Clinical relevance of corrosion patterns attributed to inflammatory cell induced corrosion: A retrieval study
-
1
University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), United Kingdom
Introduction: Cobalt Chromium (CoCr) alloys are the most commonly used materials in orthopaedics. In vitro studies have shown that human osteoclasts can corrode Stainless Steel and Titanium forming resorption pits that lead to the production of metal ions responsible for inflammatory reactions [1],[2]. Moreover, traces of cellular activities on metal orthopaedic explants have recently been reported as Inflammatory Cell-Induced (ICI) corrosion being the result of the cells sealing on the metal surfaces and releasing Reactive Oxygen Species through Fenton-like reactions [3]. We aimed to better understand the clinical relevance of this phenomenon. Our objectives were 1) to determine the extent of ICI corrosion patterns observed in a large cohort of retrieved hip implants,2) to give a 3Dimensional characterisation to the corroded regions, 3) to correlate our findings with clinical data.
Materials: This was a retrieval study involving 100 failed hip implants received at our centre with metal-on-metal (MOM) bearings. We selected Durom modular heads [n=25], Durom resurfacing [n=25] as well as ASR modular heads [n=25] and ASR resurfacing [n=25] for inspection, so that we included both metallurgical features for Co-Cr alloys: wrought and cast.
Methods: Initial screening included macroscopic and microscopic analysis of both cups and heads with the aid of a stereomicroscope (Leica) and a digital microscope (Keyence). A scanning electron microscope (Jeol) was used to perform detailed analysis of specific areas of interest highlighted from the initial visual screening. Second and Backscattered Electron Imaging ranging from low (4-5) to high (10-15) kV were used. Energy dispersive X-ray spectrometry (EDS) assessed the elemental composition and organic biocorrosion products on the implant surface. A 3D optical microscope (Bruker) showed both depth of pits and deposition of material in µm.The significance of any univariable associations between the presence of ICI corrosion and key implant and clinical variables were assessed using simple linear regression models.
Results: 59 % of implants had evidence of corrosion patterns as described by Gilbert et al. Haziness, pits and streaks were visible on the: polished regions of the heads as well as on internal edges of cups; and machining marks of acetabular rims and head rims (Figure1). Higher magnification images showed crater-like spots surrounded by a frosted pattern of a ruffled topography. SEM images confirmed the presence of the features probably derived from different cellular mechanisms . Further confirmation of this was achieved by the optical profilometer. Scaled maps gave information about depth of pits that ranged from 0.5 to 3µm. There was a significant association between ICI corrosion and aseptic loosening for the ASR modular (r=-0.488, p=0.016) and the Durom modular (r=0.454, p=0.026) explants. EDS analysis revealed the presence of iron in correspondence of corroded regions, the element was only detectable in proximity of the corrosion patterns.
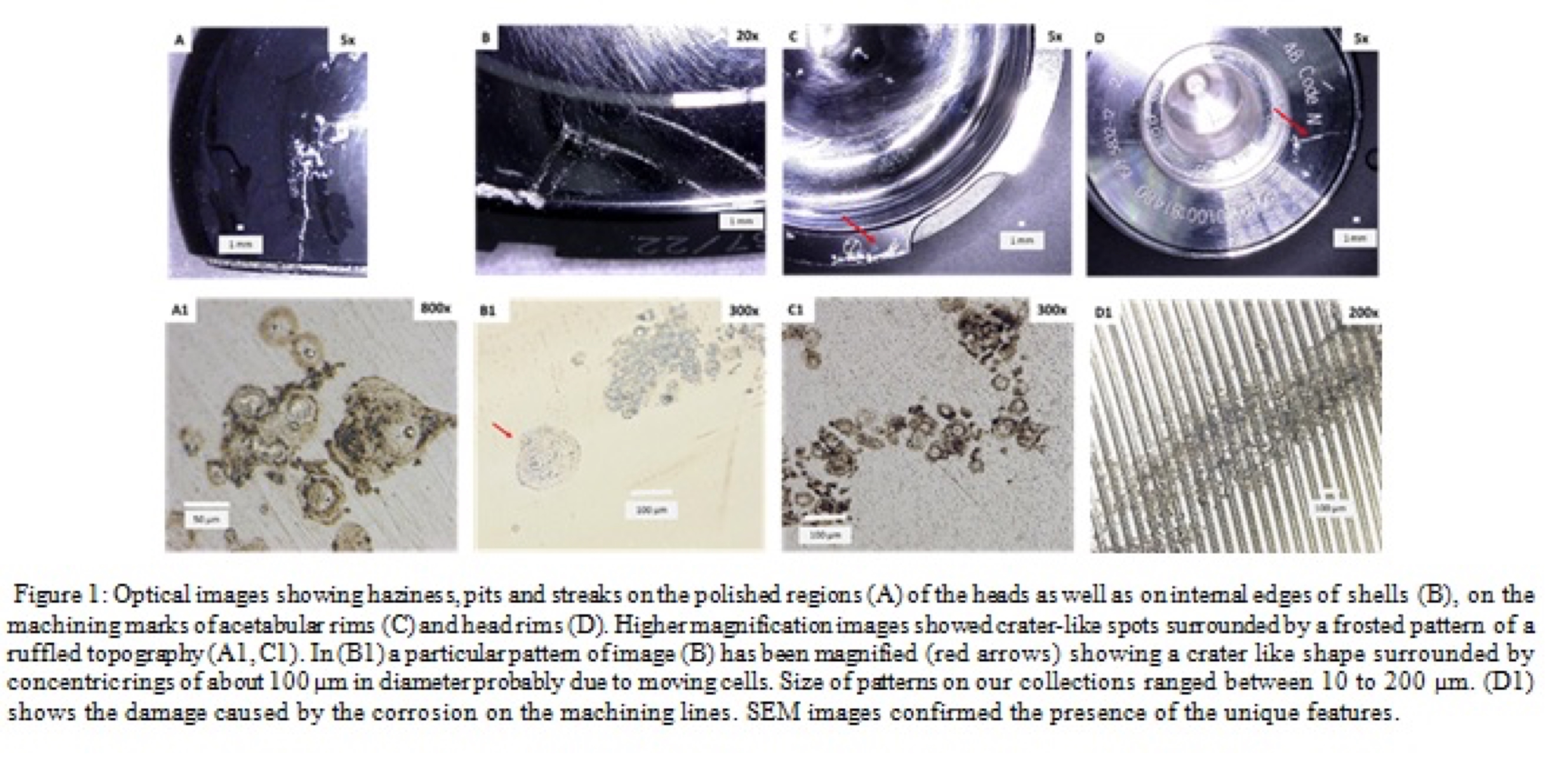
Discussion: Corrosion patterns potentially attributable to cell induced corrosion were found on 59% of retrieved hips. There was no difference between cast and wrought microstructures but there was a correlation between implants revised due to aseptic loosening and presence of cellular corrosion. Iron is also in accordance with the presence of phagocytic cells on the metal surfaces due to Fenton-like reaction taking place. We recommend further work to determine the role and type of cells in the corrosion damage patterns observed. This may help understand which patient factors are involved in hip implant failure: and whether some patients more prone to failure of CoCr implants?
References:
[1] Cadosch, D., et al., Bio‐corrosion of stainless steel by osteoclasts—in vitro evidence. Journal of Orthopaedic Research, 2009. 27(7): p. 841-846.
[2] Caicedo, M.S., et al., Soluble and particulate Co‐Cr‐Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: A novel mechanism for implant debris reactivity. Journal of Orthopaedic Research, 2009. 27(7): p. 847-854.
[3] Gilbert, J.L., et al., Direct in vivo inflammatory cell‐induced corrosion of CoCrMo alloy orthopedic implant surfaces. Journal of Biomedical Materials Research Part A, 2014.
Keywords:
joint replacement,
Implant,
Biocompatibility,
Clinical relevance
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Clinical findings for Metal-on-Metal implants.
Citation:
Di Laura
A,
Hothi
H,
Meswania
J,
Whittaker
R,
De Villiers
D,
Blunn
G,
Skinner
J and
Hart
A
(2016). Clinical relevance of corrosion patterns attributed to inflammatory cell induced corrosion: A retrieval study.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01990
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Anna Di Laura, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, Email1
Dr. Harry Hothi, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, h.hothi@ucl.ac.uk
Dr. Jay Meswania, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, j.meswania@ucl.ac.uk
Dr. Robert Whittaker, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, r.whittaker@ucl.ac.uk
Dr. Danielle De Villiers, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, d.de-villiers@ucl.ac.uk
Dr. John Skinner, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, john.skinner@ucl.ac.uk
Dr. Alister Hart, University College London, Institute of Orthopaedics and Musculoskeletal Science (IOMS), London, United Kingdom, a.hart@ucl.ac.uk