High level expression of nicotinamide nucleoside kinase from Saccharomyces cerevisiae and its purification and immobilization by one-step method
- 1Henan Provincial Key Laboratory of Funiu Mountain Insect Biology and Henan Provincal Engineering and Technology Center of Health Products for Livestock and Poultry, Nanyang Normal University, Nanyang, China
- 2College of Life Science and Agricultural Engineering, Nanyang Normal University, Nanyang, China
Nicotinamide riboside kinase (NRK) plays an important role in the synthesis of β -nicotinamide nucleotide (NMN). NMN is a key intermediate of NAD+ synthesis, and it actually contribute to the well-being of our health. In this study, gene mining technology was used to clone nicotinamide nucleoside kinase gene fragments from S. cerevisiae, and the ScNRK1 was achieved a high level of soluble expression in E. coli BL21. Then, the reScNRK1 was immobilized by metal affinity label to optimize the enzyme performance. The results showed that the enzyme activity in the fermentation broth was 14.75 IU/mL, and the specific enzyme activity after purification was 2252.59 IU/mg. After immobilization, the optimum temperature of the immobilized enzyme was increased by 10°C compared with the free enzyme, and the temperature stability was improved with little change in pH. Moreover, the activity of the immobilized enzyme remained above 80% after four cycles of immobilized reScNRK1, which makes the enzyme more advantageous in the enzymatic synthesis of NMN.
Introduction
In NAD+ biosynthesis, β-nicotinamide mononucleotide (NMN) is a bioactive nucleotide formed by the reaction between phosphate groups and nucleoside-containing ribose and nicotinamide (NAMs) (Hong et al., 2020). NMN is a key intermediate of nicotinamide adenine dinucleotide (NAD+), an essential coenzyme for cellular redox reactions (Liu et al., 2021). Generally speaking, It has two forms, alpha and beta of which only beta is active with a molecular weight of 334.221 g/mol. NAD+ plays a crucial role in redox cellular balance, catabolism, and energy production and the NAD+ molecule acts as an electron carrier in all three realms of life (Hachisuka et al., 2018; Kalia, Shi Lee et al., 2019). In addition, NAD is a cosubstrate for enzymes that create signaling metabolites or posttranslationally modify proteins (Frederick et al., 2016). There have been many studies shown that NAD+ levels decline with age, in a wide range of species (Harrison et al., 2021). NMN needs to be transformed into NAD+ in vivo to perform its physiological function (Wang et al., 2016). Recently clinical studies have found that NMN administration reduces the risk of age-related diseases Alzheimer’s disease and type 2 diabetes (Longo and Kennedy, 2006; Wang et al., 2016). NMN has been called the “elixir” and some studies have shown that oral administration of NMN in the mouse model will lead to the recovery of NAD+, which can resist aging and prolong life (Das et al., 2019; Poddar et al., 2019).
NMN is a research hotspot in health products, food and cosmetics, and more and more attentions were paid to its synthesis. NAD+ is synthesized in mammalian cells through three different pathways: 1) synthesis of new tryptophan, 2) synthesis of nicotinamide or nicotinic acid, or 3) conversion of nicotinamide ribose nicotinamide ribose (NR) (Belenky et al., 2007). In some mammals, the rescue synthesis pathway takes NR as the substrate and phosphorylates to produce NMN under the action of nicotinamide nucleoside kinase (Yoshino et al., 2018). The synthesis of NMN begins with chemical synthesis. The most effective chemical synthesis method at present is the two-step method used in the 2017 Zhang’s group report: the β-isomer was eventually obtained in 85% yield after deprotection of the ester in methanolic ammonia (Zhang and Sauve, 2017). Owing to the chemical production of NMN is time-consuming and laborious, the biosynthesis of NMN has become a hot spot (Huang et al., 2022). Compared with traditional chemical catalysis, it has unique advantages such as mild conditions, low equipment requirements and strong stereoselectivity. According to the reported biotechnological methods of producing NMN in Escherichia coli, the highest NMN yield can reach 15.42 mg/L bacterial culture (or 17.26 mg/g protein) (Marinescu et al., 2018). It is reported that some people use whole cell catalysis to produce NMN. Extracellular production of 6.79 g/L NMN with glucose and NAM as substrates. The reaction selectivity from NAM to NMN is 86% (Shoji et al., 2021). Zhou Jingwen of Jiangnan University and others transformed E. coli into nicotinamide and glucose, creating a record high yield of NMN. The NMN titer reached 16.2 g/L, and the nicotinamide conversion rate was 97.0% (Huang et al., 2022). According to the current report, both were the highest. Compared with whole cell catalysis, fed batch fermentation with direct addition of nicotinamide is more conducive to the industrial production of NMN (Huang et al., 2022). Chemical synthesis using nicotinamide nucleoside (NR) as substrate depends on excessive phosphorus oxychloride and requires strict temperature operation which is not suitable for industrial (Qian et al., 2022). A new nicotinamide nucleoside kinase (Klm-NRK) from Kluyveromyces martensii and purified it. The specific activity of Klm-NRK was 7.9 U mg−1 protein, which was in the forefront of the reported NRK (Qian et al., 2022). Therefore, optimizing the enzymatic properties of nicotinamide nucleoside kinase plays an important role in the synthesis of NMN.
Immobilization technology is a mature and commonly used method in the development of enzymes. The main motivation of enzyme immobilization has always been to improve the performance of the enzyme. This can be achieved by improving the activity, stereoselectivity or stability of the enzyme. The enzyme exists in an appropriate physical form so that it can be recycled and reused at the end of biotransformation (Federsel et al., 2021). In this study, we plan to use gene mining technology to clone nicotinamide nucleoside kinase gene fragments from Saccharomyces cerevisiae, and then use pET28a plasmid to achieve a high level of soluble expression in E. coli BL21, and analyze its free enzymology characteristics, and then the nicotinamide nucleoside kinase was immobilized by metal affinity labeling to optimize the enzyme performance. The metal affinity label is hexahistidine (6 × His), which can gently bind to the C or N terminal of the enzyme molecule, and then specifically bind to Ni2+ on the carrier, so as to achieve the directional immobilization of the enzyme on the carrier (Wu and Filutowicz, 1999). The temperature stability of immobilized enzyme was improved, and the enzyme could be reused, simplifying the experimental process, which can effectively synthesize NMN.
Materials and methods
Reagents and kits
NR, NMN and Adenosine triphosphate (ATP) were purchased from Macklin (Shanghai, China). Commonly molecular biology reagents and kits, mainly including restriction enzymes, DL 2,000 DNA Marker and PrimeSTAR® HS DNA Polymerase and so on, were purchased as previously reported (Wang et al., 2021). Methanolwere purchased from Komiou (Tianjin, China).
Strains, plasmids, and culture media
Carrying prokaryotic expression plasmid E. coli BL21 (DE3)/pET28a, S. cerevisiae strains were preserved by our research group. The culture of E. coli uses LB medium, and YPD medium is used for the culture of Saccharomyces cerevisiae (Tang et al., 2020a).
Web server and soft wares
National Center for Biotechnology Information (NCBI) was appled for searching nicotinamide nucleoside kinase sequence and structure. ProtParam tool was accustomed to predict theoretically physical and chemical parameters of target enzyme. DNAMAN was used for sequence analysis. TMHMM −2.0 was used to predict transmembrane region of the target enzyme. Origin 9.0 was used for data analysis.
Cloning and expression of nicotinamide nucleoside kinase gene
With S. cerevisiae and nicotinamide nucleoside kinase as key words, the potential nicotinamide nucleoside kinase of S. cerevisiae was searched in NCBI. Based on the reported sequence, the primers ScNRK1-F and ScNRK1-R used to amplify nicotinamide nucleoside kinase1 (NRK1) gene sequence. According to this method (Tang et al., 2020b), Take the total RNA of S. cerevisiae as the template, and use PrimeScript ™ II 1st Strand cDNA Synthesis Kit is used to synthesize the first strand of cDNA, and then ScNRK1-F and ScNRK1-R primer PCR are used to amplify the coding gene of nicotinamide nucleoside kinase. The restriction endonucleases Nde Ⅰ and Xho Ⅰ are used to double digest the target gene and pET28a plasmid respectively, and then linked and transformed into E. coli BL21 (DE3) competent cells, obtained E. Coli BL21 (DE3)/pET28a ScNRK1 recombinant strain.
Expression and purification of nicotinamide nucleoside kinase1
The E. coli BL21 (DE3)/pET28a and recombinant E. coli strains containing nicotinamide nucleoside kinase encoding genes were picked and induced as described previously (Tang et al., 2019). Recombinant E. coli was induced by the reported method (Tang et al., 2018a). Then, we collected the induced recombinant cells through cryogenic centrifuge centrifugation, and The APV2000 high-pressure homogenizer (Gatesville, New York, United States) splits the cell lysate through high-pressure homogenization, centrifuges and purifies the collected cell lysate (Ma et al., 2009; Tang et al., 2018b; Hong et al., 2020). The protein concentration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the nicotinamide nucleoside kinase1 were performed using reported previously method (Laemmli et al., 1970).
Immobilized nicotinamide riboside kinase1
The cells collected by induction centrifugation were sonicated and then centrifuged to collect the supernatant, to which 2 mM MgCl2 and 1% NaCl were added to increase the binding amount of enzyme and agarose-Ni2+ column. The new column was equilibrated by washing with 5 times the column volume of binding solution, and the collected supernatant was added to the agarose-Ni2+ column and repeatedly suspended four times to increase the amount of enzyme binding, and finally the proteins were washed with deionized water (Jia et al., 2021). The agarose-Ni2+ column media was then aspirated, freeze-dried, and the drying was completed in a four-degree refrigerator for subsequent use.
Enzyme activity and protein assays
NRK1 enzyme activity analysis: nicotinamide ribose (NR) was used as substrate, and NMN production was used as standard to calculate the enzyme activity. The determination of nicotinamide nucleoside kinase1 activity was slightly modified based on the reported method (Liu et al., 2021). The reaction system of free enzyme is 20 mmol/L pH5.8 Tris-HCL buffer, 10mmol/LMgCl2, 2 mmol/L ATP, 10 mmol/L NR, 0.1 mL diluted enzyme. The reaction system of immobilized enzyme is 20 mmol/L pH5.8 Tris-HCL buffer, 10 mmol/LMgCl2, 2 mmol/L ATP, 10 mmol/L NR, 0.03 g immobilized enzyme. Both were reacted at 40°C for 10 min, and the free enzyme was heated in boiling water for 5 min to terminate the reaction, and the reaction solution to be measured was filtered through a 0.22 μm filter membrane after the reaction was terminated. The immobilization reaction was terminated by centrifugation at 4°C and 12,000 rpm for 2 min. After centrifugation, the enzyme and liquid were separated. The supernatant was aspirated with a 1 mL syringe, the supernatant was filtered through a 0.22 μm filter membrane, and the precipitated immobilized enzyme was placed in −20°C for next use. Chromatographic conditions: Thermo Hypersil BDS C 18 column (250 mm × 4.6 mm, 5 µm), detection wavelength: 260 nm; injection volume: 10 μL. mobile phase: V (methanol): V (phosphate buffer) = 5:95 buffer; flow rate: 1 mL min−1. The amount of enzyme required to generate 1 μmol of NMN per minute under assay conditions was defined as 1 unit of enzyme activity. Then, use quantity one to estimate the apparent molecular weight of NRK1 (Tang et al., 2016).
Temperature characteristics of the recombinant enzyme
According to the reported method, the temperature and pH characteristics of the determination of recombinant nicotinamide ribokinase1 were slightly modified (Hong et al., 2020). The optimum temperature of the free and immobilization recombinant nicotinamide nucleoside kinases1 was determined under the above standard determination conditions, except temperature ranging from 25 to 55°C. To estimate the thermostability, the free and immobilization recombinant nicotinamide nucleoside kinases1 were incubated at pH 5.8 and various temperatures (25–55°C) for 1.0 h, and then, the residual enzyme activity was measured under the optimal reaction pH and temperature.
pH characteristics of the recombinant enzyme
The optimum pH of the free and immobilization recombinant nicotinamide nucleoside kinases1 was determined under the above standard determination conditions, except pH ranging from 3.4 to 8.8. To estimate the pH stability, the free and immobilization recombinant nicotinamide nucleoside kinases1 were incubated at various pH (3.4–8.8) for 1.0 h, and then, the residual enzyme activity was measured under the optimal reaction temperatures and pH.
Kinetic parameters for the recombinant enzyme
The kinetic parameters of recombinant nicotinamide riboside kinase1 were determined with slight modifications according to the reported method (Tempel et al., 2007). To determine the specificity of NR substrates, the concentration of ATP in the immobilization system was 1 mmol/L. The catalytic activity of the recombinant enzyme was measured at optimal reaction condition with varied nicotinamide riboside concentrations from 0.01 to 0.03 mg. Each measurement was executed three times. Similarly, the specificity of ATP substrate was determined. The concentration of immobilized NR was 1 mmol/L, and the ATP concentration was set to 0.04–0.2 mg to determine the catalytic activity of the recombinant enzyme. Each measurement was executed three times. The apparent kinetic data for the enzyme exhibiting no substrate inhibition were calculated using the Michaelis-Menten equation (Ziegler et al., 2009). All calculations were performed by Origin 9.0.
Reusability of reconstituted reScNRK1
To test the number of times the immobilized enzyme can be reused: the immobilized enzyme was used repeatedly to utilize the immobilized enzyme 10 times according to the above immobilized enzyme measurement method.
Results and discussion
Gene cloning and expression of nicotinamide riboside kinase1
A nicotinamide nucleoside kinase1 from S. cerevisiae was found in NCBI. The corresponding genome sequence (Genebank: AY611479.1) was designed to amplify the upstream and downstream primers of the target gene according to the corresponding gene sequence of ScNRK1. ScNRK1-F: GGAATTCCATCATGATGATGACTTCGAAAAAAAAAAAGTGATA (including Nde I digestion site) and ScNRK1-R: CCGCTCGAGCTAATCCTTACAAGCTTTAG (including Xho I digestion site), Suzhou Hongxun Biotechnology was entrusted to conduct primer synthesis. The total RNA of S. cerevisiae was used as the template, and after reverse transcription, ScNRK1-F and ScNRK1-R were used as the primers for PCR amplification. The amplified products were subjected to agarose gel electrophoresis, as shown in Figure 1A, there were obvious specific bands at about 750 bp, and the length of the PCR product fragment was basically consistent with the expected theoretical length. The PCR products were purified and recovered with the kit, and then double digested with Nde Ⅰ and Xho Ⅰ. The digested products were connected to the pET28a plasmid which was also double digested, and transformed into E. coli BL21 (DE3) competent cells were screened for kanamycin resistance and detected by double enzyme digestion and double enzyme digestion electrophoresis as shown in Figure 1B. After identification, they were sent to Shanghai Sangon for sequencing and identification to obtain the gene sequence of ScNRK1 and speculate its amino acid sequence.
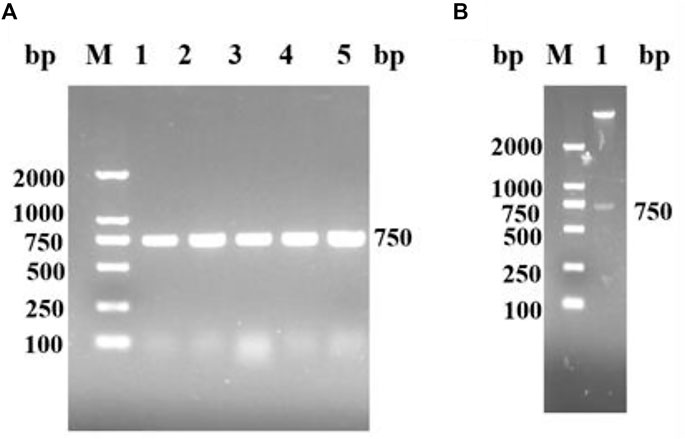
FIGURE 1. The agarose gel electrophoresis analysis for PCR products of ScNRK1. and double enzyme digestion verification of recombinant E. coli BL21 (DE3)/pET28a -ScNRK1. (A) PCR detection. Lane M, DNA marker; lane 1-5, PCR products from different strains of E. coli BL21 (DE3)/pET28a-ScNRK1; (B) Double digestion detection. Lane M, DNA marker; lane 1, Double digestion products of E. coli BL21 (DE3)/pET28a-ScNRK1 by Nde Ⅰ and Xho Ⅰ.
Sequence analysis of ScNRK1
The deduced amino acid sequence of ScNRK1 was predicted on ProtParam for theoretical physical and chemical properties. The results showed that the theoretical isoelectric point (pI) of ScNRK1 was 6.23, the theoretical molecular weight was 27689.58 Da, and the instability index II was 25.59. ScNRK1 was a relatively stable protein, and its half-life in E. coli and yeast could reach more than 10 h and 20 h respectively. The TMHMM—2.0 server was used to predict the transmembrane structure of the protein. The results showed that 240 amino acid residues of ScNRK1 were extracellular residues, indicating that ScNRK1 was theoretically easier to achieve extracellular secretory expression.
Expression, purification and immobilizition of nicotinamide nucleoside kinase 1
To evaluate the expression level and enzymatic properties of ScNRK1, the recombinant E. coli strain containing ScNRK1 encoding gene was picked and incubated as above method. The reScNRK1 was purified by affinity chromatography with His tag, and the purified results were analyzed by SDS-PAGE (Figure 2). As shown in Figure 2, the reScNRK1 was successfully expressed in soluble form. Furthermore, the results indicated that the reScNRK1 was purified to homogeneity with apparent molecular weight of 27 kDa, which was consistent with its theoretical molecular weight of 27689.58 Da. After measurement and conversion, the dehydrogenase activity in fermentation liquor was 14.75 IU/mL. And the specific activity of the reScNRK1 toward nicotinamide riboside was 2252.59 IU/mg, which was significantly higher that previously reported (Bieganowski and Brenner, 2004), and had greater potential for application. The immobilized enzyme activity was 107.80 IU/mL, the activity of immobilized enzyme is 7 times higher than that of free enzyme. In this study, 10 mL of cell lysate was collected per 100 mL LB induction, and the lysate was passed through 2 mL agarose-Ni2+ column, and 0.523 g of immobilized enzyme was finally collected by freeze-drying. The recovery of enzyme activity of immobilized enzyme was 81%.
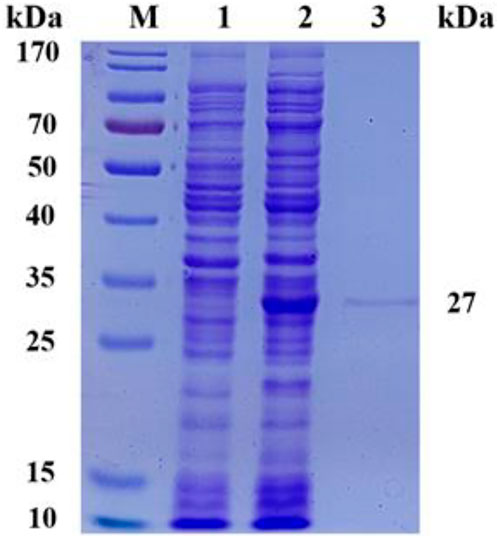
FIGURE 2. SDS-PAGE analysis of recombinant Escherichia coli expression products. SDS-PAGE analysis of recombinant Escherichia coli expression products M, PageRuler Prestained Protein Ladder; 1, expression products of E. coli BL21 (DE3)/pET28a; 2, crude expression product of E. coli BL21 (DE3)/pET28a-ScNRK1; 3, purified expression product of E. coli BL21 (DE3)/pET28a-ScNRK1.
HPLC analysis of NMN standard
The analytically pure NMN was analyzed by HPLC on ThermoHypersil C 18 column. Under this analysis condition, the retention time of NMN was about 2.79 min, as shown in Figure 3A. The substrate standard was also analyzed by HPLC, with the retention time of 4.02 min, as shown in Figure 3B. As shown in Figure 4 the immobilized standard curve is y = 6345.6x—15471, R2 = 0.9994, The results show that under the HPLC analysis conditions and concentration range, the concentration of NMN is linearly related to the peak area. Therefore, this method can be used to accurately quantify NMN.
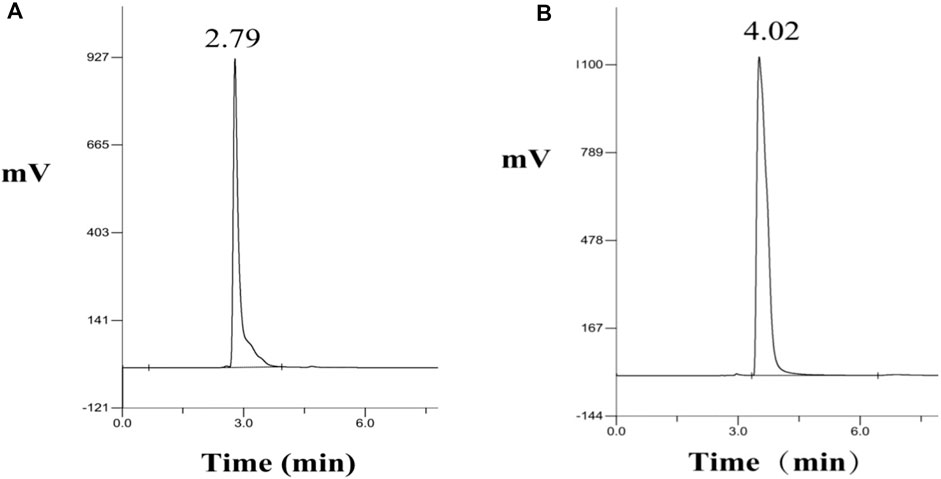
FIGURE 3. HPLC analysis of NMN standard and substrate NR. (A) Retention time of NMN standard in HPLC, (B) Retention time of NR standard in HPLC.
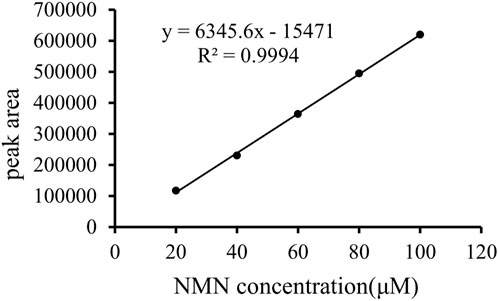
FIGURE 4. Standard curve of NMN. Prepare 100 μM NMN stock solution and dilute it to standard solutions of 20 μM, 40 μM, 60 μM, 80 μM and 100 μM respectively as standard koji of immobilized enzyme. The abscissa is the NMN standard concentration, and the ordinate is the peak area.
Temperature characteristics of the reScNRK1
The temperature characteristics of free and immobilization reScNRK1 were determined. The results are shown in Figure 5 and Figure 6 respectively. It can be seen from Figure 5 that when the free enzyme is in the temperature range of 20°C–30°C, the enzymatic reaction rate increases with the increase of temperature. When the temperature exceeds 30°C, the reaction rate decreases. The optimal reaction temperature of free enzyme is 30°C, which is not close to the physiological temperature of human body, but closer to the optimal growth temperature of bacteria. The optimum temperature of immobilization enzyme was 40°C, which was higher than that of free enzyme. It can be seen from Figure 6 that when the incubation temperature of free enzyme exceeds 40°C, its residual enzyme activity decreases significantly, only about 50%. The stability of the immobilized enzyme is relatively good, and the residual enzyme activity is 50% at 45°C. In general, the stability of the immobilized enzyme is improved. Metal affinity tag immobilization is a method to immobilize enzyme molecules on a carrier by forming coordination bonds between certain amino acids and transition metal ions (Fe2+, Co2+, Ni2+, etc.). The optimum temperature of immobilized enzymes is higher than that of free enzymes, which may be attributed to the decreased flexibility and increased rigidity of the enzyme after immobilization.
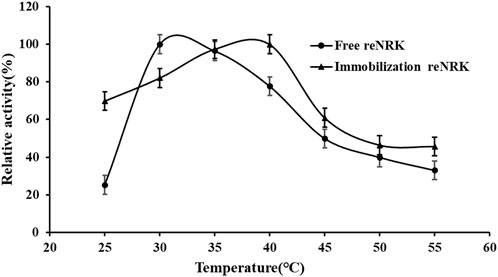
FIGURE 5. The optimal reaction temperature of the reScNRK1. The optimal reaction temperature of the reScNRK1 toward NR was determined under the standard assay conditions as above, except temperatures ranging from 25°C to 55°C.
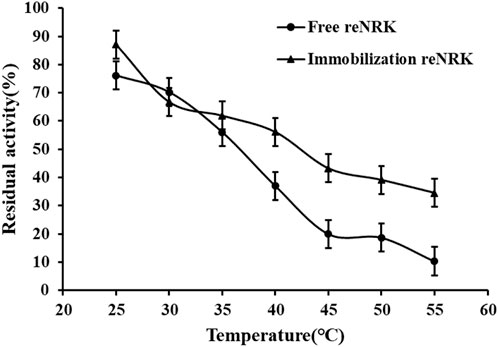
FIGURE 6. The temperature stability of the reScNRK1. The residual enzyme activity was measured at its optimal reaction temperature after being preincubated 1 h respectively.
pH characteristics of the reScNRK1
The optimum reaction pH value and pH stability of free and immobilization reScNRK1 were determined. The pH of buffer too high or too low may lead to changes in spatial structure of the enzyme, thus reducing its catalytic activity (Shortall et al., 2021). The results are shown in Figure 7 and Figure 8. It can be seen from Figure 7 that reScNRK1 is an acidophilic enzyme, and its optimal reaction pH is 5.8. When the pH is far from its optimum pH and within the acidic range, its catalytic activity decreases slightly. However, when the pH value of the system is alkaline, the catalytic activity of the recombinant enzyme will decrease significantly. Therefore, it is necessary to control the reaction system to be acidic during use. It can be seen from Figure 8 that reScNRK1 has good tolerance to acidic pH, and its residual enzyme activity can be greater than 90% under measurement conditions, but its catalytic activity is significantly reduced under alkaline conditions.
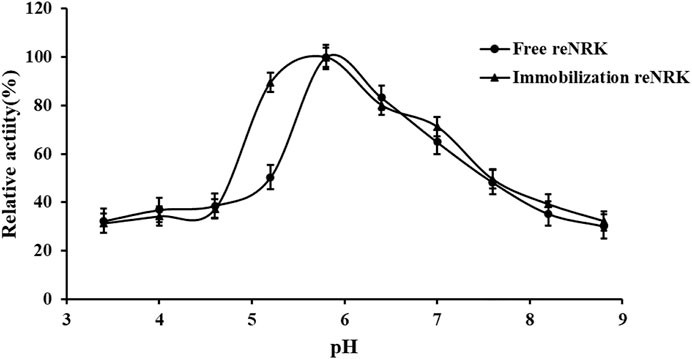
FIGURE 7. The pH optimum of the reScNRK1. The pH optimum of the r reScNRK1 was assayed by the standard activity assay method as stated above except the reaction pH values ranging from 3.4 to 8.8.
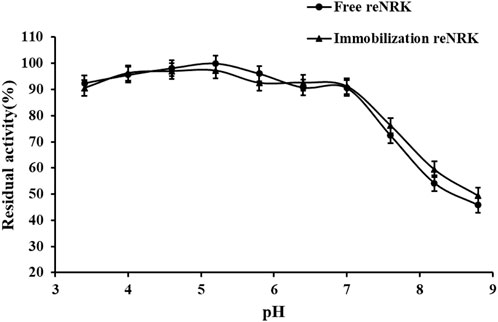
FIGURE 8. The pH stability of the reScNRK1. The residual activities were assayed under the optimal reaction temperatures and pH values after preincubating at 0°C for 1.0 h in varied pH values from 3.4 to 8.8.
Kinetic parameters of the reScNRK1
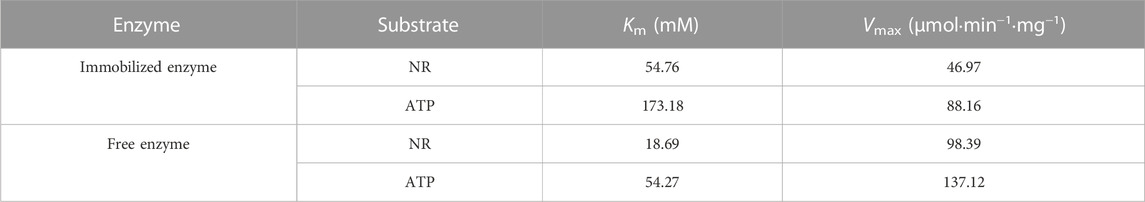
In order to determine the specificity of NR substrate, modify the system NR and ATP concentrations according to the literature (Dölle and Ziegler, 2009). The kinetic parameters of the free and immobilized reScNRK1 toward nicotinamide riboside (NR) and ATP were determined according to the above method, and the results are shown in Table1. The free reScNRK1 exhibited high catalytic efficiency and affinity toward nicotinamide riboside, its Km and Vmax values were 18.69 mM and 98.39 μmol⋅min−1⋅mg−1. Meanwhile, it exhibited high catalytic efficiency and affinity toward ATP, its Km and Vmax values were 54.27 mM and 137.12 μmol⋅min−1⋅mg−1, indicating that the reScNRK1 has great potential in the rapid removal of nicotinamide riboside and ATP. Compared with the free reScNRK1, the Km value of the immobilized enzyme increased significantly, indicating that the spatial barrier after immobilization reduced the affinity of the enzyme to the substrate.
Reusability of reconstituted reScNRK1
An important role of immobilized technology is that the enzyme can be reused. It can be seen from Figure 9 that the immobilized enzyme remains 80% active after 4 times of reuse, which is conducive to the recycling of enzyme and promotes industrial production. There are some disadvantages of metal affinity immobilization technology, such as tedious modification process of carriers and enzymes, and sometimes there are disadvantages such as easy dislodgement of metal ions, low selectivity and low affinity, which may be the reason of poor reusability. Factors affecting the adsorption and desorption of protein and Ni2+: 1) the adsorption and desorption of immobilized enzyme protein and metal Ni2+ are greatly influenced by the pH of the solution, usually the adsorption capacity is enhanced with the increase of the solution pH, because NRK1 is acidophilic enzyme and the reaction system is acidic, 2) the influence of ionic strength, at low salt concentration, the metal ions and protein mainly interact electrostatically, which will cause non-specific adsorption, but as the salt concentration increases, the positively and negatively charged salt ions cause a shielding effect between the protein and the metal ion. These two points may be the reason why the immobilized enzymes become less and less active in use.
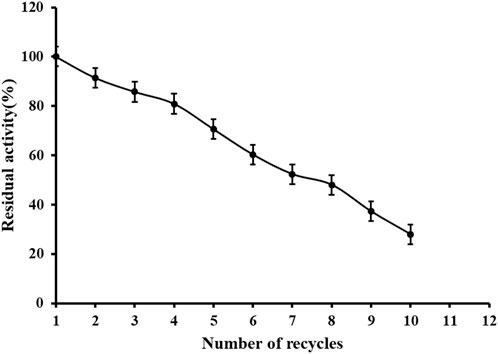
FIGURE 9. Reusability of immobilized reScNRK1. According to the above enzyme activity measurement method, repeatedly measure the activity of immobilized enzyme.
Conclusion
Nicotinamide mononucleotide is recognized as a nucleotide of NAD+ biosynthetic intermediate (Poddar et al., 2019), which is a research hotspot in food, cosmetics and health products. Genome mining technology can quickly transform hypothetical enzymes into real ones, providing more options for biocatalysis (Gong et al., 2013). In conclusion, in this study, nicotinamide ribokinase1 was successfully cloned from S. cerevisiae and solubilized for expression in E. coli, and the optimum temperature of the immobilized enzyme was increased and the temperature stability was improved. Immobilized nicotinamide riboside kinase1 can be recycled four times to maintain more than 80% activity, which implies a reduction in the cost contribution of the biocatalyst by reducing cost of the final product (Federsel et al., 2021). However, the number of times this can be recycled is small and the immobilization method will be improved subsequently.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
J-JH: conceptualization and writing original draft. X-XL: methodology and investigation. YL: investigation and data curation. ZW: investigation and software. H-LS: data curation and methodology. C-DT and L-GY: writing reviewing and editing. Y-CK: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
We are grateful to Prof. Jian-He Xu (East China University of Science and Technology) and Min-Chen Wu (Jiangnan University) for providing technical assistance. This paper was supported by the grants from Scientific and Technological Project of China (31900916), the Program for Science&Technology Innovation Talents in Universities of Henan Province (No. 21HASTIT041), Youth Talent Support Project of Henan Association for Science and Technology (No. 2021HYTP036), Henan Science and Technology Research Project (222102110260), the Key Scientific Research Projects of Higher Education Institutions in Henan Province (22A180026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Belenky, P., Racette, F. G., Bogan, K. L., McClure, J. M., Smith, J. S., and Brenner, C. (2007). Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell 129 (3), 473–484. doi:10.1016/j.cell.2007.03.024
Bieganowski, P., and Brenner, C. (2004). Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD + in fungi and humans. Cell 117 (4), 495–502. doi:10.1016/s0092-8674(04)00416-7
Das, A., Huang, G. X., Bonkowski, M. S., Longchamp, A., Li, C., Schultz, M. B., et al. (2019). Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell 176 (4), 944–945. doi:10.1016/j.cell.2019.01.026
Dölle, C., and Ziegler, M. (2009). Application of a coupled enzyme assay to characterize nicotinamide riboside kinases. Anal. Biochem. 385 (2), 377–379. doi:10.1016/j.ab.2008.10.033
Federsel, H. J., Moody, T. S., and Taylor, S. J. (2021). Recent trends in enzyme immobilization-concepts for expanding the biocatalysis toolbox. Molecules 26 (9), 2822. doi:10.3390/molecules26092822
Frederick, D. W., Loro, E., Liu, L., Davila, A., Chellappa, K., Silverman, I., et al. (2016). Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 24 (2), 269–282. doi:10.1016/j.cmet.2016.07.005
Gong, J. S., Lu, Z. M., Li, H., Zhou, Z. M., Shi, J. S., and Xu, Z. H. (2013). Metagenomic technology and genome mining: Emerging areas for exploring novel nitrilases. Appl. Microbiol. Biotechnol. 97 (15), 6603–6611. doi:10.1007/s00253-013-4932-8
Hachisuka, S. I., Sato, T., and Atomi, H. (2018). Hyperthermophilic archaeon thermococcus kodakarensis utilizes a four-step pathway for NAD(+) salvage through nicotinamide deamination. J. Bacteriol. 200 (11), 007855–e817. doi:10.1128/jb.00785-17
Harrison, D. E., Strong, R., Reifsnyder, P., Kumar, N., Fernandez, E., Flurkey, K., et al. (2021). 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell 20 (5), e13328. doi:10.1111/acel.13328
Hong, W., Mo, F., Zhang, Z., Huang, M., and Wei, X. (2020). Nicotinamide mononucleotide: A promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Front. Cell Dev. Biol. 8, 246. doi:10.3389/fcell.2020.00246
Huang, Z., Li, N., Yu, S., Zhang, W., Zhang, T., and Zhou, J. (2022). Systematic engineering of Escherichia coli for efficient production of nicotinamide mononucleotide from nicotinamide. ACS Synth. Biol. 11 (9), 2979–2988. doi:10.1021/acssynbio.2c00100
Jia, Y. Y., Xie, Y. L., Yang, L. L., Shi, H. L., Lu, Y. F., Zhang, S. P., et al. (2021). Expression of novel L-leucine dehydrogenase and high-level production of L-tert-leucine catalyzed by engineered Escherichia coli. Front. Bioeng. Biotechnol. 9, 655522. doi:10.3389/fbioe.2021.655522
Kalia, N. P., Shi Lee, B., Ab Rahman, N. B., Moraski, G. C., Miller, M. J., and Pethe, K. (2019). Carbon metabolism modulates the efficacy of drugs targeting the cytochrome bc1:aa3 in Mycobacterium tuberculosis. Sci. Rep. 9 (1), 8608. doi:10.1038/s41598-019-44887-9
Laemmli, U. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 (5259), 680–685. doi:10.1038/227680a0
Liu, Y., Yasawong, M., and Yu, B. (2021). Metabolic engineering of Escherichia coli for biosynthesis of beta-nicotinamide mononucleotide from nicotinamide. Microb. Biotechnol. 14 (6), 2581–2591. doi:10.1111/1751-7915.13901
Longo, V. D., and Kennedy, B. K. (2006). Sirtuins in aging and age-related disease. Cell 126 (2), 257–268. doi:10.1016/j.cell.2006.07.002
Ma, X. F., Yu, H. M., Wen, C., Luo, H., Li, Q., and Shen, Z. Y. (2009). Triple fusion of D-amino acid oxidase from Trigonopsis variabilis with polyhistidine and Vitreoscilla hemoglobin. World J. Microbiol. Biotechnol. 25 (8), 1353–1361. doi:10.1007/s11274-009-0022-6
Marinescu, G. C., Popescu, R. G., Stoian, G., and Dinischiotu, A. (2018). beta-nicotinamide mononucleotide (NMN) production in Escherichia coli. Sci. Rep. 8 (1), 12278. doi:10.1038/s41598-018-30792-0
Poddar, S. K., Sifat, A. E., Haque, S., Nahid, N. A., Chowdhury, S., and Mehedi, I. (2019). Nicotinamide mononucleotide: Exploration of diverse therapeutic applications of a potential molecule. Biomolecules 9 (1), 34. doi:10.3390/biom9010034
Qian, X. L., Dai, Y. S., Li, C. X., Pan, J., Xu, J. H., and Mu, B. (2022). Enzymatic synthesis of high-titer nicotinamide mononucleotide with a new nicotinamide riboside kinase and an efficient ATP regeneration system. Bioresour. Bioprocess. 9, 26. doi:10.1186/s40643-022-00514-6
Shoji, S., Yamaji, T., Makino, H., Ishii, J., and Kondo, A. (2021). Metabolic design for selective production of nicotinamide mononucleotide from glucose and nicotinamide. Metab. Eng. 65, 167–177. doi:10.1016/j.ymben.2020.11.008
Shortall, K., Djeghader, A., Magner, E., and Soulimane, T. (2021). Insights into aldehyde dehydrogenase enzymes: A structural perspective. Front. Mol. Biosci. 8, 659550. doi:10.3389/fmolb.2021.659550
Tang, C. D., Ding, P. J., Shi, H. L., Jia, Y. y., Zhou, M. z., Yu, H. l., et al. (2019). One-pot synthesis of phenylglyoxylic acid from racemic mandelic acids via cascade biocatalysis. J. Agric. Food Chem. 67 (10), 2946–2953. doi:10.1021/acs.jafc.8b07295
Tang, C. D., Li, X., Shi, H. L., Jia, Y. Y., Dong, Z. X., Jiao, Z. J., et al. (2020a). Efficient expression of novel glutamate decarboxylases and high level production of gamma-aminobutyric acid catalyzed by engineered Escherichia coli. Int. J. Biol. Macromol. 160, 372–379. doi:10.1016/j.ijbiomac.2020.05.195
Tang, C. D., Shi, H. L., Jia, Y. Y., Li, X., Wang, L. F., Xu, J. H., et al. (2020b). High level and enantioselective production of L-phenylglycine from racemic mandelic acid by engineered Escherichia coli using response surface methodology. Enzyme Microb. Technol. 136, 109513. doi:10.1016/j.enzmictec.2020.109513
Tang, C. D., Shi, H. L., Jiao, Z. J., Shi, H. F., Yao, L. G., Xu, J. H., et al. (2018a). Exploitation of cold-active cephalosporin C acylase by computer-aided directed evolution and its potential application in low-temperature biosynthesis of 7-aminocephalosporanic acid. J. Chem. Technol. Biotechnol. 93 (10), 2925–2930. doi:10.1002/jctb.5647
Tang, C. D., Shi, H. L., Tang, Q. H., Zhou, J. S., Yao, L. G., Jiao, Z. J., et al. (2016). Genome mining and motif truncation of glycoside hydrolase family 5 endo-beta-1,4-mannanase encoded by Aspergillus oryzae RIB40 for potential konjac flour hydrolysis or feed additive. Enzyme Microb. Technol. 93-94, 99–104. doi:10.1016/j.enzmictec.2016.08.003
Tang, C. D., Shi, H. L., Xu, J. H., Jiao, Z. J., Liu, F., Ding, P. J., et al. (2018b). Biosynthesis of phenylglyoxylic acid by LhDMDH, a novel d-mandelate dehydrogenase with high catalytic activity. J. Agric. Food Chem. 66 (11), 2805–2811. doi:10.1021/acs.jafc.7b05835
Tempel, W., Rabeh, W. M., Bogan, K. L., Belenky, P., Wojcik, M., Seidle, H. F., et al. (2007). Nicotinamide riboside kinase structures reveal new pathways to NAD+. PLoS Biol. 5 (10), e263. doi:10.1371/journal.pbio.0050263
Wang, H. Y., Xie, Y. L., Shi, X., Shi, H. L., Xu, J. H., Tang, C. D., et al. (2021). Directed evolution of a D-mandelate dehydrogenase toward D-o-chloromandelic acid and insight into the molecular basis for its catalytic performance. Biochem. Eng. J. 166, 107863. doi:10.1016/j.bej.2020.107863
Wang, X., Hu, X., Yang, Y., Takata, T., and Sakurai, T. (2016). Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 1643, 1–9. doi:10.1016/j.brainres.2016.04.060
Wu, J., and Filutowicz, M. (1999). Hexahistidine (His6)-tag dependent protein dimerization: A cautionary tale. Acta biochim. Pol. 46 (3), 591–599. doi:10.18388/abp.1999_4131
Yoshino, J., Baur, J. A., and Imai, S. i. (2018). NAD(+) intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 27 (3), 513–528. doi:10.1016/j.cmet.2017.11.002
Zhang, N., and Sauve, A. A. (2017). Synthesis of β-nicotinamide riboside using an efficient two-step methodology. Curr. Protoc. Nucleic Acid. Chem. 71, 14. doi:10.1002/cpnc.43
Keywords: nicotinamide nucleoside kinase, β -nicotinamide mononucleotide, expression, metal affinity tag immobilization, Saccharomyces cerevisiae
Citation: He J-J, Liu X-X, Li Y, Wang Z, Shi H-L, Kan Y-C, Yao L-G and Tang C-D (2023) High level expression of nicotinamide nucleoside kinase from Saccharomyces cerevisiae and its purification and immobilization by one-step method. Front. Bioeng. Biotechnol. 11:1134152. doi: 10.3389/fbioe.2023.1134152
Received: 30 December 2022; Accepted: 06 February 2023;
Published: 15 February 2023.
Edited by:
Yingjie Du, Tianjin University of Science and Technology, ChinaReviewed by:
Jie Cheng, Chengdu University, ChinaYanjun Jiang, Hebei University of Technology, China
Jun Ge, Tsinghua University, China
Copyright © 2023 He, Liu, Li, Wang, Shi, Kan, Yao and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Chao Kan, kanyunchao@163.com; Lun-Guang Yao, lunguangyao@163.com; Cun-Duo Tang, tcd530@126.com
 Jian-Ju He1,2
Jian-Ju He1,2  Hong-Ling Shi
Hong-Ling Shi Lun-Guang Yao
Lun-Guang Yao Cun-Duo Tang
Cun-Duo Tang