Annexin A2 and Kidney Diseases
- Department of Medicine, The Pennsylvania State University College of Medicine, Hershey, PA, United States
Annexin A2 is a Ca2+- and phospholipid-binding protein which is widely expressed in various types of cells and tissues. As a multifunctional molecule, annexin A2 is found to be involved in diverse cell functions and processes, such as cell exocytosis, endocytosis, migration and proliferation. As a receptor of plasminogen and tissue plasminogen activator, annexin A2 promotes plasmin generation and regulates the homeostasis of blood coagulation, fibrinolysis and matrix degradation. As an antigen expressed on cell membranes, annexin A2 initiates local inflammation and damage through binding to auto-antibodies. Annexin A2 also mediates multiple signaling pathways induced by various growth factors and oxidative stress. Aberrant expression of annexin A2 has been found in numerous kidney diseases. Annexin A2 has been shown to act as a co-receptor of integrin CD11b mediating NF-kB-dependent kidney inflammation, which is further amplified through annexin A2/NF-kB-triggered macrophage M2 to M1 phenotypic change. It also modulates podocyte cytoskeleton rearrangement through Cdc42 and Rac1/2/3 Rho pathway causing proteinuria. Thus, annexin A2 is implicated in the pathogenesis and progression of various kidney diseases. In this review, we focus on the current understanding of the role of annexin A2 in kidney diseases.
Introduction
Annexins are a family of different calcium-regulated phospholipids-binding proteins, which were named for their ability to “annex” meaning aggregate membranes (Creutz et al., 1978; Bharadwaj et al., 2013; Luo and Hajjar, 2013). They are widely expressed in plants, fungi, invertebrates, vertebrates and others (Bharadwaj et al., 2013), and are found to be involved in the membrane related functions such as exocytosis, endocytosis, vesicular trafficking (Creutz et al., 1978; Bharadwaj et al., 2013; Luo and Hajjar, 2013). There are 12 annexin proteins in human, named as A1-A11 and A13, of which, annexin A2 is extensively studied. Human annexin A2 is a 36-kDa protein produced from ANXA2 gene. It is expressed in endothelial cells, epithelial cells, monocytes, macrophages, dendritic cells and most cancer cells, and has been shown to be related to various human diseases (Rintala-Dempsey et al., 2008; Bharadwaj et al., 2013; Luo and Hajjar, 2013).
Structure
Structurally, annexins typically have two main domains: a variable amino-terminal head domain and a homologous carboxyl core domain. The amino-terminal head domain contains sites for post-translation modification and is unique for individual annexins, allowing annexin to bind to various ligands or other proteins, and is responsible for distinct locations and specialized functions (Bharadwaj et al., 2013; Luo and Hajjar, 2013; Madureira and Waisman, 2013). The carboxyl core domain generally has four conserved structural repeat sequences, except annexin A6 which has eight repeat sequences. Each repeat sequence is formed from five α-helices, which play an important role in the structures of annexins through hydrophobic interactions between each other (Rintala-Dempsey et al., 2008; Bharadwaj et al., 2013; Luo and Hajjar, 2013).
Annexins bind to membranes reversibly in a calcium-dependent manner to perform their functions. Calcium ions bind to annexins on the same curves side of the annexin structures and form a convex surface which promotes the interaction of annexins and phospholipid. Though, after calcium binding, the annexins do not undergo a significant structural change within their core domains, they do have higher affinity to the anionic phospholipid-containing membrane (Swairjo et al., 1995; Rintala-Dempsey et al., 2008). In some annexins such as A1 and A2, the unique N-terminal sequences are also able to dissociate from the core domain on binding of calcium and will be closely associated with the core domain in the absence of the ion (Swairjo et al., 1995; Rintala-Dempsey et al., 2008).
Annexins, such as annexin A1 and A2, exist as free monomer or as heterotetramer through binding with S100A proteins. S100 proteins are dimeric EF-hand calcium-binding proteins. After coordinating calcium ions, they will undergo a significant conformational change to expose hydrophobic residues on their surface. These hydrophobic surfaces are able to bind two N-terminal sequences of annexin proteins and form a heterotetramer, which allows two membrane-bound annexin proteins to be brought into close proximity (Smith and Shaw, 1998; Santamaria-Kisiel et al., 2006; Rintala-Dempsey et al., 2008; Luo and Hajjar, 2013). Annexin A2 (ANXA2) was first identified as a substrate for the tyrosine kinase v-Src, a gene product found in Rous sarcoma virus, which mediates cellular transformation (Erikson and Erikson, 1980). Later, the structures and functions of the heterotetramer of ANXA2 and S100 proteins were extensively investigated. Since Rety and colleagues first revealed in 1999 that the first 13 residues of N-terminus of ANXA2 is responsible for its binding to S100A10 (p11) (Rety et al., 1999), ANXA2 has been found to bind to various S100 proteins including S100A4, S100A6, S100A11 and dicalcin, an S100-like protein (Filipek et al., 1995; Semov et al., 2005; Rintala-Dempsey et al., 2006; Uebi et al., 2007). Notably, S100A10 (p11), in the absence of calcium, maintains an EF-hand dimer conformation similar to the calcium-bound states of other S100 proteins (Bharadwaj et al., 2013).
Signaling pathways and cellular functions
ANXA2 exists predominantly in cytoplasm and plasma membrane, and plays its functions through different mechanisms. ANXA2-S100A10 heterotetramer on the cell surface has been shown to be the receptor of plasminogen and tissue plasminogen activator (tPA); and plays an important role in the homeostasis of blood coagulation, fibrinolysis and matrix degradation, as well as mediating the cytokine effects of tPA in vivo (Dassah et al., 2009; Flood and Hajjar, 2011; Lin et al., 2012; Bharadwaj et al., 2013; Lin and Hu, 2017; Bharadwaj et al., 2021; Lim and Hajjar, 2021). ANXA2 is able to associate with anionic phospholipids, F-actin, and other proteins in the cell membrane and cytoskeleton, and regulates various membrane related functions including exocytosis, endocytosis, membrane bridging and tight junction formation (Yamada et al., 2005; Monastyrskaya et al., 2008; Su et al., 2010; Bharadwaj et al., 2013). The association is regulated by multiple factors including the calcium concentration, PH value and the post-translational modifications of ANXA2, implicating that ANXA2 mediates the effects of those factors in cell functions and processes. Due to the lack of intracellular domain, ANXA2, as a membrane-associate protein, can only dock onto the cell membrane in a peripheral manner (Kim and Hajjar, 2002). It remains largely unknown how ANXA2 transduces outside signals inside the cells. However, our recent work has demonstrated that ANXA2, upon binding of tPA, acts as a co-receptor of integrin CD11b, leading to their aggregation and activation of downstream integrin-linked kinase (ILK) and NF-kB signaling (Lin et al., 2012). Intriguingly, ANXA2 not only promotes NF-kB-dependent renal inflammation, but also mediates macrophage M2 to M1 phenotypic change leading to extensively enhanced inflammation (Lin and Hu, 2017). ANXA2, as an intracellular protein, modulates Rho signaling pathway through its interaction with cytoskeleton (Babbin et al., 2007; Ye et al., 2021), and regulates various membrane trafficking events (Luo and Hajjar, 2013), including calcium-mediated release of Weibel-Palade bodies (Knop et al., 2004), chromaffin granules (Umbrecht-Jenck et al., 2010), and lamellar bodies containing surfactant proteins (Wang et al., 2007). ANXA2 is also involved in the biosynthesis of cellular vesicles such as exosomes, and becomes a part of the cargos of these vesicles (Luo and Hajjar, 2013). Exosomes are excreted through an exocytotic process, and subsequently participate in intercellular interactions by contacting membrane of target cells and/or transferring their contents including ANXA2 (Margolis and Sadovsky, 2019). Thus, it is presumable that ANXA2 is involved in the extracellular vesicles (EV)-mediated cell-cell communication.
ANXA2 has several main phosphorylation sites including Tyr23, Ser25 and Ser11, which makes it a good candidate for the substrate of different kinases to participate in different signal pathways (Bharadwaj et al., 2013; Grindheim et al., 2017). Generally, Ser phosphorylation is involved in the regulation of S100A10 binding, secretory granules activity and mRNA binding, while Tyr phosphorylation is linked to actin dynamics and the endosomal pathway (Grindheim et al., 2017). Though ANXA2 is first found as a substrate of Src-family kinases, it also responds to the activation of numerous receptor tyrosine kinases by Tyr phosphorylation and involves in the signaling pathways of insulin, epithelial growth factor (EGF), platelet-derived growth factor (PDGF) and others (Brambilla et al., 1991; Biener et al., 1996; Rescher et al., 2008; Dziduszko and Ozbun, 2013; Cui et al., 2017). As a substrate of protein kinase C (PKC), ANXA2 contains sequence homology to the PKC binding site of 14-3-3 protein and may modulate PKC localization and signaling (Hoque et al., 2014). Moreover, increased Tyr 23 phosphorylation is also detected under the stress of heat, hypoxia, H2O2 and excitatory amino acids, suggesting that ANXA2 involves in the stress-induced signaling (Grindheim et al., 2017). In addition, ANXA2 contains four cysteine residues: Cys-8, Cys-132, Cys-261, and Cys-334, and is considered as a cellular redox regulatory protein and involved in redox-related functions on the cell surface, as well as in the cytoplasm and nucleus (Bharadwaj et al., 2013; Madureira and Waisman, 2013). For instance, Madureira and colleagues found that Cys-8 residue of ANXA2 is able to be oxidized by H2O2 and then reduced by the thioredoxin system, which results in the degrading of H2O2, a central redox signaling molecule mediating different signaling pathways upon its concentration in vivo (Madureira et al., 2011; Sies, 2017). On the other hand, oxidative stress-induced glutathionylation of Cys-8 and Cys-132 of the ANXA2 results in decreased binding of ANXA2-S100A10 heterotetramer with phospholipid and F-actin, and leads to related cellular function changes, suggesting that ANXA2 mediates oxidative stress-induced cellular function changes (Caplan et al., 2004).
Annexin A2 and kidney diseases
Kidney disease is getting increasing recognition in the public health problems due to its increasing prevalence and high cost of treatment. It is also an important multiplier of risk of cardiovascular disease (Xu et al., 2018). It includes heterogeneous disorders with diverse etiologies affecting kidney structures and functions (Levey and Coresh, 2012). ANXA2, a calcium regulated phospholipids-binding protein, is expressed in various cells including macrophages, dendritic cells, endothelial and epithelial cells, and mediates various functions including endocytosis, exocytosis, cell-matrix interactions, cell motility, signal transduction, transcription, mRNA transport and DNA replication (Grindheim et al., 2017). Recent studies implicated that ANXA2 is an important participant in the pathogenesis of various kidney diseases.
Annexin A2 and nephrotic syndrome
Components from a dysregulated immune system are able to mediate various acute renal injuries and play a central role in the progression of chronic kidney disease. Generally, auto-antibodies, produced through multiple mechanisms involving autoreactivity of both B and T cells, target various antigens within the kidney, lead damages directly at the local sites and finally result in the loss of kidney functions (Tecklenborg et al., 2018). ANXA2 is expressed in multiple cells in kidneys, therefore, auto-antibodies targeting ANXA2 may bind to the related cells and initiate directly renal damage through immune system.
Nephrotic syndrome is characterized by edema, proteinuria, hypoalbuminemia and hyperlipidemia, and is able to lead to end-stage kidney disease (Wang and Greenbaum, 2019; Politano et al., 2020). It may be primary or secondary to systemic disorders. The cause of primary nephrotic syndrome is not always clear, and immune dysregulation, systemic circulating factors, and structural and functional abnormalities of the podocyte can have deciding effects. Notably, some mutations in the genes encoding important podocyte proteins can cause defects in the glomerular filtration apparatus, resulting in nephrotic syndrome (Eddy and Symons, 2003; Noone et al., 2018).
Ye and colleagues has demonstrated that children with primary nephrotic syndrome (PNS) had higher level of anti-ANXA2 antibody, which significantly correlated with urine protein level. Intriguingly, BALB/c mice receiving intravenous injection of anti-ANXA2 antibody developed proteinuria as early as 24 h after injection. These mice displayed local foot-process fusion, basement membrane thickening, increased glomerular volume, narrowed balloon space, and large amount of collagen exudation. In vitro studies indicated that anti-ANXA2 antibody activates Cdc42 and Rac1/2/3 Rho pathway through phosphorylation of ANXA2 at Tyr24, leading to podocyte cytoskeleton rearrangement and eventually proteinuria (Ye et al., 2021).
Annexin A2 and lupus nephritis
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease, which is characterized by a vast array of auto-antibodies, including anti-dsDNA antibodies. Auto-antibodies against nuclear and cellular antigens produced in SLE form immune complexes, which accumulate in the tissues and contribute to local damage (Seret et al., 2012). Lupus nephritis (LN), the most common cause of kidney injury of SLE, is a major risk factor for morbidity and mortality (Parikh et al., 2020). LN is characterized by the deposition of auto-antibodies in the mesangial area and along the glomerular basement membrane, complement activation, as well as the production of local mediators related to inflammation and fibrosis (Yung et al., 2010).
There are at least four different cell types in the glomeruli: mesangial cells, endothelial cells, podocytes and parietal cells, of which, mesangial cells, together with mesangial cell-specific antibodies, are thought to play central role in the pathogenesis of lupus nephritis (Seret et al., 2012). Among the numerous auto-antibodies, anti-dsDNA antibodies present in serum of nearly 80% of patients with LN and correlate closely with LN (Yung and Chan, 2015; Wang and Xia, 2019). Yung and colleagues isolated anti-dsDNA antibodies from 32 patients with significant IgG binding to human mesangial cells (HMC), and screened the proteins of the plasma membrane fraction of HMC that can bind to the antibodies directly. ANXA2 was identified among these proteins, and was found to mediate the binding of anti-dsDNA antibodies to HMC, which, in turn, induces IL-6 and ANXA2 expression in HMC. Prior incubation of anti-dsDNA antibodies with recombinant human ANXA2 inhibited the binding ability of the anti-dsDNA to HMC and the internalization of the anti-dsDNA by HMC. Glomerular ANXA2 expression correlated with LN severity (Yung et al., 2010). Similar screening in podocytes and glomerular proteins using the serum from patients with proliferative LN also identified ANXA2 as the antigen binding to the serum auto-antibodies (Caster et al., 2015). Their results are corroborated by several clinical studies in LN patients and experimental studies in mice, which demonstrated that serum level of ANXA2-binding immunoglobins, as well as expression of glomerular ANXA2, is associated with active LN progression (Ka et al., 2006; Salle et al., 2016; Zhou et al., 2018; Tesch et al., 2020). In a recent study regarding LN and T cell anti-renal autoreactivity, Tesch and colleagues identified ANXA2 as one of the main CD4+T cell targets in kidney (Tesch et al., 2020). Therefore, ANXA2, expressed in glomerular mesangial cells, is a pathogenetic target for the auto-antibodies in the SLE. ANXA2 mediates mesangial functions and local inflammation. ANXA2 is also one of the potential biomarkers for LN because its level correlates with the LN status (Ka et al., 2006; Salle et al., 2016; Cheung et al., 2017; Zhou et al., 2018).
Annexin A2 and kidney inflammation
In the renal diseases initiated by the non-immunological mechanisms, such as acute kidney injury induced by artery infarction, the stress response pathways are activated and various cytokines and vasoactive factors are released upon the response of the renal epithelial cell damage, leading to the recruitment and activation of leukocytes and initiation of immune responses (Tecklenborg et al., 2018). Infiltrated leukocytes clear cellular debris and necrotic tissue, and promote renal healing process. However, in the presence of sustained severe damage or in the absence of anti-inflammatory factors, inflammatory process will be perpetuated and finally result in the tubular atrophy, interstitial scaring and worse kidney functions. Though the mechanisms of the regulation of the renal healing process is not completely clear, macrophages have been implicated (Rogers et al., 2014; Tecklenborg et al., 2018). Macrophage accumulation is one of the histological hallmarks of most interstitial and glomerular kidney diseases. In response to injury, macrophages differentiated into two broad but distinct categories as classically activated (M1) and alternatively activated (M2). Generally, M1 macrophages express a panoply of proinflammatory genes to promote inflammation and damage, however, M2 macrophages help to resolve inflammation and promote tissue remodeling (Ricardo et al., 2008; Wang and Harris, 2011; Lin and Hu, 2017).
Our studies have shown that tPA, a ligand of ANXA2, promotes kidney fibrosis and inflammation in the mice with unilateral ureteral obstruction (UUO), a classic chronic kidney disease mouse model. We found that tPA knockout mice had less collagen deposition, less CD11b positive macrophage infiltration, and less activation of NF-kB signaling, as indicated by lower renal p65 phosphorylation and less NF-kB-dependent chemokines, in the kidneys. In vitro studies discovered that ANXA2 mediates tPA-induced macrophage NF-kB activation through its aggregation and interaction with CD11b, followed by activation of its downstream mediator ILK (Lin et al., 2012). Further studies found that ANXA2-mediated NF-kB pathway promotes tPA-induced macrophage M2 to M1 phenotypic change, suggesting a pivotal role of ANXA2 in tPA-mediated renal inflammation (Lin and Hu, 2017).
Alternative pathway (AP) of complement is another contributor to kidney inflammation and is usually triggered by systemic illness or by tissue injury. Clinical and experimental studies implicate that AP activation contributes to the pathogenesis and promotes the injury of renal ischemia, focal segmental glomerulosclerosis (FSGS), antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis and lupus nephritis (Watanabe et al., 2000; Thurman et al., 2003; Thurman et al., 2005; Turnberg et al., 2006; Lenderink et al., 2007; Xiao et al., 2007; Xing et al., 2009; Noris et al., 2010). Factor H is a circulating protein which regulates the activation of AP of complement. Abnormal function of factor H is observed in numerous kidney diseases (Noris et al., 1999; Fremeaux-Bacchi et al., 2005; Servais et al., 2012; Sethi et al., 2012). To explore the mechanisms of factor H in the regulation of AP activation, Renner and colleagues screened the proteins from the post-ischemic kidney through immunoprecipitation with purified murine factor H, and identified ANXA2 as a factor H-binding partner. ANXA2 was shown to impair factor H function, increase complement activation on renal cell surface both in vitro and in vivo, and contribute to AP-mediated tissue inflammation (Renner et al., 2016).
Annexin A2, nephrocalcinosis and nephrolithiasis
Nephrolithiasis and nephrocalcinosis are caused by the precipitation of poor soluble waste salts, such as calcium oxalate, in the renal tubular fluid. Calcium oxalate concentration is at low level in the ultrafiltrate, and increases gradually with oxalate secretion and water absorption, which results in the crystal formation in certain conditions. Crystal retention may result in kidney stones (nephrolithiasis) in the renal calyces and pelvis, as well as the tubular calcification (tubular nephrocalcinosis). Nephrolithiasis is associated with pain and suffering, and is responsible for about 10% of urological admissions per year. It affects 12% of men and 6% of women at some point in their lives. Tubular nephrocalcinosis can lead to obstruction-induced tubular injury and sometimes end-stage renal failure (Verkoelen and Verhulst, 2007).
Attachment of newly formed crystals to renal epithelial cells is critical to the development of kidney stone. Protein with high affinity to calcium oxalate monohydrate crystal was isolated from solubilized apical membrane proteins prepared from renal epithelial cells, and ANXA2 was identified using trypsin digestion and microsequencing technique. Further cellular experiments confirmed the expression of ANXA2 in the apical surface of renal epithelial cells. Pre-incubating with ANXA2-antibody blocked the binding of the crystals to renal epithelial cells. Therefore, ANXA2 may mediate adhesion of the crystals to tubular epithelial cells, promote crystal retention and finally contribute to the pathogenesis of nephrolithiasis and nephrocalcinosis (Kumar et al., 2003).
The role of ANXA2 in the crystal adhesion is also supported by other cellular and mouse studies. Dent’s disease is a genetic disorder caused by mutation of clc-5, a chloride channel, encoding gene and is characterized by low-molecular-weight proteinuria, hypercalciuria, nephrolithiasis and nephrocalcinosis. Disruption of clc-5 by transfection of antisense clc-5 or truncated clc-5 in a collecting duct cell resulted in the translocation of ANXA2 to plasma membrane through a mechanism to be further elucidated, which is accompanied with increased crystal binding and agglomeration. Pretreatment with anti-ANXA2 antibodies attenuated the effects of clc-5 disruption on crystal binding and agglomeration (Carr et al., 2006). In a mouse model of renal crystal formation induced by glyoxylate injection, it was found that fewer renal crystal deposits, accompanied with less ANXA2, were detected in oncostatin (OSM) receptor ß (OSMRβ)-deficient mice comparing to the wildtype controls (Yamashita et al., 2020).
Annexin A2 and diabetic nephropathy
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease (ESRD) worldwide. The pathogenesis of DN development and progression is complex with many contributing pathways and mediators, and remains not fully understood. It is known that persisting hyperglycemia-induced chronic glomerular hyperfiltration and excessive matrix deposition contribute to DN pathogenesis. However, current therapy with strict blood sugar and blood pressure control is unable to halt DN progression to ESRD (Samsu, 2021). KKAy mice, a crossbred between diabetic KK and lethal yellow (Ay), an obese gene, mice, develop obesity, dyslipidemia, insulin resistance and increased urine microalbumin levels spontaneously, and are widely used in the study of type 2 diabetes and DN (Okazaki et al., 2002). Proteomic profile in glomeruli of the KKAy mice with DN was analyzed by 2-dimensional differential gel electrophoresis and compared to C57BL/6 wide-type mice. ANXA2 and prohibitin, a protein interacting with ANXA2 upon the regulation of calcium, were identified and showed to be upregulated in the DN glomeruli, suggesting that ANXA2 may be associated with DN pathogenesis (Liu et al., 2014). In vitro study in human renal glomerular endothelial cells further validated that ANXA2 mediated mitomycin A-induced expression of collagen type VI and may contribute to DN pathogenesis since collagen type VI and phosphorylated histone H2AX, a DNA damage marker, were mainly detected in diabetic nodular glomerulosclerosis (Fujii et al., 2020). Dysregulation of the coagulation system and abnormal endothelial function exist in diabetic patients and are good predictors of DN (Alsharidah, 2022). ANXA2, as a receptor for plasminogen and tPA, plays an important role in maintaining vascular and hemostatic homeostasis (Lim and Hajjar, 2021). To explore the possible role of ANXA2 in DN pathogenesis and treatment, recombinant ANXA2 protein was administered into KKAy mice for 8 weeks with PBS administration as control. DN was alleviated after the ANXA2 protein treatment, as demonstrated by less kidney weight, lower level of albuminuria and smaller glomerular lesion area. However, there was no significant difference between the two groups in bleeding time, prothrombin time and active partial thromboplastin time, suggesting that ANXA2 protein alleviated DN progression with minimal influence on the coagulation system. Thus, ANXA2 administration may be a potential therapeutic tool for DN (Ishii et al., 2007).
Annexin A2 and other kidney diseases
Acute tubular necrosis is the most common cause of the clinical state of acute renal failure. After injured by toxin, cells are damaged, cellular calcium is increased, quiescent cells undergo dedifferentiation, proliferation, and finally kidney function are restored (Toback, 1992). Calcium is an essential signaling molecular ion that controls a wide range of biological processes and functions, including cellular proliferation, differentiation and apoptosis. ANXA2, as an important calcium binding protein, was evaluated in uranyl nitrate-induced acute tubular necrosis. ANXA2 expression in renal cortex was increased in 3–7 days and attenuated on day 14. ANXA2 expression co-localized with proliferating cell nuclear antigen (PCNA). Similar ANXA2 expression pattern was observed in other acute tubular necrosis mouse models induced by folic acid and ischemic/reperfusion, though ANXA2 expression peaked at 12–24 h and declined in 72 h in the ischemic/reperfusion mouse model. It appeared that ANXA2 is the sensor of tubular injury and recovery in the acute renal failure (Cheng et al., 2005).
Mesangial proliferative glomerulonephritis is characterized by excessive mesangial cell proliferation and mesangial matrix expansion, which results in the glomeruli damage and loss of renal function. It may occur in IgA nephropathy, IgM nephropathy, lupus nephritis and other renal diseases. Proteomic analysis of renal tissue using iTRAQ technology uncovered that ANXA2 is upregulated in mesangial proliferative glomerulonephritis (Sui et al., 2013).
ANXA2 is also implicated in the cardiovascular diseases related to ESRD. Extensive and progressive vascular calcification is usually developed in ESRD patients. Lin and colleagues discovered that plasma exosomes derived from ESRD patients had higher level of ANXA2, which significantly promoted calcification of vascular smooth muscle cells (VSMCs), comparing to that from normal healthy group. Plasma exosome ANXA2 positively correlated to coronary artery calcification scores (Lin et al., 2020). Hyperphosphatemia, developed in chronic kidney disease and ESRD, is associated with increased cardiovascular risk. ANXA2 may also mediate the effect of phosphate in cardiovascular diseases, which was supported by the findings that ANXA2 was downregulated in human endothelial cells in exposure to high phosphate media or to serum from hyperphosphatemic patients, and that blockade of membrane-ANXA2 with a specific neutralizing antibody mimicked the effects of high phosphate, such as impaired endothelial cell migration and tube formation (Di Marco et al., 2013).
Annexin A2 and renal cell carcinoma
Aberrant expression of ANXA2 has been found in numerous malignancies including gastric (Zhang et al., 2012), breast (Gibbs et al., 2020), lung (Yang et al., 2015a) and colorectal (Yang et al., 2013) cancer, as well as hepatocellular (El-Abd et al., 2016) and nasopharyngeal (Chen et al., 2018) carcinoma. ANXA2 has been implicated in cell cycle regulation and regulates tumor cell growth and survival. As a receptor for plasminogen and tPA, ANXA2 promotes plasmin generation, resulting in degradation of extracellular matrix (ECM), which facilitates cellular migration, tumor cell invasion and neoangiogenesis (Andreasen et al., 2000; Xu et al., 2015; Sharma, 2019). Moreover, ANXA2 Tyr 23 phosphorylation is implicated in the initiation of endothelial-mesenchymal transition (EndMT) and regulation of cell motility, and may contribute to tumor progression (Chen et al., 2018). ANXA2 also contributes to therapeutic resistance of multiple malignancies, such as nasopharyngeal carcinoma, gastric cancer, breast cancer and pancreatic cancer by mediating various signaling pathways including p38MAPK/Akt and PI3K/Akt/NF-kB pathways (Takano et al., 2008; Gong et al., 2010; Zhang et al., 2014a; Zhang et al., 2014b; Chen et al., 2015).
Renal cell carcinoma (RCC) is the ninth most common cancer worldwide and is the most common malignancy in adult kidney. Arising from nephric epithelial cells, RCC includes a group of heterogenous disorders characterized on the basis of anatomical origin, histological features, molecular hallmarks and therapeutic outcomes. The etiology is not fully understood. Multiple factors contribute to RCC pathogenesis, such as genetic predisposition, obesity, smoking, drugs and chemicals. Most of RCC patients are diagnosed incidentally during routine examination. Only up to 10% of RCC patients present typical clinical symptoms. Based on histological manifestation, RCC falls into three major subsets including clear cell RCC, papillary RCC, chromophobe RCC and several other rare subtypes such as collecting duct carcinoma, renal medullary carcinoma and urothelial carcinoma. Current treatment strategies for metastatic RCC includes cytoreductive nephrectomy, followed by immunotherapeutic drugs, antiangiogenic agents and mTOR inhibitors since RCC is resistant to traditional therapies (Yagoda et al., 1993; Tang et al., 2006; Jonasch et al., 2014; Petejova and Martinek, 2016; Wolf et al., 2020). Mechanistic studies of RCC pathogenesis, as well as searching for new diagnostic biomarkers and novel targeted therapies, were one of the hot spots over the past decades.
Tissue-associated genes were screened in the full-length enriched cDNA libraries of clear cell RCC and normal kidney tissues. Aberrant expression of ANXA2 in RCC was identified and validated by quantitative real-time PCR and Western blot (Tang et al., 2006). Since the tumor cells usually recapitulate embryonic cells, Sadashiv and colleagues investigated the ANXA2 expression in developing renal tissues and adult RCCs. Immunostaining revealed that ANXA2 was expressed in the ureteric buds and collecting tubules of fetal kidneys and in the collecting ducts of adult normal renal tissues, but not in the papillary RCC cells. Intriguingly, ANXA2 was also expressed in the proximal convoluted tubules, which is thought to be the origin of RCC, of younger fetal kidneys, and reappeared in clear cell RCC tumor cells, but not in that of normal adult kidneys. Proximal tubular expression of ANXA2 in fetal kidneys was more profound at earlier gestational weeks, and similar pattern was observed in the clear cell RCC with higher grade in tumor progression. Therefore, the deregulation of ANXA2 may be implicated in RCC pathogenesis (Sadashiv et al., 2019).
Since most RCC patients are detected incidentally during routine examination, earlier diagnosis remains challenging. Several studies investigated the relationship between ANXA2 expression and RCC development and progression, and explored the possibility of ANXA2 as a biomarker for RCC. Domoto and colleagues found upregulated expression of ANXA2 and its binding protein, S100A10, in RCC tissues. Compared to normal tissues, S100A10 and ANXA2 gene expression was 2.5-fold and 1.6-fold higher respectively in RCC. Immunostaining revealed that both S100A10 and ANXA2 were expressed in RCC tissues, but not in the proximal tubules of normal tissues, where most RCC derived from (Domoto et al., 2007). Similar results from Yang and colleagues further confirmed that ANXA2 expression was upregulated in all three major RCC subtypes (Yang et al., 2015b). More studies linked the expression of ANXA2 with RCC clinical manifestation and prognosis. Ohno and colleagues investigated the expression of ANXA2 in clear cell RCC tissues and normal tissues, and detected upregulated expression of ANXA2 gene and protein in 14 of 18 primary RCC tissues, positive ANXA2 immunostaining in 73 of 154 primary RCC tissues, as well as 21 in 24 metastatic tumors. In primary RCC, increased ANXA2 expression was significantly associated with higher stage and higher nuclear grade. The 5-years metastasis-free rate in patients with ANXA2-positive RCC was significantly lower than those with ANXA2-negative RCC, indicating that ANXA2 may be a predictor for metastasis and a useful marker for prognosis. It was also supported by a similar study in 33 RCC patients, in which ANXA2 expression was found to be correlated with Fuhrman grade and clinical outcomes (Zimmermann et al., 2004; Ohno et al., 2009).
Although multiple studies demonstrated that increased ANXA2 expression is associated with the poor prognosis of RCC, the underlying mechanisms remain unclear. Silencing ANXA2 in RCC cell lines resulted in decreased ability of cell migration and invasion, cell polarity alteration, disruption of actin filaments formation and reduced CXCR4 expression. Inhibition of Rho/Rock axis restored cell motility suppressed by ANXA2 silencing suggesting that Rho/Rock is involved in ANXA2 signaling pathways (Yang et al., 2015b). Soluble ANXA2 was detected at higher level in renal vein serum from some RCC patients and was proved to inhibit phytohaemagglutinin-induced lymphoproliferation in vitro, suggesting immunosuppression effect of ANXA2 in the RCC progression (Aarli et al., 1997). Increased ANXA2 was detected in the rat RCC induced by ferric nitrilotriacetate, an iron chelate inducing renal oxidative damage. Further investigation showed that ANXA2 was increased time-dependently in the rat kidney after ferric nitrilotriacetate administration, as well as in H2O2-treated LLC-PK1 cells, a normal renal tubular cell line. The pattern of ANXA2 expression in rat was similar to that in clinical patients, as higher expression of ANXA2 was detected in regenerating proximal tubules, primary and metastasizing RCCs, comparing to normal kidney. Serine and tyrosine phosphorylation was also detected. Overexpression of ANXA2 induced LLC-PK1 cell proliferation, while silencing ANXA2 decreased the proliferation of FRCC cells derived from ferric nitrilotriacetate-induced primary RCCs (Tanaka et al., 2004). Therefore, it is presumable that ANXA2 mediates oxidative stress-induced renal carcinogenesis and metastasis.
Discussion
ANXA2, as a multifunctional molecule, regulates diverse cellular functions and processes through various signaling pathways. ANXA2 antibody, gene silencing techniques and ANXA2 protein administration have been used in cellular and animal experiments to explore the role of ANXA2 in kidney diseases, as well as the underlying mechanisms. As shown in Table 1, aberrant expression of ANXA2 has been detected in numerous kidney diseases, including primary and secondary nephropathy, acute kidney injury, chronic kidney disease, as well as kidney carcinoma. ANXA2, expressed in the mesangial cells and podocytes, acts as the antigen for the auto-antibodies in patients, mediates renal inflammation and injury in LN and PNS. ANXA2 also promotes kidney inflammation through mediating tPA-induced NF-kB activation and M2 to M1 phenotypic change, as well as binding to factor H to impair function of factor H and increase AP activation. ANXA2 mediates the binding of calcium oxalate crystals to renal epithelial cells and is involved in the pathogenesis of nephrolithiasis and nephrocalcinosis. ANXA2 participates in the pathogenesis and progression of RCC through its effects on immunosuppression, cell proliferation, migration and invasion. Studies revealed that ANXA2 expression correlates with the disease status of LN and RCC, implicating that ANXA2 may be a potential biomarker for RCC and LN. The fact that ANXA2 protein administration alleviated DN in the KKAy mice model suggests that it may be a potential therapeutic tool for DN. Of note, the current knowledge regarding the role of ANXA2 in kidney diseases is very limited, and many of the publications are association studies. Therefore, future more definitive and mechanistic studies on the role of ANXA2 in the pathogenesis of kidney diseases are warranted.
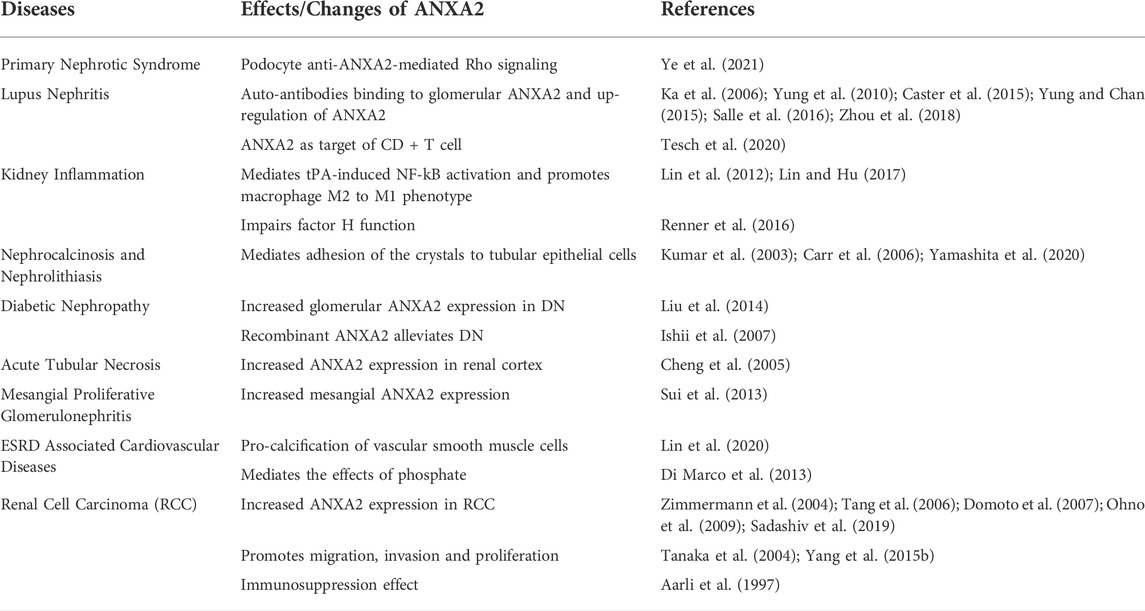
TABLE 1. Summary of the roles of annexin A2 in kidney diseases. Annexin A2 executes multiple cellular process and signaling pathways to participate in the pathogenesis and progression of various kidney diseases.
Author contributions
LL and KH developed the concept and wrote the manuscript.
Funding
KH is supported by grants from National Institutes of Health (DK102624), Pennsylvania Department of Health Tobacco CURE Funds (4100085731), and Department of Medicine Innovation Award (INNOVKHUFall2021). LL is supported by American Heart Association Career Development Award (941281), College of Medicine JFDP Scholar Award (GEN-FACDV-JFDP-Lin-L), and Department of Medicine Inspiration Pilot Award (INSPIRELINFall2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarli, A., Skeie Jensen, T., Kristoffersen, E. K., Bakke, A., and Ulvestad, E. (1997). Inhibition of phytohaemagglutinin-induced lymphoproliferation by soluble annexin II in sera from patients with renal cell carcinoma. APMIS 105 (9), 699–704. doi:10.1111/j.1699-0463.1997.tb05073.x
Alsharidah, A. S. (2022). Diabetes mellitus and diabetic nephropathy: A review of the literature on hemostatic changes in coagulation and thrombosis. Blood Res. 57, 101–105. doi:10.5045/br.2022.2021204
Andreasen, P. A., Egelund, R., and Petersen, H. H. (2000). The plasminogen activation system in tumor growth, invasion, and metastasis. Cell. Mol. Life Sci. 57 (1), 25–40. doi:10.1007/s000180050497
Babbin, B. A., Parkos, C. A., Mandell, K. J., Winfree, L. M., Laur, O., Ivanov, A. I., et al. (2007). Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am. J. Pathol. 170 (3), 951–966. doi:10.2353/ajpath.2007.060647
Bharadwaj, A., Bydoun, M., Holloway, R., and Waisman, D. (2013). Annexin A2 heterotetramer: Structure and function. Int. J. Mol. Sci. 14 (3), 6259–6305. doi:10.3390/ijms14036259
Bharadwaj, A., Kempster, E., and Waisman, D. M. (2021). The annexin A2/S100A10 complex: The mutualistic symbiosis of two distinct proteins. Biomolecules 11 (12), 1849. doi:10.3390/biom11121849
Biener, Y., Feinstein, R., Mayak, M., Kaburagi, Y., Kadowaki, T., and Zick, Y. (1996). Annexin II is a novel player in insulin signal transduction. Possible association between annexin II phosphorylation and insulin receptor internalization. J. Biol. Chem. 271 (46), 29489–29496. doi:10.1074/jbc.271.46.29489
Brambilla, R., Zippel, R., Sturani, E., Morello, L., Peres, A., and Alberghina, L. (1991). Characterization of the tyrosine phosphorylation of calpactin I (annexin II) induced by platelet-derived growth factor. Biochem. J. 278 (2), 447–452. doi:10.1042/bj2780447
Caplan, J. F., Filipenko, N. R., Fitzpatrick, S. L., and Waisman, D. M. (2004). Regulation of annexin A2 by reversible glutathionylation. J. Biol. Chem. 279 (9), 7740–7750. doi:10.1074/jbc.M313049200
Carr, G., Simmons, N. L., and Sayer, J. A. (2006). Disruption of clc-5 leads to a redistribution of annexin A2 and promotes calcium crystal agglomeration in collecting duct epithelial cells. Cell. Mol. Life Sci. 63 (3), 367–377. doi:10.1007/s00018-005-5510-8
Caster, D. J., Korte, E. A., Merchant, M. L., Klein, J. B., Wilkey, D. W., Rovin, B. H., et al. (2015). Autoantibodies targeting glomerular annexin A2 identify patients with proliferative lupus nephritis. Proteomics. Clin. Appl. 9 (11-12), 1012–1020. doi:10.1002/prca.201400175
Chen, C. Y., Lin, Y. S., Chen, C. H., and Chen, Y. J. (2018). Annexin A2-mediated cancer progression and therapeutic resistance in nasopharyngeal carcinoma. J. Biomed. Sci. 25 (1), 30. doi:10.1186/s12929-018-0430-8
Chen, C. Y., Lin, Y. S., Chen, C. L., Chao, P. Z., Chiou, J. F., Kuo, C. C., et al. (2015). Targeting annexin A2 reduces tumorigenesis and therapeutic resistance of nasopharyngeal carcinoma. Oncotarget 6 (29), 26946–26959. doi:10.18632/oncotarget.4521
Cheng, C. W., Rifai, A., Ka, S. M., Shui, H. A., Lin, Y. F., Lee, W. H., et al. (2005). Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney Int. 68 (6), 2694–2703. doi:10.1111/j.1523-1755.2005.00740.x
Cheung, K. F., Yung, S., Chau, M. K., Yap, D. Y., Chan, K. W., Lee, C. K., et al. (2017). Annexin II-binding immunoglobulins in patients with lupus nephritis and their correlation with disease manifestations. Clin. Sci. 131 (8), 653–671. doi:10.1042/CS20160732
Creutz, C. E., Pazoles, C. J., and Pollard, H. B. (1978). Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J. Biol. Chem. 253 (8), 2858–2866. doi:10.1016/s0021-9258(17)40901-x
Cui, L., Song, J., Wu, L., Cheng, L., Chen, A., Wang, Y., et al. (2017). Role of Annexin A2 in the EGF-induced epithelial-mesenchymal transition in human CaSki cells. Oncol. Lett. 13 (1), 377–383. doi:10.3892/ol.2016.5406
Dassah, M., Deora, A. B., He, K., and Hajjar, K. A. (2009). The endothelial cell annexin A2 system and vascular fibrinolysis. Gen. Physiol. Biophys. 28, F20–F28.
Di Marco, G. S., Konig, M., Stock, C., Wiesinger, A., Hillebrand, U., Reiermann, S., et al. (2013). High phosphate directly affects endothelial function by downregulating annexin II. Kidney Int. 83 (2), 213–222. doi:10.1038/ki.2012.300
Domoto, T., Miyama, Y., Suzuki, H., Teratani, T., Arai, K., Sugiyama, T., et al. (2007). Evaluation of S100A10, annexin II and B-FABP expression as markers for renal cell carcinoma. Cancer Sci. 98 (1), 77–82. doi:10.1111/j.1349-7006.2006.00355.x
Dziduszko, A., and Ozbun, M. A. (2013). Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J. Virol. 87 (13), 7502–7515. doi:10.1128/JVI.00519-13
Eddy, A. A., and Symons, J. M. (2003). Nephrotic syndrome in childhood. Lancet 362 (9384), 629–639. doi:10.1016/S0140-6736(03)14184-0
El-Abd, N., Fawzy, A., Elbaz, T., and Hamdy, S. (2016). Evaluation of annexin A2 and as potential biomarkers for hepatocellular carcinoma. Tumour Biol. 37 (1), 211–216. doi:10.1007/s13277-015-3524-x
Erikson, E., and Erikson, R. L. (1980). Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell 21 (3), 829–836. doi:10.1016/0092-8674(80)90446-8
Filipek, A., Wojda, U., and Lesniak, W. (1995). Interaction of calcyclin and its cyanogen bromide fragments with annexin II and glyceraldehyde 3-phosphate dehydrogenase. Int. J. Biochem. Cell Biol. 27 (11), 1123–1131. doi:10.1016/1357-2725(95)00096-8
Flood, E. C., and Hajjar, K. A. (2011). The annexin A2 system and vascular homeostasis. Vasc. Pharmacol. 54 (3-6), 59–67. doi:10.1016/j.vph.2011.03.003
Fremeaux-Bacchi, V., Kemp, E. J., Goodship, J. A., Dragon-Durey, M. A., Strain, L., Loirat, C., et al. (2005). The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: Evidence from two independent cohorts. J. Med. Genet. 42 (11), 852–856. doi:10.1136/jmg.2005.030783
Fujii, A., Sunatani, Y., Furuichi, K., Fujimoto, K., Adachi, H., Iwabuchi, K., et al. (2020). DNA damage in human glomerular endothelial cells induces nodular glomerulosclerosis via an ATR and ANXA2 pathway. Sci. Rep. 10 (1), 22206. doi:10.1038/s41598-020-79106-3
Gibbs, L. D., Mansheim, K., Maji, S., Nandy, R., Lewis, C. M., Vishwanatha, J. K., et al. (2020). Clinical significance of annexin A2 expression in breast cancer patients. Cancers (Basel) 13 (1), 2. doi:10.3390/cancers13010002
Gong, X. G., Lv, Y. F., Li, X. Q., Xu, F. G., and Ma, Q. Y. (2010). Gemcitabine resistance induced by interaction between alternatively spliced segment of tenascin-C and annexin A2 in pancreatic cancer cells. Biol. Pharm. Bull. 33 (8), 1261–1267. doi:10.1248/bpb.33.1261
Grindheim, A. K., Saraste, J., and Vedeler, A. (2017). Protein phosphorylation and its role in the regulation of Annexin A2 function. Biochim. Biophys. Acta. Gen. Subj. 1861 (11), 2515–2529. doi:10.1016/j.bbagen.2017.08.024
Hoque, M., Rentero, C., Cairns, R., Tebar, F., Enrich, C., and Grewal, T. (2014). Annexins - scaffolds modulating PKC localization and signaling. Cell. Signal. 26 (6), 1213–1225. doi:10.1016/j.cellsig.2014.02.012
Ishii, H., Hiraoka, M., Tanaka, A., Shimokado, K., and Yoshida, M. (2007). Recombinant annexin-2 inhibits the progress of diabetic nephropathy in a diabetic mouse model via recovery of hypercoagulability. Thromb. Haemost. 97 (1), 124–128. doi:10.1160/th06-07-0381
Jonasch, E., Gao, J., and Rathmell, W. K. (2014). Renal cell carcinoma. BMJ 349, g4797. doi:10.1136/bmj.g4797
Ka, S. M., Rifai, A., Chen, J. H., Cheng, C. W., Shui, H. A., Lee, H. S., et al. (2006). Glomerular crescent-related biomarkers in a murine model of chronic graft versus host disease. Nephrol. Dial. Transpl. 21 (2), 288–298. doi:10.1093/ndt/gfi229
Kim, J., and Hajjar, K. A. (2002). Annexin II: A plasminogen-plasminogen activator co-receptor. Front. Biosci. 7, d341–8. doi:10.2741/kim
Knop, M., Aareskjold, E., Bode, G., and Gerke, V. (2004). Rab3D and annexin A2 play a role in regulated secretion of vWF, but not tPA, from endothelial cells. EMBO J. 23 (15), 2982–2992. doi:10.1038/sj.emboj.7600319
Kumar, V., Farell, G., Deganello, S., and Lieske, J. C. (2003). Annexin II is present on renal epithelial cells and binds calcium oxalate monohydrate crystals. J. Am. Soc. Nephrol. 14 (2), 289–297. doi:10.1097/01.asn.0000046030.24938.0a
Lenderink, A. M., Liegel, K., Ljubanovic, D., Coleman, K. E., Gilkeson, G. S., Holers, V. M., et al. (2007). The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am. J. Physiol. Ren. Physiol. 293 (2), F555–F564. doi:10.1152/ajprenal.00403.2006
Levey, A. S., and Coresh, J. (2012). Chronic kidney disease. Lancet 379 (9811), 165–180. doi:10.1016/S0140-6736(11)60178-5
Lim, H. I., and Hajjar, K. A. (2021). Annexin A2 in fibrinolysis, inflammation and fibrosis. Int. J. Mol. Sci. 22 (13), 6836. doi:10.3390/ijms22136836
Lin, L., and Hu, K. (2017). Tissue-type plasminogen activator modulates macrophage M2 to M1 phenotypic change through annexin A2-mediated NF-κB pathway. Oncotarget 8 (50), 88094–88103. doi:10.18632/oncotarget.21510
Lin, L., Wu, C., and Hu, K. (2012). Tissue plasminogen activator activates NF-κB through a pathway involving annexin A2/CD11b and integrin-linked kinase. J. Am. Soc. Nephrol. 23 (8), 1329–1338. doi:10.1681/ASN.2011111123
Lin, X., Zhu, T., Xu, F., Zhong, J. Y., Li, F., Shan, S. K., et al. (2020). Plasma exosomes derived from patients with end-stage renal disease and renal transplant recipients have different effects on vascular calcification. Front. Cell Dev. Biol. 8, 618228. doi:10.3389/fcell.2020.618228
Liu, X., Yang, G., Fan, Q., and Wang, L. (2014). Proteomic profile in glomeruli of type-2 diabetic KKAy mice using 2-dimensional differential gel electrophoresis. Med. Sci. Monit. 20, 2705–2713. doi:10.12659/MSM.893078
Luo, M., and Hajjar, K. A. (2013). Annexin A2 system in human biology: Cell surface and beyond. Semin. Thromb. Hemost. 39 (4), 338–346. doi:10.1055/s-0033-1334143
Madureira, P. A., Hill, R., Miller, V. A., Giacomantonio, C., Lee, P. W., and Waisman, D. M. (2011). Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget 2 (12), 1075–1093. doi:10.18632/oncotarget.375
Madureira, P. A., and Waisman, D. M. (2013). Annexin A2: The importance of being redox sensitive. Int. J. Mol. Sci. 14 (2), 3568–3594. doi:10.3390/ijms14023568
Margolis, L., and Sadovsky, Y. (2019). The biology of extracellular vesicles: The known unknowns. PLoS Biol. 17 (7), e3000363. doi:10.1371/journal.pbio.3000363
Monastyrskaya, K., Tschumi, F., Babiychuk, E. B., Stroka, D., and Draeger, A. (2008). Annexins sense changes in intracellular pH during hypoxia. Biochem. J. 409 (1), 65–75. doi:10.1042/BJ20071116
Noone, D. G., Iijima, K., and Parekh, R. (2018). Idiopathic nephrotic syndrome in children. Lancet 392 (10141), 61–74. doi:10.1016/S0140-6736(18)30536-1
Noris, M., Caprioli, J., Bresin, E., Mossali, C., Pianetti, G., Gamba, S., et al. (2010). Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 5 (10), 1844–1859. doi:10.2215/CJN.02210310
Noris, M., Ruggenenti, P., Perna, A., Orisio, S., Caprioli, J., Skerka, C., et al. (1999). Hypocomplementemia discloses genetic predisposition to hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: Role of factor H abnormalities. Italian registry of familial and recurrent hemolytic uremic syndrome/thrombotic thrombocytopenic purpura. J. Am. Soc. Nephrol. 10 (2), 281–293. doi:10.1681/ASN.V102281
Ohno, Y., Izumi, M., Kawamura, T., Nishimura, T., Mukai, K., and Tachibana, M. (2009). Annexin II represents metastatic potential in clear-cell renal cell carcinoma. Br. J. Cancer 101 (2), 287–294. doi:10.1038/sj.bjc.6605128
Okazaki, M., Saito, Y., Udaka, Y., Maruyama, M., Murakami, H., Ota, S., et al. (2002). Diabetic nephropathy in KK and KK-Ay mice. Exp. Anim. 51 (2), 191–196. doi:10.1538/expanim.51.191
Parikh, S. V., Almaani, S., Brodsky, S., and Rovin, B. H. (2020). Update on lupus nephritis: Core curriculum 2020. Am. J. Kidney Dis. 76 (2), 265–281. doi:10.1053/j.ajkd.2019.10.017
Petejova, N., and Martinek, A. (2016). Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech. Repub. 160 (2), 183–194. doi:10.5507/bp.2015.050
Politano, S. A., Colbert, G. B., and Hamiduzzaman, N. (2020). Nephrotic syndrome. Prim. Care 47 (4), 597–613. doi:10.1016/j.pop.2020.08.002
Renner, B., Tong, H. H., Laskowski, J., Jonscher, K., Goetz, L., Woolaver, R., et al. (2016). Annexin A2 enhances complement activation by inhibiting factor H. J. Immunol. 196 (3), 1355–1365. doi:10.4049/jimmunol.1500793
Rescher, U., Ludwig, C., Konietzko, V., Kharitonenkov, A., and Gerke, V. (2008). Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J. Cell Sci. 121 (13), 2177–2185. doi:10.1242/jcs.028415
Rety, S., Sopkova, J., Renouard, M., Osterloh, D., Gerke, V., Tabaries, S., et al. (1999). The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat. Struct. Biol. 6 (1), 89–95. doi:10.1038/4965
Ricardo, S. D., van Goor, H., and Eddy, A. A. (2008). Macrophage diversity in renal injury and repair. J. Clin. Invest. 118 (11), 3522–3530. doi:10.1172/JCI36150
Rintala-Dempsey, A. C., Rezvanpour, A., and Shaw, G. S. (2008). S100-annexin complexes--structural insights. FEBS J. 275 (20), 4956–4966. doi:10.1111/j.1742-4658.2008.06654.x
Rintala-Dempsey, A. C., Santamaria-Kisiel, L., Liao, Y., Lajoie, G., and Shaw, G. S. (2006). Insights into S100 target specificity examined by a new interaction between S100A11 and annexin A2. Biochemistry 45 (49), 14695–14705. doi:10.1021/bi061754e
Rogers, N. M., Ferenbach, D. A., Isenberg, J. S., Thomson, A. W., and Hughes, J. (2014). Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat. Rev. Nephrol. 10 (11), 625–643. doi:10.1038/nrneph.2014.170
Sadashiv, R., Bannur, B. M., Shetty, P., Dinesh, U. S., Rani, H., Vishwanatha, J., et al. (2019). Differential expression pattern of annexin A2 during nephrogenesis and kidney carcinoma. Rom. J. Morphol. Embryol. 60 (3), 895–904.
Salle, V., Cordonnier, C., Schmidt, J., Maziere, C., Smail, A., Attencourt, C., et al. (2016). Vascular expression of annexin A2 in lupus nephritis. J. Clin. Pathol. 69 (6), 533–536. doi:10.1136/jclinpath-2015-203139
Samsu, N. (2021). Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. Biomed. Res. Int. 2021, 1497449. doi:10.1155/2021/1497449
Santamaria-Kisiel, L., Rintala-Dempsey, A. C., and Shaw, G. S. (2006). Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 396 (2), 201–214. doi:10.1042/BJ20060195
Semov, A., Moreno, M. J., Onichtchenko, A., Abulrob, A., Ball, M., Ekiel, I., et al. (2005). Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J. Biol. Chem. 280 (21), 20833–20841. doi:10.1074/jbc.M412653200
Seret, G., Le Meur, Y., Renaudineau, Y., and Youinou, P. (2012). Mesangial cell-specific antibodies are central to the pathogenesis of lupus nephritis. Clin. Dev. Immunol. 2012, 579670. doi:10.1155/2012/579670
Servais, A., Noel, L. H., Roumenina, L. T., Le Quintrec, M., Ngo, S., Dragon-Durey, M. A., et al. (2012). Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 82 (4), 454–464. doi:10.1038/ki.2012.63
Sethi, S., Fervenza, F. C., Zhang, Y., Zand, L., Vrana, J. A., Nasr, S. H., et al. (2012). C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 82 (4), 465–473. doi:10.1038/ki.2012.212
Sharma, M. C. (2019). Annexin A2 (ANX A2): An emerging biomarker and potential therapeutic target for aggressive cancers. Int. J. Cancer 144 (9), 2074–2081. doi:10.1002/ijc.31817
Sies, H. (2017). Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 11, 613–619. doi:10.1016/j.redox.2016.12.035
Smith, S. P., and Shaw, G. S. (1998). A change-in-hand mechanism for S100 signalling. Biochem. Cell Biol. 76 (2-3), 324–333. doi:10.1139/bcb-76-2-3-324
Su, S. C., Maxwell, S. A., and Bayless, K. J. (2010). Annexin 2 regulates endothelial morphogenesis by controlling AKT activation and junctional integrity. J. Biol. Chem. 285 (52), 40624–40634. doi:10.1074/jbc.M110.157271
Sui, W., Tang, D., Zou, T., Zou, G., Chen, J., Li, H., et al. (2013). Differential proteomic analysis of renal tissue in mesangial proliferative glomerulonephritis using iTRAQ technology. J. Nephrol. 26 (1), 191–198. doi:10.5301/jn.5000124
Swairjo, M. A., Concha, N. O., Kaetzel, M. A., Dedman, J. R., and Seaton, B. A. (1995). Ca(2+)-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 2 (11), 968–974. doi:10.1038/nsb1195-968
Takano, S., Togawa, A., Yoshitomi, H., Shida, T., Kimura, F., Shimizu, H., et al. (2008). Annexin II overexpression predicts rapid recurrence after surgery in pancreatic cancer patients undergoing gemcitabine-adjuvant chemotherapy. Ann. Surg. Oncol. 15 (11), 3157–3168. doi:10.1245/s10434-008-0061-5
Tanaka, T., Akatsuka, S., Ozeki, M., Shirase, T., Hiai, H., and Toyokuni, S. (2004). Redox regulation of annexin 2 and its implications for oxidative stress-induced renal carcinogenesis and metastasis. Oncogene 23 (22), 3980–3989. doi:10.1038/sj.onc.1207555
Tang, S. W., Chang, W. H., Chao, Y. W., Lin, C. Y., Chen, H. F., Lai, Y. H., et al. (2006). Identification of differentially expressed genes in clear cell renal cell carcinoma by analysis of full-length enriched cDNA library. J. Biomed. Sci. 13 (2), 233–240. doi:10.1007/s11373-005-9059-1
Tecklenborg, J., Clayton, D., Siebert, S., and Coley, S. M. (2018). The role of the immune system in kidney disease. Clin. Exp. Immunol. 192 (2), 142–150. doi:10.1111/cei.13119
Tesch, S., Abdirama, D., Griessbach, A. S., Brand, H. A., Goerlich, N., Humrich, J. Y., et al. (2020). Identification and characterization of antigen-specific CD4(+) T cells targeting renally expressed antigens in human lupus nephritis with two independent methods. Sci. Rep. 10 (1), 21312. doi:10.1038/s41598-020-78223-3
Thurman, J. M., Ljubanovic, D., Edelstein, C. L., Gilkeson, G. S., and Holers, V. M. (2003). Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J. Immunol. 170 (3), 1517–1523. doi:10.4049/jimmunol.170.3.1517
Thurman, J. M., Lucia, M. S., Ljubanovic, D., and Holers, V. M. (2005). Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 67 (2), 524–530. doi:10.1111/j.1523-1755.2005.67109.x
Toback, F. G. (1992). Regeneration after acute tubular necrosis. Kidney Int. 41 (1), 226–246. doi:10.1038/ki.1992.32
Turnberg, D., Lewis, M., Moss, J., Xu, Y., Botto, M., and Cook, H. T. (2006). Complement activation contributes to both glomerular and tubulointerstitial damage in adriamycin nephropathy in mice. J. Immunol. 177 (6), 4094–4102. doi:10.4049/jimmunol.177.6.4094
Uebi, T., Miwa, N., and Kawamura, S. (2007). Comprehensive interaction of dicalcin with annexins in frog olfactory and respiratory cilia. FEBS J. 274 (18), 4863–4876. doi:10.1111/j.1742-4658.2007.06007.x
Umbrecht-Jenck, E., Demais, V., Calco, V., Bailly, Y., Bader, M. F., and Chasserot-Golaz, S. (2010). S100A10-mediated translocation of annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic 11 (7), 958–971. doi:10.1111/j.1600-0854.2010.01065.x
Verkoelen, C. F., and Verhulst, A. (2007). Proposed mechanisms in renal tubular crystal retention. Kidney Int. 72 (1), 13–18. doi:10.1038/sj.ki.5002272
Wang, C. S., and Greenbaum, L. A. (2019). Nephrotic syndrome. Pediatr. Clin. North Am. 66 (1), 73–85. doi:10.1016/j.pcl.2018.08.006
Wang, P., Chintagari, N. R., Gou, D., Su, L., and Liu, L. (2007). Physical and functional interactions of SNAP-23 with annexin A2. Am. J. Respir. Cell Mol. Biol. 37 (4), 467–476. doi:10.1165/rcmb.2006-0447OC
Wang, X., and Xia, Y. (2019). Anti-double stranded DNA antibodies: Origin, pathogenicity, and targeted therapies. Front. Immunol. 10, 1667. doi:10.3389/fimmu.2019.01667
Wang, Y., and Harris, D. C. (2011). Macrophages in renal disease. J. Am. Soc. Nephrol. 22 (1), 21–27. doi:10.1681/ASN.2010030269
Watanabe, H., Garnier, G., Circolo, A., Wetsel, R. A., Ruiz, P., Holers, V. M., et al. (2000). Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J. Immunol. 164 (2), 786–794. doi:10.4049/jimmunol.164.2.786
Wolf, M. M., Kimryn Rathmell, W., and Beckermann, K. E. (2020). Modeling clear cell renal cell carcinoma and therapeutic implications. Oncogene 39 (17), 3413–3426. doi:10.1038/s41388-020-1234-3
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J., and Jennette, J. C. (2007). Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170 (1), 52–64. doi:10.2353/ajpath.2007.060573
Xing, G. Q., Chen, M., Liu, G., Heeringa, P., Zhang, J. J., Zheng, X., et al. (2009). Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J. Clin. Immunol. 29 (3), 282–291. doi:10.1007/s10875-008-9268-2
Xu, X., Nie, S., Ding, H., and Hou, F. F. (2018). Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 14 (5), 313–324. doi:10.1038/nrneph.2018.11
Xu, X. H., Pan, W., Kang, L. H., Feng, H., and Song, Y. Q. (2015). Association of annexin A2 with cancer development (Review). Oncol. Rep. 33 (5), 2121–2128. doi:10.3892/or.2015.3837
Yagoda, A., Petrylak, D., and Thompson, S. (1993). Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol. Clin. North Am. 20 (2), 303–321. doi:10.1016/s0094-0143(21)00489-4
Yamada, A., Irie, K., Hirota, T., Ooshio, T., Fukuhara, A., and Takai, Y. (2005). Involvement of the annexin II-S100A10 complex in the formation of E-cadherin-based adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 280 (7), 6016–6027. doi:10.1074/jbc.M408215200
Yamashita, S., Komori, T., Kohjimoto, Y., Miyajima, A., Hara, I., and Morikawa, Y. (2020). Essential roles of oncostatin M receptor beta signaling in renal crystal formation in mice. Sci. Rep. 10 (1), 17150. doi:10.1038/s41598-020-74198-3
Yang, J., Yang, F., Nie, J., Zou, X., Tian, H., Qin, Y., et al. (2015). Evaluation of Annexin A2 as a novel diagnostic serum biomarker for lung cancer. Cancer Biomark. 15 (2), 205–211. doi:10.3233/CBM-140455
Yang, S. F., Hsu, H. L., Chao, T. K., Hsiao, C. J., Lin, Y. F., and Cheng, C. W. (2015). Annexin A2 in renal cell carcinoma: Expression, function, and prognostic significance. Urol. Oncol. 33 (1), 22e11–e22. doi:10.1016/j.urolonc.2014.08.015
Yang, T., Peng, H., Wang, J., Yang, J., Nice, E. C., Xie, K., et al. (2013). Prognostic and diagnostic significance of annexin A2 in colorectal cancer. Colorectal Dis. 15 (7), e373–81. doi:10.1111/codi.12207
Ye, Q., Zhang, Y., Zhuang, J., Bi, Y., Xu, H., Shen, Q., et al. (2021). The important roles and molecular mechanisms of annexin A2 autoantibody in children with nephrotic syndrome. Ann. Transl. Med. 9 (18), 1452. doi:10.21037/atm-21-3988
Yung, S., and Chan, T. M. (2015). Mechanisms of kidney injury in lupus nephritis - the role of anti-dsDNA antibodies. Front. Immunol. 6, 475. doi:10.3389/fimmu.2015.00475
Yung, S., Cheung, K. F., Zhang, Q., and Chan, T. M. (2010). Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J. Am. Soc. Nephrol. 21 (11), 1912–1927. doi:10.1681/ASN.2009080805
Zhang, F., Zhang, H., Wang, Z., Yu, M., Tian, R., Ji, W., et al. (2014). P-glycoprotein associates with Anxa2 and promotes invasion in multidrug resistant breast cancer cells. Biochem. Pharmacol. 87 (2), 292–302. doi:10.1016/j.bcp.2013.11.003
Zhang, Q., Ye, Z., Yang, Q., He, X., Wang, H., and Zhao, Z. (2012). Upregulated expression of annexin II is a prognostic marker for patients with gastric cancer. World J. Surg. Oncol. 10, 103. doi:10.1186/1477-7819-10-103
Zhang, Z. D., Li, Y., Fan, Q., Zhao, B., Tan, B., and Zhao, X. F. (2014). Annexin A2 is implicated in multi-drug-resistance in gastric cancer through p38MAPK and AKT pathway. Neoplasma 61 (6), 627–637. doi:10.4149/neo_2014_078
Zhou, Y., Xiao, L., and Tang, S. (2018). Annexin A2 and FTH1 are potential biomarkers for lupus nephritis. Exp. Ther. Med. 16 (5), 3766–3776. doi:10.3892/etm.2018.6686
Keywords: annexin A2, cell signaling, kidney diseases, renal inflammation, lupus nephritis, diabetic nephropathy (DN), renal cell carcinoma, nephrolithiasis
Citation: Lin L and Hu K (2022) Annexin A2 and Kidney Diseases. Front. Cell Dev. Biol. 10:974381. doi: 10.3389/fcell.2022.974381
Received: 21 June 2022; Accepted: 08 August 2022;
Published: 02 September 2022.
Edited by:
Ursula Rescher, University of Münster, GermanyReviewed by:
Jesus Ayala-Sanmartin, Sorbonne Universités, FranceKatherine Amberson Hajjar, Cornell University, United States
Beate Vollenbröker, University Hospital Münster, Germany
Copyright © 2022 Lin and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Lin, llin1@pennstatehealth.psu.edu; Kebin Hu, kebinhu@pennstatehealth.psu.edu
 Ling Lin
Ling Lin Kebin Hu
Kebin Hu