Effects of Rederivation by Embryo Vitrification on Performance in a Rabbit Paternal Line
- 1Facultad de Zootecnia, Universidad Nacional Agraria de la Selva, Tingo María, Peru
- 2Instituto de Ciencia y Tecnología Animal, Universitat Politècnica de València, Valencia, Spain
Embryo cryopreservation is a valuable tool for maintaining genetic variability and preserving breeds and lines, allowing to assess the response to selection and enabling genetic diffusion. This study aimed to evaluate the impact of rederivation by embryo vitrification and transfer procedures on the growth and reproductive traits in a paternal rabbit line selected for average daily gain from weaning (28 days old) to fattening (63 days old). The rederived population was bred over two generations at the same time as a control population of this paternal line and, growth trait parameters (weights at weaning, end of the fattening period, and average daily gain) and reproductive performance (kindling rate, litter size at birth and at weaning) were compared with three filial generations. Moreover, fetal growth and litter size components were assessed for the second generation by ultrasonography and laparoscopy. Differences in postnatal growth traits (end of fattening weight and average daily gain) were observed in the three generations assessed. However, fetal growth, litter size components, and reproductive traits did not show significant differences. In conclusion, cryopreservation and embryo transfer processes cause changes in growth traits of reconstituted populations that influence the following generations, without changes in reproductive traits in a paternal line of rabbits.
Introduction
Estimation of response to selection in rabbit-breeding programs has been extensively described, and one alternative is the comparison between two different generations by rederivation of a control population cryopreserved generations ago (Khalil and Al-Saef, 2008), which could be stored for many years, achieving reasonable pregnancy rates and survival at birth (Lavara et al., 2011; Yao et al., 2012; Marco-Jiménez et al., 2018). The implementation of reproductive biotechnologies such as cryopreservation and embryo transfer in rabbits has been used in our research group for approximately three decades (Vicente and García-Ximénez, 1993; García-Dominguez et al., 2019). Vitrifying has proven to be one of the most cost-effective and optimal freezing technologies for rabbit embryos, achieving high-survival rates at birth and high-parturition rates (Kasai et al., 1992; Vicente and García-Ximenez, 1994), allowing the creation of an embryo bank (Vicente et al., 2003).
Rederivation of a control population has been used to estimate the response to selection in both maternal (García and Baselga, 2002a,b) and paternal lines (Piles and Blasco, 2003; Juárez et al., 2020a,b; Peiró et al., 2021). In some cases, without taking into account that the rederivation process has phenotypic effects on the growth of the fetus and newborn during the prenatal (Mocé et al., 2010; Saenz-de-Juano et al., 2014), and in the postnatal period in organ weight, such as the liver (Lavara et al., 2015; García-Dominguez et al., 2020a,b). Moreover, Lavara et al. (2014) observed effects on reproductive traits such as litter size in the derived females (F1) and transgenerational effects on the female offspring (F2).
These phenotypic changes suggested that embryo cryopreservation is not neutral. Embryo cryopreservation itself presents specific issues, embryos are exposed to osmotic, chemical, and temperature stress, to which they have to respond. Consequently, developmental alterations emerge in the embryo, fetus, and postnatal life of vitrified-transferred embryos. The recent studies have demonstrated that vitrified-transferred embryos could respond to stressful procedures by altering gene expression or even incorporating lasting epigenetic marks with effects on the epigenome, transcriptome, proteome, and metabolome of adult rabbits (García-Dominguez et al., 2018, 2020a,b,c; García-Domínguez et al., 2021; Marco-Jiménez et al., 2020). These last studies provided evidence that embryo cryopreservation induced an adaptive response resulting in phenotypic plasticity without negative consequences on health. It has been observed that this adaptive response was still present in later generations through changes in organs such as the liver and heart or bodyweight; moreover, the liver epigenetic and metabolome variations have also been found (García-Dominguez et al., 2020a,b,c; García-Domínguez et al., 2021).
In mice, vitrification and slow freezing of embryos has been shown to increase the expression of genes associated with fat mass but did not alter physiological development, movement coordination function, or brain development, even though mice become heavier at 8 weeks of age (Wang et al., 2020). This could be associated with significant dysfunction of glucose metabolism in the liver due to increased insulin resistance reported in male mice (Qin et al., 2021). Even in humans, the freezing process might cause singletons to be born with a greater risk of high birth weight and larger gestational age than singletons after fresh embryo transfer and naturally conceived (Spijkers et al., 2017).
This study aimed to evaluate the impact of embryo vitrification and transfer procedures on the growth and reproductive traits of a paternal rabbit line selected for average daily gain from weaning (28 days old) to fattening (63 days old).
Materials and Methods
Before the experimental phase began, all the animal experimentation protocols used were reviewed and approved by the Ethics and Animal Welfare Committee of the Universitat Politècnica de València (Code Number 2015/VSC/PEA/00061). Likewise, all the experiments followed the guidelines and regulations set forth in Directive 2010/63/EU/EEC. The facilities and cages used were adequate for experimentation on this species (Code: ES462500001091).
Animals and Experimental Design
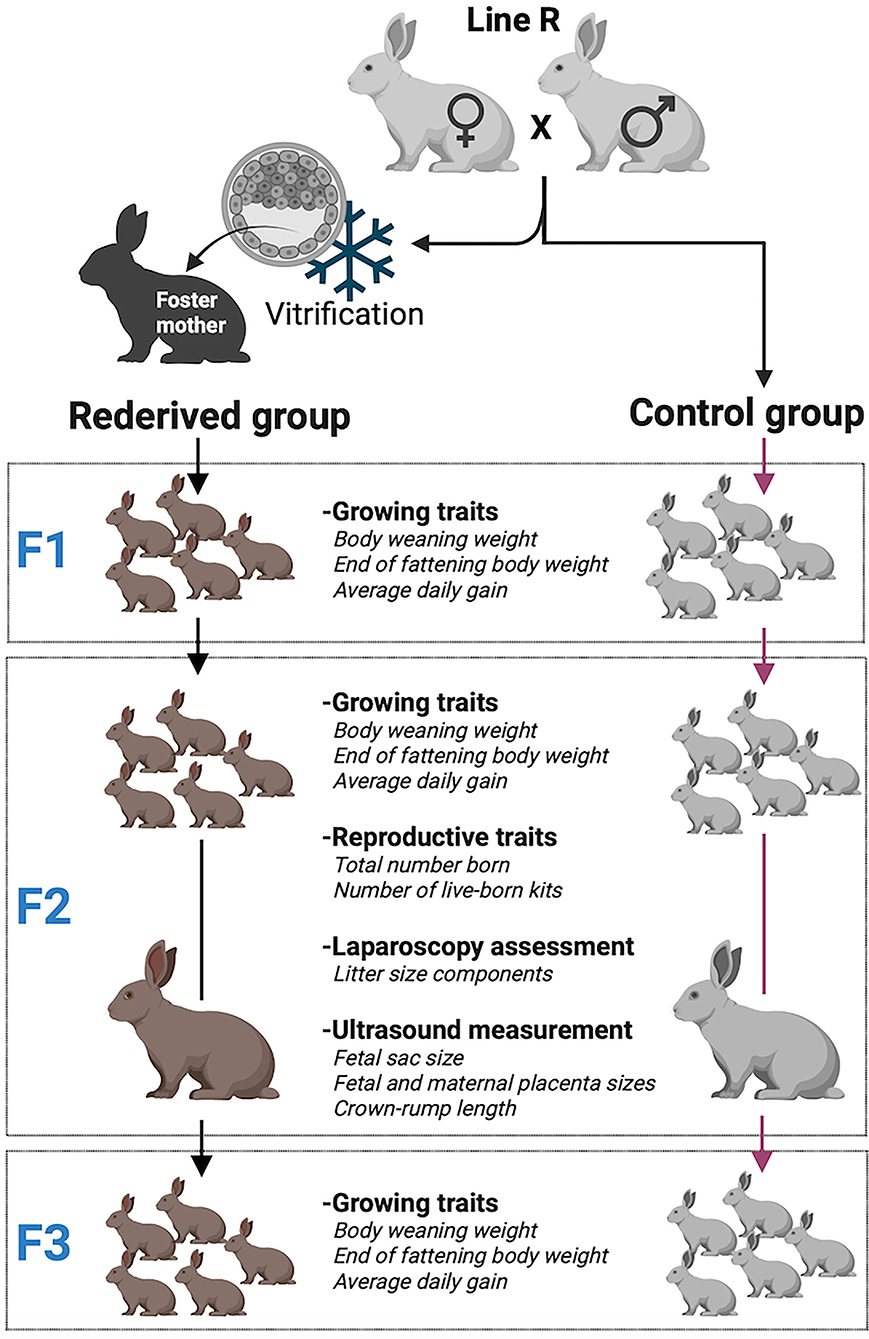
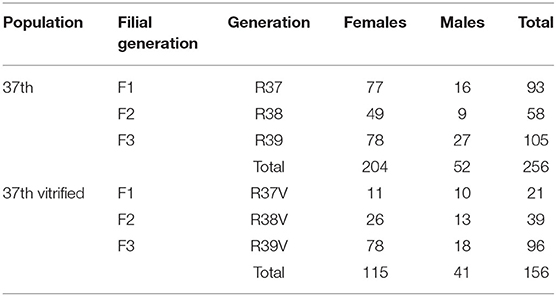
A paternal rabbit line (R) selected at the Universitat Politècnica de Valencia was used. This line was founded in 1989 from two closed paternal lines (Estany et al., 1992). Since then, the line has been selected for individual weight daily gain from 28 days (weaning) to 63 days old (end of fattening). The rederivation procedure of the population was described by Marco-Jiménez et al. (2018) from embryos of 28 donors belonging to 15 different sire families of the 36th generation. Sire families were established to avoid matings between relatives sharing a grandparent. The control population was the offspring of the 36th generation born by artificial insemination at the same time as the rederived population. Offspring from both populations were bred over two generations. The offspring of vitrified embryos were named rederived-F1 and inseminated conceived named control-F1 and the following filial generations (rederived-F2 and F3 and control-F2 and F3, Graphical Abstract and Table 1).
The animals were housed at the Universitat Politècnica de València experimental farm in flat deck indoor cages (75 × 50 × 30 cm), with free access to water and commercial pelleted diets (Cunilap, NANTA S.A., Spain, minimum of 15 g of crude protein per kg of dry matter (DM), 15 g of crude fiber per kg of DM, and 10.2 MJ of digestible energy (DE) per kg of DM). The photoperiod was 16 h of light and 8 h of dark, with a regulated room temperature between 14 and 28°C.
Growing Traits Analysis
Individual weaning weight (WW, 28 days old), individual weight at the end of the fattening period (EFW, 63 days old), and average daily weight gain (weight gained from day 28 to 63 divided by 33, ADG) during the fattening period were recorded for all the generations.
Female Reproductive Management
Reproductive management of both populations (rederived and control) and their offspring avoided mating among relatives with common grandparents, achieving non-overlapping generations. The first reproductive cycle took place at ~5 months of age, and after kindling, the new insemination was tried 10–12 days later. Briefly, two ejaculates per male were collected in each replica using an artificial vagina. Only ejaculates with total motility higher than 70% and <30% of the abnormal sperm were used. After semen evaluation, optimal ejaculates from each male were extended to 40 million/ml. All the females were synchronized by intramuscular injection with 15UI eCG (Cuniser, HIPRA S.A., Spain) 48 h before being inseminated with 0.5 ml of extended semen using a curved plastic pipette. Females were induced to ovulate by intramuscular injection of 1 μg of buserelin acetate (Suprefact, Hoechst Marion Roussel, S.A., Spain) at insemination time. Pregnancy was checked at 14 days from insemination and non-pregnant does were inseminated again at 21 days after the previous insemination. In addition, it was noted whether rabbits underwent a lactation–gestation overlap, and the dams were classified into four groups, according to the reproductive status: offspring from primiparous does without overlapping (PD), offspring from primiparous lactating does (females that were pregnant while suckling their first litter, PLD), multiparous lactating does (females with more than one parturition that were pregnant while suckling their litter, MLD) and multiparous non-lactating does (females from more than one parturition that were pregnant after lactation, MD). Total litter size, liveborn, and litter size at weaning were recorded for each female.
Evaluation of Litter Size Components
A total of 139 laparoscopies were carried out on females from the fourth and fifth parity (80 does from control-F2 and 59 does from rederived-F2). Females were sedated according to the procedure described by Juárez et al. (2021). In brief, the females were sedated with an intramuscular injection of 5 mg xylazine/kg (Rompun, Bayer AG, Leverkusen, Germany) and 3 mg/kg morphine chloride. Five minutes later, 35 mg/kg of ketamine hydrochloride (Imalgene, Merial, S.A., France) was administered intravenously. After laparoscopy, does were treated with antibiotics (200,000 IU procaine penicillin and 250 mg streptomycin, Duphapen Strep, Pfizer, S.L., Spain), 0.03 mg/kg of buprenorphine hydrochloride every 12 h and 0.2 mg/kg of meloxicam every 24 h for 3 days. The number of corpora lutea, the number of implanted embryos at 12 days (IE), and litter size at birth (LS) were recorded per female. The following variables were calculated using the aforementioned data. Ovulation rate (OR), defined as the number of corpora lutea, embryonic loss rate (ELR), estimated as (OR–IE)/OR, and fetal loss rate (FLR), estimated as (IE–LS)/IE.
Fetal Growth and Ultrasound Measurement
A total of twenty-nine pregnant does from laparoscopized females (13 from control-F2 and 16 from rederived-F2) were examined on days 12, 19, and 26 of gestation using a portable color Doppler ultrasound device (Esaote, Spain) with a 7.5 MHz linear probe (4–12 MHz range). Does were sedated according to the procedure described aforementioned and placed in a polystyrene cage where they were prevented from moving. The ultrasound examination was performed according to the procedure described by Juárez et al. (2021). After localization of different fetal sacs, 4–6 whole fetal sacs were examined per doe. The identifiable structures (fetal sac, fetus, and fetal and maternal placenta) were measured from the frozen frame pictures on the monitor, using the Esaote 16 ultrasound software.
Statistical Analyses
Growth Traits
The first analysis of growth traits was carried out to assess the effects of cryopreservation procedures on the reconstituted population:
where Yijklm was the trait to analyze, μ was the general mean, Pi was the fixed effect of rederivation (control population vs. rederived), Rj was the fixed effect of reproductive status of the doe used in the WW analysis (PD, PLD, MLD, and MD), MYk was the fixed effect of month-year in which the fattening period ended (39 levels), PRij was the effect of interaction between rederived population and reproductive status of the mothers used for WW analysis, COl was the random effect of common litter, Cov Xm was the covariate of the number born alive (BA) used for the WW trait or the covariate of WW used for weight at the end of the fattening period (EFW) and ADG traits and eijklm was the error term of the model.
A second analysis of growth traits to evaluate the effect of rederivation in each filial generation (F1, F2, and F3: control vs. rederived) was performed using the mixed linear model described aforementioned:
where Pi was the fixed effect of filial generation (control-F1 and rederived-F1 or control-F2 and rederived-F2 or control-F3 and rederived-F3). In the WW analysis of the first filial generation, the fixed effect month-year (MKk) had 33 levels, for the filial generation 2 had 31 levels and filial generation 3, had 32 levels.
Reproductive Traits
The general linear model was used to assess reproductive performance, including as fixed effects the rederived generation group (Pi, control-F2 and rederived-F2), reproductive status of does (Rj, nulliparous, primiparous lactating, multiparous lactating, and non-lactating does), month-year in which insemination was done (MKk, 9 levels) and the interaction between generation group and reproductive status of the mothers (PRij).
Litter size components (ovulation rate, implanted embryos, litter size, liveborn, and rates of the embryo and fetal losses) were analyzed by a generalized linear model, including as fixed effects the rederived generation group (Pi, control-F2 and rederived-F2) and lactating or non-lactating status (Lj) and their interactions (PLij).
To analyze fetal sac area, CRL of the fetus, fetal and maternal placenta areas at days 12, 19, and 26 of gestation, and the weight of liveborn kits, a mixed linear model was used:
where Yijkl was the trait to analyze, μ was the general media, Pi was the fixed effect of the rederived generation group (control-F2 and rederived-F2), Rj was the fixed effect of reproductive status of the doe used for analysis of weaning weight (lactating and non-lactating doe); PRij was the effect of interaction between rederived population and reproductive status of the mothers used to analyze the weaning weight, COk was the random effect of common litter, Cov Xl was the covariate of the number of implanted embryos and eijklm was the error term of the model. Values were considered statistically different at P < 0.05. Results were reported as least-square means with a standard error of the mean (SEM). All the analyses were performed with SPSS 26.0 software package (SPSS Inc., Chicago, IL, United States, 2012).
Results
Growing Traits
Growing Traits and Rederived Effect
Cryopreservation and embryo transfer procedure significantly affected EFW and ADG, but not for WW (Table 2). However, reproductive status of does affected WW, showing that young and adult mothers without lactation–gestation overlap (ND and MD, respectively) had the lowest WW (0.67 ± 0.01 and 0.68 ± 0.01 vs. 0.78 ± 0.02 and 0.78 ± 0.01 kg to ND, MD, PLD, and MLD, respectively, P < 0.05, data not shown in tables). MY fixed and common litter random effects were significant for all analyses.
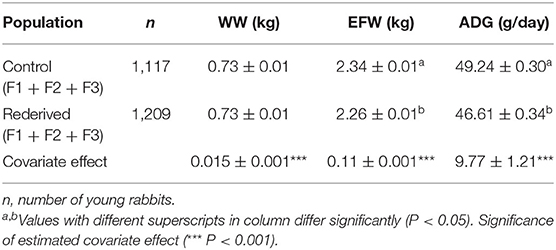
Table 2. Effect of rederivation by embryo cryopreservation on weaning weight (WW, kg), weight at end of fattening (EFW, kg), and average daily gain (ADG, g/day) in the paternal rabbit line.
Evolution of Growth Traits by Filial Generation
Differences in WW were greater for the control-F1 than rederived-F1, but in subsequent generations were greater for offspring of the rederived population (F2 and F3) than control (F2 and F3). Otherwise, for EFW and ADG, in the first generation (F1) the rederived population had higher values, but in the following generations (F2 and F3), it was greater to the control population in both the traits (Figure 1).
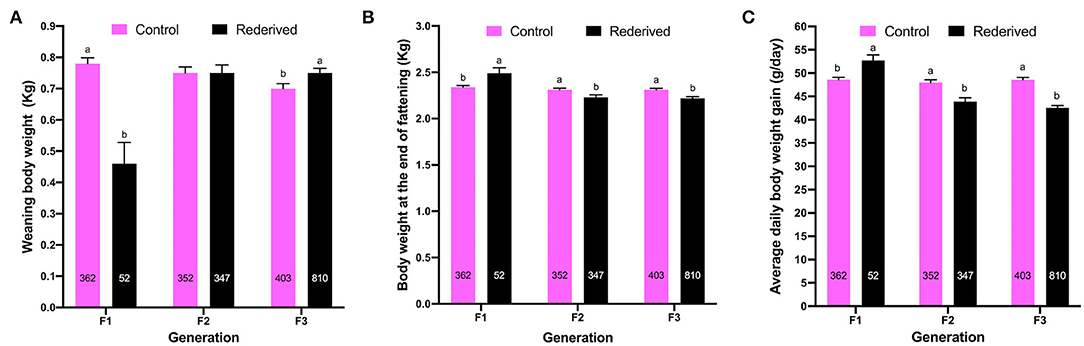
Figure 1. Effect of rederivation by embryo cryopreservation on: (A) weaning weight (WW, kg); (B) weight at end of fattening (EFW, kg) and (C) average daily gain (ADG, g/day) from filial generations (F1, F2, and F3). The number of rabbit for each population and filial generation is noted in columns. a,b Values in each treatment and filial generation with different superscripts are statistically different (P < 0.05).
Reproductive Traits
Reproductive Performance of F2 Females
No differences in litter size at birth and at weaning, perinatal and lactation mortalities, and kindling interval were found between the populations assessed (control-F2 and rederived-F2, Table 3). Likewise, no significant differences in the reproductive status of does were registered, except for the kindling interval, registering a higher KI in multiparous non-lactating does (MD), almost twice the interval (Table 3).
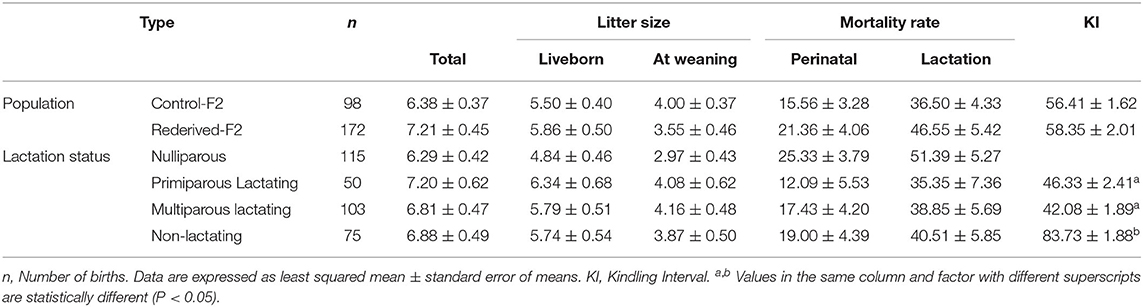
Table 3. Effect of rederivation by embryo cryopreservation on total litter size, liveborn, litter size at weaning, perinatal, and lactation mortality rates of F2 females.
Similarly, no effects of interaction between population and reproductive status of doe were reported for all the reproductive parameters evaluated.
Litter Size Components of F2 Females
In all the litter size components assessed, no significant differences were found between populations (control-F2 and rederived-F2) or between lactation status at insemination, and the interaction between generation and lactation status of the doe was not significantly different (Table 4).
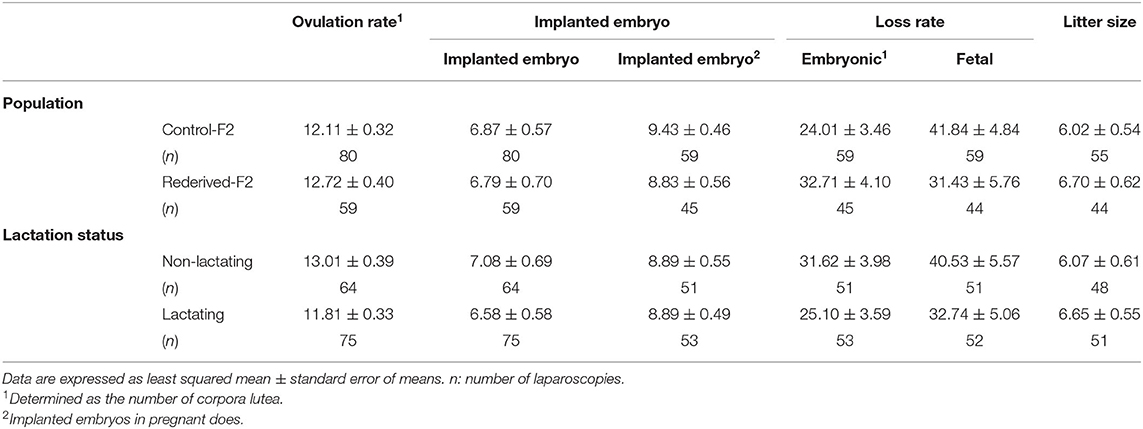
Table 4. Effect of rederivation by embryo cryopreservation on the litter size components of F2 females.
Fetal Growth During Gestation in F2 Females
No differences were observed in the size of fetal sac, maternal placenta, fetal placenta, and CRL of the fetus on any day of gestation assessed between both populations (Figure 2). Differences in the fetal sac size were observed relative to the lactational status of the doe only at 12 days of gestation, being larger in the non-lactating does. The bodyweight of the liveborn kits was no different between populations and was not affected by reproductive status (66.5 ± 2.43, data not shown in tables).
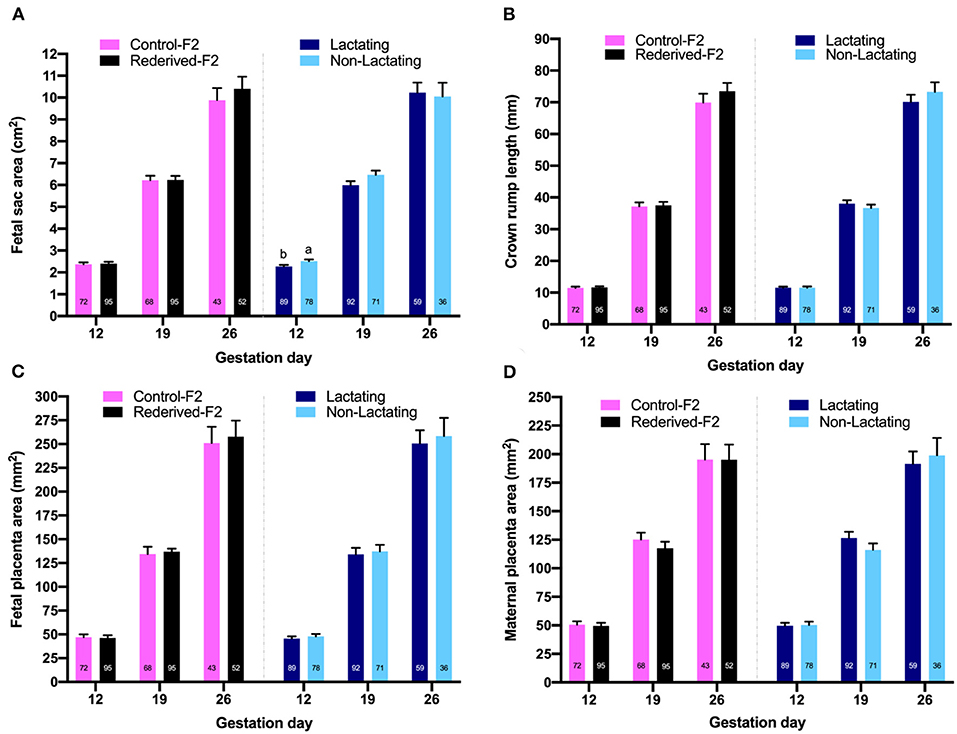
Figure 2. Effect of rederivation by embryo cryopreservation on fetal growth at 12, 19, and 26 days of gestation from a paternal rabbit line. The number of fetal traits measured is noted in columns. (A) Fetal sac area (cm2); (B) crown-rump length of fetuses (mm); (C) fetal placenta area (mm2); (D) maternal placenta area (mm2). a,b Values in each trait and age with different superscripts are statistically different (P < 0.05).
Discussion
Growth Traits
The selection objective of paternal line R is the average daily gain at fattening (Estany et al., 1992). ADG has been estimated to increase from 0.45 to 1.23 g/d per generation (Baselga, 2005), having a moderate heritability (0.17) ideal for the improvement. These estimates could be refined using an unselected, divergently selected, or a population rederived by cryopreservation as control. The latter offers a more economical tool and avoids the genetic drift of the population. However, recently, there are more and more studies showing genetic and phenotypic effects in rederivative populations that could question their use as control populations. Our study shows effects on the end of fattening weight (EFW) and average daily gain (ADG), which indicates some effect derived from the cryopreservation and transfer process performed. Both populations were coetaneous, received the same feed, and were placed at the same facilities, avoiding the environmental effect on traits under evaluation. Differences in each trait become more evident when each generation is compared, showing that growth traits are affected in all the generations. Direct or short-term effects of embryo manipulation (production, cryopreservation, and transfer) have been reported in the previous studies (Vicente et al., 2013; Saenz-de-Juano et al., 2014, 2015; Lavara et al., 2015) and by other authors in other animal species (Leme et al., 2020; López-Damián et al., 2020; Cuello et al., 2021), and even humans (Barsky et al., 2016; Beyer and Griesinger, 2016; Chen et al., 2020; Heber and Ptak, 2021), which may be involving epigenetic changes at the embryonic level (Chatterjee et al., 2017) that could cause post-implantation losses, changes in fetal and maternal placental tissues, and higher birth weight in rabbits (Saenz-de-Juano et al., 2014, 2015). Long-term (transgenerational) effects have also been reported in maternal lines (selected for litter size, Lavara et al., 2014; García-Dominguez et al., 2020d) and recently in the paternal lines (selected for growth rate, García-Dominguez et al., 2020c), where growth traits are affected more than the reproductive traits.
Differences observed from the first generation (F1) were significant in the three growth traits assessed (WW, EFW, and ADG). A higher EFW achieved by the rederived population has been previously reported associated with disturbances in the liver and adrenal gland size and lipid and fatty acid metabolism (García-Dominguez et al., 2020b; Marco-Jiménez et al., 2020). Direct effects could be due to the stress to which the embryos are subjected during recovery, vitrification, warming and transfer, in addition to the maternal effects derived from the host rabbits. Thus, in F1 WW was higher for the control population, while for EFW and ADG it was higher for the rederived population. This differentiated and lower growth before weaning could be related to the metabolism of Zn and Fe, which has been reduced in children born from ART (Xia et al., 2019), and which could influence the lower weight achieved at weaning by the rederived-F1 population, but apparently, these differences are reversed at the end of fattening where this population has the highest results. These disturbances should be taken into account when selecting animals from populations rederived by vitrification to be used as a control group, even in the subsequent generations.
It was demonstrated that cryopreservation and embryo transfer processes in lines selected for daily weight gain cause changes in the reconstituted population in weaning weight that stabilize in the following generations, but the changes in pubertal and adult weight, and liver and heart weight, are maintained, and also in some seminal characteristics and changes in the transcriptome and metabolome profile of the liver of adult rabbits (García-Dominguez et al., 2020e). These findings would indicate that the change caused in the first generation influences the following generations in the characteristics studied (WW, EFW, and ADG), causing higher WW, lower EFW, and therefore lower ADG in the descendants of the rederived population.
Reproductive Traits
Reproductive traits, such as litter size and live births, seem not to be influenced by the effects of cryopreservation either by vitrification or by slow freezing, but by the seasons and the physiological state of the dam (Cifre et al., 1996, 1999). However, Lavara et al. (2014) showed that cryopreservation and embryo transfer increased litter size, live births, and postnatal survival in the cryopreservation-born females (F1) and their female offspring (F2), whereas, in our study, no differences are observed in any of the reproductive traits examined in F2 females; differences are only reported based on the lactation status of does, being higher in non-lactating does. These discordant results could be due to the genotype of the populations studied.
Similarly, fetal sac size was smaller in lactating rabbits than in non-lactating rabbits at 12 days of gestation, but as gestation progresses these differences disappear. As in primiparous rabbits, lactation could be detrimental to receptivity, conception and ovulation rate, embryo and fetal survival, and fetal growth (Fortun-Lamothe and Prunier, 1999), possibly due to the nutritional deficit that occurs during lactation, which induces competition between the mammary glands and the pregnant uterus for the supply of nutrients (Fortun-Lamothe et al., 1999).
The effects of vitrification and embryo transfer processes may not affect the reproductive characteristics evaluated in this line because they showed lower reproductive performance than maternal lines (Baselga, 2002; García and Baselga, 2002b; Vicente et al., 2003, 2013; Naturil-Alfonso et al., 2015).
Embryo cryopreservation in rabbits has been used to estimate genetic gain by rederivation of the frozen populations from previous generations (García and Baselga, 2002a,b; Pascual et al., 2008), either in maternal lines selected for their reproductive traits or in paternal lines selected for their growth traits. It has also been found to be a valuable technique to re-establish populations cryopreserved 15 years ago (Marco-Jiménez et al., 2018). Likewise, the transgenerational effect on some reproductive traits have been noted (Lavara et al., 2014), as has the effect on birth weight, transcriptomic and metabolomic changes, and liver size of offspring of individuals born by these reproductive techniques (García-Dominguez et al., 2020e). However, our work shows for the first time the impact of these biotechnologies on the traits under selection in the paternal lines without detriment or changes in their reproductive traits two generations later, evidencing possible transgenerational epigenetic changes, suggesting that when estimating genetic gain using former generations cryopreserved years ago, this should be done with a current population subjected to the cryopreservation and embryo transfer processes.
Conclusions
Embryo vitrification and embryo transfer processes cause changes in growth traits of reconstituted populations that influence the following generations, without changes in female reproductive traits in a paternal line of rabbits. This finding should be taken into account when these populations are used as controls to estimate genetic changes in these characters. A possible solution would be a double control, rederiving the contemporary population with the same procedure.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Ethics and Animal Welfare Committee of the Universitat Politècnica de València (Code Number, 2015/VSC/PEA/00061).
Author Contributions
FM-J and JV conceptualized, designed, and obtained the financial support for this study. JD, FM-J, and JV participated in data collection and analysis and developed and discussed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by AGL2017-85162-C2-1-R research project funded by the Ministry of Economy, Industry and Competitiveness (MICINN, Spain).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
N. Macowan English Language Service revised the English text version.
References
Barsky, M., St. Marie, P., Rahil, T., Markenson, G. R., and Sites, C. K. (2016). Are perinatal outcomes affected by blastocyst vitrification and warming? Am. J. Obstetr. Gynecol. 215, 603.e1–603.e5. doi: 10.1016/j.ajog.2016.06.002
Baselga, M.. (2002). Line R (Spain). In: Rabbit Genetic Resources in Mediterranean Countries. Options Méditerranéennes: Série B, Etudes et Recherches; n. 38, eds M. H. Khalil and M. Baselga (Marseille: CIHEAM), 257–262. Available online at: http://om.ciheam.org/om/pdf/b38/02600029.pdf (accessed April 05, 2022).
Baselga, M.. (2005). Genetic improvement of meat rabbits. programmes and diffusion. In: Proc. 8th World Rabbit Congress, 7–10 September, 2004 (Puebla, Mexico), 1–13.
Beyer, D. A., and Griesinger, G. (2016). Vitrified-warmed embryo transfer is associated with mean higher singleton birth weight compared to fresh embryo transfer. Eur. J. Obstetr. Gynecol. Reprod. Biol. 203, 104–107. doi: 10.1016/j.ejogrb.2016.05.041
Chatterjee, A., Saha, D., Niemann, H., Gryshkov, O., Glasmacher, B., and Hofmann, N. (2017). Effects of cryopreservation on the epigenetic profile of cells. Cryobiology 74, 1–7. doi: 10.1016/j.cryobiol.2016.12.002
Chen, L., Ni, X., Xu, Z., Fang, J., Zhang, N., and Li, D. (2020). Effect of frozen and fresh embryo transfers on the birthweight of live-born twins. Eur. J. Obstetr. Gynecol. Reprod. Biol. 246, 50–54. doi: 10.1016/j.ejogrb.2020.01.008
Cifre, J., Baselga, M., García, M. L., and Gómez, A. (1999). Effect of embryo cryopreservation techniques on reproductive and growth traits in rabbits. Ann. Zootech. 48, 15–24.
Cifre, J., Baselga, M., García-Ximenez, F., and Vicente, J. S. (1996). A study of reproductive and growth traits of a maternal rabbit line founded by selection of hyperprolific does. In: Proc. 6th World Rabbit Congress, 9–10 July, 1996, vol 2 (Toulouse, France), 265–268.
Cuello, C., Martinez, C. A., Cambra, J. M., Parrilla, I., Rodriguez-Martinez, H., Gil, M. A., et al. (2021). Effects of vitrification on the blastocyst gene expression profile in a porcine model. Int. J. Mol. Sci. 22, 1222. doi: 10.3390/ijms22031222
Estany, J., Baselga, M., and Blasco, A. (1992). Selection response of growth rate in rabbits for meat production. Genet. Select. Evol. 24, 527–537. doi: 10.1186/1297-9686-24-6-527
Fortun-Lamothe, L., and Prunier, A. (1999). Effects of lactation, energetic deficit and remating interval on reproductive performance of primiparous rabbit does. Anim. Reprod. Sci. 55, 289–298. doi: 10.1016/S0378-4320(99)00020-2
Fortun-Lamothe, L., Prunier, A., Bolet, G., and Lebas, F. (1999). Physiological mechanisms involved in the effects of concurrent pregnancy and lactation on fetal growth and mortality in the rabbit. Livest. Prod. Sci. 60, 229–241. doi: 10.1016/S0301-6226(99)00096-2
García, M. L., and Baselga, M. (2002a). Estimation of genetic response to selection in litter size of rabbits using a cryopreserved control population. Livest. Prod. Sci. 74, 45–53. doi: 10.1016/S0301-6226(01)00280-9
García, M. L., and Baselga, M. (2002b). Estimation of correlated response on growth traits to selection in litter size of rabbits using a cryopreserved control population and genetic trends. Livest. Prod. Sci. 78, 91–98. doi: 10.1016/S0301-6226(02)00093-3
García-Dominguez, X., Diretto, G., Frusciante, S., Vicente, J. S., and Marco-Jiménez, F. (2020e). Metabolomic analysis reveals changes in preimplantation embryos following fresh or vitrified transfer. Int. J. Mol. Sci. 21, 7116. doi: 10.3390/ijms21197116
García-Dominguez, X., Diretto, G., Peñaranda, D. S., Frusciante, S., García-Carpintero, V., Cañizares, J., et al. (2021). Early embryo exposure to assisted reproductive manipulation induced subtle changes in liver epigenetics with no apparent negative health consequences in rabbit. Int. J. Mol. Sci. 22, 9716. doi: 10.3390/ijms22189716
García-Dominguez, X., Marco-Jiménez, F., Peñaranda, D. S., Diretto, G., García-Carpintero, V., Cañizares, J., et al. (2020c). Long-term and transgenerational phenotypic, transcriptional and metabolic effects in rabbit males born following vitrified embryo transfer. Sci. Rep. 10, 11313. doi: 10.1038/s41598-020-68195-9
García-Dominguez, X., Marco-Jiménez, F., Peñaranda, D. S., and Vicente, J. S. (2020b). Long-term phenotypic and proteomic changes following vitrified embryo transfer in the rabbit model. Animals 10, 1043. doi: 10.3390/ani10061043
García-Dominguez, X., Marco-Jimenez, F., Viudes-de-Castro, M. P., and Vicente, J. S. (2019). Minimally invasive embryo transfer and embryo vitrification at the optimal embryo stage in rabbit model. J. Visual. Exp. 147, e58055. doi: 10.3791/58055
García-Dominguez, X., Peñaranda, D. S., Borrás-Pérez, S., Vicente, J. S., and Marco-Jiménez, F. (2018). Embryo vitrificaction modify the proteomic profile of adult rabbit liver, and it persists across generations. Cryobiology 85:166. doi: 10.1016/j.cryobiol.2018.10.181
García-Dominguez, X., Vicente, J. S., and Marco-Jiménez, F. (2020a). Developmental plasticity in response to embryo cryopreservation: The importance of the vitrification device in rabbits. Animals 10, 804. doi: 10.3390/ani10050804
García-Dominguez, X., Vicente, J. S., Viudes-de-Castro, M. P., and Marco-Jiménez, F. (2020d). Long-term effects following fresh/vitrified embryo transfer are transmitted by paternal germline in a large size rabbit cohort. Animals 10, 1272. doi: 10.3390/ani10081272
Heber, M. F., and Ptak, E. (2021). The effects of assisted reproduction technologies on metabolic health and disease. Biol. Reprod. 104, 734–744. doi: 10.1093/biolre/ioaa224
Juárez, J. D., Marco-Jiménez, F., Lavara, R., and Vicente, J. S. (2020b). Rederivation by Cryopreservation of a paternal line of rabbits suggests exhaustion of selection for post-weaning daily weight gain after 37 generations. Animals 10, 1436. doi: 10.3390/ani10081436
Juárez, J. D., Marco-Jiménez, F., Talaván, A. M., García-Domínguez, X., Viudes-de-Castro, M. P., Lavara, R., et al. (2020a). Evaluation by re-derivation of a paternal line after 18 generations on seminal traits, proteome and fertility. Livest. Sci. 232, 103894. doi: 10.1016/j.livsci.2019.103894
Juárez, J. D., Marco-Jiménez, F., and Vicente, J. S. (2021). Evaluation of fetal growth, litter size and reproductive performance in rabbit after 18 generations of selection for growth rate using cryopreserved embryos. Livest. Sci. 253, 104702. doi: 10.1016/j.livsci.2021.104702
Kasai, M., Hamaguchi, Y., Zhu, SE., Miyake, T., Sakurai, T., and Machida, T. (1992). High survival of rabbit morulae after vitrification in an ethylene glycol-based solution by a simple method. Biol. Reprod. 46, 1042–1046. doi: 10.1095/biolreprod46.6.1042
Khalil, M. H., and Al-Saef, A. M. (2008). Methods, criteria, techniques and genetic responses for rabbit selection: a review. In: Proc. 9th World Rabbit Congress, 10–13 June, 2008 (Verona, Italy), 1–22.
Lavara, R., Baselga, M., Marco-Jiménez, F., and Vicente, J. S. (2014). Long-term and transgenerational effects of cryopreservation on rabbit embryos. Theriogenology 81, 988–992. doi: 10.1016/j.theriogenology.2014.01.030
Lavara, R., Baselga, M., Marco-Jiménez, F., and Vicente, J. S. (2015). Embryo vitrification in rabbits: consequences for progeny growth. Theriogenology 84, 674–680. doi: 10.1016/j.theriogenology.2015.04.025
Lavara, R., Baselga, M., and Vicente, J. S. (2011). Does storage time in LN2 influence survival and pregnancy outcome of vitrified rabbit embryos? Theriogenology 76, 652–657. doi: 10.1016/j.theriogenology.2011.03.018
Leme, L. O., Carvalho, J. O., Franco, M. M., and Dode, M. A. N. (2020). Effect of sex on cryotolerance of bovine embryos produced in vitro. Theriogenology 141, 219–227. doi: 10.1016/j.theriogenology.2019.05.002
López-Damián, E. P., Jiménez-Medina, J. A., Alarcón, M. A., Lammoglia, M. A., Hernández, A., Galina, C. S., et al. (2020). Cryopreservation induces higher oxidative stress levels in Bos indicus embryos compared with Bos taurus. Theriogenology 143, 74–81. doi: 10.1016/j.theriogenology.2019.12.001
Marco-Jiménez, F., Baselga, M., and Vicente, J. S. (2018). Successful re-establishment of a rabbit population from embryos vitrified 15 years ago: the importance of biobanks in livestock conservation. PLoS ONE 13, e0199234. doi: 10.1371/journal.pone.0199234
Marco-Jiménez, F., García-Dominguez, X., Domínguez-Martínez, M., Viudes-de-Castro, M. P., Diretto, G., Peñaranda, D. S., et al. (2020). Effect of embryo vitrification on the steroid biosynthesis of liver tissue in rabbit offspring. Int. J. Mol. Sci. 21, 8642. doi: 10.3390/ijms21228642
Mocé, M. L., Blasco, A., and Santacreu, M. A. (2010). In vivo development of vitrified rabbit embryos: effects on prenatal survival and placental development. Theriogenology 73, 704–710. doi: 10.1016/j.theriogenology.2009.11.010
Naturil-Alfonso, C., Marco-Jiménez, F., Jiménez-Trigos, E., Saenz-de-Juano, M., Viudes-de-Castro, M., Lavara, R., et al. (2015). Role of embryonic and maternal genotype on prenatal survival and foetal growth in rabbit. Reprod. Dom. Anim. 50, 312–320. doi: 10.1111/rda.12493
Pascual, M., Pla, M., and Blasco, A. (2008). Effect of selection for growth rate on relative growth in rabbits. J. Anim. Sci. 86, 3409–3417. doi: 10.2527/jas.2008-0976
Peiró, R., Quirino, C., Blasco, A., and Santacreu, M. A. (2021). Correlated response on growth traits and their variabilities to selection for ovulation rate in rabbits using genetic trends and a cryopreserved control population. Animals 11, 2591. doi: 10.3390/ani11092591
Piles, M., and Blasco, A. (2003). Response to selection for growth rate in rabbits estimated by using a control cryopreserved population. World Rabbit Sci. 11, 53–62. doi: 10.4995/wrs.2003.497
Qin, N., Zhou, Z., Zhao, W., Zou, K., Shi, W., Yu, C., et al. (2021). Abnormal glucose metabolism in male mice offspring conceived by in vitro fertilization and frozen-thawed embryo transfer. Front. Cell Dev. Biol. 9, 637781. doi: 10.3389/fcell.2021.637781
Saenz-de-Juano, M. D., Marco-Jimenez, F., Schmaltz-Panneau, B., Jimenez-Trigos, E., Viudes-de-Castro, M. P., Peñaranda, D. S., et al. (2014). Vitrification alters rabbit fetal placenta at transcriptomic and proteomic level. Reproduction 147, 789–801. doi: 10.1530/REP-14-0019
Saenz-de-Juano, M. D., Vicente, J. S., Hollung, K., and Marco-Jiménez, F. (2015). Effect of embryo vitrification on rabbit fetal placenta proteome during pregnancy. PLoS ONE 10, e0125157doi: 10.1371/journal.pone.0125157
Spijkers, S., Lens, J. W., Schats, R., and Lambalk, C. B. (2017). Fresh and frozen-thawed embryo transfer compared to natural conception: differences in perinatal outcome. Gynecol. Obstetr. Investig. 82, 538–546. doi: 10.1159/000468935
Vicente, J., Llobat, M., Jiménez-Trigos, E., Lavara, R., and Marco-Jiménez, F. (2013). Effect of embryonic and maternal genotype on embryo and fetal survival in rabbit. Reprod. Domest. Anim. 48, 402–406. doi: 10.1111/rda.12087
Vicente, J. S., and García-Ximénez, F. (1993). Effect of recipient doe genotype on survival rate at birth of frozen rabbit embryos. Reprod. Nutr. Dev. 33, 229–234. doi: 10.1051/rnd:19930305
Vicente, J. S., and García-Ximenez, F. (1994). Osmotic and cryoprotective effects of a mixture of DMSO and ethylene glycol on rabbit morulae. Theriogenology 42,1205–1215. doi: 10.1016/0093-691x(94)90869-9
Vicente, J. S., Viudes-de-Castro, M. P., García, M., and Baselga, M. (2003). Effect of rabbit line on a program of cryopreserved embryos by vitrification. Reprod. Nutr. Dev. 43, 137–143. doi: 10.1051/rnd:2003011
Wang, Z. Y., Chen, S., Zhu, W. J., Shen, X. T., Li, Y. B., and Zheng, J. X. (2020). Comparison of the effects of vitrification and slow freezing on the growth and development of offspring using a mouse model. Clin. Exp. Obstetr. Gynecol. 47, 701–708. doi: 10.31083/j.ceog.2020.05.2079
Xia, X. R., Jiang, S. W., Zhang, Y., Hu, Y. F., Yi, H. G., Liu, J., et al. (2019). Serum levels of trace elements in children born after assisted reproductive technology. Clin. Chim. Acta 495, 664–669. doi: 10.1016/j.cca.2018.09.032
Keywords: embryo vitrification, fetal growth, litter size, growth line, rabbit
Citation: Daniel Juárez J, Marco-Jiménez F and Vicente JS (2022) Effects of Rederivation by Embryo Vitrification on Performance in a Rabbit Paternal Line. Front. Anim. Sci. 3:909446. doi: 10.3389/fanim.2022.909446
Received: 31 March 2022; Accepted: 28 April 2022;
Published: 14 June 2022.
Edited by:
Graham Cliff Lamb, Texas A&M University College Station, United StatesReviewed by:
Jamie Larson, Mississippi State University, United StatesJoao Henrique Moreira Viana, Brazilian Agricultural Research Corporation (EMBRAPA), Brazil
Copyright © 2022 Daniel Juárez, Marco-Jiménez and Vicente. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: José Salvador Vicente, jvicent@dca.upv.es
 Jorge Daniel Juárez1
Jorge Daniel Juárez1  Francisco Marco-Jiménez
Francisco Marco-Jiménez José Salvador Vicente
José Salvador Vicente