Excellent Ultracold Molecular Candidates From Group VA Hydrides: Whether Do Nearby Electronic States Interfere?
- 1Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, China
- 2School of Chemical Sciences, University of Chinese Academy of Sciences, Beijing, China
By means of highly accurate ab initio calculations, we identify two excellent ultracold molecular candidates from group VA hydrides. We find that NH and PH are suitable for the production of ultracold molecules, and the feasibility and advantage of two laser cooling schemes are demonstrated, which involve different spin-orbit states (
Introduction
Searching for promising laser cooling candidates to produce ultracold polar molecules has attracted considerable research interests in recent years owing to their importance for a lot of promising applications in various fields such as precision measurements, quantum computing and quantum information (Hudson et al., 2011; Yan et al., 2013; Baron et al., 2014). One of the most remarkable successes is direct laser cooling of SrF to the µK level in 2010 (Shuman et al., 2010), which has initiated many research interests in molecular laser cooling. However, up to now only a very limited number of molecules have been successfully cooled to the ultracold temperatures experimentally. So there is an urgent necessity to search for more promising laser cooling candidates, and some theoretical efforts have been made to identify more candidates for laser cooling (Wells and Lane, 2011; Fu et al., 2017; Cao et al., 2019; Moussa et al., 2021). It is known (Fu et al., 2016; Yuan et al., 2019; Li et al., 2021) that, a suitable candidate for laser cooling needs to satisfy three criteria: highly diagonal Franck-Condon factors (FCFs), an extremely short radiative lifetime, and no interference from the intermediate electronic states. In our recent work, the fourth criterion for molecular laser cooling was proposed, that is, no electronic-state crossing, or the crossing point between the two states was high enough in energy (Li et al., 2020). Consequently, all electronic states close to those chosen for laser-cooling should be calculated and checked beforehand in selecting laser-cooling candidates.
Many studies have been performed for NH and PH over the past decades. Experimentally, most previous studies were based upon spectroscopic techniques. In 1959, Dixon (1959) observed the emission spectra of the
Theoretically, in 1987, Gustafsson et al. (1987) performed ab initio calculations on NH using the complete active space self-consistent field (CASSCF) method, and reported the radiative lifetimes of various rovibrational levels in the
Molecular laser cooling is achieved by a continuous scattering of a large number of photons, with each cycle of absorption and emission slowing down its translational motion by a small amount. In each cooling cycle, molecules are excited to their higher electronic state, and then return to the initial ground state through spontaneous emission. Photons are emitted in random directions with a symmetric average distribution, so their contribution to the molecule’s momentum averages to zero. Consequently, a molecule is slowed using the transfer of momentum that occurs when it absorbs a colliding photon. The emission in a molecule may populate different vibrational levels, and thus additional repump lasers must be used to bring the population back to continue the photon cycling.
So far, there have not been theoretical investigations reported on laser cooling of PH to the best of our knowledge. Very recently, the
Methods and Computational Details
In the present work, all the ab initio calculations of NH and PH are performed in the C2v point group using the MOLPRO 2012.1 program package (Werner et al., 2012). The energies of six Λ-S states of NH and PH are calculated using the CASSCF (Werner and Knowles, 1985) method followed by the icMRCI + Q (Langhoff and Davidson, 1974; Knowles and Werner, 1988; Werner and Knowles, 1988) method.
Choosing a proper active space is crucial in the CASSCF and MRCI + Q calculations (Liu et al., 2009; Yu and Bian, 2011; Yu and Bian, 2012). The full valence space is inadequate from our test calculations, thus we add additional orbitals into active space for NH and PH. The inner shell orbitals are included to account for the core-valence correlation effects, and the outer virtual orbitals are involved to give a better description on the dissociation behavior as well as Rydberg character, especially for excited electronic states (Shen et al., 2017). The best balance accuracy and computational performance is to distribute the eight electrons in ten active orbitals corresponding to N 1s2s2p3s3p and H 1s, and we use the aug-cc-pV6Z basis sets for N and H (Dunning and Peterson, 2000; van Mourik et al., 2000). The active space of PH is denoted as CAS (6e, 7o) including the P 3s3p3dπ and H 1s orbitals, and the aug-cc-pV6Z basis sets are used for P and H. In the SOC computations, the SOC effect was included by the state interaction approach with the Breit-Pauli Hamiltonian (HBP) (Berning et al., 2000), and the SO eigenstates were obtained by diagonalizing Ĥel + ĤSO in a basis of eigenfunctions of Ĥel. Moreover, the Ĥel matrix elements are obtained from the icMRCI + Q calculations, and the ĤSO matrix elements are acquired from the icMRCI + Q waves functions.
The Einstein spontaneous emission coefficient
where
For a given vibrational level ν′, the radiative lifetime
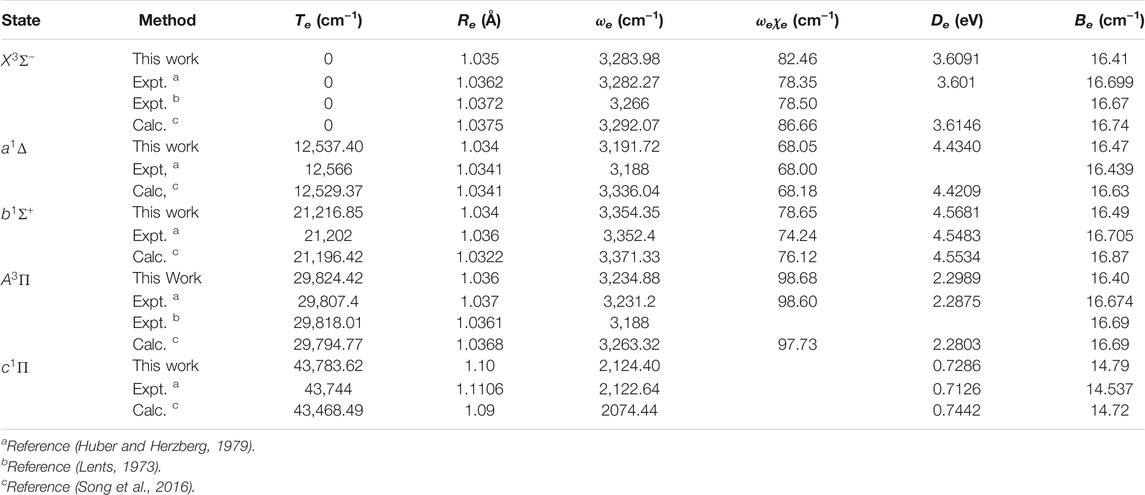
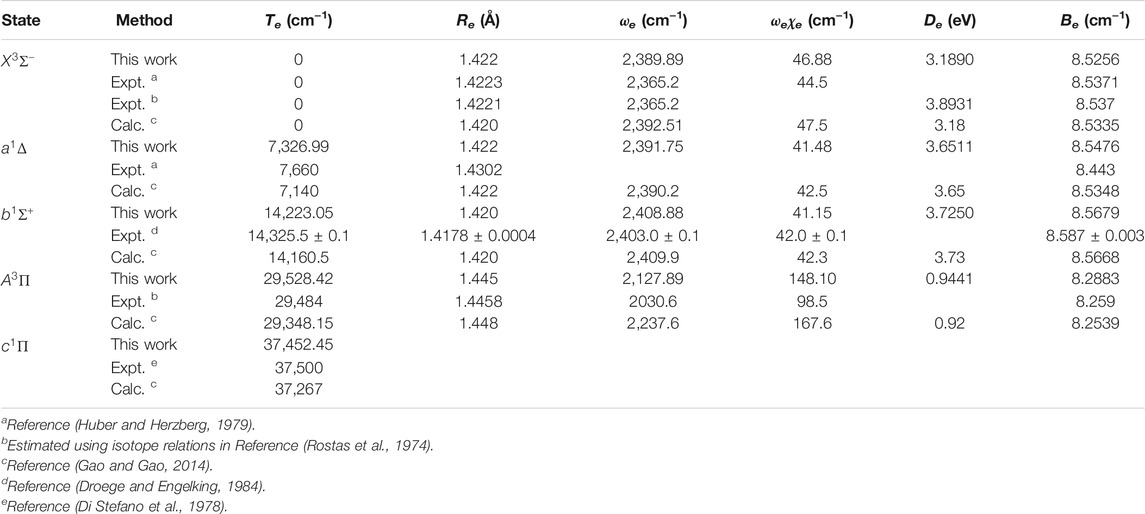
The spectroscopic constants of NH and PH, including the adiabatic relative electronic energy referred to the ground state (Te), equilibrium interatomic distance (Re), dissociation energy (De), the rotational constant (Be), the harmonic and anharmonic vibrational constants (ωe and ωeχe) are determined by solving the nuclear Schrӧdinger equation using the LEVEL 8.0 program (Le Roy, 2007).
Results and Discussion
PECs and Molecular Spectroscopic Constants
In this work, the PECs of six Λ-S electronic states of NH and PH are computed with the icMRCI + Q method. The first three low-lying electronic states (
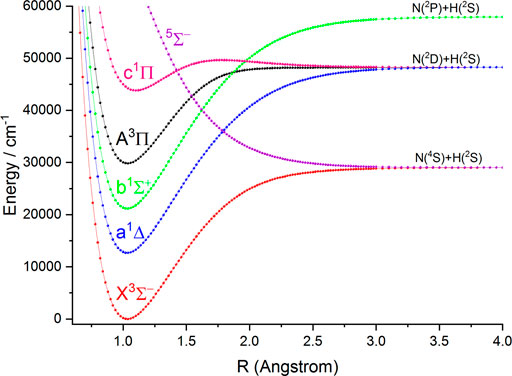
FIGURE 1. Potential energy curves of NH as a function of the interatomic distance (R) for the six Λ-S states at the icMRCI(8e, 10o)+Q/aug-cc-pV6Z level.
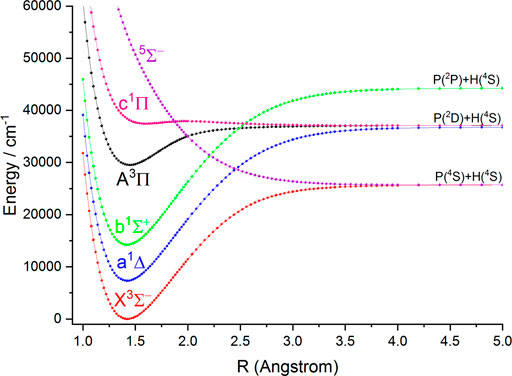
FIGURE 2. Potential energy curves of PH as a function of the interatomic distance (R) for the six Λ-S states at the icMRCI (6e, 7o) + Q/aug-cc-pV6Z level.
As seen in Table 1, for the ground state
In Table 2, our calculated Re and Be values of the
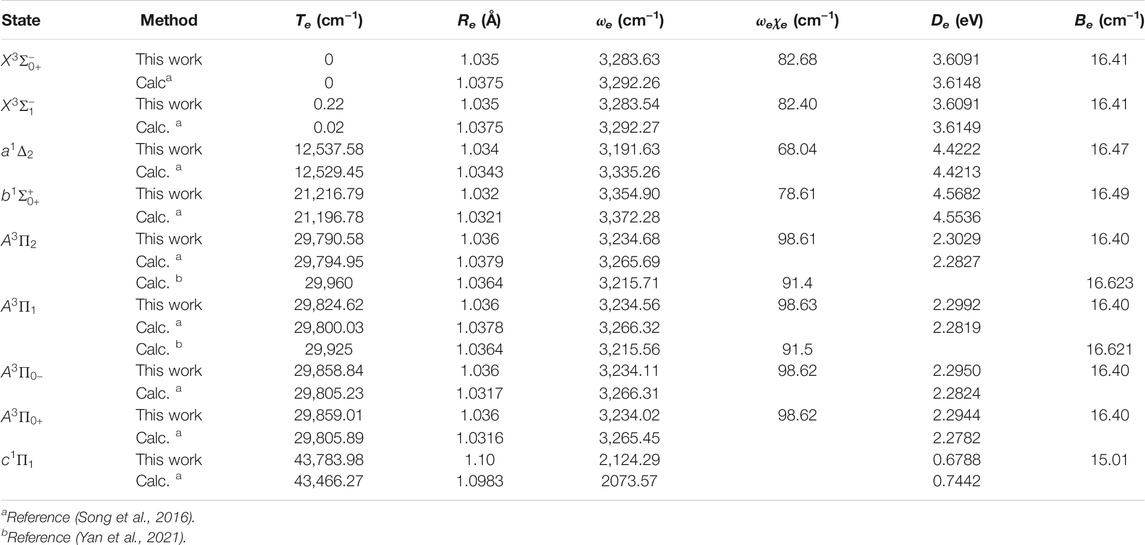
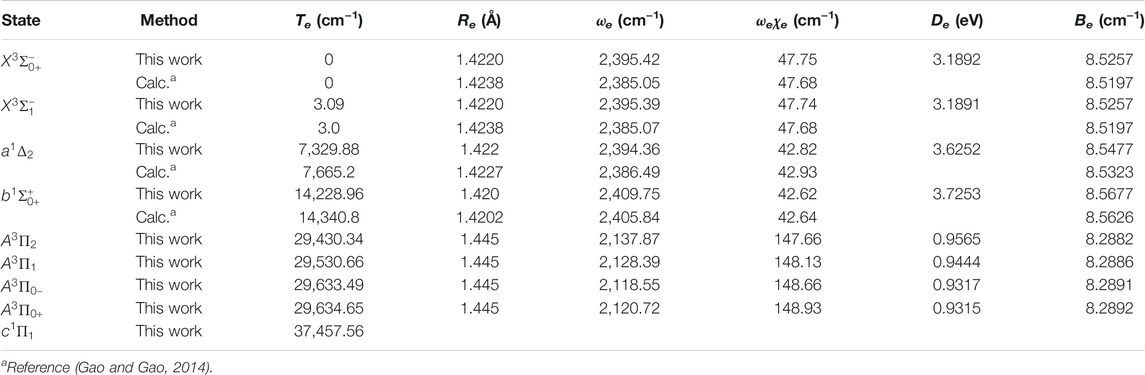
The six Λ-S states
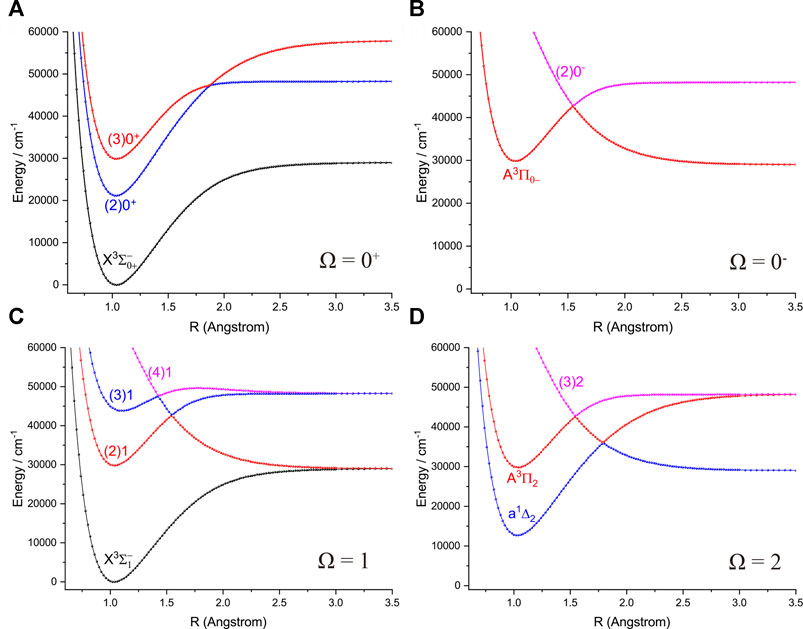
FIGURE 3. Potential energy curves of NH as a function of the interatomic distance (R) for (A) Ω = 0+, (B) Ω = 0−, (C) Ω = 1 and (D) Ω = 2 at the icMRCI + Q level.
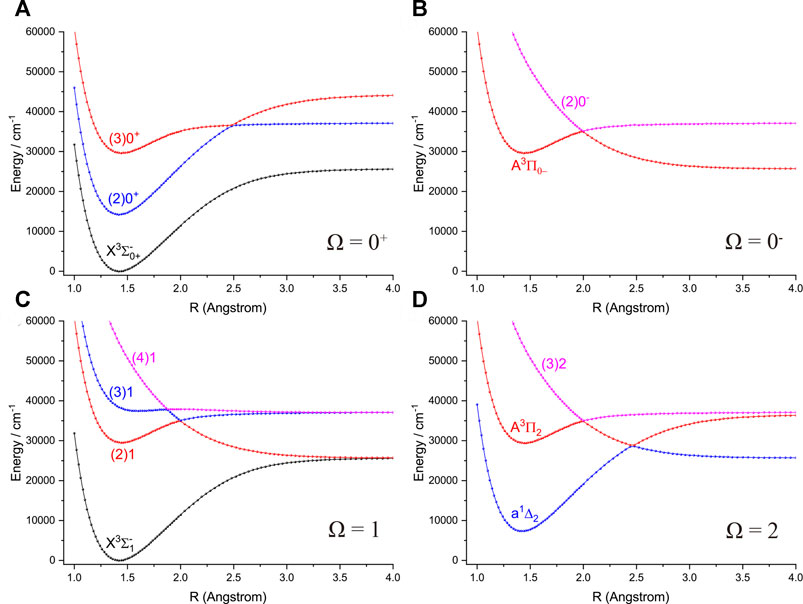
FIGURE 4. Potential energy curves of PH as a function of the interatomic distance (R) for (A) Ω = 0+, (B) Ω = 0−, (C) Ω = 1 and (D) Ω = 2 at the icMRCI + Q level.
Accurate determination of Te is very important for evaluating the pump and repump wavelengths in laser-cooling cycles, and our computed Te values, which agree very well with the corresponding experimental ones, give us confidence in the subsequent investigation on molecular laser cooling of NH and PH.
The Effects of the Extra Electronic States on Laser Cooling
Here, we discuss the effects of the extra electronic states on direct laser cooling of NH and PH. An amplified view of crossing regions of PECs of the
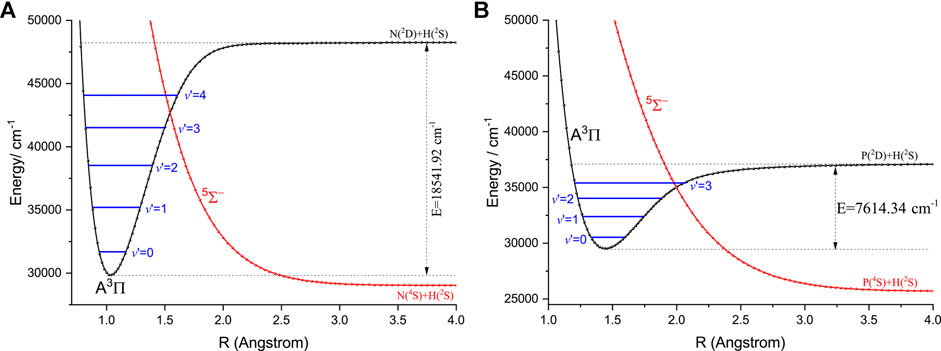
FIGURE 5. An amplified view of crossing regions of the
It should be noted that the transitions between the singlet and triplet states are allowed when the SOC effects are considered. The effects of the intermediate electronic states
Laser Cooling Schemes Proposed for NH and PH Using Specific Spin-Orbit States
Since the SOC effects are important as shown above, we construct the schemes for laser cooling of NH and PH using the spin-orbit states
The permanent dipole moments (PDMs) and TDMs for the
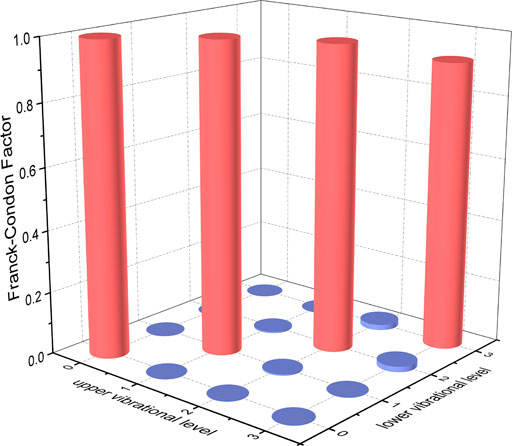
FIGURE 6. Franck-Condon factors of the
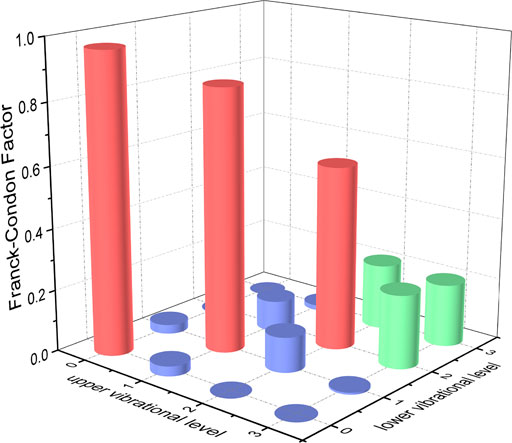
FIGURE 7. Franck-Condon factors of the
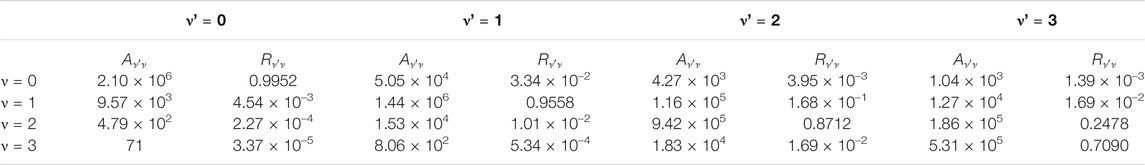
TABLE 5. Calculated Einstein A coefficients

TABLE 6. Calculated Einstein A coefficients
The
In addition, the Doppler temperatures (
The constructed laser-cooling schemes for the production of ultracold NH and PH are presented in Figures 8 and 9, respectively. As seen in Figure 8, the laser for the main cycling may drive the
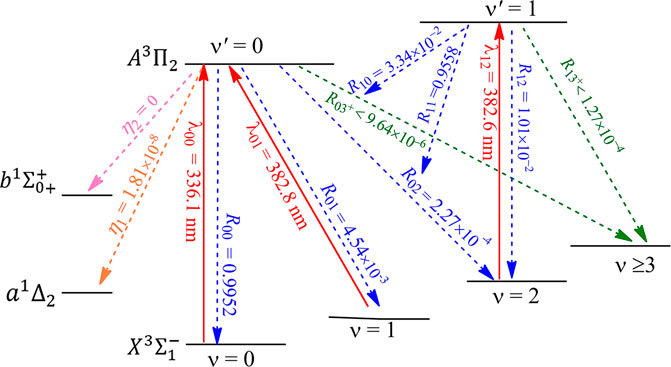
FIGURE 8. Constructed three-laser cooling scheme for NH using the
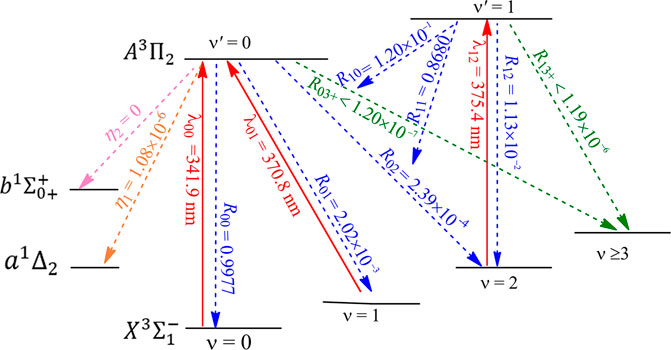
FIGURE 9. Constructed three-laser cooling scheme for PH using the
After initial cooling and trapping stages, evaporative cooling is often used to bring molecules to quantum degeneracy or Bose-Einstein condensation. The possibility of evaporative cooling of NH has been investigated (Janssen et al., 2011; Janssen et al., 2013), however, recent accurate quantum calculations (Janssen et al., 2013) indicate that chemical reactions can cause more trap loss than inelastic NH + NH collisions, and evaporative cooling is not favorable for NH. As mentioned above, the laser cooling scheme constructed here allows for 1.04 × 105 photons scattered for NH, which are sufficient for cooling to µK temperatures. In addition, PH seems to be a better candidate than NH for laser cooling. So the present work indicates that the direct laser cooling method can be used to produce magnetically trapped ultracold NH/PH molecules, and it is expected that the subsequent evaporative cooling can be avoided.
Conclusion
In this work, we identify two excellent ultracold molecular candidates from group VA hydrides using highly accurate ab initio method; in particular, NH and PH are identified as very promising laser cooling candidates, which satisfy all known criteria including the fourth one proposed in our recent work. Six low-lying Λ-S states of NH and PH are investigated with the SOC effects included. The agreement between our calculated spectroscopic constants and the available experimental data is excellent. We find that the locations of crossing point between the
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
DL carried out the ab initio and dynamical calculations. DL and WB analyzed the data, interpreted the results, developed the theoretical schemes and wrote the paper. WB supervised the research.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 21773251, 21973098).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.778292/full#supplementary-material
References
Baron, J., Baron, J., Campbell, W. C., DeMille, D., Doyle, J. M., Gabrielse, G., et al. (2014). Order of Magnitude Smaller Limit on the Electric Dipole Moment of the Electron. Science 343, 269–272. doi:10.1126/science.1248213
Berning, A., Schweizer, M., Werner, H.-J., Knowles, P. J., and Palmieri, P. (2000). Spin-orbit Matrix Elements for Internally Contracted Multireference Configuration Interaction Wavefunctions. Mol. Phys. 98, 1823–1833. doi:10.1080/00268970009483386
Brazier, C. R., Ram, R. S., and Bernath, P. F. (1986). Fourier Transform Spectroscopy of the A3Π-X3Σ− Transition of NH. J. Mol. Spectrosc. 120, 381–402. doi:10.1016/0022-2852(86)90012-3
Bruna, P. J., Hirsch, G., Peyerimhoff, S. D., and Buenker, R. J. (1981). Non-empirical CI Potential Curves for the Ground and Excited States of PH and its Positive Ion. Mol. Phys. 42, 875–898. doi:10.1080/00268978100100681
Cao, J., Li, F., Xia, W., and Bian, W. (2019). van der Waals Interactions in Bimolecular Reactions. Chin. J. Chem. Phys. 32, 157–166. doi:10.1063/1674-0068/cjcp1901007
Di Stefano, G., Lenzi, M., Margani, A., and Xuan, C. N. (1978). The (B1Σ+) State of PH in the Vacuum Ultraviolet Photolysis of Phosphine. J. Chem. Phys. 68, 959–963. doi:10.1063/1.435834
Dixon, R. N. (1959). The 0-0 and 1-0 Bands of the A3Πi - X3Σ− System of NH. Can. J. Phys. 37, 1171–1186. doi:10.1139/p59-134
Droege, A. T., and Engelking, P. C. (1984). The b1Σ+ → X3Σ− Transition in PH: A Measurement of the Term Energy, Bond Length, and Vibrational Frequency of a Phosphinidene Metastable. J. Chem. Phys. 80, 5926–5929. doi:10.1063/1.446698
Dunning, T. H., and Peterson, K. A. (2000). Approximating the Basis Set Dependence of Coupled Cluster Calculations: Evaluation of Perturbation Theory Approximations for Stable Molecules. J. Chem. Phys. 113, 7799–7808. doi:10.1063/1.1316041
Fitzpatrick, J. A. J., Chekhlov, O. V., Morgan, D. R., Burrows, R. W., and Western, C. M. (2002). Predissociation Dynamics in the A3Π State of PH: An Experimental and Ab Initio investigationElectronic Supplementary Information (ESI) Available: 6 Tables of Supporting Material. See http://www.rsc.Org/suppdata/cp/b1/b111198c/. Phys. Chem. Chem. Phys. 4, 1114–1122. doi:10.1039/b111198c
Fitzpatrick, J. A. J., Chekhlov, O. V., Western, C. M., and Ashworth, S. H. (2003). Sub-Doppler Spectroscopy of the PH Radical: Hyperfine Structure in the A3Π State. J. Chem. Phys. 118, 4539–4545. doi:10.1063/1.1543946
Fu, M., Ma, H., Cao, J., and Bian, W. (2016). Extensive Theoretical Study on Electronically Excited States of Calcium Monochloride: Molecular Laser Cooling and Production of Ultracold Chlorine Atoms. J. Chem. Phys. 144, 184302. doi:10.1063/1.4948631
Fu, M., Ma, H., Cao, J., and Bian, W. (2017). Laser Cooling of CaBr Molecules and Production of Ultracold Br Atoms: A Theoretical Study Including Spin-Orbit Coupling. J. Chem. Phys. 146, 134309. doi:10.1063/1.4979566
Gao, Y., and Gao, T. (2014). A Theoretical Study on Low-Lying Electronic States and Spectroscopic Properties of PH. Spectrochimica Acta A: Mol. Biomol. Spectrosc. 118, 308–314. doi:10.1016/j.saa.2013.07.009
Gustafsson, O., Kindvall, G., Larsson, M., Olsson, B. J., and Sigray, P. (1987). An Experimental and Theoretical Investigation of the Radiative Properties of the A3Π State of NH. Chem. Phys. Lett. 138, 185–194. doi:10.1016/0009-2614(87)80366-4
Gustafsson, O., Kindvall, G., Larsson, M., Senekowitsch, J., and Sigray, P. (1985). An Experimental Investigation of Predissociation Effects in the A3Π-X3Σ− Transition of PH. Mol. Phys. 56, 1369–1380. doi:10.1080/00268978500103101
Huber, K. P., and Herzberg, G. (1979). Molecular Spectra and Molecular Structure IV: Constants of Diatomic Molecules. New York, NY: Van Nostrand Reinhold.
Hudson, J. J., Kara, D. M., Smallman, I. J., Sauer, B. E., Tarbutt, M. R., and Hinds, E. A. (2011). Improved Measurement of the Shape of the Electron. Nature 473, 493–496. doi:10.1038/nature10104
Hummon, M. T., Yeo, M., Stuhl, B. K., Collopy, A. L., Xia, Y., and Ye, J. (2013). 2D Magneto-Optical Trapping of Diatomic Molecules. Phys. Rev. Lett. 110, 143001. doi:10.1103/PhysRevLett.110.143001
Janssen, L. M. C., van der Avoird, A., and Groenenboom, G. C. (2013). Quantum Reactive Scattering of Ultracold NH(X3Σ−) Radicals in a Magnetic Trap. Phys. Rev. Lett. 110, 063201. doi:10.1103/PhysRevLett.110.063201
Janssen, L. M. C., Żuchowski, P. S., van der Avoird, A., Groenenboom, G. C., and Hutson, J. M. (2011). Cold and Ultracold NH-NH Collisions in Magnetic fields. Phys. Rev. A. 83, 022713. doi:10.1103/PhysRevA.83.022713
Knowles, P. J., and Werner, H.-J. (1988). An Efficient Method for the Evaluation of Coupling Coefficients in Configuration Interaction Calculations. Chem. Phys. Lett. 145, 514–522. doi:10.1016/0009-2614(88)87412-8
Langhoff, S. R., and Davidson, E. R. (1974). Configuration Interaction Calculations on the Nitrogen Molecule. Int. J. Quan. Chem. 8, 61–72. doi:10.1002/qua.560080106
Le Roy, R. J. (2007). LEVEL 8.0: A Computer Program for Solving the Radial Schrӧdinger Equation for Bound and Quasibound Levels. Chemical Physics Research Report CPRR-663. Waterloo, Canada: University of Waterloo. Available online at: http://leroy.uwaterloo.ca.
Lents, J. M. (1973). An Evaluation of Molecular Constants and Transition Probabilities for the NH Free Radical. J. Quantitative Spectrosc. Radiative Transfer 13, 297–310. doi:10.1016/0022-4073(73)90061-7
Li, D., Yang, C., Sun, Z., Wang, M., and Ma, X. (2021). Theoretical Study on the Spectroscopic Properties of the Low-Lying Electronic States and the Laser Cooling Feasibility of the CaI Molecule. J. Quantitative Spectrosc. Radiative Transfer 270, 107709. doi:10.1016/j.jqsrt.2021.107709
Li, D., Fu, M., Ma, H., Bian, W., Du, Z., and Chen, C. (2020). A Theoretical Study on Laser Cooling Feasibility of Group IVA Hydrides XH (X = Si, Ge, Sn, and Pb): The Role of Electronic State Crossing. Front. Chem. 8, 20. doi:10.3389/fchem.2020.00020
Liu, C., Zhang, D., and Bian, W. (2003). Theoretical Investigation of the Reaction of Co+ with OCS. J. Phys. Chem. A. 107, 8618–8622. doi:10.1021/jp034693s
Liu, K., Yu, L., and Bian, W. (2009). Extensive Theoretical Study on Various Low-Lying Electronic States of Silicon Monochloride Cation Including Spin−Orbit Coupling. J. Phys. Chem. A. 113, 1678–1685. doi:10.1021/jp809618y
Moussa, A., El-Kork, N., and Korek, M. (2021). Laser Cooling and Electronic Structure Studies of CaK and its Ions CaK±. New J. Phys. 23, 013017. doi:10.1088/1367-2630/abd50d
Owono, L. C., Ben Abdallah, D., Jaidane, N., and Ben Lakhdar, Z. (2008). Theoretical Radiative Properties between States of the Triplet Manifold of NH Radical. J. Chem. Phys. 128, 084309. doi:10.1063/1.2884923
Owono, L. C., Jaidane, N., Kwato Njock, M. G., and Ben Lakhdar, Z. (2007). Theoretical Investigation of Excited and Rydberg States of Imidogen Radical NH: Potential Energy Curves, Spectroscopic Constants, and Dipole Moment Functions. J. Chem. Phys. 126, 244302. doi:10.1063/1.2741260
Park, J. K., and Sun, H. (1992). Dipole and Transition Moments of SiH, PH and SH by Ab Initio Effective Valence Shell Hamiltonian Method. Chem. Phys. Lett. 195, 469–474. doi:10.1016/0009-2614(92)85546-m
Ram, R. S., Bernath, P. F., and Hinkle, K. H. (1999). Infrared Emission Spectroscopy of NH: Comparison of a Cryogenic Echelle Spectrograph with a Fourier Transform Spectrometer. J. Chem. Phys. 110, 5557–5563. doi:10.1063/1.478453
Rostas, J., Cossart, D., and Bastien, J. R. (1974). Rotational Analysis of the PH and PD A3Πi-X3Σ− Band Systems. Can. J. Phys. 52, 1274–1287. doi:10.1139/p74-172
Shen, Z., Ma, H., Zhang, C., Fu, M., Wu, Y., Bian, W., et al. (2017). Dynamical importance of van der Waals saddle and excited potential surface in C(1D)+D2 complex-forming reaction. Nat. Commun. 8, 14094. doi:10.1038/ncomms14094
Shuman, E. S., Barry, J. F., and DeMille, D. (2010). Laser Cooling of a Diatomic Molecule. Nature 467, 820–823. doi:10.1038/nature09443
Smith, W. H., Brzozowski, J., and Erman, P. (1976). Lifetime Studies of the NH Molecule: New Predissociations, the Dissociation Energy, and Interstellar Diatomic Recombination. J. Chem. Phys. 64, 4628–4633. doi:10.1063/1.432046
Song, Z., Shi, D., Sun, J., and Zhu, Z. (2016). Accurate Spectroscopic Calculations of the 12 Λ-S and 25 Ω States of the NH Radical Including the Spin-Orbit Coupling Effect. Comput. Theor. Chem. 1093, 81–90. doi:10.1016/j.comptc.2016.08.017
van Mourik, T., Dunning, T. H., and Peterson, K. A. (2000). Ab Initio Characterization of the HCOx (x = −1, 0, +1) Species: Structures, Vibrational Frequencies, CH Bond Dissociation Energies, and HCO Ionization Potential and Electron Affinity. J. Phys. Chem. A. 104, 2287–2293. doi:10.1021/jp9925583
Wells, N., and Lane, I. C. (2011). Electronic States and Spin-Forbidden Cooling Transitions of AlH and AlF. Phys. Chem. Chem. Phys. 13, 19018–19025. doi:10.1039/c1cp21313j
Werner, H.-J., Knowles, P. J., Lindh, R., Manby, F. R., Schütz, M., Celani, P., et al. (2012). Molpro, Version 2012.1, A Package of Ab Initio Programs. Available online at: http://www.molpro.net.
Werner, H.-J., and Knowles, P. J. (1985). A Second Order Multiconfiguration SCF Procedure with Optimum Convergence. J. Chem. Phys. 82, 5053–5063. doi:10.1063/1.448627
Werner, H.-J., and Knowles, P. J. (1988). An Efficient Internally Contracted Multiconfiguration-Reference Configuration Interaction Method. J. Chem. Phys. 89, 5803–5814. doi:10.1063/1.455556
Wu, Y., Cao, J., Ma, H., Zhang, C., Bian, W., Nunez-Reyes, D., et al. (2019). Conical Intersection-Regulated Intermediates in Bimolecular Reactions: Insights from C(1D)+HD Dynamics. Sci. Adv. 5, eaaw0446. doi:10.1126/sciadv.aaw0446
Xing, W., Sun, J., Shi, D., and Zhu, Z. (2018). Theoretical Study of Spectroscopic Properties of 5 Λ-S and 10 Ω States and Laser Cooling for AlH+ Cation. Acta Phys. Sin. 67, 193101. doi:10.7498/aps.67.20180926
Yan, B., Moses, S. A., Gadway, B., Covey, J. P., Hazzard, K. R. A., Rey, A. M., et al. (2013). Observation of Dipolar Spin-Exchange Interactions with Lattice-Confined Polar Molecules. Nature 501, 521–525. doi:10.1038/nature12483
Yan, N., Yang, C., Sun, Z., Wang, M., and Ma, X. (2021). Direct Laser Cooling the NH Molecule with the Pseudo-closed Loop Triplet-Triplet Transition Including Intervening Electronic States. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 250, 119229. doi:10.1016/j.saa.2020.119229
Yu, L., and Bian, W. (2012). Electronically Excited-State Properties and Predissociation Mechanisms of Phosphorus Monofluoride: A Theoretical Study Including Spin-Orbit Coupling. J. Chem. Phys. 137, 014313. doi:10.1063/1.4731635
Yu, L., and Bian, W. (2011). Extensive Theoretical Study on Electronically Excited States and Predissociation Mechanisms of Sulfur Monoxide Including Spin-Orbit Coupling. J. Comput. Chem. 32, 1577–1588. doi:10.1002/jcc.21737
Yuan, X., Guo, H., Wang, Y., Xue, J., Xu, H., and Yan, B. (2019). Laser-cooling with an Intermediate Electronic State: Theoretical Prediction on Bismuth Hydride. J. Chem. Phys. 150, 224305. doi:10.1063/1.5094367
Keywords: molecular laser cooling, ab initio, spin-orbit coupling, group VA hydrides, electronic state crossing, ultracold molecules
Citation: Li D and Bian W (2021) Excellent Ultracold Molecular Candidates From Group VA Hydrides: Whether Do Nearby Electronic States Interfere?. Front. Chem. 9:778292. doi: 10.3389/fchem.2021.778292
Received: 16 September 2021; Accepted: 22 November 2021;
Published: 16 December 2021.
Edited by:
Ralph Ernstorfer, Technical University of Berlin, GermanyReviewed by:
Balakrishnan Naduvalath, University of Nevada, Las Vegas, United StatesJiri Pittner, J. Heyrovsky Institute of Physical Chemistry (ASCR), Czechia
Copyright © 2021 Li and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wensheng Bian, bian@iccas.ac.cn
 Donghui Li
Donghui Li Wensheng Bian
Wensheng Bian