The Cascade [1,5]-Hydride Shift/Intramolecular C(sp3)–H Activation: A Powerful Approach to the Construction of Spiro-Tetrahydroquinoline Skeleton
- 1Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Translational Chinese Medicine Key Laboratory of Sichuan Province, Sichuan Academy of Chinese Medicine Sciences, Sichuan Institute for Translational Chinese Medicine, Chengdu, China
- 3State Key Laboratory of Southwestern Chinese Medicine Resources, Hospital of Chengdu University of Traditional Chinese Medicine, School of Pharmacy and College of Medical Technology, Chengdu University of Traditional Chinese Medicine, Chengdu, China
The direct functionalization of inert C–H bonds is regarded as one of the most powerful strategies to form various chemical bonds and construct complex structures. Although significant advancements have been witnessed in the area of transition metal-catalyzed functionalization of inert C–H bonds, several challenges, such as the utilization and removal of expensive transition metal complexes, limited substrate scope and large-scale capacity, and poor atom economy in removing guiding groups coordinated to the transition metal, cannot fully fulfill the high standard of modern green chemistry nowadays. Over the past decades, due to its inherent advantage compared with a transition metal-catalyzed strategy, the hydride shift activation that applies “tert-amino effect” into the direct functionalization of the common and omnipresent C(sp3)–H bonds adjacent to tert-amines has attracted much attention from the chemists. In particular, the intramolecular [1,5]-hydride shift activation, as the most common hydride shift mode, enables the rapid and effective production of multifunctionally complex frameworks, especially the spiro-tetrahydroquinoline derivatives, which are widely found in biologically active natural products and pharmaceuticals. Although great accomplishments have been achieved in this promising field, rarely an updated review has systematically summarized these important progresses despite scattered reports documented in several reviews. Hence, in this review, we will summarize the significant advances in the cascade [1,5]-hydride shift/intramolecular C(sp3)-H functionalization from the perspective of “tert-amino effect” to build a spiro-tetrahydroquinoline skeleton, and the content is categorized by structure type of final spiro-tetrahydroquinoline products containing various pharmaceutical units. Besides, current limitations as well as future directions in this field are also pointed out. We hope our review could provide a quick look into and offer some inspiration for the research on hydride shift strategy in the future.
Introduction
Undoubtedly, the functionalization of inert C–H bonds is one of the most effective and powerful tools for the formation of various chemical bonds in modern organic synthesis (Su et al., 2015; Qin et al., 2017; Karimov and Hartwig, 2018; Sauermann et al., 2018). Over the past decades, tremendous advancements have been witnessed in this dynamic field, especially in the direct functionalization of unreactive C–H bonds (Hartwig, 2012; Mousseau and Charette, 2013; Yang, 2015; Hartwig, 2016; He et al., 2019). Compared to classic transition metal-catalyzed coupling reactions, the direct modification of ubiquitous C–H bonds of simple organic compounds, without pre-activation and generation of a large number of wastes such as halides and tedious synthetic procedures, has attracted intense interest from the academic and industrial community (Peng and Maulide, 2013; Zheng and You, 2014; Qin et al., 2017; Sauermann et al., 2018). Owing to its intrinsic advantages, numerous innovative and efficient synthetic methodologies have been successfully explored, offering a straightforward access to rapidly synthesize structurally complex molecules (Hazelard et al., 2017; Karimov and Hartwig, 2018; Hong et al., 2020; Junrong et al., 2021). Among these powerful strategies, the transition metal-catalyzed C–H bond activation has long dominated the top topic in this field (Cho et al., 2011; Kuhl et al., 2012; Chen et al., 2015; Gensch et al., 2016). However, in the view of green and sustainable chemistry, (1) the utilization and removal of expensive transition metals like Rh and Pd, (2) the addition of extra oxidizing agents and additives, (3) the limitation of substrate scope and large-scale capacity, and (4) the relatively poor atom economy in removing guiding groups coordinated to the transition metal have further restrained its applications nowadays. Therefore, the development of novel strategies to address these aforementioned challenges in the functionalization of inert C–H bonds, especially the common and omnipresent C(sp3)–H bonds, is increasingly significant.
With the continuing motivation towards green chemistry, the hydride shift-involved C(sp3)–H activation via a redox-neutral process, known as an ancient but effective methodology, provides new solutions to address those synthetic challenges (Wang and Xiao, 2016). In 1895, the phenomenon of redox-neutral C–H functionalization was first observed and then termed “tert-amino effect” by Meth-Cohn and Suschitzky in 1972 (Meth-Cohn and Suschitzky, 1972; Pinnow, 1895). Recognizing its great potential of selective activation and direct functionalization of unreactive C(sp3)-H bonds, enormous attention from the chemists has been paid to this magic effect, especially the most common migration mode of intramolecular [1,5]-hydride shift (Haibach and Seidel, 2014; Wang and Xiao, 2014; Kwon and Kim, 2016). Basically, both hydride donors and hydride acceptors are required in the hydride shift process (Figure 1B). The type of hydride donor involves C(sp3)–H bonds adjacent to tert-amines, ethereal oxygen and sulfur, benzylic C(sp3)–H bonds, and non-benzylic C(sp3)–H bonds, while the type of hydride acceptor contains electro-deficient alkenes, aldehydes, ketones, enals, enones, imines, alkynes, and allene derivatives. As for the specific mechanism of the hydride shift process, the scientific community has not been able to reach a consensus due to the two possible routes (Figure 1C). On the one hand (mechanism 1), the hydride shift process could undergo an intramolecular 6-endo-trig cyclization (or nucleophilic attack) to deliver the heterocycle after the generation of zwitterion A, which is formed by a [1,5]-suprafacial hydrogen shift from the α-position of carbon adjacent to heteroatom of substrate 1 to the electrophilic hydrogen acceptor in the form of a hydride (Nijhuis et al., 1987; Nijhuis et al., 1989; Meth-Cohn, 1996). On the other hand (mechanism 2), this transformation could be conducted through two sequential zwitterion B (the resonance form of substrate 1) and A (as a result of the sequent [1,5]-suprafacial hydrogen shift in the form of a sigmatropic hydride shift from zwitterion B) to provide the target product (Datta et al., 2005; Odedra et al., 2007; Shu et al., 2008). To date, a series of critical reviews from reputable groups have summarized such great progress (Haibach and Seidel, 2014; Wang and Xiao, 2014; Kwon and Kim, 2016; An and Xiao, 2021). These reviews focus mainly on the application of hydride shift-involved C(sp3)–H activation to construct five-, six-, seven-, or other-membered hetero, spiro, or fused cycles, as well as acyclic multifunctional compounds. Among them, the intramolecular cascade [1,5]-hydride shift/cyclization sequence, as the most common and useful sequential reaction, is highly effective for C(sp3)–H bond activation/C–C and C–Heteroatom formation, and proves to be a versatile method to construct six-membered cyclic compounds, especially including spirocyclic molecules like spiro-tetrahydroquinolines (Rios, 2012; Mao et al., 2013; Wang et al., 2013; Zhu et al., 2017; Xu et al., 2019).
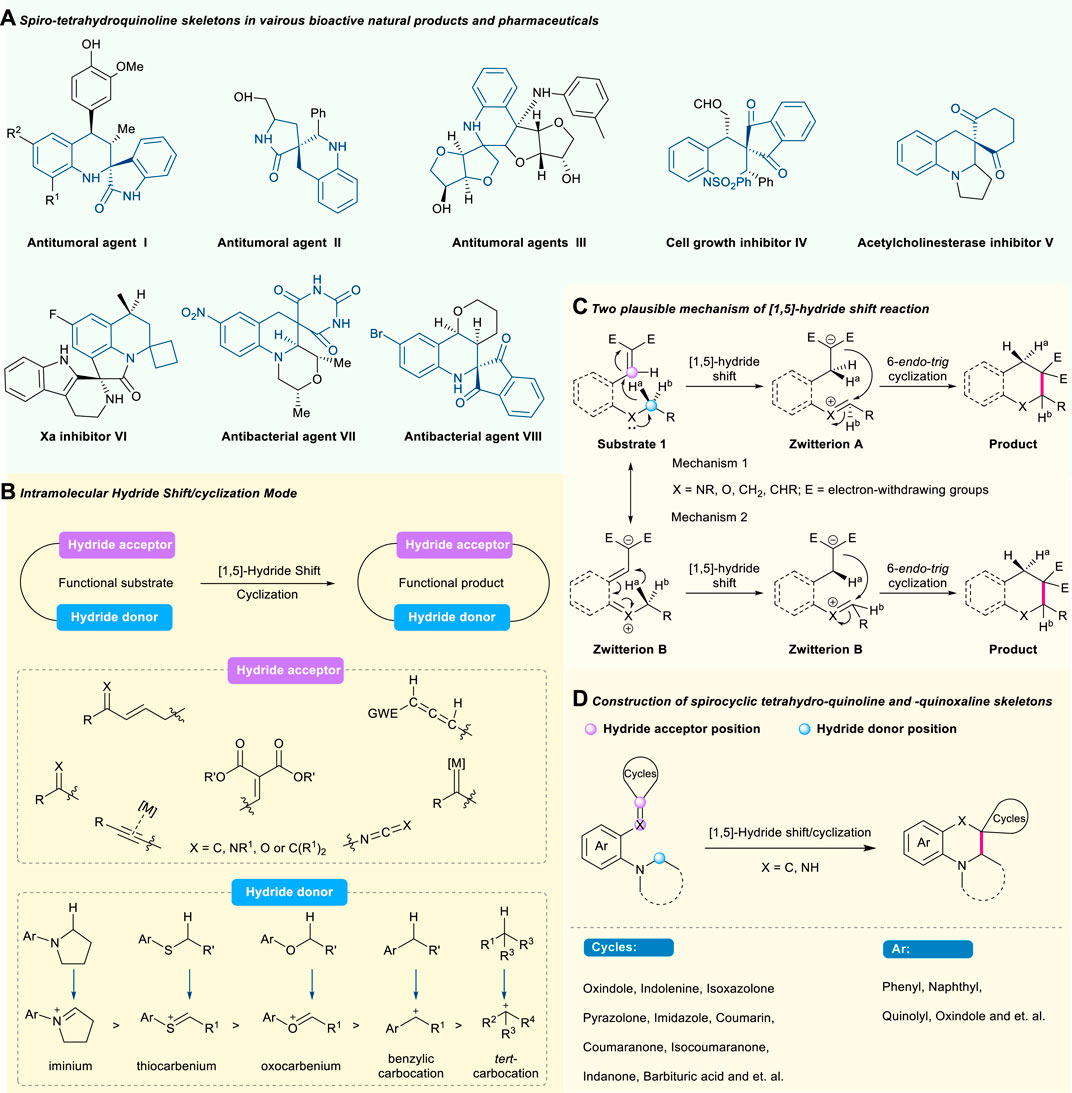
FIGURE 1. Selected bioactive natural products and pharmaceuticals with spiro-tetrahydroquinoline skeletons (A), advancement of intramolecular (B) and two plausible mechanisms (C) of [1,5]-hydride shift mode, and construction of spiro-tetrahydroquinoline framework (D).
The spiro-tetrahydroquinoline skeleton as a privileged motif widely exists in biologically active natural products and pharmaceuticals (Figure 1A). For example, antitumoral agent (I), known as a novel synthetic molecule, exhibited antitumoral and antiplasmodial activities (Kouznetsov et al., 2010). Antitumoral agent (II) possessed good bioactivity to inhibit against the HeLa and MCF-7 cell lines at micromolar concentrations (Damerla et al., 2012). Antitumoral agent (III) showed obvious in vitro immunocompetence and cytotoxicity against Hela and Eca-109 cells (Liu et al., 2005). As a synthetic compound, cell growth inhibitor (IV) displayed significant wound-healing activities (Liou et al., 2021). Compound (V) was known as a potent inhibitor against acetylcholinesterase (Toth et al., 2018). Xa inhibitor (VI) demonstrated promising inhibitory activity in the micromolar concentration against serine protease (Medvedeva et al., 2018). Antitumoral agent (VII) showed potent activity against methicillin-resistant Staphylococcus aureus (MRSA) and fluoroquinoline-resistant bacterial strains (Ruble et al., 2009). The synthetic antibacterial agent (VIII) exhibited good antibacterial activity against microorganisms (Ramesh et al., 2009). During the past years, impressive advancements have been achieved in the synthesis of these molecules through organic or metal synthesis (Han et al., 2012; Shi et al., 2013; Wang et al., 2013; Yang and Du, 2013; Li and Du, 2014; Dong et al., 2021). However, severe challenges, like the utilization and removal of expensive transition metal, addition of extra oxidizing agents and additives, and poor atom economy, still exist in this field. Therefore, the development of a novel strategy to construct these valuable spirocyclic frameworks through direct functionalization of inert chemical groups or bonds is still highly demanded. As one of the most common and effective C(sp3)-H functionalization methodologies, the cascade [1,5]-hydride shift/cyclization strategy possessed an inherent advantage in the transformation of unreactive functional groups or bonds to various chemical structures. However, building structurally complex spiro-tetrahydroquinoline via the cascade [1,5]-hydride shift/cyclization strategy has rarely been established before the limited but significant works (Pastine and Sames, 2005; Kang et al., 2010; Cao et al., 2011; Wang, 2013; Mori et al., 2015; Zhu et al., 2017; Lv et al., 2019). More importantly, rarely an updated review has systematically summarized these important progresses despite scattered reports documented in several reviews (Wang and Xiao, 2014; Wang and Xiao, 2016; Xiao Mingyan et al., 2018; An and Xiao, 2021).
In this review, we will summarize the significant advances in the cascade [1,5]-hydride shift/intramolecular C(sp3)-H functionalization from the perspective of tert-amino effect to build a spiro-tetrahydroquinoline skeleton due to its great potential in the discovery of new drugs, and also point out its current limitations as well as future directions in this field. This review is categorized by the structure type of final spirocyclic products as follows (Figure 1D): Construction of a spiro-tetrahydroquinoline skeleton containing (1) (oxo)indole, (2) (iso)coumaranone, (3) pyrazolone and imidazole, (4) isoxazolone, (5) coumarin, and (6) other units.
The Construction of a Spiro-Tetrahydroquinoline Skeleton Containing Various Pharmaceutical Cores
The spiro-tetrahydroquinoline skeleton as a privileged structure motif is quite frequently seen in a range of biologically active natural products and pharmaceuticals. In the process of constructing a spirocyclic tetrahydroquinoline framework via the [1,5]-hydride shift/cyclization strategy, the cyclic or acyclic tert-amino moiety of substrates always serves as a hydride donor, while electro-deficient alkenes containing various pharmaceutical cores (such as oxindole, indolenine, pyrazolone, coumarin, indanone, and coumaranone/isocoumaranone) and reactive imines function as a hydride acceptor, which fulfill the structural diversity of spirocyclic tetrahydroquinoline derivatives.
The Construction of a Spiro-Tetrahydroquinoline or Tetrahydroquinoxaline Skeleton Containing an (oxo)Indole Unit
In 2015, Feng’s group developed an asymmetric tandem [1,5]-hydride shift/cyclization reaction to produce chiral spirooxindole tetrahydroquinolines using chiral N, N′-dioxide/Sc(OTf)3 as a catalytic system (Scheme 1) (Cao et al., 2015). As far as we know, this is the only report for the asymmetric version of tandem [1,5]-hydride shift/cyclization. The oxindole derivative 1 as a suitable hydride acceptor triggered the intramolecular tandem [1,5]-hydride shift/ring closure reaction. With the assistance of the chiral complex of N, N′-dioxide/Sc(OTf)3, all reactions proceeded smoothly in dichloroethane (DCE) at 35°C, offering a wide range of optically active spirooxindole tetrahydroquinolines 2 with high yields of up to 97% and excellent stereoselectivies of up to 94% ee and >20:1 dr. Moreover, a gram-scale investigation of this strategy was smoothly operated with excellent reaction performance, which demonstrated the robustness of this tandem sequence toward spirocyclic tetrahydroquinolines. The author proposed a possible mechanism of chiral memory effect dominating a helical chirality in a cationic intermediate to explain the chiral information observed in optically active products.
In 2017, Tunge’s group revealed a Lewis acid-catalyzed synthesis of spiro tetrahydroquinoxalines from diamines 6 and isatins 7 (Scheme 2) (Ramakumar et al., 2017). The condensation of diamine 6 with the α-dicarbonyl substrate generated an imine intermediate 11 that is responsible for the [1,5]-hydride shift to nitrogen. Using FeCl3 as a promoter, various substituents at different positions of both substrates were all tolerated, yielding corresponding spirocyclic compounds X with accepted to outstanding yields (55%–90%) and moderate to excellent diastereoselectivities (1.4:1 to 25:1). A hypothetical mechanism for the key step of cyclocondensation is depicted in Scheme 2.
One year later, Li’s group uncovered a fluorinated alcohol-mediated cascade [1,5]-hydride shift/cyclization reaction to prepare spiro-tetrahydroquinolines bearing oxindole moiety (Scheme 3) (Chen et al., 2018). Research indicated that hexafluoroisopropanol (HFIP) demonstrated a significant influence on the efficacy of the transformation. The Knoevenagel condensation of 2-(pyrrolidin-1-yl)benzaldehyde 13 and indolin-2-one 14 triggered a [1,5]-hydride shift/cyclization sequence to generate structurally diverse spirooxindole-fused tetrahydroquinolines. With the help of HFIP as a solvent, this strategy showed good tolerance of a variety of substrates, resulting in the corresponding products having moderate to good yields (32%–89% yield) with good to high diastereoselectivities (61:39 to >20:1 dr). A plausible mechanism displaying dual hydrogen bonds of HFIP with the enol moiety of intermediate TS1/2 was proposed in their study, which is depicted in Scheme 3.
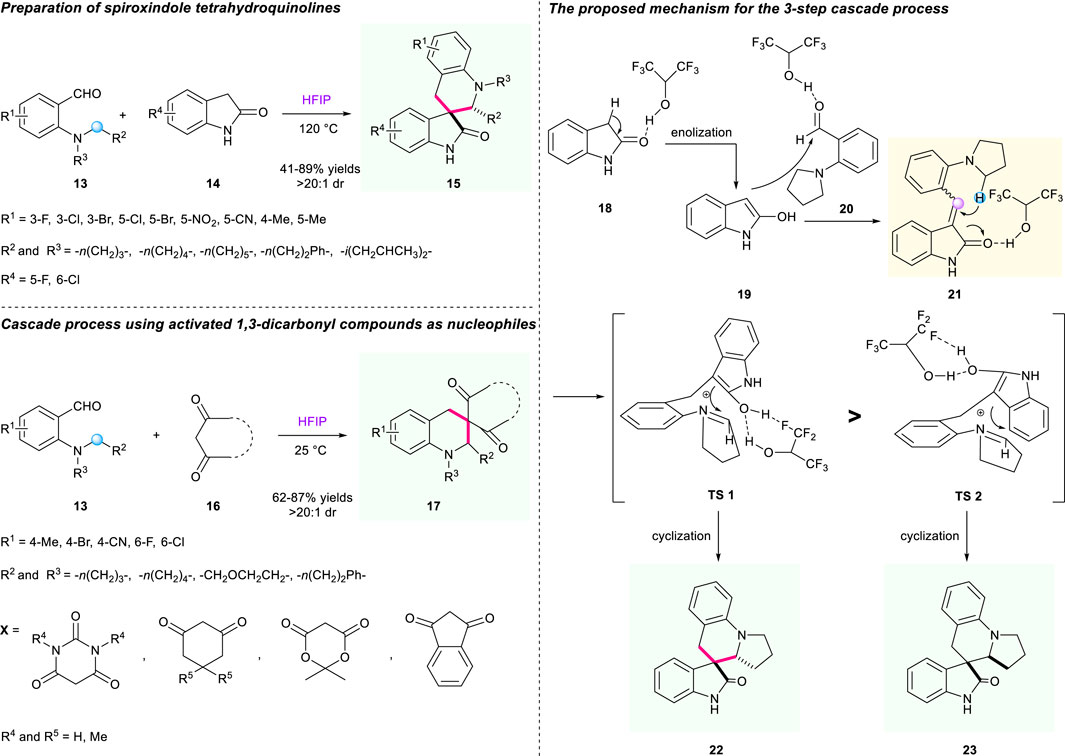
SCHEME 3. Efficient construction of tetrahydroquinolines via HFIP-mediated cascade [1,5]-hydride shift/cyclization.
Soon after, Xiao’s group uncovered the scandium-catalyzed redox-neutral cascade [1,5]-hydride shift/cyclization of C4-amine-substituted isatins 24 and 1,3-dicarbonyl compounds 25 (Scheme 4) (Zhu et al., 2019). In this process, the α,β-unsaturated 1,3-dicarbonyl intermediate 29 acted as a hydride acceptor. The optimized condition proved to be using dichloroethane (DCE) as solvent, 5-Å molecular sieves as additive, and Sc(OTf)3 as catalyst, delivering diverse product 26 with acceptable to good yields (48%–99% yield) and acceptable to excellent diastereoselectivities (1:1 to >20:1 dr). Intriguingly, the substrates containing asymmetrical acyclic N-benzyl-N-methylamine were also tolerated in this reaction, which has never been achieved before in most hydride shift sequences. Besides, the chiral control of the reaction was also investigated using chiral phosphoric acid as catalyst; however, only poor enantioselectivity was observed in this process. The plausible reaction mechanism is described in Scheme 4.
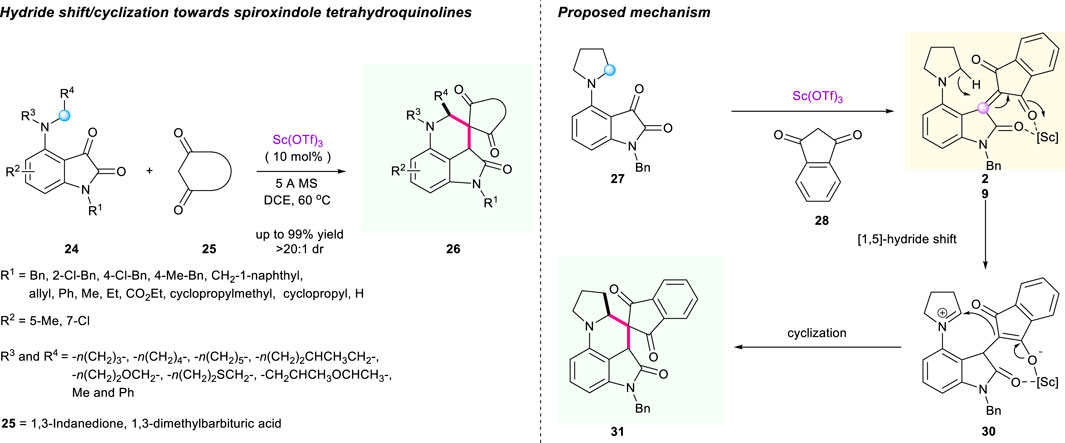
SCHEME 4. Cascade [1,5]-hydride shift/cyclization for synthesis of oxindole-fused tetrahydroquinolines.
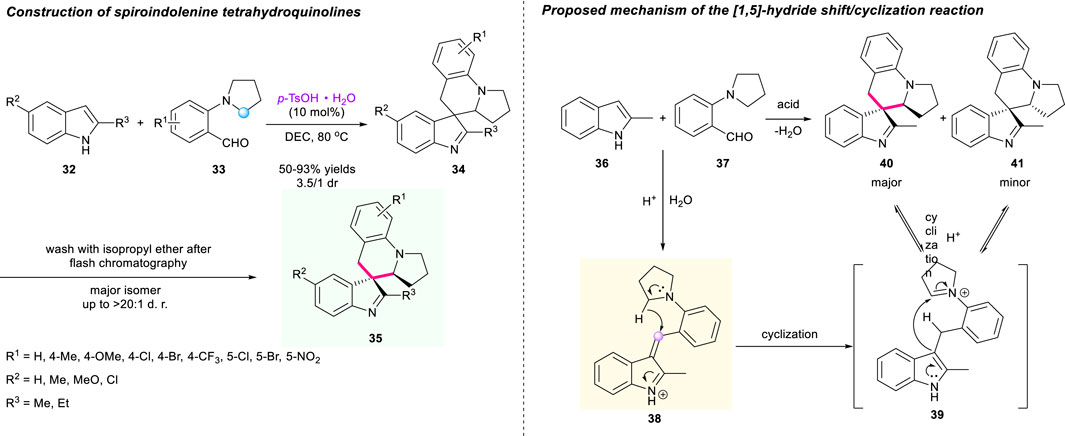
Instead of the oxindole unit, indole substrates could also participate in the [1,5]-hydride shift/cyclization sequence. In 2015, Sun and Xu’s group revealed a concise approach to construct a spiro-tetrahydroquinolines incorporated indolenine moiety 34 via the [1,5]-hydride shift/cyclization sequence (Scheme 5) (Wang P.-F. et al., 2015). The hydride acceptor iminium 38, formed through dehydration of substrates, induced the hydride shift/cyclization process under acidic conditions. Employing 2-substituted indoles 32 and 2-(pyrrolidin-1-yl)benzaldehydes 33 as substrates, p-TsOH·H2O as catalyst, and DCE as solvent, a wide range of desired target products 34 were successfully obtained with good to excellent yields and moderate diastereoselectivities. Interestingly, when the inseparable mixture of diastereoisomers was washed with isopropyl ether after rapid chromatography, the isolated products 35 could be obtained in up to >20:1 dr. An asymmetric version utilizing chiral BINOL-derived phosphoric acid was also conducted under the same condition, but only delivering the corresponding compound with low enantioselectivity. A plausible mechanism of this methodology is proposed in Scheme 5.
Recently, Xiao’s group reported the first regioselective dearomatization between 4-hydroxindoles 43 or 4-hydroxycarbazole 45 and 2-aminobenzaldehydes 42 to construct spiro-tetrahydroquinolines via an aromatization-driven hydride shift strategy (Scheme 6) (Duan et al., 2020b). Under the catalysis of scandium complex and HFIP, a variety of spirocyclic molecules incorporating indoles and carbazole moieties were provided with moderate to high yields, respectively. Meanwhile, the author found that the protection of the OH group of 4-hydroxyindole with formic ester was preferred to the generation of the spiroindolenine in HFIP. To further explore the switchable dearomatization of indoles in the carbocyclic ring and pyrrole ring, a variety of 2-aminobenzaldehydes 42 reacting with ethyl (1H-indol-4-yl) carbonate 47 were examined, giving the corresponding spiroindolenines 48 a 62%–81% yield with up to >20:1 dr. A plausible mechanism indicated that the protection of the hydroxyl group of 47 shifted the direction to another reaction site and guaranteed the conduction of this reaction at the electron-rich C-3 position of indole. Undoubtedly, this strategy provided an answer to the limitation of switchable dearomatization of fused bicyclic aromatic compounds.

SCHEME 6. The regioselective dearomatization/hydride shift sequence to build spiro-tetrahydroquinolines.
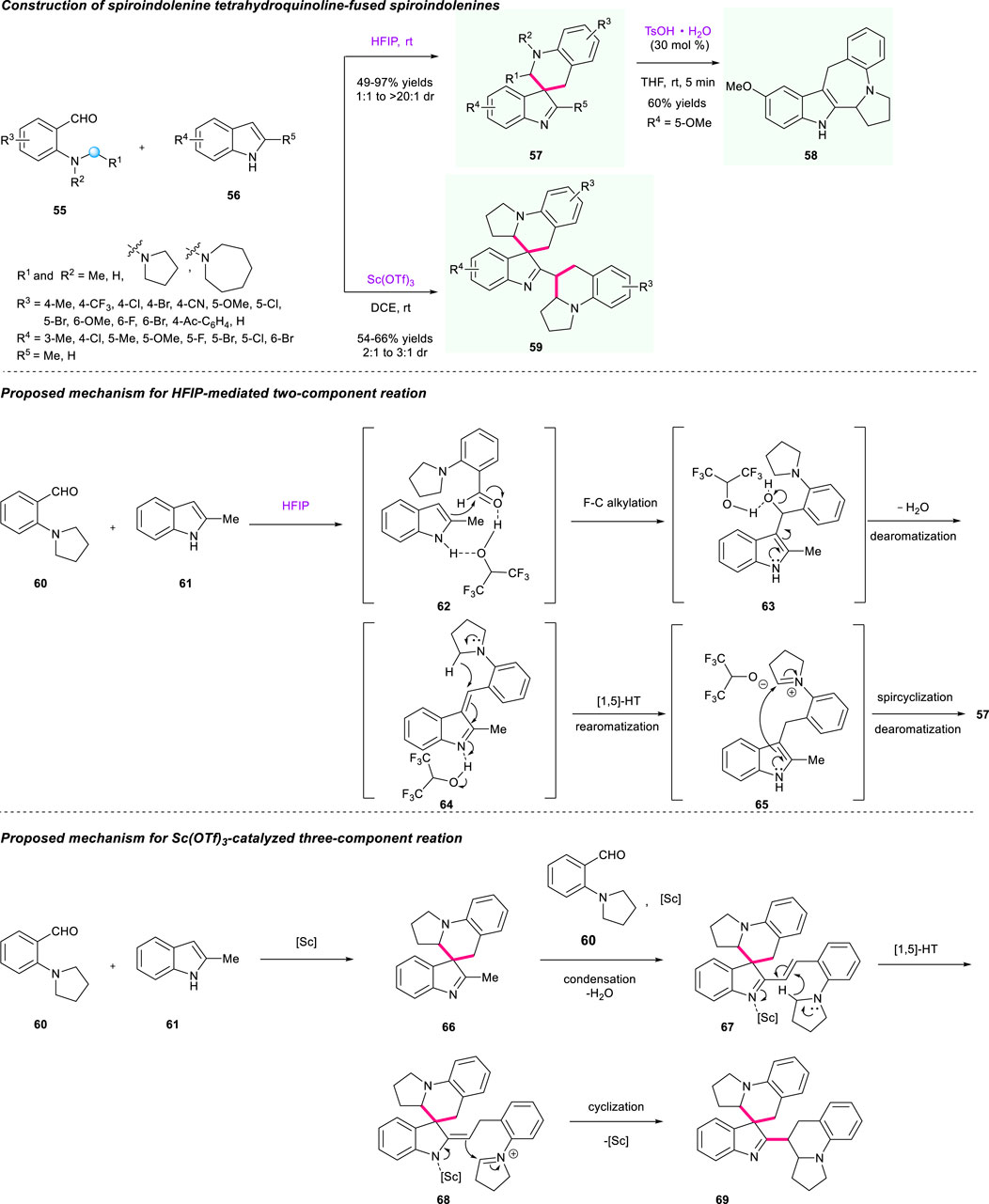
The divergent synthesis of tetrahydroquinoline-fused spiroindolenines through cascade dearomatization of indoles with ortho-aminobenzaldehydes driven by the dearomatization force was also achieved by the same group (Scheme 7) (Shen et al., 2019). The type of substrate and catalyst was a critical factor in the regulation of divergent synthesis of the final spirocyclic products. Under the catalysis of HFIP acting as both solvent and reaction promoter, an array of tetrahydroquinoline-fused spiroindolenines 57 that contain diverse electron properties on the aromatical ring of both substrates 55 and 56 were efficiently synthesized at a 47%–97% yield with up to >20:1 dr. Moreover, the addition of TsOH·H2O could further enable the transformation of THQ-fused spiroindolenine to ring-expanded derivatives 58 via the 1,2-migration process. When adding Sc(OTf)3 as catalyst into the reaction instead of HFIP in DCE at room temperature, the three-component reactions for the assembly of tetrahydroquinoline-fused indolenines 59 were successfully achieved, giving the corresponding product a 54%–66% yield with 2:1 to 3:1 dr. The plausible mechanisms were described as shown in Scheme 7 to explain this divergent synthesis. The process of synthesizing products 57 mainly contained a Friedel-Crafts alkylation/hydrolyzation/[1,5]-hydride shift/spirocyclization sequence, which was similar to a previous work by Xiao’s group with the assistance of HFIP. As for product 69, due to its strong Lewis acidity, Sc(OTf)3 was beneficial for generating the α,β-unsaturated indolenine intermediate 67 and then initiated [1,5]-hydride transfer/cyclization processes to provide the target products 69.
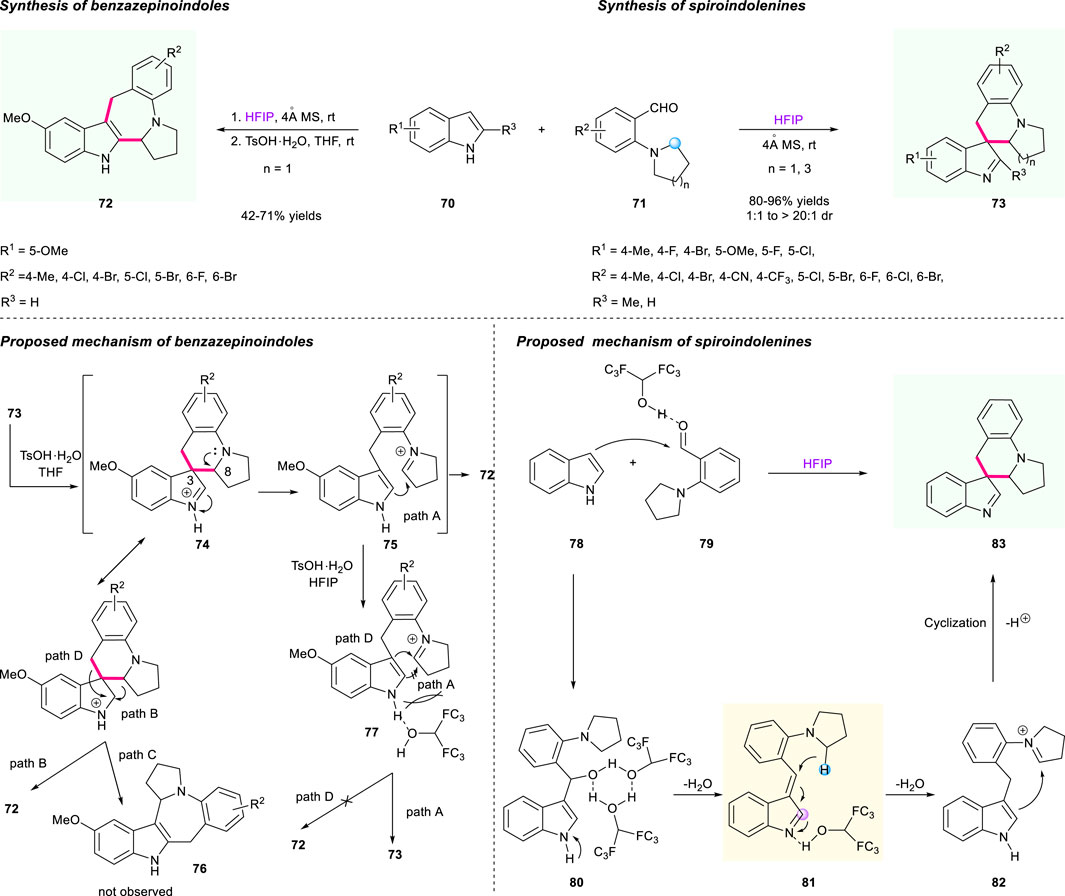
In addition to Xiao’s elegant work above, a controllable synthesis of spiroindolenines and benzazepinoindoles via HFIP-mediated cascade [1,5]-hydride shift/cyclization was successfully developed by the team of Li and Wang (Scheme 8) (Bai et al., 2019). As shown in Scheme 8 (top line), the controllable process of the two privileged skeletons 72/73 features high efficiency, mild reaction conditions, and good substrate tolerance, giving these two individual products a moderate to high yield (with moderate to excellent diastereoselectivities for product 73). The proposed mechanism of rationalizing the formation of two products 72/73 was illustrated, and the addition of TsOH·H2O played an important role in this controllable process (Scheme 8, bottom line). As for the mechanism of spiroindolenines (Scheme 8, right column), the reactive intermediate 80 was formed by the promotion of HFIP-mediated H-bonding interaction between compounds 78 and 79, followed by the result of vinylogous imine 81, which was generated through dual hydrogen bond-promoted dehydration and served as a hydride acceptor. Under the activation of HFIP, the electrophilic iminium intermediate 82 was obtained via a [1,5]-hydride shift process, and then the dearomatization product 83 was furnished after the nucleophilic attack at the C3 of the indole moiety and cyclization sequence. As for the mechanism of benzazepinoindoles, the protonation of spiroindolenine 73 gave the intermediate 74, which generated an iminium intermediate 75 after the bond cleavage of C3–C8 promoted by rearomatization. Then, the final thermodynamic benzazepinoindole 72 was offered after an attack of iminium moiety on the C2 position of the indole ring (path A). The author also proposed two alternative competitive migration processes based on the observed phenomenon (pathways B and C), which might furnish two possible products 72 and 76 via the “three-center-two-electron” transition state. However, in fact, there was no possible product 76 observed in this reaction. Notably, the N−H bond in intermediate 77 could chelate the OH group of HFIP with the addition of TsOH·H2O in the reaction medium, which served as a significant steric hindrance to block the nucleophilic attack of C2 of indole to the iminium moiety, and only delivered spiroindolenines 73 rather than benzazepinoindoles 72 (pathway D).
The Construction of a Spiro-Tetrahydroquinoline Skeleton Containing an (iso)Coumaranone Unit
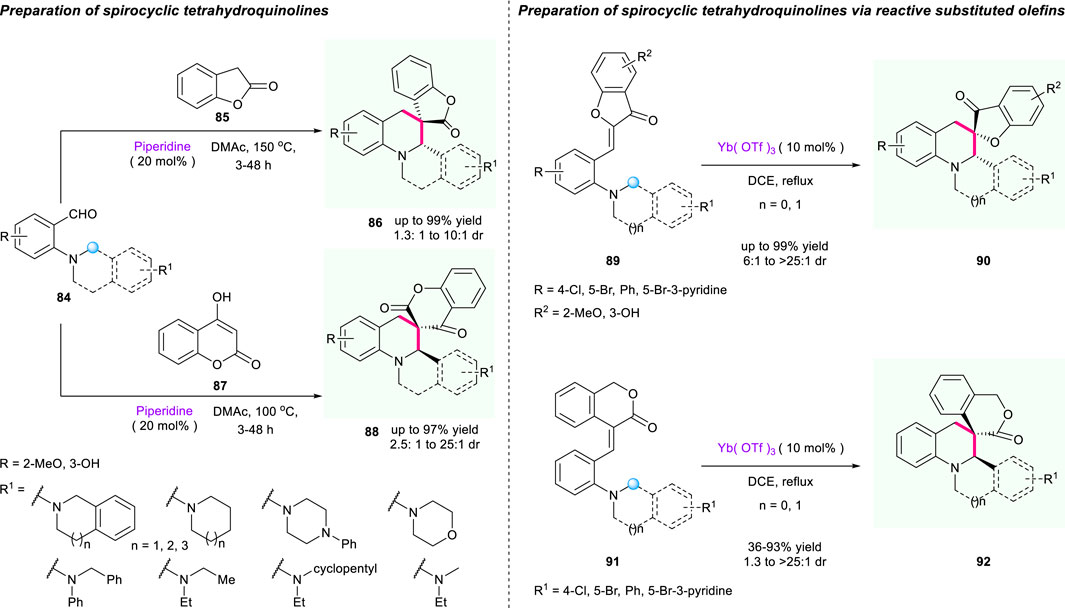
In 2020, Deb’s group reported a diastereoselective olefination/[1,5]-hydride shift/cyclization sequence to synthesize spiroheterocycles from reaction of ortho amino benzaldehydes 84 or olefins 85/87 with active methylene compounds 86/88 (Scheme 9) (Bhowmik et al., 2021). The α,β-unsaturated electron-deficient alkene used as a hydride acceptor enabled the synthesis of novel spiro tetrahydroquinolines bearing 2- or 3-coumaranone moieties with good to excellent yields (up to 99% yield). Moreover, the employment of 4-hydroxycoumarin or 3-isochromanone substituted olefins as substrates in the presence of Yb(OTf)3 successfully provided access to a wide range of spiro-tetrahydroquinolines 90/92 containing chromanone or 3-isochromanone moieties with excellent to good yields and diastereoselectivities.
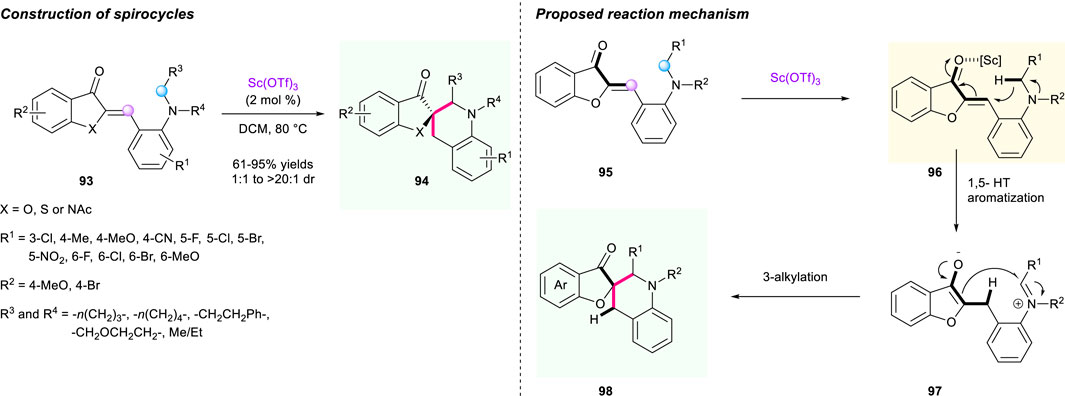
Soon after, an efficient access to tetrahydroquinoline spiro-heterocycles via the hydride shift cyclizations of aurones 94 was developed by Xiao’s group (Scheme 10) (Duan et al., 2020a). With low loading of Sc(OTf)3 in 2 mol%, a series of biologically important spiro-heterocycles were achieved with good yield (up to 95%) and good diastereoselectivities (up to >20:1 dr) under mild conditions. The researchers proposed a plausible mechanism for this reaction, as described in Scheme 10. Again, this work shows great potential of the driving force of aromatization in hydride shift cyclization strategy.
The Construction of a Spiro-Tetrahydroquinoline Skeleton Containing Pyrazolone and Imidazole Units
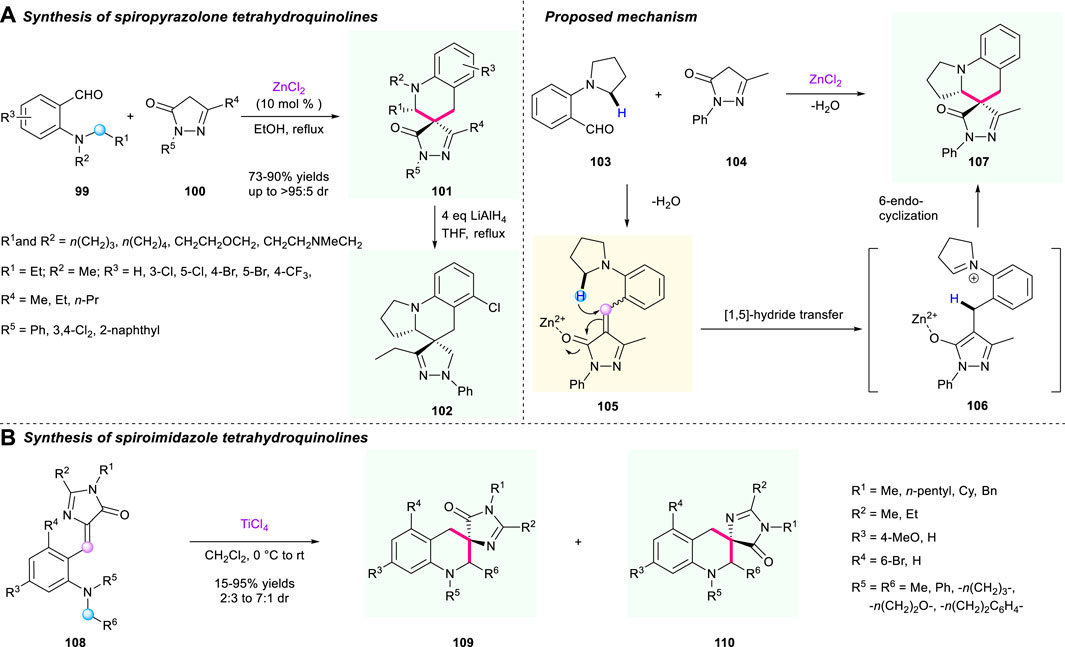
The structure of pyrazolone derivatives is widely seen in pharmaceuticals and drugs; hence, merging this valuable unit into a spiro-tetrahydroquinoline skeleton by a [1,5]-hydride shift/cyclization strategy could be a promising direction for the discovery of new drugs. In 2015, a zinc chloride-catalyzed protocol to synthesize a range of pyrazolone-fused spiro-terahydroquinolines via a tandem [1,5]-hydride shift/cyclization process was documented by Wang’s group (Scheme 11A) (Zhao et al., 2015). The α,β-unsaturated pyrazolone intermediate 101 served as a hydride acceptor and engaged in the 1,5-hydride shift/cyclization sequence. This methodology features broad substrate scope, high yields (up to 95% yield), good to excellent diastereoselectivities (up to >95:5 dr), as well as gram-scale capacity. Also, the reduction of one of the spirocyclic compounds 101 using LiAlH4 in refluxing THF condition was successfully realized, resulting in the corresponding novel spiro-terahydroquinoline 102 having a good reaction performance. However, efforts to explore an enantioselective version of this reaction are still being developed. The proposed mechanism for the construction of spiro-tetrahydroquinoline is shown in Scheme 11A.
Very recently, Smirnov’s group reported an intramolecular tandem [1,5]-hydride shift and cyclization to form spirocyclic tetrahydroquinoline derivatives 107 under the promotion of TiCl4 (Scheme 11B) (Zaitseva et al., 2021). The hydride shift process was triggered by reactive α,β-unsaturated imidazole fragments 105. This reaction demonstrated impressive substrate tolerance, giving the desirable spirocyclic compounds a 25%–95% yield under mild conditions. Moreover, a gram-scale reaction was successfully conducted with up to 93% yield, paving the way to potential research of antibacterial activity of those bioactive molecules.
The Construction of a Spiro-Tetrahydroquinoline Skeleton Containing an Isoxazolone Unit
As important heterocyclic structures, isoxazol-5-one and tetrahydroquinoline scaffolds are found in a wide range of medicines and bioactive natural products (Sridharan et al., 2011). Hence, merging these two structures to synthesize novel spirocyclic molecules could be attractive for the discovery of lead compounds. In 2013, an intramolecular tandem 1,5-hydride transfer/cyclization process catalyzed by Lewis acid Sc(OTf)3 to construct isoxazolone-tetrahydroquinolines and 3-amino-3-carboxytetrahydroquinoline derivatives has been established by the group of Yuan (Scheme 12) (Han et al., 2013). In this method, the (Z)-alkylidene azlactone 111 served as both a hydride donor and an acceptor, offering an array of tetracyclic and pentacyclic heterocycles containing two stereogenic centers and spirocyclic skeletons with up to 99% yield with diastereoselectivities ranging from 57:43 to 73:27. To demonstrate the synthetic utility of this method, transformation of several spirocyclic products to 3-amino-3-carboxytetrahydroquinoline derivatives 113 was also demonstrated through an efficient ring opening process using MeONa as base in MeOH with up to 97% yield, and 70:30 to 75:25 dr.
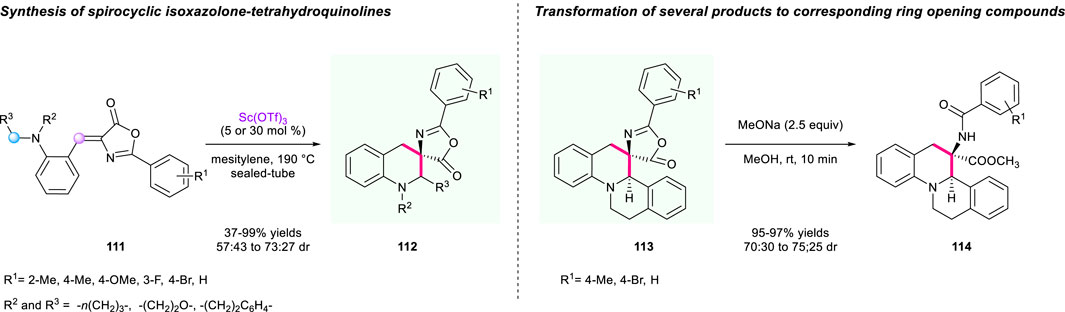
SCHEME 12. A tandem 1,5-hydride transfer/cyclization process to construct isoxazolone-tetrahydroquinolines and 3-amino-3-carboxytetrahydroquinoline derivatives.
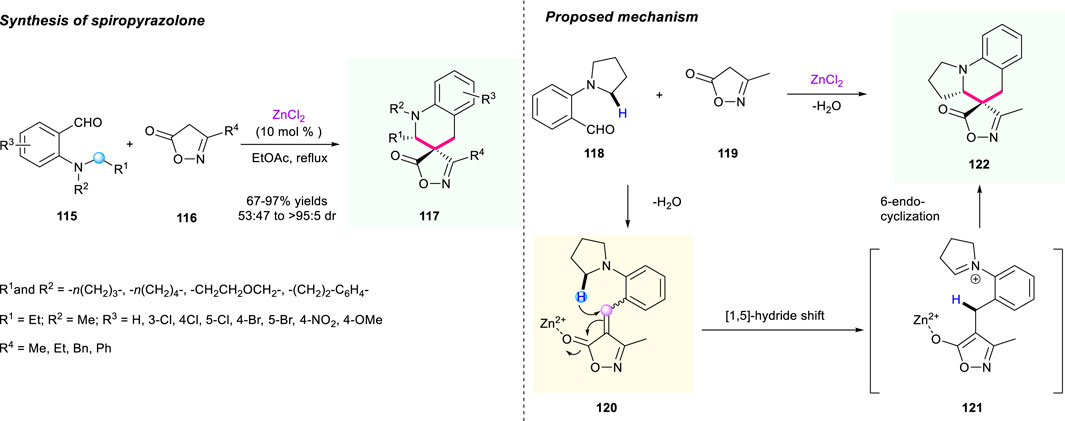
Three years later, Wang’s group designed a ZnCl2-tatalyzed Knoevenagel condensation/[1,5]-hydride shift/cyclization sequence to synthesize a series of novel spiroisoxazol-5-one tetrahydroquinolines (Scheme 13) (Zhao et al., 2016). In their strategy, the condensation of 2-(pyrrolidin-1-yl)benzaldehyde 118 and 3-methylisoxazol-5(4H)-one 119 changed the reactive intermediate 120 into a hydride donor and acceptor, which underwent a subsequent [1,5]-hydride shift/cyclization process to furnish the final product 122. As a result, this reaction featured a broad substrate scope and a simple reaction operation, providing the target spirocyclic products 117 with up to 97% yield and up to >95:5 dr, which demonstrated the high efficiency of this methodology.
The Construction of a Spiro-Tetrahydroquinoline Skeleton Containing a Coumarin Unit
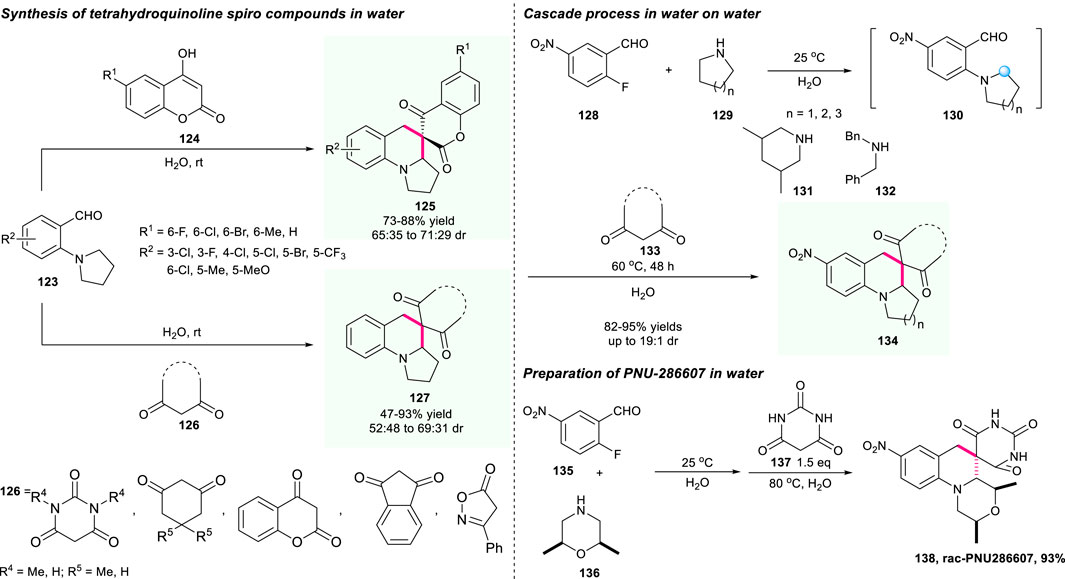
In 2017, Xiao’s group developed an innovative and operationally practical on-water catalysis to efficiently construct important spiro-tetrahydroquinoline compounds through a novel cascade SNAr/Knoevenagel condensation/[1,5]-hydride shift/cyclization sequence (Scheme 14) (Zhu et al., 2017). Compared with previous work, this reaction offered an example of cascade C(sp3)–H functionalization sequence operated on water under mild conditions instead of complex and harsh reaction conditions. The Knoevenagel condensation of 2-aminobenzaldehydes 123 and 1,3-dicarbonyl 124/126 compounds generated the reactive electron-deficient alkenes, which further initialized the subsequent [1,5]-hydride shift/cyclization route. Most of the substrates could be well-tolerated and produce the required spirocyclic compounds 128/129 with good yield and acceptable dr values as well as good atom and step economy in one operation. Moreover, the construction of the anti-bacterial agent (−)-PNU-286607 was also smoothly conducted with an excellent yield of 93%, which further demonstrated the power of this strategy.
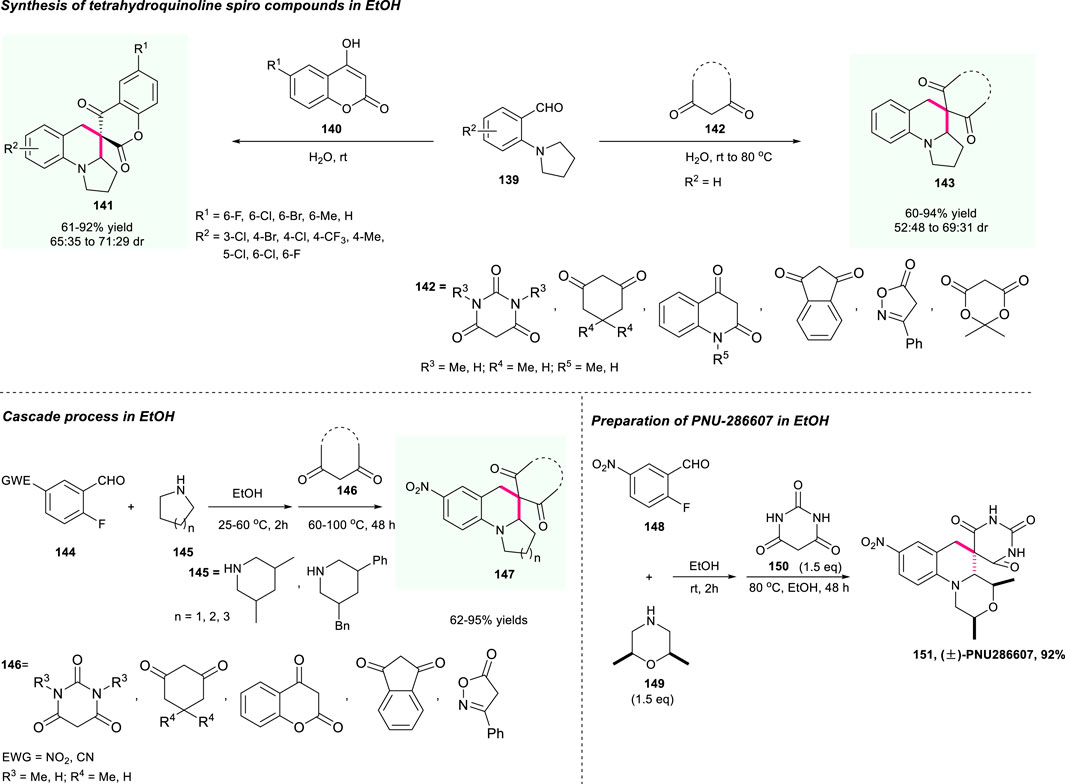
To further expand the potential of that practical strategy, in 2021, the same group developed a similar work that used environmental-friendly EtOH as solvent for the efficient construction of the pharmaceutically significant spirocyclic tetrahydroquinolines 147 (Scheme 15) (Yu et al., 2022). This strategy featured high efficiency, mild reaction conditions, high step and atom economy, and good substrate tolerance as well, producing the target spirocyclic tetrahydroquinolines containing different pharmaceutically interesting moieties with impressive results. Moreover, this strategy has been smoothly applied for the preparation of PUN-286607, affording the target molecule 151 with up to 92% yield, which demonstrated the powerful applicability of this method.
In 2020, Wang’s group developed a catalyst-free tandem 1,5-hydride shift/cyclization process to form polycyclic spiro skeletons (Scheme 16) (Liu et al., 2020). The generated α,β-unsaturated chroman intermediate 157 acted as a hydride acceptor in the reaction process. This reaction features high atom and step economy, and mild conditions, providing access to a series of new spiro benzoquinolizidine-chromanones 154 with satisfactory yields (up to 91% yield) and excellent diastereoselectivities (up to >20:1 dr). Notably, both the gram-scale reaction and derivatization of the spirocyclic products were smoothly conducted with satisfactory reaction performance, which demonstrated the robustness of this methodology. A plausible mechanistic pathway was proposed by Wang and co-workers in Scheme 16.
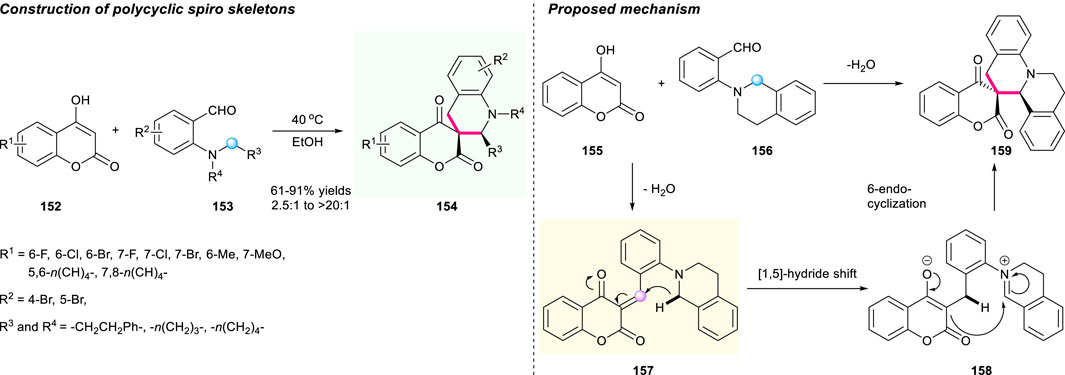
SCHEME 16. [1,5]-Hydride shift reaction toward spirocyclic tetrahydroquinolines bearing chromanone moieties.
The Construction of a Spiro-Tetrahydroquinoline Skeleton Containing Other Units
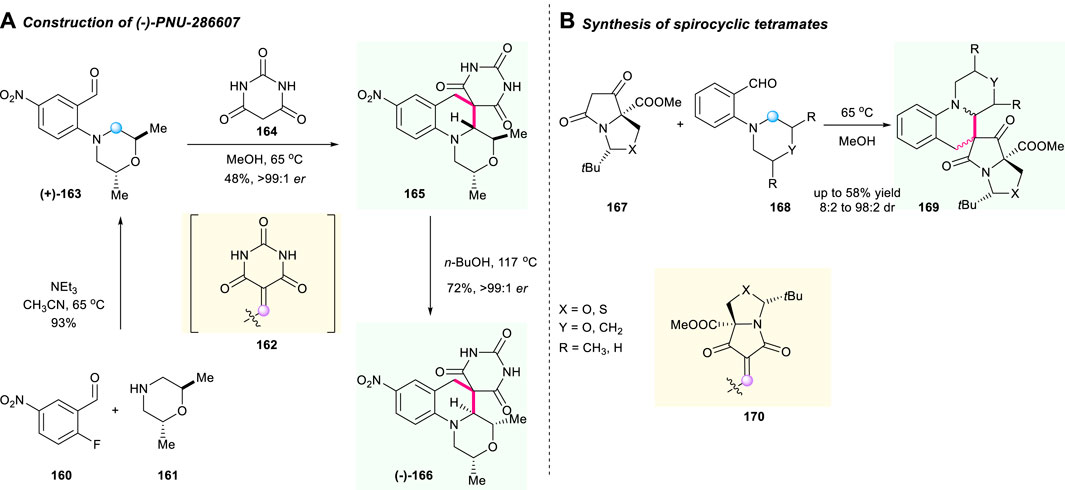
As early as 2009, the Kamilar research group developed a practical two-step route for the asymmetric synthesis of the (-)-PNU-286607 166, a promising spirocyclic tetrahydroquinoline compound bearing barbituric acid moiety (Scheme 17A) (Ruble et al., 2009). This is a limited case that applied the cascade [1,5]-hydride shift/cyclization sequence to prepare the chiral barbituric acid-fused spiro tetrahydroquinoline. The whole reaction route started with chiral trans-dimethylmorpholine 161 in MeCN as a reaction mediated at a temperature of 65°C, and resulted in desirable chiral molecule 163 with excellent stereochemical control after a comprehensive study on the stereochemical process. Notably, the isomerization of 166 using n-BuOH as solvent delivered the diastereoisomer 165 with excellent reaction performance and excellent ee value. The condensation of aldehyde 160 and barbituric acid 164 led to the production of unsaturated barbituric acid intermediate 162 as a hydride acceptor. This exploration demonstrated the potential of [1,5]-hydride shift/cyclization-involved C(sp3)–H activation to construct valuable spiro-tetrahydroquinoline molecules.
Inspired by Kamilar’s unprecedented work, a sequential Knoevenagel condensation/[1,5]-prototropic shift of tetramates 167 with aminobenzaldehydes 168 to furnish the functionalized spirocyclic tetramates 170 was reported by Moloney’s group in 2019 (Scheme 17B) (Josa-Cullere et al., 2019). The α,β-unsaturated 1,3-dicarbonyl intermediate 169 served as a hydride acceptor and started the sequential reaction under optimized conditions. Interestingly, the stability of isolated major products was dependent on the solvent and on the nature of the azacycle, and the final products were obtained with low to good yields and up to 98:2 dr.
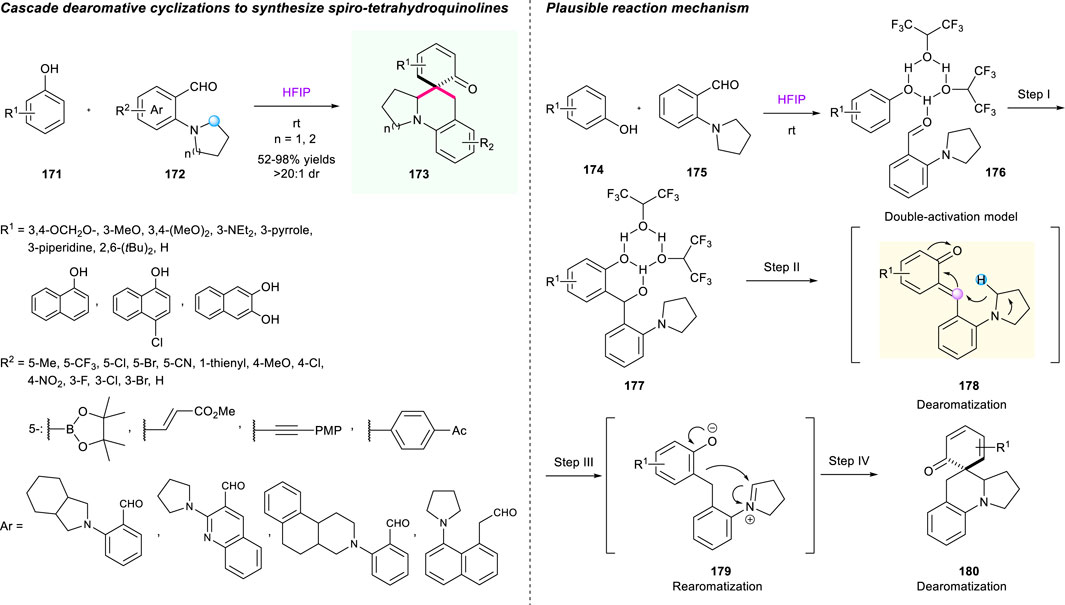
One year later, Xiao’s group developed a rapid buildup of polycyclic skeleton directly from phenols 171 and ortho-aminobenzaldehydes 172 via cascade [1,5]-hydride shift/dearomative cyclizations (Scheme 18) (Li et al., 2018a). HFIP was used as both reaction promoter and solvent, enabling one-step construction of structurally diverse spiro-tetrahydroquinolines with a good yield (up to 98%) and with high diastereoselectivities (up to >20:1), good functional group compatibilities, as well as gram-scale capacity. Importantly, this is an unprecedented strategy that employed in situ generated o-QMs 173 as novel hydride acceptors and aromatization as the driving force to initiate the hydride shift/cyclization sequence. Undoubtedly, this novel method opens a new avenue for the assembly of complex molecules via a cascade hydride shift/cyclization strategy. The researchers proposed a possible mechanism to point out the significance of HFIP (Scheme 18).
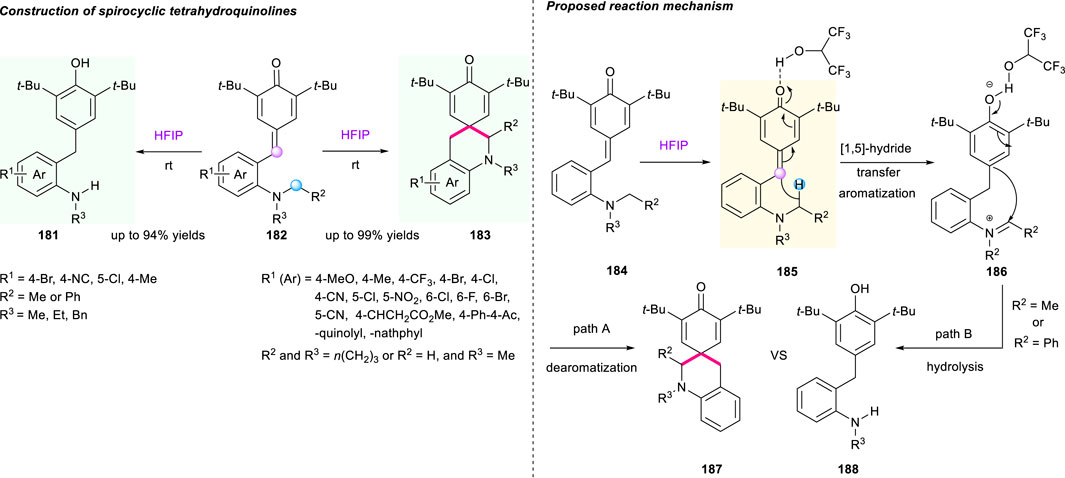
In 2019, based on previous works, the same group continued to use HFIP as the solvent to develop p-QMs-triggered cascade [1,5]-hydride shift/spirocyclization and hydrolysis reaction. This strategy enabled the synthesis of spirocyclic products 181 with good to high yields (52%–99%) under mild conditions, featuring room temperature, additive-free, and good functional group tolerance (Scheme 19) (Lv et al., 2019). Interestingly, an array of ortho-benzylated anilines 183 were obtained with high yields when using acyclic amines incorporating N,N′-dibenzyl, N-methyl-N′-benzyl, and N,N′-diethyl groups. The plausible reaction mechanism indicated that the rearomatic complex 185 with iminium ion generated by the intramolecular [1,5]-hydride transfer underwent two reaction process to produce the dearomatic product 187 (path A) and the hydrolysis process product 188 (path B, R2 = Me or Ph) based on the properties of R2 groups. Undoubtedly, Xiao’s work demonstrated that aromatization serving as a powerful driving force could trigger hydride shift-involved cascade reactions for the buildup of architecturally complex molecules.
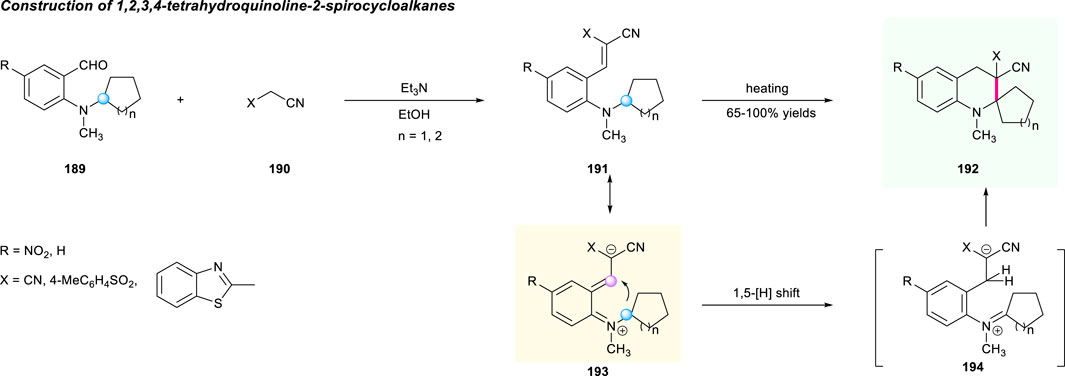
In addition to the above works, the [1,5]-hydride shift strategy can also be used for the construction of spirocyclic tetrahydroquinolines containing cycloalkane units. In 2006, Tverdokhlebov and co-authors disclosed an interaction of ortho-aminobenzaldehydes 189 with substituted acetonitriles 190 promoted by Et3N in EtOH to yield tetrahydroquinoline-2-spirocycloalkanes 192 with high yields (Scheme 20) (Tverdokhlebov et al., 2006). According to the tert-amino effect mechanism, the reaction was assumed to move forward via sequential Knoevenagel condensation/[1,5]-hydrogen shift/ring closure of the formed adduct.
Summary and Prospect
Spiro-tetrahydroquinolines are unique molecules in medicinal chemistry and pharmaceuticals that have attracted considerable attention from the industrial and academic community. In the past years, remarkable advancements have been achieved in the construction of these useful compounds via the cascade [1,5]-hydride shift-involved C(sp3)–H activation reaction. In this review, we have systematically highlighted the utility and versatility of the cascade [1,5]-hydride shift/cyclization reaction for constructing spiro-tetrahydroquinoline derivatives. These valuable spirocyclic molecules have been well categorized according to the structural type of final products. Despite the significant developments that have been made in this growing field, some challenges still need to addressed: (1) The limitation of substrate scope, structural diversity of product, complex reaction conditions, and problem of large-scale capacity still limit its potential in organic synthesis (Mori et al., 2014; Liu et al., 2018; Xing et al., 2020; Yuan et al., 2020; Guo et al., 2021; Sakai et al., 2021; Yang X. et al., 2021; Xie et al., 2022). (2) The current application of the cascade [1,5]-hydride shift/cyclization strategy mainly focuses on the construction of five- and six-membered spiro-tetrahydroquinoline. However, exploration of building a spiro-architecture with a challenging ring size (like divergent synthesis of medium ring size) as well as conducting total synthesis of a structurally complex natural product remain elusive (Li et al., 2018b; Wang et al., 2018; Kataoka et al., 2019; Hu et al., 2020; Shen et al., 2020; Hu et al., 2021a; Hu et al., 2021b; Wang et al., 2021; Yang S. et al., 2021). (3) Notably, the application of [1,5]-hydride shift/cyclization strategy in stereoselective chemistry was barely reported (Mori et al., 2018). Considerable efforts should be focused on the synthesis of chiral spirocyclic molecules with the application of this powerful strategy. (4) Finally, the bio-evaluation of target spirocyclic products for new drug discovery and research is quite far behind its synthetic chemistry (Sridharan et al., 2011; Wang Y. et al., 2015; Muthukrishnan et al., 2019). Further medicinal research of those bioactive compounds should be devoted to this field in the near future. We hope our review could provide a quick look into and offer some inspiration for the research on hydride shift strategy in the future.
Author Contributions
YYQ, XX, and HML contributed to the conception and design of the study. YYQ and LX collected the articles and performed the statistical analysis, YYQ, LX, and XL wrote the first draft of the manuscript. XX and HML contributed to the manuscript revision. All authors read and approved the submitted version.
Funding
We are grateful for the financial support from the National Natural Science Foundation of China (82104376); the China Postdoctoral Science Foundation (2020M673295 and 2020T130273); the Fundamental Research Funds for Sichuan Provincial Scientific Research Institutes (A-2021N-Z-2); the Xinglin Scholar Research Promotion Project of Chengdu University of TCM (YXRC2019004, ZRYY1922, and BSH2020018); and the Open Research Foundation of State Key Laboratory of Southwestern Chinese Medicine Resources of Chengdu University of TCM (BSH2020018 and 2020BSH008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
An, X.-D., and Xiao, J. (2021). Recent Advances in Hydride Transfer-Involved C(sp3)-H Activation Reactions. Org. Chem. Front. 8 (6), 1364–1383. doi:10.1039/d0qo01502d
Bai, G., Dong, F., Xu, L., Liu, Y., Wang, L., and Li, S.-S. (2019). Controllable Syntheses of Spiroindolenines and Benzazepinoindoles via Hexafluoroisopropanol-Mediated Redox-Neutral Cascade Process. Org. Lett. 21 (16), 6225–6230. doi:10.1021/acs.orglett.9b02051
Bhowmik, A., Das, S., Sarkar, W., Saidalavi, K. M., Mishra, A., Roy, A., et al. (2021). Diastereoselective Spirocyclization via Intramolecular C( Sp 3 )−H Bond Functionalization Triggered by Sequential [1,5]‐Hydride Shift/Cyclization Process: Approach to Spiro‐tetrahydroquinolines. Adv. Synth. Catal. 363 (3), 826–832. doi:10.1002/adsc.202001011
Cao, W., Liu, X., Guo, J., Lin, L., and Feng, X. (2015). Asymmetric Tandem 1,5-Hydride Shift/Ring Closure for the Synthesis of Chiral Spirooxindole Tetrahydroquinolines. Chem. Eur. J. 21 (4), 1632–1636. doi:10.1002/chem.201404327
Cao, W., Liu, X., Wang, W., Lin, L., and Feng, X. (2011). Highly Enantioselective Synthesis of Tetrahydroquinolines via Cobalt(II)-Catalyzed Tandem 1,5-Hydride Transfer/Cyclization. Org. Lett. 13 (4), 600–603. doi:10.1021/ol1028282
Chen, C., Xu, L., Wang, L., and Li, S.-S. (2018). Efficient Construction of Tetrahydroquinolines via Fluorinated Alcohol Mediated cascade [1,5]-hydride Transfer/cyclization. Org. Biomol. Chem. 16 (39), 7109–7114. doi:10.1039/c8ob02012d
Chen, Z., Wang, B., Zhang, J., Yu, W., Liu, Z., and Zhang, Y. (2015). Transition Metal-Catalyzed C-H Bond Functionalizations by the Use of Diverse Directing Groups. Org. Chem. Front. 2 (9), 1107–1295. doi:10.1039/c5qo00004a
Cho, S. H., Kim, J. Y., Kwak, J., and Chang, S. (2011). Recent Advances in the Transition Metal-Catalyzed Twofold Oxidative C-H Bond Activation Strategy for C-C and C-N Bond Formation. Chem. Soc. Rev. 40 (10), 5068–5083. doi:10.1039/c1cs15082k
Damerla, V. S. B., Tulluri, C., Gundla, R., Naviri, L., Adepally, U., Iyer, P. S., et al. (2012). Reagent-Based DOS: Developing a Diastereoselective Methodology to Access Spirocyclic- and Fused Heterocyclic Ring Systems. Chem. Asian J. 7 (10), 2351–2360. doi:10.1002/asia.201200385
Datta, S., Odedra, A., and Liu, R.-S. (2005). Ruthenium-Catalyzed Cycloisomerization of Cis-3-En-1-Ynes to Cyclopentadiene and Related Derivatives through a 1,5-Sigmatropic Hydrogen Shift of Ruthenium−Vinylidene Intermediates. J. Am. Chem. Soc. 127 (33), 11606–11607. doi:10.1021/ja053674o
Dong, T., Wei, P., Li, M., Gao, F., and Qin, Y. (2021). Highly Diastereoselective Synthesis of Tetrahydroquinoline Derivatives via [4 + 2] Annulation of Ortho-Tosylaminophenyl-Substituted Para-Quinone Methides and Cyanoalkenes. Front. Chem. 9, 764866. doi:10.3389/fchem.2021.764866
Duan, K., An, X.-D., Li, L.-F., Sun, L.-L., Qiu, B., Li, X.-J., et al. (2020a). Hydride Transfer Initiated Redox-Neutral Cascade Cyclizations of Aurones: Facile Access to [6,5] Spirocycles. Org. Lett. 22 (7), 2537–2541. doi:10.1021/acs.orglett.0c00309
Duan, K., Shi, H., Wang, L.-X., Li, S.-S., Xu, L., and Xiao, J. (2020b). Hydride Transfer Enabled Switchable Dearomatization of Indoles in the Carbocyclic Ring and the Pyrrole Ring. Org. Chem. Front. 7 (17), 2511–2517. doi:10.1039/d0qo00658k
Gensch, T., Hopkinson, M. N., Glorius, F., and Wencel-Delord, J. (2016). Mild Metal-Catalyzed C-H Activation: Examples and Concepts. Chem. Soc. Rev. 45 (10), 2900–2936. doi:10.1039/c6cs00075d
Guo, M., Dong, F., Yin, X., Xu, L., Wang, L., and Li, S.-S. (2021). Facile Syntheses of Tetrahydroquinolines and 1,2-dihydroquinolines via Vinylogous cascade Hydride Transfer/cyclization. Org. Chem. Front. 8 (10), 2224–2231. doi:10.1039/D0QO01622E
Haibach, M. C., and Seidel, D. (2014). C–H Bond Functionalization through Intramolecular Hydride Transfer. Angew. Chem. Int. Ed. 53 (20), 5010–5036. doi:10.1002/anie.201306489
Han, W.-Y., Zuo, J., Wu, Z.-J., Zhang, X.-M., and Yuan, W.-C. (2013). Lewis Acid Catalyzed Formation of 3-Amino-3-Carboxy-Tetrahydroquinoline Derivatives via Tandem 1,5-hydride Transfer/cyclization Process. Tetrahedron 69 (34), 7019–7025. doi:10.1016/j.tet.2013.06.047
Han, Y.-Y., Han, W.-Y., Hou, X., Zhang, X.-M., and Yuan, W.-C. (2012). FeCl3-Catalyzed Stereoselective Construction of Spirooxindole Tetrahydroquinolines via Tandem 1,5-Hydride Transfer/Ring Closure. Org. Lett. 14 (16), 4054–4057. doi:10.1021/ol301559k
Hartwig, J. F. (2012). Borylation and Silylation of C-H Bonds: A Platform for Diverse C-H Bond Functionalizations. Acc. Chem. Res. 45 (6), 864–873. doi:10.1021/ar200206a
Hartwig, J. F. (2016). Evolution of C-H Bond Functionalization from Methane to Methodology. J. Am. Chem. Soc. 138 (1), 2–24. doi:10.1021/jacs.5b08707
Hazelard, D., Nocquet, P.-A., and Compain, P. (2017). Catalytic C-H Amination at its Limits: Challenges and Solutions. Org. Chem. Front. 4 (12), 2500–2521. doi:10.1039/c7qo00547d
He, C., Whitehurst, W. G., and Gaunt, M. J. (2019). Palladium-Catalyzed C(sp3)-H Bond Functionalization of Aliphatic Amines. Chem 5 (5), 1031–1058. doi:10.1016/j.chempr.2018.12.017
Hong, B., Luo, T., and Lei, X. (2020). Late-Stage Diversification of Natural Products. ACS Cent. Sci. 6 (5), 622–635. doi:10.1021/acscentsci.9b00916
Hu, F., Li, X., Ding, Z., Wang, L., Ge, C., Xu, L., et al. (2021a). Divergent Synthesis of [3,4]-Fused 3-Alkenyl-Oxindoles via Propargyl Alcohol-Triggered C(sp3)-H Functionalization. ACS Catal. 12 (2), 943–952. doi:10.1021/acscatal.1c04931
Hu, F., Wang, L., Ge, C., Li, X., Ding, Z., Xu, L., et al. (2021b). Divergent α-functionalization of Cyclic Amines via Ring Construction by Molecular O2 Oxidized Dearomatization and Ring Deconstruction by Aromatization-Driven C-C σ-bond Cleavage. Green. Chem. 23 (15), 5535–5541. doi:10.1039/d1gc00941a
Hu, F., Wang, L., Xu, L., and Li, S.-S. (2020). Aromatization-driven Deconstruction/refunctionalization of Unstrained Rings. Org. Chem. Front. 7 (12), 1570–1575. doi:10.1039/D0QO00344A
Josa-Culleré, L., Hirst, M. G., Lockett, J. P., Thompson, A. L., and Moloney, M. G. (2019). Spirocyclic Tetramates by Sequential Knoevenagel and [1,5]-Prototropic Shift. J. Org. Chem. 84 (15), 9671–9683. doi:10.1021/acs.joc.9b01345
Junrong, H., Min, Y., Chuan, D., Yajun, Z., Huilong, F., Lizhi, Z., et al. (2021). Novel Strategies in C-H Oxidations for Natural Product Diversification-A Remote Functionalization Application Summary. Front. Chem. 9, 737530. doi:10.3389/fchem.2021.737530
Kang, Y. K., Kim, S. M., and Kim, D. Y. (2010). Enantioselective Organocatalytic C−H Bond Functionalization via Tandem 1,5-Hydride Transfer/Ring Closure: Asymmetric Synthesis of Tetrahydroquinolines. J. Am. Chem. Soc. 132 (34), 11847–11849. doi:10.1021/ja103786c
Karimov, R. R., and Hartwig, J. F. (2018). Transition-Metal-Catalyzed Selective Functionalization of C(sp3 )−H Bonds in Natural Products. Angew. Chem. Int. Ed. 57 (16), 4234–4241. doi:10.1002/anie.201710330
Kataoka, M., Otawa, Y., Ido, N., and Mori, K. (2019). Highly Diastereoselective Synthesis of Medium-Sized Carbocycle-Fused Piperidines via Sequential Hydride Shift Triggered Double C(sp3)-H Bond Functionalization. Org. Lett. 21 (23), 9334–9338. doi:10.1021/acs.orglett.9b03498
Kuhl, N., Hopkinson, M. N., Wencel-Delord, J., and Glorius, F. (2012). Beyond Directing Groups: Transition-Metal-Catalyzed C–H Activation of Simple Arenes. Angew. Chem. Int. Ed. 51 (41), 10236–10254. doi:10.1002/anie.201203269
Kwon, S. J., and Kim, D. Y. (2016). Organo- and Organometallic-Catalytic Intramolecular [1,5]-Hydride Transfer/Cyclization Process through C(sp3)-H Bond Activation. Chem. Rec. 16 (3), 1191–1203. doi:10.1002/tcr.201600003
Li, J.-H., and Du, D.-M. (2014). Organocatalyzed Cascade Aza-Michael/Michael Addition for the Asymmetric Construction of Highly Functionalized Spiropyrazolone Tetrahydroquinolines. Chem. Asian J. 9 (11), 3278–3286. doi:10.1002/asia.201402706
Li, S.-S., Lv, X., Ren, D., Shao, C.-L., Liu, Q., and Xiao, J. (2018a). Redox-triggered cascade Dearomative Cyclizations Enabled by Hexafluoroisopropanol. Chem. Sci. 9 (43), 8253–8259. doi:10.1039/c8sc03339k
Li, S.-S., Zhou, L., Wang, L., Zhao, H., Yu, L., and Xiao, J. (2018b). Organocatalytic C(sp3)-H Functionalization via Carbocation-Initiated Cascade [1,5]-Hydride Transfer/Cyclization: Synthesis of Dihydrodibenzo[b,e]azepines. Org. Lett. 20 (1), 138–141. doi:10.1021/acs.orglett.7b03492
Liou, Y.-C., Lin, Y.-A., Wang, K., Yang, J.-C., Jang, Y.-J., Lin, W., et al. (2021). Synthesis of Novel Spiro-Tetrahydroquinoline Derivatives and Evaluation of Their Pharmacological Effects on Wound Healing. Int. J. Mol. Sci. 22 (12), 6251. doi:10.3390/ijms22126251
Liu, H.-M., Liu, F.-W., Zou, D.-P., and Dai, G.-F. (2005). Asymmetric Synthesis of Novel Tetrahydroquinoline Derivatives with a Sugar Building Block and Their Bioactivities. Bioorg. Med. Chem. Lett. 15 (7), 1821–1824. doi:10.1016/j.bmcl.2005.02.024
Liu, S., Wang, H., and Wang, B. (2020). Catalyst-free Construction of spiro [benzoquinolizidine-Chromanones] via a Tandem Condensation/1,5-Hydride Transfer/cyclization Process. Org. Biomol. Chem. 18 (43), 8839–8843. doi:10.1039/d0ob01887b
Liu, S., Zhao, T., Qu, J., and Wang, B. (2018). Expedient Synthesis of 1,4‐Benzodiazepines via a Tandem Condensation/[1,5]‐Hydride Transfer/Cyclization Process. Adv. Synth. Catal. 360 (21), 4094–4098. doi:10.1002/adsc.201800781
Lv, X., Hu, F., Duan, K., Li, S.-S., Liu, Q., and Xiao, J. (2019). Aromatization-Driven Cascade [1,5]-Hydride Transfer/Spirocyclization Promoted by Fluorinated Alcohols. J. Org. Chem. 84 (4), 1833–1844. doi:10.1021/acs.joc.8b02754
Mao, H., Lin, A., Tang, Y., Shi, Y., Hu, H., Cheng, Y., et al. (2013). Organocatalytic Oxa/aza-Michael-Michael Cascade Strategy for the Construction of Spiro [Chroman/Tetrahydroquinoline-3,3′-Oxindole] Scaffolds. Org. Lett. 15 (16), 4062–4065. doi:10.1021/ol401595g
Medvedeva, S. M., Potapov, A. Y., Gribkova, I. V., Katkova, E. V., Sulimov, V. B., and Shikhaliev, K. S. (2018). Synthesis, Docking, and Anticoagulant Activity of New Factor-Xa Inhibitors in a Series of Pyrrolo[3,2,1-ij]Quinoline-1,2-Diones. Pharm. Chem. J. 51 (11), 975–979. doi:10.1007/s11094-018-1726-4
Meth-Cohn, O., and Suschitzky, H. (1972). Heterocycles by Ring Closure of Ortho-Substituted T-Anilines (The T-Amino Effect). Adv. Heterocycl. Chem. 14, 211–278. doi:10.1016/S0065-2725(08)60954-X
Meth-Cohn, O. (1996). The t-Amino Effect: Heterocycles Formed by Ring Closure of Ortho-Substituted t-Anilines*. Adv. Heterocycl. Chem. 65, 1–37. doi:10.1016/s0065-2725(08)60294-9
Mori, K., Isogai, R., Kamei, Y., Yamanaka, M., and Akiyama, T. (2018). Chiral Magnesium Bisphosphate-Catalyzed Asymmetric Double C(sp3)-H Bond Functionalization Based on Sequential Hydride Shift/Cyclization Process. J. Am. Chem. Soc. 140 (20), 6203–6207. doi:10.1021/jacs.8b02761
Mori, K., Kurihara, K., Yabe, S., Yamanaka, M., and Akiyama, T. (2014). Double C(sp3)-H Bond Functionalization Mediated by Sequential Hydride Shift/Cyclization Process: Diastereoselective Construction of Polyheterocycles. J. Am. Chem. Soc. 136 (10), 3744–3747. doi:10.1021/ja412706d
Mori, K., Umehara, N., and Akiyama, T. (2015). Synthesis of 3-Aryl-1-Trifluoromethyltetrahydroisoquinolines by Brønsted Acid-Catalyzed C(sp3)–H Bond Functionalization. Adv. Synth. Catal. 357 (5), 901–906. doi:10.1002/adsc.201400775
Mousseau, J. J., and Charette, A. B. (2013). Direct Functionalization Processes: A Journey from Palladium to Copper to Iron to Nickel to Metal-free Coupling Reactions. Acc. Chem. Res. 46 (2), 412–424. doi:10.1021/ar300185z
Muthukrishnan, I., Sridharan, V., and Menéndez, J. C. (2019). Progress in the Chemistry of Tetrahydroquinolines. Chem. Rev. 119 (8), 5057–5191. doi:10.1021/acs.chemrev.8b00567
Nijhuis, W. H. N., Verboom, W., Abu El-Fadl, A., Van Hummel, G. J., and Reinhoudt, D. N. (1989). Stereochemical Aspects of the "Tert-Amino Effect". 2. Enantio- and Diastereoselectivity in the Synthesis of Quinolines, Pyrrolo[1,2-A]quinolines, and [1,4]oxazino[4,3-A]quinolines. J. Org. Chem. 54 (1), 209–216. doi:10.1021/jo00262a044
Nijhuis, W. H. N., Verboom, W., Reinhoudt, D. N., and Harkema, S. (1987). Self-reproduction of Chirality in Carbon-Carbon Bond Formation via Dipolar Intermediates Generated In Situ by [1,5] Hydrogen Transfer. J. Am. Chem. Soc. 109 (10), 3136–3138. doi:10.1021/ja00244a041
Odedra, A., Datta, S., and Liu, R.-S. (2007). Ruthenium-Catalyzed Cyclization of 2-Alkyl-1-Ethynylbenzenes via a 1,5-Hydrogen Shift of Ruthenium−Vinylidene Intermediates. J. Org. Chem. 72 (9), 3289–3292. doi:10.1021/jo062573l
Pastine, S. J., and Sames, D. (2005). Room Temperature Intramolecular Hydro-O-Alkylation of Aldehydes: Sp3 C−H Functionalization via a Lewis Acid Catalyzed Tandem 1,5-Hydride Transfer/Cyclization. Org. Lett. 7 (24), 5429–5431. doi:10.1021/ol0522283
Peng, B., and Maulide, N. (2013). The Redox-Neutral Approach to C-H Functionalization. Chem. Eur. J. 19 (40), 13274–13287. doi:10.1002/chem.201301522
Pinnow, J. (1895). Ueber Derivate des Dimethyl‐ p ‐toluidins. Ber. Dtsch. Chem. Ges. 28, 3039–3045. doi:10.1002/cber.189502803129
Qin, Y., Zhu, L., and Luo, S. (2017). Organocatalysis in Inert C-H Bond Functionalization. Chem. Rev. 117 (13), 9433–9520. doi:10.1021/acs.chemrev.6b00657
Ramakumar, K., Maji, T., Partridge, J. J., and Tunge, J. A. (2017). Synthesis of Spirooxindoles via the Tert-Amino Effect. Org. Lett. 19 (15), 4014–4017. doi:10.1021/acs.orglett.7b01752
Ramesh, E., Manian, R. D. R. S., Raghunathan, R., Sainath, S., and Raghunathan, M. (2009). Synthesis and Antibacterial Property of Quinolines with Potent DNA Gyrase Activity. Bioorg. Med. Chem. 17 (2), 660–666. doi:10.1016/j.bmc.2008.11.058
Rios, R. (2012). Enantioselective Methodologies for the Synthesis of spiro Compounds. Chem. Soc. Rev. 41 (3), 1060–1074. doi:10.1039/c1cs15156h
Ruble, J. C., Hurd, A. R., Johnson, T. A., Sherry, D. A., Barbachyn, M. R., Toogood, P. L., et al. (2009). Synthesis of (−)-PNU-286607 by Asymmetric Cyclization of Alkylidene Barbiturates. J. Am. Chem. Soc. 131 (11), 3991–3997. doi:10.1021/ja808014h
Sakai, D., Machida, M., and Mori, K. (2021). Stereoselective Synthesis of Highly Congested Tetralin-Fused Spirooxindoles with Hydroxy Group: Pseudo Oxygen Atom Induced Hydride Shift/cyclization Process. Tetrahedron Lett. 83, 153408. doi:10.1016/j.tetlet.2021.153408
Sauermann, N., Meyer, T. H., Qiu, Y., and Ackermann, L. (2018). Electrocatalytic C-H Activation. ACS Catal. 8 (8), 7086–7103. doi:10.1021/acscatal.8b01682
Shen, Y.-B., Li, L.-F., Xiao, M.-Y., Yang, J.-M., Liu, Q., and Xiao, J. (2019). Redox-Neutral Cascade Dearomatization of Indoles via Hydride Transfer: Divergent Synthesis of Tetrahydroquinoline-Fused Spiroindolenines. J. Org. Chem. 84 (21), 13935–13947. doi:10.1021/acs.joc.9b02110
Shen, Y.-B., Wang, L.-X., Sun, Y.-M., Dong, F.-Y., Yu, L., Liu, Q., et al. (2020). Hexafluoroisopropanol-Mediated Redox-Neutral α-C(sp3)-H Functionalization of Cyclic Amines via Hydride Transfer. J. Org. Chem. 85 (4), 1915–1926. doi:10.1021/acs.joc.9b02606
Shi, F., Xing, G.-J., Zhu, R.-Y., Tan, W., and Tu, S. (2013). A Catalytic Asymmetric Isatin-Involved Povarov Reaction: Diastereo- and Enantioselective Construction of Spiro[indolin-3,2′-quinoline] Scaffold. Org. Lett. 15 (1), 128–131. doi:10.1021/ol303154k
Shu, X.-Z., Ji, K.-G., Zhao, S.-C., Zheng, Z.-J., Chen, J., Lu, L., et al. (2008). Synthesis of Naphthalenyl Acetate by Platinum-Catalyzed [1,5]-Sigmatropic Hydrogen Shift of Propargylic Esters. Chem. Eur. J. 14 (34), 10556–10559. doi:10.1002/chem.200801591
Sridharan, V., Suryavanshi, P. A., and Menéndez, J. C. (2011). Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev. 111 (11), 7157–7259. doi:10.1021/cr100307m
Su, B., Cao, Z.-C., and Shi, Z.-J. (2015). Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 48 (3), 886–896. doi:10.1021/ar500345f
Tóth, L., Mándi, A., Váradi, D., Kovács, T., Szabados, A., Kiss-Szikszai, A., et al. (2018). HPLC-ECD and TDDFT-ECD Study of Hexahydropyrrolo[1,2-A]quinoline Derivatives. Chirality 30 (7), 866–874. doi:10.1002/chir.22969
Tverdokhlebov, A. V., Gorulya, A. P., Tolmachev, A. A., Kostyuk, A. N., Chernega, A. N., and Rusanov, E. B. (2006). A Novel Tert-Amino Effect Based Approach to 1,2,3,4-Tetrahydroquinoline-2-Spirocycloalkanes. Tetrahedron 62 (39), 9146–9152. doi:10.1016/j.tet.2006.07.042
V. Kouznetsov, V., R. Merchan Arenas, D. D., Arvelo, F., S. Bello Forero, J., Munoz, A., and Munoz, A. (2010). 4-Hydroxy-3-methoxyphenyl Substituted 3-Methyl-Tetrahydroquinoline Derivatives Obtained through Imino Diels-Alder Reactions as Potential Antitumoral Agents. Lett. Drug Des. Discov. 7 (9), 632–639. doi:10.2174/157018010792929577
Wang, L.-X., Qiu, B., An, X.-D., Dong, P.-Z., Liu, R.-B., and Xiao, J. (2021). Organocatalytic cascade Aldimine Condensation/[1,6]-Hydride transfer/Mannich-type Cyclization: Sustainable Access to Indole-2,3-Fused Diazocanes. Green. Chem. 23 (20), 8181–8186. doi:10.1039/D1GC02570H
Wang, L., and Xiao, J. (2014). Advancement in Cascade [1,n]-Hydrogen Transfer/Cyclization: A Method for Direct Functionalization of Inactive C(sp3)—H Bonds. Adv. Synth. Catal. 356 (6), 1137–1171. doi:10.1002/adsc.201301153
Wang, L., and Xiao, J. (2016). Hydrogen-Atom Transfer Reactions. Top. Curr. Chem. (Z) 374 (2), 17. doi:10.1007/s41061-016-0018-2
Wang, M. (2013). Enantioselective Intramolecular 1,5-Hydride Transfer/Cyclization through the Direct Functionalization of C(sp3)-H Bonds Adjacent to a Heteroatom: From Nitrogen to Oxygen. Chemcatchem 5 (6), 1291–1293. doi:10.1002/cctc.201200692
Wang, P.-F., Jiang, C.-H., Wen, X., Xu, Q.-L., and Sun, H. (2015). C-H Bond Functionalization via [1,5]-Hydride Shift/Cyclization Sequence: Approach to Spiroindolenines. J. Org. Chem. 80 (2), 1155–1162. doi:10.1021/jo5026817
Wang, S.-G., Zhang, W., and You, S.-L. (2013). Construction of Spiro-tetrahydroquinolines via Intramolecular Dearomatization of Quinolines: Free of a Preinstalled Activation Group. Org. Lett. 15 (7), 1488–1491. doi:10.1021/ol4002416
Wang, S., An, X.-D., Li, S.-S., Liu, X., Liu, Q., and Xiao, J. (2018). Hydride Transfer Initiated Ring Expansion of Pyrrolidines toward Highly Functionalized Tetrahydro-1-Benzazepines. Chem. Commun. 54 (98), 13833–13836. doi:10.1039/C8CC08238C
Wang, Y., Lu, H., and Xu, P.-F. (2015). Asymmetric Catalytic Cascade Reactions for Constructing Diverse Scaffolds and Complex Molecules. Acc. Chem. Res. 48 (7), 1832–1844. doi:10.1021/acs.accounts.5b00217
Xiao, M., Zhu, S., Shen, Y., Wang, L., and Xiao, J. (2018). Construction of Chiral Cyclic Compounds via Asymmetric Cascade[1,n]-Hydride Transfer/Cyclization. Chin. J. Org. Chem. 38 (2), 328–340. doi:10.6023/cjoc201708024
Xie, R., Chen, S., Xiang, X., Yin, X., Xu, L., Li, S.-S., et al. (2022). Diastereoselective Construction of Structurally Diverse 2,3-Dihydroquinolin-4-One Scaffolds via Redox Neutral cascade [1,7]-hydride Transfer/cyclization. Org. Chem. Front. 9 (3), 660–666. doi:10.1039/D1QO01530C
Xing, Y., Dong, F., Yin, X., Wang, L., and Li, S. S. (2020). Facile Construction of 3,4‐dihydro‐2H‐1,2,4‐Benzothiadiazine 1,1‐Dioxides via Redox‐Neutral Cascade Condensation/[1,7]‐Hydride Transfer/Cyclization. Asian J. Org. Chem. 9 (11), 1787–1792. doi:10.1002/ajoc.202000485
Xu, P.-W., Yu, J.-S., Chen, C., Cao, Z.-Y., Zhou, F., and Zhou, J. (2019). Catalytic Enantioselective Construction of Spiro Quaternary Carbon Stereocenters. ACS Catal. 9 (3), 1820–1882. doi:10.1021/acscatal.8b03694
Yang, J. (2015). Transition Metal Catalyzed Meta-C-H Functionalization of Aromatic Compounds. Org. Biomol. Chem. 13 (7), 1930–1941. doi:10.1039/c4ob02171a
Yang, S., An, X.-D., Qiu, B., Liu, R.-B., and Xiao, J. (2021). Access to Polycyclic Indole-3,4-Fused Nine-Membered Ring via Cascade 1,6-Hydride Transfer/Cyclization. Org. Lett. 23 (23), 9100–9105. doi:10.1021/acs.orglett.1c03389
Yang, W., and Du, D.-M. (2013). Cinchona-based Squaramide-Catalysed cascade Aza-Michael-Michael Addition: Enantioselective Construction of Functionalized Spirooxindole Tetrahydroquinolines. Chem. Commun. 49 (78), 8842–8844. doi:10.1039/c3cc44930k
Yang, X., Wang, L., Hu, F., Xu, L., Li, S., and Li, S.-S. (2021). Redox-Triggered Switchable Synthesis of 3,4-Dihydroquinolin-2(1h)-One Derivatives via Hydride Transfer/N-Dealkylation/N-Acylation. Org. Lett. 23 (2), 358–364. doi:10.1021/acs.orglett.0c03863
Yu, S., Xiao, J., Yu, L., Qiu, B., and Dong, P. (2022). Facile Synthesis of Spirocyclic Tetrahydroquinolines via C(sp3)-H Functionalization in a Cascade Redox Process. Synthesis 54 (05), 1309–1320. doi:10.1055/s-0040-1720890
Yuan, K., Dong, F., Yin, X., Li, S.-S., Wang, L., and Xu, L. (2020). The Dual Alkylation of the C(sp3)-H Bond of Cyclic α-methyl-N-sulfonyl Imines via the Sequential Condensation/hydride Transfer/cyclization Process. Org. Chem. Front. 7 (23), 3868–3873. doi:10.1039/D0QO00972E
Zaitseva, E. R., Smirnov, A. Y., Myasnyanko, I. N., Mineev, K. S., Sokolov, A. I., Volkhina, T. N., et al. (2021). Imidazol-5-ones as a Substrate for [1,5]-hydride Shift Triggered Cyclization. New J. Chem. 45 (4), 1805–1808. doi:10.1039/d0nj05738j
Zhao, T., Zhang, H., Cui, L., Qu, J., and Wang, B. (2015). Zinc Chloride Catalyzed Stereoselective Construction of Spiropyrazolone Tetrahydroquinolines via Tandem [1,5]-hydride Shift/cyclization Sequence. RSC Adv. 5 (105), 86056–86060. doi:10.1039/c5ra18471a
Zhao, T., Zhang, H., Cui, L., Wang, C., Qu, J., and Wang, B. (2016). A ZnCl2-Catalyzed Knoevenagel Condensation/1,5-Hydride Shift/Cyclization Sequence: Synthesis of Novel Spiroisoxazol-5-One Tetrahydroquinolines. ChemistrySelect 1 (13), 3713–3717. doi:10.1002/slct.201600823
Zheng, C., and You, S.-L. (2014). Recent Development of Direct Asymmetric Functionalization of Inert C-H Bonds. RSC Adv. 4 (12), 6173–6214. doi:10.1039/c3ra46996d
Zhu, S., Chen, C., Duan, K., Sun, Y.-M., Li, S.-S., Liu, Q., et al. (2019). Cascade [1,5]-Hydride Transfer/Cyclization for Synthesis of [3,4]-Fused Oxindoles. J. Org. Chem. 84 (13), 8440–8448. doi:10.1021/acs.joc.9b00489
Keywords: cascade reaction, [1.5]-hydrogen transfer, intramolecular C(sp3)-H activation, spiro-tetrahydroquinoline, tert-amino effect
Citation: Liu H, Quan Y, Xie L, Li X and Xie X (2022) The Cascade [1,5]-Hydride Shift/Intramolecular C(sp3)–H Activation: A Powerful Approach to the Construction of Spiro-Tetrahydroquinoline Skeleton. Front. Chem. 10:840934. doi: 10.3389/fchem.2022.840934
Received: 21 December 2021; Accepted: 10 February 2022;
Published: 07 April 2022.
Edited by:
Teresa M. V. D. Pinho e Melo, University of Coimbra, PortugalReviewed by:
Indubhusan Deb, Indian Institute of Chemical Biology (CSIR), IndiaNuno R. Candeias, University of Aveiro, Portugal
Copyright © 2022 Liu, Quan, Xie, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Liu, liuhongmei@uestc.edu.cn; Xiang Li, lixiang2@cdutcm.edu.cn; Xin Xie, xiexin@cdutcm.edu.cn
†These authors have contributed equally to this work
 Hongmei Liu
Hongmei Liu Yunyun Quan
Yunyun Quan Long Xie3†
Long Xie3†  Xiang Li
Xiang Li Xin Xie
Xin Xie