperature under N2 for 20 min, isolated yields are given. b) Using 0.6 mmol NIS react for 2 min, isolated yields are given.
Of course, current three-component aminohalogenation is particularly practical and useful because N-iodoamine is too unstable to be separated as an initial reactant. In addition, we also investigated the capacity of different alkenes, such as styrene, cinnamonitrile, 2-benzylidenemalononitrile and acrylic ester, and no aminohalogenation products were detected, which suggested that the autoxidation and unique reactivity of 1,4-naphthoquinone was the essential for this transformation.
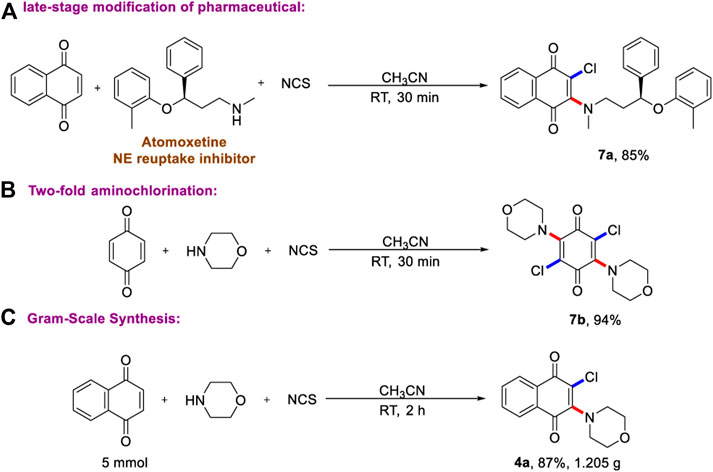
Given the operational simplicity of this protocol and practical application, we next prepared the drug molecular analogs using late-stage modification of pharmaceuticals. As described in Scheme 4A, good chemo-selective vinylchlorination of atomoxetine (trade name: Strattera) on N-Me group has been achieved, and the corresponding product has been obtained in excellent yield. Most importantly, the two-fold aminochlorination of benzoquinone has been accomplished (Scheme 4B), and the molecular symmetric product was produced in 94% yield. This result highlights the power of the current multi-component aminohalogenation reaction, which could not be realized by any known synthetic methods. Finally, as shown in Scheme 4C, the gram-scale reaction proves the reliability and repeatability of two-step one-pot aminohalogenation reaction.
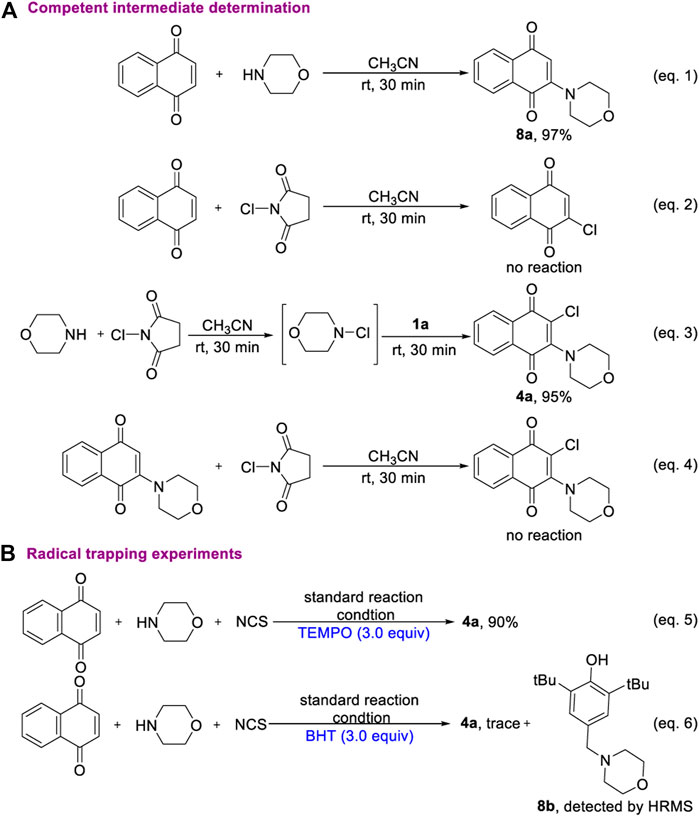
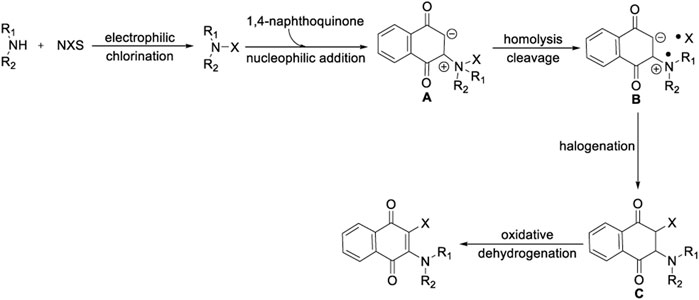
To shed light on the mechanism of oxidative aminohalogenation of 1,4-naphthoquinone, some consideration and control experiments were designed and performed (Scheme 5). First, the reaction of 1,4-naphthoquinone with morpholine produces a completely converted oxidative amination product 8a (eq. 1). In contrast, there was no reaction between 1,4-naphthoquinone and NCS (eq. 2). This result suggests that the amination of 1,4-naphthoquinone initiated a mutli-component reaction owing to the nucleophilicity of nitrogen. Next, we aim to elucidate the chlorination of the intermediate 8a. Two blank experiments were conducted, in which the mixture of morpholine and NCS was stirred in CH3CN for 30 min, and then 1,4-naphthoquinone was added to furnish the corresponding product with excellent yield (eq. 3). This result clearly shows that three-component aminohalogenation reaction is initiated by the formation of N-X bonds. However, the electrophilic chlorination reaction of enaminone 8a did not occur (eq. 4). Based on the experimental result, the progress of an initial oxidative amination followed by elctrophilic capture of the resulting enaminone 8a with electrophilic chlorination reagent could be excluded. A possible intermolecular radical cascade mechanism includes hemolytic cleavage of the nitrogen-halogen bonds and addition to electron-deficient 1,4-naphthoquinone. Interestingly, the aminochlorination of 1,4-naphthoquinone was hardly affected when 3.0 equivalent TEMPO was added (eq. 5). In addition, the BHT-captured product was detected by HRMS, which indicated that N-radical species might be generated during the transformation process of current reaction (eq. 6).
According to these experimental results and related literature, a reasonable reaction mechanism of 1,4-naphthoquinone oxidative aminohalogenation is proposed in Scheme 6. The initial electrophilic halogenation of alkylamine with NXS generated the N-X reagents (Ruffoni et al., 2019). Then, the resulting N-X and electron-deficient 1,4-naphthoquinone undergo nucleophilic addition (Yang et al., 2018) to produce intermediate A, and followed by homolytically cleaving to generate aminium and halide radical B (Hendrick and Wang, 2015), Finally, the key substance C is formed by rapid halogenation, and the corresponding products are obtained by oxidative dehydrogenation (Deng et al., 2015).
Conclusion
In summary, we have developed a straightforward and feasible method of oxidative aminohalogenation of quinones with alkyamines and NXS. The prominent feature of multi-component reactions is that N-haloamines are used as bifunctional reagents, the reaction conditions are mild and simple, the reaction time is short, and the functional group are excellent tolerated. Furthermore, the two-fold aminochlorination and late-stage functionalization of pharmaceuticals demonstrated the highly synthetic value and great potential in the field of medicinal chemistry and discovery of novel drugs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JL, XZ, and Y-AL performed this project. GW prepared and revised this manuscript.
Funding
This work was supported by Public Welfare Science and Technology Project of Wenzhou (No. Y20190132, Y20180233).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.917371/full#supplementary-material
References
Calil, F. A., David, J. S., Chiappetta, E. R. C., Fumagalli, F., Mello, R. B., and Leite, F. H. A. (2019). Ligand-based Design, Synthesis and Biochemical Evaluation of Potent and Selective Inhibitors of Schistosoma Mansoni Dihydroorotate Dehydrogenase. Eur. J. Med. Chem. 167, 357–366. doi:10.1016/j.ejmech.2019.02.018
Deng, H.-P., Wang, D., and Szabó, K. J. (2015). Direct Allylation of Quinones with Allylboronates. J. Org. Chem. 80, 3343–3348. doi:10.1021/acs.joc.5b00264
Dong, Y., Yang, J., Zhang, H., Zhan, X.-Y., He, S., Shi, Z.-C., et al. (2020). Cobalt-catalyzed Cycloamination: Synthesis and Photophysical Properties of Polycyclic N-Heterocycles. Org. Lett. 22, 5151–5156. doi:10.1021/acs.orglett.0c01753
Fang, Z., Ning, Y., Mi, P., Liao, P., and Bi, X. (2014). Catalytic C-H α-trifluoromethylation of α,β-unsaturated Carbonyl Compounds. Org. Lett. 16, 1522–1525. doi:10.1021/ol5004498
Fujiwara, Y., Domingo, V., Seiple, I. B., Gianatassio, R., Bel, M. D., and Baran, P. S. (2011). Practical C-H Functionalization of Quinones with Boronic Acids. J. Am. Chem. Soc. 133, 3292–3295. doi:10.1021/ja111152z
Galloway, J. D., Mai, D. N., and Baxter, R. D. (2017). Silver-catalyzed Minisci Reactions Using Selectfluor as a Mild Oxidant. Org. Lett. 19, 5772–5775. doi:10.1021/acs.orglett.7b02706
Hendrick, C. E., and Wang, Q. (2015). Synthesis of Ortho-Haloaminoarenes by Aryne Insertion of Nitrogen-Halide Bonds. J. Org. Chem. 80, 1059–1069. doi:10.1021/jo502541t
Ilangovan, A., Saravanakumar, S., and Malayappasamy, S. (2013). γ-Carbonyl Quinones: Radical Strategy for the Synthesis of Evelynin and its Analogues by C-H Activation of Quinones Using Cyclopropanols. Org. Lett. 15, 4968–4971. doi:10.1021/ol402229m
Jardim, G. A. M., Bower, J. F., and Júnior, E. N. S. (2016a). Rh-catalyzed Reactions of 1,4-benzoquinones with Electrophiles: C-H Iodination, Bromination, and Phenylselenation. Org. Lett. 18, 4454–4457. doi:10.1021/acs.orglett.6b01586
Jardim, G. A. M., Silva J´unior, E. N., and Bower, J. F. (2016b). Overcoming Naphthoquinone Deactivation: Rhodium-Catalyzed C-5 Selective C–H Iodination as a Gateway to Functionalized Derivatives. Chem. Sci. 7, 3780–3784. doi:10.1039/C6SC00302H
Lisboa, C. S., Santos, V. G., Vaz, B. G., Lucas, N. C., Eberlin, M. N., and Garden, S. J. (2011). C-H Functionalization of 1, 4-naphthoquinone by Oxidative Coupling with Anilines in the Presence of a Catalytic Quantity of Copper (II) Acetate. J. Org. Chem. 76, 5264–5273. doi:10.1021/jo200354u
Liu, R.-H., He, Y.-H., Yu, W., Zhou, B., and Han, B. (2019). Silver-catalyzed Site-Selective Ring-Opening and C-C Bond Functionalization of Cyclic Amines: Access to Distal Aminoalkyl-Substituted Quinones. Org. Lett. 21, 4590–4594. doi:10.1021/acs.orglett.9b01496
Prachayasittikul, V., Pingaew, R., Worachartcheewan, A., Nantasenamat, C., Prachayasittikul, S., Ruchirawat, S., et al. (2014). Synthesis, Anticancer Activity and QSAR Study of 1,4-naphthoquinone Derivatives. Eur. J. Med. Chem. 84, 247–263. doi:10.1016/j.ejmech.2014.07.024
Ruffoni, A., Juliá, F., Svejstrup, T. D., Mcmillan, A. J., Douglas, J. J., and Leonori, D. (2019). Practical and Regioselective Amination of Arenes Using Alkyl Amines. Nat. Chem. 11, 426–433. doi:10.1038/s41557-019-0254-5
Shi, S., Katz, T. J., Yang, B. V., and Liu, L. (1996). Use of Thiazyl Chlorides, Alkyl Carbamates, and Thionyl Chloride to Fuse 1,2,5-thiadiazoles to Quinones and to Oxidize, Chlorinate, and Aminate Them. J. Org. Chem. 60, 1285–1297. doi:10.1021/jo00110a036
Shvartsberg, M. S., Kolodina, E. A., Lebedeva, N. I., and Fedenok, L. G. (2009). Synthesis of Benz[f]indole-4,9-Diones via Acetylenic Derivatives of 1,4-naphthoquinone. Tetrahedron Lett. 50, 6769–6771. doi:10.1016/j.tetlet.2009.09.110
Sieveking, I., Thomas, P., Estévez, J. C., Quiñones, N., Cuéllar, M. A., Villena, J., et al. (2014). 2-Phenylaminonaphthoquinones and Related Compounds: Synthesis, Trypanocidal and Cytotoxic Activities. Bioorg. Med. Chem. 22, 4609–4620. doi:10.1016/j.bmc.2014.07.030
Vaidya, S. D., Toenjes, S. T., Yamamoto, N., Maddox, S. M., and Gustafson, J. L. (2020). Catalytic Atroposelective Synthesis of N-Aryl Quinoid Compounds. J. Am. Chem. Soc. 142, 2198–2203. doi:10.1021/jacs.9b12994
Varricchio, C., Beirne, K., Aeschlimann, P., Heard, C., Rozanowska, M., Votruba, M., et al. (2020). Discovery of Novel 2-Aniline-1,4-Naphthoquinones as Potential New Drug Treatment for Leber’s Hereditary Optic Neuropathy (LHON). J. Med. Chem. 63, 13638–13655. doi:10.1021/acs.jmedchem.0c00942
Wang, X., Ye, Y., Ji, G., Xu, Y., Zhang, S., Feng, J., et al. (2013). Copper-catalyzed Direct C-H Trifluoromethylation of Quinones. Org. Lett. 15, 3730–3733. doi:10.1021/ol4016095
Yang, J., Zhao, B., Xi, Y., Sun, S., Yang, Z., Ye, Y., et al. (2018). Construction of Benzene Rings by Copper-Catalyzed Cycloaddition Reactions of Oximes and Maleimides: an Access to Fused Phthalimides. Org. Lett. 20, 1216–1219. doi:10.1021/acs.orglett.8b00141
Yu, W., Hjerrild, P., Jacobsen, K. M., Tobiesen, H. N., Clemmensen, L., and Poulsen, T. B. (2018). A Catalytic Oxidative Quinone Heterofunctionalization Method: Synthesis of Strongylophorine-26. Angew. Chem. Int. Ed. 57, 9805–9809. doi:10.1002/anie.201805580
Zeng, F.-L., Chen, X.-L., He, S.-Q., Sun, K., Liu, Y., Fu, R., et al. (2019). Copper-catalyzed One-Pot Three-Component Thioamination of 1,4-naphthoquinone. Org. Chem. Front. 6, 1476–1480. doi:10.1039/C9QO00091G
Zhang, C., McClure, J., and Chou, C. J. (2015). Silver-catalyzed Direct Thiolation of Quinones by Activation of Aryl Disulfides to Synthesize Quinonyl Aryl Thioethers. J. Org. Chem. 80, 4919–4927. doi:10.1021/acs.joc.5b00247
Keywords: metal-free, aminohalogenation, quinones, NXS, radical reactions
Citation: Li J, Li Y-A, Wu G and Zhang X (2022) Metal-Free Aminohalogenation of Quinones With Alkylamines and NXS at Room Temperature. Front. Chem. 10:917371. doi: 10.3389/fchem.2022.917371
Received: 12 April 2022; Accepted: 19 April 2022;
Published: 20 May 2022.
Edited by:
Guangzhao Wang, Yangtze Normal University, ChinaReviewed by:
Bing Yu, Zhengzhou University, ChinaYongchao Ma, Qingdao Agricultural University, China
Copyright © 2022 Li, Li, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-An Li, liyuan@wzhospital.cn; Ge Wu, wuge@wmu.edu.cn
†These authors have contributed equally to this work
 Jia Li
Jia Li Yu-An Li
Yu-An Li Ge Wu
Ge Wu Xu Zhang1
Xu Zhang1